Abstract
Erythrina velutina is a species of arboreal leguminous that occurs spontaneously in the northeastern states of Brazil. Leguminous seeds represent an abundant source of peptidase inhibitors, which play an important role in controlling peptidases involved in essential biological processes. The aim of this study was to purify and characterize a novel Kunitz-type peptidase inhibitor from Erythrina velutina seeds and evaluate its anti-proliferative effects against cancer cell lines. The Kunitz-type chymotrypsin inhibitor was purified from Erythrina velutina seeds (EvCI) by ammonium sulphate fractionation, trypsin– and chymotrypsin–sepharose affinity chromatographies and Resource Q anion-exchange column. The purified EvCI has a molecular mass of 18 kDa with homology to a Kunitz-type inhibitor. Inhibition assays revealed that EvCI is a competitive inhibitor of chymotrypsin (with Ki of 4 × 10–8 M), with weak inhibitory activity against human elastase and without inhibition against trypsin, elastase, bromelain or papain. In addition, the inhibitory activity of EvCI was stable over a wide range of pH and temperature. Disulfide bridges are involved in stabilization of the reactive site in EvCI, since the reduction of disulfide bridges with DTT 100 mM abolished ~ 50% of its inhibitory activity. The inhibitor exhibited selective anti-proliferative properties against HeLa cells. The incubation of EvCI with HeLa cells triggered arrest in the cell cycle, suggesting that apoptosis is the mechanism of death induced by the inhibitor. EvCI constitutes an interesting anti-carcinogenic candidate for conventional cervical cancer treatments employed currently. The EvCI cytostatic effect on Hela cells indicates a promised compound to be used as anti-carcinogenic complement for conventional cervical treatments employed currently.
Keywords: EvCI, HeLa cell, Cervical cancer, Apoptosis, Chymotrypsin inhibitor, Leguminous seeds
Introduction
Peptidases catalyze indispensable reactions in all living organisms, contributing to cellular homeostasis in events, such as digestion, apoptosis, blood coagulation and inflammatory responses (dos Santos et al. 2012). The uncontrolled activity of peptidases contributes to the development of several pathologies, among them cancer (Martin and List 2019). The control of peptidase activity is mediated by peptidase inhibitors (PIs). Several families of PIs are known, and they display specificity for enzymes from different families, such as serine, cysteine, aspartic and metallopeptidases (Bacha et al. 2019). Among PIs from serine peptidase, the Kunitz inhibitors comprise the most investigated group, composed of proteins with different molecular weight, number of polypeptide chains, content of disulfide bridges, number of reactive sites and specificity of inhibition (Richardson 1991; dos Santos et al. 2012).
Plant PIs have been studied for decades. Several biological properties have been proposed for these PIs including, particularly, their insecticidal properties (Migliore et al. 2007). Biomedical research has made great progress in shedding light on the importance of PIs in cardiovascular diseases (Pathak et al. 2013), osteoporosis (Delaisse et al. 1987), Alzheimer’s disease (Nguyen et al. 2014), AIDS (Naggie and Hicks 2010), and cancer (Bacha et al. 2019; Martin and List 2019). Among the plant families, Leguminosae is widely investigated and the genus Erythrina is known as a source of several PIs. Previously, PIs have been purified and characterized from E. variegata (Kouzuma et al. 1992), E. latíssima (Joubert et al. 1981), E. caffra (Joubert 1982a) and E. acanthocarpa (Joubert 1982b). Erythrina velutina Willd (Leguminosae: Papilionoideae) popularly known as “mulungu”, is a tree native to Brazil (Lorenzi 2014). Previously, we described the purification and characterization of a trypsin inhibitor of E. velutina seeds with anti-inflammatory and anticoagulant activities (Machado et al. 2013). In this study, we reported the purification and characterization of a novel Kunitz-type chymotrypsin inhibitor, isolated from E. velutina seeds, named EvCI. Furthermore, we describe the anti-proliferative properties of EvCI against HeLa cells, highlighting the relevance of basic research for carcinogenesis and cancer promotion studies.
Materials and methods
Chemicals
Papain (EC 3.4.22.2), Bromelain (EC 3.4.22.33), Bovine α-chymotrypsin (EC 3.4.21.1), Bovine Trypsin (EC 3.4.22.33), porcine elastase (EC 3.4.21.36), and human leukocyte elastase (EC 3.4.21.37); Substrates: BApNA, BApNA SAAVpNA, and Azocasein were purchased from Sigma Chemical Co. (St. Louis, MO); dimethyl sulfoxide (DMSO), DL-dithiothreitol (DTT), and iodoacetamide (IAA) were acquired from Sigma-Aldrich (Sao Paulo, Brazil); and SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis were purchased from Sigma (St. Louis, MO, USA).
Plant material
Erythrina velutina Willd seeds were donated by the FLONA/ICMBio (Floresta Nacional de Nísia Floresta, Instituto Chico Mendes de Conservação da Biodiversidade) seed bank, RN, Brazil.
Purification of E. velutina chymotrypsin inhibitor (EvCI)
Erythrina velutina seeds were finely ground and homogenized at 1:10 (w/v) with 0.05 M Tris–HCl buffer, pH 7.5, for 3 h at room temperature. Following a centrifugation at 8000×g for 30 min at 4 °C, the supernatant was fractionated with ammonium sulfate in 0–30% (F1), 30–60% (F2), and 60–90% (F3). The fractions were dialyzed against distilled water, lyophilized and submitted to enzymatic assays. F2 showed the highest inhibitory activity against chymotrypsin and was applied onto a trypsin–sepharose column (4.0 × 2.5 cm) equilibrated with 50 mM Tris–HCl buffer, pH 7.5. Fractions without affinity for trypsin were collected during the washing step, pooled and applied onto a chymotrypsin–sepharose column (10 × 1.5 cm), equilibrated with 50 mM Tris–HCl buffer, pH 7.5 at flow of 2 mL min−1. Proteins adsorbed to column were eluted with 5 mM HCl and applied onto a Resource Q anion-exchange chromatography column, coupled in a AKTA purifier system equilibrated with buffer A (20 mM Tris–HCl, pH 8.0), and the elution step was carried out with a linear gradient of buffer B (20 mM Tris–HCl, pH 8.0 containing 1 M NaCl) at flow of 2 mL min−1. The absorbance was monitored at 280 nm. The peak eluted from Resource Q anion-exchange column was named EvCI—E. velutina chymotrypsin inhibitor.
Inhibitory activity assays
The methodology with modification for inhibitory activity against chymotrypsin was performed using azocasein as substrate (Kunitz 1947). Ten microliters of bovine chymotrypsin (0.3 mg mL−1) was pre-incubated with 50 mM Tris–HCl, pH 7.5 containing 20 mM CaCl2 and EvCI for 15 min at 37 °C. Reaction was initiated by addition of 200 μL of 1% azocasein. After 30 min at 37 °C, the reaction was stopped by the addition of 300 µL of 20% TCA, centrifuged at 12,000×g for 10 min and the supernatant was alkalinized with 2 M NaOH 1:1 (v:v). The absorbance was determined at 540 nm. One unit of inhibitory activity was defined as the amount of inhibitor that decreased absorbance by 0.01 at 540 nm. Assays were performed in triplicate and three independent experiments were conducted.
The inhibitory activity against trypsin was determined using BApNA as substrate (Erlanger et al. 1961). Ten microliters of trypsin (0.3 mg mL−1 in 50 mM Tris–HCl, pH 7.5 buffer containing 20 mM CaCl2) solution was pre-incubated for 10 min at 37 °C with 100 μL of EvCI and 390 μL of 50 mM Tris–HCl, pH 7.5. The reaction started with the addition of 250 μL of 1.25 mM BApNA, prepared in 1% DMSO and 50 mM Tris–HCl buffer, pH 7.5. After 15 min at 37 °C, the reaction was stopped by the addition of 60 μL of 30% acetic acid solution. The absorbance was determined at 410 nm.
The inhibition of human neutrophil elastase (HNE) was determined using SAAVpNA as substrate, following the methodology described by Johansson and collaborators (Johansson et al. 2002), with some modifications. A volume of 20 μL HNE (0.5 µg mL−1), 100 µL of EvCI and 390 µL of 0.1 M PBS buffer, pH 7.4 was pre-incubated for 30 min at 37 °C. Then the reaction was started by the addition of 5 μL of 0.15 M SAAVpNA solution. After 60 min at 37 °C, the reaction was stopped with 250 μL of a 2% citric acid. The absorbance was determined at 410 nm.
The inhibition of elastase was analyzed using 1% azocasein as substrate (Kunitz 1947). A volume of 20 μL of pig pancreas elastase (0.1 mg mL−1 in 0.05 M Tris–HCl buffer, pH 7.5) was pre-incubated with 380 μL of 0.05 M Tris–HCl buffer, pH 7.5 and 100 µL of EvCI for 15 min at 37 °C. Then the reaction was started by adding 200 µL of 1% azocasein. After 30 min, the reaction was stopped with the addition of 300 μL of 20% TCA. The reaction was centrifuged at 12,000×g for 10 min and the supernatant was alkalinized with 2 M NaOH 1:1 (v:v). The absorbance was determined at 540 nm.
The inhibition of bromelain was carried out using 1% azocasein as substrate (Kunitz 1947). Briefly, 30 µL of bromelain solution (1 mg mL−1 dissolved in 0.3 M sodium acetate buffer, pH 5.5) was pre-incubated with 290 µL activation buffer (0.3 M sodium acetate buffer, pH 5.5, containing 0.02 M EDTA and 0.03 M DTT) and 20 µL of EvCI for 20 min at 45 °C. After this period, 500 µL of 1% azocasein was added. After 30 min incubation, the reaction was stopped by adding 150 µL of 20% TCA solution. The reaction was centrifuged at 12,000×g for 10 min, and the supernatant was alkalinized with 2 M NaOH 1:1 (v:v). The absorbance was determined at 540 nm.
The inhibition of papain was determined using BANA as substrate (Zhao et al. 1996). Ten microliters of papain (0.1 mg mL−1 in 0.025 M sodium phosphate buffer, pH 6.0) solution was pre-incubated for 10 min at 37 °C with 20 µL of activation solution (0.02 M EDTA and 0.03 M DTT, pH 6.0) 20 µL of EvCI, and 250 µL of 0.25 M sodium phosphate buffer, pH 6.0. The reaction was started by addition of 100 µL of 0.001 M BANA solution, prepared in 1% DMSO and 0.025 M sodium phosphate buffer, pH 6.0. After 20 min at 37 °C, the reaction was stopped by adding 250 µL of 2% HCl in ethanol. The color product was developed by the addition of 250 µL of 0.06% p-di-methyl-amino-cinnamaldehyde in ethanol and measured by absorbance at 540 nm.
Protein determination and polyacrylamide gel electrophoresis
The protein content was determined according to Bradford (Bradford 1976), using bovine serum albumin as a standard. The sodium dodecyl sulphate polyacrylamide gel electrophoresis (12% SDS-PAGE) was conducted as described by Laemmli (Laemmli 1970). Following electrophoresis, the gels were stained with Coomassie Blue R-250. Protein molecular weight markers, alcohol dehydrogenase (150 kDa), β-galactosidase (116 kDa), bovine serum albumin (BSA) (66 kDa), ovalbumin (45 kDa), lactate dehydrogenase (35 kDa), restriction endonuclease Bsp981 (25 kDa) and β-lactoalbumin (18.4 kDa) were purchased from Fermentas Inc. (Burlington, CA).
Matrix-assisted laser desorption-ionization time-of-flight mass spectrometric analysis (MALDI-ToF MS)
The Coomassie Blue R-250-stained protein spots were excised from gels, washed with 25% (v:v) methanol and 7% (v:v) acetic acid for 12 h at 20 °C, and de-stained with 50 mM NH4HCO3 in 50% (v:v) methanol for 1 h at 40 °C. Protein was reduced with 10 mM DTT in 100 mM NH4HCO3 for 1 h at 60 °C and incubated with 40 mM iodoacetamide in 100 mM NH4HCO3 for 30 min. The gel pieces were minced and allowed to dry and then rehydrated in 100 mM NH4HCO3 with 1 pmol of trypsin at 37 °C overnight.
The digested peptides were extracted from the gel slices with 0.1% tri-fluoro-acetic acid (TFA) in 50% (v:v) acetonitrile:water three times. The peptide solution, thus obtained, was dried and reconstituted with 3 mL of 0.1% TFA in 5% acetonitrile/water, and then desalted by C18 ZipTip pipette tips (Millipore, Bedford, MA, USA). MALDI-ToF MS was performed using a Voyager time-of-flight mass spectrometer (Applied Biosystems, Framingham, MA, USA). The peptide solution was mixed with the matrix saturated with α-cyano-4-hydroxycinnamic acid and air-dried. Calibrations were carried out using a standard peptide mixture. The mass spectra were subjected to sequence database search with Mascot software (Matrix Science Ltd, London, UK).
Stability of EvCI
The thermal stability of EvCI (1 µg mL−1) was assayed at different temperatures (Gomes et al. 2005). Samples of EvCI (1 mg mL−1) were incubated at temperatures of 37, 40, 60, 70, 90 and 100 °C, for 30 min. Following incubation of 10 min at 4 °C, the inhibitory assays against chymotrypsin were performed. The stability of EvCI at different pH values was also investigated. Samples of EvCI (1 mg mL−1) were prepared in buffers 100 mM glycine–HCl (pH 2–3), 100 mM sodium phosphate (pH 6–8) and 100 mM glycine–NaOH (pH 11–12). After incubation in each buffer for 1 h at 37 °C, the inhibitory activity against chymotrypsin was analyzed using 1% azocasein as substrate. The assays were carried out in triplicate and expressed as mean ± SD.
Kinetic studies of EvCI
Inhibition curve of chymotrypsin with different inhibitor concentrations was carried out. The stoichiometric ratio between EvCI and chymotrypsin was determined by the titration curve plotting the residual enzyme activity against the inhibitor–enzyme molar ratio. Increasing concentrations of EvCI (2.6 and 5.3 × 10–8 M; 1, 2.1, 2.6, 3.7, 4.8, and 6.4 × 10–7 M) were incubated with 8.0 × 10–8 M chymotrypsin. The mechanism of inhibition of EvCI on chymotrypsin was determined using two different concentrations of azocasein (0.025 and 0.085 mM) and increasing concentrations of EvCI (1.1, 2.2, 3.3, and 4.4 × 10–7 M). The kinetic parameters (Vmax and Km) were established to determine the value of dissociation constant (Ki).
Cancer cell lines
The cancer cells HeLa (human cervical cancer), MDA (human breast cancer), K562 (chronic myeloid leukemia) and PC3 (human prostate carcinoma) were grown in Eagle’s minimal essential medium (DMEM) with Earle’s salts or RPMI-1640 medium (Nutricell, Campinas, SP, Brazil), supplemented with 10% fetal bovine serum (FBS, Sigma), penicillin (1000 U mL−1) and streptomycin (250 mg mL−1). The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Treatment of cells with EvCI
The PC3, HeLa and MDA cells were seeded (103 cells mL−1) in 96-well microplates until they reached a semi-confluent density. Different concentrations of EvCI (0.0005–200 µg mL−1) were incubated with cells and treatment carried out for 72 h. The K562 cells were incubated (100,000 cells mL−1) with different concentrations of EvCI (0.0005–200 µg mL−1) for 48 h. The cell viability was determined using MTT. The analysis of cell cycle and cell death were evaluated by annexin V-FITC and propidium iodide (PI) labelling by flow cytometry using a FACSCalibur flow cytometer (BD Biosciences).
MTT reduction assays
Following the treatment with EvCI at respective incubation times, the cells were incubated in 200 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg mL−1 in culture medium) for 3 h at 37 °C. The formazan was dissolved using isopropanol and the absorbance was measured at 540 nm in a microplate reader (Bio-Rad, Philadelphia, PA, USA). The results of cell viability were used to obtain the half-maximum inhibition concentration (IC50).
Analysis by flow cytometry
Following the treatment with EvCI for 72 h, HeLa cells were washed with cold PBS and re-suspended in binding buffer (0.01 M HEPES, pH 7.4, 0.14 M NaCl and 2.5 mM CaCl2) at a concentration of 1 × 106 cells mL−1. The cells were concentrated in 5 mL tubes and mixed with 5 µL of Annexin V-FITC and 5 µg mL−1 PI. Following an incubation of 20 min at room temperature, 300 µL of binding buffer was added. In total, 10,000 events were collected in a FACSCalibur Flow Cytometer (BD Biosciences) and analyzed in CellQuest software. Control cells were treated with medium only.
To investigate the effects of EvCI on cell cycle, HeLa cells were synchronized at G0 by 24 h of incubation in serum-free RPMI 1640 medium, and then treated with 50 µg mL−1 of EvCI for 72 h. Afterwards, cells were harvested, washed with cold PBS, fixed and permeabilized. Then, cells were treated with 4 µg mL−1 RNase type I for 1 h at 37 °C and re-suspended in PBS. Cells were stained with 25 µg mL−1 PI, and 10,000 events were obtained in FACSCalibur Flow Cytometer (BD Biosciences) and analyzed in Cell Quest software. The DNA content was evaluated using a FL2H detector in a logarithmic scale.
Statistical analysis
The data were expressed as means ± SD, except when indicated in another way. Differences among the treatments were analyzed by ANOVA, Tukey’s test and Kruskal–Wallis test with Dunn’s post test. Significant differences occurred when p < 0.05.
Results and discussion
Purification of EvCI
PIs are potential candidates for biotechnological and medical applications (Srikanth and Chen 2016). PIs from the Erythrina genus possess reactive sites with specificity for trypsin, chymotrypsin, or both (Joubert et al. 1981; Joubert 1982a, b; Kouzuma et al. 1992). To purify EvCI, an initial ammonium sulphate precipitation was used. Among the three fractions, the F2 fraction showed the highest inhibitory activity against chymotrypsin. To avoid collecting fractions with activity against trypsin, we used a trypsin–sepharose column as the capture step. Thus, the fractions with activity against trypsin were adsorbed to resin while the other fractions were pooled, depleted of trypsin inhibitors. Following our purification strategy, a chymotrypsin–sepharose column was used to capture EvCI (Fig. 1A). This procedure allowed us to separate EvCI from most of the contaminants. The fraction eluted from the chymotrypsin–sepharose column showed strong inhibitory activity against chymotrypsin. Finally, a Resource Q column was used in the polishing step to obtain EvCI (Fig. 1B). A minor contaminant was separated from the EvCI peak. At the end of process, we obtained a purification of 104.3-fold and a yield of 1.1% (Tables 1, 2).
Fig. 1.
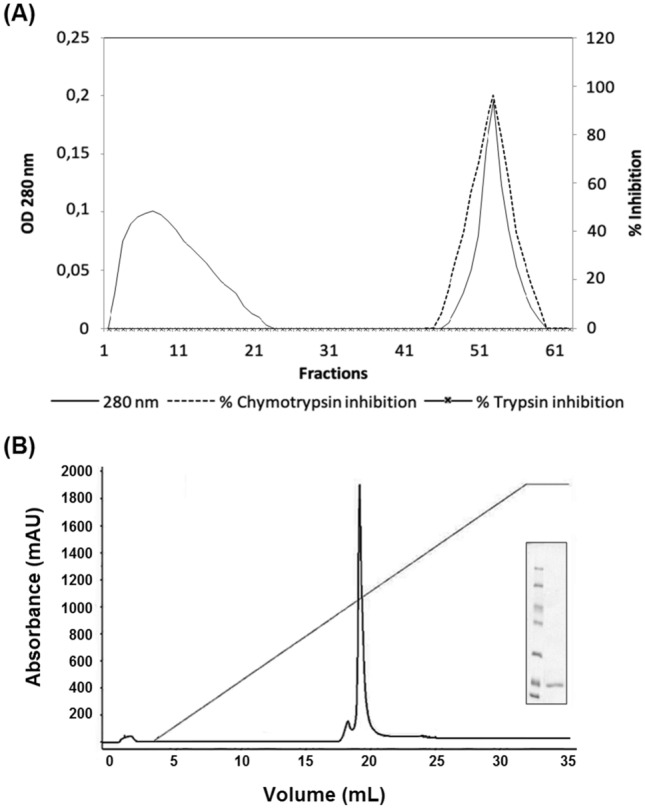
Purification of chymotrypsin inhibitor from E. velutina seeds (EvCI). A Chromatogram of F2 fraction applied onto chymotrypsin-Sepharose column (10 × 1.5 cm), equilibrated with 50 mM Tris–HCl buffer, pH 7.5. Fractions were eluted at constant flow of 2 mL min−1. The adsorbed proteins were eluted with 5 mM HCl. Fractions were monitored at 280 nm and assayed against trypsin and chymotrypsin. B Chromatogram of fractions from chymotrypsin-sepharose column applied onto Resource Q column (AKTA purifier system), equilibrated with 20 mM Tris–HCl buffer, pH 8.0 and eluted with a NaCl linear gradient (1 M final) at 2 mL min−1. Fractions were monitored at 280 nm. (Inset) SDS-PAGE (12%) of EvCI from Resource Q. Proteins were stained with Coomassie blue. Molecular weight markers: β-galactosidase (116 kDa); bovine serum albumin (66 kDa); ovalbumin (45 kDa); lactate dehydrogenase (35 kDa); restriction endonuclease Bsp981 (25 kDa); β-lactoglobulin (18.4 kDa) and lysozyme (14.4 kDa)
Table 1.
Purification steps of EvCI
| Purification steps | Volume (mL) | Total protein (mg) | Total inhibitor activity (U) | Specific activity (U mg−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Crude extract | 160.0 | 1862.40 | 83,520 | 44.9 | 1.0 | 100.0 |
| F2 (30–60%) | 51.0 | 407.18 | 25,806 | 63.4 | 1.4 | 30.9 |
| Trypsin-sepharose (not retained) | 47.0 | 16.60 | 21,949 | 1322.3 | 29.5 | 26.3 |
| Chymotrypsin-sepharose | 9.0 | 1.26 | 4185 | 3321.0 | 74.0 | 5.0 |
| Resource Q | 2.0 | 0.20 | 936 | 4680.0 | 104.3 | 1.1 |
One chymotrypsin inhibition unit (1 U) was defined as the inhibitor amount that decreased the absorbance at 540 nm by 0.01 OD in the chymotrypsin assay conditions
Table 2.
Inhibitory activity of EvCI towards peptidases
| Enzyme | Inhibition (%)a |
|---|---|
| Chymotrypsin | 89.16 ± 6.31 |
| Neutrophil elastase | 35.91 ± 3.91 |
| Trypsin | 6.30 ± 3.92 |
| Porcine elastase | ND |
| Papain | 2.27 ± 1.21 |
| Bromelain | ND |
ND not detectable
aValues are means ± standard error
The combination of bio-affinity and ion-exchange chromatographies is widely used in the purification process of PIs, such as the chymotrypsin and trypsin inhibitors purified from Caesalpinia bonduc (Bhattacharyya et al. 2007), Archidendron ellipticum (Bhattacharyya et al. 2006) and Entada scandens (Lingaraju and Gowda 2008). The purification of EvCI was equivalent to or smaller than those found for other similar inhibitors reported in the literature, such as the Kunitz-type inhibitor from Pithecellobium dumosum seeds, purified 139.2-fold (Rufino et al. 2013), the trypsin inhibitors from Crotalaria pallida seeds (Gomes et al. 2005) and A. ellipticum (Bhattacharyya et al. 2006), purified 180- and 124-fold, respectively. The recovery of 1.1% is in accordance with those found for other Kunitz inhibitors (Gomes et al. 2005; Bhattacharyya et al. 2007; Cruz et al. 2013; Machado et al. 2013).
Characterization of EvCI
The analysis of EvCI by SDS-PAGE was described by (Bradford 1976) and the presence of EvCI was revealed as a single protein band with an apparent molecular weight (MW) of about 18 kDa (Fig. 1B, inset). The identification of tryptic digestion of two EvCI peptides showed 100% identity with the sequence of the chymotrypsin inhibitor from E. variegata, ECI, which possesses 179 amino acids (NCBI identity access gi:2129823). The coverage of the two sequenced peptides corresponds to 13.96% of the whole ECI (Fig. 2). The molecular mass of EvCI is close to already purified chymotrypsin inhibitors from Lens culinaris (16 kDa) (Cheung and Ng 2007), Schizolobium parahyba (20 kDa) (Teles et al. 2004) and Psophocarpus tetragonolobus (21 kDa) (Kortt 1980).
Fig. 2.
Sequencing of EvCI peptide fingerprint analysis. Amino-acid sequence of chymotrypsin inhibitor ECI from E. variegata seeds (NCBI identity access gi 2129823) was used as template. Doubly underlined segments correspond to the sequenced EvCI peptides
Inhibitory activity of EvCI
To determine the specificity of EvCI, inhibition assays were assayed against serine and cysteine peptidases. EvCI was highly active against chymotrypsin (89.16% ± 6.31). A moderate inhibition against neutrophil elastase was noticed (35.91% ± 7.91). Other serine (trypsin and pancreatic elastase) or cysteine (papain) peptidases were not inhibited by EvCI. PIs specific against chymotrypsin without activity against trypsin have been purified from other plant species, such as Psophocarpus tetragonolobus (Kortt 1980) and Schizolobium parahyba (Souza et al. 1995). PIs from Caesalpinia bonduc (Bhattacharyya et al. 2007), Archidendron ellipticum (Bhattacharyya et al. 2006) and Piptadenia moniliformis (Cruz et al. 2013) have the ability to inhibit both trypsin and chymotrypsin. Despite selectivity, EvCI did not inhibit cysteine peptidase (papain), so it does not have the characteristic of bi-functionality, present in some PIs, such as PmTKI (Cruz et al. 2013) and ITC (Gomes et al. 2005). Therefore, EvCI is a chymotrypsin and neutrophil elastase inhibitor, indicating that it may play a potential role in healing and/or prevention of various diseases (Champ 2002; Duranti 2006) due to its specificity.
Stability of EvCI
The effects of temperature on EvCI activity were investigated until 100 °C. The inhibitor was stable at all temperatures assayed (Fig. 3A). The pH range also did not affect the chymotrypsin inhibitory activity of EvCI (Fig. 3B). EvCI was also pre-incubated with increasing concentrations of DTT (1, 10 and 100 mM) for different times (15, 30, 60 and 120 min). At the highest concentration of DTT (100 mM) and time (120 min), a reduction in inhibitory activity of 50% was noticed (data not shown). As regards the effect of DTT on EvCI, a similar result was found for the inhibitor of Inga laurina, ILTI, which lost 56% of the inhibitory activity in the same conditions (Macedo et al. 2007). However, the stability in DTT observed for EvCI and ILTI is higher than other PIs, such as Entada scandens (Lingaraju and Gowda 2008), C. pallida (Gomes et al. 2005) and P. moniliformis (Cruz et al. 2013). Typically, the stability of PIs in denaturing agents is related to presence, localization and the number of intramolecular disulfide bonds. Considering the occurrence of disulfide bonds in EvCI, a possible explanation associated with its stability in DTT would be the localization of the disulfide bonds (Garcia et al. 2004; Macedo et al. 2007). The trypsin inhibitor from E. caffra, ETI, is able to maintain its inhibitory activity unaffected under reduction conditions, because its disulfide bridge is not involved in stabilization of the reactive site (Lehle et al. 1994), which is maintained by hydrogen bonds. The Kunitz trypsin inhibitor from Catanduva (Piptadenia moniliformis) seeds (PmTKI) was not affected by the reducing agent and lost only 30% of activity after 2 h, demonstrating that the stability of this inhibitor does not depend exclusively on the disulfide bonds (Cruz et al. 2013). The trypsin inhibitor from Entada acaciifolia (EATI) is another example of the importance of the disulfide bridge for further functions beyond the stabilization of the reactive site (de Oliveira et al. 2014). When the disulfide bridges of EATI were reduced, the inhibitor lost part of its inhibitory activity with variations in temperature and pH, suggesting that disulfide bridges could contribute with the overall folding of PIs.
Fig. 3.

Stability of EvCI in a range of (A) temperature and (B) pH
Kinetics of inhibition
The concentration of EvCI that inhibits 50% of the chymotrypsin activity (IC50) was 1.3 × 10–7 M, according to the linear regression of data from the dose–response curve (Fig. 4A). Another important point determined by titration is the maximum percentage of inhibition of chymotrypsin achieved by EvCI, which was around 89%. From the titration curve with increasing concentrations of EvCI and a fixed concentration of chymotrypsin (Fig. 4B), the stoichiometric ratio of 2:1 was established between the inhibitor and the enzyme.
Fig. 4.

Kinetic analysis of EvCI with chymotrypsin. A Inhibition curve of chymotrypsin by EvCI. Increasing concentrations of EvCI (0.027, 0.053, 0.27, 0.58 and 0.9 µM) were incubated with fixed concentration of chymotrypsin (0.08 µM) to calculate the IC50. B Chymotrypsin activity under different molar relationships EvCI-enzyme. C EvCI Dixon’s plot. Increasing concentrations (0.11, 0.22, 0.33 and 0.44 µM) of EvCI were pre-incubated with chymotrypsin and residual enzyme activities were determined with two different concentrations of azocasein (0.025 and 0.085 mM)
To determine the inhibition mechanism of EvCI against chymotrypsin, the inhibition kinetics data were analyzed by the Dixon plot (Fig. 4C). The Dixon plot revealed that lines corresponding to the concentrations of azocasein converge to a common point above the x-axis. The analysis showed a competitive inhibition mechanism of EvCI for chymotrypsin with a Ki value of 4 × 10–8 M. Kinetic assays demonstrated that EvCI is a competitive inhibitor for chymotrypsin. Similar results were found for inhibitors Archidrendon ellipticum (Bhattacharyya et al. 2006) and Derris foliata Lour. (Bhattacharyya and Babu 2009). The stoichiometry 1:1 inhibitor:enzyme was also reported for Schizolobium parayba (Souza et al. 1995) and Derris trifoliate Lour PIs (Bhattacharyya and Babu 2009), but was different from Psophocarpus tetragonolobus, which forms complexes in a ratio of 1:2 inhibitor:chymotrypsin (Kortt 1980).
The value of dissociation constant (Ki) calculated for EvCI (4 × 10–8 M) is in accordance with other inhibitors, such as Derris trifoliata Lour (1.25 × 10–10 M) (Bhattacharyya and Babu 2009), Plathymenia foliosa (1.4 × 10–6 M) (da Silveira et al. 2008) and Schizolobium parahyba (5.85 × 10–8 M) (Souza et al. 1995).
Cell-based EvCI tests
The viability of HeLa, MDA, K562, HepG2 and PC3 cells was analyzed after an exposure period of 72 h with increasing concentrations of EvCI (0.0005–200 µg mL−1). A dose-dependent reduction of HeLa viability was noticed (Fig. 5A). The reduction of viability reached about 60% with EvCI at 50 µg mL−1. No cytotoxic effect was observed on the other cells treated with EvCI (Fig. 5A). Several plant-purified compounds are also being evaluated for their biochemical and therapeutic applications. Thus, the cytotoxic potential of EvCI was analyzed on different tumor cell lines: HeLa, MDA, PC3 and K562. EvCI inhibited HeLa cell proliferation with an IC50 of 50 µg mL−1; however, it showed no cytotoxic effect against the other ones. EvCI showed a specific antitumor activity, which is similar to other PIs that have been analyzed (Lam and Ng 2010; Chan et al. 2013). Given the cytotoxic effect of EvCI in HeLa cells, we investigated the mechanism of death triggered by EvCI using flow cytometry. The results of flow cytometry assay using annexin V-FITC/PI double staining to distinguish living cells (An−/PI−), dead cells (An−/PI+), early apoptotic cells (An+/PI−), and late apoptotic/dead cells (An+/PI+) showed an increase in percentage of cells undergoing apoptosis regardless of types, both apoptosis early (An+PI−) and late apoptosis (An+PI+) when submitted to the highest dose of 50 µg mL−1 of EvCI for 72 h and compared to the experimental control (Fig. 5B).
Fig. 5.

Effects of EvCI on viability of cancer cell lines. A The cells were treated with EvCI (0.0005–200 µg mL−1) for 72 h. B Determination of cell death mechanism using HeLa cells treated with EvCI. HeLa cells (1 × 106 UFC) were exposed to 0.0005 and 50 µg mL−1 of EvCI for 72 h and analyzed by flow cytometry using annexin V-FITC/PI. C Effects of EvCI on HeLa cell cycle. The cells were treated with 50 µg mL−1 of EvCI for 72 h and analyzed by flow cytometer
Changes in the distribution of HeLa cells were checked at different stages of the cell cycle. Treatment of HeLa cells with 50 µg mL−1 of EvCI promoted reduction of the cell population (p < 0.05) in G0/G1 after 72 h, accompanied by a small increase in the S/G2/M population (p < 0.05) (Fig. 5C). No significant changes were observed in the sub-G1phase after treatment, confirming the results obtained on induction of cell death by flow cytometry after double-staining with Annexin V-FITC/PI. Furthermore, exposure of cells to a concentration four times higher than IC50 used in the MTT assay (200 µg mL−1) caused no significant reduction in cell proliferation when compared to treatment with 50 µg mL−1 for the same period of exposure. Taken together, these results indicate that the primary effect of EvCI is induction of arrest in the cell cycle and, therefore, a cytostatic effect specific for HeLa cells.
Tumor cells exhibit various alterations in cell cycle regulation. Changes in signal transduction pathways are required for the establishment of all tumor types (Evan and Vousden 2001). The control of cell proliferation is considered an effective strategy to prevent or delay tumor growth (Molinari 2000). In this study, we observed a cytostatic effect of EvCI on HeLa cells, indicating that this inhibitor could be an alternative to or complement for conventional treatments, such as surgery, radiotherapy and chemotherapy, which have moderate efficiency and side effects. EvCI reduced cell viability and proliferation and induced arrest in the cell cycle of HeLa tumor cells. Flow cytometry analysis showed a significant reduction of cells in the G0/G1 phase and an increase in the proportion of cells in S and G2/M phases (p < 0.05) of the cell cycle, suggesting a cytostatic effect in HeLa cells for arrest at these points in the cycle. Similarly, Bowman–Birk PIs presented a cytostatic effect on the osteosarcoma cell cycle (Saito et al. 2007). Another example is the classic Bowman–Birk inhibitor from Vigna unguiculata, which had a significant cytostatic effect on reducing the proliferation of MFC-7 cells, presenting arrest in S/G2/M and a significant increase in annexin-V+ cell number (Joanitti et al. 2010). Cytostatic effects of Bowman–Birk PIs have been described in ovary (Wan et al. 1998), breast, mouth (Zhang et al. 1999), colon (Clemente et al. 2005), osteosarcoma (Saito et al. 2007) and other tumor cells (Kennedy 1998).
Conclusion
EvCI, a member of the Kunitz chymotrypsin inhibitors from E. velutina seeds, is a competitive inhibitor of chymotrypsin and displays high functional stability regarding pH, temperature, and exposure to DTT. Furthermore, EvCI displayed a selective anti-proliferative effect on HeLa cells, and the primary effects on cell viability and cell cycle indicate arrest in the cell cycle followed by apoptosis. These data suggest that EvCI may be a promising compound to be studied as a potential anticancer agent in the future, but other morphological and biochemical studies should be performed to elucidate the mechanism of EvCI action in HeLa cell lines.
Acknowledgements
This paper is dedicated to Prof. Maurício Pereira de Sales (in memoriam), who initiated this work and created the bioactive proteins research group in the Department of Biochemistry at UFRN.
Funding
This work was supported by grants of Brazilian agencies: CAPES, CNPq and FINEP/RENORBIO.
Declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Ethics approval and consent to participate
Not applicable.
Human and animal rights
Not applicable.
Footnotes
Sheyla V. Lucena and Fabíola P. Rufino contributed equally to this manuscript.
References
- Bacha AB, Jemel I, Moubayed NMS, et al. Peptide-based protease inhibitors from plants. 3 Biotech. 2019;24:1877–1889. doi: 10.1007/s13205-017-0764-z. [DOI] [Google Scholar]
- Bhattacharyya A, Babu CR. Purification and biochemical characterization of a serine proteinase inhibitor from Derris trifoliata Lour. seeds: insight into structural and antimalarial features. Phytochemistry. 2009;70:703–712. doi: 10.1016/j.phytochem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Mazumdar S, Leighton SM, Babu CR. A Kunitz proteinase inhibitor from Archidendron ellipticum seeds: purification, characterization, and kinetic properties. Phytochemistry. 2006;67:232–241. doi: 10.1016/j.phytochem.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Rai S, Babu CR. A trypsin and chymotrypsin inhibitor from Caesalpinia bonduc seeds: isolation, partial characterization and insecticidal properties. Plant Physiol Biochem. 2007;45:169–177. doi: 10.1016/j.plaphy.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Champ MM-J. Non-nutrient bioactive substances of pulses. Br J Nutr. 2002;88:307. doi: 10.1079/BJN2002721. [DOI] [PubMed] [Google Scholar]
- Chan YS, Zhang Y, Ng TB. Brown kidney bean Bowman–Birk trypsin inhibitor is heat and pH stable and exhibits anti-proliferative activity. Appl Biochem Biotechnol. 2013;169:1306–1314. doi: 10.1007/s12010-012-9998-8. [DOI] [PubMed] [Google Scholar]
- Cheung AH, Ng TB. Isolation and characterization of a trypsin-chymotrypsin inhibitor from the seeds of green lentil (Lens culinaris) Protein Pept Lett. 2007;14:859–864. doi: 10.2174/092986607782110310. [DOI] [PubMed] [Google Scholar]
- Clemente A, Gee JM, Johnson IT, Mackenzie DA, Domoney CP. Pisum sativum L. protease inhibitors from the Bowman–Birk class influence the growth of human colorectal adenocarcinoma HT29 cells in vitro. J Agric Food Chem. 2005;53:8979–8986. doi: 10.1021/jf051528w. [DOI] [PubMed] [Google Scholar]
- Cruz ACB, Massena FS, Migliolo L, et al. Bioinsecticidal activity of a novel Kunitz trypsin inhibitor from Catanduva (Piptadenia moniliformis) seeds. Plant Physiol Biochem. 2013;70:61–68. doi: 10.1016/j.plaphy.2013.04.023. [DOI] [PubMed] [Google Scholar]
- da Silveira RV, de Souza SG, das Graças Machado Freire M, et al. Purification and characterization of a trypsin inhibitor from Plathymenia foliolosa seeds. J Agric Food Chem. 2008;56:11348–11355. doi: 10.1021/jf802778b. [DOI] [PubMed] [Google Scholar]
- de Oliveira CFR, Marangoni S, Macedo MLR. The trypsin inhibitor from Entada acaciifolia seeds affects negatively the development of Mediterranean flour moth, Anagasta kuehniella. Pestic Biochem Physiol. 2014;108:74–79. doi: 10.1016/j.pestbp.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Delaisse JM, Boyde A, Maconnachie E, et al. The effects of inhibitors of cysteine-proteinases and collagenase on the resorptive activity of isolated osteoclasts. Bone. 1987;8:305–313. doi: 10.1016/8756-3282(87)90007-X. [DOI] [PubMed] [Google Scholar]
- dos Santos EA, de Oliveira AS, Arajo Rablo LM, et al. Affinity chromatography as a key tool to purify protein protease inhibitors from plants. Affin Chromatogr. 2012 doi: 10.5772/34982. [DOI] [Google Scholar]
- Duranti M. Grain legume proteins and nutraceutical properties. Fitoterapia. 2006;77:67–82. doi: 10.1016/j.fitote.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Erlanger BF, Kokowski N, Cohen W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Garcia V, Freire MGM, Novello JC, et al. Trypsin inhibitor from Poecilanthe parviflora seeds: purification, characterization, and activity against pest proteases. Protein J. 2004;23:343–350. doi: 10.1023/B:JOPC.0000032654.67733.d5. [DOI] [PubMed] [Google Scholar]
- Gomes CEM, Barbosa AED, Macedo LLP, et al. Effect of trypsin inhibitor from Crotalaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (fruit fly) Plant Physiol Biochem. 2005;43:1095–1102. doi: 10.1016/j.plaphy.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Joanitti GA, Azevedo RB, Freitas SM. Apoptosis and lysosome membrane permeabilization induction on breast cancer cells by an anticarcinogenic Bowman–Birk protease inhibitor from Vigna unguiculata seeds. Cancer Lett. 2010;293:73–81. doi: 10.1016/j.canlet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Johansson S, Göransson U, Luijendijk T, et al. A neutrophil multitarget functional bioassay to detect anti-inflammatory. J Nat Prod. 2002;65:32–41. doi: 10.1021/np010323o. [DOI] [PubMed] [Google Scholar]
- Joubert FJ. Purification and properties of the proteinase inhibitors from Erythrina caffra (coast erythrina) seed. Int J Biochem. 1982;14:187–193. doi: 10.1016/0020-711X(82)90137-9. [DOI] [PubMed] [Google Scholar]
- Joubert FJ. Purification and some properties of two proteinase inhibitors from Erythrina acanthocarpa seed. J Nat Prod. 1982;45:427–433. doi: 10.1021/np50022a011. [DOI] [PubMed] [Google Scholar]
- Joubert FJ, Carlsson FH, Haylett T. Purification and some properties of two proteinase inhibitors (DE-1 and DE-3) from Erythrina latissima (broad-leaved erythrina) seed. Hoppe Seylers Z Physiol Chem. 1981;362:531–538. doi: 10.1515/bchm2.1981.362.1.531. [DOI] [PubMed] [Google Scholar]
- Kennedy AR. Chemopreventive agents: protease inhibitors. Pharmacol Ther. 1998;78:167–209. doi: 10.1016/s0163-7258(98)00010-2. [DOI] [PubMed] [Google Scholar]
- Kortt AA. Isolation and properties of a chymotrypsin inhibitor from winged bean seed (Psophocarpus tetragonolobus (L.) Dc) Biochim Biophys Acta. 1980;624:237–248. doi: 10.1016/0005-2795(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Kouzuma Y, Suetake M, Kimura M, Yamasaki N. Isolation and primary structure of proteinase inhibitors from Erythrina variegata (Linn.) var. orientalis seeds. Biosci Biotechnol Biochem. 1992;56:1819–1824. doi: 10.1271/bbb.56.1819. [DOI] [PubMed] [Google Scholar]
- Kunitz M. Crystalline soybean trypsin inhibitor: II. General properties. J Gen Physiol. 1947;30:291–310. doi: 10.1085/jgp.30.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam SK, Ng TB. A dimeric high-molecular-weight chymotrypsin inhibitor with antitumor and HIV-1 reverse transcriptase inhibitory activities from seeds of Acacia confusa. Phytomedicine. 2010;17:621–625. doi: 10.1016/j.phymed.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Lehle K, Wrba A, Jaenicke R. Erythrina caffra trypsin inhibitor retains its native structure and function after reducing its disulfide bonds. J Mol Biol. 1994;239:276–284. doi: 10.1006/jmbi.1994.1367. [DOI] [PubMed] [Google Scholar]
- Lingaraju MH, Gowda LR. A Kunitz trypsin inhibitor of Entada scandens seeds: another member with single disulfide bridge. Biochim Biophys Acta Proteins Proteom. 2008;1784:850–855. doi: 10.1016/j.bbapap.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Lorenzi H (2014) Árvores Brasileiras vol 1: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Nova Odessa
- Macedo MLR, Garcia VA, das Freire MGM, Richardson M. Characterization of a Kunitz trypsin inhibitor with a single disulfide bridge from seeds of Inga laurina (SW.) Willd. Phytochemistry. 2007;68:1104–1111. doi: 10.1016/j.phytochem.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Machado RJA, Monteiro NKV, Migliolo L, et al. Characterization and pharmacological properties of a novel multifunctional Kunitz inhibitor from Erythrina velutina seeds. PLoS One. 2013 doi: 10.1371/journal.pone.0063571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, List K. Cell surface–anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 2019;38:357–387. doi: 10.1007/s10555-019-09811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Rotini A, Randazzo D, et al. Phenols content and 2-D electrophoresis protein pattern: a promising tool to monitor Posidonia meadows health state. BMC Ecol. 2007;7:6. doi: 10.1186/1472-6785-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggie S, Hicks C. Protease inhibitor-based antiretroviral therapy in treatment-naive HIV-1-infected patients: the evidence behind the options. J Antimicrob Chemother. 2010;65:1094–1099. doi: 10.1093/jac/dkq130. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Macaubas C, Truong P, et al. Serum amyloid A induces mitogenic signals in regulatory T cells via monocyte activation. Mol Immunol. 2014;59:172–179. doi: 10.1016/j.molimm.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak M, Wong SS, Dreveny I, Emsley J. Structure of plasma and tissue kallikreins. Thromb Haemost. 2013;110:423–433. doi: 10.1160/TH12-11-0840. [DOI] [PubMed] [Google Scholar]
- Richardson M (1991) Seed storage proteins: the enzyme inhibitor. In: Methods in plants. Biochemistry. New York
- Rufino FPS, Pedroso VMA, Araujo JN, et al. Inhibitory effects of a Kunitz-type inhibitor from Pithecellobium dumosum (Benth) seeds against insect-pests’ digestive proteinases. Plant Physiol Biochem. 2013;63:70–76. doi: 10.1016/j.plaphy.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Saito T, Sato H, Virgona N, et al. Negative growth control of osteosarcoma cell by Bowman–Birk protease inhibitor from soybean; involvement of connexin 43. Cancer Lett. 2007;253:249–257. doi: 10.1016/j.canlet.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Souza EMT, Mizuta K, Sampaio MU, Sampaio CM. Purification and partial characterization of a Schizolobium parahyba chymotrypsin inhibitor. Phytochemistry v. 1995;39:521–525. doi: 10.1016/0031-9422(94)00921-F. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Chen Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front Pharmacol. 2016 doi: 10.3389/fphar.2016.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles RCL, de Souza EMT, Calderon LD, de Freitas SM. Purification and pH stability characterization of a chymotrypsin inhibitor from Schizolobium parahyba seeds. Phytochemistry. 2004;65:793–799. doi: 10.1016/j.phytochem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wan XS, Hamilton TC, Ware JH, et al. Growth inhibition and cytotoxicity induced by Bowman-Birk inhibitor concentrate in cisplatin-resistant human ovarian cancer cells. Nutr Cancer. 1998;31:8–17. doi: 10.1080/01635589809514672. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wan XS, Donahue JJ, et al. Effects of the Bowman–Birk inhibitor on clonogenic survival and cisplatin- or radiation-induced cytotoxicity in human breast, cervical, and head and neck cancer cells. Nutr Cancer. 1999;33:165–173. doi: 10.1207/S15327914NC330208. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Botella MA, Subramanian L, et al. Two wound-inducible soybean cysteine proteinase inhibitors have greater insect digestive proteinase inhibitory activities than a constitutive homolog. Plant Physiol. 1996;111:1299–1306. doi: 10.1104/pp.111.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]



