Abstract
Background and Aim
International consensus on the definition and classification of post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) has been reached. However, the diagnosis and severity of PEP are often assessed according to the diagnostic criteria and classification for acute pancreatitis (AP). This study determined the incidence, severity, and risk factors of PEP diagnosed according to the diagnostic criteria and classification for AP in a large cohort.
Methods
This prospective, multicenter, observational cohort study conducted at five high‐volume centers included 1932 patients who underwent ERCP‐related procedures. The incidence, severity, and risk factors for PEP were evaluated.
Results
PEP occurred in 142 patients (7.3%); it was mild in 117 patients (6.0%) and severe in 25 patients (1.3%). According to the Cotton criteria, PEP occurred in 87 patients (4.5%); it was mild in 54 patients (2.8%), moderate in 20 patients (1.0%), and severe in 13 patients (0.7%). In the multivariate analysis, female sex (odds ratio [OR] 2.239; 95% confidence interval [CI] 1.546–3.243), naïve papilla (OR 3.047; 95% CI 1.803–5.150), surgically‐altered gastrointestinal anatomy (OR 2.538; 95% CI 1.342–4.802), procedure time after reaching the papilla (OR 1.009; 95% CI 1.001–1.017), pancreatic duct injection (OR 2.396; 95% CI 1.565–3.669), and intraductal ultrasonography (OR 1.641; 95% CI 1.024–2.629) were independent risk factors.
Conclusion
According to the diagnostic criteria and classification for AP, the incidence of PEP was higher than that according to the Cotton criteria and the severity of PEP tended to be severe.
Keywords: acute pancreatitis, cohort studies, endoscopic retrograde cholangiopancreatography, intraductal ultrasonography, post‐endoscopic retrograde cholangiopancreatography pancreatitis
According to the diagnostic criteria for acute pancreatitis (AP), the incidence of post‐endoscopic retrograde cholangiopancreatography pancreatitis (PEP) was higher than that with the Cotton criteria; the incidence rate of PEP was equivalent to that in previous reports. More patients were diagnosed with severe PEP using the Japanese severity criteria for AP. The risk factors of PEP were female sex, naïve papilla, surgically altered gastrointestinal anatomy, procedure time after reaching the papilla, pancreatic duct injection, and intraductal ultrasonography.
Introduction
Procedures related to endoscopic retrograde cholangiopancreatography (ERCP) play a major role in the diagnosis and treatment of pancreatobiliary diseases. Post‐ERCP pancreatitis (PEP) remains the most common and severe complication of ERCP. The condition of PEP ranges from mild, requiring only intravenous fluid therapy, to severe, requiring intensive care. Even if PEP is initially diagnosed as mild, it may become severe during the clinical course. It is important to accurately diagnose and evaluate the severity of PEP, promptly start appropriate treatment, and repeatedly evaluate its severity during the course. Previous studies have reported that the incidence of PEP is 3.5–9.7%. 1 , 2 At present, there are no established diagnostic criteria for PEP, and most previous studies used the diagnostic and classification criteria proposed by Cotton et al. 3 : (i) new or worsened abdominal pain; (ii) serum amylase at least three times the upper limit of normal, measured more than 24 h after the procedure; and (iii) new or prolongation of hospitalization for at least 2 days. The Cotton criteria have obtained international consensus and persistent upper abdominal pain and elevated serum amylase are important findings of PEP.
Nevertheless, it has been reported that when lipase levels and image findings that are usually used for the diagnosis of acute pancreatitis (AP) are added to the criteria, the number of diagnosed PEP cases increased, and 41.9% of the PEP cases were overlooked with the consensus criteria alone. 4 Another study reported that 37% of patients who had hyperamylasemia without abdominal pain after ERCP was diagnosed with pancreatitis based on computed tomography (CT). 5 In addition, regarding severity assessment, the Cotton criteria have another problem in that they do not accurately evaluate severity in the early phase or allow repeated assessment in a short period. Therefore, the severity assessment for AP proposed by the Japanese Ministry of Health, Labour, and Welfare (Japanese severity criteria for AP) (Table 1), 6 which allows the evaluation of the severity of pancreatitis in the early phase and repeated assessment, is usually used for severity assessment of PEP in Japan. Although the diagnostic criteria and severity classification of AP are often used to assess PEP in clinical practice, few studies 7 , 8 have used these clinical criteria in the epidemiological study of PEP.
Table 1.
Japanese severity scoring system for acute pancreatitis of the Ministry of Health, Labour, and Welfare of Japan (2008 revision)
|
|
|
1 + 2 = total score Total score = 0 or 1, Grade 1 Total score = 2, Grade 2 Total score = 3 or more, Grade 3 |
|
Assessment of severity If prognostic factor score is ≧3, or CT grade is ≧2, the acute pancreatitis is evaluated as ‘severe’ | |
Measures in SIRS criteria include body temperature >38 or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or PaCO2 <32 torr, and white blood cell counts >12 000 cells/mm3, <4000 cells/mm3, or >10% immature (band) forms.
BUN, blood urea nitrogen; CRP, C‐reactive protein; CT, computed tomography; LDH; lactate dehydrogenase, SIRS, systemic inflammatory response syndrome.
Therefore, in this study, we considered PEP as AP that occurred after ERCP. We conducted this prospective multicenter study to determine the incidence, severity, and risk factors of PEP that is diagnosed according to the diagnostic criteria and classification for AP in a large cohort.
Methods
This is a prospective multicenter observational cohort study conducted at five high‐volume centers in Kyoto and Shiga prefecture of western Japan. The incidence and severity of PEP that had been diagnosed according to diagnostic and severity criteria for AP were investigated and compared to those, which were obtained according to the consensus criteria proposed by Cotton et al. Risk factors for PEP were statistically analyzed using multivariate analysis. The variables investigated in this study are shown in Table 2. The protocol of this study was approved by the ethics committees of all participating centers and registered in the University hospital Medical Information Network (UMIN) clinical trial registration system (UMIN000024813). This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Table 2.
Investigated risk factors of PEP
| Patient‐related factors | ||
| Female sex | Previous acute pancreatitis | Naïve papilla |
| Younger age (<50 years of age) | Normal serum bilirubin | Peripapillary diverticulum |
| ASA grade | Acute cholangitis | Surgically altered gastrointestinal anatomy |
| Procedure‐related factors | ||
| Therapeutic ERCP | Minor papilla cannulation | Precut sphincterotomy |
| Emergency ERCP | Pancreatic duct injection | Endoscopic stone extraction |
| Procedure time after reaching papilla | Pancreatic guidewire passage | Endoscopic biliary drainage |
| ERCP by trainee | Intraductal ultrasonography | Pancreatic duct brushing cytology |
| Contrast‐guided cannulation | Endoscopic sphincterotomy | Pancreatic stenting |
| Cannulation attempts (≧10) | Endoscopic papillary balloon dilatation | Prophylactic pancreatic stent placement |
ASA; American Society of Anesthesiologists; ERCP; endoscopic retrograde cholangiopancreatography; PEP, post‐endoscopic retrograde cholangiopancreatography pancreatitis.
Patients
All patients who underwent ERCP‐related procedures at the centers between February 2015 and May 2016 were enrolled in a registry and the data on patient characteristics, indications for ERCP, findings of procedures, and adverse events were collected prospectively. We excluded patients who had AP at the time of ERCP, who had undergone biliary reconstruction, or in whom the endoscopist failed to reach the major papilla during ERCP. ERCP‐related procedures were performed in accordance with the strategies of each center, and blood tests were performed 2 h after ERCP and 18 h (the following morning) in all patients. Written informed consent was obtained from all the patients prior to registration.
Definitions
PEP was defined as the presence of at least two of the following three manifestations based on diagnostic criteria for AP 9 , 10 : (i) elevated levels of serum amylase; (ii) abdominal pain lasting more than 24 h; and (iii) characteristics findings of AP on CT. The elevation of serum amylase levels was considered significant when it was elevated to more than three times the upper limit of normal according to the consensus criteria. 3 CT findings that confirm the diagnosis of AP include focal or diffuse enlargement of the pancreas, heterogeneity of pancreatic parenchyma, peripancreatic stranding, pancreatic or peripancreatic fluid collections, pancreatic necrosis, and peripancreatic fat necrosis. Two expert radiological diagnosticians who were blinded to clinical information independently assessed CT images and confirmed the presence or absence of AP. 11 , 12 , 13 , 14 The severity of PEP was assessed according to the Japanese severity assessment for AP. 6
Statistical analysis
A multivariable logistic regression analysis was used to identify the independent risk factors for PEP. First, Variables in Table 2 were assessed using univariate analysis with the χ2 test for categorical variables and Mann–Whitney U test for continuous variables. The variables entered into the logistic regression model were chosen referring to prior reports and results of the preceding univariable analyses and considering the scientific plausibility and the clinical meaningfulness of the association. The number of variables entered into the logistic regression model was determined according to the rule of thumb that a logistic model should be used with a minimum of 10 events per predictor variable. P‐values <0.05 were considered significant. Statistical analyses were performed using IBM SPSS statistics 22 (IBM Corp, Armonk, NY, USA).
Results
Overall, 2078 patients were enrolled in the registry. We excluded 74 patients who already had AP, 63 who had undergone biliary reconstruction, and 9 in whom the endoscope could not reach the papilla of Vater; 1932 patients were finally analyzed (Fig. 1).
Figure 1.
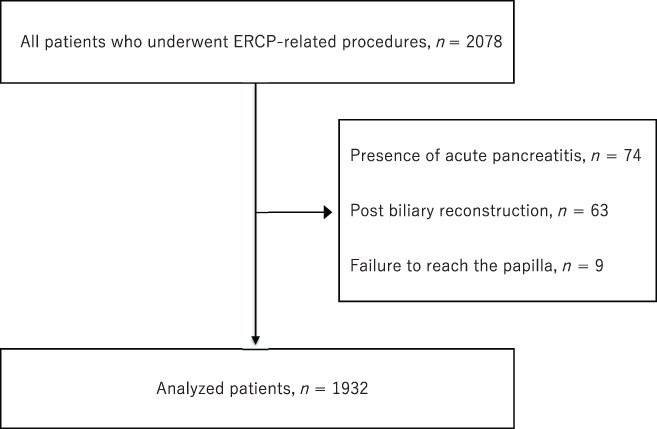
Flowchart of the registered patients. There were 2078 patients who received endoscopic retrograde cholangiopancreatography (ERCP)‐related procedures. We excluded 74 patients who already had acute pancreatitis, 63 who had undergone biliary reconstruction, and 9 in whom the endoscope could not reach the papilla of Vater. We analyzed 1932 patients.
Table 3 displays the patient characteristics. The mean age of the patients was 72.9 years, and 774 (40.1%) were female; 87.4% of the patients had American Society of Anesthesiologists (ASA) grade I or II, 13.5% had a history of AP, 34.7% had obstructive jaundice, 54.6% had naïve papillae, and 5.5% had a surgically‐altered gastrointestinal (GI) anatomy. The most common indication for ERCP was choledocholithiasis, accounting for 46.0% of the total. The proportion of patients with malignant diseases was 36.5%.
Table 3.
Patient characteristics
| n (%) | ||
|---|---|---|
| Mean age (years old) | 72.9 | |
| Female | 774 | (40.1) |
| ASA grade | ||
| 1 | 924 | (47.8) |
| 2 | 766 | (39.6) |
| 3 | 227 | (11.7) |
| 4 | 15 | (0.8) |
| Previous acute pancreatitis | 261 | (13.5) |
| Obstructive jaundice | 671 | (34.7) |
| Acute cholangitis | 591 | (30.6) |
| Naïve papilla | 1054 | (54.6) |
| Peripapillary diverticulum | 498 | (25.8) |
| Surgically altered gastrointestinal anatomy | 107 | (5.5) |
| Billroth‐I | 44 | (2.3) |
| Billroth‐II | 22 | (1.1) |
| Roux‐en‐Y | 41 | (2.1) |
| Indication of ERCP | ||
| Choledocholithiasis | 888 | (46.0) |
| Chronic pancreatitis/pancreatic stone | 131 | (6.8) |
| Benign biliary stenosis | 66 | (3.4) |
| Acute cholangitis | 50 | (2.6) |
| Pancreatic cancer | 261 | (13.5) |
| Bile duct cancer | 248 | (12.8) |
| Gallbladder cancer | 36 | (1.9) |
| Other malignant tumor | 63 | (3.3) |
| Intraductal papillary mucinous neoplasm | 49 | (2.5) |
| Papillary tumor | 29 | (1.5) |
| Others | 111 | (5.7) |
ASA, American Society of Anesthesiologists; ERCP, endoscopic retrograde cholangiopancreatography.
Table 4 displays the characteristics of ERCP‐related procedures. Therapeutic ERCP accounted for large proportion of patients at 89.1%. The proportion of patients undergoing emergency ERCP was 22.7% and that of those undergoing ERCP by trainees with less than 5 years of ERCP experience was 60.4%. The average procedure time was 37.9 min.
Table 4.
Characteristics of procedures
| n (%) | ||
|---|---|---|
| Therapeutic ERCP | 1722 | (89.1) |
| Emergency ERCP | 438 | (22.7) |
| ERCP by trainees | 1166 | (60.4) |
| Mean procedure time (min) | 37.9 | |
| Cannulation method | ||
| Wire‐guided | 1041 | (53.9) |
| Contrast‐guided | 869 | (45.0) |
| Others | 22 | (1.1) |
| Cannulation attempts | ||
| 1–3 | 1243 | (64.3) |
| 4–9 | 376 | (19.5) |
| ≧10 | 313 | (16.2) |
| Minor papilla cannulation | 19 | (1.0) |
| Pancreatic duct injection | 595 | (30.8) |
| Pancreatic guidewire passage | 469 | (24.3) |
| Intraductal ultrasonography | 225 | (11.6) |
| Endoscopic sphincterotomy | 484 | (25.1) |
| Endoscopic papillary balloon dilatation | 136 | (7.0) |
| Precut sphincterotomy | 31 | (1.6) |
| Endoscopic stone extraction | 587 | (30.4) |
| Endoscopic biliary drainage | 1135 | (58.7) |
| Pancreatic duct brushing cytology | 30 | (1.6) |
| Pancreatic stenting | 234 | (12.1) |
| Prophylactic pancreatic stenting | 99 | (5.1) |
ERCP, endoscopic retrograde cholangiopancreatography.
Wire‐guided cannulation was the first choice for cannulation at three of the five centers and contrast‐guided cannulation was the first choice at two centers. Wire‐guided cannulation was performed in 1041 patients (53.9%) and contrast‐guided cannulation was used in 869 (45.0%). In approximately two‐thirds of the patients, cannulation was successful within 1–3 attempts. Including unintentional contrast material injection and guidewire insertion into the pancreatic duct, pancreatic duct injection was performed in 595 patients (30.8%) and pancreatic guidewire passage was performed in 469 patients (24.3%). Intraductal ultrasonography (IDUS) was performed in 225 patients (11.6%). The morning after ERCP, serum amylase levels were elevated to more than the upper limit of normal in 576 patients (29.8%) and to more than three times the upper limit of normal in 207 patients (10.7%). Abdominal CT scan was performed in 444 of 576 patients (77.1%) with high amylase levels.
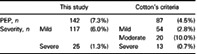
PEP occurred in 142 of 1932 patients (7.3%). The severity was mild in 117 patients (6.0%) and severe in 25 patients (1.3%) according to the Japanese severity criteria for AP. Using the Cotton consensus criteria, PEP was diagnosed in 87 patients (4.5%), and the severity was mild in 54 patients (2.8%), moderate in 20 patients (1.0%), and severe in 13 patients (0.7%) (Table 5). Overall, among the 117 patients diagnosed with mild PEP according to the Japanese severity criteria for AP, 50 patients were diagnosed as having nonpancreatitis, 65 patients mild–moderate PEP, and two patients severe PEP according to the Cotton criteria. Among 25 patients diagnosed with severe PEP according to the Japanese severity criteria for AP, five patients were diagnosed with nonpancreatitis, nine patients mild–moderate PEP, and 11 patients severe PEP according to the Cotton criteria.
Table 5.
Incidence rate of PEP
| This study | Cotton's criteria | |||||
|---|---|---|---|---|---|---|
| PEP, n | 142 | (7.3%) | 87 | (4.5%) | ||
| Severity, n | Mild | 117 | (6.0%) | Mild | 54 | (2.8%) |
| Moderate | 20 | (10.0%) | ||||
| Severe | 25 | (1.3%) | Severe | 13 | (0.7%) | |
PEP, post‐endoscopic retrograde cholangiopancreatography pancreatitis.
Regarding risk factors of PEP, in univariate analysis, female sex, naïve papilla, surgically altered GI anatomy, no coexistence of acute cholangitis, diagnostic ERCP, elective ERCP, procedure time after reaching the papilla, number of cannulation attempts, precut sphincterotomy, IDUS, pancreatic duct injection, pancreatic guidewire passage, and prophylactic pancreatic stenting were significant risk factors (Table 6).
Table 6.
Risk factors of post‐ERCP pancreatitis
| Univariate analysis † | Multivariate analysis ‡ | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Patient‐related risk factors | ||||||
| Female sex | 2.237 | 1.583–3.161 | <0.001 | 2.239 | 1.546–3.243 | <0.001 |
| Younger age (<50 years of age) | 0.732 | 0.292–1.834 | 0.504 | 0.703 | 0.271–1.822 | 0.468 |
| ASA grade III or IV | 0.879 | 0.516–1.497 | 0.638 | — | — | — |
| Previous acute pancreatitis | 0.626 | 0.349–1.125 | 0.114 | 0.881 | 0.462–1.683 | 0.702 |
| Normal serum bilirubin | 0.915 | 0.642–1.303 | 0.623 | 1.032 | 0.689–1.546 | 0.878 |
| No coexistence of acute cholangitis | 1.630 | 1.084–2.451 | 0.019 | — | — | — |
| Naïve papilla | 5.286 | 3.296–8.480 | <0.001 | 3.047 | 1.803–5.150 | <0.001 |
| Surgically altered gastrointestinal anatomy | 2.371 | 1.352–4.156 | 0.002 | 2.538 | 1.342–4.802 | 0.004 |
| Peripapillary diverticulum | 0.908 | 0.608–1.356 | 0.637 | — | — | — |
| Procedure‐related risk factors | ||||||
| Diagnostic ERCP | 2.170 | 1.396–3.372 | <0.001 | — | — | — |
| Elective ERCP | 1.750 | 1.087–2.817 | 0.020 | 1.364 | 0.801–2.324 | 0.254 |
| Procedure time after reaching the papilla | <0.001 § | 1.009 | 1.001–1.017 | 0.035 | ||
| ERCP by trainee | 0.978 | 0.690–1.386 | 0.901 | — | — | — |
| Contrast‐guided cannulation | 1.273 | 0.903–1.794 | 0.168 | — | — | — |
| Cannulation attempts (≧10) | 3.155 | 2.183–4.561 | <0.001 | 1.118 | 0.700–1.786 | 0.641 |
| Minor papilla cannulation | 1.490 | 0.341–6.514 | 0.646 | — | — | — |
| Pancreatic duct injection | 3.992 | 2.805–5.681 | <0.001 | 2.396 | 1.565–3.669 | <0.001 |
| Pancreatic guidewire passage | 2.989 | 2.111–4.233 | <0.001 | 1.340 | 0.854–2.102 | 0.202 |
| Intraductal ultrasonography | 2.191 | 1.426–3.366 | <0.001 | 1.641 | 1.024–2.629 | 0.040 |
| Endoscopic sphincterotomy | 1.189 | 0.812–1.740 | 0.373 | — | — | — |
| Endoscopic papillary balloon dilatation | 0.563 | 0.244–1.301 | 0.173 | — | — | — |
| Precut sphincterotomy | 3.815 | 1.615–9.016 | 0.006 | 1.028 | 0.389–2.717 | 0.956 |
| Endoscopic stone extraction | 0.704 | 0.473–1.049 | 0.083 | — | — | — |
| Endoscopic biliary drainage | 0.820 | 0.581–1.155 | 0.255 | — | — | — |
| Pancreatic duct brushing cytology | 2.577 | 0.971–6.837 | 0.064 | — | — | — |
| Pancreatic stenting | 1.285 | 0.791–2.087 | 0.310 | — | — | — |
| Prophylactic pancreatic stenting | 2.399 | 1.345–4.277 | 0.002 | 0.935 | 0.478–1.827 | 0.843 |
χ2 test.
Logistic regression analysis.
Mann–Whitney U test.
ASA, American Society of Anesthesiologists; CI; confidence interval; ERCP, endoscopic retrograde cholangiopancreatography; OR, odds ratio.
Referring to this result and prior reports, 14 variables were entered into a logistic regression model to assess risk factor for PEP: female sex, younger age, previous AP, normal serum bilirubin, naïve papilla, surgically altered GI anatomy, elective ERCP, procedure time, number of cannulation attempts, precut sphincterotomy, IDUS, pancreatic duct injection, pancreatic guidewire passage, and prophylactic pancreatic stenting. In multivariate analysis, female sex (odds ratio [OR] 2.239; 95% confidence interval [CI] 1.546–3.243), naïve papilla (OR 3.047; 95% CI 1.803–5.150), surgically‐altered GI anatomy (OR 2.538; 95% CI 1.342–4.802), procedure time after reaching the papilla (OR 1.009; 95% CI 1.001–1.017), pancreatic duct injection (OR 2.396; 95% CI 1.565–3.669), and IDUS (OR 1.641; 95% CI 1.024–2.629) were independent risk factors (Table 6).
Discussion
According to the diagnostic criteria and classification for AP, the incidence rate of PEP was 7.3% and that of severe PEP was 0.7%. Female sex, naïve papilla, surgically‐altered GI anatomy, procedure time after reaching the papilla, pancreatic duct injection, and IDUS were found to be the risk factors in multivariate analysis.
We diagnosed PEP based on diagnostic criteria for AP. 9 , 10 PEP was diagnosed in 142 patients (7.3%), while 87 patients (4.5%) were diagnosed as having PEP according to the Cotton consensus criteria. These incidence rates of PEP were not significantly different from that of previous reports (3.5–9.7%). 1 , 2 A total of 38.7% of patients with PEP were not diagnosed with PEP according to the consensus criteria, which was similar to the results presented by Artifon et al. 4 Of the 55 patients who were not diagnosed with PEP based on the consensus criteria alone, 45 patients did not have persistent abdominal pain and 10 patients did not have significantly elevated serum amylase levels. Adding CT findings to the diagnostic criteria, PEP with poor clinical symptoms can be identified. This may lead to early detection of PEP and appropriate initial management to improve clinical course, but further studies are needed to confirm that.
We assessed the severity of PEP using Japanese severity criteria for AP. These criteria have been reported to correlate with in‐hospital mortality and to be useful for severity assessment of AP at the early stage of hospital admission. 15 The severity of PEP was mild in 6.0% and severe in 1.3%, and severe PEP was occurred in 0.7% (13 patients) according to the Cotton criteria in this study. The incidence of severe PEP has been reported to be 0.3–0.5%. 1 , 2 The Japanese severity criteria for AP potentially deemed more cases as severe in contrast to the Cotton criteria. Another possibility is that PEP was diagnosed as severe in the early phase and appropriate initial treatment improved the clinical course. The revised Atlanta Classification 9 has been also proposed as criteria for assessing the severity of AP. However, the revised classification that includes persistent organ failure as the key determinant of severity also poses difficulties for evaluating the severity in the early phase and repeated assessment as with the Cotton criteria. Because the pathophysiology of PEP may differ from that of AP, unique severity criteria need to be established for the pathophysiology of PEP.
We also assessed risk factors of PEP in this study. “No coexistence of acute cholangitis” and “diagnostic ERCP” were significant risk factors in the univariate analysis but these factors were not entered into a logistic regression analysis, because we considered that these associations are due to the association of these findings with procedure time and naive papilla, respectively, and are scientifically implausible.
In the multivariable analysis of this study, female sex, naïve papilla, surgically altered GI anatomy, procedure time after reaching the papilla, pancreatic duct injection, and IDUS were found to be independent risk factors. Female sex and pancreatic duct injection were considered risk factors for PEP in the American Society for Gastrointestinal Endoscopy guidelines 16 and European Society Gastrointestinal Endoscopy guidelines 17 among other reports. 18 , 19 , 20 IDUS has been reported to be an independent risk factor for PEP in a retrospective study. 21 In the report, Meister et al. suggested that papilledema of Vater caused by physical stimulation with an IDUS probe induces PEP. In our study, a naïve papilla was defined as a major papilla that has not undergone a prior endoscopic procedure such as endoscopic sphincterotomy (EST) or endoscopic papillary balloon dilation (EPBD) at the time of ERCP. Deep cannulation in patients with a naïve papilla is usually more difficult than in patients who have already undergone EST or EPBD and physical stimulation of a naive papilla is often stronger. Moreover, in many cases of naïve papillae, because the bile duct orifice and pancreatic duct orifice are not separated as in a post‐EST case, physical stimulation of the papilla can easily influence the pancreatic orifice. This may explain why the incidence of PEP increased in patients with a naïve papilla. A conventional forward‐viewing endoscope or a balloon enteroscope is usually used for ERCP in patients with Billroth II reconstruction or Roux‐en‐Y reconstruction. In such cases, when reaching the papilla of Vater, the papilla is often on the left or upper side of the screen and the view is upside down as compared to an ordinary ERCP view. 22 Thus, physical stimulation of the papilla is likely to be more extensive because of the difficult positioning of the papilla and the lack of the elevator that facilitates delicate maneuvers of devices. Furthermore, a duodenoscope is used in patients with Billroth I reconstruction; however, deformation of the duodenum and common bile duct often makes cannulation difficult and increases the risk of developing PEP. Moreover, physical stimulation of the papilla of Vater can occur not only during cannulation but also during the entire procedure. This suggests that longer procedure times after reaching the papilla correlate with stronger irritation to the papilla. It has been reported that procedure time of 30 min or more is a risk factor of PEP. 23 Previous pancreatitis, younger age, normal serum bilirubin, and pancreatic guidewire passage have been reported to be independent risk factors for PEP 16 , 17 ; however, in the present study, we did not identify these as significant risk factors for PEP.
This study had some limitations that need to be taken into account while interpreting the results. This study is limited by its multicenter prospective observational design, and there was no standardized protocol for ERCP‐related procedures. Therefore, incidence of PEP and a correlation between each risk factor and PEP might be confounded by unmeasured factors. For example, we did not investigate the amount of hydration in our study; however, it has been suggested that aggressive hydration with lactated Ringer's solution reduces the incidence of PEP. 24 , 25 , 26 , 27 The amount of hydration possibly confounded the development of PEP. In addition, rectal administration of nonsteroidal anti‐inflammatory drugs (NSAIDs) and prophylactic pancreatic stent placement, both of which have been shown to be effective in preventing PEP, 20 , 28 , 29 , 30 , 31 , 32 was performed only in high‐risk patients according to the judgment of the operator. As a result, the number of patients who received rectal NSAIDs was small and rectal NSAIDs were excluded from the variables investigated. Prophylactic pancreatic stenting has been recognized as a risk factor for PEP in univariate analysis. Finally, selection bias may have influenced the results. However, it was confirmed that the amount of hydration before and after ERCP and the selection of high‐risk cases for PEP did not differ significantly among the five institutions at a pre‐study meeting, and thus, the effects of confounding are likely to be small. In order to evaluate the preventive effects of these factors, it is necessary to investigate PEP using a protocol for prophylactic treatment in patients other than those at high risk and diagnose PEP based on the criteria for AP.
In conclusion, according to the diagnostic criteria for AP, the incidence of PEP was higher than that with the Cotton criteria; the incidence rate of PEP was equivalent to that in previous reports. More patients were diagnosed with severe PEP using the Japanese severity criteria for AP. The risk factors of PEP were female sex, naïve papilla, surgically altered GI anatomy, procedure time after reaching the papilla, pancreatic duct injection, and IDUS.
Acknowledgments
We would like to thank all colleagues of the SOSUI study group for their support in clinical practice.
Declaration of conflict of interest: All authors disclosed no financial relationships relevant to this study, and there are no conflicts of interest in this study.
References
- 1. Andriulli A, Loperfido S, Napolitano G et al. Incidence rates of post‐ERCP complications: a systematic survey of prospective studies. Am. J. Gastroenterol. 2007; 102: 1781–8. [DOI] [PubMed] [Google Scholar]
- 2. Kochar B, Akshintala VS, Afghani E et al. Incidence severity, and mortality of post‐ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest. Endosc. 2015; 81: 143–9. [DOI] [PubMed] [Google Scholar]
- 3. Cotton PB, Lehman G, Vennes J et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest. Endosc. 1991; 37: 383–93. [DOI] [PubMed] [Google Scholar]
- 4. Artifon ELA, Chu A, Freeman M, Sakai P, Usmani A, Kumar A. A comparison of the consensus and clinical definitions of pancreatitis with a proposal to redefine post‐endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2010; 39: 530–5. [DOI] [PubMed] [Google Scholar]
- 5. Uchino R, Sasahira N, Isayama H et al. Detection of painless pancreatitis by computed tomography in patients with post‐endoscopic retrograde cholangiopancreatography hyperamylasemia. Pancreatology. 2014; 14: 17–20. [DOI] [PubMed] [Google Scholar]
- 6. Takeda K, Yokoe M, Takada T et al. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J. Hepatobiliary Pancreat. Sci. 2010; 17: 37–44. [DOI] [PubMed] [Google Scholar]
- 7. Smeets XJNM, Bouhouch N, Buxbaum J et al. The revised Atlanta criteria more accurately reflect severity of post‐ERCP pancreatitis compared to the consensus criteria. United European Gastroenterol. J. 2019; 7: 557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim EJ, Cho JH, Oh KY, Kim SY, Kim YS. The risk factors for moderately severe and severe post‐endoscopic retrograde cholangiopancreatography pancreatitis according to the revised Atlanta classification. Pancreas. 2017; 46: 1208–13. [DOI] [PubMed] [Google Scholar]
- 9. Banks PA, Bollen TL, Dervenis C et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62: 102–11. [DOI] [PubMed] [Google Scholar]
- 10. Yokoe M, Takeda T, Mayumi T et al. Japanese guidelines for the management of acute pancreatitis: Japanese guidelines 2015. J. Hepatobiliary Pancreat. Sci. 2015; 22: 405–32. [DOI] [PubMed] [Google Scholar]
- 11. Banks PA. Freeman ML and the Practice Parameters Committee of American College of Gastroenterology. Practice Guidelines in Acute Pancreatitis. Am. J. Gastroenterol. 2006; 101: 2379–400. [DOI] [PubMed] [Google Scholar]
- 12. Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990; 174: 331–6. [DOI] [PubMed] [Google Scholar]
- 13. Balthazar EJ, Freeny PC, van Sonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994; 193: 297–306. [DOI] [PubMed] [Google Scholar]
- 14. Mortele KJ, Wiesner W, Intriere L et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am. J. Roentgenol. 2004; 183: 1261–5. [DOI] [PubMed] [Google Scholar]
- 15. Hamada T, Yasunaga H, Nakai Y et al. Japanese severity score for acute pancreatitis well predicts in‐hospital mortality: a nationwide survey of 17,901 cases. J. Gastroenterol. 2013; 48: 1384–91. [DOI] [PubMed] [Google Scholar]
- 16. Chandrasekhara V, Khashab MA, Muthusamy VR et al. Adverse events associated with ERCP. Gastrointest. Endosc. 2017; 85: 32–47. [DOI] [PubMed] [Google Scholar]
- 17. Dumonceau JM, Andriulli A, Elmunzer BJ et al. Prophylaxis of post‐ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – updated June 2014. Endoscopy. 2014; 46: 799–815. [DOI] [PubMed] [Google Scholar]
- 18. Freeman ML, DiSario JA, Nelson DB et al. Risk factors for post‐ERCP pancreatitis: a prospective, multicenter study. Gastrointest. Endosc. 2001; 54: 425–34. [DOI] [PubMed] [Google Scholar]
- 19. Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta‐analysis. Endoscopy. 2003; 35: 830–4. [DOI] [PubMed] [Google Scholar]
- 20. Sun HL, Han B, Zhai HP, Cheng XH, Ma K. Rectal NSAIDs for the prevention of post‐ERCP pancreatitis: a meta‐analysis of randomized controlled trials. Surgeon. 2014; 12: 141–7. [DOI] [PubMed] [Google Scholar]
- 21. Meister T, Heinzow H, Heinecke A, Hoehr R, Domschke W, Domagk D. Post‐ERCP pancreatitis in 2364 ERCP procedures: is intraductal ultrasonography another risk factor? Endoscopy. 2011; 43: 331–6. [DOI] [PubMed] [Google Scholar]
- 22. Shimatani M, Matsushita M, Takaoka M et al. Effective “short” double‐balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy. 2009; 41: 849–54. [DOI] [PubMed] [Google Scholar]
- 23. Sofuni A, Maguchi H, Mukai T et al. Endoscopic pancreatic duct stents reduce the incidence of PEP in high―risk patients. Clin. Gastroenterol. Hepatol. 2011; 9: 851–8. [DOI] [PubMed] [Google Scholar]
- 24. Buxbaum J, Yan A, Yeh K, Lane C, Nguyen N, Laine L. Aggressive hydration with lactated Ringer's solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin. Gastroenterol. Hepatol. 2014; 12: 303–307.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaygan‐Nejad A, Masjedizadeh AR, Ghavidel A, Ghojazadeh M, Khoshbaten M. Aggressive hydration with Lactated Ringer's solution as the prophylactic intervention for post endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled double‐blind clinical trial. J. Res. Med. Sci. 2015; 20: 838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi JH, Kim HJ, Lee BU, Kim TH, Song IH. Vigorous periprocedural hydration with lactated ringer's solution reduces the risk of pancreatitis after retrograde cholangiopancreatography in hospitalized patients. Clin. Gastroenterol. Hepatol. 2017; 15: 86–92.e1. [DOI] [PubMed] [Google Scholar]
- 27. Mok SRS, Ho HC, Shah P, Patel M, Gaughan JP, Elfant AB. Lactated Ringer's solution in combination with rectal indomethacin for prevention of post‐ERCP pancreatitis and readmission: a prospective randomized, double‐blinded, placebo‐controlled trial. Gastrointest. Endosc. 2017; 85: 1005–13. [DOI] [PubMed] [Google Scholar]
- 28. Sethi S, Sethi N, Wadhwa V, Garud S, Brown A. A meta‐analysis on the role of rectal diclofenac and indomethacin in the prevention of post‐endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2014; 43: 190–7. [DOI] [PubMed] [Google Scholar]
- 29. Shen C, Shi Y, Liang T, Su P. Rectal NSAIDs in the prevention of post‐endoscopic retrograde cholangiopancreatography pancreatitis in unselected patients: systematic review and meta‐analysis. Dig. Endosc. 2017; 29: 281–90. [DOI] [PubMed] [Google Scholar]
- 30. Patai A, Solymosi N, Mohacsi L, Patai AV. Indomethacin and diclofenac in the prevention of post‐ERCP pancreatitis: a systematic review and meta‐analysis of prospective controlled trials. Gastrointest. Endosc. 2017; 85: 1144–1156.e1. [DOI] [PubMed] [Google Scholar]
- 31. Mazaki T, Mado K, Masuda H, Shiono M. Prophylactic pancreatic stent placement and post‐ERCP pancreatitis: an updated meta‐analysis. J. Gastroenterol. 2014; 49: 343–55. [DOI] [PubMed] [Google Scholar]
- 32. Choudhary A, Bechtold ML, Arif M et al. Pancreatic stents for prophylaxis against post‐ERCP pancreatitis: a meta‐analysis and systematic review. Gastrointest. Endosc. 2011; 73: 275–82. [DOI] [PubMed] [Google Scholar]


