Abstract
NiII cyclam (cyclam = 1,4,8,11-tetraazacyclotetradecane) is an efficient catalyst for the selective reduction of CO2 to CO. A crucial elementary step in the proposed catalytic cycle is the coordination of CO2 to a NiI cyclam intermediate. Isolation and spectroscopic characterization of this labile NiI species without solvent has proven to be challenging, however, and only partial IR spectra have previously been reported using multiple photon fragmentation of ions generated by gas phase electron transfer to the NiII cyclam dication at 300 K. Here, we report a chemical reduction method that efficiently prepares NiI cyclam in solution. This enables the NiI complex to be transferred into a cryogenic photofragmentation mass spectrometer using inert gas-mediated electrospray ionization. The vibrational spectra of the 30 K ion using both H2 and N2 messenger tagging over the range 800 – 4000 cm−1 were then measured. The resulting spectra were analyzed with the aid of electronic structure calculations, which show strong method dependence in predicted band positions and small molecule activation. The conformational changes of the cyclam ligand induced by binding of the open shell NiI cation were compared with those caused by the spherical, closed-shell LiI cation, which has a similar ionic radius. We also report the vibrational spectrum of a NiI cyclam complex with a strongly bound O2 ligand. The cyclam ligand supporting this species exhibits a large conformational change compared to the complexes with weakly bound N2 and H2, which is likely due to significant charge transfer from Ni to the coordinated O2.
Graphical Abstract

I. Introduction
A major challenge in chemistry is to study the structure and reactivity of intermediates formed in catalysis1–7. These species often have short lifetimes and are too unstable to analyze using traditional techniques, such as X-ray diffraction or NMR spectroscopy. For example, the coordination and activation of CO2 by reactive transition metal complexes is proposed to be a crucial elementary step in the catalytic conversion of CO2 to a range of more valuable chemicals including CO and methanol8–13. The present literature, however, rarely explores the binding of CO2 to the highly reactive and unstable complexes that are proposed as intermediates in catalysis and typically focusses on more stable model complexes14–17. Here, we investigate Ni complexes supported by the planar macrocyclic cyclam (cyclam = 1,4,8,11- tetraazacyclotetradecane, hereafter denoted “L1”) ligand (Figure 1). Complexes of the this type are efficient and selective electrocatalysts for the reduction of CO2 to CO18 with the NiI-L1 intermediate hypothesized to play a key role in the high selectivity19–21, but experimental information about the structure of NiI-L1 is limited22.
Figure 1.
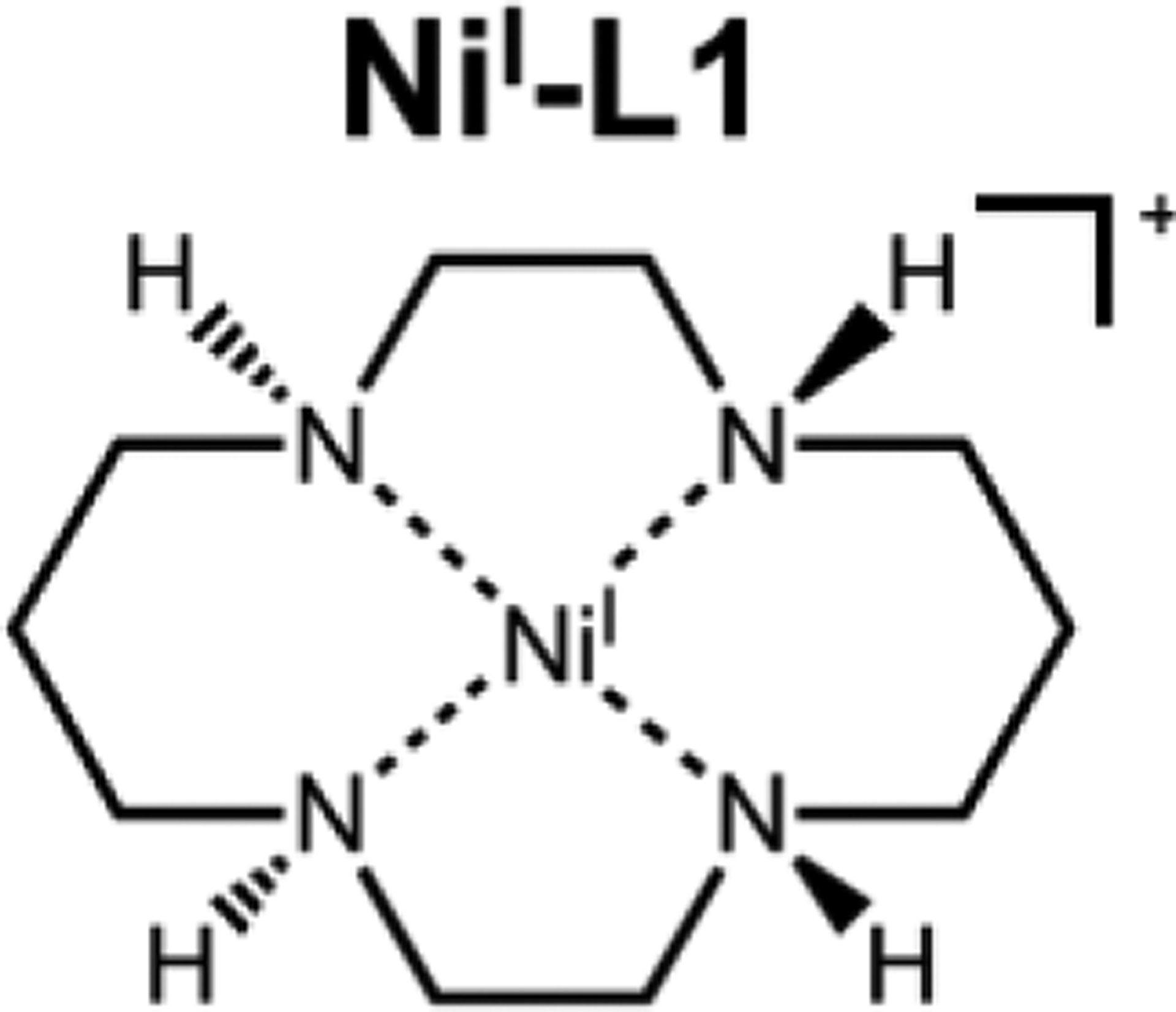
Schematic structure of the trans-III isomer of NiI-L1.
A recently developed method for studying the activation of small molecules in compounds such as NiI-L1 involves isolating the singly charged molecular cations in the gas phase with open coordination sites on the metal center and then attaching substrates in a temperature-controlled ion trap23–28. This approach follows earlier methods for the study of intermediates that employed an infrared-ultraviolet double-resonance scheme for acquiring the infrared spectra of cold ions29. Although the present method was successful for studying CO2 binding to a NiI complex with a specifically designed, non-catalytic ligand system30–31, it has been difficult to efficiently prepare the key NiI-L1 complex in a manner compatible with cryogenic ion spectroscopy. The only reported characterization of the NiI-L1 cation in isolation involved a scheme that employed exothermic gas-phase electron transfer to the stable NiII-L1 complex32–35 followed by buffer gas cooling to 300 K and did not enable a full IR spectrum to be measured.
Here, we describe an alternative method involving chemical reduction of the stable NiII-L1 compound with decamethylcobaltocene ((C5(CH3)5)2CoII), hereafter denoted (Cp*)2Co) to generate NiI-L1 cations in solution. These ions are extracted into the gas phase using electrospray ionization (ESI) and characterized at low temperature via vibrational spectroscopy. Spectra are recorded using the messenger tagging approach, where weakly bound H2 and N2 complexes prepared in a cryogenic (30 K) radiofrequency ion trap undergo infrared photodissociation (IRPD). The resulting band patterns in the 800 – 4000 cm−1 range are compared with predictions from a variety of computational methods. Surprisingly, the computational predictions of small-molecule activation by NiI-L1 show strong method dependence, which indicates there are challenges associated with calculating the structure of these complexes. We compared the experimental structure of NiI-L1 with LiI-L1, which contains a closed-shell metal center. This enabled us to determine the impact of the Ni d electrons on the binding of the L1 ligand and determine that cyclam binding is primarily governed by electrostatic factors. Finally, using O2 as a substrate, we demonstrate that it is possible to bind a more reactive molecule than N2 or H2 to the NiI center by carrying out a three-body attachment of O2 in an ion guide. The resulting complex was characterized using cryogenic vibrational spectroscopy.
II. Experimental Methods
A. Oxidation state control.
The stable NiII-L1 PF6 salt was reduced with a slight excess of (Cp*)2Co in dry and deoxygenated acetonitrile to generate NiI-L1. The reduction was performed in a glove box and the products were transferred to a gas-tight syringe (Hamilton Company) for transport and injection into the ion source. This method generates a solution containing the NiI-L1 cation that is stable for several hours under the oxygen- and water-free conditions maintained by the syringe. Samples were analyzed with the Yale hybrid photofragmentation mass spectrometer, which has been described previously26. In short, the instrument combines a commercial ThermoFisher Orbitrap Velos Pro mass spectrometer (hereafter denoted “Orbitrap”) with a custom-built, double-focusing tandem time-of-flight photofragmentation mass spectrometer. Electrospray ionization of the NiI-L1 solution described above carried out with the Orbitrap’s ion source using N2 sheath gas yields the mass spectrum (hereafter denoted “MS”) in Figure 2b. This is compared with the MS from the NiII-L1 sample solution prior to reduction with (Cp*)2Co in panel Figure 2a. These results highlight the utility of the Orbitrap’s high resolution capability by confirming successful synthesis of the labile NiI intermediate before transfer to the photofragmentation spectrometer. Specifically, this capability allowed unambiguous identification of a minor deprotonated NiII-L1 contaminant, which is formed during generation of NiI-L1 (Figure 2b).
Figure 2.

High resolution mass spectrometric preparation and isolation of the NiI-L1 cation. a) Mass spectrum of unreduced NiII-L1 and b) NiI-L1 generated by reduction with (Cp*)2Co in solution. Isotopic pattern simulations are color-coded to the inset chemical structures and inverted underneath the experimental data. The isotopic pattern of the reduced sample b) is in excellent agreement with the expected combined mass spectrum for NiI-L1 and a lower abundance of the deprotonated NiII-L1 complex. The NiII species is expected to contribute less than 5% to the peak around m/z 258 that was isolated for spectroscopic characterization. The green-shaded inset in panel b) expands the m/z axis scale to demonstrate the Orbitrap’s capability to resolve the features of NiI-L1 and deprotonated NiII-L1, which are separated by less than 0.05 m/z.
III. Results and Discussion
A. Mass isolation of NiI complexes and spectroscopic verification of product oxidation state
To verify that the present solution-phase preparation yields the same structures as the gas-phase approaches employed in our previous work30–31, we applied the synthesis described above to another NiI complex whose IRPD spectrum we earlier acquired following gas-phase synthesis: NiI-L-N4Me2 (L-N4Me2 = N,N’-dimethyl-2,11-diaza[3,3](2,6)pyridinophane, hereafter denoted “L2”). Those experiments generated the coordinatively unsaturated NiI-L2 center by collisional dissociation of a stable NiI precursor ion in the gas phase30–31. The solution chemistry applied to the generation of NiI-L1 indeed provides an alternative route to this NiI-L2 species: comparison of NiI-L2 infrared spectra taken as part of the present study and those reported previously (Figure S1) confirms that both solution- and gas-phase methods produce the same forms of the cation. We note that isolating the desired NiI-L2 (m/z = 326.104) cation from this reaction mixture with ESI is challenging because the spray yields an intense peak at m/z = 329 due to the oxidized reducing agent, [(Cp*)2CoIII]+ (329.168 m/z), which saturates the ion trap required for the spectroscopy experiments, thus suppressing the yield of the target NiI-L2 cation. This problem was overcome using the Orbitrap’s mass isolation function to select m/z = 326 prior to injection into the photofragmentation mass spectrometer, as illustrated by the mass spectra before and after isolation in Figure S2.
B. Vibrational spectrum of NiI-L1 at 30 K using H2 tagging
The ability to generate robust yields of the Ni-L1 in the critical NiI oxidation state enables the acquisition of its vibrational spectrum in a linear action regime using cryogenic IR photodissociation of complexes with weakly bound “mass tags”. This represents a significant advance because the previous spectroscopic study of this system32 was limited to the 800–1600 cm−1 range, and was obtained using 300 K infrared multiphoton dissociation (IRMPD) of the bare ion. Here, we report the spectrum of the NiI-L1-2H2 cluster ion, where the H2 tags are expected to bind to the NiI metal center, over the range 800–4100 cm−1 (Figures 3a and 4b). This is compared with the predicted harmonic spectrum (Figure 3b) and previously acquired IRMPD spectrum (Figure 3c) of bare NiI-L1, whose minimum energy structure is displayed in the Figure 3 inset. The doubly-tagged (2H2) complex was chosen to avoid mass overlap between the 60NiI-L1 isotopologue of the bare cation and 58NiI-L1-H2. The H2 molecules were observed to attach at approximately 40 K, which is typical for operation in the tagging regime where the spectra of the H2 complexes are observed to be very close to those of the isolated, cold parent ions36. Further evidence of the weak interaction between NiI-L1 and H2 is the fact that the H2 stretching fundamental is weak and observed at 4137 cm−1 (Figure 4b, blue line), very close to the value (4161 cm−1, grey line in Figure 4) in isolated H237. The H2 molecule is predicted, at the M06-2X/SDD level of theory38–41, to attach by primarily electrostatic interaction with the metal center in an “end-on” arrangement as indicated in the structure displayed in Figure S3. Activation of the IR-forbidden diatomic stretching transitions is attributable to polarization of, and charge transfer to, the diatomic tag by the molecular ion. This behavior and the expected relationship between transition strength and red shift (transition strength and red-shift increase together) has been treated in previous work and agrees with the trends observed here42–47. Red shifts of the H2 stretching transitions observed in this work are summarized in Figures S5 and S6.
Figure 3.

Comparison between the vibrational spectra of the NiI-L1 cation obtained by a) vibrational predissociation of the NiI-L1–2H2 cluster at 30 K and c) 300 K IRMPD of the bare ion reproduced from an earlier report32. The calculated spectrum for the bare NiI-L1 cation (M06–2X/SDD) is displayed in b) along with qualitative descriptions of the modes involved in the various fundamentals. The corresponding minimum-energy structure is shown as an inset. The asterisk marks a feature which we do not assign. The observed tag-dependent multiplet structure in the region expected for the NH stretching modes (see Figure S4) is not predicted at the harmonic level.
Figure 4.

CIVP spectra of a) NiI-L1-N2 cation, b) NiI-L1–2H2 cation, c) LiI-L1-N2 cation, d) LiI-L1-H2 cation, and e) NiI-L1-O2N2 cation. The structure of NiI-L1-O2 predicted at the M06–2X/SDD level of theory is included in f).
The spectrum of the NiI-L1-2H2 ion at 30 K in Figure 3a generally displays features in the same locations as those observed with IRMPD of room temperature ions (Figure 3c). As expected, the relative intensities are somewhat different – and the features better resolved – in the linear spectrum of the cold ion. However, this technique also introduces the possibility that the tag molecules perturb the intrinsic structure and vibrational band pattern of the isolated NiI-L1 cation. To address this issue, we noted that N2 attachment to form the NiI-L1-N2 cluster ion occurred at a similarly low temperature, suggesting that N2 is also very weakly bound46–47 to the molecular cation. The observed IRPD spectra of the H2- and N2-tagged ions are compared in Figure 4. These spectra are essentially identical with the exception of tag-dependent multiplet structure (labeled *) in the NH stretching region (Figure S4), which is evident in the features near 3300 cm−1 in Figure 4a and 4b. This overall similarity, combined with the fact that the N2 stretch is extremely weak and occurs at 2328 cm−1, very close to the 2330 cm−1 value of the N-N stretching vibration48 in free N2 (red vertical line in Figure 4), lends strong support to the conclusion that the tag molecules do not significantly affect the band pattern, and hence structure, of the isolated ion.
The greatest advantage of the tagging method for acquiring vibrational spectra is that the band patterns can be directly compared with (scaled) harmonic predictions of the absorption spectra predicted for candidate minimum energy structures. In the case of NiI-L1, there are two dominant isomeric forms20 that differ according to the orientation of the NH groups (the so-called trans-I and trans-III isomers, shown in Figure S7). The spectrum calculated (M06-2X/SDD) for the trans-III isomer, which is predicted to lie ~3.5 kJ/mol lower in energy than the trans-I form, is presented in Figure 3b and is in excellent agreement with the observed pattern. A comparison of the observed spectrum with those calculated for the trans-I and trans-III isomers is presented in Figure S7. The agreement in the lower energy region near 1000 cm−1 for the trans-III isomer is particularly compelling. Specifically, the strong narrow peak corresponding to the ring deformation in the trans-I spectrum is predicted to degrade into a plethora of bands in the trans-III spectrum. We note that the trans-III form was identified as the isomer generated in the gas phase in the earlier report32. Finally, it is valuable to acknowledge how strongly the calculated spectra depend on the choice of DFT functionals and basis sets. Figure S8 illustrates the surprisingly large variations in the harmonic spectra of NiI-L1-N2 calculated using 28 different combinations of functionals and basis sets. Remarkably, the harmonic predictions for N2 stretching frequencies span a range of ~1200 cm−1. This work does not seek to identify the cause of this variation, but rather to report the linear experimental spectrum and thus provide an experimental benchmark to guide future theoretical efforts. It is useful to note, however, that the large variation only occurs in the frequency of the N2 stretch; the general pattern of bands from scaffold are largely consistent across the methods surveyed.
C. Investigation of metal-ion-dependent scaffold deformation
The large variation in theoretical predictions for the interactions between NiI-L1 and N2 highlights the challenges for widely accessible electronic structure theory to accurately predict the behavior of the open shell (nominally d9) NiI ion. To empirically explore the extent to which the response of the cyclam ligand is primarily electrostatic in character, we recorded the vibrational spectra of the LiI analogues LiI-L1-H2, and LiI-L1-N2 (Figures 5a, 4c and 4d), which are compared to the NiI-L1-2H2 and NiI-L1-N2 spectra in Figure 4a–d, respectively. The bands associated with the complexes tagged by either H2 and N2 are once again essentially identical: the positions of all bands remain within the ~3 cm−1 bandwidth of our laser on moving from the H2 to N2 tags. A survey of predicted LiI-L1-N2 spectra (Figure S9) using the same methods applied to the NiI-L1-N2 complex (Figure S8) shows that the calculated N2 stretching transitions are much more consistent in intensity and somewhat more accurate in terms of frequency, spanning a ~700 cm−1 range. The experimental spectrum for LiI-L1-H2 is compared with the harmonic prediction in Figure 5 to illustrate the performance of the M06-2X/SDD level of theory. The predicted bands in the CH region are very accurate, and the CH/NH bending and ring deformation features in the fingerprint region are also satisfactory. Weaker bands in the CH rock/NH bend region involving collective motions are in worse agreement, however, as are the NH stretches near 3300 cm−1. Thus, the poor agreement with the NH stretching bands mentioned above cannot be solely attributed to complications arising from the open shell d9 configuration of the NiI cation.
Figure 5.

Comparison of the a) experimental and b) predicted (M06–2X/SDD, scaled by 0.95) vibrational spectra of LiI-L1-H2. The predicted minimum energy structure is shown in c).
The most important result from this study is that we have established a robust method to generate catalytically relevant NiI-L1. In principle, this enables the investigation of the reactivity of NiI-L1 with small molecules, which has previously been challenging. As a demonstration of this capability, we attached O2 to NiI-L1 by introducing O2 into an ion guide that transfers the cation to the cryogenic ion trap at ambient temperature. The fact that O2 binds at such high temperature confirms that the O2 is very strongly bound to the metal center. Since the resulting NiI-L1-O2 complex is too tightly bound to allow the collection of photofragmentation spectra below ~2500 cm−1 in a linear regime, the O2 complex was tagged with N2 in the cryogenic ion trap at 40 K. The vibrational spectrum of NiI-L1-O2-N2 ion is displayed in Figure 4e. As evidenced by the dramatic changes in the fingerprint-region band pattern relative to those of the isolated ion Figure 4a–d, attachment of O2 indeed induces substantial rearrangement of the ligand. The calculated structure of the O2 complex is displayed in Figure 4f and features substantial charge transfer to the O2. These large changes in the ligand bands are easily rationalized by considering the substantial charge redistribution between the NiI-L1 complex and the O2 substrate. Calculations at the same level of theory applied to the bare cation indicate about 0.5 e− is transferred from Ni to the O2 moiety. The band associated with the O2 stretch, which is predicted to be weak and fall in the congested fingerprint region at around 1100 cm−1, was not readily identified by inspection49.
Unfortunately, our attempts to isolate CO2 bound to NiI-L1 using this approach were not successful, indicating that this interaction is weak compared to the behavior of the nominally similar NiI-L2 system that readily attaches CO2 at elevated temperature30. This difference raises the issue of how the local coordination environment around the NiI ion drives chemical interactions with small molecules. This aspect of NiI coordination chemistry can be addressed by introducing a solvent molecule to one of the two open sites on the metal center in the planar cyclam scaffold before condensing CO2 onto the remaining open site. Modifications to the instrument are currently underway to explore how substrate activation responds to such manipulations of the coordination environment around the NiI metal center.
IV. Conclusion
We have demonstrated an efficient method to isolate NiI-cyclam with the metal ion in the labile NiI oxidation state. This approach enables the characterization of NiI-cyclam with high-resolution mass spectrometry and cryogenic vibrational spectroscopy. The NiI cyclam cation was generated by chemical reduction of the stable NiII cyclam dication in solution, then isolated by extraction into a photofragmentation mass spectrometer. Isolated NiI-cyclam displayed relatively inert chemical behavior toward N2 and H2 and appears similar in terms of both structure and chemical activity to LiI-cyclam, which was chosen to preserve the charge and size of the central ion, while eliminating contributions from d-orbitals. The isolated NiI-cyclam cation binds O2 very strongly, and its vibrational spectrum reveals substantial distortion of the cyclam ligand, consistent with partial charge transfer to the O2 substrate. The methods reported here provide a useful protocol for investigating the intrinsic interactions of this system with catalytically relevant substrates, and further work is ongoing to explore the role of solvent coordination in the activation of small molecule substrates, such as CO2.
Supplementary Material
Acknowledgements
MAJ thanks the Air Force Office of Scientific Research (AFOSR) under grant FA9550-17-1-0267 and acknowledges that the study was carried out using an instrument developed under (DURIP) FA9550-18-1-0213. NH acknowledges support from the NIHGMS under Award Number R01GM120162. We thank Prof. Hans-Jӧrg Krueger (Univ. Kaiserslautern) for providing a sample of the L2 ligand. LCMS based chemical analyses critical to this study regarding the purity of the ligands was obtained using a high-resolution mass spectrometer provided under NSF MRI grant CHE-1828190.
Footnotes
Supporting Information
Experimental methods; computational methods; summary of molecular names, formulae, and masses; comparison of present NiI-L2 CIVP spectra and predicted harmonic spectra with previously published results; demonstration of hybrid instrument mass isolation capability; minimum energy structure of NiI-L1-H2; demonstration of tag-dependent multiplet structure in NiI-L1 NH stretching region; comparison of NiI-L1 CIVP spectra with predicted harmonic spectra of trans-III and trans-I isomers; comparison of NiI-L1 and LiI-L1 CIVP spectra with harmonic spectra predicted for same using different functional/basis set combinations; summary of H2 stretching fundamental red shifts in H2-tagged complexes.
References
- 1.Neri G; Walsh JJ; Teobaldi G; Donaldson PM; Cowan AJ Detection of catalytic intermediates at an electrode surface during carbon dioxide reduction by an earth-abundant catalyst. Nat. Catal 2018, 1 (12), 952–959. [Google Scholar]
- 2.Hemberger P; Custodis VBF; Bodi A; Gerber T; van Bokhoven JA Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat. Commun 2017, 8 (1), 15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goswami M; Chirila A; Rebreyend C; de Bruin B EPR Spectroscopy as a Tool in Homogeneous Catalysis Research. Top. Catal 2015, 58 (12), 719–750. [Google Scholar]
- 4.Tsang ASK; Sanhueza IA; Schoenebeck F Combining Experimental and Computational Studies to Understand and Predict Reactivities of Relevance to Homogeneous Catalysis. Chem. Eur. J 2014, 20 (50), 16432–16441. [DOI] [PubMed] [Google Scholar]
- 5.Vikse KL; Ahmadi Z; Scott McIndoe J The application of electrospray ionization mass spectrometry to homogeneous catalysis. Coord. Chem. Rev 2014, 279, 96–114. [Google Scholar]
- 6.Gustafson KPJ; Guðmundsson A; Bajnóczi ÉG; Yuan N; Zou X; Persson I; Bäckvall J-E In Situ Structural Determination of a Homogeneous Ruthenium Racemization Catalyst and Its Activated Intermediates Using X-Ray Absorption Spectroscopy. Chem. Eur. J 2020, 26 (15), 3411–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMillan SN; Lancaster KM X-ray Spectroscopic Interrogation of Transition-Metal-Mediated Homogeneous Catalysis: Primer and Case Studies. ACS Catal 2017, 7 (3), 1776–1791. [Google Scholar]
- 8.Burkart MD; Hazari N; Tway CL; Zeitler EL Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal 2019, 9 (9), 7937–7956. [Google Scholar]
- 9.Ra EC; Kim KY; Kim EH; Lee H; An K; Lee JS Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal 2020, 10 (19), 11318–11345. [Google Scholar]
- 10.Artz J; Müller TE; Thenert K; Kleinekorte J; Meys R; Sternberg A; Bardow A; Leitner W Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev 2018, 118 (2), 434–504. [DOI] [PubMed] [Google Scholar]
- 11.Álvarez A; Bansode A; Urakawa A; Bavykina AV; Wezendonk TA; Makkee M; Gascon J; Kapteijn F Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev 2017, 117 (14), 9804–9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sordakis K; Tang C; Vogt LK; Junge H; Dyson PJ; Beller M; Laurenczy G Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. (Washington, DC, U. S.) 2018, 118 (2), 372–433. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann P; Kilpatrick AFR; Ar D; Demeshko S; Cula B; Limberg C Electron transfer within beta-diketiminato nickel bromide and cobaltocene redox couples activating CO2. Chem. Commun 2021, 57 (7), 875–878. [DOI] [PubMed] [Google Scholar]
- 14.Francke R; Schille B; Roemelt M Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts. Chem. Rev 2018, 118 (9), 4631–4701. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz H Metal-Mediated Activation of Carbon Dioxide in the Gas Phase: Mechanistic Insight Derived from a Combined Experimental/Computational Approach. Coord. Chem. Rev 2017, 334, 112–123. [Google Scholar]
- 16.Yang HB; Hung SF; Liu S; Yuan KD; Miao S; Zhang LP; Huang X; Wang HY; Cai WZ; Chen R, et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3 (2), 140–147. [Google Scholar]
- 17.Dodson LG; Thompson MC; Weber JM Characterization of Intermediate Oxidation States in CO2 Activation. Annu. Rev. Phys. Chem 2018, 69, 231–252. [DOI] [PubMed] [Google Scholar]
- 18.Beley M; Collin J-P; Ruppert R; Sauvage J-P Nickel(II)-cyclam: an extremely selective electrocatalyst for reduction of CO2 in water. J. Chem. Soc., Chem. Commun 1984, (19), 1315–1316. [Google Scholar]
- 19.Froehlich JD; Kubiak CP The Homogeneous Reduction of CO2 by [Ni(cyclam)]+: Increased Catalytic Rates with the Addition of a CO Scavenger. J. Am. Chem. Soc 2015, 137 (10), 3565–3573. [DOI] [PubMed] [Google Scholar]
- 20.Froehlich JD; Kubiak CP Homogeneous CO2 Reduction by Ni(cyclam) at a Glassy Carbon Electrode. Inorg. Chem 2012, 51 (7), 3932–3934. [DOI] [PubMed] [Google Scholar]
- 21.Song J; Klein EL; Neese F; Ye S The Mechanism of Homogeneous CO2 Reduction by Ni(cyclam): Product Selectivity, Concerted Proton–Electron Transfer and C–O Bond Cleavage. Inorg. Chem 2014, 53 (14), 7500–7507. [DOI] [PubMed] [Google Scholar]
- 22.Behnke SL; Manesis AC; Shafaat HS Spectroelectrochemical investigations of nickel cyclam indicate different reaction mechanisms for electrocatalytic CO2 and H+ reduction. Dalton Trans 2018, 47 (42), 15206–15216. [DOI] [PubMed] [Google Scholar]
- 23.Wolk AB; Leavitt CM; Garand E; Johnson MA Cryogenic Ion Chemistry and Spectroscopy. Acc. Chem. Res 2014, 47 (1), 202–210. [DOI] [PubMed] [Google Scholar]
- 24.Roithová J; Gray A; Andris E; Jašík J; Gerlich D Helium Tagging Infrared Photodissociation Spectroscopy of Reactive Ions. Acc. Chem. Res 2016, 49 (2), 223–230. [DOI] [PubMed] [Google Scholar]
- 25.Roithová J Characterization of reaction intermediates by ion spectroscopy. Chem. Soc. Rev 2012, 41 (2), 547–559. [DOI] [PubMed] [Google Scholar]
- 26.Menges FS; Perez EH; Edington SC; Duong CH; Yang N; Johnson MA Integration of High-Resolution Mass Spectrometry with Cryogenic Ion Vibrational Spectroscopy. J. Am. Soc. Mass. Spectrom 2019, 30 (9), 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz H; Asmis KR Identification of Active Sites and Structural Characterization of Reactive Ionic Intermediates by Cryogenic Ion Trap Vibrational Spectroscopy. Chem. Eur. J 2019, 25 (9), 2112–2126. [DOI] [PubMed] [Google Scholar]
- 28.Kamrath MZ; Rizzo TR Combining Ion Mobility and Cryogenic Spectroscopy for Structural and Analytical Studies of Biomolecular Ions. Acc. Chem. Res 2018, 51 (6), 1487–1495. [DOI] [PubMed] [Google Scholar]
- 29.Fedorov A; Couzijn EPA; Nagornova NS; Boyarkin OV; Rizzo TR; Chen P Structure and Bonding of Isoleptic Coinage Metal (Cu, Ag, Au) Dimethylaminonitrenes in the Gas Phase. J. Am. Chem. Soc 2010, 132 (39), 13789–13798. [DOI] [PubMed] [Google Scholar]
- 30.Menges FS; Craig SM; Totsch N; Bloomfield A; Ghosh S; Kruger HJ; Johnson MA Capture of CO2 by a Cationic Nickel(I) Complex in the Gas Phase and Characterization of the Bound, Activated CO2 Molecule by Cryogenic Ion Vibrational Predissociation Spectroscopy. Angew. Chem. Int. Ed 2016, 55 (4), 1282–1285. [DOI] [PubMed] [Google Scholar]
- 31.Craig SM; Menges FS; Johnson MA Application of Gas Phase Cryogenic Vibrational Spectroscopy to Characterize the CO2, CO, N2 and N2O Interactions With the Open Coordination Site on a Ni(I) Macrocycle Using Dual Cryogenic Ion Traps. J. Mol. Spectrosc 2017, 332, 117–123. [Google Scholar]
- 32.Munshi MU; Craig SM; Berden G; Martens J; DeBlase AF; Forman DJ; McLuckey SA; Oomens J; Johnson MA Preparation of Labile Ni+(cyclam) Cations in the Gas Phase Using Electron Transfer Reduction through Ion-ion Recombination in an Ion Trap and Structural Characterization with Vibrational Spectroscopy. J. Chem. Phys. Lett 2017, 8, 5047–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rijs AM; Oomens J IR Spectroscopic Techniques to Study Isolated Biomolecules. Top Curr. Chem 2015, 364, 1–42. [DOI] [PubMed] [Google Scholar]
- 34.Oomens J; Sartakov BG; Meijer G; Helden G. v. Gas Phase Infrared Mulitiple Photon Dissociation Spectroscopy of Mass Selected Molecular Ions. Intl. J. Mass Spec 2006, 254, 1–19. [Google Scholar]
- 35.Martens J; Berden G; Gebhardt CR; Oomens J Infrared ion spectroscopy in a modified quadrupole ion trap mass spectrometer at the FELIX free electron laser laboratory. Rev. Sci. Instrum 2016, 87 (10), 103108. [DOI] [PubMed] [Google Scholar]
- 36.Yang N; Duong CH; Kelleher PJ; Johnson MA; McCoy AB Isolation of site-specific anharmonicities of individual water molecules in the I−·(H2O)2 complex using tag-free, isotopomer selective IR-IR double resonance. Chem. Phys. Lett 2017, 690, 159–171. [Google Scholar]
- 37.Dickenson GD; Niu ML; Salumbides EJ; Komasa J; Eikema KSE; Pachucki K; Ubachs W Fundamental Vibration of Molecular Hydrogen. Phys. Rev. Lett 2013, 110 (19), 193601. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y; Truhlar DG The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc 2008, 120 (1), 215–241. [Google Scholar]
- 39.Dolg M; Wedig U; Stoll H; Preuss H Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys 1987, 86 (2), 866–872. [Google Scholar]
- 40.Dunning TH; Hay PJ Gaussian Basis Sets for Molecular Calculations. In Methods of Electronic Structure Theory, Schaefer HF, Ed. Springer US: Boston, MA, 1977; pp 1–27. [Google Scholar]
- 41.Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Petersson GA, et al. Gaussian 09, Revision D.01, Gaussian, Inc.: Wallingford, CT, 2009. [Google Scholar]
- 42.Kamrath MZ; Garand E; Jordan PA; Leavitt CM; Wolk AB; Van Stipdonk MJ; Miller SJ; Johnson MA Vibrational Characterization of Simple Peptides Using Cryogenic Infrared Photodissociation of H2-Tagged, Mass-Selected Ions. J. Am. Chem. Soc 2011, 133 (16), 6440–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marsh BM; Zhou J; Garand E Vibrational spectroscopy of small hydrated CuOH+ clusters. J. Phys. Chem. A 2014, 118 (11), 2063–2071. [DOI] [PubMed] [Google Scholar]
- 44.Mitra S; Duong CH; McCaslin LM; Gerber RB; Johnson MA Isomer-Specific Cryogenic Ion Vibrational Spectroscopy of the D2-Tagged Cs+(HNO3)(H2O)(n = 0–2) Complexes: Ion-Driven Enhancement of the Acidic H-Bond to Water. Phys. Chem. Chem. Phys 2020, 22 (8), 4501–4507. [DOI] [PubMed] [Google Scholar]
- 45.Kamrath MZ; Relph RA; Guasco TL; Leavitt CM; Johnson MA Vibrational Predissociation Spectroscopy of the H2-tagged Mono- and Dicarboxylate Anions of Dodecanedioic Acid. Int. J. Mass Spectrom 2011, 300, 91–98. [Google Scholar]
- 46.Johnson CJ; Wolk AB; Fournier JA; Sullivan EN; Weddle GH; Johnson MA Communication: He-Tagged Vibrational Spectra of the SarGlyH+ and H+(H2O)2,3 Ions: Quantifying Tag Effects in Cryogenic Ion Vibrational Predissociation (CIVP) Spectroscopy. J. Chem. Phys 2014, 140 (22), 221101. [DOI] [PubMed] [Google Scholar]
- 47.Duong CH; Yang N; Johnson MA; DiRisio RJ; McCoy AB; Yu Q; Bowman JM Disentangling the Complex Vibrational Mechanics of the Protonated Water Trimer by Rational Control of Its Hydrogen Bonds. J. Phys. Chem. A 2019, 123 (37), 7965–7972. [DOI] [PubMed] [Google Scholar]
- 48.Bendtsen J The rotational and rotation-vibrational Raman spectra of 14N2, 14N15N and 15N2. J. Raman Spectrosc 1974, 2 (2), 133–145. [Google Scholar]
- 49.Wolk AB; Leavitt CM; Fournier JA; Kamrath MZ; Wijeratne GB; Jackson TA; Johnson MA Isolation and characterization of a peroxo manganese (III) dioxygen reaction intermediate using cryogenic ion vibrational predissociation spectroscopy. Int. J. Mass Spec 2013, 354, 33–38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


