ABSTRACT
Muscle weakness has been recognized as a hallmark feature of vitamin D deficiency for many years. Until recently, the direct biomolecular effects of vitamin D on skeletal muscle have been unclear. Although in the past, some reservations have been raised regarding the expression of the vitamin D receptor in muscle tissue, this special issue review article outlines the clear evidence from preclinical studies for not only the expression of the receptor in muscle but also the roles of vitamin D activity in muscle development, mass, and strength. Additionally, muscle may also serve as a dynamic storage site for vitamin D, and play a central role in the maintenance of circulating 25‐hydroxy vitamin D levels during periods of low sun exposure. © 2021 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: MUSCLE, VITAMIN D, VITAMIN D RECEPTOR, SARCOPENIA, SATELLITE CELLS, DEVELOPMENT
Introduction
By the 1970s it had become clear that the vitamin D status of populations was largely determined by exposure to solar UVB light driving photochemical synthesis in the skin rather than by dietary intake. Vitamin D is produced in the skin when UV photons convert 7‐dehydrocholesterol through a two‐step process into vitamin D3. This is followed by 25‐hydroxylation in the liver and 1α‐hydroxylation in the kidney (predominantly) to form the metabolically active 1,25‐dihydroxyvitamin D3 (1,25(OH)2D3). The principal role of vitamin D in the body is to signal the absorption of calcium and phosphate from the gut. Calcium absorption across the intestinal absorptive cells, the enterocytes, occurs both via active transport, facilitated by 1,25(OH)2D3, and via paracellular diffusion through tight junctions.( 1 ) It is well established that the genomic actions of 1,25(OH)2D3 in the enterocytes result in the influx of calcium through the apical calcium channel known as the transient receptor potential vanilloid type 6 (TRPV6), followed by translocation through the cell by calbindin and finally, release across the plasma membrane via the calcium adenosine triphosphatase (ATPase) pump. The passive diffusion of calcium is less reliable and has traditionally been considered a function of the electrochemical gradient across the lumen. Increasingly, however, evidence also suggests that 1,25(OH)2D3 plays a role in improving the ion permeability of the tight junctions, and thus this passive diffusion of calcium as well.( 2 , 3 ) Vitamin D likewise improves phosphate absorption.( 1 ) Vitamin D also increases renal calcium reabsorption resulting in a net increase in serum calcium levels. Overall, vitamin D is necessary for the maintenance of mineral homeostasis and is vital for the health of the musculoskeletal system.( 4 )
The role of vitamin D in bone health has been well established for many years, although it has more recently come to light that there may also be a strong link between vitamin D and skeletal muscle health that lies outside of the circulating levels of phosphate and calcium.( 5 , 6 , 7 ) There are still many questions regarding the direct actions of 1,25(OH)2D3 in muscle. Severe vitamin D deficiency results in muscle weakness and vitamin D status is a predictor of muscle strength and performance in older adults,( 8 ) but whether these observations are linked to the knock‐on effects on circulating calcium and phosphate levels alone or whether vitamin D directly modulates these parameters of muscle is still hotly debated.( 9 ) This review focuses on the current literature that indicates an independent role for vitamin D in the maintenance of skeletal muscle quality, the central role of the vitamin D receptor (VDR) in muscle function as well as a summary of new research that posits the muscle as an important storage site for vitamin D and its binding protein, VDBP, to aid in the maintenance of circulating levels during winter. The various functions of vitamin D in skeletal muscle cells have been summarized in Fig. 1.
Fig 1.
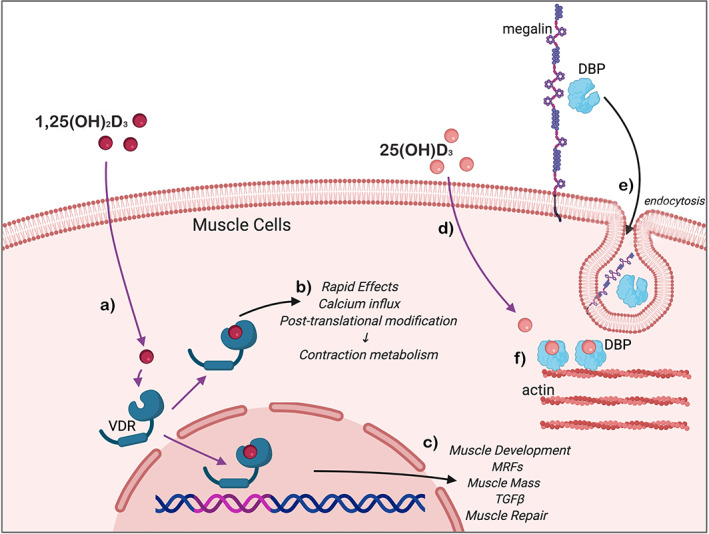
(a) Active 1,25(OH)2D3 diffuses across the muscle cell membrane and binds the intracellular VDR. This ligand‐receptor complex then drives either (b) rapid, nongenomic effects on intracellular calcium signaling pathways; or (c) transcriptional activity of genes involved in the differentiation of myocytes into mature contractile myotubes, maintenance of skeletal muscle quality, and protection from muscle atrophy. (d) Circulating 25(OH)D3 diffuses across the muscle cell membrane, while (e) VDBP requires endocytosis via megalin activity at the cell surface. (f) VDBP attaches to a binding site on intracellular actin filaments and binds the 25(OH)D3, aiding in the maintenance of circulating levels of this metabolite during winter. VDBP = vitamin D binding protein; VDR = vitamin D receptor.
VDR Expression in Skeletal Muscle
Following the discovery of VDR in 1974,( 10 ) our knowledge of its structural composition, biological activity, and ligand interactions have expanded considerably.( 11 ) The late Anthony W. Norman shed immense light on the complexity of the VDR, defining its protein composition through X‐ray crystallography and expounding key conformational changes that determine genomic versus rapid effects of VDR on cellular activity.( 12 ) An appreciation of structure–function interactions of VDR, and its activity as an orphan nuclear receptor, in addition to established effects through ligand binding (1,25D‐VDR‐retinoid X receptor [RXR]), provide strong mechanistic bases for vitamin D's expanding nonclassical effects beyond bone and mineral homeostasis.( 12 )
The VDR is present in invertebrates, fish, birds, and mammals, with a wide repertoire of expression in different organs and tissues. Until recently, however, the presence of VDR in skeletal muscle was unclear. There had been conflicting reports on VDR detection by Western blot and immunohistochemistry in murine and adult human muscle.( 13 , 14 , 15 ) Using a specific VDR antibody, VDR was found to be present at low levels( 16 ) or absent.( 15 , 17 ) Relative to classical sites of VDR activity, such as the duodenum, its expression in muscle is substantially lower. Low baseline levels, however, do not preclude a biological role for VDR in muscle. Transcription factors may exert genomic effects even at low levels of expression, dependent on their binding affinity to DNA.( 18 )
Approaches that examine systems‐wide VDR distribution, such as the use of a luciferase‐expressing VDR transgene in a murine model,( 19 ) miss the relatively low level of expression in skeletal muscle. Protein detection methods may also not be sensitive enough to detect low baseline levels of VDR, and induction by key physiologic stimuli may be necessary for detection. In support of this, priming VDR by treatment with its ligand increased expression levels in older human subjects.( 20 , 21 ) In another study, muscle injury relating to severe knee osteoarthritis (OA) was associated with higher VDR levels in quadriceps,( 22 ) although the VDR was clearly detected in muscles from both OA and control subjects.
VDR expression within muscle is also time‐dependent and modulated throughout muscle development and aging. In vitro, muscle precursor cells display substantially greater levels of VDR compared to fully differentiated myotubes and whole muscle fibers.( 16 , 17 ) This is consistent with the early expression of VDR in the mesoderm, the embryonic tissue that gives rise to the musculoskeletal system.( 23 ) Mice show similar age‐dependent changes in muscle VDR with significantly higher levels in newborn mice compared to 3‐week‐old mice and adult mice.( 16 ) Muscle injury in adult mice leads to a recapitulation of embryonic myogenesis, and VDR is increased under these circumstances.( 24 , 25 ) Interestingly, VDR is specifically expressed in satellite cells, muscle stem cells, and in vitro; 1,25(OH)D3 modulates myogenic cell differentiation in these primordial cells.( 26 ) Therefore, VDR's predominant expression in muscle within primordial cells, newborn mice, and regenerating muscle fibers supports a pleiotropic role of vitamin D in muscle development and repair.
In summary, a number of experimental and biological issues have confounded the clear demonstration of VDR in muscle. These include wide differences in muscle models used, nonspecificity of VDR antibodies, and protein detection methods that are insufficiently sensitive to detect low baseline levels of biologically active VDR, although most of these obstacles have now been overcome. Muscle VDR may also sequester within a specific cell population, such as satellite cells, and thus evade detection by methods examining whole tissue (satellite cells comprise ~5% cells in adult muscle).( 27 ) The weight of evidence indicates that VDR is indeed expressed in muscle but at very low baseline levels in adults, and that injury upregulates its expression. VDR in muscle predominates in precursor cells and in developing and regenerating muscle fibers. Thus, its activity in this tissue appears related to muscle development and pleiotropy.
Vitamin D and Muscle Cells: In Vitro Models
In vitro studies using isolated myotubes and cultured muscle cell lines are one way of testing whether vitamin D and its metabolites have direct effects in muscle, bypassing the modulation of circulating calcium and phosphate mineral levels and the subsequent effects these changes may elicit in muscle. Various studies have investigated the direct effects of vitamin D using cell culture models, the majority of which have analyzed myotube formation and size,( 9 , 26 , 28 , 29 , 30 , 31 ) the expression of proteins involved in muscle formation and function, insulin sensitivity,( 32 , 33 , 34 , 35 ) glucose( 35 ) and lipid uptake and metabolism,( 36 , 37 ) as well as mitochondrial activity.( 36 )
Most of the cell studies have employed similar methods that include incubating either isolated mouse or human skeletal myoblasts or commonly used immortalized mouse or rat myoblast cell lines with active 1,25(OH)2D3. The results of these studies demonstrate positive effects on myoblast differentiation, fusion and myotube formation,( 9 , 26 , 28 , 29 , 30 ) reduced proliferation of early myoblasts in preparation for fusion,( 29 , 30 , 31 ) and improved myotube maintenance and size.( 28 )
Two broad mechanisms are responsible for these pleiotropic effects including: (i) posttranslational modification of signaling molecules involved in inhibition of myoblast proliferation, Rb, JNK, Raf‐1, and CREB( 9 , 29 , 38 ); and (ii) genomic regulation of myogenic regulatory factors (MRF) responsible for muscle differentiation.( 9 , 29 ) Knockdown studies have confirmed VDR as requisite to the effects of 1,25(OH)D3 on MRF expression.( 39 )
In vitro studies also report a vitamin D–protective effect against muscle atrophy via the downregulation of muscle atrophy F‐box (MAFbx/Atrogin 1) and muscle RING finger 1 (MuRF1) proteins as well as inhibition of ubiquitin ligases involved in catabolism and muscle atrophy.( 32 )
Corroborative findings in studies on human myocytes were reported with VDR‐mediated modulation of age‐related pathways including ubiquitin ligases, inflammatory markers tumor necrosis factor α (TNF‐α) and interleukin 6 (IL6), and phosphatidylinositol‐3′‐kinase (PI3K)/protein kinase B (AKT) signaling.( 21 , 40 ) Mitochondrial gene modulation and increased mitochondrial volume and oxygen consumption in muscle cells treated with 1,25(OH)D3 were demonstrated.( 41 ) Thus, vitamin D may reduce oxygen free radicals in aging muscle and mitigate the effects of mitochondrial dysfunction, thereby counteracting sarcopenia.
Studies also report increases in intracellular calcium movement in muscle in response to vitamin D.( 6 ) In cell models, 1,25(OH)2D3 exposure leads to the rapid movement of calcium from the sarcoplasmic reticulum into cytosol,( 42 ) followed by more sustained calcium flux from the extracellular compartment via activation of store‐operated calcium entry (SOCE) and L‐type voltage‐dependent channels.( 6 ) Intricate intracellular signaling mechanisms for these effects have been elucidated, including the activation of the protein kinase C pathway( 42 ) and increases in intracellular cyclic AMP (cAMP).( 43 ) Interestingly, one study reported the expression of CYP27B1, suggesting local muscle metabolism of 25(OH)D3 into 1,25(OH)2D3.( 31 )
Thus, a body of in vitro work provides proof of concept that vitamin D modulates myogenesis, through the inhibition of myoblast proliferation, promotion of myocyte differentiation and myotube formation, and by exerting anabolic effect on myotube size.( 9 , 28 , 29 , 38 , 44 ) Vitamin D also modulates the response to muscle injury and inflammation, with effects on intracellular calcium handling.
Insights on Vitamin D's Role in Muscle From Animal Models
A number of different animal models have shed light on key aspects of vitamin D physiology and skeletal muscle.
The classical model, namely the whole‐body vitamin D receptor knockout (VDRKO) mouse model has provided key insights in the pleiotropic effects of vitamin D,( 38 , 45 ) but the systemic defects in this model, and aberrant calcium/mineral signaling, confound the elucidation of tissue specific effects of the VDR. More recently, two muscle‐specific knockout mouse models have been generated.( 46 , 47 ) In the first model, myosin light chain 1 (MLC1f) was the promoter gene used to ablate VDR in the muscle of these mice. MLC1f is expressed in embryonic life, making this model appropriate to assess effects on muscle differentiation. Changes in muscle morphology and insulin sensitivity were noted, with a reduction in type II muscle fiber diameter and overexpression of forkhead Box O1 (FOXO1) protein resulting in glucose intolerance.( 46 ) In the second model, significant reductions in lean mass, voluntary physical function, and grip strength in muscle‐specific VDRKO mice, underpinned by key morphologic changes in muscle fibers, were demonstrated.( 47 ) The presence of central nucleoli in muscle fibers of knockout mice suggested an underlying defect in muscle repair in the absence of VDR.
A model overexpressing VDR in the tibialis anterior muscle of Wistar rats was recently developed.( 48 ) Significant increases in muscle fiber size were reported with upregulation of key genomic pathways on RNA‐Seq relating to extracellular matrix (ECM) remodeling, satellite cell activity, and markers of proliferation.( 48 ) In addition, humans were found to upregulate VDR mRNA in skeletal muscle following hypertrophy‐inducing exercise, suggesting a central role in muscle conditioning to exercise and recovery.( 48 )
Animal models of vitamin D deficiency have also been developed. Vitamin D–deficient mice had reduced locomotor ability and by 5 weeks on a diet lacking vitamin D, mice had significantly lower neuromuscular innervation in the tibialis anterior muscle compared to calcium and vitamin D–replete animals.( 49 ) Muscle atrophy was accentuated by vitamin D deficiency in aged mice with an increase in muscle protein catabolism via activation of TGF‐β, FOXO, and the ubiquitin‐proteasome system.( 45 , 50 ) Conversely, vitamin D supplementation improved muscle recovery following freeze‐crush injury or high‐intensity exercise.( 51 , 52 ) These effects were explained by vitamin D–mediated reductions in oxidative stress and inflammation, together with an effect on stress‐related proteins (ERK1/2, p38, and MAPK).( 51 , 53 )
Murine models of energy dysmetabolism have also been used to investigate the role of vitamin D in muscle health; the prospective muscle atrophy, increased lipid accumulation within skeletal muscle, and overall reduced muscle structure and function render these models useful for studying muscle maintenance, function, and form. Overall, these investigations report that vitamin D treatment in animals fed high‐fat and high‐sugar diets, diabetic rodent models, and sedentary fatty rats preserved skeletal muscle form and function by: increasing myoblast determination protein 1 (MyoD) expression( 54 ); suppressing the expression of catabolic proteins atrogin‐1 and MuRF1( 54 ); upregulating the expression of protein fibronectin‐type III domain‐containing 5 (FNDC5) and irisin—genes involved in the conversion of white adipocytes into brown adipocytes and increasing energy expenditure( 55 ); and the downregulation of the lipogenic activation pathway that includes sterol regulatory element binding protein‐1c (SREBP1c) and SREBP cleavage activating protein (SCAP).( 56 )
Despite these positive findings, one study reported that dosing mice with extremely high levels of vitamin D in a single bolus treatment resulted in reduced contraction force and reduced recovery periods following fatigue exercises,( 57 ) supporting data from humans in which comparable single high doses increase the risk of falls and fractures.( 58 )
These animal studies have laid the foundation for further examination of vitamin D effects in human muscle tissue. A correlation between VDR protein and interleukin‐6 (IL6) expression in human muscle suggests integrated effects in the inflammatory response to muscle damage.( 59 ) Vitamin D supplementation modulated cytokine levels following exercise, including IL‐10, IL‐13, and reduced inflammatory mediators TNF‐α and interferon γ (IFN‐γ).( 60 , 61 ) PCR array analysis in healthy human subjects identified 24 skeletal muscle genes associated with pathways involving muscle contraction, myogenesis, cell stress, and muscle repair that correlate with serum 25(OH)D3 status.( 62 )
In summary, vitamin D modulates muscle morphology and pleoitropy with effects on muscle size, repair, and aging. Effects on oxidative stress, inflammatory cytokines, and muscle protein turnover via the ubiquitin‐proteasome have been reported in both rodent and human studies. Vitamin D supplementation may reverse these effects, and further research is needed on the potential anti‐aging, anabolic, and reparative effects of vitamin D on skeletal muscle.
Muscle as a Storage Site for Vitamin D
Other recent research has asked not what vitamin D can do for muscle but what muscle can do for vitamin D. At somewhere between 50 to 100 days,( 63 , 64 ) 25(OH)D3 has an unusually long half‐life for a steroid hormone, particularly a seco‐steroid with a broken carbon ring, whereas the half‐life of 1,25(OH)2D3 is more similar to other steroid hormones, at only a few days.( 65 ) The half‐life of the vitamin D binding protein (VDBP), which transports both the endocrine metabolite and its parent molecule, is also only a few days.( 66 ) These characteristics of 1,25(OH)2D3, alongside the well‐known dependence on UVB exposure, and thus seasonal variation for the synthesis of vitamin D, make a reasonable argument for an extravascular storage site for vitamin D within the body.( 67 ) Given the lipophilic nature of free vitamin D and that it is purportedly sequestered in the fat mass of obese patients, it is unlikely that the vitamin D in adipose can be mobilized and released as required into the circulation.
In cell culture, radiolabeled 25(OH)D3 is taken up into mature myotubes but not mature adipocytes.( 68 ) The levels in liver are very low,( 69 ) and in rats, radioactively labeled vitamin D was found mostly as 25(OH)D3 in the skeletal muscle of newborn pups when it had been administered to the pregnant mothers.( 69 ) Therefore, muscle appears to be a newly recognized site of accessible 25(OH)D storage.
Although 25(OH)D3 can passage into and out of muscle cells unaided, the binding protein, VDBP has been shown to depend on the presence of megalin, a member of the low‐density lipoprotein receptor family.( 70 ) VDBP was shown to bind to intracellular skeletal muscle actin,( 71 ) in the same manner as had been reported in hepatocytes.( 71 ) Thus, it is likely that vitamin D is stored in muscle by actin‐bound VDBP that is proteolyzed to allow release of free 25(OH)D3 back into the circulation when UVB exposure is low. Finally, uptake of 25(OH)D3 into muscle is inhibited by parathyroid hormone (PTH) acting on PTH receptors in the sarcolemma,( 72 ) indicating the interplay between these calciotropic hormones to maintain calcium homeostasis even at the skeletal muscle. Because PTH is a major inducer of CYP27B1 in the kidneys, it is conceivable that during low calcium situations PTH directs 25(OH)D3 away from muscle storage to promote renal conversion of the CYP27B1 substrate to 1,25(OH)2D3. Thus, skeletal muscle represents an active storage site for vitamin D, playing a novel role in the maintenance of physiological circulating levels during periods of hibernation and low UVB exposure.
Conclusion
In his 2006 review on the “already busy receptor,” the late Anthony Norman described skeletal muscle as a new frontier in VDR's assignments beyond its genomic effects at classical sites.( 73 ) This special issue article in memory of this pioneer of vitamin D research summarizes the current understanding of its effects on skeletal muscle—in development, metabolism, repair, and the modulation of muscle fiber size. A body of preclinical research in support of these functions has been presented from in vitro cell models, animal models, and biomolecular studies on human muscle tissue (summarized in Fig. 1).
Morphologically, muscle mass, fiber size, and the reparative response to muscle injury are altered by vitamin D.( 25 , 45 ) Genomic effects in muscle involve myogenic regulatory factors, transforming growth factor β (TGF‐β) signaling, myostatin, and the ubiquitin‐proteasome.( 9 , 29 , 62 ) Vitamin D may also exert age‐related changes in skeletal muscle by altering oxidative stress, atrophy signaling, and protein turnover.( 45 , 50 ) Nongenomic effects of vitamin D have been elucidated, including the rapid activation of second messenger systems to modulate intramuscular calcium flux, vital for muscle contraction and strength.( 43 , 74 , 75 ) Skeletal muscle may also represent a dynamic depot for vitamin D, regulating circulating levels and facilitating the release of vitamin D during periods of low UVB exposure such as hibernation.( 70 )
Until recently, VDR's expression in skeletal muscle has been controversial. Technical challenges, differences in muscle models, and muscle cell‐specific and age‐specific differences in VDR expression gave rise to this controversy. The body of evidence now indicates that VDR is indeed expressed in muscle, but at levels that may elude some detection methods. The expression of VDR in muscle occurs primarily in primordial muscle cells, such as satellite cells, and in regenerating muscle fibers.( 16 , 17 ) VDR's predominant expression in these early muscle cells indicates a mostly pleiotropic role in this tissue. At a functional level, a direct action of VDR in muscle is supported by the phenotype of murine models of muscle‐specific knockout( 47 ) and overexpression of this protein.( 48 )
However, questions regarding the activity of vitamin D in muscle remain. Although preclinical studies support a robust role of vitamin D in this tissue, the clinical translation of these effects to human subjects requires further assessment. Direct changes in muscle function relating to vitamin D status have not been a uniform finding in human studies. On a molecular level, the presence of vitamin D response elements (VDRE) have not been clearly demonstrated in genes purportedly regulated by vitamin D in muscle. Chromatin immunoprecipitation (ChIP) studies are required to elucidate the specific VDR binding sites across the genome (or cistrome) in skeletal muscle and will establish a clearer picture of direct genomic activity. Nongenomic effects of vitamin D on calcium flux have been reported by many in vitro studies but the extrapolation of these findings to in vivo muscle physiology is not a fait accompli. Future studies using intracellular calcium imaging are needed to characterize real‐time calcium flux in skeletal muscle in vivo in response to vitamin D. The role of Vitamin D in muscle repair also raises the possibility that vitamin D directly regulates the response of satellite cells to injury, enhancing regeneration. The ongoing development of animal models of aberrant VDR signaling in muscle will help to clarify cell‐specific effects in this tissue.
Future strategies targeting vitamin D signaling in muscle and modulating the VDR “metabolome” may also hold important clues for future treatments of musculoskeletal disorders, congenital myopathies, and sarcopenia.
Conflict of Interest
The authors have no conflict of interest to report.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jbm4.10575.
Acknowledgment
Authors’ roles: CMG received support from a Westmead Hospital Early Career Charitable Trust Fund Grant.
JBMR Plus Vitamin D Special Issue
In memoriam the late Anthony W. Norman
References
- 1. Mason RS, Sequeira VB, Gordon‐Thomson C. Vitamin D: the light side of sunshine. Eur J Clin Nutr. 2011;65(9):986‐993. [DOI] [PubMed] [Google Scholar]
- 2. Kutuzova GD, Deluca HF. Gene expression profiles in rat intestine identify pathways for 1,25‐dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys. 2004;432(2):152‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujita H, Sugimoto K, Inatomi S, et al. Tight junction proteins claudin‐2 and ‐12 are critical for vitamin D‐dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19(5):1912‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouillon R, Van Schoor NM, Gielen E, et al. Optimal vitamin D status: a critical analysis on the basis of evidence‐based medicine. J Clin Endocrinol Metab. 2013;98(8):E1283‐E1304. [DOI] [PubMed] [Google Scholar]
- 5. Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Girgis CM, Clifton‐Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33‐83. [DOI] [PubMed] [Google Scholar]
- 7. Girgis CM, Clifton‐Bligh RJ, Turner N, Lau SL, Gunton JE. Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clin Endocrinol (Oxf). 2014;80(2):169‐181. [DOI] [PubMed] [Google Scholar]
- 8. Bischoff‐Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25‐hydroxyvitamin D concentrations are associated with better lower‐extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752‐758. [DOI] [PubMed] [Google Scholar]
- 9. Girgis CM, Clifton‐Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology. 2014;155(2):347‐357. [DOI] [PubMed] [Google Scholar]
- 10. Brumbaugh PF, Haussler MR. 1 Alpha,25‐dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25‐dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974;249(4):1251‐1257. [PubMed] [Google Scholar]
- 11. Girgis CM. Vitamin D and skeletal muscle. In Feldman DJ, Pike W, Bouillon R, Giovanucci E, Goltzman D, Hewison M, eds. Vitamin D. 4th ed. Elsevier; 2018. pp 597‐613. [Google Scholar]
- 12. Norman AW, Mizwicki MT, Norman DP. Steroid‐hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3(1):27‐41. [DOI] [PubMed] [Google Scholar]
- 13. Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25‐dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 14. Buitrago C, Boland R. Caveolae and caveolin‐1 are implicated in 1alpha,25(OH)2‐vitamin D3‐dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010;121(1‐2):169‐175. [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152(2):354‐363. [DOI] [PubMed] [Google Scholar]
- 16. Girgis CM, Mokbel N, Minn Cha K, et al. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25‐hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155(9):3227‐3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olsson K, Saini A, Stromberg A, et al. Evidence for vitamin D receptor expression and direct effects of 1alpha,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology. 2016;157(1):98‐111. [DOI] [PubMed] [Google Scholar]
- 18. Cheng C, Alexander R, Min R, et al. Understanding transcriptional regulation by integrative analysis of transcription factor binding data. Genome Res. 2012;22(9):1658‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee SM, Bishop KA, Goellner JJ, O'Brien CA, Pike JW. Mouse and human BAC transgenes recapitulate tissue‐specific expression of the vitamin D receptor in mice and rescue the VDR‐null phenotype. Endocrinology. 2014;155(6):2064‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ceglia L, Niramitmahapanya S, Morais MD, et al. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98(12):E1927‐E1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pojednic RM, Ceglia L, Olsson K, et al. Effects of 1,25‐dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif Tissue Int. 2015;96(3):256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brennan‐Speranza TC, Mor D, Mason RS, et al. Skeletal muscle vitamin D in patients with end stage osteoarthritis of the knee. J Steroid Biochem Mol Biol. 2017;173:180‐184. [DOI] [PubMed] [Google Scholar]
- 23. Johnson JA, Grande JP, Roche PC, Kumar R. Ontogeny of the 1,25‐dihydroxyvitamin D3 receptor in fetal rat bone. J Bone Miner Res. 1996;11(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 24. Makanae Y, Ogasawara R, Sato K, et al. Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp Physiol. 2015;100(10):1168‐1176. [DOI] [PubMed] [Google Scholar]
- 25. Srikuea R, Zhang X, Park‐Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol. 2012;303(4):C396‐C405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braga MSZ, Norris KC, Ferrini MG, Artaza JN. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr Connect. 2017;6(3):139‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia LA, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)(2)vitamin D(3) enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C(2)C(12) skeletal muscle cells. J Steroid Biochem Mol Biol. 2013;133:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011;152(8):2976‐2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Romeu Montenegro K, Carlessi R, Cruzat V, Newsholme P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J Steroid Biochem Mol Biol. 2019;193:105423. [DOI] [PubMed] [Google Scholar]
- 31. van der Meijden K, Bravenboer N, Dirks NF, et al. Effects of 1,25(OH)2 D3 and 25(OH)D3 on C2C12 myoblast proliferation, differentiation, and myotube hypertrophy. J Cell Physiol. 2016;231(11):2517‐2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hayakawa N, Fukumura J, Yasuno H, Fujimoto‐Ouchi K, Kitamura H. 1α,25(OH)2 D3 downregulates gene expression levels of muscle ubiquitin ligases MAFbx and MuRF1 in human myotubes. Biomed Res. 2015;36(2):71‐80. [DOI] [PubMed] [Google Scholar]
- 33. Tamilselvan B, Seshadri K, Venkatraman G. Role of vitamin D on the expression of glucose transporters in L6 myotubes. Indian J Endocrinol Metab. 2013;17(7):326‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salles J, Chanet A, Giraudet C, et al. 1,25(OH)2‐vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol Nutr Food Res. 2013;57(12):2137‐2146. [DOI] [PubMed] [Google Scholar]
- 35. Manna P, Achari AE, Jain SK. 1,25(OH)2‐vitamin D3 upregulates glucose uptake mediated by SIRT1/IRS1/GLUT4 signaling cascade in C2C12 myotubes. Mol Cell Biochem. 2018;444(1):103‐108. [DOI] [PubMed] [Google Scholar]
- 36. Chang E. 1,25‐Dihydroxyvitamin D decreases tertiary butyl‐hydrogen peroxide‐induced oxidative stress and increases AMPK/SIRT1 activation in C2C12 muscle cells. Molecules. 2019;24(21):3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang E, Kim Y. Vitamin D ameliorates fat accumulation with AMPK/SIRT1 activity in C2C12 skeletal muscle cells. Nutrients. 2019;11(11):2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Endo I, Inoue D, Mitsui T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144(12):5138‐5144. [DOI] [PubMed] [Google Scholar]
- 39. Tanaka M, Kishimoto KN, Okuno H, Saito H, Itoi E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve. 2014;49(5):700‐708. [DOI] [PubMed] [Google Scholar]
- 40. Antinozzi C, Corinaldesi C, Giordano C, et al. Potential role for the VDR agonist elocalcitol in metabolic control: evidences in human skeletal muscle cells. J Steroid Biochem Mol Biol. 2017;167:169‐181. [DOI] [PubMed] [Google Scholar]
- 41. Ryan ZC, Craig TA, Folmes CD, et al. 1alpha,25‐dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J Biol Chem. 2016;291(3):1514‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Capiati DA, Vazquez G, Tellez Iñón MT, Boland RL. Role of protein kinase C in 1,25(OH)(2)‐vitamin D(3) modulation of intracellular calcium during development of skeletal muscle cells in culture. J Cell Biochem. 2000;77(2):200‐212. [DOI] [PubMed] [Google Scholar]
- 43. Vazquez G, Boland R, de Boland AR. Modulation by 1,25(OH)2‐vitamin D3 of the adenylyl cyclase/cyclic AMP pathway in rat and chick myoblasts. Biochim Biophys Acta. 1995;1269(1):91‐97. [DOI] [PubMed] [Google Scholar]
- 44. Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200(2):207‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Girgis CM, Cha KM, Houweling PJ, et al. Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int. 2015;97(6):602‐610. [DOI] [PubMed] [Google Scholar]
- 46. Chen S, Villalta SA, Agrawal DK. FOXO1 mediates vitamin D deficiency‐induced insulin resistance in skeletal muscle. J Bone Miner Res. 2016;31(3):585‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Girgis CM, Cha KM, So B, et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle. 2019;10(6):1228‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bass JJ, Nakhuda A, Deane CS, et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol Metab. 2020;42:101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gifondorwa DJ, Thompson TD, Wiley J, et al. Vitamin D and/or calcium deficient diets may differentially affect muscle fiber neuromuscular junction innervation. Muscle Nerve. 2016;54(6):1120‐1132. [DOI] [PubMed] [Google Scholar]
- 50. Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A. Vitamin D deficiency induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology. 2013;154(11):4018‐4029. [DOI] [PubMed] [Google Scholar]
- 51. Choi M, Park H, Cho S, Lee M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high‐intensity exercise in SD rats. Cytokine. 2013;63(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 52. Stratos I, Li Z, Herlyn P, et al. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am J Pathol. 2013;182(3):895‐904. [DOI] [PubMed] [Google Scholar]
- 53. Ke CY, Yang FL, Wu WT, et al. Vitamin D3 reduces tissue damage and oxidative stress caused by exhaustive exercise. Int J Med Sci. 2016;13(2):147‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Akagawa M, Miyakoshi N, Kasukawa Y, et al. Effects of activated vitamin D, alfacalcidol, and low‐intensity aerobic exercise on osteopenia and muscle atrophy in type 2 diabetes mellitus model rats. PLoS One. 2018;13(10):e0204857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nadimi H, Djazayery A, Javanbakht MH, et al. The effect of vitamin D supplementation on serum and muscle Irisin levels, and FNDC5 expression in diabetic rats. Rep Biochem Mol Biol. 2019;8(3):236‐243. [PMC free article] [PubMed] [Google Scholar]
- 56. Benetti E, Mastrocola R, Chiazza F, et al. Effects of vitamin D on insulin resistance and myosteatosis in diet‐induced obese mice. PLoS One. 2018;13(1):e0189707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayes A, Rybalka E, Debruin DA, Hanson ED, Scott D, Sanders K. The effect of yearly‐dose vitamin D supplementation on muscle function in mice. Nutrients. 2019;11(5):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high‐dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815‐1822. [DOI] [PubMed] [Google Scholar]
- 59. Pojednic RM, Ceglia L, Lichtenstein AH, Dawson‐Hughes B, Fielding RA. Vitamin D receptor protein is associated with interleukin‐6 in human skeletal muscle. Endocrine. 2015;49(2):512‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barker T, Henriksen VT, Martins TB, et al. Higher serum 25‐hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients. 2013;5(4):1253‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barker T, Martins TB, Hill HR, et al. Vitamin D sufficiency associates with an increase in anti‐inflammatory cytokines after intense exercise in humans. Cytokine. 2014;65(2):134‐137. [DOI] [PubMed] [Google Scholar]
- 62. Hassan‐Smith ZK, Jenkinson C, Hernandez I, et al. 25‐hydroxyvitamin D3 and 1,25‐dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS One. 2017;12(2):e0170665. 10.1371/journal.pone.0170665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Clements MR, Davies M, Fraser DR, Lumb GA, Mawer EB, Adams PH. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin Sci (Lond). 1987;73(6):659‐664. [DOI] [PubMed] [Google Scholar]
- 64. Datta P, Philipsen PA, Olsen P, et al. The half‐life of 25(OH)D after UVB exposure depends on gender and vitamin D receptor polymorphism but mainly on the start level. Photochem Photobiol Sci. 2017;16(6):985‐995. [DOI] [PubMed] [Google Scholar]
- 65. Mason RS, Lissner D, Posen S, Norman AW. Blood concentrations of dihydroxylated vitamin D metabolites after an oral dose. Br Med J. 1980;280(6212):449‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haddad JG, Fraser DR, Lawson DE. Vitamin D plasma binding protein. Turnover and fate in the rabbit. J Clin Invest. 1981;67(5):1550‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mason RS, Rybchyn MS, Abboud M, Brennan‐Speranza TC, Fraser DR. The role of skeletal muscle in maintaining vitamin D status in winter. Curr Dev Nutr. 2019;3(10):nzz087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abboud M, Gordon‐Thomson C, Hoy AJ, et al. Uptake of 25‐hydroxyvitamin D by muscle and fat cells. J Steroid Biochem Mol Biol. 2014;144:232‐236. [DOI] [PubMed] [Google Scholar]
- 69. Clements MR, Fraser DR. Vitamin D supply to the rat fetus and neonate. J Clin Invest. 1988;81(6):1768‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Abboud M, Puglisi DA, Davies BN, et al. Evidence for a specific uptake and retention mechanism for 25‐hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154(9):3022‐3030. [DOI] [PubMed] [Google Scholar]
- 71. Gressner OA, Lahme B, Gressner AM. Gc‐globulin (vitamin D binding protein) is synthesized and secreted by hepatocytes and internalized by hepatic stellate cells through Ca(2+)‐dependent interaction with the megalin/gp330 receptor. Clin Chim Acta. 2008;390(1‐2):28‐37. [DOI] [PubMed] [Google Scholar]
- 72. Abboud M, Rybchyn MS, Liu J, et al. The effect of parathyroid hormone on the uptake and retention of 25‐hydroxyvitamin D in skeletal muscle cells. J Steroid Biochem Mol Biol. 2017;173:173‐179. [DOI] [PubMed] [Google Scholar]
- 73. Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147(12):5542‐5548. [DOI] [PubMed] [Google Scholar]
- 74. Capiati DA, Vazquez G, Boland RL. Protein kinase C alpha modulates the Ca2+ influx phase of the Ca2+ response to 1alpha,25‐dihydroxy‐vitamin‐D3 in skeletal muscle cells. Horm Metab Res. 2001;33(4):201‐206. [DOI] [PubMed] [Google Scholar]
- 75. Boland RL. VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol. 2011;347(1‐2):11‐16. [DOI] [PubMed] [Google Scholar]


