Abstract
Background
Symptoms experienced by adult patients with attention-deficit/hyperactivity disorder (ADHD) frequently result in functional impairment across academic/occupational functioning, daily life, and social functioning. A substantial proportion of undiagnosed and untreated ADHD has been suggested in Japan. This study aims to better understand the potential undiagnosed ADHD population in Japan by quantifying the burden associated with ADHD symptoms through a comparison of the prevalence of comorbidities, health-related quality of life (HRQoL), work productivity and activity impairment (WPAI), and healthcare resource utilization (HRU) between undiagnosed potential ADHD respondents who were screened positive and negative using Adult ADHD Self-Report Scale (ASRS)-v1.1.
Methodology
Respondents from Japan National Health and Wellness Survey 2016 who answered ASRS-v1.1 without an ADHD diagnosis were included. Respondents checking ≥4 items from ASRS-A and ≥9 from ASRS-A+B were classified as ASRS A+ (n = 309) and ASRS AB+ (n = 227), respectively. ASRS negative (n = 9,280) were respondents who were neither ASRS A+ nor ASRS AB+. Data on the presence of comorbidities, HRQoL, WPAI, and HRU were compared.
Results
ASRS A+ and ASRS AB+ respondents reported higher coexistence of mental comorbidities (depression, generalized anxiety disorder, bipolar disorder, obsessive-compulsive disorder, etc.), sleep problems (insomnia, narcolepsy, sleep apnea, etc.), and physical comorbidities (non-alcoholic steatohepatitis, allergy, and asthma). They also reported greater WPAI and HRU and lower HRQoL than matched ASRS-negative respondents.
Conclusions
A significantly higher burden was identified among undiagnosed adults with potential ADHD symptoms. Appropriate diagnosis may help those at risk or those who present with symptoms overlapping with ADHD.
Keywords: undiagnosed adhd, resource utilization, work productivity, quality of life, adhd symptoms with comorbidities, asrs
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder that is characterized by inattention and/or excessive activity and impulsivity symptoms [1]. Symptoms of ADHD are usually observed in childhood and some symptoms, especially inattention rather than hyperactivity and impulsivity, can persist into adulthood [2]. These symptoms experienced by patients with ADHD frequently result in functional impairment across academic/occupational functioning, daily life, and social functioning [3].
ADHD prevalence was reported to be 6-10% in US children and adolescents between 1997 and 2016 [4]. In adults, the global prevalence of persistent ADHD and symptomatic ADHD was estimated at 2.58% and 6.76%, respectively [5]. Although the prevalence of ADHD in adults in the United States has been estimated to be 5.2% [6], the rates of diagnosed/treated patients have been reported as only 0.8% [7]. ADHD prevalence among Japanese adults was estimated to be around 1.65% [8], which is lower than the estimated 2.5% prevalence of ADHD in adults from a meta-analysis [9]. This could potentially indicate a sizable proportion of undiagnosed and consequently untreated ADHD patients in Japan.
Several studies have reported that diagnosed adult ADHD patients have high rates of comorbidities associated with psychiatric diseases, including depression and generalized anxiety disorder, and somatic diseases, including allergic diseases, obesity, and metabolic disorders, and are suffering from the disease burden of ADHD [10-13]. However, the burden of adults with ADHD symptoms without formal ADHD diagnosis has not been clarified yet.
ADHD in adults is diagnosed according to the guidelines of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [1]. The Adult ADHD Self-Report Scale version 1.1 (ASRS-v1.1) Symptom Checklist is an extensively used 18-item screening tool that comprises 18 Category A symptoms from the DSM-Fourth Edition (DSM-IV) to identify potential ADHD adults and the severity of their symptoms [14,15]. The ASRS-v1.1 Symptom Checklist comprises a six-item Part A and a 12-item Part B [16]. Part A forms the basis for the ASRS-v1.1 Screener (ASRS-A) and has been validated as a pre-diagnostic screening tool to help recognize at-risk ADHD adults with a sensitivity of 91.4% and a specificity of 96.0% in the general population [14]. Part B provides additional information regarding the ADHD symptoms that respondents experienced [16]. Together, Part A and Part B form the full ASRS-v1.1 Symptom Checklist, which helps healthcare providers assess adult ADHD symptom profile and the level of impairment. The ASRS-v1.1 Symptom Checklist could also be categorized into the symptom domains of inattention and hyperactivity/impulsivity comprising nine items each, which is consistent with the DSM-IV ADHD criteria [15]. Updates based on the DSM-5 version of the screening scale of the ASRS-v1.1 instrument included the reduction of the minimum number of symptoms from six to five in either symptom domain (inattention or hyperactive/impulsive). This reduction in symptoms expanded the application of the screening scale to older adolescents and adults, allowing for more effective identification of adults with ADHD [17].
Although the ASRS-v1.1 instrument has been commonly used as an initial self-assessment tool and changes in the DSM-5 criteria increase the likelihood of identifying ADHD in adults, it remains unknown how the disease burden and baseline characteristics differ between respondents who had not been diagnosed with ADHD but were screened positive and negative. Knowing the potential disease burden and baseline characteristics of at-risk ADHD adults would help better inform and navigate them for proper diagnosis and treatment.
This study aims to address this by quantifying the burden associated with potential ADHD symptoms through a comparison of the prevalence of comorbidities, health-related quality of life (HRQoL), work productivity and activity impairment (WPAI), and healthcare resource utilization (HRU) between respondents screened ASRS-v1.1 positive and negative but not clinically diagnosed with ADHD in Japan.
Materials and methods
Data source
The study utilized existing data from the Japan National Health and Wellness Survey (NHWS) conducted in 2016. The NHWS is a cross-sectional, internet-based, self-administered survey of a nationwide sample of respondents aged 18 or above. Respondents for the NHWS were recruited from the existing members of web-based opt-in consumer panels, and only those who provided informed consent were included in the study. Panel members were recruited through opt-in e-mails, e-newsletters, banner placements, and co-registration with panel partners. A stratified random sampling procedure, stratified by age and gender, was implemented to ensure that the demographic composition of the NHWS respondents was representative of the general adult population in Japan.
The NHWS survey was approved with exemption status upon review by Pearl Institutional Review Board (Indianapolis, IN, IRB Study Number: 16-KAN-124). All NHWS respondents provided informed consent prior to participating.
Study population
A random subset among all 2016 Japan NHWS respondents was selected and asked to complete the ASRS-v1.1 Symptom Checklist. Only respondents who responded to the ASRS-v1.1 Symptom Checklist were included in the study. Those who self-reported a diagnosis of ADHD by a physician were excluded. Respondents would indicate in the boxes of the copyrighted two-part ASRS-v1.1 Symptom Checklist that most closely represent the frequency of the occurrence for each symptom [15,16]. Responses for each question in each part of the ASRS-v1.1 Symptom Checklist were categorized into the number of darkly shaded boxes marked after the survey. The respondents were subsequently defined as the following: (1) “ASRS-A+” if at least four out of six questions were in the darkly shaded boxes in Part A; (2) “ASRS-AB+” if at least nine out of eighteen questions were in the darkly shaded boxes in Part A and Part B; and (3) “Control (ASRS-negative respondents)” if respondents were screened neither ASRS-A+ nor ASRS-AB+.
Measures, survey instruments, and outcome assessment
Demographic and general health characteristics measured in this study included gender, age, marital status, education, household income, employment status, Charlson Comorbidity Index (CCI) [18-20], body mass index (BMI), smoking status, alcohol use, and exercise behavior. Experience of ADHD-related symptoms was self-reported using the Japanese version of the ASRS-v1.1 Symptom Checklist [21].
Experience of comorbidities (anxiety, depression, generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, phobias, post-traumatic stress disorder, social anxiety disorder, schizophrenia, bipolar disorder, insomnia, narcolepsy, sleep apnea, sleep difficulties, allergies, asthma, chronic obstructive pulmonary disease, high cholesterol, type 2 diabetes mellitus (T2DM), obesity, hypertension, alcoholism, and non-alcoholic steatohepatitis) was self-reported in the NHWS.
HRQoL was assessed using the Medical Outcomes Study Short-Form 36-Item Health Survey Version 2 (SF-36v2), an instrument used to measure an individual’s general health status [22]. The instrument is designed to report on eight health domains (physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health). The respondents completed this using the validated Japanese version of the SF-36v2 [23]. Scores from these domains can be used to calculate two summary scores: physical component summary (PCS) and mental component summary (MCS) scores. Summary scores were calculated using norm-based scoring algorithms. Higher scores indicate better quality of life. Health state utilities were evaluated using the EuroQol five dimension five-level (EQ-5D-5L) instrument that provides a simple, general measure of health through five questions [24]. The EQ-5D Index is a single number index value using a validated standard Japan value set [25]. An individual had better health status if they had higher scores.
The six-item WPAI validated questionnaire [26] was used to measure the impact of any health impairments on work and activities. In the WPAI, four measures of percentages (absenteeism, presenteeism, overall work productivity loss, and activity impairment) are generated, with higher percentages indicating more impairment due to health problems in the past seven days. Absenteeism measures percentage work time missed due to health, presenteeism measures the percentage of impairment at work due to health, overall work productivity loss measures impairment due to health using absenteeism and presenteeism measures, and activity impairment measures the percentage of impairment in daily activities due to health. Activity impairment measure is provided for all respondents but all other measures in the WPAI are only provided for respondents who reported work.
HRU was measured by the reported number of visits in the past six months to the emergency room (ER), healthcare providers (e.g., general and specialized physicians, internists, and dentists), and for hospitalizations because of the patient’s medical condition.
Analysis
To understand any underlying differences between respondents screened positive using ASRS-v1.1 Part A and controls, as well as between respondents screened positive using ASRS-v1.1 Part A+B and controls, a descriptive summary and comparison of their baseline demographic and health characteristics were made. Crude odds ratio (OR) of experiencing selected comorbidities among respondents screened positive using Part A and Part A+B compared to respondents screened negative were described.
A greedy matching algorithm was used to form the matched control groups for ASRS-A+ and ASRS-AB+ groups, respectively. Age, gender, marital status, education, employment status, household income, health insurance, CCI, BMI, alcohol consumption, and whether a respondent was a smoker were used in 1:4 propensity score matching. These factors were used in matching as previous studies have identified an association between the factors and health outcomes [27-29]. Bivariate comparisons were conducted after matching to ensure the covariates were balanced. Using 1:4 propensity score matching, the proportion of self-reported experience of comorbidities and health outcomes were compared between ASRS-v1.1-positive respondents and matched controls. Categorical variables and continuous variables were assessed using the chi-square test and one-way analyses of variance (ANOVA), respectively.
Statistical significance was evaluated at the 0.05 significance level during data analysis using SPSS Statistics Version 25 (IBM Corp., Armonk, NY, USA) [30] and R Version 3.4.4 [31].
Results
Participants
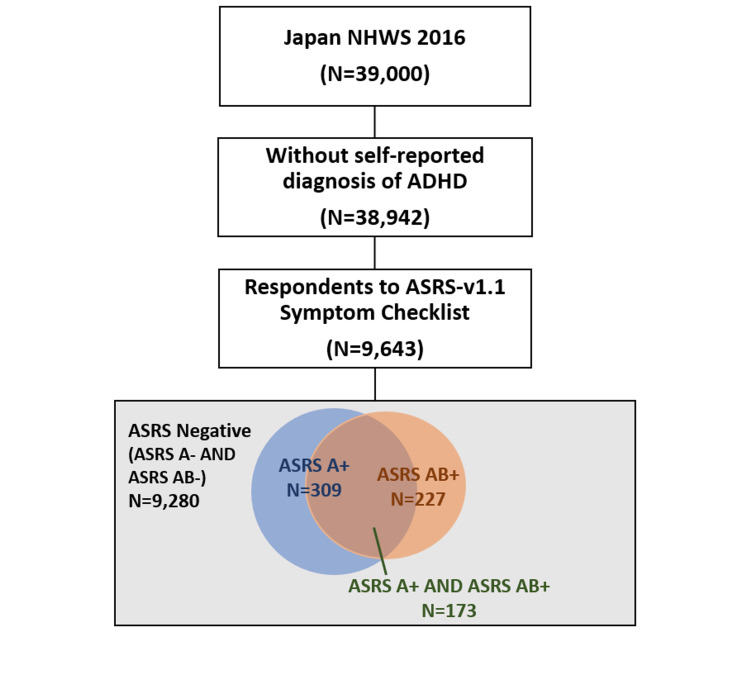
A total of 9,643 respondents without a diagnosis of ADHD who responded to the ASRS-v1.1 Symptom Checklist were included in the study. Of those, 9,280 (96.2% of all who responded to the ASRS-v1.1) were screened negative using Part A and Part A+B (control), 309 (3.2% of all who responded to ASRS-v1.1) were screened positive for Part A (ASRS-A+), and 227 (2.4%) were screened positive for Part A+B (ASRS-AB+). A total of 173 (1.8%) respondents were screened positive for both Part A and Part A+B (Figure 1). A total of 1,236 matched controls were identified for ASRS-A+ (matched ASRS-A-) and 908 matched controls for ASRS-AB+ (matched ASRS-AB-).
Figure 1. Respondent flow chart.
Screened positive figures using Part A and using Part A+B are not exclusive. A total of 173 respondents were screened positive using both Part A and Part A+B. ASRS-negative are those who were screened negative for both Part A and Part A+B.
ADHD: attention-deficit hyperactivity disorder; ASRS-v1.1: Adult ADHD Self-Report Scale version 1.1; NHWS: National Health and Wellness Survey
Demographic and general health characteristics
Compared to the respective ASRS-negative controls, ASRS-A+ and ASRS-AB+ respondents tended to be younger (mean [standard deviation: SD]: 42.91 [15.43] and 41.56 [15.23] vs. 50.74 [16.24]) and had a higher CCI (0.26 [0.80] and 0.23 [0.85] vs. 0.19 [0.56]) (Table 1). Compared to ASRS-negative controls, fewer ASRS-A+ and ASRS-AB+ respondents were married/living with partner (43.04% and 37.44% vs. 64.44%) and had completed university (39.48% and 40.09% vs. 45.04%). ASRS-A+ and ASRS-AB+ respondents also had lower household income compared to ASRS-negative respondents.
Table 1. Demographic and general health characteristics among ASRS-negative, ASRS Part A positive (score ≥4) (ASRS-A+), and ASRS Part A+B positive (score ≥9) (ASRS-AB+) prior to matching.
ASRS: Adult ADHD Self-Report Scale; BMI: body mass index; SD: standard deviation
| ASRS negative (N = 9,280) | ASRS-A+ (N = 309) | ASRS-AB+ (N = 227) | |||||
| Continuous variable | Mean | SD | Mean | SD | Mean | SD | |
| Age | 50.74 | 16.24 | 42.91 | 15.43 | 41.56 | 15.23 | |
| Charlson Comorbidity Index | 0.19 | 0.56 | 0.26 | 0.80 | 0.23 | 0.85 | |
| ASRS score | ASRS Part A score | 0.36 | 0.83 | 4.73 | 0.84 | 4.55 | 1.36 |
| ASRS Part B score | 0.41 | 1.04 | 5.77 | 3.98 | 8.37 | 2.70 | |
| ASRS Part A+B score | 0.77 | 1.70 | 10.50 | 4.61 | 12.92 | 3.49 | |
| Categorical variable | Count | % | Count | % | Count | % | |
| Gender | Male | 4,888 | 52.67% | 164 | 53.07% | 115 | 50.66% |
| Female | 4,392 | 47.33% | 145 | 46.93% | 112 | 49.34% | |
| Marital status | Married or living with partner | 5,980 | 64.44% | 133 | 43.04% | 85 | 37.44% |
| Divorced/separated/widowed | 3,273 | 35.27% | 171 | 55.34% | 137 | 60.35% | |
| Decline to answer | 27 | 0.29% | 5 | 1.62% | 5 | 2.20% | |
| Level of education | Not completed university | 5,014 | 54.03% | 180 | 58.25% | 129 | 56.83% |
| Completed university education | 4,180 | 45.04% | 122 | 39.48% | 91 | 40.09% | |
| Decline to answer | 86 | 0.93% | 7 | 2.27% | 7 | 3.08% | |
| Household income | Less than ¥3,000,000 | 1,509 | 16.26% | 71 | 22.98% | 57 | 25.11% |
| ¥3,000,000 to | 2,246 | 24.20% | 80 | 25.89% | 50 | 22.03% | |
| ¥5,000,000 to | 2,259 | 24.34% | 76 | 24.60% | 48 | 21.15% | |
| ¥8,000,000 or more | 1,958 | 21.10% | 46 | 14.89% | 40 | 17.62% | |
| Decline to answer | 1,308 | 14.09% | 36 | 11.65% | 32 | 14.10% | |
| Employment status | Currently not employed | 3,534 | 38.08% | 122 | 39.48% | 83 | 36.56% |
| Currently employed | 5,746 | 61.92% | 187 | 60.52% | 144 | 63.44% | |
| BMI | Underweight (BMI < 18.5) | 951 | 10.25% | 28 | 9.06% | 25 | 11.01% |
| Normal weight (18.5 ≤ BMI ≤ 22.9) | 4,590 | 49.46% | 143 | 46.28% | 101 | 44.49% | |
| Pre-obese (23 ≤ BMI ≤ 24.9) | 1,615 | 17.40% | 47 | 15.21% | 32 | 14.10% | |
| Obese (BMI ≥ 25) | 1,651 | 17.79% | 71 | 22.98% | 49 | 21.59% | |
| Decline to answer | 473 | 5.10% | 20 | 6.47% | 20 | 8.81% | |
| Smoking status | Current smoker | 1,616 | 17.41% | 51 | 16.50% | 43 | 18.94% |
| Former smoker | 2,440 | 26.29% | 77 | 24.92% | 40 | 17.62% | |
| Never smoker | 5,224 | 56.29% | 181 | 58.58% | 144 | 63.44% | |
| Alcohol use | No use | 3,092 | 33.32% | 119 | 38.51% | 95 | 41.85% |
| Currently use alcohol | 6,188 | 66.68% | 190 | 61.49% | 132 | 58.15% | |
| Exercise status | No exercise | 4,329 | 46.65% | 169 | 54.69% | 129 | 56.83% |
| Currently exercise | 4,951 | 53.35% | 140 | 45.31% | 98 | 43.17% | |
After matching, all demographic and general health characteristics were balanced between matched ASRS-A- and ASRS-A+ respondents, as well as between matched ASRS-AB- and ASRS-AB+ respondents (Table 4 in the Appendices).
Prevalence of comorbidities among ASRS-positive and negative respondents
Prior to propensity score matching, significantly more ASRS-A+ and ASRS-AB+ respondents reported experiencing mental and neurological disorders, sleep problems, and physical illnesses (Table 2).
Table 2. Self-reported experience of comorbidities between unmatched ASRS-negative control group and ASRS-positive groups, between matched ASRS-A-negative and ASRS-A+ (score ≥4 for Part A), and between matched ASRS-AB-negative and ASRS-AB+ (score ≥9 for both Parts A and B).
ap < 0.05 comparing to ASRS-negative prior matching; bp < 0.05 comparing to matched ASRS A-; cp < 0.05 comparing to matched ASRS AB-.
ASRS: Adult ADHD Self-Report Scale
| Prior matching | Matched ASRS-A- versus ASRS-A+ | Matched ASRS-AB- versus ASRS-AB+ | ||||||||
| ASRS negative (N = 9,280) | Matched ASRS-A- (N = 1,236) | ASRS-A+ (N=309) | Matched ASRS-AB- (N = 908) | ASRS-AB+ (N = 227) | ||||||
| Mental and neurological disorders | Count | % | Count | % | Count | % | Count | % | Count | % |
| Anxiety | 1,328 | 14.31% | 234 | 18.93% | 119 | 38.51%ab | 179 | 19.71% | 80 | 35.24%ac |
| Depression | 295 | 3.18% | 53 | 4.29% | 65 | 21.04%ab | 38 | 4.19% | 56 | 24.67%ac |
| Generalized anxiety disorder | 33 | 0.36% | 5 | 0.40% | 15 | 4.85%ab | 2 | 0.22% | 16 | 7.05%ac |
| Obsessive-compulsive disorder | 43 | 0.46% | 12 | 0.97% | 10 | 3.24%ab | 6 | 0.66% | 10 | 4.41%ac |
| Panic disorder | 99 | 1.07% | 16 | 1.29% | 19 | 6.15%ab | 18 | 1.98% | 16 | 7.05%ac |
| Phobias | 51 | 0.55% | 14 | 1.13% | 19 | 6.15%ab | 11 | 1.21% | 16 | 7.05%ac |
| Post-traumatic stress disorder | 45 | 0.48% | 7 | 0.57% | 9 | 2.91%ab | 6 | 0.66% | 6 | 2.64%ac |
| Social anxiety disorder | 97 | 1.05% | 25 | 2.02% | 36 | 11.65%ab | 14 | 1.54% | 27 | 11.89%ac |
| Schizophrenia | 75 | 0.81% | 17 | 1.38% | 21 | 6.80%ab | 11 | 1.21% | 18 | 7.93%ac |
| Bipolar disorder | 45 | 0.48% | 10 | 0.81% | 18 | 5.83%ab | 4 | 0.44% | 16 | 7.05%ac |
| Sleep problems | Count | % | Count | % | Count | % | Count | % | Count | % |
| Insomnia | 637 | 6.86% | 96 | 7.77% | 87 | 28.16%ab | 78 | 8.59% | 63 | 27.75%ac |
| Narcolepsy | 21 | 0.23% | 4 | 0.32% | 5 | 1.62%ab | 6 | 0.66% | 5 | 2.20%ac |
| Sleep apnea | 249 | 2.68% | 32 | 2.59% | 24 | 7.77%ab | 18 | 1.98% | 12 | 5.29%ac |
| Sleep difficulties (other than insomnia, narcolepsy or sleep apnea) | 99 | 1.07% | 19 | 1.54% | 16 | 5.18%ab | 21 | 2.31% | 16 | 7.05%ac |
| Physical conditions | Count | % | Count | % | Count | % | Count | % | Count | % |
| Allergies | 951 | 10.25% | 144 | 11.65% | 58 | 18.77%ab | 115 | 12.67% | 51 | 22.47%ac |
| Asthma | 297 | 3.20% | 41 | 3.32% | 26 | 8.41%ab | 32 | 3.52% | 23 | 10.13%ac |
| Chronic obstructive pulmonary disease | 32 | 0.34% | 4 | 0.32% | 3 | 0.97% | 2 | 0.22% | 4 | 1.76%ac |
| High cholesterol | 1,114 | 12.00% | 113 | 9.14% | 37 | 11.97% | 69 | 7.60% | 26 | 11.45% |
| Type 2 diabetes mellitus | 379 | 4.08% | 55 | 4.45% | 14 | 4.53% | 35 | 3.85% | 13 | 5.73% |
| Obesity | 1,651 | 17.79% | 270 | 21.84% | 71 | 22.98%a | 198 | 21.81% | 49 | 21.59% |
| Hypertension | 1,370 | 14.76% | 140 | 11.33% | 36 | 11.65% | 72 | 7.93% | 28 | 12.33%c |
| Alcoholism | 58 | 0.63% | 1 | 0.08% | 9 | 2.91%ab | 5 | 0.55% | 5 | 2.20%ac |
| Non-alcoholic steatohepatitis | 43 | 0.46% | 4 | 0.32% | 6 | 1.94%ab | 6 | 0.66% | 5 | 2.20%ac |
From an association perspective, ASRS-A+ and ASRS-AB+ respondents were significantly more likely to experience depression (OR [95% confidence interval: CI]: 8.11 [6.03, 10.92] and 9.97 [7.22, 13.78]), generalized anxiety disorder (14.30 [7.68, 26.61] and 21.25 [11.52, 39.20]), bipolar disorder (12.69 [7.26, 22.20] and 15.56 [8.66, 27.98]), insomnia (5.32 [4.10, 6.90] and 5.21 [3.86, 7.05]), allergies (2.02 [1.51, 2.71] and 2.54 [1.85, 3.49]), and asthma (2.78 [1.83, 4.22] and 3.41 [2.18, 5.33]) than ASRS-negative controls (Figure 2).
Figure 2. Crude odds ratio of experiencing selected comorbidities among respondents screened positive using ASRS-v1.1 compared to respondents screened negative.
Error bar represents 95% confidence interval for the crude odds ratio.
ASRS-v1.1: Adult ADHD Self-Report Scale version 1.1
After propensity score matching, a significantly higher proportion of ASRS-A+ respondents experienced mental and neurological disorders, including anxiety, depression, generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, phobias, post-traumatic stress disorder, social anxiety disorder, schizophrenia, and bipolar disorder, compared to matched ASRS-A- respondents. Significantly more ASRS-A+ respondents also experienced sleep problems, including insomnia, narcolepsy, sleep apnea, and other sleep difficulties (other than insomnia, narcolepsy, or sleep apnea), compared to matched ASRS-A- respondents. In addition, significantly more ASRS-A+ respondents also reported experiencing some physical illnesses, including allergies, asthma, alcoholism, and non-alcoholic steatohepatitis, compared to matched ASRS-A- respondents (Table 2). Similar to ASRS-A+ respondents, significantly more respondents screened ASRS-AB+ also reported experiencing similar mental and neurological disorders, sleep problems, and physical illnesses compared to matched ASRS-AB- respondents (Table 2).
Health outcomes assessment among ASRS-positive and negative respondents
After propensity score matching, ASRS-A+ respondents reported significantly lower HRQoL relative to matched ASRS-A- respondents in PCS (48.02 [8.27] vs. 52.72 [6.55], p < 0.001), MCS (34.44 [11.69] vs. 45.90 [9.66], p < 0.001), and EQ-5D-5L index (0.68 [0.21] vs. 0.84 [0.16], p < 0.001) (Table 3). ASRS-A+ respondents reported significantly increased absenteeism (12.01% [23.32%] vs. 4.10% [14.99%], p < 0.001), presenteeism (46.42% [29.77%] vs. 20.94% [24.85%], p < 0.001), total work productivity impairment (49.82% [30.37%] vs. 22.60% [26.24%], p < 0.001), and total activity impairment (48.64% [28.88%] vs. 23.34% [25.56%], p < 0.001) compared to matched ASRS A- respondents. The impairment was more than two times that of the matched ASRS-A- respondents. In terms of HRU, compared to matched ASRS A- respondents, ASRS A+ respondents had significantly more visits to the HCP (7.85 [11.83] vs. 4.59 [7.40], p < 0.001) and reported more numbers of hospitalizations (2.16 [15.03] vs. 0.82 [6.79], p = 0.020) in the past six months (Table 3).
Table 3. HRQoL, WPAI, and HRU between matched ASRS-negative and ASRS-A+ (score ≥4 for Part A) and between matched ASRS-negative and ASRS-AB+ (score ≥9 for both Parts A and B).
ASRS: Adult ADHD Self-Report Scale; EQ-5D-5L: ER: emergency room; EuroQol five dimension five-level; HCP: healthcare provider; HRU: healthcare resource utilization; SD: standard deviation; WPAI: work productivity and activity impairment
| Matched ASRS-A- versus ASRS-A+ | Matched ASRS-AB- versus ASRS-AB+ | |||||||||
| Matched ASRS A- (N = 1,236) | ASRS A+ (N = 309) | P-value | Matched ASRS AB- (N = 908) | ASRS AB+ (N = 227) | P-value | |||||
| Health-related quality of life | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Physical component summary | 52.72 | 6.55 | 48.02 | 8.27 | <0.001 | 52.94 | 6.29 | 47.42 | 8.22 | <0.001 |
| Mental component summary | 45.90 | 9.66 | 34.44 | 11.69 | <0.001 | 45.82 | 9.66 | 32.60 | 11.31 | <0.001 |
| EQ-5D-5L index | 0.84 | 0.16 | 0.68 | 0.21 | <0.001 | 0.85 | 0.16 | 0.66 | 0.21 | <0.001 |
| Work productivity and activity impairment | Mean | SD | Mean | SD | P-value | Mean | SD | Mean | SD | P-value |
| Absenteeism | 4.10% | 14.99% | 12.01% | 23.32% | <0.001 | 3.57% | 12.59% | 15.14% | 24.37% | <0.001 |
| Presenteeism | 20.94% | 24.85% | 46.42% | 29.77% | <0.001 | 22.07% | 24.49% | 51.25% | 29.15% | <0.001 |
| Total work productivity impairment | 22.60% | 26.24% | 49.82% | 30.37% | <0.001 | 23.99% | 25.86% | 55.84% | 29.16% | <0.001 |
| Total activity impairment | 23.34% | 25.56% | 48.64% | 28.88% | <0.001 | 23.99% | 25.83% | 53.13% | 27.56% | <0.001 |
| Healthcare resource utilization | Mean | SD | Mean | SD | P-value | Mean | SD | Mean | SD | P-value |
| Number of HCP visits in past 6 months | 4.59 | 7.40 | 7.85 | 11.83 | <0.001 | 3.99 | 6.90 | 8.03 | 11.52 | <0.001 |
| Number of ER visits in the past 6 months | 0.08 | 0.62 | 0.37 | 5.13 | 0.056 | 0.04 | 0.31 | 0.48 | 5.98 | 0.029 |
| Number of hospitalizations in the past 6 months | 0.82 | 6.79 | 2.16 | 15.03 | 0.020 | 0.60 | 5.00 | 2.41 | 17.15 | 0.006 |
Similarly, after propensity score matching, ASRS-AB+ respondents reported significantly lower HRQoL relative to matched ASRS-AB- respondents in PCS (47.42 [8.22] vs. 52.94 [6.29], p < 0.001), MCS (32.60 [11.31] vs. 45.82 [9.66], p < 0.001), and EQ-5D-5L index (0.66 [0.21] vs. 0.85 [0.16], p < 0.001) (Table 3). ASRS AB+ respondents reported significantly increased absenteeism (15.14% [24.37%] vs. 3.57% [12.59%], p < 0.001), presenteeism (51.25% [29.15%] vs. 22.07% [24.49%], p < 0.001), work productivity impairment (55.84% [29.16%] vs. 23.99% [25.86%], p < 0.001), and activity impairment (53.13% [27.56%] vs. 23.99% [25.83%], p < 0.001) compared to matched ASRS-AB- respondents. The impairment was also more than two times that of the matched ASRS-AB- respondents. In terms of HRU, compared to matched ASRS-AB- respondents, ASRS-AB+ respondents had significantly more HCP visits (8.03 [11.52] vs. 3.99 [6.90], p < 0.001) and ER visits (0.48 [5.98] vs. 0.04 [0.31], p = 0.029) and reported more hospitalizations (2.41 [17.15] vs. 0.60 [5.00], p = 0.006) in the past six months (Table 3).
Discussion
In this study, we investigated the physical and mental burden among the population in Japan who remained undiagnosed with ADHD but were screened to have potential ADHD symptoms using the ASRS-v1.1 Screener and Symptom Checklist. Among the 9,643 respondents who were undiagnosed with ADHD, 3.2% (n = 309) and 2.4% (n = 227) were found to have been screened ASRS-A+ and ASRS-AB+, respectively. Only 1.8% (n = 173) were screened positive for both ASRS-A and ASRS-AB. In Japan, the prevalence of diagnosed ADHD was previously reported to be 1.65% [8], which is lower than the 2.5-3.4% average prevalence among adults worldwide [6,9]. If we consider our study population, that is, those who were undiagnosed with ADHD but were screened positive using the ASRS-v1.1 instrument, the prevalence of ADHD in Japan may be higher and closer to the current worldwide prevalence.
In our study, those who were undiagnosed for ADHD but were screened ASRS-positive (irrespective of ASRS-A or ASRS-AB) tended to be younger, had lower income levels, and were significantly less likely to be married or living with a partner or have completed a university education compared to non-ADHD controls (Table 1). The sociodemographic differences in income levels and marital status observed between the two groups could have been because the ASRS-A+ and ASRS-AB+ groups were significantly younger than the matched control group. This is also consistent with previously published findings demonstrating poorer academic, social, and occupational functioning among adults diagnosed with ADHD [3,12].
In terms of health characteristics, previous studies have reported a higher risk of substance use and dependence, for example, consumption of alcohol and smoking, in patients with ADHD [32,33]. In addition, a possible genetic basis for the co-occurrence of substance use disorder and ADHD has been reported [34]. Our study findings supported this, though fewer ASRS+ respondents reported currently drinking alcohol, alcoholism occurred at an approximately 4-36-fold greater rate among ASRS-positive respondents compared to ASRS-negative respondents.
Our study showed that ASRS-positive respondents with potential ADHD symptoms experienced significantly more mental and neurological comorbidities (e.g., depression, generalized anxiety disorder, bipolar disorder, insomnia, social anxiety disorder) and physical comorbidities (e.g., allergies, asthma, sleep apnea, non-alcoholic steatohepatitis), even after propensity score matching. This is in line with previous studies reporting that ADHD patients have higher rates of other comorbid mental disorders with symptoms overlapping ADHD [12,35]. Compared to the study by Kirino et al., our study found a smaller difference in the proportion of respondents with comorbid mental disorders compared to matched controls, that is, depression, generalized anxiety disorder, and sleep difficulties. This may be attributed to the possibility that other comorbid conditions of the undiagnosed ADHD population also remain undiagnosed compared to diagnosed patients. This also highlights the importance of appropriate diagnosis of ADHD and other comorbidities.
While Kooij et al. only focused on central nervous system (CNS)-related conditions, other studies have investigated the association between ADHD and physical disorders such as inflammation, allergic diseases [13,36], obesity, T2DM, and hypertension [10,37]. For example, Wang et al. reported a significant association between ADHD risk and the presence of rhinitis and eczema symptoms among children [36]. Likewise, our study showed that allergies were approximately two-fold more common compared to matched controls. Although causal mechanisms remain unknown, this may further support the theory of a shared etiology, linking an increase in proinflammatory biochemical mechanisms to physical comorbidities as well as brain circuits associated with emotional and behavior control in patients with ADHD [13]. These psychiatric symptoms have been reported to present more severely with the co-occurrence of ADHD and other psychiatric disorders [38]. It has been reported that adults with ADHD also have a higher prevalence of T2DM, obesity, and hypertension compared to those without ADHD [10,37]. Although a significant association between ADHD and obesity has been reported [39], a higher prevalence of obesity was not evident among adults with ADHD symptoms in this study. This might be due to the lower rate and/or milder level of obesity in Japan compared to Western populations [40], or due to the adjustment of BMI used in matching. It could be that obesity might be lower in undiagnosed adults with ADHD symptoms compared to diagnosed ADHD patients. In our study, the prevalence for T2DM and hypertension among those with ADHD symptoms were 4.5-5.7% and 11.7-12.3% respectively, compared to 3.9% and 8.5%, respectively, in adults with ADHD from previous reports [37]. Matched ASRS-negative controls in our study also had a higher prevalence of 3.9-4.4% for T2DM and 7.9-11.3% for hypertension compared to 1.6% and 4.5%, respectively, for adults without ADHD in previous reports [37]. While our analysis showed there were no statistically significant differences in T2DM and hypertension between ASRS-positive and negative groups, it is possible that the higher prevalence of somatic comorbidities and other differences observed between ASRS-positive and negative groups in our study was due to the higher mean age of the control group.
It is well established that ADHD with or without the presence of comorbidities contributes to poorer HRQoL [11,12]. Our study findings showed that this was true even among those undiagnosed for ADHD but were screened ASRS-positive. After propensity score matching, our study showed that the presence of ADHD symptoms itself was significantly associated with poorer HRQoL, not just in the MCS, but also poorer physical component scores. As for EQ-5D-5L, it has been reported as 0.74 among diagnosed adults with ADHD [11], and it was even lower (0.59) among those with psychiatric comorbidities [41]. In our study, EQ-5D-5L of 0.66 and 0.68 among undiagnosed adults with ADHD symptoms fell within the range above and was significantly lower than ASRS-negative respondents (0.84 and 0.85). However, how the EQ-5D-5L score is associated with comorbidities in undiagnosed adults with ADHD still needs to be investigated.
While our study could not evaluate the severity of ADHD symptoms, the ASRS+ group had more health problems, as shown by their significantly greater healthcare resource use compared to controls. The ASRS+ group reported approximately 1.7-2.0 times more physician visits, 4.6-12.0 times more emergency room visits, and 2.6-4.0 times more hospitalizations than their respective matched controls (Table 3). Because the study participants of this study have never been diagnosed and treated for ADHD, it is assumed that HRU was due to comorbidities other than ADHD or due to their increased risk of injuries [42]. Moreover, despite treatments for comorbidities, lower QoL and impaired work productivity were observed in undiagnosed adults with ADHD symptoms.
Similar to what has been reported for patients with ADHD [12], poorer occupational and social functioning was observed in ASRS+ respondents. The mean overall WPAI scores for the ASRS+ groups were over two times higher compared to the control group. Compared to matched controls, they reported a greater degree of absenteeism and presenteeism and impaired ability to engage in general daily activities due to health problems. It is known that overall work impairment was high (45.65%) in undiagnosed adults with ADHD symptoms [11]. It has also been reported that overall work impairment was higher (60.8%) in diagnosed ADHD patients with comorbidities [41]. In this study which included respondents with various comorbidities, overall work impairment was 49.82-55.84%. This was as severe as that reported for diagnosed ADHD patients with/without comorbidities. Absenteeism and presenteeism for major depressive disorder (MDD) patients in Canada were also reported as 22.2% and 61.5%, respectively [43]. As for MDD patients in Japan, absenteeism and presenteeism have been reported as 14.8% and 38.0%, respectively [44], contributing to significant disability and socioeconomic burden in Japan. Although some respondents with comorbid MDD were included in this study, the results showed that absenteeism (12.0-15.1%) and presenteeism (46.4-51.3%) were sizable in undiagnosed adults with ADHD symptoms. Moreover, having ADHD symptoms has been associated with similar impairment reported previously among patients with MDD.
While most studies highlighted the burden of ADHD among diagnosed adults compared to adults without ADHD, there remains a paucity of information regarding the potential burden associated with missed ADHD symptoms among the undiagnosed population. A cross-sectional, retrospective analysis of 247 patients revealed that the presence of any psychiatric symptom, in the presence or absence of a formal diagnosis, had a negative impact on the patient’s quality of life [45]. Here, the results from the study revealed that adults with potential ADHD symptoms experienced significant humanistic burden, although undiagnosed. It has been established that treatment for ADHD improves long-term outcomes in ADHD patients, which would remain poor otherwise [46]. A future consideration is to evaluate how proper diagnosis and appropriate treatment for ASRS-positive individuals may improve their outcomes.
Limitations
Due to the cross-sectional nature of the NHWS data, no causal inference can be made. As the data were provided by self-administered questionnaires, it is subject to recall bias, and no verification was done for patient-reported outcomes. While the NHWS sample was made to be predominantly representative of the adult population in Japan, the representativeness of ASRS+ respondents to the larger population is uncertain. As the NWHS was an online self-administered questionnaire, respondents with Internet access and those better-versed in technology might be relatively well-educated or have high socioeconomic status compared to those without access to this survey. Therefore, generalizability might be considered. Our study did not evaluate the severity of ADHD symptoms nor did it explore the potential presentation type of ADHD symptoms (i.e., inattention or hyperactive/impulsive or combined); therefore, no comparison can be made with patients diagnosed with ADHD nor any association of the observed burden among ASRS+ respondents with the ADHD presentation type. Although ASRS is a screener of ADHD, it is possible that symptoms of comorbidities can increase the likelihood of screening ASRS positive. Thus, it is worth noting that ASRS-positive individuals may not necessarily be clinically diagnosed with ADHD. Our study did not exclude the possibility of the involvement of comorbidities in ADHD symptoms.
Conclusions
Undiagnosed adults who demonstrate potential ADHD symptoms in Japan experience a significant personal and professional burden. They reported a substantial prevalence of comorbidities and suffer decreased QoL, impaired work productivity, and greater HRU, similar to patients diagnosed with ADHD, compared to the ASRS-negative population. These preliminary results raise considerations to take note of to ensure the appropriate diagnosis of those at risk for ADHD or for those who present with symptoms overlapping with ADHD. Future studies that assess comorbidities, HRQOL, WPAI, and HRU focusing specifically on clinically diagnosed ADHD patients could provide further insights into the disease burden of ADHD in Japan.
Acknowledgments
The authors would like to thank Dr. Hiroshi Tsuda from Takeda Pharmaceutical Company Limited for providing valuable suggestions to the study design. The authors would also like to acknowledge Cheryl Teoh for the assistance in the medical writing of the manuscript. Sungeun Jung was an employee of the Health Division, Kantar, South Korea at the time the study was conducted. Sungeun Jung is currently affiliated with Novo Nordisk Pharmaceuticals, Korea. Chika Sakai was an employee of Shionogi & Co. Ltd., Osaka, Japan at the time the study was conducted. Chika Sakai is currently affiliated with Janssen Pharmaceutical K.K., Tokyo, Japan.
Appendices
Table 4. Demographic and general health characteristics between matched ASRS-negative and ASRS Part A positive (score ≥4) (ASRS-A+) and between matched ASRS-negative and ASRS Part A+B positive (score ≥9) (ASRS-AB+).
ASRS: Adult ADHD Self-Report Scale; BMI: body mass index; SD: standard deviation
| Matched ASRS-A- versus ASRS-A+ | Matched ASRS-AB- versus ASRS-AB+ | ||||||||||||
| Matched ASRS-A- (N = 1,236) | ASRS-A+ (N = 309) | P-value | Standardized mean difference | Matched ASRS-AB- (N = 908) | ASRS-AB+ (N = 227) | P-value | Standardized mean difference | ||||||
| Continuous variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Age | 41.92 | 16.19 | 42.91 | 15.43 | 0.332 | 0.063 | 41.55 | 15.69 | 41.56 | 15.23 | 0.992 | 0.001 | |
| Charlson comorbidity index | 0.24 | 0.73 | 0.26 | 0.80 | 0.681 | 0.025 | 0.19 | 0.62 | 0.23 | 0.85 | 0.377 | 0.059 | |
| Categorical variable | Count | % | Count | % | P-value | Standardized mean difference | Count | % | Count | % | P-value | Standardized mean difference | |
| Gender | Male | 639 | 51.70% | 164 | 53.07% | 0.665 | 0.028 | 481 | 52.97% | 115 | 50.66% | 0.533 | 0.046 |
| Female | 597 | 48.30% | 145 | 46.93% | 427 | 47.03% | 112 | 49.34% | |||||
| Marital status | Married or living with partner | 522 | 42.23% | 133 | 43.04% | 0.867 | 0.033 | 327 | 36.01% | 85 | 37.44% | 0.549 | 0.076 |
| Divorced/separated/widowed | 698 | 56.47% | 171 | 55.34% | 569 | 62.67% | 137 | 60.35% | |||||
| Decline to answer | 16 | 1.29% | 5 | 1.62% | 12 | 1.32% | 5 | 2.20% | |||||
| Level of education | Not completed university | 727 | 58.82% | 180 | 58.25% | 0.738 | 0.047 | 507 | 55.84% | 129 | 56.83% | 0.955 | 0.023 |
| Completed university education | 489 | 39.56% | 122 | 39.48% | 374 | 41.19% | 91 | 40.09% | |||||
| Decline to answer | 20 | 1.62% | 7 | 2.27% | 27 | 2.97% | 7 | 3.08% | |||||
| Household income | Less than ¥3,000,000 | 275 | 22.25% | 71 | 22.98% | 0.972 | 0.046 | 233 | 25.66% | 57 | 25.11% | 0.954 | 0.061 |
| ¥3,000,000 to | 308 | 24.92% | 80 | 25.89% | 193 | 21.26% | 50 | 22.03% | |||||
| ¥5,000,000 to | 320 | 25.89% | 76 | 24.60% | 181 | 19.93% | 48 | 21.15% | |||||
| ¥8,000,000 or more | 178 | 14.40% | 46 | 14.89% | 179 | 19.71% | 40 | 17.62% | |||||
| Decline to answer | 155 | 12.54% | 36 | 11.65% | 122 | 13.44% | 32 | 14.10% | |||||
| Employment status | Currently not employed | 488 | 39.48% | 122 | 39.48% | 1.000 | <0.001 | 322 | 35.46% | 83 | 36.56% | 0.757 | 0.023 |
| Currently employed | 748 | 60.52% | 187 | 60.52% | 586 | 64.54% | 144 | 63.44% | |||||
| BMI | Underweight (BMI < 18.5) | 124 | 10.03% | 28 | 9.06% | 0.952 | 0.053 | 92 | 10.13% | 25 | 11.01% | 0.960 | 0.059 |
| Normal weight (18.5 ≤ BMI ≤ 22.9) | 590 | 47.73% | 143 | 46.28% | 425 | 46.81% | 101 | 44.49% | |||||
| Pre-obese (23 ≤ BMI ≤ 24.9) | 176 | 14.24% | 47 | 15.21% | 122 | 13.44% | 32 | 14.10% | |||||
| Obese (BMI ≥ 25) | 270 | 21.84% | 71 | 22.98% | 198 | 21.81% | 49 | 21.59% | |||||
| Decline to answer | 76 | 6.15% | 20 | 6.47% | 71 | 7.82% | 20 | 8.81% | |||||
| Smoking status | Current smoker | 198 | 16.02% | 51 | 16.50% | 0.974 | 0.015 | 174 | 19.16% | 43 | 18.94% | 0.924 | 0.029 |
| Former smoker | 313 | 25.32% | 77 | 24.92% | 150 | 16.52% | 40 | 17.62% | |||||
| Never smoker | 725 | 58.66% | 181 | 58.58% | 584 | 64.32% | 144 | 63.44% | |||||
| Alcohol use | No use | 466 | 37.70% | 119 | 38.51% | 0.793 | 0.017 | 379 | 41.74% | 95 | 41.85% | 0.976 | 0.002 |
| Currently use alcohol | 770 | 62.30% | 190 | 61.49% | 529 | 58.26% | 132 | 58.15% | |||||
| Exercise status | No exercise | 667 | 53.96% | 169 | 54.69% | 0.818 | 0.015 | 534 | 58.81% | 129 | 56.83% | 0.588 | 0.040 |
| Currently exercise | 569 | 46.04% | 140 | 45.31% | 374 | 41.19% | 98 | 43.17% | |||||
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Funding Statement
This research was funded by Shionogi & Co. Ltd., Osaka, Japan and Takeda Pharmaceutical Company Limited, Tokyo, Japan.
The authors have declared financial relationships, which are detailed in the next section.
Noriyuki Naya and Toshinaga Tsuji declare(s) employment and stock/stock options from Shionogi & Co. Ltd, Osaka, Japan. Chika Sakai declare(s) employment from Shionogi & Co. Ltd, Osaka, Japan. Toshinaga Tsuji declare(s) stock/stock options from Takeda Pharmaceutical Company Limited. Nobuhiro Nishigaki declare(s) employment from Takeda Pharmaceutical Company Limited, Tokyo, Japan. Sungeun Jung declare(s) employment from Kantar, Health Division, Seoul, South Korea. Kantar received funding from Shionogi & Co. Ltd. and Takeda Pharmaceutical Company Limited for conducting the analysis and manuscript development. Hirotaka Kosaka declare(s) personal fees from Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Daiichi Sankyo Company, Limited, Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., MSD K.K., Eisai Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Yoshitomiyakuhin Corporation, Tsumura & Co., Shionogi & Co., Ltd., Pfizer Japan Inc. and Takeda Pharmaceutical Company Limited.. Yirong Chen declare(s) employment from Kantar, Health Division, Singapore. Kantar received funding from Shionogi & Co. Ltd. and Takeda Pharmaceutical Company Limited for conducting the analysis and manuscript development.
Human Ethics
Consent was obtained or waived by all participants in this study. Pearl Institutional Review Board issued approval 16-KAN-124. The 2016 NHWS surveys were cross-sectional surveys conducted in a total of eight nations, including Japan. The Japan survey was approved with exemption status upon review by Pearl Institutional Review Board (Indianapolis, IN, IRB Study Number: 16-KAN-124). All NHWS respondents provided informed consent prior to participating.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. [Google Scholar]
- 2.Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Lara C, Fayyad J, de Graaf R, et al. Biol Psychiatry. 2009;65:46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. Spencer TJ, Biederman J, Mick E. Ambul Pediatr. 2007;7:73–81. doi: 10.1016/j.ambp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among US children and adolescents, 1997-2016. Xu G, Strathearn L, Liu B, Yang B, Bao W. JAMA Netw Open. 2018;1:0. doi: 10.1001/jamanetworkopen.2018.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. Song P, Zha M, Yang Q, Zhang Y, Li X, Rudan I. J Glob Health. 2021;11:4009. doi: 10.7189/jogh.11.04009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Fayyad J, De Graaf R, Kessler R, et al. Br J Psychiatry. 2007;190:402–409. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 7.Trends in medication treatment for ADHD. Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. J Atten Disord. 2007;10:335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- 8.Epidemiological survey of adult attention deficit hyperactivity disorder (ADHD) in Japan [In Japanese] Nakamura S, Ohnishi M, Uchiyama S. http://www.seiwa-pb.co.jp/search/bo01/bo0102/bn/28/02index.html Jpn J Psychiatr Treat. 2013;155:62. [Google Scholar]
- 9.Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Br J Psychiatry. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 10.Adult ADHD and comorbid somatic disease: a systematic literature review. Instanes JT, Klungsøyr K, Halmøy A, Fasmer OB, Haavik J. J Atten Disord. 2018;22:203–228. doi: 10.1177/1087054716669589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Health-related quality of life and work productivity of adults with ADHD: a U.K. web-based cross-sectional survey. Joseph A, Kosmas CE, Patel C, Doll H, Asherson P. J Atten Disord. 2019;23:1610–1623. doi: 10.1177/1087054718799367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sociodemographics, comorbidities, healthcare utilization and work productivity in Japanese patients with adult ADHD. Kirino E, Imagawa H, Goto T, Montgomery W. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0132233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proinflammatory mediators and their associations with medication and comorbid traits in children and adults with ADHD. Yang LL, Stiernborg M, Skott E, Söderström Å, Giacobini M, Lavebratt C. Eur Neuropsychopharmacol. 2020;41:118–131. doi: 10.1016/j.euroneuro.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Screening for adult ADHD. Anbarasan D, Kitchin M, Adler LA. Curr Psychiatry Rep. 2020;22:72. doi: 10.1007/s11920-020-01194-9. [DOI] [PubMed] [Google Scholar]
- 15.The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Kessler RC, Adler L, Ames M, et al. Psychol Med. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization: Adult ADHD Self-Report Scale-V1.1 (ASRS-V1.1) Symptoms Checklist from WHO Composite International Diagnostic Interview. [ Sep; 2020 ];https://www.hcp.med.harvard.edu/ncs/ftpdir/adhd/18Q_ASRS_English.pdf 2003
- 17.Changes in the definition of ADHD in DSM-5: subtle but important. Epstein JN, Loren RE. Neuropsychiatry (London) 2013;3:455–458. doi: 10.2217/npy.13.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Charlson ME, Pompei P, Ales KL, MacKenzie CR. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. J Clin Epidemiol. 2008;61:1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Quan H, Li B, Couris CM, et al. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 21.Psychometric properties of the Japanese version of the Adult Attention-deficit hyperactivity disorder (ADHD) Self-Report Scale (ASRS-J) and its short scale in accordance with DSM-5 diagnostic criteria. Takeda T, Tsuji Y, Kurita H. Res Dev Disabil. 2017;63:59–66. doi: 10.1016/j.ridd.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 22.The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Ware JE Jr, Sherbourne CD. https://www.jstor.org/stable/3765916. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 23.Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. J Clin Epidemiol. 1998;51:1037–1044. doi: 10.1016/s0895-4356(98)00095-x. [DOI] [PubMed] [Google Scholar]
- 24.EuroQol--a new facility for the measurement of health-related quality of life. EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 25.Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Shiroiwa T, Fukuda T, Ikeda S, Igarashi A, Noto S, Saito S, Shimozuma K. Qual Life Res. 2016;25:707–719. doi: 10.1007/s11136-015-1108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The validity and reproducibility of a work productivity and activity impairment instrument. Reilly MC, Zbrozek AS, Dukes EM. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 27.The influence of alcohol consumption on sickness presenteeism and impaired daily activities. the WIRUS screening study. Aas RW, Haveraaen L, Sagvaag H, Thørrisen MM. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0186503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adult obesity and health-related quality of life, patient activation, work productivity, and weight loss behaviors in the United States. Rozjabek H, Fastenau J, LaPrade A, Sternbach N. Diabetes Metab Syndr Obes. 2020;13:2049–2055. doi: 10.2147/DMSO.S245486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Examining the association of smoking with work productivity and associated costs in Japan. Suwa K, Flores NM, Yoshikawa R, Goto R, Vietri J, Igarashi A. J Med Econ. 2017;20:938–944. doi: 10.1080/13696998.2017.1352507. [DOI] [PubMed] [Google Scholar]
- 30.IBM Corp: IBM SPSS Statistics for Windows. [ Sep; 2020 ];IBM Corp: IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. https://www.ibm.com/products/spss-statistics 2017
- 31.R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. [ Sep; 2020 ];https://www.r-project.org/ 2018
- 32.Screened attention deficit/hyperactivity disorder as a predictor of substance use initiation and escalation in early adulthood and the role of self-reported conduct disorder and sensation seeking: a 5-year longitudinal study with young adult Swiss men. Moggi F, Schorno D, Soravia LM, Mohler-Kuo M, Estévez-Lamorte N, Studer J, Gmel G. Eur Addict Res. 2020;26:233–244. doi: 10.1159/000508304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The nature of the relationship between attention-deficit/hyperactivity disorder and substance use. Wilens TE. https://www.psychiatrist.com/jcp/neurodevelopmental/adhd/nature-relationship-between-attention-deficit-hyperactivity/?CLICK=1. J Clin Psychiatry. 2007;68 Suppl 11:4–8. [PubMed] [Google Scholar]
- 34.Genetic overlap and causality between substance use disorder and attention-deficit and hyperactivity disorder. Vilar-Ribó L, Sánchez-Mora C, Rovira P, et al. Am J Med Genet B Neuropsychiatr Genet. 2021;186:140–150. doi: 10.1002/ajmg.b.32827. [DOI] [PubMed] [Google Scholar]
- 35.Distinguishing comorbidity and successful management of adult ADHD. Kooij JJ, Huss M, Asherson P, et al. J Atten Disord. 2012;16:3–19. doi: 10.1177/1087054711435361. [DOI] [PubMed] [Google Scholar]
- 36.Attention deficit-hyperactivity disorder is associated with allergic symptoms and low levels of hemoglobin and serotonin. Wang LJ, Yu YH, Fu ML, et al. Sci Rep. 2018;8:10229. doi: 10.1038/s41598-018-28702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: a population-based cross-sectional study. Chen Q, Hartman CA, Haavik J, et al. PLoS One. 2018;13:0. doi: 10.1371/journal.pone.0204516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psychiatric comorbidity in ADHD symptom subtypes in clinic and community adults. Sprafkin J, Gadow KD, Weiss MD, Schneider J, Nolan EE. J Atten Disord. 2007;11:114–124. doi: 10.1177/1087054707299402. [DOI] [PubMed] [Google Scholar]
- 39.Association between ADHD and obesity: a systematic review and meta-analysis. Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, Faraone SV. Am J Psychiatry. 2016;173:34–43. doi: 10.1176/appi.ajp.2015.15020266. [DOI] [PubMed] [Google Scholar]
- 40.Trajectories of body mass index and their associations with mortality among older Japanese: do they differ from those of Western populations? Murayama H, Liang J, Bennett JM, et al. Am J Epidemiol. 2015;182:597–605. doi: 10.1093/aje/kwv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. van Hout B, Janssen MF, Feng YS, et al. Value Health. 2012;15:708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 42.The risk of injury in adults with attention-deficit hyperactivity disorder: a nationwide, matched-cohort, population-based study in Taiwan. Chien WC, Chung CH, Lin FH, et al. Res Dev Disabil. 2017;65:57–73. doi: 10.1016/j.ridd.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Improvements in workplace productivity in working patients with major depressive disorder: results from the AtWoRC study. Chokka P, Tvistholm AH, Bougie J, Clerzius G, Ettrup A. J Occup Environ Med. 2020;62:0–101. doi: 10.1097/JOM.0000000000001805. [DOI] [PubMed] [Google Scholar]
- 44.The incremental burden of pain in patients with depression: results of a Japanese survey. Vietri J, Otsubo T, Montgomery W, Tsuji T, Harada E. BMC Psychiatry. 2015;15:104. doi: 10.1186/s12888-015-0488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The prevalence and impact of psychiatric symptoms in an undiagnosed diseases clinical program. Waserstein G, Partin C, Cohen D, Schettler P, Kinkead B, Rapaport MH. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0216937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, Arnold LE. BMC Med. 2012;10:99. doi: 10.1186/1741-7015-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]