Key Points
Question
Are all pulmonary clots seen on chest imaging after trauma pulmonary emboli (PE), or are some de novo pulmonary thrombosis (PT) with distinct risk factors?
Findings
In this cohort study of 7880 injured patients, 277 had deep venous thrombosis, 40 had PE, and 117 had PT. Factors significantly associated with the development of PT but not deep venous thrombosis or PE include the presence of shock on admission and major chest injury.
Meaning
This cohort study provides further evidence that not all pulmonary clots are PE and sets the stage for future studies into the pathophysiology and treatment of de novo PT.
Abstract
Importance
Pulmonary clots are seen frequently on chest computed tomography performed after trauma, but recent studies suggest that pulmonary thrombosis (PT) and pulmonary embolism (PE) after trauma are independent clinical events.
Objective
To assess whether posttraumatic PT represents a distinct clinical entity associated with the nature of the injury, different from the traditional venous thromboembolic paradigm of deep venous thrombosis (DVT) and PE.
Design, Setting, and Participants
This prospective, observational, multicenter cohort study was conducted by the Consortium of Leaders in the Study of Traumatic Thromboembolism (CLOTT) study group. The study was conducted at 17 US level I trauma centers during a 2-year period (January 1, 2018, to December 31, 2020). Consecutive patients 18 to 40 years of age admitted for a minimum of 48 hours with at least 1 previously defined trauma-associated venous thromboembolism (VTE) risk factor were followed up until discharge or 30 days.
Exposures
Investigational imaging, prophylactic measures used, and treatment of clots.
Main Outcomes and Measures
The main outcomes of interest were the presence, timing, location, and treatment of any pulmonary clots, as well as the associated injury-related risk factors. Secondary outcomes included DVT. We regarded pulmonary clots with DVT as PE and those without DVT as de novo PT.
Results
A total of 7880 patients (mean [SD] age, 29.1 [6.4] years; 5859 [74.4%] male) were studied, 277 with DVT (3.5%), 40 with PE (0.5%), and 117 with PT (1.5%). Shock on admission was present in only 460 patients (6.2%) who had no DVT, PT, or PE but was documented in 11 (27.5%) of those with PE and 30 (25.6%) in those with PT. Risk factors independently associated with PT but not DVT or PE included shock on admission (systolic blood pressure <90 mm Hg) (odds ratio, 2.74; 95% CI, 1.72-4.39; P < .001) and major chest injury with Abbreviated Injury Score of 3 or higher (odds ratio, 1.72; 95% CI, 1.16-2.56; P = .007). Factors associated with the presence of PT on admission included major chest injury (14 patients [50.0%] with or without major chest injury with an Abbreviated Injury Score >3; P = .04) and major venous injury (23 [82.1%] without major venous injury and 5 [17.9%] with major venous injury; P = .02). No deaths were attributed to PT or PE.
Conclusions and Relevance
To our knowledge, this CLOTT study is the largest prospective investigation in the world that focuses on posttraumatic PT. The study suggests that most pulmonary clots are not embolic but rather result from inflammation, endothelial injury, and the hypercoagulable state caused by the injury itself.
This cohort study of patients with trauma-associated venous thromboembolism risk factors assesses whether posttraumatic pulmonary clots are all pulmonary embolisms or whether some represent pulmonary thrombosis.
Introduction
Venous thromboembolism (VTE) remains a leading cause of morbidity and mortality after trauma.1,2 The long-standing assumption that deep vein thrombosis (DVT) and pulmonary emboli (PE) are manifestations of the same pathology has driven prophylactic guidelines with the hypothesis that preventing DVT will lead to a reduction in PE. However, in recent years, many have questioned the association between DVT and pulmonary clots in patients with major injury, concluding instead that pulmonary thrombosis (PT) can arise de novo without DVT and may be a manifestation of local inflammation.2,3,4,5,6,7 In fact, pulmonary clots are frequently detected early after injury on computed tomographic scans (CTs) of the chest in both military and civilian patients with trauma and many are asymptomatic, raising the question of whether posttraumatic clots arise in situ and are not embolic in nature.8,9,10 Given the implications that this could have on prophylactic measures (specifically prophylactic vena cava filters) and potentially even on treatment, a large-scale, multicenter investigation characterizing posttraumatic PT was needed.
To address this important issue, a number of experts in the field of posttraumatic VTE came together to form the Consortium of Leaders in the Study of Traumatic Thromboembolism (CLOTT).11 The 17 CLOTT trauma surgeons, each of whom represents a level I American College of Surgeons–verified trauma center, agreed to work collaboratively in this prospective study with the main objective of defining the pathogenesis of and risk factors for posttraumatic PT. We hypothesized that posttraumatic PT represents a distinct clinical entity with identifiable risk factors primarily associated with the nature and the severity of the injury, different from the traditional venous thromboembolic paradigm of DVT and PE.
Methods
Study Design and Data Collection
This prospective, multicenter, observational cohort study (CLOTT 1) was conducted at 17 major trauma centers in the US. Permission to conduct the study was obtained at all 17 institutional review boards and included a waiver of informed consent given the observational nature and minimal risk to the patients related to this research. The study was also approved by the US Department of Defense Human Research Protection Office. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The treating surgeon was at liberty to order any imaging studies related to VTE and to choose the methods used for prophylaxis and the treatment of any clots detected on imaging. The comprehensive electronic case report form was designed exclusively for this study. Patient data were entered into the secure central Research Electronic Data Capture (REDCap) system located at the University of California, San Francisco. Each patient was given a unique record identification number in the database to ensure patient confidentiality. Each site has access to its own data; however, because potentially identifying data (age, sex, and date of admission) were included, only the main study principal investigator (Dr Knudson) had access to the entire data set as well as the codes for each participating trauma center. Before the start of the study, webinars were conducted to train clinical research coordinators at each site on the use of the case report form and entry into REDCap. A data dictionary and a standard operating manual were also developed and distributed. Periodic quality checks of the data entered into REDCap were performed at the main principal investigator site and included identification of missing data points as well as data errors.
Patients were screened for inclusion in this study if they were admitted to any of the participating trauma centers during the study period (January 1, 2018, to December 31, 2020). Because this study was funded by the US Department of Defense, the age criteria were matched to military personnel in the deployed setting (18-40 years of age). Patients who remained in the hospital for at least 48 hours and who had at least 1 previously identified posttraumatic VTE risk factor were considered eligible for the study12 (Table 1). Data collected prospectively from the electronic medical record and other hospital sources included patient demographic characteristics, admission vital signs and laboratory data, mechanism of injury, blood products and procoagulants administered during the first 24 hours, preexisting and postinjury VTE risk factors, Abbreviated Injury Scale (AIS) and Injury Severity Scale scores, length of stay, days in the intensive care unit, days receiving ventilatory support, discharge disposition, and cause of any deaths. Extensive data were collected on VTE prophylaxis used, including when it was initiated, chemical prophylactic drugs ordered, missed doses (including the reason for holding doses), and any complications associated with prophylaxis. Data were also collected on the use of vena cava filters as prophylactic devices. The primary outcome of interest was the presence of any pulmonary clot seen on computed tomography. We collected dates of all scans performed and the indications for the scan (ie, initial posttrauma evaluation, suspected PE, retained hemothorax, pneumonia or pneumothorax evaluation). Of particular interest was the location of pulmonary clots (side of the chest, main pulmonary artery, lobar artery, segmental artery, or subsegmental pulmonary artery). The treatment of pulmonary clots and any complications associated with treatment were also entered into the database.
Table 1. Patient Characteristics and Potential Risk Factors of Venous Thromboembolisma.
| Variable | Overall (N = 7880) | DVT only (n = 277) | PE (n = 40) | PT (n = 117) | None (n = 7446) |
|---|---|---|---|---|---|
| Age at admission, mean (SD), y | 29.1 (6.4) | 29.8 (6.2) | 27.7 (5.3) | 29.9 (6.4) | 29.1 (6.4) |
| Sex | |||||
| Male | 5859 (74.4) | 218 (78.7) | 28 (70.0) | 91 (77.8) | 5522 (74.2) |
| Female | 2021 (25.7) | 59 (21.3) | 12 (30.0) | 26 (22.4) | 1924 (25.8) |
| Head injury | 2341 (29.7) | 137 (49.5) | 10 (25.0) | 35 (29.9) | 2159 (29.0) |
| Spinal cord injury | 343 (4.4) | 30 (10.8) | 3 (7.5) | 11 (9.4) | 299 (4.0) |
| Shock on admission (SBP <90 mm Hg) | 543 (6.9) | 42 (15.2) | 11 (27.5) | 30 (25.6) | 460 (6.2) |
| Chest injury | 2487 (31.6) | 123 (44.4) | 22 (55.0) | 62 (53.0) | 2280 (30.6) |
| Abdominal injury | 1716 (21.8) | 95 (34.3) | 17 (42.5) | 46 (39.3) | 1558 (20.9) |
| Pelvic fracture | 1408 (17.9) | 68 (24.5) | 15 (37.5) | 31 (26.5) | 1294 (17.4) |
| Long bone fracture | 2521 (32.0) | 87 (31.4) | 16 (40.0) | 46 (39.3) | 2372 (31.9) |
| Venous injury | 462 (5.9) | 36 (13.0) | 9 (22.5) | 15 (12.8) | 402 (5.4) |
| Femoral venous catheter | 521 (6.6) | 71 (25.6) | 12 (30.0) | 26 (22.2) | 412 (5.5) |
| Major operative procedure (≥1 h) | 5815 (73.8) | 229 (82.7) | 38 (95.0) | 103 (88.0) | 5445 (73.1) |
| ≥4 d of ventilatory support | 1190 (15.1) | 137 (49.5) | 26 (65.0) | 45 (38.5) | 982 (13.2) |
| Tranexamic acid administration | 648 (8.2) | 48 (17.3) | 10 (25.0) | 11 (9.4) | 579 (7.8) |
| Coagulopathy (INR>1.5) | 224 (2.8) | 25 (9.0) | 5 (12.5) | 9 (7.7) | 185 (2.5) |
| History of VTE | 31 (0.4) | 0 (0.0) | 0 (0.0) | 2 (1.7) | 29 (0.4) |
| Current cancer | 26 (0.3) | 2 (0.7) | 0 (0.0) | 0 (0.0) | 24 (0.3) |
| Pregnancy or recently post partum (within last 3 mo) | 85 (1.1) | 1 (0.4) | 0 (0.0) | 1 (0.9) | 83 (1.1) |
| Hormone therapy | 163 (2.1) | 6 (2.2) | 2 (5.0) | 3 (2.6) | 152 (2.0) |
| Inflammatory bowel disease | 25 (0.3) | 1 (0.4) | 0 (0.0) | 1 (0.9) | 23 (0.3) |
| Heparin | |||||
| 5000 U 2 times daily | 137 (1.7) | 10 (3.6) | 3 (7.5) | 5 (4.3) | 119 (1.6) |
| 5000 U 3 times daily | 1142 (14.5) | 69 (24.9) | 10 (25.0) | 20 (17.1) | 1043 (14.0) |
| Enoxaparin | |||||
| 30 mg Twice daily | 3541 (44.9) | 76 (27.4) | 10 (25.0) | 46 (39.3) | 3409 (45.8) |
| 40 mg Once daily | 1235 (15.7) | 54 (19.5) | 3 (7.5) | 9 (7.7) | 1169 (15.7) |
| Prophylaxis | |||||
| Other | 93 (1.2) | 3 (1.1) | 0 (0.0) | 1 (0.9) | 89 (1.2) |
| No | 750 (9.5) | 22 (7.9) | 6 (15.0) | 15 (12.8) | 707 (9.5) |
Abbreviations: DVT, deep vein thrombosis; INR, international normalized ratio; PE, pulmonary emboli; PT, pulmonary thrombosis; SBP, systolic blood pressure; VTE, venous thromboembolism.
Data are presented as number (percentage) of patients unless otherwise indicated. No prophylaxis indicates not receiving prophylaxis when DVT, PT, or PE was detected.
For the secondary outcome of interest, DVT, detailed data points included the date of any duplex ultrasound examinations performed (or CT where DVT was noted), symptoms precipitating the examination (if not part of a surveillance program), the location of any clots detected, and their treatment (including complications associated with treatment). Because some of the centers conducted routine duplex examinations as part of a surveillance program, the number of studies at each of the 17 centers varied.13 Enrolled patients were followed up until discharge or for up to 30 days from admission.
The number of patients to be included in the study was based on an estimated 4% overall incidence of pulmonary clots. The number of years to complete the study was estimated at 2 given the admission rate of injured patients to these trauma centers in the allowable age range of 18 to 40 years.
Definitions
On the basis of previous work,1,2,3,4,11,12 the CLOTT group developed the following set of definitions for each of the variables of interest: shock: systolic blood pressure less than 90 mm Hg; major head injury: AIS score of 3 or higher; major chest injury: AIS score of 3 or higher; major abdominal injury: AIS score of 3 or higher; long bone fracture: lower-extremity fracture above the ankle; venous injury: a major named vein that required operative repair or ligation; major operation: posttrauma procedure that lasted at least 1 hour and required general anesthesia; and coagulopathy: international normalized ratio greater than 1.5 on admission. Of importance, for the purposes of this study, pulmonary clots seen in association with any DVT that developed within the 30 days were considered PE, whereas isolated clots without known DVT were considered de novo PT.
Data Analysis
For the purposes of this investigation, the patients were assigned to 1 of 4 categories: no VTE, DVT only, PE, and PT only. Risk factor stratification was based on previous work12 that included 1602 episodes captured retrospectively from the American College of Surgeons National Trauma Data Bank in 2004. In that study (which did not separate pulmonary clots from DVT but rather focused on the conglomerate of VTE, which included DVT and PE and/or PT), Knudson et al12 identified 6 high-risk factors for the development of VTE (odds ratio [OR] of 2-3): age of 40 years or older, a pelvic fracture, lower-extremity fracture (above the ankle), shock on admission, spinal cord injury, and major head injury. Characteristics associated with a very high risk of VTE (OR for VTE of 4-10) were a major operative procedure, the presence of a major venous injury, the need for mechanical ventilatory support for more than 3 days, and the presence of 2 or more of the high-risk factors.
Statistical Analysis
Tabulations or summary statistics of potential risk factors for VTE were described by each outcome category (DVT only, PE, PT only, or none). Each candidate risk factor was included in unadjusted multinomial logistic regression models, with the none category as the reference group for the outcome. Candidate variables that had associations with P < .10 from the unadjusted analyses were included in multivariable binary logistic regression models for each outcome category, using the reference group none, and covariates not attaining significance at the P < .05 level were sequentially eliminated until all covariates were significantly associated with each outcome category. High-risk and very high-risk factors were defined as listed above.
All primary analysis variables had complete data for the 7880 study participants. Multicollinearity issues were addressed if any variable had a correlation coefficient of 0.6 or higher or a variance inflation factor of 2 or higher. Hypothesis tests were 2-sided and the significance threshold was set to P < .05. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc). A secondary analysis to identify factors associated with the presence of PT on the admitting CT was performed using Fisher exact tests.
Results
During the 2-year period, 7880 patients (mean [SD] age, 29.1 [6.4] years; 5859 [74.4%] male) were enrolled in this study (Table 1). Data on race and ethnicity as well as insurance status were collected because such data are generally required on any federal grant to ensure lack of bias in enrollment. However, because these categories do not have any known association with VTE, we did not analyze these data. A total of 277 patients (3.5%) were diagnosed with DVT only during their hospitalization for trauma, 40 (0.5%) with both DVT and pulmonary clot (presumed PE), and 117 (1.5%) with pulmonary clot only (presumed PT). Shock on admission was present in only 460 patients (6.2%) who had no DVT, PT, or PE but was documented in 11 (27.5%) of those with PE and 30 (25.6%) in those with PT (Table 1). Compared with those with no pulmonary or deep venous clots (n = 7446), those with DVT (n = 277), PE (n = 40), or PT (n = 117) were more significantly injured, with higher rates of major chest injury (123 [44.4%] in the DVT group, 22 [55.0%] in the PE group, and 62 [53.0%] in the PT group vs 2280 [30.6%] in those with no pulmonary or deep venous clots), abdominal injury (95 [34.3%] in the DVT group, 17 [42.5%] in the PE group, and 46 [39.3%] in the PT group vs 1558 [20.9%] in those with no pulmonary or deep venous clots), pelvic fracture (68 [24.5%] in the DVT group, 15 [37.5%] in the PE group, and 31 [26.5%] in the PT group vs 1294 [17.4%] in those with no pulmonary or deep venous clots), long bone fracture (87 [31.4%] in the DVT group, 16 [40.0%] in the PE group, and 46 [39.3%] in the PT group vs 2372 [31.9%] in those with no pulmonary or deep venous clots), and venous injury (36 [13.0%] in the DVT group, 9 [22.5%] in the PE group, and 15 [12.8%] in the PT group vs 402 [5.4%] in those with no pulmonary or deep venous clots). Those who developed venous or pulmonary clots after injury were also more likely to require ventilatory support for 4 days or longer (1190 [15.1%] vs 982 [13.2%]). Tranexamic acid was administered to 648 patients and 48 developed DVT, 10 PE, and 11 PT.
Preexisting risk factors for VTE (prior history of VTE, current cancer, pregnancy, hormone therapy, or inflammatory bowel disease) were rare among these relatively young patients. Various chemical VTE prophylactic measures were used during the study, but 8 of the 40 patients with PE (20.0%) and 28 of the 117 patients with PT (23.9%) had clots visible on the admitting CTs before prophylaxis could be initiated (Table 1; eTables 1 and 2 in the Supplement).
A number of variables appeared to be significantly associated with the diagnoses of DVT alone, PE, and PT in the unadjusted regression model (Table 2). For each year increase in patient age older than 18 years, the odds of DVT vs none increased by 1.8% (P = .06), and the odds of PT only vs none increased by 2.1% (P = .16). Of interest, all 9 high-risk and very high-risk factors that had previously been identified were still significant in this prospective study as well.12
Table 2. Results of the Unadjusted Multinomial Logistic Regression Modelsa.
| Variable | DVT only vs none (n = 277) | PE vs none (n = 40) | PT vs none (n = 117) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at admission | 1.02 (1.00-1.04) | .06 | 0.97 (0.92-1.02) | .19 | 1.02 (0.99-1.05) | .16 |
| Sex (female vs male) | 0.78 (0.58-1.04) | .09 | 1.23 (0.62-2.42) | .55 | 0.83 (0.53-1.29) | .40 |
| Head injury | 2.40 (1.88-3.05) | <.001 | 0.82 (0.40-1.67) | .58 | 1.05 (0.70-1.56) | .83 |
| Spinal cord injury | 2.90 (1.95-4.31) | <.001 | 1.94 (0.59-6.32) | .27 | 2.48 (1.32-4.66) | .005 |
| Shock on admission (SBP <90 mm Hg) | 2.71 (1.93-3.82) | <.001 | 5.76 (2.86-11.6) | <.001 | 5.24 (3.42-8.01) | <.001 |
| Chest injury | 1.81 (1.42-2.31) | <.001 | 2.77 (1.48-5.17) | .001 | 2.55 (1.77-3.68) | <.001 |
| Abdominal injury | 1.97 (1.53-2.54) | <.001 | 2.79 (1.49-5.24) | .001 | 2.45 (1.68-3.56) | <.001 |
| Pelvic fracture | 1.55 (1.17-2.05) | .002 | 2.85 (1.50-5.43) | .001 | 1.71 (1.13-2.60) | .01 |
| Long bone fracture | 0.98 (0.76-1.27) | .88 | 1.43 (0.76-2.69) | .27 | 1.39 (0.95-2.02) | .09 |
| Venous injury | 2.62 (1.82-3.77) | <.001 | 5.09 (2.41-10.8) | <.001 | 2.58 (1.48-4.47) | <.001 |
| Femoral venous catheter | 5.88 (4.41-7.84) | <.001 | 7.32 (3.69-14.5) | <.001 | 4.88 (3.12-7.63) | <.001 |
| Major operative procedure (≥1 h) | 1.75 (1.28-2.40) | <.001 | 6.98 (1.68-28.9) | .007 | 2.70 (1.54-4.74) | <.001 |
| ≥4 d of ventilatory support | 6.44 (5.04-8.23) | <.001 | 12.2 (6.36-23.5) | <.001 | 4.11 (2.82-6.01) | <.001 |
| Tranexamic administration | 2.49 (1.80-3.43) | <.001 | 3.96 (1.92-8.13) | <.001 | 1.23 (0.66-2.30) | .52 |
| Coagulopathy (INR >1.5) | 3.89 (2.52-6.02) | <.001 | 5.61 (2.17-14.5) | <.001 | 3.27 (1.63-6.56) | <.001 |
| History of VTE | 0 | .15 | 0 | .58 | 4.45 (1.05-18.9) | .04 |
| Current cancer | 2.25 (0.53-9.56) | .27 | 0 | .61 | 0 | .39 |
| Pregnancy or recently post partum (within last 3 mo) | 0.32 (0.04-2.32) | .26 | 0 | .34 | 0.76 (0.11-5.54) | .79 |
| Hormone therapy | 1.06 (0.47-2.42) | .89 | 2.53 (0.60-10.6) | .20 | 1.26 (0.40-4.02) | .69 |
| Inflammatory bowel disease | 1.17 (0.16-8.69) | .88 | 0 | .62 | 2.78 (0.37-20.8) | .32 |
| Heparin | ||||||
| 5000 U 2 times daily | 2.31 (1.20-4.45) | .01 | 4.99 (1.52-16.4) | .008 | 2.75 (1.10-6.86) | .03 |
| 5000 U 3 times daily | 2.04 (1.54-2.69) | <.001 | 2.05 (1.00-4.20) | .05 | 1.27 (0.78-2.06) | .34 |
| Enoxaparin | ||||||
| 30 mg Twice daily | 0.45 (0.34-0.58) | <.001 | 0.39 (0.19-0.81) | .01 | 0.77 (0.53-1.11) | .16 |
| 40 mg Once daily | 1.30 (0.96-1.76) | .09 | 0.44 (0.13-1.41) | .17 | 0.45 (0.23-0.89) | .02 |
| Prophylaxis | ||||||
| Other | 0.91 (0.28-2.88) | .87 | 0 | .33 | 0.71 (0.10-5.16) | .74 |
| No | 0.82 (0.53-1.28) | .39 | 1.68 (0.70-4.02) | .24 | 1.40 (0.81-2.42) | .23 |
Abbreviations: DVT, deep vein thrombosis; INR, international normalized ratio; OR, odds ratio; PE, pulmonary emboli; PT, pulmonary thrombosis; SBP, systolic blood pressure; VTE, venous thromboembolism.
For associations with P < .10, these variables entered the backward elimination selection process for multivariable logistic regression model development. Likelihood ratio test P value is reported if there were 0 events in a variable category.
Table 3 contains the results of the multivariable logistic regression analysis. In addition to advancing age (OR, 1.02; 95% CI, 1.00-1.04), injury variables associated with an increased risk of DVT included the presence of major injury to the head (OR, 1.41; 95% CI, 1.05-1.89), spinal cord injury (OR, 1.74; 95% CI, 1.14-2.67), abdominal injury (OR, 1.60; 95% CI, 1.20-2.12), pelvic fracture (OR, 1.36; 95% CI, 1.01-1.84), and a major venous injury (OR, 1.64; 95% CI, 1.09-2.48). Placement of a femoral vein catheter for resuscitation (OR, 2.25; 95% CI, 1.61-3.14), the need for major surgery (OR, 1.56; 95% CI, 1.12-2.21), the need for prolonged ventilatory support (OR, 3.68; 95% CI, 2.72-4.98), and administration of tranexamic acid (OR, 1.65; 95% CI, 1.16-2.35) were all significantly associated with the development of DVT. Risk factors independently associated with the development of PT but not DVT or PE include shock on admission (OR, 2.74; 95% CI, 1.72-4.39), major chest injury (OR, 1.72; 95% CI, 1.16-2.56), and a history of VTE (OR, 5.39; 95% CI, 1.19-24.4). Of the 40 patients with PE, 25 patients (62.5%) developed PE during the first week after injury and 23 (57.0%) had their DVT detected during that first week as well(eTable 2 in the Supplement).
Table 3. Results of the Multivariable Binary Logistic Regression Models.
| Variable | DVT only vs none (n = 277) | PE vs none (n = 40) | PT vs none (n = 117) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at admission | 1.02 (1.00-1.04) | .048 | NA | NA | NA | NA |
| Head injury | 1.41 (1.05-1.89) | .02 | NA | NA | NA | NA |
| Spinal cord injury | 1.74 (1.14-2.67) | .01 | NA | NA | NA | NA |
| Shock on admission (SBP <90 mm Hg) | NA | NA | NA | NA | 2.74 (1.72-4.39) | <.001 |
| Chest injury | NA | NA | NA | NA | 1.72 (1.16-2.56) | .007 |
| Abdominal injury | 1.60 (1.20-2.12) | .001 | NA | NA | 1.58 (1.06-2.36) | .03 |
| Pelvic fracture | 1.36 (1.01-1.84) | .046 | 2.52 (1.31-4.86) | .006 | NA | NA |
| Long bone fracture | NA | NA | NA | NA | ||
| Venous injury | 1.64 (1.09-2.48) | .02 | 3.49 (1.60-7.62) | .002 | NA | NA |
| Femoral venous catheter | 2.25 (1.61-3.14) | <.001 | NA | NA | 2.19 (1.32-3.62) | .002 |
| Major operative procedure (≥1 h) | 1.56 (1.12-2.21) | .009 | NA | NA | 2.09 (1.18-3.71) | .01 |
| ≥4 d of ventilatory support | 3.68 (2.72-4.98) | <.001 | 10.1 (5.20-19.7) | <.001 | 2.21 (1.44-3.39) | <.001 |
| Tranexamic acid administration | 1.65 (1.16-2.35) | .005 | 2.48 (1.17-5.26) | .02 | NA | NA |
| Coagulopathy (INR >1.5) | NA | NA | NA | NA | NA | NA |
| History of VTE | NA | NA | NA | NA | 5.39 (1.19-24.4) | .03 |
| Heparin | ||||||
| 5000 U 2 times daily | NA | NA | NA | NA | 3.26 (1.27-8.35) | .01 |
| 5000 U 3 times per day | NA | NA | NA | NA | NA | NA |
| Enoxaparin | ||||||
| 30 mg Twice daily | 0.52 (0.39-0.70) | <.001 | NA | NA | NA | NA |
| 40 mg Once daily | 1.55 (1.10-2.18) | .01 | NA | NA | NA | NA |
Abbreviations: DVT, deep vein thrombosis; INR, international normalized ratio; NA, not applicable; OR, odds ratio; PE, pulmonary emboli; PT, pulmonary thrombosis; SBP, systolic blood pressure; VTE, venous thromboembolism.
On further examination of patients with PT only (n = 117), 61 (52.1%) had clots on the same side as a chest injury. Of these patients, 28 (23.9%) had clot evident on the admitting chest CT, and an additional 55 scans (47.0%) were positive for PT between days 1 and 7. Of the 89 patients who developed delayed PT, 84 (94.3%) had a prior CT or comparison. Eighty-eight patients (75.2%) with PT in this study were treated with full dose anticoagulation, 14 patients (11.9%) received an inferior vena cava filter, and 15 patients (12.8%) with PT were simply observed. Eight clots were in the main pulmonary artery, 26 were lobar, 28 were segmental, 21 were subsegmental, and 34 were multiple (eTable 1 in the Supplement). Factors associated with the presence of PT on admission included major chest injury (14 patients [50.0%] with or without major chest injury with an Abbreviated Injury Score >3; P = .04) and major venous injury (23 [82.1%] without major venous injury and 5 [17.9%] with major venous injury; P = .02) (Table 4).
Table 4. Presence or Absence of Pulmonary Thrombosis on Admission in Patients Who Also Had VTE Risk Factors.
| Variable | Overall (N = 7880) | Outcome | P value | |
|---|---|---|---|---|
| No PT at admission (n = 7852) | PT at admission (n = 28) | |||
| Shock (SBP <90 mm Hg) | ||||
| Absent | 7338 (93.1) | 7314 (93.1) | 24 (85.7) | .12 |
| Present | 543 (6.9) | 539 (6.9) | 4 (14.3) | |
| Coagulopathy (INR >1.5) | ||||
| Absent | 7656 (97.1) | 7629 (97.1) | 27 (96.4) | .56 |
| Present | 225 (2.9) | 224 (2.9) | 1 (3.6) | |
| Chest injury (AIS score >3) | ||||
| Absent | 5393 (68.4) | 5379 (68.5) | 14 (50.0) | .04 |
| Present | 2488 (31.6) | 2474 (31.5) | 14 (50.0) | |
| Head injury (AIS score >3) | ||||
| Absent | 5540 (70.3) | 5523 (70.3) | 17 (60.7) | .30 |
| Present | 2341 (29.7) | 2330 (29.7) | 11 (39.3) | |
| Abdominal injury (AIS score >3) | ||||
| Absent | 6165 (78.2) | 6146 (78.3) | 19 (67.9) | .18 |
| Present | 1716 (21.8) | 1707 (21.7) | 9 (32.1) | |
| Spinal cord injury | ||||
| Absent | 7538 (95.6) | 7512 (95.7) | 26 (92.9) | .35 |
| Present | 343 (4.4) | 341 (4.3) | 2 (7.1) | |
| Pelvic fracture | ||||
| Absent | 6273 (82.1) | 6453 (82.2) | 20 (71.4) | .14 |
| Present | 1408 (17.9) | 1400 (17.8) | 8 (28.6) | |
| Long bone fracture | ||||
| Absent | 5360 (68) | 5344 (68.1) | 16 (57.1) | .23 |
| Present | 2521 (32.0) | 2509 (31.9) | 12 (42.9) | |
| Venous injury | ||||
| Absent | 7419 (94.1) | 7396 (94.2) | 23 (82.1) | .02 |
| Present | 462 (5.9) | 457 (5.8) | 5 (17.9) | |
| Major operative procedure (≥1 h) | ||||
| Absent | 2065 (26.2) | 2061 (26.2) | 4 (14.3) | .20 |
| Present | 5816 (73.8) | 5792 (73.8) | 24 (85.7) | |
| >4 d of ventilatory support | ||||
| Absent | 6690 (84.9) | 6674 (85) | 16 (57.1) | <.001 |
| Present | 1190 (15.1) | 1190 (15.0) | 12 (42.9) | |
Abbreviations: AIS, abbreviated injury score; INR, international normalized ratio; PT, pulmonary thrombosis; SBP, systolic blood pressure.
Discussion
The most important finding in this cohort study is that major chest trauma and shock significantly increase the risk of developing PT but not DVT or PE, confirming our hypothesis that PT represents a distinct clinical entity. Chest trauma likely results in inflammation manifested by pulmonary clots that appear to develop early after injury. Severely injured patients who present in shock rapidly develop a hypercoagulable state, which likely contributes to the risk of PT.14,15,16,17,18 Previous studies19,20,21 suggest that fibrinolytic shutdown occurs frequently after severe injury, preventing clot lysis and contributing to the hypercoagulable state. The association between fibrinolytic shutdown and posttraumatic DVT, PE, and PT is the subject of an ongoing study by CLOTT investigators.
The association between trauma and pulmonary clots was established almost a century ago based primarily on autopsy studies of patients with fractures.22 The past 20 years of military operations in Afghanistan and Iraq has precipitated a renewed interest in VTE because rates of pulmonary clots among those who have been injured in combat are reported as high as 9%.8,23,24 In both civilian and military medical communities, the traditionally held dogma that DVT leads to pulmonary embolism has been recently challenged.2,3,4,5,6,7,8,9,10 Perhaps the most compelling evidence to support the development of de novo pulmonary clots was published by Velmahos et al,4 who performed computed tomographic venography and computed tomographic pulmonary angiography simultaneously in 247 patients with trauma, finding that 19% had pulmonary clots but only 7% had DVT.
Five of the independent risk factors for DVT identified in this large prospective study (age, major head injury, pelvic fractures, major venous injury, and femoral vein catheters) have been previously reported and are not discussed further in this article.1,2,12,14,25,26,27 The association between PT on admission and the presence of a major venous injury deserves further study. It is potentially possible that these patients had a clot that embolized before the admitting chest CT. A more likely hypothesis is that the venous injury caused shock and an early hypercoagulable state precipitating the PT. The use of the antifibrinolytic agent tranexamic acid after major trauma has increased since the publication of the CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2)28 and MATTERs (Military Application of Tranexamic Acid in Trauma Emergency Resuscitation)29,30,31,32,33 studies, but its association with VTE is hotly debated. In the current study, tranexamic acid administration was associated with increases in the risk of development of DVT by 1.65 times and of PE by 2.48 times (Table 3), but the true risk will require elucidation of the timing and dosing of tranexamic acid. Although the best prophylactic regimen to prevent VTE after trauma continues to be debated, our preliminary results suggest that the 2 doses of enoxaparin used in this study may not offer equivalent protection against DVT, whereas all 4 prophylactic regimens appeared to be effective in preventing PE (Table 3).34,35,36,37,38,39,40,41,42 However, the agent and dose analyzed for the analysis were the combination that the patient was receiving at the time DVT, PE, or PT was diagnosed. We did not account for the timing of initiation of prophylaxis or missed doses. In addition, it might well be that different prophylactic regimens are more effective for certain types of injuries but not for others.
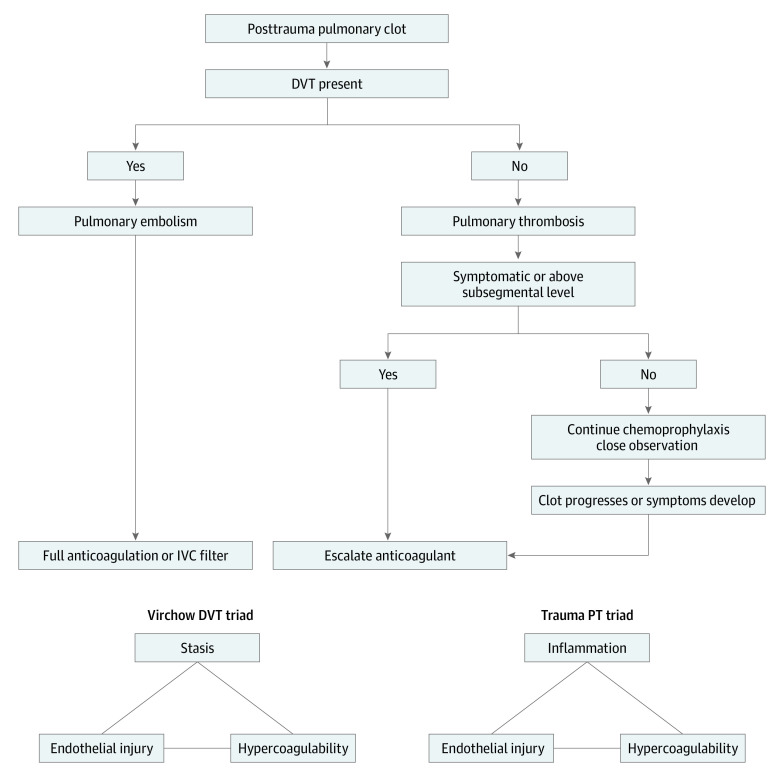
Finally, based on our evolving understanding of the pathogenesis of PT, we recommend consideration of observation of posttraumatic, subsegmental PT that are discovered incidentally and not associated with proximal DVT, a position supported in the recent American College of Chest Physicians guidelines.43 This recommendation may be particularly relevant to patients whose clots are detected on admission or early in their hospital course or in those who are at risk for hemorrhagic complications with anticoagulation. However, the safety of this approach deserves further study. The Figure outlines a treatment approach to consider for posttraumatic PT and PE.
Figure. Algorithm for the Treatment of Posttraumatic Pulmonary Thromboembolism (PT) and Proposed New Triad for Posttraumatic PT.
DVT indicates deep venous thrombosis; IVC, inferior vena cava.
Limitations
This study has limitations, including the relatively young age of the patients included, which will affect its applicability in older patients with trauma. In addition, because this was an observational study, the use of prophylactic measures, DVT screening procedures, and the treatment of any clots discovered varied considerably across the 17 centers. Most importantly, we assumed that pulmonary clots not associated with DVT are not embolic, but only 64.2% of patients with DVT and pulmonary clot had a duplex DVT scan close to their CT with positive findings in this study. It is possible that DVT that developed before the PE was simply missed. In addition, most venous duplex examinations are limited in the area of the pelvis and will not detect clots in major abdominal veins, which may also be the source of emboli. On the other hand, the presence of both DVT and pulmonary clot in the same patient during hospitalization does not prove that the pulmonary clot is embolic. The presence of PT on the admitting CT in one-quarter of the patients with PT in our study lends support to the premise that many pulmonary clots are de novo PT and not PE. Despite these limitations, this is, to our knowledge, the largest prospective study to date focused on posttraumatic PT.
Conclusions
This cohort study found that not all pulmonary clots seen on computed tomography of the chest after injury are embolic but instead represent a combination of PE and PT. On the basis of this study’s findings, PT has independent risk factors different than those traditionally associated with VTE. With a focus on the original Virchow DVT triad, this study offers a new PT triad that can guide future research into the role of injury, inflammation, and a hypercoagulable state as factors associated with the development of posttraumatic PT.
eTable 1. Summary of Timing, Symptoms, Location and Treatment of Isolated Pulmonary Thrombosis in 117 Patients
eTable 2. Timing of PE and DVT Detection in Patients With Pulmonary Embolism and Indications for CT Scans
References
- 1.Rogers FB. Venous thromboembolism in trauma patients: a review. Surgery. 2001;130(1):1-12. doi: 10.1067/msy.2001.114558 [DOI] [PubMed] [Google Scholar]
- 2.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254(4):625-632. doi: 10.1097/SLA.0b013e3182300209 [DOI] [PubMed] [Google Scholar]
- 3.Brakenridge SC, Henley SS, Kashner TM, et al. ; Inflammation and the Host Response to Injury Investigators . Comparing clinical predictors of deep venous thrombosis versus pulmonary embolus after severe injury: a new paradigm for posttraumatic venous thromboembolism? J Trauma Acute Care Surg. 2013;74(5):1231-1237. doi: 10.1097/TA.0b013e31828cc9a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144(10):928-932. doi: 10.1001/archsurg.2009.97 [DOI] [PubMed] [Google Scholar]
- 5.Van Gent JM, Zander AL, Olson EJ, et al. Pulmonary embolism without deep venous thrombosis: de novo or missed deep venous thrombosis? J Trauma Acute Care Surg. 2014;76(5):1270-1274. doi: 10.1097/TA.0000000000000233 [DOI] [PubMed] [Google Scholar]
- 6.Tadlock MD, Konstantinos C, Kennedy M, et al. The origin of fatal pulmonary emboli: a postmortem analysis of 500 death from pulmonary embolism in trauma. Am J Surg. 2015;209(6):959-968. doi: 10.1016/j.amjsurg.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 7.Schutzman LM, Rigor RR, Khosravi N, Galante JM, Brown IE. P-selectin is critical for de novo pulmonary artery thrombosis following blunt thoracic trauma. J Trauma Acute Care Surg. 2019;86(4):583-591. doi: 10.1097/TA.0000000000002166 [DOI] [PubMed] [Google Scholar]
- 8.Lundy JB, Oh JS, Chung KK, et al. Frequency and relevance of acute peritraumatic pulmonary thrombus diagnosed by computed tomographic imaging in combat casualties. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S215-S220. doi: 10.1097/TA.0b013e318299da66 [DOI] [PubMed] [Google Scholar]
- 9.Schultz DJ, Brasel KJ, Washington L, et al. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J Trauma. 2004;56(4):727-731. doi: 10.1097/01.TA.0000119687.23542.EC [DOI] [PubMed] [Google Scholar]
- 10.Menaker J, Stein DM, Scalea TM. Incidence of early pulmonary embolism after injury. J Trauma. 2007;63(3):620-624. doi: 10.1097/TA.0b013e31812f60aa [DOI] [PubMed] [Google Scholar]
- 11.Bandle J, Shackford SR, Sise CB, Knudson MM; CLOTT Study Group . Variability is the standard: the management of venous thromboembolic disease following trauma. J Trauma Acute Care Surg. 2014;76(1):213-216. doi: 10.1097/TA.0b013e3182aa2fa9 [DOI] [PubMed] [Google Scholar]
- 12.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3):490-496. doi: 10.1097/01.sla.0000137138.40116.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shackford SR, Cipolle MD, Badiee J, et al. Determining the magnitude of surveillance bias in the assessment of lower extremity deep venous thrombosis: a prospective observational study of two centers. J Trauma Acute Care Surg. 2016;80(5):734-739. doi: 10.1097/TA.0000000000001009 [DOI] [PubMed] [Google Scholar]
- 14.Sumislawski JJ, Kornblith LZ, Conroy AS, Callcut RA, Cohen MJ. Dynamic coagulability after injury: is delaying chemoprophylaxis worth the wait? J Trauma Acute Care Surg. 2018;85(5):907-914. doi: 10.1097/TA.0000000000002048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meissner MH, Chandler WL, Elliott JS. Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability? J Trauma. 2003;54(2):224-231. doi: 10.1097/01.TA.0000046253.33495.70 [DOI] [PubMed] [Google Scholar]
- 16.McCully BH, Connelly CR, Fair KA, et al. ; PROPPR Study Group . Onset of coagulation function recovery is delayed in severely injured trauma patients with venous thromboembolism. J Am Coll Surg. 2017;225(1):42-51. doi: 10.1016/j.jamcollsurg.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harr JN, Moore EE, Chin TL, et al. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74(3):756-762. doi: 10.1097/TA.0b013e3182826d7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76(2):255-256. doi: 10.1097/TA.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Liras IN, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222(4):347-355. doi: 10.1016/j.jamcollsurg.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured patients. J Am Coll Surg. 2017;224(4):575-582. doi: 10.1016/j.jamcollsurg.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 21.Harr JN, Moore EE, Chin TL, et al. Postinjury hyperfibrinogenemia compromises efficacy of heparin-based venous thromboembolism prophylaxis. Shock. 2014;41(1):33-39. doi: 10.1097/SHK.0000000000000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coon WW. Risk factors in pulmonary embolism. Surg Gynecol Obstet. 1976;143(3):385-390. [PubMed] [Google Scholar]
- 23.Hutchison TN, Krueger CA, Berry JS, Aden JK, Cohn SM, White CE. Venous thromboembolism during combat operations: a 10-y review. J Surg Res. 2014;187(2):625-630. doi: 10.1016/j.jss.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 24.Gillern SM, Sheppard FR, Evans KN, et al. Incidence of pulmonary embolus in combat casualties with extremity amputations and fractures. J Trauma. 2011;71(3):607-612. doi: 10.1097/TA.0b013e3182282574 [DOI] [PubMed] [Google Scholar]
- 25.Knudson MM, Collins JA, Goodman SB, McCrory DW. Thromboembolism following multiple trauma. J Trauma. 1992;32(1):2-11. doi: 10.1097/00005373-199201000-00002 [DOI] [PubMed] [Google Scholar]
- 26.Meredith JW, Young JS, O’Neil EA, Snow DC, Hansen KJ. Femoral catheters and deep venous thrombosis: a prospective evaluation with venous duplex sonography. J Trauma. 1993;35(2):187-190. doi: 10.1097/00005373-199308000-00003 [DOI] [PubMed] [Google Scholar]
- 27.Farrell MS, Knudson MM, Stein DM. Venous ligation versus repair: does the procedure impact the venous thromboembolism risk? Trauma Surg Acute Care Open. 2021;6:e000687. doi: 10.1136/tsaco-2021-000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. West Indian Med J. 2010;59(6):612-624. [PubMed] [Google Scholar]
- 29.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119. doi: 10.1001/archsurg.2011.287 [DOI] [PubMed] [Google Scholar]
- 30.Napolitano LM. Prehospital tranexamic acid: what is the current evidence? Trauma Surg Acute Care Open. 2017;2(1):e000056. doi: 10.1136/tsaco-2016-000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers SP, Kutcher ME, Rosengart MR, et al. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg. 2019;86(1):20-27. doi: 10.1097/TA.0000000000002061 [DOI] [PubMed] [Google Scholar]
- 32.Johnston LR, Rodriguez CJ, Elster EA, Bradley MJ. Evaluation of military use of tranexamic acid and associated thromboembolic events. JAMA Surg. 2018;153(2):169-175. doi: 10.1001/jamasurg.2017.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taeuber I, Weibel S, Herrmann E, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis and meta-regression. JAMA Surg. 2021;156(6):e210884. doi: 10.1001/jamasurg.2021.0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holley AB, Petteys S, Mitchell JD, Holley PR, Collen JF. Thromboprophylaxis and VTE rates in soldiers wounded in Operation Enduring Freedom and Operation Iraqi Freedom. Chest. 2013;144(3):966-973. doi: 10.1378/chest.12-2879 [DOI] [PubMed] [Google Scholar]
- 35.Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701-707. doi: 10.1056/NEJM199609053351003 [DOI] [PubMed] [Google Scholar]
- 36.Knudson MM, Morabito D, Paiement GD, Shackleford S. Use of low molecular weight heparin in preventing thromboembolism in trauma patients. J Trauma. 1996;41(3):446-459. doi: 10.1097/00005373-199609000-00010 [DOI] [PubMed] [Google Scholar]
- 37.Velmahos GC, Toutouzas KG, Brown C, Vassiliu P, Gkiokas G, Rhee P. Thromboprophylaxis does not protect severely injured patients against pulmonary embolism. Am Surg. 2004;70(10):893-896. [PubMed] [Google Scholar]
- 38.Louis SG, Van PY, Riha GM, et al. Thromboelastogram-guided enoxaparin dosing does not confer protection from deep venous thrombosis: a randomized controlled pilot trial. J Trauma Acute Care Surg. 2014;76(4):937-942. doi: 10.1097/TA.0000000000000165 [DOI] [PubMed] [Google Scholar]
- 39.Ko A, Harada MY, Barmparas G, et al. Association between enoxaparin dosage adjusted by anti–factor Xa through level and clinically evident venous thromboembolism after trauma. JAMA Surg. 2016;151(11):1006-1013. doi: 10.1001/jamasurg.2016.1662 [DOI] [PubMed] [Google Scholar]
- 40.Berndtson AE, Costantini TW, Lane J, Box K, Coimbra R. If some is good, more is better: an enoxaparin dosing strategy to improve pharmacologic venous thromboembolism prophylaxis. J Trauma Acute Care Surg. 2016;81(6):1095-1100. doi: 10.1097/TA.0000000000001142 [DOI] [PubMed] [Google Scholar]
- 41.Jacobs BN, Cain-Nielsen AH, Jakubus JL, et al. Unfractionated heparin versus low-molecular-weight heparin for venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg. 2017;83(1):151-158. doi: 10.1097/TA.0000000000001494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee TH, Alonzo BJ, Differding J, et al. The effects of location and low-molecular-weight heparin administration on deep vein thrombosis outcomes in trauma patients. J Trauma Acute Care Surg. 2013;74(2):476-481. doi: 10.1097/TA.0b013e31827c5f66 [DOI] [PubMed] [Google Scholar]
- 43.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of Timing, Symptoms, Location and Treatment of Isolated Pulmonary Thrombosis in 117 Patients
eTable 2. Timing of PE and DVT Detection in Patients With Pulmonary Embolism and Indications for CT Scans