Abstract
Although isolates of the “Streptococcus milleri” group (SMG) of bacteria are regarded as members of the commensal microflora of the body, they are frequently encountered in purulent infections from a range of body sites. The genetic diversity of 91 epidemiologically unrelated SMG isolates (including 37 commensal strains and 49 disease-associated strains) was analyzed by macrorestriction fingerprinting (MF). The genomes were digested with SmaI and ApaI independently, and fragments were resolved by pulsed-field gel electrophoresis. Similarities between banding profiles were determined, and strains were clustered on this basis into dendrograms. In common with other commensal species that have been examined by MF, considerable genetic diversity was revealed. In addition, the clustering of strains tended to support the current taxonomic position of this heterogeneous group. The present study has shown that MF is a powerful tool for characterization of SMG strains and that its use is likely to be of great value in epidemiological and population genetic studies of this group of bacteria.
Isolates of the “Streptococcus milleri” group (SMG) of bacteria are generally regarded as members of the commensal microflora of the body and are found at various sites including the oral cavity, genitourinary system, and gastrointestinal tract (10, 34, 38). However, SMG bacteria are also frequently encountered in suppurative infections at a range of clinical sites, including liver and brain abscesses, dentoalveolar infections, and infective endocarditis (10, 17, 24, 33, 34). It has been suggested that these microaerophilic anaerobes are initiators of infection and, as such, prepare the environment for subsequent colonization by the strict anaerobes with which they are frequently isolated (1, 15, 21, 33).
The taxonomy of this bacterial group has been a cause for much debate, largely due to the great heterogeneity of the microorganisms. Although regarded as closely related species on the basis of rRNA sequencing data, three species, S. intermedius, S. constellatus, and S. anginosus, distinguishable by phenotypic methods have been described (35, 37, 39, 40, 41). It has been suggested on the basis of DNA homology and sodium dodecyl sulfate-polyacrylamide gel electrophoresis studies that a further species may exist in addition to a distinct subspecies within the S. anginosus group (39, 40).
It is assumed that the endogenous microflora is the source of strains of SMG that are encountered in clinical infection involving SMG. However, little is known about the epidemiology of infection, and individual strain characterization or the comparison of the commensal and disease-associated microflora has rarely been performed.
Macrorestriction analysis of the genome is a technique which has been used extensively for strain characterization mainly in typing studies for epidemiological purposes but less frequently for taxonomic and population genetics studies, particularly of autochthonous bacteria (22, 32).
The aim of the present study was to develop a reproducible technique for genotypic characterization of SMG strains by analysis of macrorestriction fingerprints obtained by pulsed-field gel electrophoresis (PFGE) of genomic fragments.
MATERIALS AND METHODS
Test strains.
The isolates of SMG were obtained from dentoalveolar abscesses (n = 21), extraoral infections (n = 28), and healthy oral sites (n = 37); and 5 isolates were of unknown origin. Strains isolated from extraoral sites originated from blood, brain, lung, abdominal, perianal, urogenital, skin, soft tissue, bone, and miscellaneous infections (Table 1). The majority of the nonoral isolates were kindly provided by R. Whiley (Oral Microbiology, St. Bartholomew's and the Royal London, United Kingdom). Commensal oral strains of SMG (Table 2) were isolated from the mixed saliva, supra- and subgingival plaques, dorsum of the tongue, and throat swabs obtained from unrelated healthy individuals and were cultured by use of a selective medium (4). The isolates were routinely maintained either in brain heart infusion broth (Oxoid Ltd., Basingstoke, United Kingdom) or on blood base agar (Oxoid Ltd.) containing 5% (vol/vol) defibrinated horse blood (TCS Microbiology, Botolph Claydon, United Kingdom). Cultures were incubated at 37°C in an anaerobic workstation (Don Whitley Scientific Ltd., Shipley, United Kingdom) containing an atmosphere of 10% hydrogen, 10% carbon dioxide, 80% nitrogen.
TABLE 1.
Sources and identities of 54 infection-related SMG isolates used in this study
| Reference strain | Species identificationa | Clinical source |
|---|---|---|
| 54244/95 | S. constellatus | Pilonidal abscess |
| 54252/95 | S. intermedius | Ethmoidal fluid |
| 4515/96 | S. constellatus | Perianal abscess |
| 25358/96 | S. anginosus | High vaginal swab |
| 27647/96 | S. constellatus | Wound swab, appendix |
| 43586/96 | S. anginosus | High vaginal swab |
| 240A/95 | S. anginosus | Dentoalveolar abscess |
| 240B/95 | S. intermedius | Dentoalveolar abscess |
| 313A/95 | S. intermedius | Dentoalveolar abscess |
| 313B/95 | S. constellatus | Dentoalveolar abscess |
| 322/95 | S. constellatus | Dentoalveolar abscess |
| 428/95 | S. constellatus | Dentoalveolar abscess |
| 447/95 | S. intermedius | Dentoalveolar abscess |
| 500/95 | S. anginosus | Dentoalveolar abscess |
| 670/95 | S. anginosus | Dentoalveolar abscess |
| 743/95 | S. constellatus | Dentoalveolar abscess |
| 762/95 | S. constellatus | Dentoalveolar abscess |
| 870/95 | S. intermedius | Dentoalveolar abscess |
| 904/95 | S. anginosus | Dentoalveolar abscess |
| 910/95 | S. anginosus | Dentoalveolar abscess |
| 48/96 | S. intermedius | Dentoalveolar abscess |
| 127/96 | S. intermedius | Dentoalveolar abscess |
| 183/96 | S. constellatus | Dentoalveolar abscess |
| 229/96 | S. constellatus | Dentoalveolar abscess |
| 232/96 | S. intermedius | Finger abscess |
| 274/96 | S. constellatus | Dentoalveolar abscess |
| 322/96 | S. anginosus | Dentoalveolar abscess |
| 350/96 | S. constellatus | Dentoalveolar abscess |
| HW69 | S. intermedius | Brain abscess |
| ‘HW13’ | S. intermedius | Unknown |
| 7K | S. anginosus | Brain abscess |
| CDC 2236-81 | S. anginosus | Blood |
| CDC 2405-81 | S. anginosus | Blood |
| R87/3795 | S. constellatus | Blood |
| R87/3802 | S. constellatus | Blood |
| R87/3972 | S. intermedius | Blood |
| A2940 | S. anginosus | Blood |
| W414 | S. constellatus | Abdominal mass |
| F458L | S. intermedius | Abdominal mass |
| ‘M6561’ | S. constellatus | Unknown |
| SL34/W | S. anginosus | Subphrenic abscess |
| H-D T2 | S. intermedius | Acute sinusitis |
| C1792 | S. constellatus | Spinal osteomyelitis |
| F436 | S. constellatus | Pleural empyema |
| NCTC 10713 | S. anginosus | Throat (ATCC 12395) |
| 39/2/14A | S. anginosus | Unknown |
| 55371/96 | S. constellatus | Wound swab trachea |
| 4216/24A | S. anginosus | Unknown |
| 3244/97 | S. intermedius | Swab, pilonidal abscess |
| 3287/97 | S. anginosus | Ear swab |
| 3395/97 | S. constellatus | Perianal swab |
| 16/3/11N | S. constellatus | Bacteremia |
| NCTC 11325 | S. constellatus | Purulent pleurisy (ATCC 27823) |
| NCTC 11324 | S. intermedius | Unknown (ATCC 27335) |
Species were identified by the method of Whiley et al. (37).
TABLE 2.
Sources and identities of 37 SMG commensal strains isolated from healthy sites
| Reference strain | Species identification | Clinical source |
|---|---|---|
| 10c | S. anginosus | Plaque |
| 18c | S. anginosus | Plaque |
| 20c | S. anginosus | Plaque |
| 28c | S. intermedius | Plaque |
| 33c | S. intermedius | Plaque |
| 30c | S. intermedius | Tongue |
| 5′c | S. constellatus | Plaque |
| 23′c | S. intermedius | Plaque |
| 38c | S. anginosus | Tongue |
| 41c | S. anginosus | Tongue |
| 45c | S. intermedius | Tongue |
| 46c | S. anginosus | Tongue |
| 40c | S. intermedius | Tongue |
| 42c | S. anginosus | Tongue |
| 43c | S. anginosus | Tongue |
| 25′c | S. intermedius | Plaque |
| 69C | S. intermedius | Plaque |
| 48C | S. constellatus | Tongue |
| 75C | S. anginosus | Plaque |
| 72C | S. constellatus | Tongue |
| 35C | S. intermedius | Plaque |
| 34C | S. constellatus | Tongue |
| 83C | S. intermedius | Plaque |
| 84C | S. intermedius | Plaque |
| 57C | S. constellatus | Tongue |
| 56C | S. anginosus | Tongue |
| 52C | S. anginosus | Throat |
| 31C | S. intermedius | Plaque |
| 11C | S. intermedius | Tongue |
| 10C | S. anginosus | Tongue |
| 19C | S. anginosus | Plaque |
| 20C | S. intermedius | Plaque |
| 17C | S. anginosus | Tongue |
| 16C | S. anginosus | Plaque |
| 62r | S. anginosus | Plaque |
| 23r | S. anginosus | Plaque |
| 4dw | S. intermedius | Plaque |
A total of 91 clinical SMG isolates were examined in the study, including 3 type strains, S. anginosus NCTC 10713 (ATCC 12395); S. constellatus NCTC 11325 (ATCC 27823), and S. intermedius NCTC 11324 (ATCC 27335) (Table 1). Each strain was assigned to either S. anginosus, S. constellatus, or S. intermedius according to a differential phenotypic identification scheme (37). Briefly, strains were tested for a panel of six glycosidase reactions by using 4-methylumbelliferyl-linked fluorogenic substrates and hyaluronidase production by a modified plate assay (37). The identity of each isolate was confirmed with the Rapid ID 32 Strep API system (bioMérieux sa, Marcy l'Etoile, France). All isolates were tested for the presence of Lancefield group antigens A, C, F, and G by using the Streptex kit (Murex Biotech Ltd., Dartford, United Kingdom). Carbohydrate fermentation and esculin and arginine hydrolysis tests were miniaturized to a microtiter plate assay.
DNA preparation.
The genomic DNA of the test strains was prepared in agarose plugs by the rapid DNA extraction procedure described by Matsuhek et al. (23), with minor modifications. A 5-ml volume of an overnight bacterial culture in brain heart infusion broth was harvested by centrifugation. The bacterial pellet was resuspended in 0.5 ml of 2× lysis solution (12 mM Tris HCl [pH 7.4], 2 M NaCl, 20 mM EDTA [pH 7.5], 1.0% Brij, 0.4% sodium deoxycholate, 1.0% sodium lauroyl sarcosine) containing lysozyme at 1.0 mg/ml, mutanolysin at 10 U/ml, and RNase A at 40 μg/ml. This suspension was mixed with an equal volume of 2% low-melting-point agarose (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom) tempered at 56°C. The agarose mixture was placed in a 100-μl plug mold agarose (Bio-Rad). After solidification, the resulting agarose blocks were incubated sequentially in the following solutions for the indicated times: 3 ml of 1× lysis buffer (6 mM Tris HCl [pH 7.4], 1 M NaCl, 10 mM EDTA [pH 7.5], 0.5% Brij, 0.2% sodium deoxycholate, 0.5% sodium lauroyl sarcosine) containing lysozyme at 0.5 mg/ml, mutanolysin at 5 U/ml, and RNase A at 20 μg/ml for 2 h at 37°C; 3 ml of proteinase K buffer (10 mM Tris HCl [pH 7.4], 1 mM EDTA) to which proteinase K at 100 μg/ml and 1% sodium dodecyl sulfate was freshly added before incubation for 1 h at 50°C; and 5 ml of dilute TE (10 mM Tris HCl [pH 7.4], 0.1 mM EDTA) for 1 h at 50°C. The plugs were washed three times in 5 ml of TE buffer containing 1 mM phenylmethylsulfonyl fluoride (stock solution freshly made at 100 mM in absolute ethanol) for 1 h at room temperature in order to inactivate the proteinase K. The agarose blocks were finally washed in 5 ml of TE buffer and were stored at 4°C until restriction digestion.
Restriction digestion of DNA.
Prior to DNA restriction, a slice of an agarose plug was cut with sterilized coverslips (Chance Propper Ltd., Warley, United Kingdom) and was equilibrated in distilled water for 1 h at 4°C. The selection of a restriction endonuclease was based on the recognition sequence of the enzyme and the G+C DNA base composition of 38 to 40 mol% reported for SMG isolates (35). In a preliminary experiment, four restriction endonucleases that recognize eight G+C-rich sites (SfiI [GGCCN5GGCC] and NotI [GCGGCCGC]) or 6 bp SmaI [CCCGGG] and ApaI [GGGCCC]) were tested with 10 strains. The restriction profiles of NotI and SfiI produced too few fragments. SmaI and ApaI were found to be most suitable in their ability to produce highly resolved genomic restriction fragments of sufficient complexity. These two enzymes were consequently used to analyze all of the strains in the study. The reactions were performed in a 100-μl volume containing 10 U of the restriction enzyme according to the manufacturer's instructions (New England Biolabs, Hitchin, United Kingdom). Each digestion was carried out for 18 h. After the digestion, the plug slices were placed in dilute TE for 1 h at 4°C before loading onto the gel.
PFGE.
The gel slices of one-fourth to one-half of a plug were loaded into a 20-well 1% agarose gel (Bio-Rad). Typically, each PFGE gel consisted of 17 DNA sample lanes. A bacteriophage concatemer with 48.5-kb increments (Bio-Rad) was used as a molecular size marker and was run on the outermost and middle lane in each electrophoresis run to facilitate band alignment. The macrorestriction fragments were separated with a contour-clamped homogeneous field electrophoresis apparatus (CHEF-DRII; (Bio-Rad). PFGE was performed in 0.5× TBE buffer (1× TBE buffer is 89 mM Tris HCl [pH 7.4], 89 mM boric acid, 25 mM EDTA [pH 8.0]) at 4°C. The pulsed-field parameters for SmaI and ApaI were 20 h at 6 V/cm (200 V), with switch times ramped from 5 to 35 s and 50 to 90 s for NotI and SfiI, respectively. After electrophoresis, the nucleic acid present in the gel was stained in 300 ml of distilled water containing 0.5 μg of ethidium bromide per ml and was destained in distilled water for at least 3 h. The DNA bands were visualized under UV illumination, and the gel image was captured and digitized by Windows, version 1.5, Molecular Analyst software (Bio-Rad).
Data analysis.
The macrorestriction fingerprints generated by PFGE were analyzed by Windows, version 3.0, Gelcompar software (Applied Maths, Kortrijk, Belgium). The gels were normalized in order to avoid intergel and intragel variations and were aligned by associating bands of the internal reference pattern on each gel with the matching reference positions. The similarities of the restriction fragment length polymorphisms (RFLPs) of pairs of isolates were determined by using the Dice coefficient of similarity (5) according to the formula Sxy = 2nxy/(nx + ny), where the similarity (Sxy) for isolates x and y is the number of common fragments in the DNA profiles (nxy) divided by the average number of fragments exhibited by both patterns (nx and ny).
Matrices of similarity coefficients between all possible pairs of strains were obtained and clustered by the unweighted pair group method with arithmetic averages (UPGMA) (26). Dendrograms were constructed to reflect the similarities between the strains in the matrix. The optimization feature was enabled, which allowed small global shifts of up to 4% in patterns that were not perfectly aligned. A tolerance in the band position of 1.5% was applied during the comparison of PFGE fingerprinting patterns.
The discriminatory power of the PFGE technique was evaluated by the use of Simpson's index of diversity, as defined by Hunter and Gaston (14), after exclusion of the nontypeable strains. This expresses the probability that two unrelated stains will be placed into different typing groups. The discrimination index (D) depends on the number of types defined by the test method and the relative frequencies of these types: D = 1 − {[1/N(N − 1)] × [Σnj(nj − 1)]}, where N is the total number of unrelated strains and nj is the number of strains that belong to the jth type.
RESULTS
Determination of species of isolates.
Biochemical and enzymatic analyses enabled determination of the species of all the test strains by the proposed scheme of Whiley et al. (37). Almost half of the isolates (n = 40) possessed Lancefield group antigens, the most frequent being F (n = 29). The remainder of strains belonged to Lancefield group A (n = 1), group C (n = 7), and group G (n = 3). Although the majority (83%) of the isolates were nonhemolytic, 16 strains (mainly of the S. constellatus species) showed beta-hemolysis. Strains 25358/98, 43586/96, and 3287/97 produced biochemically atypical results and were shown to ferment mannitol. S. constellatus strain F436 was unable to produce hyaluronidase. All isolates except five strains of S. constellatas were shown to hydrolyze esculin and arginine after 5 days of incubation.
Macrorestriction fingerprint analysis.
Eight-six of the 91 isolates tested were typeable following digestion with either enzyme, SmaI or ApaI (Table 3). The remaining five isolates were resistant to digestion with one of the two enzymes. Of these strains, four were S. constellatus (strains 762/95, “M6561”, 3395/97, and 5′c) and resisted digestion with SmaI and one strain was S. intermedius, strain 232/96, and was not amenable to digestion with ApaI. The discriminatory index of the PFGE method was calculated to be 0.999 for both restriction endonucleases. The reproducibility of the technique was found to be 100% by the repeated testing of the isolates. The fingerprints of 10 strains were shown to be stable over 1 year of repeated triweekly subculturing (data not shown).
TABLE 3.
Pulsotypes obtained by macrorestriction fingerprint analysis of 91 strains of SMG following restriction with either SmaI or ApaI
| Restriction enzyme | No. of untypeable strains | No. of typeable strains | No. of identical pairs | No. of pulsotypes |
|---|---|---|---|---|
| SmaI | 4 | 87 | 4 | 83 |
| ApaI | 1 | 90 | 5 | 85 |
SmaI fingerprinting.
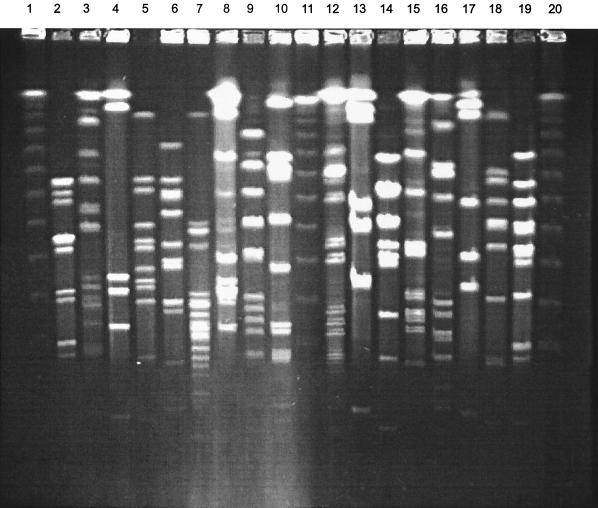
Electrophoresis of SmaI digests yielded well-resolved patterns of 4 to 16 fragments that ranged in size from 10 kb to >1 Mb. The DNA bands were well spaced throughout the gel under the parameters used. A total of 83 macrorestriction patterns were generated among the 87 clinical isolates analyzed. Four pairs of commensal strains from different subjects, isolated either from the dorsum of the tongue or from plaque samples, had identical restriction patterns and could not be differentiated from each other; rough variant S. intermedius strains 28c and 30c, 40c and 45c (both S. intermedius), 16C and 10C (S. anginosus strains), and group C 43c and 17C (group C S. anginosus strains). The remaining isolates yielded unique profiles. Representative restriction profiles are shown in Fig. 1.
FIG. 1.
PFGE of SmaI restriction fragments from 17 strains of SMG isolated from infected sites. Lanes: 1, 11, and 20, bacteriophage lambda 50-kb ladder which served as a molecular size marker; 2, 27647/96; 3, 43586/96; 4, 39/2/14A; 5, 55371/96; 6, 4216/24A; 7, 2405-81; 8, 2236-81; 9, HW13; 10, NCTC 11324; 12, HW69; 13, 25358/96; 14, R87/3795; 15, A2940; 16, 3244/97; 17, 3287/97; 18, NCTC 11325; 19, 4515/96.
ApaI fingerprinting.
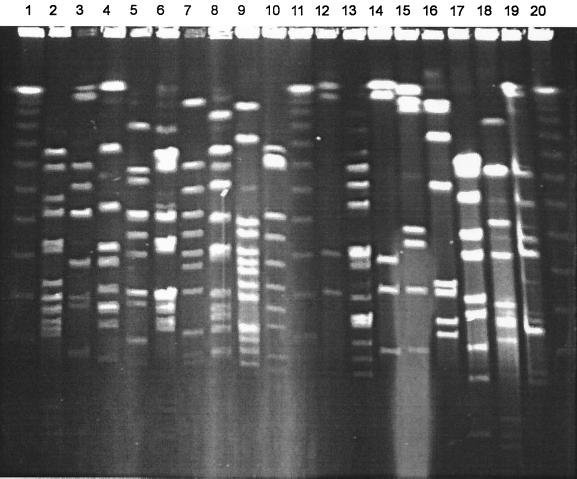
ApaI macrorestriction patterns consisted of 7 to 19 clearly discernible bands of between 20 kb and >1 Mb (Fig. 2). ApaI fragments tended to be more closely spaced in the lower-molecular-size region of <250 kb than the SmaI profiles. Eighty-five distinctive restriction profiles were apparent for the 90 isolates that yielded profiles. Five pairs of strains yielded identical profiles and are indicated in Table 4. From Table 4 it can be seen that three pairs of strains yielded identical patterns with both enzymes tested. Again, the pairs of strains were from apparently unrelated epidemiological sites. Two pairs of strains, although apparently identical in profile with one enzyme, produced different profiles when analyzed with a second enzyme (Table 4).
FIG. 2.
PFGE of ApaI restriction fragments from 17 strains of SMG isolated from healthy oral sites. Lanes: 1, 11, and 20, bacteriophage lambda 50-kb ladder; 2, 69C; 3, 48C; 4, 75C; 5, 72C; 6, 35C; 7, 34C; 8, 83C; 9, 57C; 10, 31C; 12, 19C, 13, 20C; 14, 16C; 15, 18c; 16, 20c; 17, 28c; 18, 33c; 19, 23′c.
TABLE 4.
Percent similarities (Dice coefficient) of six pairs of SMG strains yielding identical banding patterns with one or both enzymes (SmaI and ApaI) following PFGE
| Strains compared | Species |
SmaI
|
ApaI
|
||
|---|---|---|---|---|---|
| % Similarity | No. of band differences | % Similarity | No. of band differences | ||
| 28c & 30c | S. intermedius | 100 | 0 | 100 | 0 |
| 16C & 10C | S. anginosus | 100 | 0 | 100 | 0 |
| 43c & 17C | S. anginosus | 100 | 0 | 100 | 0 |
| 40c & 45c | S. intermedius | 100 | 0 | 90 | 6 |
| 41c & A2940 | S. anginosus | 82 | 3 | 100 | 0 |
| 5′c & 3395/97 | S. constellatus | NTa | 100 | 0 | |
NT, nontypeable.
Cluster analysis.
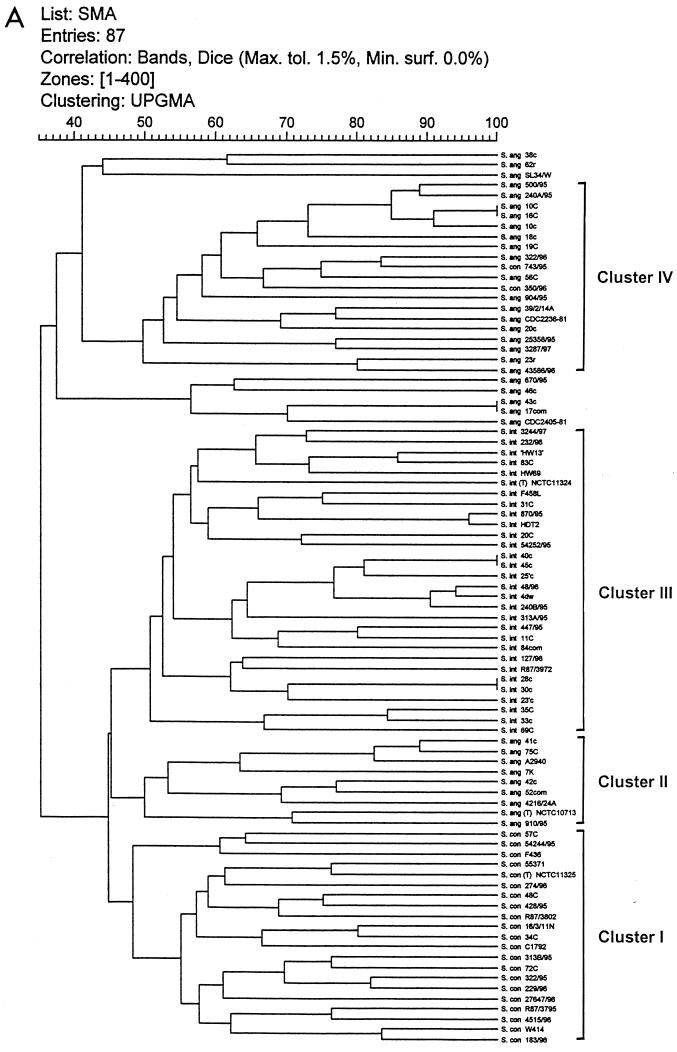
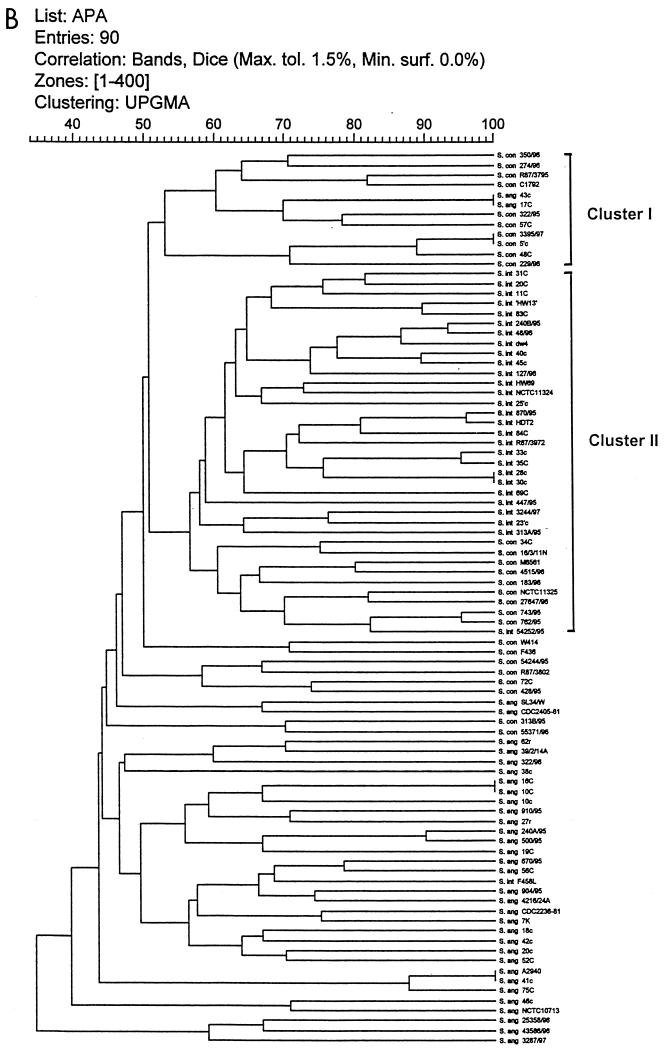
The dendrograms obtained from numerical analysis of the SmaI and ApaI DNA restriction PFGE profiles of the strains are shown in Fig. 3A and B, respectively. The cluster analysis correlated with visual inspection in detecting identical patterns of 100% similarity for the strains listed in Table 4. In addition, the analysis revealed considerable genomic diversity among SMG isolates, with similarities between strains ranging from 35 to 100%. The isolates were divided into clusters of strains that showed at least 47% similarity for SmaI and 52% similarity for ApaI (Fig. 3A and B). Only major clusters are labeled.
FIG. 3.
Dendrograms for SMG isolates digested with SmaI (A) and ApaI (B) after cluster analysis. Strain reference numbers and the sources and species identification of the SMG isolates are shown on the vertical axis. The numbers on the horizontal axis indicate the percent similarities as determined with the Dice correlation coefficient.
SmaI.
Cluster I contained 21 of the 23 S. constellatus strains analyzed and includes the type strain NCTC 11325. The other S. constellatus strains clustered deep within a group of S. anginosus strains that showed similarities of 67 and 83% to their nearest neighbors. Cluster III contained all 30 strains identified as S. intermedius. Five further clusters were present, with each containing strains of S. anginosus. The S. anginosus type strain clustered within cluster II, which as a group appeared to be more closely related to the S. intermedius and S. constellatus clusters than the other clusters of S. anginosus.
ApaI.
Percent similarity values for the strains analyzed with ApaI ranged from 35 to 100%. Two large clusters were formed at the 52% similarity level. Cluster I contained 10 isolates of S. constellatus, in addition to two strains of S. anginosus. Cluster II consisted of almost all of the strains of S. intermedius, with only one strain (strain F 458L) clustering in a different group. In addition, cluster II was seen to contain nine strains of S. constellatus, including the type strain NCTC 11325. The remaining strains of S. anginosus clustered in groups other than the two main clusters described.
For both enzymes it was apparent that S. intermedius strains formed a cohesive cluster. This was also true for strains of S. constellatus as analyzed by SmaI. However, the S. constellatus strains were not so closely related in the ApaI analysis. In general, both analyses indicated that S. intermedius and S. constellatus were more similar to each other than either was to S. anginosus. In both cases, S. anginosus isolates showed the greatest diversity of the three species groups. Interestingly, the three atypical strains of S. anginosus, which were mannitol positive, grouped closely together in the ApaI analysis.
DISCUSSION
The present study has demonstrated the characterization of SMG strains from a range of epidemiologically unrelated sites by macrorestriction fingerprinting (MF). The majority of strains of SMG were amenable to analysis by MF, which was shown to yield reproducible profiles on repeated testing. Five isolates were refractory to digestion with one or other of the restriction enzymes tested. Studies of other species have reported a similar failure of a minority of strains to undergo digestion, and the reasons for this are not entirely clear (13, 22, 32).
The approach used in the present study proved to be highly discriminatory, with 95% of strains yielding distinct fingerprints. This high level of discrimination has been observed previously in the analysis of a wide range of species, and indeed, MF has been proposed as the “gold standard” in typing studies for some bacterial groups, such as enterococci (25). MF profiles reflect the structural organization of the bacterial chromosome, detecting the distribution of restriction sites throughout the entire length of the genome (22). As such, MF has a distinct advantage over other genotypic methods of strain characterization, and it is therefore surprising that, to date, there have been very few population studies with epidemiologically unrelated strains. Snopkova et al. (29) studied 47 isolates of coagulase-negative staphylococci and revealed high levels of diversity. Considerable genetic diversity has also been revealed in S. aureus subsp. aureus, with strains showing levels of similarity ranging from 30 to 100% (27). It would appear that only one other study has examined a large number of strains of streptococci frequently encountered in the oropharyngeal flora. It was found that although the index of diversity for PFGE analysis of strains of S. dysgalactiae was high at 0.99, when genetic similarity values were determined, the isolates as a group were much more similar than the streptococcal isolates examined in the present study, sharing more than 86% genetic similarity (3).
In the present study, both commensal and clinical isolates were examined. It was interesting to observe that the four pairs of strains that yielded identical fingerprints were of commensal origin from apparently unrelated sources, raising the possibility that commensal isolates are less diverse than clinical isolates. While there is no approved method of summarizing the diversity of a collection of isolates, in this study, the means of the similarity values for commensal and clinical isolates as two independent groups were calculated and were not found to be markedly different (ranging from 45 to 49% and 44 to 47% for commensal and clinical isolates, respectively). Therefore, the significance of the small numbers of pairs of commensal strains with identical profiles is unclear.
For most bacterial pathogens, little information is available regarding the frequency with which distinctive clones are recovered from asymptomatic persons. In the present study, two individuals were studied longitudinally, and MF profiles of SMG isolates from plaque samples were studied. In each case strains with identical pulsotypes were isolated between two and three times over a 12-month period. These findings confirm the reported stability of other species within the endogenous oral microflora (6).
It would have been useful to have been able to analyze duplicate isolates (commensal and disease related) from patients with clinical disease. In a separate study, saliva samples and throat swabs were collected from patients presenting with a bacteremia involving SMG. However, SMG isolates were not recovered from any of the oral or throat specimens of these patients.
Although the reasons for the extensive genomic diversity within the SMG are unclear, the findings are consistent with the commensal nature of these organisms. The ability to generate genotypic and hence phenotypic variety within bacterial populations is essential for the colonization or survival of the pathogen within the host and, in fact, is the driving force of microbial evolution (11, 28). Moreover, it has been suggested that a high degree of genetic diversity is a useful mechanism for avoidance of immune elimination of such bacteria (6). Although, in theory, a number of genetic events can affect the fingerprint profiles (8, 13, 22, 31), the factors contributing to the heterogeneity of RFLPs revealed by MF have not been studied extensively. However, the information available for the enterococci and S. pneumoniae suggests that DNA rearrangements rather than point mutations are responsible for the RFLPs observed in these particular bacterial groups (12, 13). It is not possible from the present analysis to infer which mechanisms for the generation of diversity might be in operation. As the SMG is part of a complex microflora, it would theoretically be possible for the exchange of genetic information to contribute to diversity, and indeed, the in vitro transformation of cariogenic strains of SMG has been demonstrated (16).
In addition to supporting a nonclonal structure, the high level of genetic diversity, the branched nature of the dendrograms, and the general lack of phenotypic associations within the clusters observed here may reflect a high recombination rate within the SMG (31). It is possible that the greater diversity observed for the S. anginosus group of strains and the lower level of concordance of the two dendrograms for members of this group reflect a greater amount of gene exchange within this taxon. The marked heterogeneity of the S. anginosus species has been shown by other methods, including ribotyping, and has been reflected in a wide range of DNA homology values (36, 39).
The manner in which banding patterns are interpreted is as important as the original choice of typing method (22). The intention is to correlate the relatedness based on RFLP patterns to the overall genetic similarity of isolates. As discussed, the process is to a degree arbitrary as the actual genetic basis underlying the RFLPs is lacking (22, 32). Attempts have been made to quantitatively correlate the number of band differences with genetic events. Tenover et al. (32) proposed that two or three band differences represented one independent genetic event and that seven or more band differences represented three or more independent genetic events. However, these guidelines are likely to vary depending on the nature of the genetic rearrangements present. Further care in interpretation is required since studies have shown that differences of up to seven different bands can result by insertion of a single mobile element such as Tn916 (31). Therefore, strains may have major banding differences and still be closely related. It has been reported that the interpretation of relationships at lower levels of similarity are often inaccurate and clustering becomes less reliable (31).
Although identical and highly similar strains were discernible by eye, software-assisted analysis was found to be useful due to the diversity of the patterns. However, it is not possible to dispense with visual analysis entirely since a high degree of manual and visual analysis is required for optimal performance of the software, particularly for patterns as diverse as those obtained in the current study.
In order to reduce the chances of misinterpretation of strain affinities (7), two restriction enzymes were used in the present study. Although there is some concordance, discrepancies were also apparent. ApaI cuts more frequently than SmaI, producing greater numbers of bands, and, in effect, more information about the genome is obtained. However, due to deficiencies in the current algorithms in calculating similarities for distant relationships as assessed by restriction profiles, it is possible that the analysis of diverse strains may be more prone to errors.
Even with the limitations discussed above, it is still possible to make taxonomic inferences from the results obtained here. Despite a high degree of intraspecies polymorphism, in general, strains of the same species were more similar to each other than to strains that belonged to a different species. Type strains of each of the SMG species clustered within their appropriate groups. The clusters that contained the majority of S. constellatus and S. intermedius strains were more closely related to each other than to S. anginosus strains. This is in agreement with previous 16S rRNA sequence analysis and DNA hybridization studies (2, 19, 20). It is important that the species of the test strains were determined by phenotypic means, and although the discriminatory tests used have been extensively validated, it may be the case that some of the discrepancies seen reflect the limitations of reliance on phenotypic traits.
Of the three taxa, the greatest diversity was shown for S. anginosus strains. In previous studies sufficient heterogeneity was revealed within a group of beta-hemolytic, group C, hyaluronidase-negative strains to represent a new species and within hyaluronidase-positive strains to represent a new subspecies (39, 40). It would be useful to examine such strains by PFGE.
The results of MF would tend to further support the division of the SMG into three taxa. However, there are no guidelines regarding thresholds of genetic similarities based on MF useful for species distinction. In the study of coagulase-negative staphylococci by Snopkova et al. (29), it was assumed that strains that had more than 25% similarity to and that shared similar phenotypic traits with the type strains belonged to the same species and were probably of common phylogenetic origin (29). Genetic similarity data derived from MF profiles is required for a greater number of bacterial groups to address this issue.
In summary, the analysis of the SMG by MF has provided a highly discriminatory tool for strain characterization useful for studies in population genetics and epidemiology. Considerable genetic diversity was revealed for both commensal and disease-associated isolates. In addition, the current findings tended to support the current taxonomic status of the SMG. A protocol for the rapid extraction of DNA was followed, taking only 3 days from beginning to end, and although specialized operator expertise is required, the technique soon became routine. As a method of genotypic strain characterization, MF provides a highly discriminatory nonarbitrary approach and should be reproducible in interlaboratory studies.
REFERENCES
- 1.Aderhold L, Knothe H, Frenkel G. The bacteriology of dentogenous pyogenic infections. Oral Surg. 1981;52:583–587. doi: 10.1016/0030-4220(81)90072-4. [DOI] [PubMed] [Google Scholar]
- 2.Bentley R W, Leigh J A, Collins M D. Intragenic structure of Streptococcus based on comparative analysis of small subunit rRNA sequences. Int J Syst Bacteriol. 1991;41:487–494. doi: 10.1099/00207713-41-4-487. [DOI] [PubMed] [Google Scholar]
- 3.Bert F, Branger C, Poutrel B, Lambert-Zechovsky N. Differentiation of human and animal strains of Streptococcus dysgalactiae by pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1997;150:107–112. doi: 10.1111/j.1574-6968.1997.tb10357.x. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson J. A medium for isolation of Streptococcus mutans. Arch Oral Biol. 1967;12:1657–1658. doi: 10.1016/0003-9969(67)90201-4. [DOI] [PubMed] [Google Scholar]
- 5.Dice L R. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 6.Fitzsimmons S, Evans M, Pearce C, Sheridan M J, Wientzen R, Bowden G, Cole M F. Clonal diversity of Streptococcus mitis biovar 1 isolates from the oral cavity of human neonates. Clin Diagn Lab Immunol. 1996;3:517–522. doi: 10.1128/cdli.3.5.517-522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson J, Lorenz E, Owen R J. Lineages within Campylobacter jejuni defined by numerical analysis of pulsed-field gel electrophoresis DNA profiles. J Med Microbiol. 1997;46:157–163. doi: 10.1099/00222615-46-2-157. [DOI] [PubMed] [Google Scholar]
- 8.Gordillo M E, Singh K V, Baker C J, Murray B E. Typing of group B streptococci: comparison of pulsed-field gel electrophoresis and conventional electrophoresis. J Clin Microbiol. 1993;31:1430–1434. doi: 10.1128/jcm.31.6.1430-1434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordillo M E, Singh K V, Murray B E. Comparison of ribotyping and pulsed-field gel electrophoresis for subspecies differentiation of strains of Enterococcus faecalis. J Clin Microbiol. 1993;31:1570–1574. doi: 10.1128/jcm.31.6.1570-1574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10:257–285. doi: 10.1093/clinids/10.2.257. [DOI] [PubMed] [Google Scholar]
- 11.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 12.Hall L M. Are point mutations or DNA arrangements responsible for the restriction fragment length polymorphisms that are used to type bacteria? Microbiology. 1994;140:197–204. doi: 10.1099/13500872-140-1-197. [DOI] [PubMed] [Google Scholar]
- 13.Hall L M, Duke B. Conservation of restriction sites in isolates of Streptococcus pneumoniae with diverse restriction fragment patterns. J Clin Microbiol. 1998;36:1805–1807. doi: 10.1128/jcm.36.6.1805-1807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter P R, Gaston M. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwu C, Macfarlane T W, MacKenzie D, Stenhouse D. The microbiology of periapical granulomas. Oral Surg Med Pathol. 1990;69:502–505. doi: 10.1016/0030-4220(90)90386-7. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs A E, Horton W A, Drucker D B. Genetic transformation in some cariogenic Streptococcus milleri. Microbios. 1989;60:167–175. [PubMed] [Google Scholar]
- 17.Jacobs J A, Peterson H G, Stobberingh E E, Soeters P B. Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. Clinical relevance, hemolytic and serologic characteristics. Am J Clin Pathol. 1995;104:547–553. doi: 10.1093/ajcp/104.5.547. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs J A, Scholt C S, Bunschoten A E, Schouls L M. Rapid species identification of “Streptococcus milleri” strains by line dot hybridization: identification of a distinct 16S rRNA population closely related to Streptococcus constellatus. J Clin Microbiol. 1996;34:1717–1721. doi: 10.1128/jcm.34.7.1717-1721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamura Y, Hou X-G, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 20.Kilpper-Balz R, Williams B L, Lutticken R, Schleifer K H. Relatedness of “Streptococcus milleri” with Streptococcus anginosus and Streptococcus constellatus. Syst Appl Microbiol. 1984;5:494–500. [Google Scholar]
- 21.Lewis M A O, Macfarlane T W, McGowan D A, Macdonald D G. Assessment of the pathogenicity of bacterial species isolated from acute dentoalveolar abscesses. J Med Microbiol. 1988;27:109–116. doi: 10.1099/00222615-27-2-109. [DOI] [PubMed] [Google Scholar]
- 22.Maslow J N, Mulligan M E, Arbeit R D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993;17:153–162. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- 23.Matsuhek M G, Bonten M J M, Hayden M K. Rapid preparation of bacterial DNA for pulsed-field gel electrophoresis. J Clin Microbiol. 1996;34:2598–2600. doi: 10.1128/jcm.34.10.2598-2600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina J M, Leport C, Bure A, Wolff M, Michon C, Vilde J L. Clinical and bacterial features of infections caused by Streptococcus milleri. Scand J Infect Dis. 1991;23:659–666. doi: 10.3109/00365549109024289. [DOI] [PubMed] [Google Scholar]
- 25.Murray B E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nei M, Li W H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantucek R, Gotz F, Doskar J, Rosypal S. Genomic variability of Staphylococcus aureus and other coagulase-positive staphylococcus species estimated by macro restriction analysis using pulsed-field gel electrophoresis. Int J Syst Bacteriol. 1997;35:25–32. doi: 10.1099/00207713-46-1-216. [DOI] [PubMed] [Google Scholar]
- 28.Robertson B D, Meyer T F. Genetic variation in pathogenic bacteria. Trends Genet. 1992;8:422–427. doi: 10.1016/0168-9525(92)90325-x. [DOI] [PubMed] [Google Scholar]
- 29.Snopkova S, Gotz S, Doskar J, Rosypal J. Pulsed-field gel electrophoresis of the genomic restriction fragments of coagulase-negative staphylococci. FEMS Microbiol Lett. 1994;124:131–140. doi: 10.1016/0378-1097(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 30.Talon D, Bailly P, Leprat R, Godard C, Deconnick E, Cahn J Y, Michel-Briand Y. Typing of hospital strains of Xanthomonas maltophilia by pulsed-field gel electrophoresis. J Hosp Infect. 1994;27:209–217. doi: 10.1016/0195-6701(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 31.Tibayrenc M. Towards a unified evolutionary genetics of microorganisms. Annu Rev Microbiol. 1996;50:401–429. doi: 10.1146/annurev.micro.50.1.401. [DOI] [PubMed] [Google Scholar]
- 32.Tenover F C, Arbeit R D, Goering R V, Micklesen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA resolution patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2333–2339. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Auwera P. Clinical significance of Streptococcus milleri. Eur J Clin Microbiol. 1985;4:386–390. doi: 10.1007/BF02148688. [DOI] [PubMed] [Google Scholar]
- 34.Whiley R A, Beighton D, Winstanley T G, Fraser H Y, Hardie J M. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J Clin Microbiol. 1992;30:243–244. doi: 10.1128/jcm.30.1.243-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiley R A, Beighton D. Emended descriptions and recognition of Streptococcus constellatus, Streptococcus intermedius and Streptococcus anginosus as distinct species. Int J Syst Bacteriol. 1991;41:1–5. doi: 10.1099/00207713-41-1-1. [DOI] [PubMed] [Google Scholar]
- 36.Whiley R A, Duke B, Hardie J M, Hall L M C. Heterogeneity among 16S-23S rRNA spacers within the “Streptococcus milleri group.”. Microbiology. 1995;141:1461–1467. doi: 10.1099/13500872-141-6-1461. [DOI] [PubMed] [Google Scholar]
- 37.Whiley R A, Fraser H, Hardie J M, Beighton D. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the “Streptococcus milleri group”. J Clin Microbiol. 1990;28:1497–1501. doi: 10.1128/jcm.28.7.1497-1501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiley R A, Freemantle L, Beighton D, Radford J R, Hardie J M, Tillotsen G. Isolation, identification and prevalence of Streptococcus anginosus, S. intermedius and S. constellatus from the human mouth. Microb Ecol Health Dis. 1993;6:285–291. [Google Scholar]
- 39.Whiley R A, Hall L M C, Hardie J M, Beighton D. Genotypic and phenotypic diversity within Streptococcus anginosus. Int J Syst Bacteriol. 1997;47:645–650. [Google Scholar]
- 40.Whiley R A, Hall L M C, Hardie J M, Beighton D. A study of small-colony, β haemolytic, Lancefield group C streptococci within the anginosus group: description of Streptococcus constellatus subsp. pharyngis subsp. nov., associated with the human throat and pharyngitis. Int J Syst Bacteriol. 1999;49:1443–1449. doi: 10.1099/00207713-49-4-1443. [DOI] [PubMed] [Google Scholar]
- 41.Whiley R A, Hardie J M. DNA-DNA hybridisation studies and phenotypic characteristics for strains within the “Streptococcus milleri group.”. J Gen Microbiol. 1989;135:2623–2633. doi: 10.1099/00221287-135-10-2623. [DOI] [PubMed] [Google Scholar]