Abstract
Ischaemia with non-obstructed coronary artery disease (INOCA) remains a diagnostic and therapeutic challenge. An anatomical investigationbased approach to ischaemic heart disease fails to account for disorders of vasomotion. The main INOCA endotypes are microvascular angina, vasospastic angina, mixed (both) or non-cardiac symptoms. The interventional diagnostic procedure (IDP) enables differentiation between clinical endotypes, with linked stratified medical therapy leading to a reduced symptom burden and a better quality of life. Interventionists are therefore well placed to make a positive impact with more personalised care. Despite adjunctive tests of coronary function being supported by contemporary guidelines, IDP use in daily practice remains limited. More widespread adoption should be encouraged. This article reviews a stratified approach to INOCA, describes a streamlined approach to the IDP and highlights some practical and safety considerations.
Keywords: Ischaemia with no obstructive coronary artery disease, ischaemic heart disease, coronary, angina, coronary microvascular dysfunction, vasospastic angina, interventional diagnostic procedure
The classic cause of ischaemic heart disease is epicardial coronary atherosclerosis, but disorders of coronary vasomotion are being increasingly identified.[1–4] Anatomical imaging with CT coronary angiography (CTCA) is the first-line test for patients with known or suspected angina and no prior history of coronary artery disease (CAD).[5] Anatomical imaging is useful to clarify the presence and extent of coronary atherosclerosis, but does not evaluate microvascular function or coronary artery spasm. This presents a diagnostic gap for patients with ischaemia with non-obstructed coronary artery disease (INOCA).
The strengths of invasive angiography are its high sensitivity for revealing coronary atherosclerosis, providing haemodynamic measurements and the option of following on with percutaneous coronary intervention (PCI). Its limitations are, first, that its spatial resolution of approximately 0.3 mm is insensitive in visualising resistance arterioles that help to modulate myocardial blood flow.[2] Second, angiography does not directly assess vascular function. Despite adjunctive tests of coronary function now being recommended (class IIa) by contemporary guidelines, its uptake in daily practice remains limited.[4]
Up to 50% of patients with known or suspected angina have INOCA.[3] Of these, 80% are found to have microvascular and vasospastic angina when studied using specific tests.[3,6,7] These conditions are associated with an impaired quality of life, as well as greater morbidity and health resource utilisation.[8,9,10] Dismissing these patients would be doing them a disservice. Interventional cardiologists are uniquely placed in the care pathway to offer gold-standard assessment of the coronary circulation via the interventional diagnostic procedure (IDP). While disorders of coronary function may coexist in patients with epicardial stenosis, diagnostic testing remains limited in the presence of obstructive CAD. This review focuses on the diagnosis of INOCA, describes a streamlined approach to the IDP, and highlights some practical and safety considerations.
Importance of Diagnosis
Illness perception tends to be more threatening in patients with diagnostic uncertainty, which in turn leads to disability and absence from work.[11] A conclusive diagnosis can be therapeutic, encouraging compliance with medication and lifestyle interventions. The CorMicA study demonstrated that an IDP with linked stratified medical therapy improved symptom burden, quality of life, treatment satisfaction and illness perception at 6 and 12 months.[6,12] Invasive coronary angiography could therefore be considered incomplete without assessment of coronary vascular function.
Alternative Approaches
Some clinicians propose that ex juvantibus criteria for the management of these patients could convey the same benefits without an IDP, by instituting a therapeutic trial of vasomotor-specific antianginals in all patients. The arguments for this approach include a shorter procedural duration and reduced upfront cost from diagnostic guidewires, adenosine and acetylcholine.
Stratified Medicine
Stratified medicine is the identification of key subgroups within a heterogeneous population of patients, each with differing disease mechanisms and/or responses to treatment. Mechanistically linked treatments should be prescribed based on clinical endotypes, as determined from results of the IDP. Unnecessary medication should be discontinued, minimising polypharmacy and its possible side effects. This concept aligns with precision medicine – offering the ‘right treatment for the right patient at the right time’.[13] Stratification can improve mechanistic understanding of disease to enable development of biomarkers and identify new disease-specific targets for treatment.[13]
Ultimately, many of these patients are significantly affected by their symptoms, such that they may be willing to undergo invasive angiography with its small risk of life-threatening complications. We owe it to them to inform them about the risks and benefits and offer opportunity for further assessment.
Defining INOCA by Clinical Endotypes
Physiological coronary vascular function reflects a balance between vascular tone, vasodilator reserve and resistance. Vascular function may vary between coronary artery territories. Disorders cause a relative supply-demand mismatch of blood flow relative to requirements, resulting in ischaemia.
INOCA is considered after obstructive epicardial disease has been excluded via standard angiography, with or without measurement of non-hyperaemic pressure ratios and/or fractional flow reserve (FFR). The main INOCA endotypes to consider are described below. The Coronary Vasomotor Disorders International Study Group (COVADIS) has published standardised definitions to aid clinical diagnosis (Table 1).[14]
Table 1: INOCA Diagnosis.
| Clinical Endotype | Diagnostic Criteria | Evidence |
|---|---|---|
| Microvascular angina | 1. Symptoms of myocardial ischaemia | Effort or rest angina/angina-equivalent, including dyspnoea |
| 2. Absence of obstructive coronary artery disease (<50% stenosis or FFR >0.80) | CTCA or invasive coronary angiography ± FFR | |
| 3. Objective evidence of myocardial ischaemia | Ischaemic ECG during episode of chest pain and/or abnormal functional tests (e.g. myocardial perfusion or regional wall motion abnormality). | |
| 4. Evidence of impaired coronary microvascular function | CFR ≤2.0 (2.1–2.5 grey-zone result), IMR ≥25, HMR ≥2.5 mmHg/cm/s, and/or microvascular spasm (TIMI flow ≤2) during vasoreactivity testing | |
| Vasospastic angina | 1. ≥90% epicardial vasoconstriction | ≥90% reduction in coronary luminal diameter versus baseline during vasoreactivity testing |
| 2. Reproduction of usual anginal symptoms | Angina/ angina-equivalent symptoms including chest discomfort and dyspnoea | |
| 3. Ischaemic ECG changes | ST-segment deviation ≥0.1 mV or new negative U waves | |
| Mixed (microvascular and vasospastic) | Overlap condition meeting criteria for both microvascular angina and vasospastic angina | Evidence of microvascular dysfunction in the presence of significant (≥90%) epicardial vasoconstriction |
| Non-cardiac symptoms | Unobstructed coronary arteries with normal coronary function test results | <50% stenosis or FFR >0.80, with normal CFR, IMR/HMR and vasoreactivity testing |
Coronary Vasomotor Disorders International Study Group Diagnostic Criteria for INOCA. CFR = coronary flow reserve; CTCA = CT coronary angiography; FFR = fractional flow reserve; HMR = hyperaemic microvascular resistance; IMR = index of microcirculatory resistance; TIMI = thrombolysis in MI. Source: Ong et al. 2018.[14] Adapted with permission from Elsevier.
Microvascular Angina
Microvascular angina (MVA) may involve one or more distinct mechanisms. This concept has also been described in heart failure with preserved ejection fraction.[15] MVA is caused by microcirculatory disorders that may be functional, structural or both. Functional disorders impair coronary arteriolar vasodilation or vasoconstriction. Structural disorders relate to inflammation, inward remodelling of the vascular wall or interstitial matrix and/or capillary rarefaction.[16] Remodelled arterioles have also demonstrated hypersensitivity to vasoconstricting stimuli.[17]
Epicardial Vasospastic Angina
Focal epicardial spasm was first described by Prinzmetal et al. in 1959 as ‘variant angina’.[18] The defining feature is hyper-reactivity to vasoconstrictor stimuli. This includes both spontaneous spasm and episodes during provocation testing. It may exhibit a circadian pattern and be precipitated by stress or hyperventilation. Vasospastic angina (VSA) typically occurs at rest but may also be induced by exertion in a minority of people.
Mixed Microvascular and Vasospastic Angina
VSA may coexist with microvascular disorders and/or structural epicardial disease. There is evidence that patients with mixed MVA/VSA have worse overall quality of life.[19]
Non-Cardiac Symptoms
When coronary arteries are unobstructed and coronary function results are normal, alternative aetiologies for symptoms should be considered.
Functional Coronary Angiography and the Interventional Diagnostic Procedure
Functional coronary angiography integrates anatomical imaging with functional tests. The IDP increases the diagnostic yield and differentiative power of invasive angiography among patients with unobstructed epicardial coronaries. The additional information provided reduces diagnostic uncertainty and related variations in treatment.[6]
The assessment of INOCA comprises two main components: diagnostic, guidewire-based assessment comparing flow at rest versus during hyperaemia; and pharmacological provocation testing for epicardial and/or microvascular vasoconstriction.
There is some debate regarding which test should be performed first. The authors advocate performing guidewire/adenosine studies first because of the risk of profound vasoconstriction during provocation testing, which can in turn elevate sympathetic drive and disrupt resting haemodynamic physiology. This accepts the small risk of incomplete nitrate metabolism before vasoreactivity testing.
Current diagnostic guidewires use either a pressure/temperature sensor for thermodilution or a Doppler/pressure sensor. Thermodilution-based wires are safe and straightforward to use.[6] Doppler/pressure wires may have higher accuracy and better correlation with non-invasive testing results, but at the expense of wire manoeuvrability.[20] This review focuses on thermodilution-based guidewires and acetylcholine provocation testing. A streamlined workflow with its practical considerations is summarised in Figure 1.
Figure 1: Interventional Diagnostic Procedure Workflow.
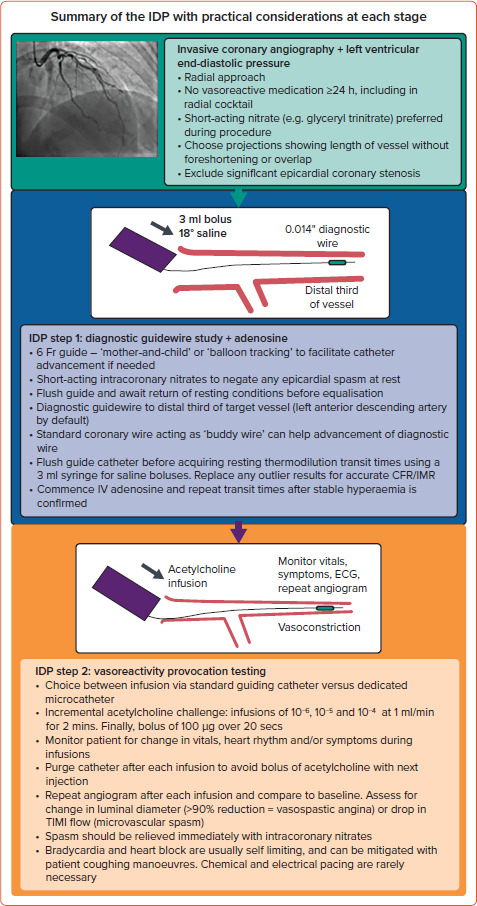
CFR = coronary flow reserve; IDP = interventional diagnostic procedure; IMR = index of microvascular resistance; TIMI = thrombolysis in MI.
In practice, the IDP takes approximately 20 minutes, similar to single-vessel PCI.[6] However, the benefits of complete diagnostic assessment should be acknowledged, including the reduced need for repeat referrals in the future.
General Considerations
Patient comfort and safety are paramount. A radial approach is recommended as per contemporary guidelines.[4] An anxiolytic sedative facilitates a favourable patient experience but may, theoretically, reduce the propensity for inducing coronary spasm during acetylcholine provocative testing. All vasoactive medication should be withheld >24 hours beforehand, including abstinence from caffeine. This reduces the risk of false-negative results during vasoreactivity testing. Calcium channel blockers and long-acting nitrates should be avoided in the radial cocktail. Short-acting nitrates such as glyceryl trinitrate (which has a half-life of ~2 minutes) are preferred. Rapidly metabolised nitrates minimise the risk of false-negative results during vasoreactivity testing.[21]
Measuring left ventricular end-diastolic pressure is recommended as elevated pressures might suggest alternative explanations for symptoms (e.g. decompensated heart failure), which may themselves be associated with microvascular dysfunction.
Diagnostic Guidewire Assessment
Resting thermodilution indices should be obtained in all patients undergoing FFR studies. This allows the option of completing hyperaemic indices during a single adenosine infusion after the FFR result is acquired. A bolus of intracoronary short-acting nitrate at the start mitigates any confounding epicardial spasm at rest. The guiding catheter is flushed with normal saline to avoid pressure-damping from contrast or blood. Allow 30 seconds for resting conditions to return before performing pressure wire equalisation. Meticulous initial set-up reduces the risk of pressure wire signal drift downstream.
The choice of guiding catheter should be personalised, taking account of the patient’s stature. A 6 Fr or 5 Fr guiding catheter represents an ideal balance between size, support and patient tolerability. ‘Balloon tracking’ or, alternatively, a ‘mother-and-child’ technique of advancing a 5 Fr diagnostic pigtail catheter inside a 6 Fr guide catheter can help overcome issues with radial spasm and shearing injury during catheter advancement. The benefits of good catheter support are threefold: first, it reduces variation in the volume delivered from each saline bolus; second, less in-and-out recoil of the catheter during rapid bolus injections reduces the risk of vessel injury; and third, reduced catheter movement stabilises the diagnostic guidewire position within the vessel, thereby reducing variability in transit times.
Heparin (50–70 U/kg) should be administered to achieve an activated clotting time (ACT) of approximately 250 seconds before coronary instrumentation. The left anterior descending artery is the default artery of choice because of its subtended myocardial mass. The pressure wire should be advanced to the distal third of the target vessel. If advancing the diagnostic guidewire is difficult, pathfinding with a standard coronary guidewire that acts as a ‘buddy wire’ can be helpful. This is particularly pertinent if using a combination Doppler/pressure wire.
With the diagnostic guidewire positioned, the guiding catheter is again flushed to expel any saline warmed by the patient’s body temperature. After resting haemodynamic conditions have returned, resting transit times are acquired with brisk 3 ml injections of room-temperature saline, administered via a coaxial guiding catheter to avoid hydro-trauma. In large left-dominant circulations, 5 ml injections may be required. The manifold should be immediately switched back to live pressure recording after each injection to allow simultaneous measurement of aortic and distal coronary pressures. Movement of the manifold should be minimised to avoid artefactual pressure recordings. Thermodilution deflection curves and transit times should be inspected, with outlier and artefactual values replaced. The distribution of transit times should be within 0.1 second of each other.
Adenosine can be administered as an IV infusion (140–210 µg/kg/min) or intracoronary bolus (50–200 µg). The intracoronary dose for the dominant coronary artery should be approximately halved to limit the possibility of transient heart block. Markers of hyperaemia should be confirmed: ‘ventricularisation’ and disappearance of the dicrotic notch in the distal pressure waveform; separation of the aortic and distal pressures; and symptoms (typically chest discomfort and/or dyspnoea). Changes in heart rate and blood pressure are less reliable indicators of hyperaemia. When stable hyperaemia with adenosine infusion is confirmed, FFR is measured and thermodilution transit times repeated. The same principles apply if using a Doppler/pressure wire to measure Doppler-derived average peak velocities at rest and hyperaemia.
Coronary Flow Reserve
Coronary flow reserve (CFR) reflects the vasodilator capacity of the whole coronary circulation, including the epicardial coronaries and microcirculation. The WISE study demonstrated that low CFR was an independent predictor for the composite endpoint of death, non-fatal MI, non-fatal stroke or hospitalisation for heart failure (HR 1.20; 95% CI [1.05–1.38]; p=0.008).[22]
CFR can be impaired if basal coronary flow is high, diastolic time is reduced or intra-myocardial pressure is increased.[23] Therefore, fluctuations in the resting haemodynamic status can lead to variability during repeat measurement. CFR is calculated from the principles of thermodilution by dividing the resting mean transit time by the hyperaemic mean transit time. A CFR of ≤2.0 is abnormal, with 2.1–2.5 reflecting a ‘grey-zone’ result.
Index of Microvascular Resistance
Coronary vascular resistance is mainly determined within the microcirculation. The apparent index of microvascular resistance (IMR) is the product of distal coronary pressure and hyperaemic mean transit time. An IMR if ≥25 is considered abnormal, with a few caveats: there is a weak correlation between IMR and subtended myocardial mass; and there is variation between ethnicities, with a lower cut-off suggested in Asians.[24]
Pharmacological Vasoreactivity Testing
Acetylcholine is now the default agent for provocation testing.[25] Okumura et al. demonstrated that intracoronary acetylcholine had 90% sensitivity and 99% specificity for diagnosing epicardial spasm.[26]
Acetylcholine is an endogenous neurotransmitter acting on muscarinic and nicotinic receptors. In blood vessels, its effects reflect responses from both the endothelium and smooth muscle cells. Acetylcholine is not licensed for parenteral administration so gaining informed consent should take account of the rationale for its use. Acetylcholine testing assesses the physiological response, which, in a healthy artery, should be vasodilatation. In the presence of endothelial dysfunction, the response may be micro- or macrovascular spasm. The hospital pharmacy should facilitate drug access, storage, transport and reconstitution.
For the final stage of the IDP, intracoronary acetylcholine is infused via either the guiding catheter or a dedicated microcatheter. The benefit of a microcatheter is the theoretical avoidance of pan-coronary spasm. The CorMicA study demonstrated direct infusion using the guiding catheter was safe, and avoid the cost and risk of additional coronary instrumentation.[6]
Our protocol for acetylcholine provocative testing involves intracoronary infusion of incremental concentrations (10-6, 10-5, 10-4 mol/l) at 1 ml/min for 2 minutes using a mechanical pump, followed by a bolus of 100 µg (half dose for the dominant coronary artery) given over 20 seconds, with continuous monitoring of the ECG and patient.
An alternative approach is manual infusions of 2 µg, 20 µg, 100 µg and 200 µg. If infusing into a ‘dominant’ coronary artery, consider halving the dose to limit potential bradyarrhythmia. Before starting each infusion, first fill the guiding catheter lumen with infusate to reduce any 'dead space' effect when the infusion begins. Afterwards, ensure gentle purging of the catheter following each infusion to avoid a sudden bolus of acetylcholine with the next injection of contrast. Vigilance is required during infusions, with intracoronary nitrates at hand to reverse any spasm. The half-life of acetylcholine is <15 seconds, with effects ceasing rapidly once the infusion is stopped.[27]
Coronary angiography at a projection without vessel foreshortening is acquired at baseline, then repeated after each infusion. The vessel luminal diameter is compared to its size at baseline. A diameter reduction of >20% suggests endothelial dysfunction (MVA), while ≥90% reduction suggests VSA. Semiquantitative analysis of antegrade contrast flow may be undertaken by calculating the TIMI (thrombolysis in MI) frame count, with >27 (images acquired at 30 frames/second) suggesting MVA (coronary slow-flow phenomenon from microvascular spasm).[3]
A pressure wire pullback should be performed at the end of the IDP to exclude significant (>0.03) signal drift. The IDP can be repeated in different coronary artery territories if false-negative results are suspected. Figure 2 highlights typical examples for each clinical endotype.
Figure 2: Flowchart for Diagnosis of Different INOCA Endotypes, with Typical Examples.
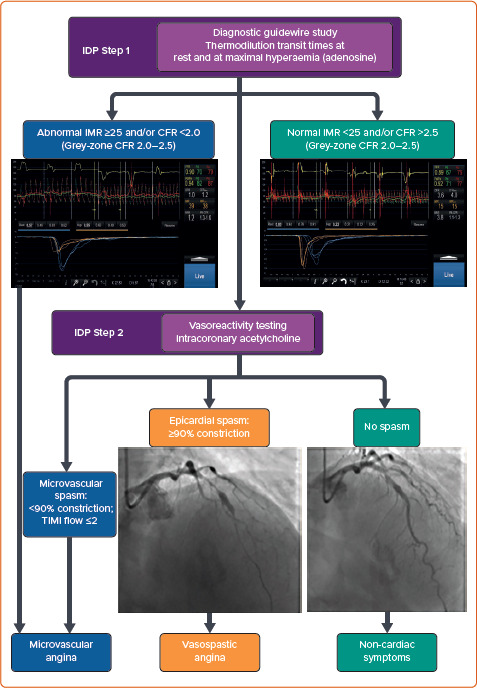
CFR = coronary flow reserve; IDP = interventional diagnostic procedure; IMR = index of microvascular resistance; TIMI = thrombolysis in MI.
Risks and Safety Considerations
The main risks of the IDP are those relating to standard coronary angiography, use of a guidewire and the physiological effects of acetylcholine including refractory coronary spasm, bradycardia and/or AF.
The CorMicA study demonstrated that the IDP is feasible, safe and low risk in trained hands.[6] Likewise, other studies demonstrated an incidence of cardiac arrhythmias during provocation testing comparable with that observed during spontaneous spasm (6.8% versus 7.0%).[28] The monitoring set-up during invasive angiography facilitates safety. Vasospasm and bradycardia are usually transient. Patient coughing manoeuvres can help maintain haemodynamic perfusion in the event of asystole.[29] Atropine and isoprenaline should be readily available, with electrical pacing rarely necessary.
Treatments
The goals of therapy are twofold: amelioration of symptoms; and reduction in cardiovascular risk. Linked stratified medical therapy guided by the IDP can improve angina and quality of life within a previously undifferentiated, heterogenous population.[6,12] Guideline-recommended treatments based on clinical endotype are summarised in Table 2.[3] Bairey Merz et al. have published a review of current and upcoming treatments in INOCA.[30] Endothelial dysfunction typically precedes atherosclerosis, which mandates aggressive treatment of cardiovascular risk factors.[31]
Table 2: Guideline-recommended Treatments Based on Clinical Endotype.
| Diagnosis | Treatment | Mechanism of Effect |
|---|---|---|
| Microvascular angina | β-blockers (nebivolol 2.5–10 mg once daily) |
|
| Calcium-channel blockers (amlodipine 10 mg once daily) |
|
|
| Nicorandil (10–20 mg twice daily) |
|
|
| Ranolazine (375–750 mg twice daily) |
|
|
| Trimetazidine (35 mg twice daily) |
|
|
| ACE inhibitors (ramipril 2.5–10 mg once daily); angiotensin-receptor blockers if intolerant |
|
|
| Vasospastic angina | Calcium channel blockers (verapamil 240 mg once daily or diltiazem 120–360 mg daily) |
|
| Nitrates (isosorbide mononitrate 20–120 mg daily) |
|
|
| Nicorandil (10–20 mg twice daily) |
|
|
| Mixed microvascular and vasospastic angina | Calcium channel blockers (amlodipine 10 mg once daily, diltiazem 90 mg twice daily or verapamil 240 mg once daily) |
|
| Nicorandil (10–20 mg twice daily) |
|
|
| Trimetazidine (35 mg twice daily) |
|
|
| ACE inhibitors (ramipril 2.5–10 mg); angiotensin-receptor blockers if intolerant |
|
|
| Non-cardiac symptoms | Discontinue antianginal medication; consider continuing cardiovascular risk reduction medication (e.g. statin) if coronary artery disease present |
|
| Cardiovascular risk reduction | Statins (e.g. atorvastatin 20–80 mg) |
|
| Antihypertensives |
|
|
| Lifestyle: smoking cessation, exercise, cardiac rehabilitation, Mediterranean diet, cognitive behavioural therapy |
|
Summary of European Association of Percutaneous Cardiovascular Interventions expert consensus[3] and European Society of Cardiology chronic coronary syndromes 2019 guideline[4] for recommended treatment based on clinical endotype. CFR = coronary flow reserve. Adapted from: Kunadian et al. 2020.[3] Used with permission from Oxford University Press.
Challenges in INOCA
INOCA testing, particularly the off-label use of acetylcholine, remains the remit of specific specialist centres at present, perpetuating limited experience in the wider community. Local arrangements are required for the supply of acetylcholine at each site, either as vials for reconstitution or as infusion-ready solutions from the pharmacy. The former option requires enhanced staff training, while the latter is more expensive. Patient reminders for abstinence of caffeine and vasoactive medication are needed during allocation of angiography appointments, to facilitate the option of vasoreactivity testing on the day.
The management of INOCA often requires a period of optimisation; there is no one-size-fits-all panacea. Repeated clinical follow-up is necessary for individualised assessment and titration of medication to achieve the optimal patient-specific management plan.
Future Work
The true prevalence of INOCA in the general population remains unclear. Studies in cardiac catheterisation settings represent a highly selected and symptomatic subset, and fail to account for patients who are medically managed. While the CorMicA pilot was successful in demonstrating the utility of the IDP, larger-scale, international studies are needed to strengthen practice guidelines. To this end, the iCorMicA trial (NCT04674449) is currently recruiting.
Studies are under way for microvascular-specific therapies, including endothelin receptor antagonist zibotentan (PRIZE study; NCT04097314); coronary sinus reducer stent (NCT02710435); and pressure-controlled intermittent coronary sinus occlusion (NCT03625869).
Conclusion
INOCA remains a diagnostic and therapeutic challenge. There is growing evidence to support the use of adjunctive IDP in the assessment of INOCA, as reflected in the latest guidelines.[4] Today’s interventionists are well placed to make a positive impact with more personalised care. The theoretical risk and added time of performing the IDP is outweighed by its benefits. More widespread adoption should be encouraged, as should its incorporation in training and professional standards.
Clinical Perspective
Up to half of patients undergoing coronary angiography for known or suspected angina have ischaemia with non-obstructed coronary artery disease, which is associated with impaired quality of life, higher morbidity and healthcare resource utilisation.
Interventionists are uniquely placed to offer gold-standard assessment of microvascular function and more personalised care through the interventional diagnostic procedure (IDP).
The IDP with linked stratified medical therapy improves symptom burden, quality of life, treatment satisfaction and illness perception.
Acknowledgments
The authors thank the patients and colleagues who have contributed to the body of knowledge cited in this article.
References
- 1.Ford TJ, Berry C. Angina. contemporary diagnosis and management. Heart. 2020;106:387–98. doi: 10.1136/heartjnl-2018-314661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford TJ, Corcoran D, Berry C. Stable coronary syndromes: pathophysiology, diagnostic advances and therapeutic need. Heart. 2018;104:284–92. doi: 10.1136/heartjnl-2017-311446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunadian V, Chieffo A, Camici PG et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology working group on coronary pathophysiology & microcirculation endorsed by Coronary Vasomotor Disorders International. Eur Heart J. 2020;41:3504–20. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann FJ, Sechtem U, Banning AP et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. London: NICE; 2016. https://www.nice.org.uk/cg95 (accessed 4 October 2021).
- 6.Ford TJ, Stanley B, Good R et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–55. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Aziz A, Hansen HS, Sechtem U et al. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J Am Coll Cardiol. 2017;70:2349–58. doi: 10.1016/j.jacc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Tavella R, Cutri N, Tucker G et al. Natural history of patients with insignificant coronary artery disease. Eur Hear J Qual Care Clin Outcomes. 2016;2:117–24. doi: 10.1093/ehjqcco/qcv034. [DOI] [PubMed] [Google Scholar]
- 9.Maddox TM, Stanislawski MA, Grunwald GK et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–63. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespersen L, Hvelplund A, Abildstrøm SZ et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 11.Petrie KJ, Weinman J, Sharpe N, Buckley J. Role of patients’ view of their illness in predicting return to work and functioning after myocardial infarction: longitudinal study. BMJ. 1996;312:1191–4. doi: 10.1136/bmj.312.7040.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford TJ, Stanley B, Sidik N et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33–45. doi: 10.1016/j.jcin.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medical Research Council. The MRC Framework for the Development, Design and Analysis of Stratified Medicine Research. Swindon, UK: MRC, 2018. https://mrc.ukri.org/research/initiatives/precision-medicine/stratified-medicine-methodology-framework (accessed 4 October 2021).
- 14.Ong P, Camici PG, Beltrame JF et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 15.Crea F, Bairey Merz CNB, Beltrame JF et al. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38:473–7. doi: 10.1093/eurheartj/ehw461. [DOI] [PubMed] [Google Scholar]
- 16.Berry C, Sidik N, Pereira AC et al. Small-vessel disease in the heart and brain: current knowledge, unmet therapeutic need, and future directions. J Am Heart Assoc. 2019;8:e011104. doi: 10.1161/JAHA.118.011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorop O, Merkus D, De Beer VJ et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res. 2008;102:795–803. doi: 10.1161/CIRCRESAHA.108.172528. [DOI] [PubMed] [Google Scholar]
- 18.Prinzmetal M, Kennamer R, Merliss R et al. Angina pectoris I. A variant form of angina pectoris. Preliminary report. Am J Med. 1959;27:375–88. doi: 10.1016/0002-9343(59)90003-8. [DOI] [PubMed] [Google Scholar]
- 19.Ford TJ, Yii E, Sidik N et al. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv. 2019;12:e008126. doi: 10.1161/CIRCINTERVENTIONS.119.008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams RP, De Waard GA, De Silva K et al. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am J Cardiol. 2017;121:1–8. doi: 10.1016/j.amjcard.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glyceryl trinitrate 5 mg/ml sterile concentrate. Summary of product characteristics. EMC. 18 January 2021. https://www.medicines.org.uk/emc/product/3792/smpc (accessed 4 October 2021).
- 22.Pepine CJ, Anderson RD, Sharaf BL et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia. Results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould KL, Johnson NP. Coronary physiology beyond coronary flow reserve in microvascular angina: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2642–62. doi: 10.1016/j.jacc.2018.07.106. [DOI] [PubMed] [Google Scholar]
- 24.Suda A, Takahashi J, Hao K et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol. 2019;74:2350–60. doi: 10.1016/j.jacc.2019.08.1056. [DOI] [PubMed] [Google Scholar]
- 25.Beltrame JF, Crea F, Kaski JC et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–8. doi: 10.1093/eurheartj/ehv351. [DOI] [PubMed] [Google Scholar]
- 26.Okumura K, Yasue H, Matsuyama KK et al. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988;12:883–8. doi: 10.1016/0735-1097(88)90449-4. [DOI] [PubMed] [Google Scholar]
- 27.El-Tamimi H, Mansour M, Wargovich TJ et al. Constrictor and dilator responses to intracoronary acetylcholine in adjacent segments of the same coronary artery in patients with coronary artery disease endothelial function revisited. Circulation. 1994;89:45–51. doi: 10.1161/01.cir.89.1.45. [DOI] [PubMed] [Google Scholar]
- 28.Takagi Y, Yasuda S, Takahashi J et al. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258–67. doi: 10.1093/eurheartj/ehs199. [DOI] [PubMed] [Google Scholar]
- 29.Jafary FH. Cough-assisted maintenance of perfusion during asystole. Can J Cardiol. 2008;24:e76. doi: 10.1016/S0828-282X(08)70693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116:856–70. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109((23 Suppl 1)):III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]


