Abstract
Anti-spike monoclonal antibodies have proven invaluable in preventing severe outcomes from COVID-19, including hospitalization and death. The rise of the SARS-CoV-2 delta variant begs the question of whether monoclonal antibodies maintain similar efficacy now as they had when the alpha and beta variants predominated, when they were first assessed and approved. We used a retrospective cohort to compare rates of severe outcomes in an epoch in which alpha and beta were predominant compared with delta. A total of 5356 patients were infused during the alpha/beta variant–predominant (n=4874) and delta variant–predominant (n=482) era. Overall, odds of severe infection were 3.0% of patients in the alpha/beta-predominant era compared with 4.9% in the delta-predominant cohort. The unadjusted odds ratio (OR) was higher for severe disease in the delta era (OR, 1.67; 95% CI, 0.96 to 2.89), particularly when adjusted for Charlson Comorbidity Index (adjusted OR, 2.04; 95% CI, 1.30 to 3.08). The higher odds of severe infection could be due to a more virulent delta variant, although the possibility of decreased anti-spike monoclonal antibody effectiveness in the clinical setting cannot be excluded. Research into the most effective strategies for using and improving anti-spike monoclonals for the treatment of emerging variants is warranted.
Abbreviations and Acronyms: CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; EUA, emergency use authorization; FDA, Food and Drug Administration; MASS, Monoclonal Antibody Screening Score; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Anti-spike monoclonal antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have provided some of the first highly effective treatments for outpatients with mild to moderate coronavirus disease 2019 (COVID-19). These therapies have convincingly demonstrated reductions in progression to severe disease and hospitalization,1, 2, 3 and more recent data suggest mortality reduction.4 , 5
Mayo Clinic and the Mayo Clinic Health System established infusion centers for anti-spike monoclonal antibody therapy in Arizona, Florida, Minnesota, and Wisconsin. As of October 28, 2021, the program has infused more than 14,000 patients since the first product, bamlanivimab, was made available in November19, 2020, under Food and Drug Administration (FDA) emergency use authorization (EUA).6 In the analysis of initial real-world experience, bamlanivimab monotherapy was associated with 40% to 60% reductions in hospitalization along with significant reductions in the rates of intensive care unit admissions and mortality.7 Treatments have evolved over time because of the evolution of SARS-CoV-2 variants. Currently, our centers are infusing casirivimab/imdevimab to outpatients with mild to moderate COVID-19 who meet the FDA EUA eligibility criteria. In another analysis, there was a 70% reduction in hospitalization among high-risk patients who received casirivimab/imdevimab therapy compared with a propensity score–matched untreated control group.8 There was no significant difference in the rates of hospitalization between patients treated with bamlanivimab monotherapy and those who received casirivimab/imdevimab combination.7
The SARS-CoV-2 alpha (B.1.1.7) variant emerged in the United States in November 2020 and became dominant in March 2021. During that time, the beta (B.1.351) variant contributed a smaller proportion of cases by early spring. Since July 2021, the delta (B.1.617.2) variant has rapidly become the predominant lineage, associated with higher rates of hospitalization and health care utilization than prior strains.9 As the delta variant demonstrates a greater ability to evade neutralizing antibodies,10 this leads to the question of whether the clinical benefits of reduced hospitalization associated with anti-spike monoclonal antibodies are maintained. We compared the clinical outcomes of monoclonal antibody infusion during 2 time periods—an earlier period when alpha and beta were the predominant variants, and a later period when delta was predominant.
Patients and Methods
We conducted a retrospective cohort study analyzing the outcomes of patients infused with anti-spike monoclonal antibodies in 2 broad epochs stratified by geography for times and spaces when the delta variant was predominant vs when it was less common. The Mayo Clinic Institutional Review Board determined the study to be exempt. Eligible study participants both received an anti-spike monoclonal antibody infusion for COVID-19 at a Mayo Clinic site before July 31, 2021, and had not opted out of inclusion of their medical records for research purposes.
Patients were eligible to receive anti-spike monoclonal antibody if they were diagnosed with mild to moderate COVID-19, were within 10 days of symptom onset, and met the FDA EUA criteria. These state that patients should have characteristics that put them at high risk for severe COVID-19, including age older than 65 years; body mass index (BMI) above 35 kg/m2; diabetes, chronic kidney disease, and immunosuppressive therapy or condition; and, among patients older than 55 years, hypertension, cardiovascular disease, or chronic lung disease. A Monoclonal Antibody Screening Score (MASS) was developed with these criteria to stratify patients on the basis of their risk of hospitalization, as previously described.7 On May 14, 2021, the FDA EUA criteria expanded to include all patients with a BMI above 25 kg/m2; the removal of age restriction for hypertension, cardiovascular disease, and pulmonary diseases; and the inclusion of liver disease, neurodevelopmental disorders, and dependence on medical devices, among other criteria.
This study employed an electronic health record–based registry tool to identify patients with COVID-19 and to determine pertinent demographic characteristics (age, sex, race, ethnicity) and comorbidities defined by the Charlson Comorbidity Index (CCI) and MASS. Electronic health record data were used to abstract date and type of monoclonal antibody infusions and the region in which the infusion was performed. Registry data included the highest score on the World Health Organization ordinal scale for clinical improvement within 30 days of infection.11 Outcomes were stratified as those having a score of 4 or more, “severe” disease (hospitalized, requiring oxygen therapy or worse); and those with a score below 4, “non-severe” 30-day outcome. Patients with infection occurring before April 30, 2021, at any site were identified as likely not having the delta variant on the basis of circulating trends at that time. Patients from any site infected after July 1, 2021, were classified as likely to be delta, given the predominant circulating strains in that time and geography. Infections from the months of May and June were excluded as variant mix in that time period would not be clearly attributable to either alpha/beta or delta.
Analyses were performed using RStudio version 1.4.1106 (PBC) and the packages dplyr,12 epitools,13 sjplot,14 and ggplot2.15 Standard descriptive statistics were used. In addition, we conducted a sensitivity analysis calculating adjusted odds to adjust risk of severe outcome for comorbidities.
Results
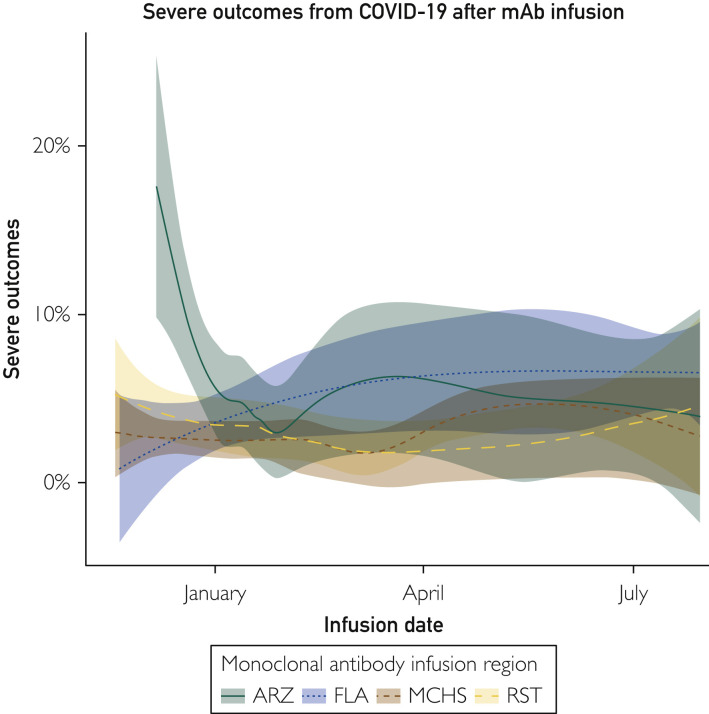
Between November 19, 2020, and July 30, 2021, Mayo Clinic infused a total of 5663 patients who met inclusion criteria with anti-spike monoclonal antibodies. Regions demonstrated different rates of severe outcomes initially but had begun to converge by April 2021 (Figure 1 ). Notably, Arizona started infusions in December, later than other sites and during a local peak in admissions.
Figure 1.
Trends in severe COVID-19 after anti-spike monoclonal antibody therapy over time and by geography. ARZ, Arizona; FLA, Florida; mAb, monoclonal antibody; MCHS, Mayo Clinic Health System; RST, Rochester.
After exclusion of patients infused in May and June, a total of 5356 patients were infused during the alpha/beta variant–predominant (n=4874) and delta variant–predominant (n=482) era. Although there were some demographic differences at baseline between sites when alpha and beta were predominant, these were not significant in the delta era (Table ; Supplemental Table, available online at http://www.mayoclinicproceedings.org). Patients during the delta variant–predominant era had a lower average BMI (because of changes in the EUA criteria that reduced the BMI eligibility from >35 kg/m2 to >25 kg/m2) and possessed more comorbidity (as assessed by higher CCI) but did not differ in terms of age or sex characteristics. Fewer Whites and more African Americans were infused in the delta era, which was notable, given the worse outcomes reported in African Americans infected with COVID-19. Interestingly, despite the higher CCI, the median MASS was lower in the delta cohort (P<.01). This may result from the expanded criteria for eligibility in May 2021, allowing younger and less comorbid patients to be infused. COVID-19 vaccination was expectedly higher in the delta than in the alpha/beta era (46.9% vs 2.1%). The period between polymerase chain reaction testing and antibody infusion was similar in the delta-predominant era compared with the alpha/beta-predominant era. In the delta-predominant era, casirivimab/imdevimab was the only anti-spike monoclonal antibody being infused.
Table.
| Alpha/beta predominant (n=4874) | Delta predominant (n=482) | Total (N=5356) | P value | |
|---|---|---|---|---|
| Body mass index (kg/m2) | 32.28 (27.31-38.26) | 28.72 (25.77-33.36) | 31.93 (27.11-37.89) | <.01 |
| No. missing | 543 | 64 | 607 | |
| Age (y) | 62.92 (51.34-71.53) | 58.91 (45.85-68.44) | 62.57 (50.61-71.41) | <.01 |
| No. missing | 120 | 16 | 136 | |
| Sex | 2413 (49.5) | 230 (47.7) | 2643 (49.3) | .45 |
| Race | <.01 | |||
| American Indian/Pacific Islander | 19 (0.4) | 0 (0.0) | 19 (0.4) | |
| Asian | 69 (1.4) | 4 (0.8) | 73 (1.4) | |
| Black/African American | 108 (2.2) | 32 (6.6) | 140 (2.6) | |
| Unknown | 134 (2.7) | 23 (4.8) | 157 (2.9) | |
| White | 4544 (93.2) | 423 (87.8) | 4967 (92.7) | |
| Ethnicity | .84 | |||
| Hispanic/Latino | 251 (5.1) | 27 (5.6) | 278 (5.2) | |
| Not Hispanic or Latino | 4540 (93.1) | 448 (92.9) | 4988 (93.1) | |
| Unknown | 83 (1.7) | 7 (1.5) | 90 (1.7) | |
| CCI total | 77.48 (21.36-90.15) | 90.15 (53.39-95.87) | 77.48 (21.36-90.15) | <.01 |
| MASS total points | 3.00 (2.00-5.00) | 3.00 (0.00-5.00) | 3.00 (2.00-5.00) | <.01 |
| Monoclonal antibody infused | NA | |||
| Bamlanivimab | 3392 (69.6) | 0 (0.0) | 3392 (63.3) | |
| Bamlanivimab/etesevimab | 460 (9.4) | 0 (0.0) | 460 (8.6) | |
| Casirivimab/imdevimab | 1022 (21.0) | 482 (100.0) | 1504 (28.1) | |
| Monoclonal antibody infusion site | NA | |||
| ARZ | 639 (13.1) | 67 (13.9) | 706 (13.2) | |
| FLA | 670 (13.7) | 268 (55.6) | 938 (17.5) | |
| MCHS | 2399 (49.2) | 98 (20.3) | 2497 (46.6) | |
| RST | 1166 (23.9) | 49 (10.2) | 1215 (22.7) | |
| Time to infusion | 2.00 (2.00-3.00) | 2.00 (1.00-3.00) | 2.00 (2.00-3.00) | <.01 |
| No. missing | 120 | 16 | 136 | |
| Severe COVID-19 outcome | 160 (3.3) | 24 (5.0) | 184 (3.4) | .05 |
| Required intensive care unit–level intervention | 19 (0.4) | 3 (0.6) | 22 (0.4) | .45 |
| Completed vaccination | 102 (2.10) | 226 (46.9) | 328 (6.1) | <.01 |
ARZ, Arizona; CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; FLA, Florida; MASS, Monoclonal Antibody Screening Score; MCHS, Mayo Clinic Health System; NA, not applicable; RST, Rochester.
Categorical variables are presented as number (percentage). Continuous variables are presented as median (Q1-Q3).
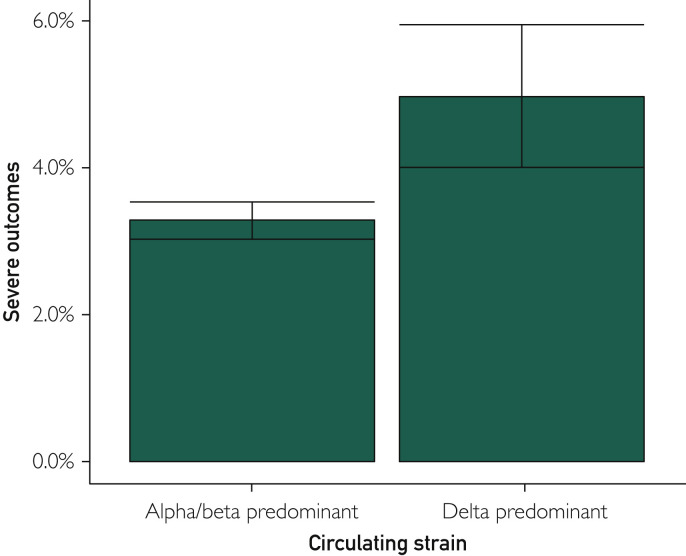
Overall, 184 of 5356 patients (3.4%) progressed to severe infection after monoclonal antibody infusion. The odds of severe infection were 3.0% of patients in the alpha/beta-predominant era compared with 4.9% in the delta-predominant cohort (Figure 2 ). The unadjusted odds ratio (OR) was not significantly higher for severe disease in the delta era compared with the alpha/beta era (OR, 1.67; 95% CI, 0.96 to 2.89); however, this became significant when adjusted for CCI (adjusted OR, 2.04; 95% CI, 1.30 to 3.08). Restricting the analysis to only those who received casirivimab/imdevimab in both eras did not change the directionality or significance of results (unadjusted OR, 1.74 [95% CI, 1.02 to 2.96]; adjusted OR, 2.09 [95% CI, 1.19 to 3.66]; Supplemental Table). The overall number of patients who required admission to the intensive care unit was 22 (0.4%), which was not significantly different across the epochs.
Figure 2.
Rates of severe COVID-19 after anti-spike monoclonal antibody therapy during the likely alpha/beta vs delta epochs.
Discussion
We observed increased risk of severe disease and poor outcomes after anti-spike antibody infusion during the period when the delta variant predominated compared with the earlier strains. Whereas the absolute difference is slight, it remained significant after adjustment for pertinent covariates. This finding suggests that despite anti-spike monoclonal antibody treatment, delta variant infection remains more virulent than alpha/beta variant infection. This observation is in line with data from other countries that suggest increased virulence of delta variant infection compared with other variants, noting an increased risk of hospitalization, intensive care unit stay, and death with delta variant, primarily in unvaccinated persons.16, 17, 18
The difference in outcomes is particularly notable in the context of the more liberal nature of the revised EUA, which allowed infusion of patients with fewer comorbidities than in the previous version. Despite a lower MASS, there was a significant increase in the CCI in the delta cohort. This is surprising, although the conditions accounted for in the MASS are limited to those in the original EUA eligibility, whereas the CCI provided a more global assessment and considered other comorbidities not in the MASS. The MASS elements were also developed and validated in the prevaccine era, and some elements overlapped with earlier vaccine eligibility, a potential confounder. Nonetheless, more cases with severe outcomes were observed despite this lower MASS and similar time to infusion in the delta-predominant era.
Our study has several limitations. First, we used epochs and geography to correlate with circulating strains as opposed to linking delta variant cases to specific outcomes. Whereas the omission of May and June data meant the predominant circulating strains in the periods assessed were considerably more homogeneous, this may not fully account for regional variation. Second, we did not account for the impact of specific anti-spike monoclonal antibody used. The shifts in practice toward the use of casirivimab/imdevimab meant we necessarily compared with bamlanivimab/etesevimab and bamlanivimab in prior eras. In our subset analysis, however, restricting the analysis only to those who received casirivimab/imdevimab in both periods did not change the overall results. Moreover, our previous work that compared bamlanivimab vs casirivimab/imdevimab outcomes during the alpha/beta-predominant era did not show any significant difference in efficacy.7 Third, the FDA EUA criteria for eligibility for anti-spike monoclonal antibody were expanded between the 2 eras, but the outcome should favor the delta period because the criteria were expanded to allow infusion of patients with lower comorbidity scores (as indicated by the MASS). Despite this, we observed higher comorbidity scoring by the CCI in the delta period. Finally, the comparisons of different epochs and locations reflect different practices with regard to hospital admission policies as well as vaccination rates, which may have reduced the need for hospitalization in the delta cohort. However, the proportion of vaccinated persons was expectedly higher during the delta period. As the directionality was reversed (ie, more patients in the later period required hospital admission and were thus classified as severe disease), these factors, including vaccination status, did not appear to have decreased severe outcomes in our study population. Accordingly, casirivimab/imdevimab treatment of breakthrough COVID-19 should be recommended even among vaccinated persons.
Conclusion
Severe COVID-19 outcomes are more common after anti-spike monoclonal antibody infusions in the cohort of patients more likely to have been infected with the delta variant than with the alpha/beta variant. This difference may be due to the overall greater virulence of the delta variant. Whereas experimental studies indicate that casirivimab/imdevimab demonstrates persistent activity against delta, the possibility of decreased anti-spike monoclonal antibody effectiveness in the clinical setting cannot be totally excluded. Controlled clinical trials are needed to further delineate this. Further research should also explore the confounders to determine the most effective way to use anti-spike monoclonal antibodies in the delta variant–predominant era.
Footnotes
Grant Support:Mayo Clinic.
Potential Competing Interests: J.C.O. has received consulting fees from Bates College and small grants from Nference, Inc outside of the present work. R.R.R. received research grants (funds given to the institution) from Gilead, Regeneron, and Roche, outside of the present work. R.R.R. is a member of the Data and Safety Monitoring Board of Novartis, outside of this work. R.R.R. received research internal funds from the Mayo Clinic on COVID-19 monoclonal antibody research. All other authors have no conflicts.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb R.L., Nirula A., Chen P., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bariola J.R., McCreary E.K., Wadas R.J., et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2021;8(7):ofab254. doi: 10.1093/ofid/ofab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group R.C., Horby P.W., Mafham M., et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.06.15.21258542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Razonable R.R., Aloia N.C.E., Anderson R.J., et al. A framework for outpatient infusion of antispike monoclonal antibodies to high-risk patients with mild-to-moderate coronavirus disease-19: the Mayo Clinic Model. Mayo Clin Proc. 2021;96(5):1250–1261. doi: 10.1016/j.mayocp.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganesh R., Philpot L.M., Bierle D.M., et al. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis. 2021;224(8):1278–1286. doi: 10.1093/infdis/jiab377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razonable R.R., Pawlowski C., O'Horo J.C., et al. Casirivimab–imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40:101102. doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twohig K.A., Nyberg T., Zaidi A., et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2021 Aug 27;S1473-3099(21) doi: 10.1016/S1473-3099(21)00475-8. 00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 11.Novel Coronavirus . World Health Organization; 2020. COVID-19 Therapeutic Trial Synopsis. [Google Scholar]
- 12.dplyr: a grammar of data manipulation [computer program] 2021. [Google Scholar]
- 13.epitools: epidemiology tools [computer program] 2020. [Google Scholar]
- 14.sjPlot: data visualization for Statistics in Social Science [computer program. 2021. [Google Scholar]
- 15.Wickham H. Springer-Verlag; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 16.Fisman D.N., Tuite A.R. Progressive increase in virulence of novel SARS-CoV-2 variants in Ontario, Canada. medRxiv. 2021 doi: 10.1101/2021.07.05.21260050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikh A., McMenamin J., Taylor B., Robertson C. SARS-CoV-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong S.W.X., Chiew C.J., Ang L.W., et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (alpha), B.1.315 (beta), and B.1.617.2 (delta) Clin Infect Dis. 2021 Aug 23 doi: 10.1093/cid/ciab721. ciab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.