Abstract
Recognition of suspected ischaemia with no obstructive coronary artery disease – termed INOCA – has increased over the past decades, with a key contributor being microvascular angina. Patients with microvascular angina are at higher risk for major adverse cardiac events including MI, stroke, heart failure with preserved ejection fraction and death but to date there are no clear evidence-based guidelines for diagnosis and treatment. Recently, the Coronary Vasomotion Disorders International Study Group proposed standardised criteria for diagnosis of microvascular angina using invasive and non-invasive approaches. The management strategy for remains empirical, largely due to the lack of high-levelevidence- based guidelines and clinical trials. In this review, the authors will illustrate the updated approach to diagnosis of microvascular angina and address evidence-based pharmacological and non-pharmacological treatments for patients with the condition.
Keywords: Microvascular angina, coronary microvascular dysfunction, coronary function test, diagnosis, management
The recognition of suspected ischaemia with no obstructive coronary artery disease (INOCA) has increased over the past decades, with a key contributor being microvascular angina (MVA).[1] Evidence of MVA was previously thought to be benign.[2–4] Previous studies have demonstrated that patients with suspected MVA are often found to have coronary microvascular dysfunction (CMD) and are at higher risk of developing major adverse cardiac events (MACE), including MI, stroke and heart failure with preserved ejection fraction (HFpEF).[5–7] Data from the Women’s Ischaemic Syndrome Evaluation (WISE) suggest there may be at least 3–4 million women and men with vasomotion dysfunction.[8]
The prevalence of MVA appears to have increased over the past several years because of increased diagnostic imaging, with a recent study reporting that of 400,000 individuals undergoing diagnostic angiography for suspected coronary artery disease (CAD), over 50% were found to have either no CAD or non-obstructive CAD.[9–11] Recent studies also showed that CMD is identified in four in five patients with suspected MVA or vasospastic angina.[12–14] Anatomically, the coronary vasculature is classified into large calibre vessels (≥500 μm; the epicardial coronary arteries) and smaller vessels (<500 μm; the arterioles that feed the capillaries). The term INOCA encompasses a large number of clinical scenarios characterised by reduced coronary flow reserve (CFR) in the absence of anatomical obstructive epicardial disease.[10,11,15]
Pathophysiology
The pathophysiological mechanism contributing to MVA is multifactorial. It was previously described by Camici and Crea as CMD with no obstructive CAD or myocardial disease, CMD with the presence of myocardial disease, CMD with the presence of obstructive CAD or iatrogenic.[16] Early studies in patients with CMD and no CAD (formerly known as cardiac syndrome X) provided evidence of reduced endothelial-dependent (e.g. acetylcholine) and non-endothelial-dependent (e.g. nitroglycerin) coronary vasodilation as well as metabolic evidence of myocardial ischaemia.[17,18] Maseri et al. proposed that these patients might have focal epicardial or microvascular ischaemia scattered throughout the myocardium, therefore CMD with no CAD can be defined as epicardial, microvascular endothelial or non-endothelial dysfunction leading to reduced myocardial perfusion, most often detected by reduced CFR.[1,19] Additionally, the data from WISE-CVD showed that myocardial scarring was prevalent (8%) in women with MVA and vasospastic angina with an added annual incidence of 1%.[20] Recently, Rahman et al. physiologically described two endotypes of CMD – functional and structural.[21] In structural CMD, the systemic endothelial dilatory function leads to systemic hypertension and increased myocardial work, whereas coronary blood flow augmentation is impaired and associated with inefficient cardiac-coronary coupling.[21] The functional CMD endotype is related to inefficient cardiac-coronary coupling during peak exercise and during rest leads to higher myocardial oxygen demand in the setting of exhausted vasodilatory reserve.[21]
CMD with no obstructive CAD or myocardial disease represent the functional aspect of the classical CAD risk factors such as smoking, hypertension, hyperlipidaemia, diabetes and others.[16] Ageing can result in CMD by increasing arterial wall stiffness, medial thickening and lumen enlargement. Over time, these changes may lead to increased pulse pressure and hypertrophy of arteries, which can to subsequently result in endothelial dysfunction and subendocardial hypoperfusion.[22] Cigarette smoking is a known risk factor for CAD and impairs endothelial-dependent vasodilation in chronic smokers.[23,24] Uncontrolled hypertension is associated with remodelling of the coronary arteries and can lead to arteriolar thickening and reduced myocardial perfusion.[25] Diabetes and chronic hyperglycaemia reduce endothelial-dependent and non-endothelial-dependent coronary vasodilation.[19,26] Reduced CFR has been documented in asymptomatic patients with hypercholesterolaemia and non-obstructive CAD.[27] Additionally, data from the WISE study showed that myocardial-ischaemia-related steatosis appears to be linked mechanistically to impaired left-ventricular relaxation in women with CMD evidenced by magnetic resonance spectroscopy.[28]
Predictors and Adverse Outcomes
Similar to obstructive CAD, non-obstructive CAD risk factors including age, hypertension, diabetes and smoking are associated with increased mortality.[29] Data from the WISE study showed that abnormal coronary function testing (CFT) used to diagnose women with MVA predicted adverse outcomes, including cardiac-related deaths, non-fatal MI, non-fatal stroke and hospitalisations related to heart failure.[7] CFR <2.32 best predicted adverse outcomes in women with MVA, with a 5-year MACE rate of 27% versus 9.3% for those with a CFR >2.32 (p=0.01).[7] Upon longer-term follow-up (median 9.7 years), CFR continued to be a predictor of increased MACE (HR 1.06; 95% CI [1.01–1.12]; p=0.02).[30] Similar findings were observed in a previous study and summarised by Bairey Merz et al.[1] Furthermore, Seitz et al. showed that long-term follow-up in patients with epicardial or microvascular spasm in the absence of obstructive CAD was associated with 7.5% cardiac and non-cardiac-related death, 1.4% non-fatal MI and 2.2% stroke over a median of 7.2 years.[31] Non-invasive testing in patients with MVA also predicted MACE and showed consistent results with invasive testing.
A recent report from the iPOWER study showed that echocardiography-derived coronary flow velocity reserve (CFVR) of 2.33 predicted MACE including non-fatal MI and heart failure (HR 1.07; 95% CI [1.03–1.11] per 0.1 unit decrease; p<0.001) over a median of 4.5 years follow-up.[32] Zhou et al. showed that stress perfusion cardiac magnetic resonance (CMR)-derived myocardial perfusion reserve index (MPRI) <1.47 predicted MACE including all-cause death, acute coronary syndrome, epicardial CAD development, heart failure hospitalisation and non-fatal stroke (HR 3.14; 95% CI [1.58–6.25]; p=0.001) over a median of 5.5 years follow-up.[33] Additionally, Murthy et al. showed that cardiac-PET-derived CFR <2.0 predicted MACE including cardiac death, MI, late revascularisation or heart failure hospitalisation at 3 years compared to patients with higher CFR.[34]
A meta-analysis by Gdoski et al. of patients with CMD detected through invasive or non-invasive testing showed that CMD patients had an OR of 5.16 (95% CI [2.81–9.47]; p<0.001) to develop MACE compared to patients without CMD.[35] Endpoints were all-cause mortality and MACE including cardiac or cardiovascular death, nonfatal MI, cardiac hospitalisation or coronary revascularisation.[35]
Diagnosis
The Coronary Vasomotion Disorders International Study Group proposed standardised criteria for diagnosis of MVA including the following: symptoms suggestive of ischaemia in the absence of epicardial CAD (>50% diameter reduction or fractional flow reserve [FFR] <0.80), objective evidence of myocardial ischaemia and evidence of vasomotor dysfunction (abnormal index of microvascular resistance [IMR], CFR or microvascular spasm to acetylcholine).[13,15]
Symptoms
Patients with MVA can present with exertional retrosternal chest pain/pressure or discomfort or have exertional dyspnoea.[15] Symptoms may also develop during exercise, after exercise or even at rest and are relatively less likely to be relieved by nitrates compared with obstructive CAD.[15,36] Duration of symptoms are variable, tend to be prolonged and may differ in nature, i.e. stabbing pain, jaw pain or back pain.[15] CMD can occur in both men and women but recent reports and studies indicate that it is more prevalent in women (especially post-menopause).[15] Although symptoms can be the initial presentation in patients with MVA, the results from the Cardiac Autonomic Nervous System study showed a high prevalence of silent ischaemia in women with CMD.[37] In total, 39% of women with CMD had a total of 26 silent ischaemia episodes versus no episodes in the reference group (p=0.002). Among these women, 93% had silent ischaemia documented by ambulatory ECG tracing.[37] The long-term prognosis of silent ischaemia in women with MVA is yet to be elucidated.
Evidence of Myocardial Ischaemia
Current guidelines recommend that patients with stable angina with an intermediate pre-test probability to have obstructive CAD should have non-invasive diagnostic testing for detection of myocardial ischaemia.[15,38,39] Patients with stable angina can have evidence of myocardial ischaemia through rest/stress ECG and/or non-invasive imaging by reduced myocardial perfusion with single photon emission CT, PET or cardiac rest/stress magnetic resonance or testing cardiac function using stress echocardiography. Patients with MVA can demonstrate ST-segment changes suggestive of myocardial ischaemia and may exhibit reduced perfusion on other non-invasive testing, while only a minority of patients demonstrate wall motion abnormalities.[15,40]
Absence of Obstructive or Flow-limiting Coronary Artery Stenosis
The absence of obstructive or flow-limiting CAD is necessary to diagnose MVA. Anatomically coronary arteries can be classified to no CAD (mild) with <20% stenosis, non-obstructive CAD (moderate) with ≥20% but <50% and obstructive CAD (severe) with ≥50% in any epicardial coronary artery using coronary angiography.[15,41] Limiting flow to the coronary arteries can be defined as FFR <0.8.[15] Anatomical illustration of the absence of obstructive CAD may be insufficient in certain instances such as diffused non-obstructive CAD, so it is necessary to demonstrate if there is any haemodynamically flow-limiting lesion of the epicardial coronaries using FFR defined as <0.8.[15] Coronary CT angiography is a useful tool to exclude obstructive CAD epicardial disease defined as <50% stenosis and CT-derived FFR is an emerging promising tool to measure FFR but not yet proven for use in routine practice.[15,42]
Non-invasive Testing
The presence of symptoms of angina or angina-like symptoms and evidence of myocardial ischaemia is sufficient evidence to consider MVA in the absence of obstructive CAD. Further details regarding imaging use are described in Table 1.[36,43]
Table 1: Invasive and Non-invasive Testing Approaches to Diagnose Microvascular Angina.
| Advantages | Disadvantages | |
|---|---|---|
| Non-invasive Testing | ||
| Transthoracic Doppler, contrast echocardiography |
|
|
| Cardiac PET |
|
|
| CMR |
|
|
| Cardiac CT |
|
|
| Invasive Testing | ||
| Coronary function test |
|
|
CBF = coronary blood flow; CFR = coronary flow reserve; CMD = coronary microvascular dysfunction; CMR = cardiac magnetic resonance; LVEDP = left ventricular end diastolic pressure; MVA = microvascular angina.
Contrast and Doppler Transthoracic Echocardiography
Contrast or Doppler echocardiography can be used in the evaluation of CMD. They are considered safe, albeit operator-dependent and as yet lacking reproducibility and validity. [43] In one study, patients with symptoms of angina and no obstructive CAD diagnosed by coronary angiography, CFVR was assessed using Doppler of the left anterior descending coronary artery at rest and after dipyridamole use.[44] Twenty-six per cent of patients had CFVR <2 with greater physical limitation and disease perception scores on the Seattle Angina Questionnaire.[1,44] The CEVENT study used Doppler-derived coronary flow reserve (TDE-CFR).[45] In brief, a basic transthoracic echocardiography protocol was performed and the mid to distal part of the left anterior descending coronary artery was identified using colour Doppler in the interventricular sulcus in a modified two-chamber view.[45] Pulsed Doppler was used to sample flow velocity signals at rest and during adenosine infusion. Mean diastolic flow velocity at baseline and during peak hyperaemia was measured by manual tracing of the diastolic Doppler flow signals.[45] CFR was calculated as the ratio between the hyperaemic and baseline flow velocity values.[45] A CFR ratio of ≤2 was considered reduced. Recently, CFVR has demonstrated a prognostic value, with data from the iPOWER study showing that CFVR <2.3 predicted MACE and heart failure hospitalisation.[32] Furthermore, Gan et al. showed that TDE-CFR value of ≤2.0 was an independent predictor of MACE (HR 4.63; 95% CI [2.78–7.69]; p<0.001) over a median of 4.5 years follow-up.[45]
Cardiac PET
Cardiac PET is the most validated, reliable and accurate non-invasive method in diagnosing CMD in patients with suspected MVA, although it is costly and with limited use in certain institutions.[1,36,43] PET-derived myocardial perfusion measurement is based on myocardial flow (MBF) quantification in millilitres per minute per gram using intravenous positron-emitting tracers such as [13]N-ammonia, [82]rubidium, [18]F-flurpiridaz and [15]O-water.[36,46] PET-derived myocardial perfusion imaging in conjunction with tracer-kinetic modelling allows accurate assessment of rest and post hyperaemic flow using adenosine, dobutamine, or dipyridamole and enables the determination of CFR through quantification of MBF and further characterisation of CMD.[1,36,46] Furthermore, PET-derived CFR carries a prognostic value.
In a study by Taqueti et al., CFR was an independent predictor for MACE including non-fatal MI and HFpEF hospitalisations (CFR < 2 had HR 2.38; 95% [CI 1.21–4.67]; p=0.01 for MACE and HR 2.47; 95% CI [1.09–5.62]; p=0.03 for HFpEF hospitalisation) after adjusting for age and history of AF.[47] CFR was also found to be associated with impaired left ventricular myocardial relaxation or elevated filling pressures among patients with no obstructive CAD and minimally elevated troponin levels.[48] Among patients with resistant hypertension, cardiac PET derived myocardial perfusion reserve was a predictor for diastolic dysfunction and cardiovascular adverse outcomes.[49]
Cardiac MRI
The assessment of CMD using CMR is validated against invasive tests and cardiac PET and does not involve radiation exposure.[36,43,50] Myocardial perfusion assessment using rest/post-vasodilator stress (adenosine) allows semi-quantification of MPRI to evaluate CMD.[36,50] Data from WISE-CVD showed that an MPRI of ≤1.84 predicted invasive CFT abnormality with a sensitivity of 73% and specificity of 74% using 1.5T magnet and CAAS MRV 3.4 as a post-processing software.[50] In addition to its role in diagnosing CMD, CMR has greater spatial and temporal resolution compared to cardiac PET, although it is expensive, is not widely available and prognostic data are limited.[36,43] Recently, an automated pixel-wise perfusion mapping technique was used to detect significant CAD lesion compared to invasive testing FFR, CMD defined by IMR, and to differentiate MVA from multivessel coronary disease. MBF ≤1.94 ml/g/min accurately detected obstructive CAD on a regional basis (area under the curve [AUC] 0.90; p <0.001).[51] In patients without regional perfusion defects, global stress MBF <1.82 ml/g/min accurately detected CMD (AUC 0.94; p<0.001).[51]
CT Perfusion
CT perfusion has the potential to diagnose CMD and can simultaneously assess for CAD on the same study.[36,43] Dynamic first-pass vasodilator stress and rest perfusion imaging allows perfusion quantification.[36,43] Recent work by Rossi et al. used adenosine-stress dynamic CT myocardial perfusion imaging, and semiquantitative perfusion parameters, such as blood flow, were calculated by parametric deconvolution for each myocardial voxel.[52] Semiquantitative perfusion predicted subendocardial myocardial ischaemia (AUC 0.87). CT perfusion also provides greater spatial and temporal resolution compared to cardiac PET.[36,43] The perfusion quantification requires repeated imaging and exposes patients to high radiation doses and the need for iodinated contrast can be associated with higher risk of renal injury.[36,43] Although CT perfusion has a potential to diagnose CMD, its validation is limited in patients with MVA and it is not regularly used to diagnose CMD.[43]
Invasive Testing
Coronary angiography can accurately exclude obstructive CAD defined as (<50% stenosis in epicardial artery or an FFR of >0.8). CFT is a standardised invasive test to diagnose and confirm different pathways of CMD.[1,15,36,43] It is the confirmatory test to identify certain pathways and phenotypes in patients with suspected MVA.[15,36,43] The coronary microcirculation is modulated further by physical and neural factors.[53] CFT is performed by infusing vasoactive substances through a guiding catheter placed in the left main coronary artery, then a Doppler guide wire is positioned in the proximal left anterior descending coronary artery.[53] Another approach is through using a thermodilution wire that can be positioned in the distal third of the targeted coronary artery.[54–56] CMD pathway identification is performed through measurements of coronary blood flow (CBF) and the change in epicardial coronary artery diameter with 1) endothelium-dependent probes–acetylcholine), bradykinin, substance-P, l-NGmonomethyl arginine citrate, and shear stress – and predominantly, 2) endothelium-independent probes, adenosine and sodium nitroprusside.
The CFT preparation and protocol along with calculation of CFR and CBF are illustrated in Figure 1. Graded infusion of different vasoactive substances including adenosine, acetylcholine and nitroglycerin are illustrated in Figure 2. After injecting the vasoactive substances, one or more pathway dysfunctions can be identified as shown in Figure 2 with interpretation. Because acetylcholine requires certain infusion concentrations and safety precautions, the infusion rate and concentrations of acetylcholine are standardised as shown in Table 2. Data from WISE-CVD also showed a good correlation between acetylcholine and cold pressor test to evaluate coronary artery diameter changes in women with MVA.[57]
Figure 1: Coronary Function Test Preparation and Protocol.
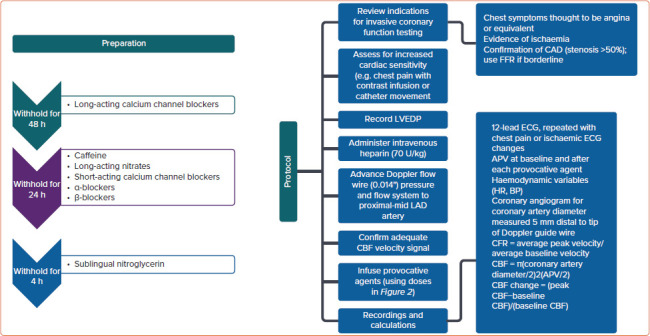
APV = arterial pulse velocity; BP = blood pressure; CAD = coronary artery disease; CBF = coronary blood flow; FFR = fractional flow reserve; HR = heart rate; LAD = left anterior descending; LVEDP = left ventricular end diastolic pressure. Source: Merz et al. 2017.[111] Adapted with permission from Wolters Kluwer Health.
Figure 2: CMD Pathway Definitions on Administration of Different Vasoactive Substances.
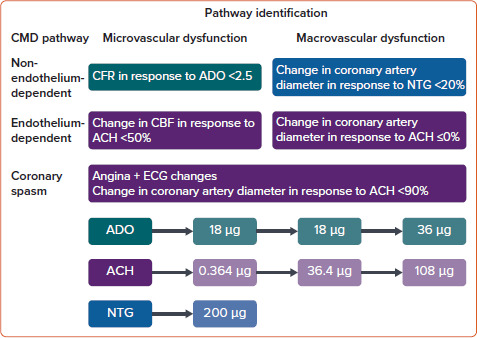
ACH = acetylcholine; ADO = adenosine; CBF= coronary blood flow; CFR = coronary flow reserve; CMD = coronary microvascular dysfunction; NTG = nitroglycerin. Source: Merz et al. 2017.[111] Reproduced with permission from Wolters Kluwer Health.
Table 2: Intracoronary Acetylcholine Concentration and Infusion.
| Prepared Concentration, mol/l (μg/ml) | Infusion Rate (ml/h) | Infusion Duration (min) | Infused Dose (μg) |
|---|---|---|---|
| 10−6 (0.182) | 48 | 3 | 0.364 |
| 10−4 (18.2) | 48 | 3 | 36.4 |
| 10−4 (18.2) | 120 | 3 | 108 |
Source: Merz et al. 2017.[111] Reproduced with permission from Wolters Kluwer Health.
Using the above protocol, four pathways contributing to CMD are being tested (Figure 2).[7,53,58] A CFR ≤2.5 in response to adenosine is considered abnormal.[7,53,58] Endothelial-dependent microvascular function using intermediate dose of acetylcholine to calculate CBF increase.[53] CBF <50% increase in response to the highest dose of acetylcholine was considered abnormal.[53] Endothelial-dependent macrovascular coronary function was defined as coronary artery dilation >5% in response to the acetylcholine infusion.[53] Non-endothelial-dependent macrovascular function using a single dose of nitroglycerin.[53] A diameter increase <20% was considered abnormal.[53] Excellent safety data from previous reports were published with <1% adverse events and no deaths.[53,59] Other methods used to identify CMD include calculating the IMR, which is calculated as the product of distal coronary pressure at maximal hyperaemia multiplied by the hyperaemic mean transit time.[60] The normal range of IMR is considered to be <25.[54] Another method for assessment of CMD is by semiquantitative analysis by calculating the thrombolysis in myocardial infarction (TIMI) frame count.[54] In patients with suspected MVA the corrected TIMI frame count >25 (images acquired at 30 frames/second) suggests CMD.[54] Advantages and limitations of invasive testing illustrated in Table 1.
Criteria for Diagnosis
Recently, Kunadian et al. described that INOCA is explained by the mismatch between blood supply and myocardial oxygen demands, which can be caused by CMD and/or epicardial vasospastic angina.[55] Definitive MVA is only diagnosed if all four criteria are present including presence of symptoms, absence of obstructive/flow limiting coronary stenosis, objective evidence of myocardial ischaemia on non-invasive testing and evidence of CMD on CFT (Figure 3).[15] Suspected MVA is diagnosed if the following criteria are present: symptoms; absence of obstructive/flow limiting coronary stenosis; and at least one of the following: objective evidence of myocardial ischaemia on non-invasive testing; or coronary microvascular spasm, defined as reproducibility of symptoms with ECG changes suggestive of ischaemia but no epicardial spasm during CFT (Figure 3).[13,15]
Figure 3: COVADIS Criteria to Diagnose Patients with Microvascular Angina.
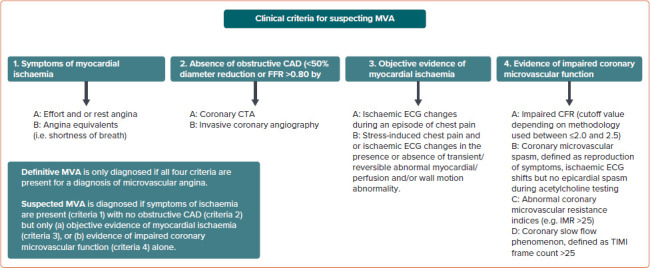
CAD = coronary artery disease; CFR = coronary flow reserve; COVADIS = Coronary Vasomotion Disorders International Study Group; CTA = CT angiography; FFR = fractional flow reserve; IMR = index of microvascular resistance; MVA = microvascular angina; TIMI = thrombolysis in myocardial infarction. Source: Ong et al. 2018.[15] Adapted with permission from Elsevier.
Management
Due to the lack of large randomised clinical trials addressing the treatment of MVA there are no definitive guidelines for treatment. The Japanese Circulation Society provided a low level of evidence for treatment of vasospastic angina, and US guidelines currently do not specifically address angina and CMD management.[38,61–63]
The European Society of Cardiology guidelines for diagnosis and management of chronic coronary syndromes, published in 2019, recommended testing for suspected MVA using a guidewire-based CFR and/or IMR as a Class IIa recommendation with level B evidence.[62,64] Furthermore, the guidelines proposed the use of transthoracic Doppler echocardiography, CMR or cardiac PET as non-invasive test for assessment of CFR (Class IIb recommendation with level B evidence).[62,64] The guidelines also recommended that the treatment should address the mechanism or pathway dysfunction contributing to MVA.[62] In patients with abnormal CFR <2.0 or IMR ≥25 units and a negative acetylcholine provocation test, β-blockers, angiotensin-converting enzyme inhibitors (ACE-I), and statins, along with lifestyle changes and weight loss, are indicated.[62] Microvascular spasm can also be treated like vasospastic angina.[62]
The effectiveness of a tailored treatment strategy was based on the findings from the CORMICA trial, which randomised 151 patients to a stratified medical treatment (based on the results of CFR, IMR, and acetylcholine testing) versus a usual treatment group (including a sham interventional diagnostic procedure).[13,62,65] After 1 year of follow up there was a significant difference in angina scores estimated using Seattle Angina Questionnaire favouring patients assigned to the stratified medical treatment arm.[13,62,65]
Pharmacological Therapy
Statins, Angiotensin Converting Enzyme Inhibitors and Aspirin (Anti-atherosclerotic Therapy)
Statins, ACE-I and aspirin have anti-atherosclerotic and anti-thrombotic effects, can counteract oxidative stress on cellular level, have anti-inflammatory effects and can improve both endothelial and microvascular function.[66,67] These agents are known to improve angina and myocardial perfusion.
Statins
In previous clinical trials using intravascular ultrasound (IVUS), statins have been shown to alter the progression and even promote the regression of atherosclerosis and improve vascular endothelial function.[68,69] In addition to their cholesterol-lowering properties, statins have powerful anti-inflammatory effects.[70] In two pilot studies, atorvastatin improved CFR at 2 and 6 months.[71,72] A recent systematic review and meta-analysis of randomised controlled trials assessing the effect of statins on coronary and peripheral endothelial function showed treatment with statins was associated with a significant improvement in endothelial function with a standardised mean difference of 0.66 (95% CI [0.46–0.85]; p<0.001).[73]
Angiotensin Converting Enzyme Inhibitors
Data from the WISE study showed that after 16 weeks, treatment of women with MVA (CFR <3.0) with quinapril 80 mg/day was significantly associated with improvement of angina symptoms and CFR compared with the placebo group.[74] Consistent with the WISE and the WISE-CVD studies, another study showed improvement in CFR, plasma nitrite and exercise duration after 8 weeks with enalapril 5 mg twice daily compared with the placebo group.[75] Furthermore, in patients with hypertensive disease who were treated for 12 weeks with cilazapril, cardiac PET showed 42% improvement in their CFR.[76] Studies have shown that the benefits of ACE-I are not limited to the objective measurements of CFT, endothelial function and symptoms but extend to improvement in other circulating biomarkers.[64,77]
Antiplatelet Agents
In an IVUS study, coronary atherosclerosis was detected in most patients with microvascular dysfunction.[78] Thus, thromboxane A2 (TXA2) inhibitors (low-dose aspirin and P2Y12 platelet inhibitors) are likely helpful in preventing adverse outcomes in patients with MVA. The proposed mechanism is that TXA2 can cause arterial vasoconstriction, platelet aggregation, and vascular injury. Therefore, inhibition of TXA2 pathway may prevent further microvascular damage.[1]
β-blockers, Calcium Channel Blockers and Nitrates (Anti-anginal Therapy)
β-blockers
Certain β-blockers, including atenolol carvedilol and nebivolol, have been evaluated in small clinical studies,.[79–81] Intracoronary nebivolol was associated with a significant increase in CFR as well as a decrease in collateral flow index, a finding that is parallel to reduction in myocardial oxygen consumption.[81] β-blockers increase diastolic coronary filling time and reduce myocardial oxygen consumption.
Calcium Channel Blockers
Calcium channel blockers are widely used in vasospastic angina and have the effect of improving vasodilatory response, further episodes of vasospasm and reducing cardiac afterload.[1] Although calcium channel blockers have a predominant vasodilatory effect on the epicardial arteries, one study demonstrated that intracoronary diltiazem administration did not improve CFR in patients with MVA.[82] In another study, amlodipine did not improve anginal chest pain episodes.[79] On the other hand, in patients with impaired vasodilator reserve, verapamil and long-acting nifedipine have been reported to be associated with improved symptoms and exercise tolerance.[83]
Nitrates
Nitrates can ameliorate anginal pain through venodilation to reduce preload. They may also have some coronary vasodilatory effect, but this effect is greater in patients with obstructive CAD compared to MVA.[1,15] Generally, patients with MVA do not have rapid or sufficient symptom relief in response to sublingual nitroglycerin.[15] Kanatsuka et al. demonstrated that steady-state infusion of nitroglycerin selectively dilates coronary arterial vessels >200 μm.[84] This selectivity may explain the inefficient relief of angina symptoms in patients with MVA.
Other Strategies
Ranolazine
Ranolazine inhibits late sodium current and reduces intracellular calcium levels in cardiac myocytes, hence improving ventricle relaxation and oxygen consumption.[85] Variable results on symptoms and CFR have been reported in pilot studies.[86,87] In one large randomised cross-over clinical trial of ranolazine and placebo, there was no difference in symptoms and myocardial perfusion reserve as measured by CMR.[88] However, when stratified by baseline CFR, those with reduced CFR showed improvement with ranolazine.[89]
Ivabradine
Ivabradine reduces heart rate via its blocking effect on the If channels in the sinoatrial node.[15,64] In patients with stable CAD, it is found to improve CFR.[90] Another study showed improvement of angina symptoms but no effect on coronary microvascular function, suggesting the improvement may be attributed to the effect of decreased heart rate.[87] Given the previous evidence, ivabradine may play a role in the treatment of MVA.
Low-dose Tricyclic Antidepressants (Abnormal Cardiac Nociception)
Abnormal cardiac nociception is a condition primarily studied in women with suspected MVA and characterised by abnormal cardiac pain perception.[91] Tricyclic antidepressants can modulate norepinephrine uptake and anticholinergic effects, which may induce analgesia.
Aminophylline
Aminophylline is a nonselective adenosine-receptor antagonist and blocks the mediation of nociception. The suggested mechanism to improve symptoms in patients with CMD is by attenuating the excess dilation of the microvasculature in relatively well-perfused areas, thus shunting blood to a poorly perfused areas.[1] Imipramine has also been studied, and shown to reduce frequency of symptoms, albeit with no significant improvement in quality of life.[92]
Mineralocorticoid Inhibitors
Mineralocorticoid inhibitors although it is one of potential therapies for CMD but it provided no additional of anginal improvement when combined with ACE-I in previous randomised clinical trial.[93]
Phosphodiesterase Inhibitors
The effect of the phosphodiesterase (PDE) type 3 inhibitor cilostazol has been assessed in patients with coronary vasospasm.[64] In one study in patients with coronary vasospasm refractory to calcium channel blockers and nitrates, the addition of cilostazol appeared effective.[94] The PDE type 5 inhibitor sildenafil has demonstrated acute increases in CFR, especially in women with MVA with CFR <2.5.[95]
Rho-kinase Inhibitors
Rho-kinase plays an important role in coronary vasospasm.[96] Fasudil has been found to be effective in preventing acetylcholine induced coronary spasm.[97] Intracoronary fasudil is effective not only in patients with epicardial coronary spasm but also approximately two-thirds of those with MVA.[98]
L-arginine
L-arginine is a precursor of nitric oxide. L-arginine supplementation has been shown to improve both symptoms and endothelial function, but increases the risk of MI in patients with obstructive CAD.[99]
Glycaemic Active Agents
Sodium–glucose co-transporter (SGLT) 1 and 2 inhibitors improve cardiovascular outcomes in studies of patients with diabetes. Inhibition of the endothelial SGLT2 improves hyperglycaemia-induced vascular dysfunction in vitro.[100] Any benefit on CMD and outcome in patients with diabetes with MVA has not yet been demonstrated. Metformin is an insulin sensitiser and may improve endothelial function in nondiabetic women with suspected MVA.[101]
Endothelin Receptor Antagonists
Endothelin (ET)-1 contributes to coronary endothelial dysfunction and may increase atherosclerosis risk factor burden.[102,103] ET-1 is a small peptide produced primarily in the endothelium that is a potent constrictor of human blood vessels.[104] ET-1 is mediated by two receptors: ETA and ETB ETA activation by ET-1 mediates coronary vasoconstriction.[104] In a randomised clinical trial evaluating the ET receptor antagonist atrasentan in patients with CMD, microvascular coronary endothelial function after 6 months was improved.[105] The CORMICA investigators found that peripheral arterioles from patients with MVA showed enhanced constriction to ET-1 compared with reference controls, which may be a target for future therapies.[106] The on-going clinical trial PRIZE aims to test efficacy of the potent ET inhibitor zibotentan as an adjunct therapy in patients with MVA.[104]
Non-pharmacological Therapy
Exercise training is beneficial in stimulating nitric oxide pathways, which can result in exercise capacity with less anginal pain.[107] Spinal cord stimulation improves anginal pain perception, and increases exercise tolerance.[108,109] Enhanced external counter pulsation uses pneumatic cuffs applied to a patient’s legs with simultaneous inflation and deflation synchronised to the cardiac cycle to improve haemodynamics.[110]
Future Directions
Continued work is needed to refine diagnostic and management strategies for MVA and CMD. On-going trials are exploring whether the intensive treatment of coronary atherosclerosis with high-intensity statins, ACE-I or angiotensin receptor blockers (ARBs) and low-dose aspirin improves angina and ischaemia. The WARRIOR trial (NCT03417388) is testing if such treatment translates to improved outcomes, while the MINOCA-BAT study (NCT03686696) is evaluating a β-blocker and ACE/ARB intervention on MACE.
Conclusion
Recognition of suspected INOCA has increased over the past decades, with a key contributor being MVA. Patients with MVA are at higher risk for MACE including MI, stroke, HFpEF and death. Guidelines for diagnosis and management of patients with MVA are still evolving. However, on-going clinical trials are testing strategies that will inform the management of patients with MVA.
Funding Statement
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23 HL105787, K23 HL125941, K23 HL127262, K23HL151867 T32 HL69751, R01 HL090957, 1R03 AG032631, R01 HL146158, R01HL124649, PR150224P1 (CDMRP-DoD) and U54 AG065141; GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences grant UL1TR000124; and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ; the Women’s Guild of Cedars- Sinai Medical Center, Los Angeles, CA; the Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA; QMED, Inc, Laurence Harbor, NJ; the Edythe L Broad and Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, CA; the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, CA; the Society for Women’s Health Research, Washington, DC; the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, CA, US.
References
- 1.AlBadri A, Lai K, Wei J et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE). PloS One. 2017;12:e0177684. doi: 10.1371/journal.pone.0177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaski JC, Rosano GM, Collins P et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–14. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 3.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7:479–83. doi: 10.1016/S0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 4.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol. 1995;25:1013–8. doi: 10.1016/0735-1097(94)00519-V. [DOI] [PubMed] [Google Scholar]
- 5.Gulati M, Cooper-DeHoff RM, McClure C et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson BD, Shaw LJ, Buchthal SD et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 7.Pepine CJ, Anderson RD, Sharaf BL et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldiwani H, Zaya M, Suppogu N et al. Angina Hospitalization Rates in Women With Signs and Symptoms of Ischemia But no Obstructive Coronary Artery Disease: A Report from the WISE (Women's Ischemia Syndrome Evaluation) Study. J Am Heart Assoc. 2020;9:e013168. doi: 10.1161/JAHA.119.013168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel MR, Peterson ED, Dai D et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong P, Athanasiadis A, Borgulya G et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arterie: the ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59:655–62. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Reis SE, Holubkov R, Conrad Smith AJ et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 12.Aziz A, Hansen HS, Sechtem U et al. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J Am Coll Cardiol. 2017;70:2349–58. doi: 10.1016/j.jacc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Ford TJ, Stanley B, Good R et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–55. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Sara JD, Widmer RJ, Matsuzawa Y et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–453. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Ong P, Camici PG, Beltrame JF et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 16.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 17.Cannon RO, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation. 1992;85:883–92. doi: 10.1161/01.CIR.85.3.883. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan A, Mullins PA, Taylor G et al. Both endothelium-dependent and endothelium-independent function is impaired in patients with angina pectoris and normal coronary angiograms. Eur Heart J. 1997;18:60–8. doi: 10.1093/oxfordjournals.eurheartj.a015119. [DOI] [PubMed] [Google Scholar]
- 19.Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol. 1991;17:499–506. doi: 10.1016/S0735-1097(10)80122-6. [DOI] [PubMed] [Google Scholar]
- 20.Wei J, Bakir M, Darounian N et al. myocardial scar is prevalent and associated with subclinical myocardial dysfunction in women with suspected ischemia but no obstructive coronary artery disease: from the Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction study. Circulation. 2018;137:874–6. doi: 10.1161/CIRCULATIONAHA.117.031999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman H, Demir OM, Khan F et al. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol. 2020;75:2538–49. doi: 10.1016/j.jacc.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau P, d’Uscio LV, Lüscher TF. Structure and reactivity of small arteries in aging. Cardiovasc Res. 1998;37:247–53. doi: 10.1016/S0008-6363(97)00225-3. [DOI] [PubMed] [Google Scholar]
- 23.Sackett DL, Gibson RW, Bross ID, Pickren JW. Relation between aortic atherosclerosis and the use of cigarettes and alcohol. An autopsy study. N Engl J Med. 1968;279:1413–20. doi: 10.1056/NEJM196812262792602. [DOI] [PubMed] [Google Scholar]
- 24.Zeiher AM, Schächinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–100. doi: 10.1161/01.CIR.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Huo T, Delia Johnson B et al. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI-sponsored WISE Study. J Am Heart Assoc. 2014;3:e000660. doi: 10.1161/JAHA.113.000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387–93. doi: 10.1016/S0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 27.Dayanikli F, Grambow D, Muzik O et al. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994;90:808–17. doi: 10.1161/01.CIR.90.2.808. [DOI] [PubMed] [Google Scholar]
- 28.Wei J, Nelson MD, Szczepaniak EW et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol. 2016;310:h14–9. doi: 10.1152/ajpheart.00612.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie JX, Eshtehardi P, Varghese T et al. Prognostic significance of nonobstructive left main coronary artery disease in women versus men: Long-term outcomes from the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: an International Multicenter) registry. Circ Cardiovasc Imaging. 2017;10:e006246. doi: 10.1161/CIRCIMAGING.117.006246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AlBadri A, Bairey Merz CN, Johnson BD et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–93. doi: 10.1016/j.jacc.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitz A, Gardezy J, Pirozzolo G et al. Long-term follow-up in patients with stable angina and unobstructed coronary arteries undergoing intracoronary acetylcholine testing. JACC Cardiovasc Interv. 2020;13:1865–76. doi: 10.1016/j.jcin.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Schroder J, Michelsen MM, Mygind ND et al. Coronary flow velocity reserve predicts adverse prognosis in women with angina and no obstructive coronary artery disease: results from the iPOWER study. Eur Heart J. 2021;42:228–39. doi: 10.1093/eurheartj/ehaa944. [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Lee JCY, Leung ST et al. Long-term prognosis of patients with coronary microvascular disease using stress perfusion cardiac magnetic resonance. JACC Cardiovasc Imaging. 2021;14:602–11. doi: 10.1016/j.jcmg.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 34.Murthy VL, Naya M, Taqueti VR et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gdowski MA, Murthy VL, Doering M Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc 2020;9:e014954. [DOI] [PMC free article] [PubMed]
- 36.Schindler TH, Dilsizian V. Coronary microvascular dysfunction: Clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging. 2020;13:140–55. doi: 10.1016/j.jcmg.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 37.Roy R, Aldiwani H, Darouian N et al. Ambulatory and silent myocardial ischemia in women with coronary microvascular dysfunction: results from the Cardiac Autonomic Nervous System study (CANS). Int J Cardiol. 2020;316:1–6. doi: 10.1016/j.ijcard.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126:e354–471. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Smeeth L, Skinner JS, Ashcroft J et al. NICE clinical guideline: chest pain of recent onset. Br J Gen Pract. 2010;60:607–10. doi: 10.3399/bjgp10X515124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panza JA, Laurienzo JM, Curiel RV et al. Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography. J Am Coll Cardiol. 1997;29:293–301. doi: 10.1016/S0735-1097(96)00481-0. [DOI] [PubMed] [Google Scholar]
- 41.Sharaf B, Wood T, Shaw L et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–41. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hulten E, Di Carli MF. FFRCT: Solid PLATFORM or thin ice? J Am Coll Cardiol. 2015;66:2324–8. doi: 10.1016/j.jacc.2015.09.065. [DOI] [PubMed] [Google Scholar]
- 43.Mathew RC, Bourque JM, Salerno M, Kramer CM. Cardiovascular imaging techniques to assess microvascular dysfunction. JACC Cardiovascular Imaging. 2020;13:1577–90. doi: 10.1016/j.jcmg.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mygind ND, Michelsen MM, Pena A et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Am Heart Assoc. 2016;5:e003064. doi: 10.1161/JAHA.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gan LM, Svedlund S, Wittfeldt A et al. Incremental value of transthoracic Doppler echocardiography-assessed coronary flow reserve in patients with suspected myocardial ischemia undergoing myocardial perfusion scintigraphy. J Am Heart Assoc. 2017;6:e004875. doi: 10.1161/JAHA.116.004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–40. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Taqueti VR, Solomon SD, Shah AM et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2017;39:840–49. doi: 10.1093/eurheartj/ehx721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taqueti VR, Everett BM, Murthy VL et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–35. doi: 10.1161/CIRCULATIONAHA.114.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaudieri V, Mannarino T, Zampella E et al. Prognostic value of coronary vascular dysfunction assessed by rubidium-82 PET/CT imaging in patients with resistant hypertension without overt coronary artery disease. Eur J Nucl Med Mol Imaging. 2021;48:3162–71. doi: 10.1007/s00259-021-05239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson LEJ, Wei J, Agarwal M et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. Circ Cardiovasc Imaging. 2015;8:e002481. doi: 10.1161/CIRCIMAGING.114.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotecha T, Martinez-Naharro A, Boldrini M et al. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. 2019;12:1958–69. doi: 10.1016/j.jcmg.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi A, Wragg A, Klotz E et al. Dynamic computed tomography myocardial perfusion imaging: comparison of clinical analysis methods for the detection of vessel-specific ischemia. Circ Cardiovasc Imaging. 2017;10:e005505. doi: 10.1161/CIRCIMAGING.116.005505. [DOI] [PubMed] [Google Scholar]
- 53.Wei J, Mehta PK, Johnson BD et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5:646–53. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ford TJ, Ong P, Sechtem U et al. Assessment of vascular dysfunction in patients without obstructive coronary artery disease: why, how, and when. JACC Cardiovasc Interv. 2020;13:1847–64. doi: 10.1016/j.jcin.2020.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunadian V, Chieffo A, Camici PG et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504–20. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niccoli G, Morrone D, De Rosa S et al. The central role of invasive functional coronary assessment for patients with ischemic heart disease. Int J Cardiol. 2021;331:17–25. doi: 10.1016/j.ijcard.2021.01.055. [DOI] [PubMed] [Google Scholar]
- 57.AlBadri A, Wei J, Mehta PK et al. Acetylcholine versus cold pressor testing for evaluation of coronary endothelial function. PLoS One. 2017;12:e0172538. doi: 10.1371/journal.pone.0172538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suwaidi JA, Hamasaki S, Higano ST et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 59.Lee BK, Lim HS, Fearon WF et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–60. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fearon WF, Balsam LB, Farouque HM et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–32. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 61.JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–801. doi: 10.1253/circj.CJ-66-0098. [DOI] [PubMed] [Google Scholar]
- 62.Knuuti J, Wijns W, Saraste A et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 63.Fihn SD, Blankenship JC, Alexander KP et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. J Thorac Cardiovasc Surg. 2015;149:e5–23. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116:856–70. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ford TJ, Stanley B, Sidik N et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33–45. doi: 10.1016/j.jcin.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kayikcioglu M, Payzin S, Yavuzgil O et al. Benefits of statin treatment in cardiac syndrome-X1. Eur Heart J. 2003;24:1999–2005. doi: 10.1016/S0195-668X(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 67.Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–8. doi: 10.1161/01.CIR.0000100722.34034.E4. [DOI] [PubMed] [Google Scholar]
- 68.Ballantyne CM, Raichlen JS, Nicholls SJ et al. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation. 2008;117:2458–66. doi: 10.1161/CIRCULATIONAHA.108.773747. [DOI] [PubMed] [Google Scholar]
- 69.Dupuis J, Tardif JC, Cernacek P, Théroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (Reduction of Cholesterol in Ischemia and Function of the Endothelium) trial. Circulation. 1999;99:3227–33. doi: 10.1161/01.CIR.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 70.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Am J Cardiol. 2010;106:204–9. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Caliskan M, Erdogan D, Gullu H et al. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol. 2007;30:475–9. doi: 10.1002/clc.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eshtehardi P, McDaniel MC, Dhawan SS et al. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J Invasive Cardiol. 2012;24:522–9. [PubMed] [Google Scholar]
- 73.Reriani MK, Dunlay SM, Gupta B et al. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2011;18:704–16. doi: 10.1177/1741826711398430. [DOI] [PubMed] [Google Scholar]
- 74.Pauly DF, Johnson BD, Anderson RD et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–84. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen JW, Hsu NW, Wu TC et al. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–82. doi: 10.1016/S0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 76.Masuda D, Nohara R, Tamaki N et al. Evaluation of coronary blood flow reserve by 13N-NH3 positron emission computed tomography (PET) with dipyridamole in the treatment of hypertension with the ACE inhibitor (cilazapril). Ann Nucl Med. 2000;14:353–60. doi: 10.1007/BF02988695. [DOI] [PubMed] [Google Scholar]
- 77.Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. 1989;63:286–90. doi: 10.1016/0002-9149(89)90332-9. [DOI] [PubMed] [Google Scholar]
- 78.Khuddus MA, Pepine CJ, Handberg EM et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. 2010;23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–6 a8. doi: 10.1016/S0002-9149(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 80.Matsuda Y, Akita H, Terashima M et al. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140:753–9. doi: 10.1067/mhj.2000.110093. [DOI] [PubMed] [Google Scholar]
- 81.Togni M, Vigorito F, Windecker S et al. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovasc Drugs Ther. 2007;21:99–108. doi: 10.1007/s10557-006-0494-7. [DOI] [PubMed] [Google Scholar]
- 82.Sütsch G, Oechslin E, Mayer I, Hess OM. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol. 1995;52:135–43. doi: 10.1016/0167-5273(95)02458-9. [DOI] [PubMed] [Google Scholar]
- 83.Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56:242–6. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- 84.Kanatsuka H, Eastham CL, Marcus ML, Lamping KG. Effects of nitroglycerin on the coronary microcirculation in normal and ischemic myocardium. J Cardiovasc Pharmacol. 1992;19:755–63. [PubMed] [Google Scholar]
- 85.Hasenfuss G, Maier LS. Mechanism of action of the new anti-ischemia drug ranolazine. Clin Res Cardiol. 2008;97:222–6. doi: 10.1007/s00392-007-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta PK, Goykhman P, Thomson LE et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4:514–22. doi: 10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villano A, Di Franco A, Nerla R et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13. doi: 10.1016/j.amjcard.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 88.Bairey Merz CN, Handberg EM, Shufelt CL et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37:1504–13. doi: 10.1093/eurheartj/ehv647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rambarat CA, Elgendy IY, Handberg EM et al. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction: Women's Ischemia Syndrome Evaluation – Coronary Vascular Dysfunction ancillary study. Int J Cardiol. 2019;276:8–13. doi: 10.1016/j.ijcard.2018.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skalidis EI, Hamilos MI, Chlouverakis G et al. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215:160–5. doi: 10.1016/j.atherosclerosis.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 91.Phan A, Shufelt C, Merz CN. Persistent chest pain and no obstructive coronary artery disease. JAMA. 2009;301:1468–74. doi: 10.1001/jama.2009.425. [DOI] [PubMed] [Google Scholar]
- 92.Cox ID, Hann CM, Kaski JC. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur Heart J. 1998;19:250–4. doi: 10.1053/euhj.1997.0615. [DOI] [PubMed] [Google Scholar]
- 93.Bavry AA, Handberg EM, Huo T et al. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation. Am Heart J. 2014;167:826–32. doi: 10.1016/j.ahj.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoo SY, Song SG, Lee JH et al. Efficacy of cilostazol on uncontrolled coronary vasospastic angina: a pilot study. Cardiovasc Ther. 2013;31:179–85. doi: 10.1111/j.1755-5922.2012.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Denardo SJ, Wen X, Handberg EM et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women’s Ischemia Syndrome Evaluation (WISE) ancillary study. Clin Cardiol. 2011;34:483–7. doi: 10.1002/clc.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nihei T, Takahashi J, Hao K et al. Prognostic impacts of Rho-kinase activity in circulating leucocytes in patients with vasospastic angina. Eur Heart J. 2018;39:952–959. doi: 10.1093/eurheartj/ehx657. [DOI] [PubMed] [Google Scholar]
- 97.Masumoto A, Mohri M, Shimokawa H et al. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–7. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 98.Mohri M, Shimokawa H, Hirakawa Y et al. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–19. doi: 10.1016/S0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 99.Lerman A, Burnett JC Jr., Higano ST et al. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–8. doi: 10.1161/01.CIR.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 100.Pulakazhi Venu VK, El-Daly M, Saifeddine M et al. Minimizing hyperglycemia-induced vascular endothelial dysfunction by inhibiting endothelial sodium-glucose cotransporter 2 and attenuating oxidative stress: implications for treating individuals with type 2 diabetes. Can J Diabetes. 2019;43:510–4. doi: 10.1016/j.jcjd.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 101.Jadhav S, Ferrell W, Greer IA et al. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2006;48:956–63. doi: 10.1016/j.jacc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 102.MacCarthy PA, Pegge NC, Prendergast BD et al. The physiological role of endogenous endothelin in the regulation of human coronary vasomotor tone. J Am Coll Cardiol. 2001;37:137–43. doi: 10.1016/S0735-1097(00)01042-1. [DOI] [PubMed] [Google Scholar]
- 103.Mather KJ, Lteif AA, Veeneman E et al. Role of endogenous ET-1 in the regulation of myocardial blood flow in lean and obese humans. Obesity (Silver Spring) 2010;18:63–70. doi: 10.1038/oby.2009.196. [DOI] [PubMed] [Google Scholar]
- 104.Morrow AJ, Ford TJ, Mangion K et al. Rationale and design of the Medical Research Council's Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J. 2020;229:70–80. doi: 10.1016/j.ahj.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reriani M, Raichlin E, Prasad A et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122:958–66. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ford TJ, Corcoran D, Padmanabhan S et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J. 2020;41:3239–52. doi: 10.1093/eurheartj/ehz915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eriksson BE, Tyni-Lennè R, Svedenhag J et al. Physical training in Syndrome X: physical training counteracts deconditioning and pain in Syndrome X. J Am Coll Cardiol. 2000;36:1619–25. doi: 10.1016/S0735-1097(00)00931-1. [DOI] [PubMed] [Google Scholar]
- 108.Sestito A, Lanza GA, Le Pera D et al. Spinal cord stimulation normalizes abnormal cortical pain processing in patients with cardiac syndrome X. Pain. 2008;139:82–9. doi: 10.1016/j.pain.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 109.Lanza GA, Sestito A, Sandric S et al. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital Heart J. 2001;2:25–30. [PubMed] [Google Scholar]
- 110.Kitsou V, Xanthos T, Roberts R et al. Enhanced external counterpulsation: mechanisms of action and clinical applications. Acta Cardiol. 2010;65:239–47. doi: 10.2143/AC.65.2.2047060. [DOI] [PubMed] [Google Scholar]
- 111.Merz CNB, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–92. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]


