Abstract
The tumor suppressor protein p53 limits mutagenesis in response to ultraviolet-B (UVB) light exposure by activating the transcription of genes that mitigate the damaging effects of UVB radiation on DNA. Because most nonmelanoma skin cancers (NMSCs) occur in older individuals, it is important to understand the process of mutagenesis in the geriatric skin microenvironment. Based on previous studies demonstrating that geriatric skin expresses lower levels of the growth factor insulin-like growth factor-1 (IGF-1) than young adult skin, a role for IGF-1 in the regulation of p53 target genes was investigated in both human keratinocytes in vitro and human skin explants ex vivo. The products of the p53 target genes p21 and DNA polymerase eta (pol η) were found to be increased by UVB exposure in both experimental systems, and this induction was observed to be partially abrogated by depriving keratinocytes of IGF-1 in vitro or by the treatment of keratinocytes in vitro and human skin explants with an IGF-1 receptor antagonist. Because p21 and pol η function to limit mutagenic DNA replication following UVB exposure, these results suggest that NMSC risk in geriatric populations may be due to age-dependent decreases in IGF-1 signaling that disrupt p53 function in the skin.
INTRODUCTION
Nonmelanoma skin cancers (NMSCs) are growing in incidence and are primarily caused by exposures to ultraviolet-B (UVB) wavelengths of sunlight that induce photoproducts in epidermal keratinocyte genomic DNA. If not efficiently removed by the nucleotide excision repair (NER) system (1), UVB photoproducts may lead to mutagenesis and eventual carcinogenesis. However, the fact that the majority of NMSCs are found in people over the age of 60 (2) indicates that advanced age is a second risk factor for skin cancer development. Though there are likely multiple factors that contribute to the risk of skin carcinogenesis in older individuals, previous work has demonstrated a strong correlation between increased dermal fibroblast senescence, decreased expression of insulin-like growth factor-1 (IGF-1) and altered keratinocyte responses to UVB radiation (3-5).
Within the skin microenvironment in humans, IGF-1 is thought to be primarily supplied by fibroblasts in the dermal layer of the skin (6,7). In geriatric and photoaged skin, a decreased synthesis of IGF-1 was found to be associated with an increased number of senescent fibroblasts (8). Moreover, fibroblasts induced to undergo replicative or stress-induced senescence in vitro exhibit decreased IGF-1 production (5,9). Though human epidermal keratinocytes express IGF-1 receptors (IGF-1Rs) (6,7), they do not synthesize IGF-1 and thus are dependent on the production of this important growth factor by dermal fibroblasts in the skin.
Several lines of evidence indicate that IGF-1/IGF-1R signaling impacts how epidermal keratinocytes respond to UVB radiation (3). Early work demonstrated that the lack of activated IGF-1Rs in cultured keratinocytes prevented the cells from undergoing senescence after UVB exposure (10) and to instead undergo apoptosis (5,11). More recent studies have demonstrated that IGF-1-deprived cultured keratinocytes show a slower rate of UVB photoproduct removal from the genome (12) and fail to properly activate ATR-CHK1 kinase signaling (13,14), which normally functions to suppress DNA synthesis after DNA damage (15-17). De-regulation of some of these DNA damage response systems by IGF-1R inactivation has also been found in human skin explants ex vivo (12,13) and in human skin xenografted onto immunodeficient mice in vivo (12). Furthermore, experimental studies with young adult and geriatric human subjects have demonstrated that skin from older individuals shows a reduced rate of UVB photoproduct removal, both throughout the total epidermis (18) and specifically within proliferating (Ki67-positive) basal keratinocytes (5). Additional studies showed that the injection of IGF-1 into geriatric skin (5) and the use of skin rejuvenation approaches that increase dermal IGF-1 expression (8,19,20) reduce the number of proliferating basal keratinocytes with unrepaired UVB photoproducts. Thus, together these data argue for an important role for IGF-1/IGF-1R signaling in the response of epidermal keratinocytes to UVB radiation.
The tumor suppressor protein p53 plays an integral role in all aspects of the DNA damage response via its ability to transactivate the expression of various pro-survival and pro-apoptotic target genes (21-23). Transcriptional targets of p53 include genes involved in the removal of UVB photoproducts by nucleotide excision repair (XPC, DDB2) (24-26), the arrest of the cell cycle (p21) (27) and the replication of UVB photoproducts by the translesion synthesis (TLS) DNA polymerase pol eta (pol η) (28,29) (Fig. 1a). Though a previous study provided evidence that the activation of p53 and the expression of its canonical substrate p21 were altered in cultured keratinocytes with inactive IGF-1Rs after exposure to high doses of UVB radiation (10), little is known about how IGF-1 impacts other transcriptional targets of p53 and at lower, less toxic and potentially more environmentally relevant doses of UVB radiation. Moreover, the experimental data on p53 function in cultured keratinocytes in vitro needs to be examined in the context of human skin epidermis to better understand the likelihood that deregulation of p53 function contributes to mutagenesis and NMSC initiation in IGF-1-deficient skin tissue. Here, using cultured keratinocytes in vitro and human skin explants ex vivo, IGF-1 signaling is shown to be critical for the induction of both the cell cycle regulator protein p21 and the TLS DNA polymerase pol eta (pol η). Pol η is capable of accurately synthesizing DNA across UVB-induced thymine dimers (30), and mutations in pol η are responsible for the variant form of the skin cancer-prone disease xeroderma pigmentosum (31-33). Thus, these results show that IGF-1 deficiency interferes with activation of the p53 target genes p21 and pol η, which prevent mutagenic DNA synthesis after UVB exposure. This increased mutagenic burden in IGF-1-deficient geriatric skin may partially explain the increased frequency of NMSCs in older individuals.
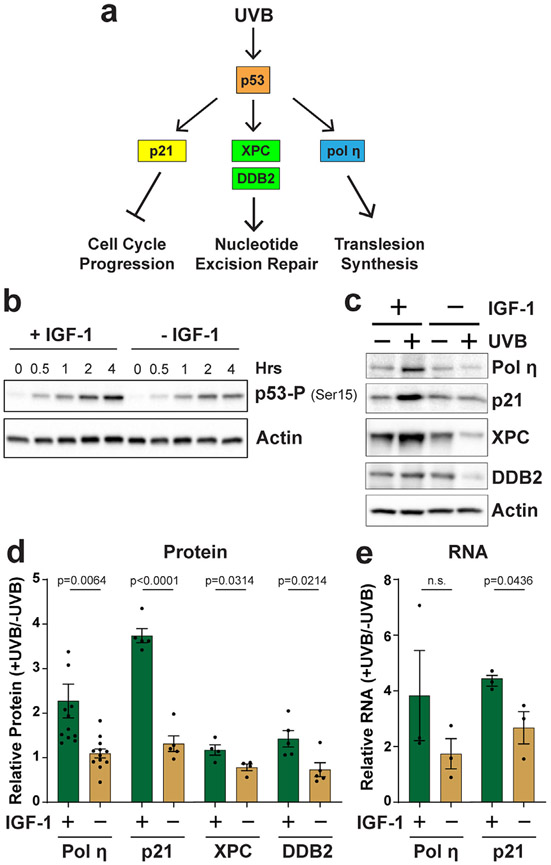
Figure 1.
IGF-1 is required for the induction of p53 target genes in UVB-irradiated human keratinocytes in vitro. (a) In response to UVB radiation, p53 transactivates the expression of genes involved in slowing cell cycle progression, nucleotide excision repair and translesion synthesis across UVB-induced thymine dimers. (b) p53 phosphorylation on Ser15 was monitored by protein immunoblotting at the indicated time points after exposure to 100 J m−2 in N-TERT cells grown in the presence (+ IGF-1) or absence (− IGF-1) of IGF-1. (c) Cells were treated as in (b) except that cells were harvested 24 h after UVB to examine protein induction. (d) Quantitation (average and SEM) of relative protein levels (+UVB/−UVB) from several independent experiments (n = 4–11, as indicated) performed as in (b). (e) Quantitative reverse transcription PCR analysis of pol η and p21 RNA levels 12 h after UVB exposure in cells treated as in (b) (n = 3). Unpaired, two-tailed t-tests were performed to determine whether the difference in relative gene product levels (+UVB/−UVB) was significant between the two treatment groups, and the p-values are indicated (n.s., not significant).
MATERIALS AND METHODS
Cell culture.
Telomerase-immortalized human neonatal foreskin keratinocytes (N-TERTs) (34) were cultured in EpiLife medium containing human keratinocyte growth supplement (HKGS) (Thermo Fisher Scientific) and penicillin/streptomycin. Cells were maintained in a 5% CO2 humidified incubator at 37°C and monitored periodically for mycoplasma contamination (Sigma Venor GeM Kit). For experiments involving IGF-1 deprivation, the IGF-1 component of the growth supplement was omitted from the culture medium for 24 h before UVB exposure with a Philips F20T12 broadband UVB bulb at a dose rate of 5 J m−2 s−1. Unless otherwise indicated, N-TERTs were exposed to a UVB dose of 100 J m−2. Cells were treated with DMSO, the IGF-1R antagonist AG538 (Sigma; 10 μm) or the p53 inhibitor pifithrin-α (Sigma; 20 μm) for 30 min prior to UVB exposure. With the exception of the cell survival assays, cells were incubated with the indicated culture medium throughout the experiments. To harvest cells for RNA and protein analyses, cells were scraped from the plate into cold PBS, pelleted and stored at −80°C until ready for analysis.
Skin explants.
De-identified human skin discarded during panniculectomy procedures was cut into small pieces and placed into 6 cm round cell culture dishes containing a small volume of basal EpiLife medium. The skin sections were treated topically with either DMSO or 20 μm AG538 for 30 min before exposure to 800 J m−2 of UVB radiation. The skin sections were then incubated for 24 h in a 37°C water bath, after which the epidermis was removed by heat shock and a curette, snap frozen in liquid nitrogen and stored at −80°C. For protein analyses, 6 mm punch biopsies were obtained from the treated skin, snap frozen in liquid nitrogen, and then epidermis was separated by heat shock. For experiments examining IGF-1 expression in discarded skin samples from donors of different ages, punch biopsies were stored in RNAlater solution at −20°C until sample processing to examine IGF-1 levels in the dermal fraction of the skin biopsy.
Protein immunoblotting.
Cells and epidermal tissues were resuspended in ice-cold RIPA buffer, disrupted by sonication and vortexing, and then, soluble lysates were obtained by high-speed centrifugation. Lysates were separated by SDS-PAGE, transferred to a nitrocellulose membrane and then stained with Ponceau S to ensure equal protein loading. Following washing with TBST (Tris-buffered saline containing 0.1% Tween-20) and blocking in 5% milk in TBST, blots were probed with 1:2000 or 1:5000 dilutions of antibodies against actin (Bethyl A300-485), pol η (Santa Cruz sc-17770 or Bethyl A301-231), XPC (Santa Cruz sc-74410), p21 (Santa Cruz sc-2646), sc-DDB2 (Cell Signaling 5416) or phospho-p53 (Ser15; Cell Signaling 8284) overnight in TBST. After washing, the blots were probed with HRP-coupled anti-mouse or anti-rabbit IgG (Thermo Fisher) secondary antibodies for 1 h at room temperature. Chemiluminescence was visualized with either Clarity Western ECL substrate (Bio-Rad) or SuperSignal West Femto substrate (Thermo Scientific) using a Molecular Imager Chemi-Doc XRS+ imaging system (Bio-Rad). Signals in the linear range of detection were quantified by densitometry using Image Lab (Bio-Rad) and normalized as previously described (35) or calculated as fold changes in UVB-irradiated samples relative to the nonirradiated sample in each experiment. Unpaired t-tests were used to compare the UVB-dependent changes in protein levels between the treatment groups (+IGF-1 vs −IGF-1 or DMSO vs inhibitor). A one-sample t-test was performed to compare the relative levels of p21 protein in experiments carried out with human skin.
Reverse transcriptase quantitative PCR (RT-qPCR).
Cell pellets and epidermal tissues were placed on ice and homogenized in 1 mL of QIAzol or TRIzol by pipetting up and down several times and then vortexing. After addition of chloroform (1/5th volume) and mixing, samples were centrifuged at 15 min at maximum speed. The top layer was transferred to a new tube, and an equal volume of 70% RNase-free ethanol was then added. RNA was then purified using RNeasy columns (Qiagen) and eluted with nuclease-free water. RNA concentrations were determined with a NanoDrop One spectrophotometer (Thermo Fisher). Equal amounts of RNA were reverse transcribed to cDNA using a QuantiTect Reverse Transcription Kit (Qiagen). PCRs were prepared using 2X TaqMan Fast Universal PCR Master Mix and TaqMan probes targeting p21 (Hs00355782), pol η (Hs00197814) and beta-2-microglobulin (B2M) (Hs0187842) (Applied Biosystems). PCRs were run on a Bio-Rad CFX96 Real-Time PCR Detection System using an initial 3 min melting step at 95°C followed by 40 cycles of 95°C for 10 s and 55°C for 30 s. The ΔΔCt method was used to calculate fold changes in gene expression using B2M as an internal housekeeping gene. Analyses of IGF-1 expression in skin of different aged donors involved normalizing the IGF-1 Ct value to the B2M Ct value, which was compared to a set of B2M cDNA standards (Addgene plasmid #12099; pBJ1-human B2M) of known copy number.
Cell cycle analyses.
Ethanol-fixed cells were stained with propidium iodide after RNase treatment and analyzed for DNA content using an Accuri C6 flow cytometer. To determine the percentage of cells in S phase, cells were pulsed with 100 μm EdU during the final hour of the 24 h incubation period after UVB exposure. Cells were fixed in 2% paraformaldehyde, permeabilized with saponin and labeled with AlexaFluor647-azide for 30 min using a Click-iT EdU Cell Proliferation Kit (Thermo Fisher). After rinsing and labeling with 300 nm DAPI, imaging was performed on a Cytation 5 Cell Imager Multi-Mode Reader (Bio-Tek) to acquire 4–5 images per sample at 4X magnification using the 405 nm, 630 nm and phase contrast channels. Within the population of DAPI nuclei recognized using Gen5 (Bio-Tek) software, an EdU-positive nucleus was defined as a DAPI-positive region larger than 5 μm, brighter than the 99.9th percentile of the EdU-negative control in the 630 nm channel (n = 9 images).
Cell survival.
Acute cell survival was determined using an MTT assay, in which methylthiazolyldiphenyl-tetrazolium bromide (MTT) was added to cell culture medium for 45 min before solubilization with DMSO and absorbance measurements at 570 nm on a Synergy H1 spectrophotometer (Bio-Tek) (36). Absorbance values for the UVB-irradiated samples were normalized to the nonirradiated samples. Clonogenic survival was determined by plating between 200 and 800 treated cells onto 10 cm plates 5 days after treatment in EpiLife medium containing IGF-1. Cells were allowed to grow and form colonies for 10–14 days, after which cells were stained with crystal violet and counted. Unpaired, two-tailed t-tests were used to determine whether UVB exposure resulted in changes in survival.
RESULTS
IGF-1 is required for the induction of p53 target genes in UVB-irradiated human keratinocytes in vitro
The tumor suppressor protein p53 is a transcription factor that activates the expression of gene products that are important for cellular responses to UV-induced DNA damage (Fig. 1a). A classical p53 target gene is p21 (37), which mediates cell cycle arrest via inhibition of cyclin-dependent kinases (27,38). The nucleotide excision repair (NER) factors XPC and DDB2, which promote UV photoproduct recognition during NER (39), have similarly been reported to be regulated by p53 in various cell types (24-26). Lastly, the translesion synthesis (TLS) DNA polymerase pol η has also been shown to be induced by UV in a p53-dependent manner in human skin fibroblasts (28,29). Pol η can accurately synthesize DNA across thymine dimers (30), which are the major photoproduct induced in DNA by UV radiation. Mutations in this gene are found in a variant form of the skin cancer-prone disorder xeroderma pigmentosum (31,40). No information is currently available on the regulation of pol η expression in human keratinocytes or human skin epidermis.
Based on previous data demonstrating that decreases in IGF-1 expression in the skin are correlated with advanced age (4,5,41) and defective cellular responses to UVB-induced DNA damage (3,5,12,13), the expression of p53 gene products was investigated in telomerase-immortalized human neonatal skin keratinocytes (N-TERTs) grown for 24 h in the presence or absence of IGF-1 and then exposed to UVB radiation. Consistent with a previous report (10), keratinocytes with inactive IGF-1 receptor signaling due to withdrawal of IGF-1 show a reduced level of p53 phosphorylation on Ser15 after UVB exposure (Fig. 1b), which indicates that p53 is not properly activated in the absence of IGF-1/IGF-1R signaling. As shown in Fig. 1c, protein levels of both pol η and p21 were increased 24 h after exposure 100 J m−2 of UVB radiation in cells containing IGF-1 in the growth medium. In contrast, cells deprived of IGF-1 for 24 h before UVB exposure failed to show the same level of induction of either pol η or p21. Quantification of protein induction from multiple independent experiments is shown in Fig. 1d. Because protein levels of the NER factors XPC and DDB2 were only modestly increased by UVB exposure in N-TERT cells (Fig. 1c,d), these gene products were not examined further. To support the hypothesis that the increases in pol η and p21 protein were associated with transcriptional induction of these genes, the RNA levels of pol η and p21 were next examined. As shown in Fig. 1e, RNA levels of both pol η and p21 were increased to a greater extent in N-TERTs grown in the presence of IGF-1 than in the absence of IGF-1.
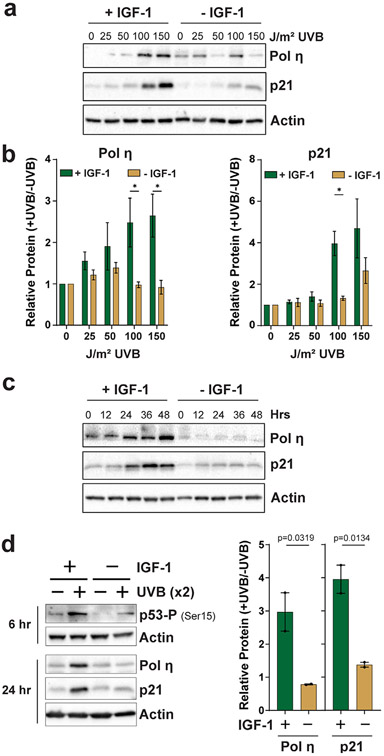
UVB dose–response experiments were next performed to further validate these findings using a range of UVB doses that do not lead to significant acute lethality. A representative experiment is shown in Fig. 2a. Results from several independent experiments are quantified in Fig. 2b, which shows that the protein levels of both pol η and p21 increase in a UVB dose-dependent manner and that N-TERTs deprived of IGF-1 contain lower levels of these proteins in comparison to cells cultured with IGF-1. Time course experiments revealed that the failure of IGF-1-deprived cells to significantly increase the expression of pol η and p21 protein after UVB exposure can be observed at several time points after UVB irradiation (Fig. 2c). Interestingly, this failure to increase the expression of these p53 target genes and to promote p53 phosphorylation also holds true when cells are exposed to UVB radiation twice over a 48 h period (Fig. 2d), which may have implications for chronic UVB exposures associated with routine, daily exposures of human skin to the sun.
Figure 2.
Characterization of IGF-1 function in pol η and p21 induction in UVB-irradiated keratinocytes. (a) N-TERTs were treated as in Fig. 1 except that cells were exposed to the indicated doses of UVB radiation and harvested 24 h later. (b) Quantitation of protein levels from at least three independent experiments as shown in (a). A multiple t-test analysis was carried out to determine whether the difference between treatment groups was significant at each UVB dose. Asterisks indicate a significant difference (P < 0.05). (c) N-TERTs were cultured in the presence or absence of IGF-1, exposed to 100 J m−2 UVB and then harvested at the indicated time points for protein immunoblotting. (d) N-TERTs were exposed twice (x2) to 100 J m−2 UVB 24 h and 48 h after beginning incubation in medium containing or lacking IGF-1. Cells were harvested 6 h after the second UVB exposure to examine p53 phosphorylation on Ser15 and 24 h after the second UVB exposure to examine pol η and p21 induction. The graphs show the relative level of protein induction from two independent experiments. One-tailed t-tests were used to determine whether the relative protein induction in cells grown with IGF-1 was higher than in the absence of IGF-1.
IGF-1 receptor and p53 inhibition abrogate the UVB-dependent induction of p21 and pol η in keratinocytes in vitro
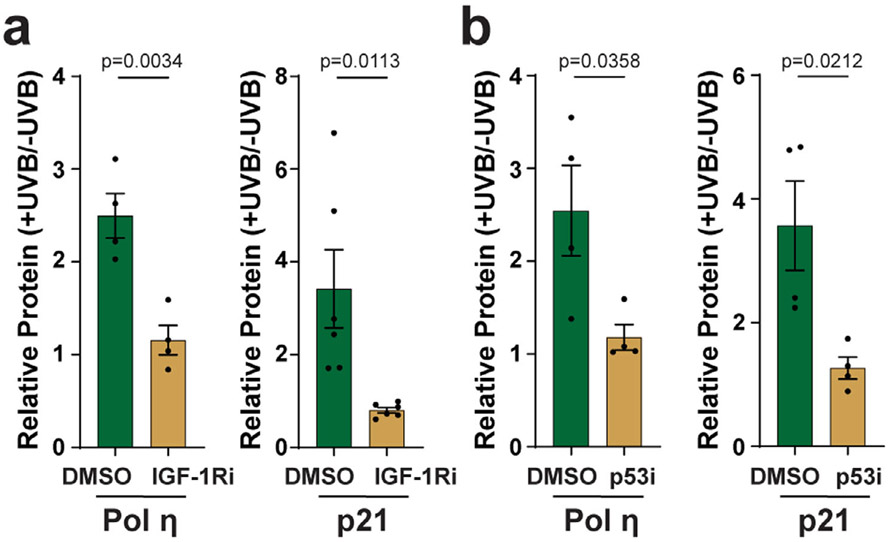
To further validate the effects of the loss of IGF-1 on keratinocyte responses to UVB radiation, N-TERTs were treated with the IGF-1 receptor antagonist (IGF-1Ri) AG538 and then exposed to UVB. Similar to the effects of IGF-1 withdrawal from the culture medium, IGF-1Ri treatment blocked the UVB-dependent increase in both pol η and p21 protein (Fig. 3a). Based on the hypothesis that the increase in pol η and p21 expression is due to p53, N-TERTs were treated with the p53 inhibitor (p53i) pifithrin-α and then exposed to UVB radiation. As shown in Fig. 3b, p53i treatment prevented pol η and p21 levels from increasing after UVB exposure. These results further confirm that the UVB-dependent induction of pol η and p21 protein in N-TERT keratinocytes is dependent on p53 and IGF-1/IGF-1R signaling.
Figure 3.
Inhibition of IGF-1R and p53 abrogates the UVB-dependent induction of pol η and p21 in keratinocytes in vitro. (a) N-TERT keratinocytes were treated with vehicle (DMSO) or IGF-1R inhibitor (IGF-1Ri, 10 μm AG538) for 30 min before exposure to 100 J m−2 UVB. Cells were harvested 24 h later for protein immunoblot analysis. (b) Cells were treated as in (a) except that the p53 inhibitor (p53i; 20 μm pifithrin-α) was used. The relative level of protein induction was determined from 4 to 6 independent experiments, as indicated. Unpaired, two-tailed t-tests were performed to determine whether the difference in relative gene product levels (+UVB/−UVB) was significant between the two treatment groups.
Loss of IGF-1 negatively impacts clonogenic growth potential following UVB exposure
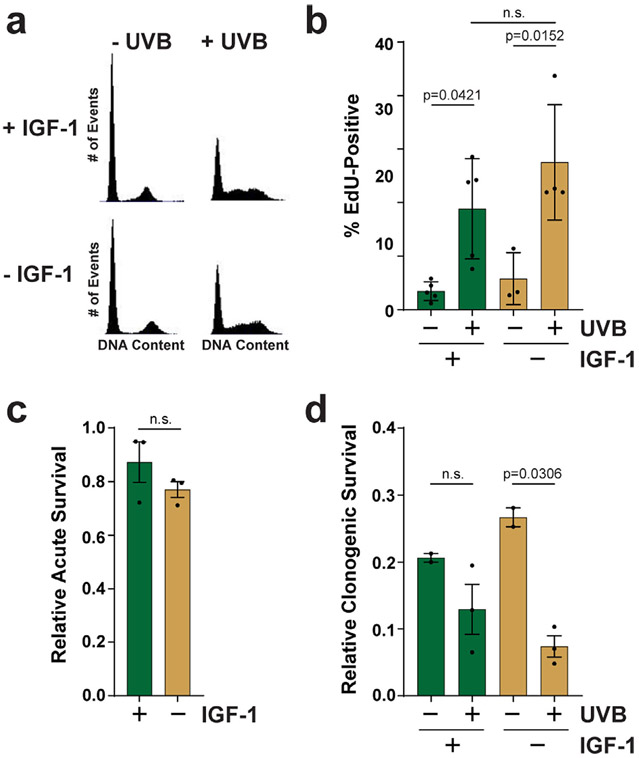
The failure to induce p21 and pol η in cells lacking IGF-1 may be expected to lead to a failure to arrest the cell cycle or efficiently replicate UVB-damaged DNA. However, flow cytometric analysis of propidium iodide-stained cells failed to reveal a significant difference in cell cycle distribution 24 h after UVB exposure (Fig. 4a). Moreover, fluorescence microscopic analysis of EdU incorporation showed that N-TERTs contained similar numbers of replicating cells whether grown in either the presence or absence of IGF-1 (Fig. 4b). MTT assays indicated a small, but nonsignificant reduction in acute cell survival 24 h after UVB exposure in cells deprived of IGF-1 (Fig. 4c). However, clonogenic survival assays revealed a significant reduction in growth potential in keratinocytes deprived of IGF-1 (Fig. 4d), which indicates that the absence of IGF-1 during the period of UVB exposure has negative long-term consequences on keratinocytes. Importantly, the reduction in clonogenic growth potential in IGF-1-deprived cells is likely not due to increased senescence (10).
Figure 4.
The loss of IGF-1 signaling impacts clonogenic growth potential in response to a low dose of UVB radiation. (a) Cells were treated as described in Fig. 1 except that cells were fixed in ethanol, stained with propidium iodide and analyzed by flow cytometry for DNA content. (b) Cells were treated as in (a) but were pulsed with EdU and processed with a Click-iT EdU staining kit to monitor S phase cells by fluorescence microscopy (n = 4–5 images per condition). (c) Cells were treated as described in (a) except that MTT assays were performed 24 h after UVB exposure. The results show the relative level of survival from 3 independent experiments. (d) Clonogenic survival assays were carried out with N-TERTs grown in the presence or absence of IGF-1 and exposed or not to 100 J m−2 UVB. The results show the fraction of cells that formed colonies in 2–3 independent experiments. One-tailed t-tests were used to determine whether the percentage of EdU-positive cells or surviving cells was higher after UVB exposure or in the absence of IGF-1 (n.s., not significant).
IGF-1 signaling is required for p21 and pol η induction in UVB-irradiated human skin epidermis ex vivo
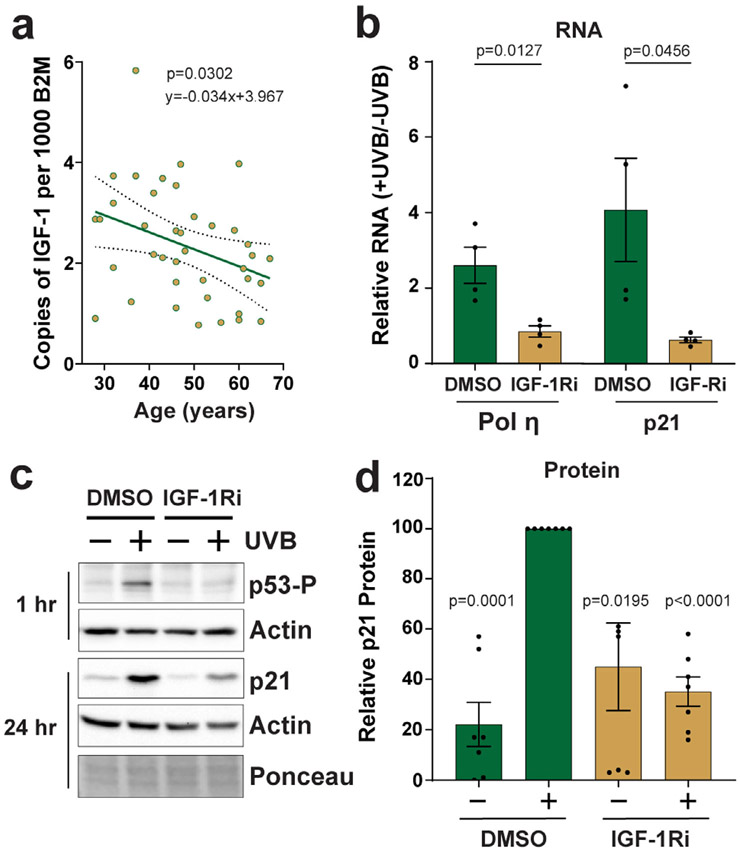
Though studies of cultured keratinocytes in vitro are valuable for understanding how the loss of IGF-1 may impact cellular responses to UVB radiation, studies with human skin explants ex vivo may provide an experimental system that is more physiologically relevant to human skin in vivo. RT-qPCR analyses of IGF-1 mRNA expression in human skin discarded during routine panniculectomies revealed that IGF-1 levels declined in an age-dependent manner, with an approximate yearly decrease of 3.4% on average (Fig. 5a). To determine how IGF-1 signaling impacts the induction of p53 target genes, discarded skin samples were then treated topically with either vehicle or the IGF-1Ri AG538 to mimic the status of IGF-1/IGF-1R signaling in geriatric skin (12,13). Skin sections were then exposed to 800 J m−2 of UVB radiation, which corresponds to approximately twice a minimal erythemal dose of UVB in lightly pigmented skin. Skin epidermis was then harvested 24 h later for analysis of gene expression by RT-qPCR. As shown in Fig. 5b, the RNA levels of both pol η and p21 were found to be increased by UVB exposure in the control skin. Though p21 has previously been reported to be elevated in UVB-irradiated human skin (42-44), these data provide the first evidence that pol η expression can similarly be increased by UVB exposure in human skin epidermis. Interestingly, and consistent with results with keratinocytes in vitro (Fig. 3a), treatment of the skin epidermis with the IGF-1Ri failed to show an increase in either p21 or pol η. Correlated with the reduced p21 and pol η, RNA induction was a reduction in p53 phosphorylation on Ser15 after UVB exposure when the IGF-1R was inhibited (Fig. 5c). Additional experiments monitoring UVB photoproduct formation in epidermal genomic DNA revealed similar levels of cyclobutane pyrimidine dimer content in both vehicle and IGF-1Ri-treated skin (data not shown), and thus, the IGF-1Ri does not appear to be acting as a sunscreen to block DNA damage formation by UVB.
Figure 5.
IGF-1 signaling is required for p21 and pol η induction in UVB-irradiated human skin epidermis ex vivo. (a) RT-qPCR was used to determine IGF-1 mRNA expression in skin samples (n = 39) discarded during routine surgeries. IGF-1 was normalized to B2M and then compared to a set of B2M standards of known copy number. A linear regression was performed, and the line of best-fit is plotted along with the 95% confidence bands. (b) Human skin explants were treated topically with either DMSO or 20 μm AG538 (IGF-1Ri) for 30 min before exposure to 800 J m−2 UVB. Epidermis was isolated 24 h later for RNA analysis by RT-qPCR. The graphs show the induction of pol η and p21 in UVB-irradiated skin relative to nonirradiated skin from 4 different skin donors (36–62 years of age). Unpaired, two-tailed t-tests were performed to determine whether the difference in relative gene product levels (+UVB/−UVB) was significant. (c) A representative experiment in which skin was treated as in (a) except that the epidermis was analyzed by protein immunoblotting. (d) Quantitation of 7 independent experiments performed as in (c). For each experiment, the level of p21 protein (normalized to actin) in the DMSO + UVB sample was set to an arbitrary value of 100. All other signals were then compared to this value. A one-sample t-test was used to compare the treatment values to the DMSO + UVB control mean set to 100.
To determine whether the changes in RNA are also apparent at the protein level, protein immunoblotting was performed using epidermal skin samples treated as described in Fig. 5a. A representative immunoblotting result is shown in Fig. 5c, and the results of experiments from seven different skin samples are quantified in presented in Fig. 5d. Consistent with the RNA analyses, p21 protein levels became increased by approximately five-fold on average in control skin after UVB exposure. Moreover, this induction was partially blocked by treatment of the skin with the IGF-1Ri (Fig. 5c,d). Currently available antibodies lacked the sensitivity and specificity necessary for reliably detecting pol η in human skin epidermis, and thus, it is not known if the changes in pol η RNA observed by UVB irradiation (Fig. 5a) are also manifested at the protein level. Nonetheless, the experiments using human skin explants ex vivo validate the results with cultured keratinocytes in vitro demonstrating that the loss of IGF-1 signaling prevents the induction of the p53 target genes p21 and pol η following UVB exposure.
DISCUSSION
Due to the growing incidence of NMSCs in geriatric populations, the identification of factors that predispose geriatric skin to mutagenesis and carcinogenesis is important to identify and to examine experimentally. The low levels of IGF-1 expression associated with increased fibroblast senescence in geriatric skin (5,8) coupled with the defective repair of UVB photoproducts and deficient DNA damage checkpoint signaling in IGF-1-deficient keratinocytes (12-14) offer a potential mechanism for increased NMSC risk in older individuals. However, additional work is necessary to understand the specific DNA damage response systems and pathways that are impacted in geriatric skin and by deficient IGF-1 signaling. As a master regulator of genome integrity, the tumor suppressor protein p53 impacts cellular responses to UVB radiation (21-23) and is frequently found to be mutated in both NMSCs and in regions of sun-exposed skin (45,46). Thus, defective p53 function likely contributes to mutagenesis and carcinogenesis in epidermal keratinocytes (47).
To better understand how IGF-1 signaling impacts p53 function in the skin, IGF-1/IGF-1R signaling was inhibited in both cultured keratinocytes in vitro and human skin ex vivo prior to UVB exposure. The results showed that the loss of IGF-1 signaling is associated with a failure to induce the p53 target genes p21 and pol η following UVB exposure in both cultured keratinocytes (Figs. 1-3) and human skin epidermis (Fig. 5). Because these gene products function to limit the likelihood of mutagenic DNA synthesis from taking place by preventing S phase entry (27,38) and promoting accurate DNA synthesis across thymine dimers (30,48), the failure to properly induce these products may lead to mutagenesis or cell death. However, the data here show that the loss of IGF-1 signaling has no discernable effect on bulk measurements of cell cycle distribution, the percentage of S phase cells or acute survival after exposure to a single and relatively low dose (100 J m−2) of UVB radiation (Fig. 4a-c). In contrast, the more sensitive clonogenic survival assay revealed that the loss of IGF-1 negatively impacted growth potential (Fig. 4d), which suggests that IGF-1 signaling is important for the long-term proliferative potential of UVB-irradiated keratinocytes. Additionally, it is possible that the failure to induce these p21 and pol η becomes more important in the context of repeated exposures to UVB radiation (49-51). Therefore, in order to understand exposure of human skin to the sun on a daily basis, it will be important to determine how chronic exposures to low doses of UVB radiation impacts the process of mutagenesis and cell transformation in cultured keratinocytes and human skin.
Lastly, the replicative clamp protein PCNA (proliferating cell nuclear antigen) plays a critical role in translesion synthesis across UV photoproducts (52). Mono-ubiquitination of PCNA is generally considered to be a signal to recruit TLS polymerases such as pol η to sites of DNA damage to allow lesion bypass. Moreover, p21 binding to PCNA has been reported to influence TLS (53). In preliminary studies, we have found that PCNA mono-ubiquitination is elevated by the loss of IGF-1 signaling in both keratinocytes in vitro and human skin ex vivo (Hutcherson and Kemp, unpublished). Consistent with a role for the IGF-1R in TLS, a previous study showed that the IGF-1R binds to and phosphorylates PCNA to impact PCNA ubiquitination (54). Thus, it will be important to understand how the interplay between IGF-1/IGF-1R signaling, PCNA mono-ubiquitination and the induction of p21 and pol η impact UVB mutagenesis in IGF-1-deficient geriatric skin.
It will also be valuable to extend the analyses of p21 and pol η RNA/protein induction in UVB-irradiated human skin ex vivo to work with human subjects. Because geriatric skin expresses less IGF-1 than young adult skin (5), the data presented here predict that geriatric individuals will show less induction of these p53 target genes in the skin epidermis than young adults. Mutations in pol η lead to a variant form of the skin cancer-prone disease xeroderma pigmentosum (XP-V) (33), and thus, the lower expression of pol η protein in sunlight-exposed geriatric skin may explain the elevated risk of skin cancer in these individuals. Interestingly, skin rejuvenation approaches such as fractionated laser resurfacing demonstrated the ability to restore IGF-1 expression in geriatric skin to levels found in young adult skin (8,19,20,55) and may prevent the development of actinic keratoses (56). Thus, it will be interesting to determine whether these clinical interventions similarly restore the capacity for proper p53 signaling in the skin of older individuals.
Acknowledgements—
The authors thank the WSU Proteome Analysis Laboratory and Center for Genomics Research for the use of equipment to carry out this work. We wish to acknowledge the manuscript editing and valuable discussion from Dr. Jeffrey Travers (WSU). This work was supported by start-up funding provided by Wright State University and by a grant from the National Institute of General Medical Sciences (GM130583 to MGK).
REFERENCES
- 1.Sancar A (2016) Mechanisms of DNA repair by photolyase and excision nuclease (Nobel Lecture). Angew. Chem. Int. Ed Engl 55, 8502–8527. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer KH (1997) Sunlight and skin cancer: Another link revealed. Proc. Natl. Acad. Sci. U. S. A 94, 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemp MG, Spandau DF and Travers JB (2017) Impact of age and insulin-like growth factor-1 on DNA damage responses in UV-irradiated human skin. Molecules 22, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JB, Travers JB and Spandau DF (2009) A new paradigm for the role of aging in the development of skin cancer. J. Invest. Dermatol 129, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JB, Travers A, Somani K and Spandau DF (2010) The IGF-1/IGF-1R signaling axis in the skin: A new role for the dermis in aging-associated skin cancer. Oncogene 29, 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavakkol JT, Elder CE, Griffiths KD, Cooper H, Talwar GJ, Fisher GJ, Keane KM, Foltin SK and Voorhees JJ (1992) Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J. Invest. Dermatol 99, 343–349. [DOI] [PubMed] [Google Scholar]
- 7.Ando Y and Jensen PJ (1993) Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J. Invest. Dermatol 100, 633–639. [DOI] [PubMed] [Google Scholar]
- 8.Spandau DF, Lewis DA, Somani AK and Travers JB (2012) Fractionated laser resurfacing corrects the inappropriate UVB response in geriatric skin. J. Invest. Dermatol 132, 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferber A, Chang C, Sell C, Ptasznik A, Cristofalo VJ, Hubbard K, Ozer HL, Adamo M, Roberts CT and LeRoith D (1993) Failure of senescent human fibroblasts to express the insulin-like growth factor-1 gene. J. Biol. Chem 268, 17883–17888. [PubMed] [Google Scholar]
- 10.Lewis DA, Yi Q, Travers JB and Spandau DF (2008) UVB-induced senescence in human keratinocytes requires a functional insulin-like growth factor-1 receptor and p53. Mol. Biol. Cell 19, 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn SA, Hurwitz MG, Cotton Kumar J and Spandau DF (1999) Activation of the insulin-like growth factor-1 receptor promotes the survival of human keratinocytes following ultraviolet B irradiation. Int. J. Cancer 80, 431–438. [DOI] [PubMed] [Google Scholar]
- 12.Loesch MM, Collier AE, Southern DH, Ward RE, Tholpady SS, Lewis DA, Travers JB and Spandau DF (2016) Insulin-like growth factor-1 receptor regulates repair of ultraviolet B-induced DNA damage in human keratinocytes in vivo. Mol. Oncol 10, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp MG, Spandau DF, Simman R and Travers JB (2017) Insulin-like growth factor 1 receptor signaling is required for optimal ATR-CHK1 kinase signaling in ultraviolet B (UVB)-irradiated human keratinocytes. J. Biol. Chem 292, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez TL, Van Lonkhuyzen DR, Dawson RA, Kimlin MG and Upton Z (2015) Insulin-like growth factor-I and UVB photoprotection in human keratinocytes. Exp. Dermatol 24, 235–238. [DOI] [PubMed] [Google Scholar]
- 15.Heffernan TP, Simpson DA, Frank AR, Heinloth AN, Paules RS, Cordeiro-Stone M and Kaufmann WK (2002) An ATR- and Chk1-dependent S checkpoint inhibits replicon initiation following UVC-induced DNA damage. Mol. Cell. Biol 22, 8552–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chastain PD 2nd, Heffernan TP, Nevis KR, Lin L, Kaufmann WK, Kaufman DG, Kaufman DG and Cordeiro-Stone M (2006) Checkpoint regulation of replication dynamics in UV-irradiated human cells. Cell Cycle 5, 2160–2167. [DOI] [PubMed] [Google Scholar]
- 17.Saldivar JC, Cortez D and Cimprich KA (2017) The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol 18, 622–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada M, Udono MU, Hori M, Hirose R, Sato S, Mori T and Nikaido O (2006) Aged human skin removes UVB-induced pyrimidine dimers from the epidermis more slowly than younger adult skin in vivo. Arch. Dermatol. Res 297, 294–302. [DOI] [PubMed] [Google Scholar]
- 19.Lewis DA, Travers JB, Machado C, Somani A-K and Spandau DF (2011) Reversing the aging stromal phenotype prevents carcinoma initiation. Aging (Albany NY) 3, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travers JB, Kemp MG, Weir NM, Cates E, Alkawar AM, Mahajan AS and Spandau DF (2020) Wounding with a micro-needling device corrects the inappropriate ultraviolet B radiation response in geriatric skin. Arch. Dermatol. Res 312, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin L, Ullrich SE, Kripke ML and Ananthaswamy HN (2008) p53 tumor suppressor gene: A critical molecular target for UV induction and prevention of skin cancer. Photochem. Photobiol 84, 55–62. [DOI] [PubMed] [Google Scholar]
- 22.Lakin ND and Jackson SP (1999) Regulation of p53 in response to. DNA Damage 18, 7644–7655. [DOI] [PubMed] [Google Scholar]
- 23.Fischer M (2017) Census and evaluation of p53 target genes. Oncogene 36, 3943–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford JM (2005) Regulation of DNA damage recognition and nucleotide excision repair: another role for p53. Mutat. Res 577, 195–202. [DOI] [PubMed] [Google Scholar]
- 25.Adimoolam S and Ford JM (2002) p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc. Natl. Acad. Sci. U. S. A 99, 12985–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang J, Ford JM, Hanawalt PC and Chu G (1999) Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc. Natl. Acad. Sci. U. S. A 96, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Deiry S (2016) p21(WAF1) mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 76, 5189–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerner LK, Francisco G, Soltys DT, Rocha CR, Quinet A, Vessoni AT, Castro LP, David TIP, Bustos SO, Strauss BE, Gottifredi V, Stary A, Sarasin A, Chammas R and Menck CFM (2017) Predominant role of DNA polymerase eta and p53-dependent translesion synthesis in the survival of ultraviolet-irradiated human cells. Nucleic Acids Res. 45, 1270–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G and Chen X (2006) DNA polymerase eta, the product of the xeroderma pigmentosum variant gene and a target of p53, modulates the DNA damage checkpoint and p53 activation. Mol. Cell. Biol 26, 1398–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washington MT, Johnson RE, Prakash L and Prakash S (2001) Accuracy of lesion bypass by yeast and human DNA polymerase eta. Proc. Natl. Acad. Sci. U. S. A 98, 8355–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordonnier M and Fuchs RP (1999) Replication of damaged DNA: molecular defect in xeroderma pigmentosum variant cells. Mutat. Res 435, 111–119. [DOI] [PubMed] [Google Scholar]
- 32.Kannouche P and Stary A (2003) Xeroderma pigmentosum variant and error-prone DNA polymerases. Biochimie 85, 1123–1132. [DOI] [PubMed] [Google Scholar]
- 33.Masutani FH and Ahmad SI (2008) Xeroderma pigmentosum variant, XP-V: Its product and biological roles. Adv. Exp. Med. Biol 637, 93–102. [DOI] [PubMed] [Google Scholar]
- 34.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP and Rheinwald JG (2000) Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol 20, 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kemp MG, Krishnamurthy S, Kent MN, Schumacher DL, Sharma P, Excoffon KJDA and Travers JB (2019) Spironolactone depletes the XPB protein and inhibits DNA damage responses in UVB-irradiated human skin. J. Invest. Dermatol 139, 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaj K, Hutcherson RJ and Kemp MG (2020) ATR kinase activity limits mutagenesis and promotes the clonogenic survival of quiescent human keratinocytes exposed to UVB radiation. Photochem. Photobiol 96, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825. [DOI] [PubMed] [Google Scholar]
- 38.Petrocelli T, Poon R, Drucker DJ, Slingerland JM and Rosen CF (1996) UVB radiation induces p21Cip1/WAF1 and mediates G1 and S phase checkpoints. Oncogene 12, 1387–1396. [PubMed] [Google Scholar]
- 39.Sugasawa K (2019) Mechanism and regulation of DNA damage recognition in mammalian nucleotide excision repair. Enzymes. 45, 99–138. [DOI] [PubMed] [Google Scholar]
- 40.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K and Hanaoka F (1999) The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399, 700–704. [DOI] [PubMed] [Google Scholar]
- 41.Travers JB, Spandau DF, Lewis DA, Machado C, Kingsley M, Mousdicas N and Somani AK (2013) Fibroblast senescence and squamous cell carcinoma: How wounding therapies could be protective. Dermatol. Surg 39, 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brash E, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ and Ponten J (1991) A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A 88, 10124–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonason S, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, Leffell DJ, Tarone RE and Brash DE (1996) Frequent clones of p53-mutated keratinocytes in normal human skin. Proc. Natl. Acad. Sci. U. S. A 93, 14025–14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brash DE (2006) Roles of the transcription factor p53 in keratinocyte carcinomas. Br. J. Dermatol 154(Suppl 1), 8–10. [DOI] [PubMed] [Google Scholar]
- 45.Cruet-Hennequart S, Gallagher K, Sokol AM, Villalan S, Prendergast AM and Carty MP (2010) DNA polymerase eta, a key protein in translesion synthesis in human cells. Subcell. Biochem 50, 189–209. [DOI] [PubMed] [Google Scholar]
- 46.Decraene D, Smaers K, Maes D, Matsui M, Declercq L and Garmyn M (2005) A low UVB dose, with the potential to trigger a protective p53-dependent gene program, increases the resilience of keratinocytes against future UVB insults. J. Invest. Dermatol 125, 1026–1031. [DOI] [PubMed] [Google Scholar]
- 47.Berube R, Drigeard Desgarnier MC, Douki T, Lechasseur A and Rochette PJ (2018) Persistence and tolerance of DNA damage induced by chronic UVB irradiation of the human genome. J. Invest. Dermatol 138, 405–412. [DOI] [PubMed] [Google Scholar]
- 48.Drigeard Desgarnier MC and Rochette PJ (2018) Enhancement of UVB-induced DNA damage repair after a chronic low-dose UVB pre-stimulation. DNA Repair (Amst) 63, 56–62. [DOI] [PubMed] [Google Scholar]
- 49.Ponten B, Berne ZP, Nister Ren M and Ponten J (1995) Ultraviolet light induces expression of p53 and p21 in human skin: Effect of sunscreen and constitutive p21 expression in skin appendages. J. Invest. Dermatol 105, 402–406. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed NU, Ueda M and Ichihashi M (1999) Induced expression of p16 and p21 proteins in UVB-irradiated human epidermis and cultured keratinocytes. J. Dermatol. Sci 19, 175–181. [DOI] [PubMed] [Google Scholar]
- 51.O’Grady A, Kay EW, McKenna DB, Bennett MA, Murphy GM and Leader MB (1998) Altered expression of the p53-regulated proteins, p21Waf1/Cip1, MDM2, and Bax in ultraviolet-irradiated human skin. Hum. Pathol 29, 559–564. [DOI] [PubMed] [Google Scholar]
- 52.Kanao R and Masutani C (2017) Regulation of DNA damage tolerance in mammalian cells by post-translational modifications of PCNA. Mutat. Res 803-805, 82–88. [DOI] [PubMed] [Google Scholar]
- 53.Avkin S, Sevilya Z, Toube L, Geacintov N, Chaney SG, Oren M and Livneh Z (2006) p53 and p21 regulate error-prone DNA repair to yield a lower mutation load. Mol. Cell 22, 407–413. [DOI] [PubMed] [Google Scholar]
- 54.Waraky A, Lin Y, Warsito D, Haglund F, Aleem E and Larsson O (2017) Nuclear insulin-like growth factor 1 receptor phosphorylates proliferating cell nuclear antigen and rescues stalled replication forks after DNA damage. J. Biol. Chem 292, 18227–18239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krbanjevic JB, Travers JB and Spandau DF (2016) How wounding via lasers has potential photocarcinogenic preventative effects via dermal remodeling. Curr. Dermatol. Rep 5, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen R, Wargo JJ, Williams A, Cates E, Spandau DF, Knisely C and Travers JB (2020) single ablative fractional resurfacing laser treatment for forearm actinic keratoses: 6-month follow-up data from an intrapatient comparison between treated and untreated sites. Lasers Surg. Med 52, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]