Abstract
Background
There are increasing global data relating to prevalence of food allergy and food-induced anaphylaxis; however, this is often based on surrogate measures of sensitization rather than objective symptoms at food challenge. In terms of protecting food-allergic consumers from reactions, to our knowledge, there has been no global survey assessing geographic differences in the proportion of anaphylaxis triggered by specific foods.
Objective
We sought to identify common triggers for food-induced anaphylaxis and how these vary from country to country.
Methods
Systematic review of relevant reports published between January 2010 and November 2020. Results were reported following PRISMA guidelines. Publications were screened and data extracted by 2 independent reviewers, and the risk of bias was assessed.
Results
Sixty-five studies (encompassing 41 countries and all 6 regions as defined by the Food and Agriculture Organization of the United Nations) were included. Significant regional variations in the most common triggers of food anaphylaxis were seen; however, in general, there was good agreement between local legislative requirements for allergen disclosure and the most common allergens for each region or nation.
Conclusions
Local legislation for allergen disclosure generally reflects those allergens commonly responsible for food anaphylaxis. Cow’s milk and crustaceans appear to cause a higher proportion of anaphylaxis compared to peanut in some regions.
Key words: Allergen labeling, anaphylaxis, Codex, epidemiology, food allergy, prevalence
Abbreviations used: LTP, Lipid transfer protein; NASWP, North America/Southwest Pacific
Graphical abstract
Food supply increasingly involves supply chains across multiple countries. The Codex Alimentarius (often abbreviated to Codex) is a set of international food standards, guidelines, and codes of practice established by the Food and Agricultural Organization of the United Nations and World Health Organization to facilitate the safety of global trade in food supply. Currently, the Codex requires disclosure for ingredients relating to 8 food groups: cereals containing gluten, crustaceans, egg, fish, peanut and soybean, milk, and tree nuts; sulfites (where present at concentrations of ≥10 mg/kg) must also be declared.1
The Codex list includes food allergens that are generally considered to cause over 90% of food-induced allergic reactions in most regions. However, anaphylaxis has been reported to almost all foods, and there are significant geographic differences in the prevalence of allergen-specific food allergies worldwide,2 presumably as a result of differences in dietary consumption and/or exposure. Some countries/regions therefore include additional allergens that must be declared on food labels.3
There are increasing data globally relating to the relative prevalence of food allergy due to specific foods; however, these epidemiologic data may not correspond to the list of foods that commonly cause anaphylaxis.4 Prevalence data should ideally be derived from unselected populations, but this often results in very small numbers of individuals allergic to a specific food and thus a high level of uncertainty over the resulting estimated prevalence data generated. More information relating to specific food triggers can be obtained from less rigorous methodologies (for example, diagnosis based on self-report, or the presence of sensitization with or without clinical history). However, this may not correspond to real-world data relating to the occurrence of food-induced allergic reactions due to accidental exposure. This may be because some food allergies resolve over time (for example, the majority of younger children allergic to cow’s milk and hen’s egg), or because some allergens (such as those implicated in pollen–food allergy syndrome) are not generally considered to cause systemic reactions in most affected individuals.5 In terms of assessing the risk posed to food-allergic consumers, to our knowledge, there has been no global survey assessing geographic differences in the relative proportions of anaphylaxis due to specific foods. We therefore undertook a systematic review to address this evidence gap.
Methods
We undertook a systematic review of the literature to identify studies reporting proportions of anaphylaxis in different countries/regions due to specific food triggers. This was undertaken and reported in accordance with the PRISMA statement.6
Search strategy
We used the search strategy from a systematic review of global anaphylaxis epidemiology4 (but limited to food allergens as the trigger for anaphylaxis) to perform a systematic search on the following electronic databases: Medline (Ovid), PubMed, and Embase (Ovid). There was no registered protocol for this review, but the methods and analyses were planned a priori. No language restrictions were made, and we planned to include non-English papers if they met our inclusion criteria. Abstracts were independently screened by 2 authors, and disagreements were resolved by discussion. We also reviewed reference lists of included studies and review articles to identify other relevant studies.
Study selection
We included all studies that provided details as to specific triggers for food anaphylaxis, either cases in patients presenting to a medical facility or reported to a central registry. We also included case series recording more than 10 fatalities due to food anaphylaxis. Risk of bias was assessed according to Hoy et al.7 Studies at high risk of bias were excluded unless there were no other data sets to provide information for that specific country. Where multiple publications were identified for the same data set with overlapping time periods, we included the report with the largest number of individuals where we could be certain that no duplication was present.
Data extraction and analyses
Data were extracted in duplicate, and any discrepancies identified were resolved by discussion and/or by contacting authors for clarifications. The different definitions used for anaphylaxis in individual studies were noted accordingly, along with an indication of the completeness of the data (proportion of cases where a specific food trigger was identified). Data were expressed as the proportion of anaphylaxis cases due to a specified food trigger compared to all cases of food anaphylaxis reported in that case series. Heat maps were used to identify the most common food allergens in each data series and to facilitate between-country comparisons.
In order to compare the proportion of anaphylaxis to reported prevalence for that allergen by region, both prevalence rates and anaphylaxis frequencies for individual allergens were pooled across studies using a generalized linear mixed model in R 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/) (metaprop function, metafor package, logit transformation with a random intercept logistic regression model for the summary estimate). This approach avoids many of the issues surrounding the use of transformations when undertaking meta-analyses of proportions.8,9 We conducted meta-analyses even if significant heterogeneity was seen between study estimates, as is the norm when conducting meta-analysis of proportions. Additional information regarding the data sets used to determine prevalence is available in the Online Repository at www.jacionline.org and Table E1.
Results
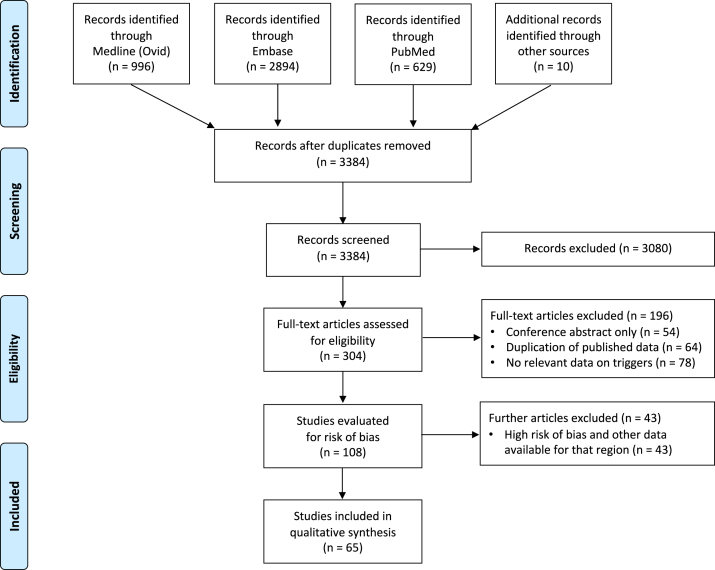
Sixty-five studies (encompassing 41 countries and all 6 regions as defined by the Food and Agriculture Organization of the United Nations) were identified for inclusion (Fig 1). Details of the individual studies appear in Fig E1 in the Online Repository at www.jacionline.org and Fig 2, along with the definition of anaphylaxis used and an indication of data completeness and risk of bias assessment.
Fig 1.
PRISMA flow diagram.
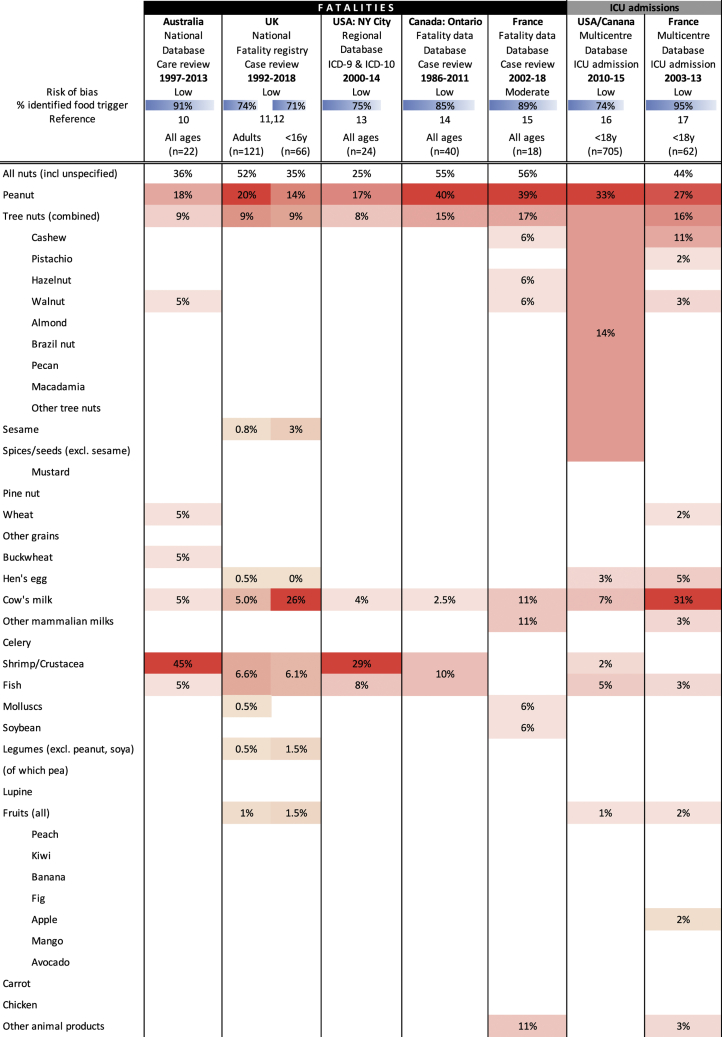
Fig E1.
Studies reporting fatalities and intensive care unit (ICU) admissions for food anaphylaxis. Shown is the percentage of all cases attributed to a food allergen caused by a specified food trigger. Heat map colors indicate relative (rather than absolute) prevalence of specific foods within each case series.
Fig 2.
Studies reporting food anaphylaxis events presenting to medical facilities (ie, emergency department [ED] visits, hospitalizations, clinics). Data are presented as the proportion of all reported cases of food anaphylaxis due to the specified food trigger. Heat map colors indicate relative (rather than absolute) prevalence of specific foods within each case series.
In total, 6 studies reported food anaphylaxis fatalities (covering Australia,10 the United Kingdom,11,12 the United States [New York City],13 Canada [Ontario],14 and France15), while an additional 2 studies reported intensive care admissions due to food-induced anaphylaxis.16,17 These studies are reported in Fig E1. The most common triggers reported for severe reactions were peanut, tree nuts, cow's milk and crustaceans. Fifty-seven other studies were included: 10 reports from anaphylaxis registries, 21 reporting visits to emergency departments, and 4 reporting hospitalizations due to food anaphylaxis, 4 surveys, 1 report of emergency medical services usage, and 17 describing clinic referrals for food anaphylaxis. All but 2 studies provided details regarding the specific triggers for food anaphylaxis; 2 (one from Chile, another from Morocco) included non-anaphylaxis reactions, but they were included in this analysis as a result of an absence of alternative data for these countries. These studies are reported in Fig 2.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74
Major causes of food-induced anaphylaxis by Codex region
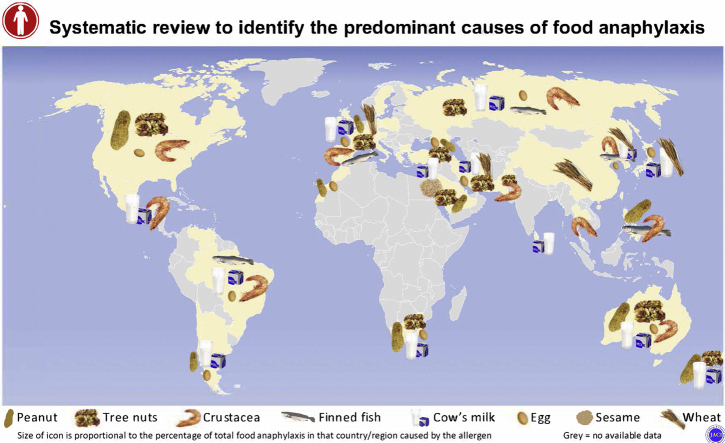
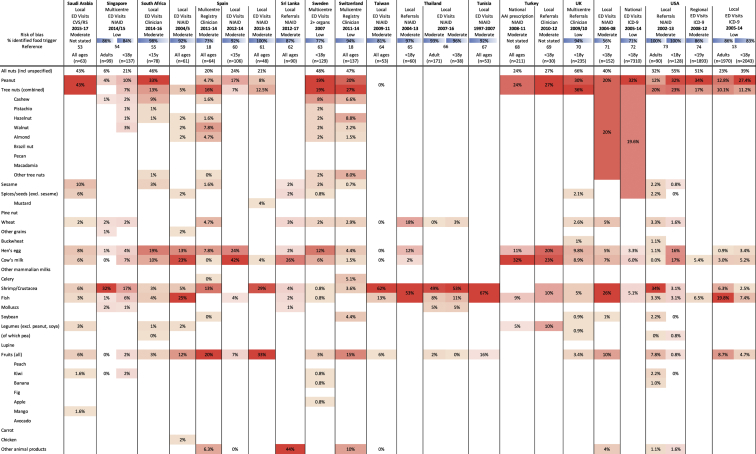
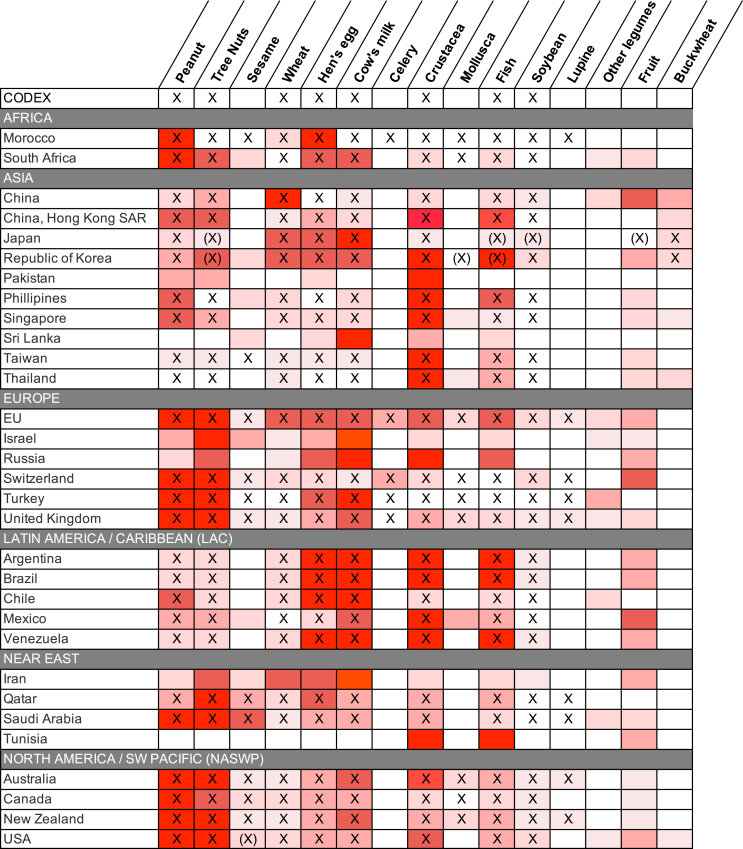
To further assess geographic variations in the most common food allergens reported to cause anaphylaxis, the data from Fig 2 were tabulated by Codex region (Fig 3) and plotted on a global map (Fig 4). These data demonstrated that while there are some allergens that are a common cause of anaphylaxis in multiple regions, there are also some foods that seem to be limited as a common trigger to just 1 or 2 regions. Of note, soya was not a major cause of food anaphylaxis in any region.
Fig 3.
Common food allergens reported to cause anaphylaxis by Codex Alimentarius (Codex) region and country. X indicates local legislation requiring disclosure for that allergen; (X), more limited or voluntary disclosure recommended.3 Heat map colors indicate relative (rather than absolute) prevalence of that allergen (group) as a common cause of food anaphylaxis in that region.
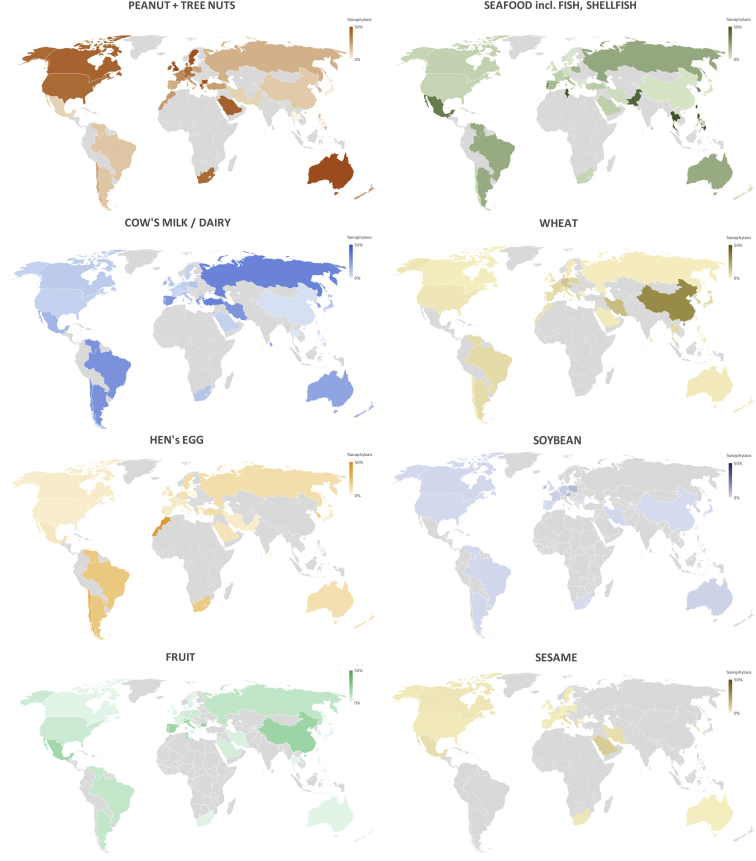
Fig 4.
Global maps showing variations in the relative proportion of reported food anaphylaxis cases due to a specific food trigger (peanut and tree nuts [combined], seafood, cow’s milk, wheat, egg, soybean, fruit [combined] and sesame), by country.
Common food triggers for anaphylaxis compared to prevalence
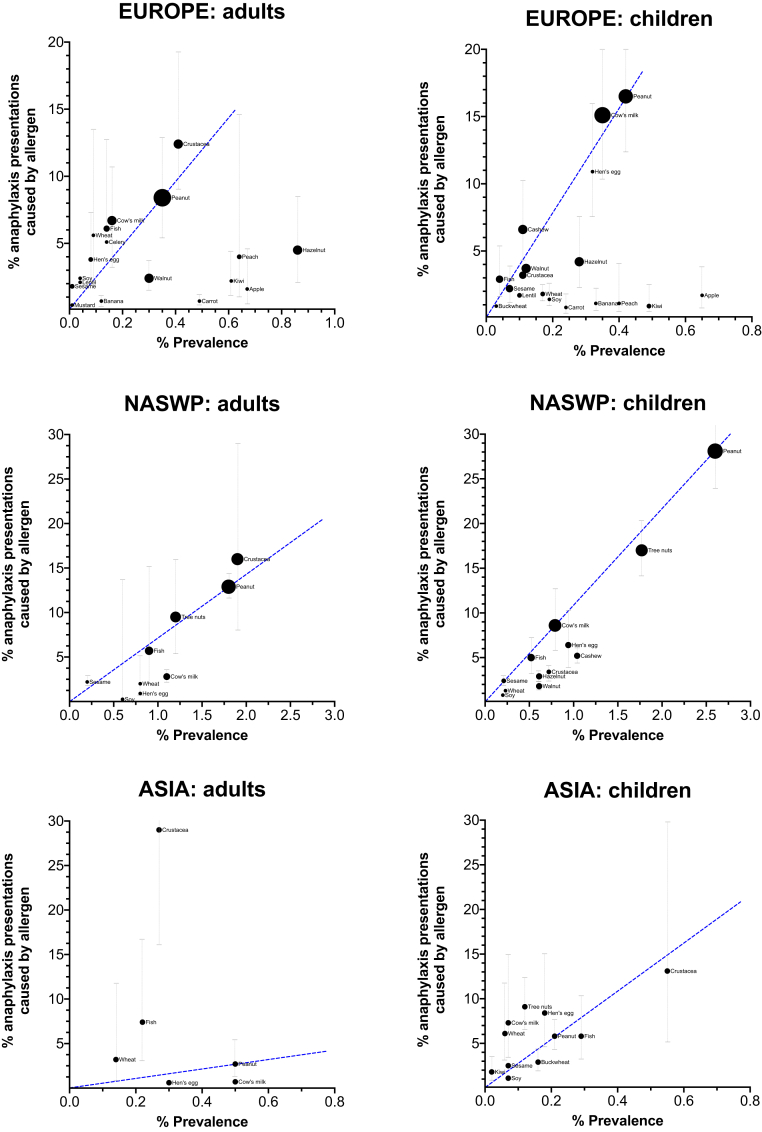
Finally, prevalence data were obtained for Europe, North America/Southwest Pacific (NASWP), and Asia from the literature, and the estimated pooled prevalence (derived from meta-analysis, and reported in Table E1 in the Online Repository at www.jacionline.org) for a specified food trigger plotted against the proportion of reported anaphylaxis reactions caused by that food (Fig 5). For Europe, crustaceans and cow’s milk appeared to cause a higher proportion of anaphylaxis in adults compared to peanut given the reported prevalence of allergy to those triggers. Hazelnut and some fruits caused a lower proportion of anaphylaxis for their reported prevalence compared to peanut; this could be due to their role as triggers for pollen–food allergy syndrome. Fish and crustaceans were common causes of anaphylaxis in adults in Asia, although this may be exaggerated by the relatively lower proportion of peanut anaphylaxis in this region.
Fig 5.
Comparison of the proportion of total food anaphylaxis caused by a specific food trigger in any given region compared to its prevalence as a cause of food allergy. Dotted lines represent 95% confidence interval (CI). The 95% CIs for prevalence estimates are reported in Table E1 in the Online Repository at www.jacionline.org. For NASWP, the bubble size represents the relative number of fatalities reported due to food anaphylaxis for the specific food trigger. (These data were not available for the Asia region.) The blue dashed line is included to facilitate comparisons of these data to peanut.
Discussion
As the food supply becomes increasingly globalized, there is a need to identify which foods should be singled out on food labels for disclosure in order to help protect food-allergic consumers. Epidemiologic data relating to prevalence and incidence of food allergy are limited by the impracticality of conducting food challenges in those with suspected allergy to distinguish between nonallergic adverse reactions to food, IgE sensitization without clinical reactivity, and true IgE-mediated food allergy with associated risk of anaphylaxis. For example, pollen–food allergy syndrome is thought to affect up to 35% of individuals in some regions,75 but such patients are considered to be at lower risk of anaphylaxis compared to those with primary food sensitization.5 In addition, the Codex requirements for allergen disclosure are for the scenario where the presence of the allergen may not be obvious (for example, in processed foods) rather than for fresh foods; because fruits and vegetables are generally visible and typically not consumed as highly processed foods, they do not currently feature as specified allergens in the Codex (although this may change in the future with the increased use of “vegetable protein concentrates”). To our knowledge, this analysis is the first in the literature to report a global assessment of the most common food triggers for anaphylaxis using a systematic approach. Rather than rely of reports of prevalence to specific food allergens that are limited by a lack of robust data,4 we instead used real-world data as to the most common causes of anaphylaxis presenting to medical facilities, as a surrogate measure to inform the choice of priority allergens for inclusion in legislation.
We found significant interregional and intraregional differences in the most common triggers for food anaphylaxis. Significant variations in the prevalence of allergy to different food triggers have been reported in Europe;76,77 it is therefore perhaps not surprising that similar differences were also evident for anaphylaxis, both within and between Codex regions. Peanut and tree nuts are a common cause of anaphylaxis in the European and NASWP regions, but less so in Asia. Wheat is generally less common as a cause of anaphylaxis, but it accounts for a disproportionate number of anaphylaxis presentations in China. These differences can potentially present a challenge for the regulation of food allergens within the supply chain, as food products produced and packaged in one country are often consumed in another; in addition, tourism can also significantly impact the specific food allergies that consumers might present with. In this respect, it is reassuring that in general, there was good agreement between local legislative requirements for allergen disclosure and the most common allergens causing anaphylaxis in that locality.
It was also revealing to compare the relative frequencies of food triggers causing anaphylaxis compared to their reported prevalence in causing food allergy. Data were available for this comparison for Europe, NASWP, and Asia. Using peanut as a reference allergen, our data indicate that crustaceans appear to cause a disproportionate number of anaphylaxis reactions in all 3 regions in adults. Interestingly, cow’s milk allergy also appears to cause a greater-than-expected proportion of anaphylaxis in children in Europe and Asia. Cow’s milk allergy may be considered to be a less serious food allergy, as it is commonly outgrown in early childhood. However, there are increasing data that in older children with persisting allergy to cow’s milk, it is a common cause of not just anaphylaxis but also near-fatal and fatal anaphylaxis.11,12,16 For example, in Greece, cow’s-milk allergy is relatively uncommon compared to the rest of Europe,76,77 yet it still accounts for around one quarter of anaphylaxis presentations.18 This may be due to a lower awareness of cow’s milk as a potential cause of severe reactions, as well as its ubiquitous use in Western-style diets, particularly in processed foods.
Conversely, at least in Europe, some fruits and tree nuts appeared to be less likely to cause anaphylaxis, presumably because these data do not distinguish between allergy due to primary food sensitization (with higher risk of anaphylaxis) and pollen–food allergy syndrome. Fruit as a food group was a common cause of anaphylaxis globally. However, the likely impact of differences in patterns of cross-sensitization and cross-reactivity are not obvious from these data. In Northern Europe, allergy to fruit is commonly associated with birch pollen sensitization; in Mediterranean regions, lipid transfer protein (LTP), particularly peach LTP, is also a common cause, which appears to be independent of pollen sensitization.76 However, in China, peach is also a relatively common cause of anaphylaxis, but this is usually associated with cross-reactivity to mugwort pollen; in contrast to European LTP allergy, LTP-related anaphylaxis in China is often due to primary sensitization to mugwort.78 More research is needed to better understand the clinical implications of geographic differences in sensitization patterns between different plant-derived allergens.
Strengths and limitations of this study
The inclusion of global data sets identified through a systematic search of the literature is a key strength of this analysis. However, it is important to note the limitations of this analysis: different definitions were used to assign both anaphylaxis and the causative trigger, including International Classification of Disease (9th or 10th revision) codes, which are subject to miscoding.79 We believe that even with this limitation, the data would still represent the more severe end of the spectrum of allergic symptoms. The proportion of anaphylaxis due to any given specific food trigger is dependent on multiple factors, including underlying prevalence of allergy to that trigger within the population, consumption patterns, inherent ability of that allergen to cause more severe reactions, and host factors such as IgE sensitization. While these factors are all potential confounders, the use of real-world data provides an additional dimension to better understand which allergens are more likely to cause anaphylaxis than others. It is therefore not surprising that there is a clear correlation between prevalence of allergy to a specific food and the proportion of anaphylaxis cases it causes (Fig 3). This comparison was limited by the high uncertainty in data relating to food allergy prevalence and the very limited data from some regions. This is particularly a concern for North America, where challenge-based epidemiologic data are lacking; despite using systematic methodologies to estimate prevalence using household sampling approaches, allergy to cow’s milk in adults is apparently more common than peanut allergy (perhaps due to a lack of distinction between lactose intolerance and IgE-mediated allergy).80 The use of real-world anaphylaxis data may therefore provide less uncertainty as to the major causes of food anaphylaxis, compared to relying on estimates of food allergy prevalence alone.
Conclusion
Using a systematic approach, we identified important and often region-specific differences in the most common food allergens causing anaphylaxis across the globe. However, legislative requirements for food allergen disclosure generally mirrored the local allergens most commonly responsible for food anaphylaxis events. Cow’s milk and shellfish/crustaceans are important causes of anaphylaxis globally, in addition to peanut and tree nuts. These data support the use of location-specific epidemiology to guide both public health policy and research with respect to food allergy.
Clinical implications.
In addition to peanut and tree nuts, cow’s milk and shellfish/crustaceans are important causes of anaphylaxis globally.
Acknowledgments
We thank Moshe Ben-Shoshan and Greg Shand for providing additional data for this analysis, as well as Joseph Baumert, Simon Brooke-Taylor, Geert Houben, Stefano Luccioli, Benjamin Remington, and Stephen Taylor for useful comments on the data analysis.
Footnotes
Funded by a UK Medical Research Council Clinician Scientist award to P. J. Turner (reference MR/K010468/1). P. J. Turner is supported through the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
Disclosure of potential conflict of interest: P. J. Turner reports grants from UK Medical Research Council, NIHR/Imperial BRC, UK Food Standards Agency, and J. M. Charitable Foundation during the conduct of the study; and personal fees from UK Food Standards Agency, Aimmune Therapeutics, Allergenis, and ILSI Europe outside the submitted work. The rest of the authors declare that they have no relevant conflicts of interest.
Methods
Estimated prevalence of allergy to specific foods
Prevalence rates for individual allergens were pooled across included studies using a generalized linear mixed model in R 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/) (metaprop function, metafor package, logit transformation with a random intercept logistic regression model for the summary estimate, with a continuity correction of 0.5). This approach avoids many of the issues surrounding the use of transformations when undertaking meta-analyses of proportions.E1, E2 We conducted meta-analysis even if significant heterogeneity was seen between study estimates, as is the norm when conducting meta-analysis of proportions. Normal approximation was used for calculating confidence intervals.
For Europe, prevalence was estimated on the basis of reported rates of challenge-positive food allergy reported in a systematic reviewE3 and also generated by the EuroPrevall studies (on the basis of study-defined probable food allergy).E4, E5
For the North America/Southwest Pacific (NASWP) region, there are no prevalence data for adults that are based on food challenges in unselected populations. Instead, adult prevalence data were extracted from Gupta et alE6 and compared to equivalent data for Canada.E7 As outlined in the Discussion, as a result of concerns that the reported prevalence of cow’s-milk allergy in adults by Gupta et al is likely to be an overestimate, the equivalent figure for Canada was used instead. For prevalence of food allergy in children in the NASWP region, rates were pooled from studies reporting prevalence for the United States,E8 Canada,E7 and AustraliaE9 (only the latter incorporated food challenges to assess prevalence).
Limited data exist for the prevalence of food allergy in the Asia region.E10 For adults, data were pooled from studies conducted in TaiwanE11 and India.E12 For children, data for China and India were extracted from the EuroPrevall-INCO SurveysE13 and 2 studies from ThailandE14, E15 (all of which included food challenges to confirm food allergy), as well as published data for JapanE16 and KoreaE17 that did not rely on challenge-positive outcomes.
The pooled estimates for reported prevalence to specific food allergens are shown in Table E1.
Table E1.
Estimated prevalence of allergy to specific foods
| Characteristic | Europe |
North America/Southwest Pacific |
Asia |
|||
|---|---|---|---|---|---|---|
| Adults | Children | Adults | Children | Adults | Children | |
| Reference | E3, E4 | E3, E5 | E6, E7 | E7, E8, E9 | E11, E12 | E13, E14, E15, E16, E17 |
| Peanut, % (95% CI) | 0.35 (0.20-0.60) | 0.42 (0.25-0.70) | 1.8 (0.6-1.9) | 2.60 (2.17-3.11) | 0.46 (0.36-0.58) | 0.21 (0.17-0.27) |
| Tree nuts (combined), % (95% CI) | 1.2 (1.1-1.3) | 1.77 (1.26-2.47) | 0.12 (0-6.63) | |||
| Cashew, % (95% CI) | 0.11 (0-0.62) | 0.5 (0.5-0.6) | 1.04 (0.59-1.84) | |||
| Hazelnut, % (95% CI) | 0.86 (0.39-1.90) | 0.28 (0.10-0.77) | 0.6 (0.5-0.7) | 0.61 (0.54-0.69) | ||
| Walnut, % (95% CI) | 0.30 (0.14-0.66) | 0.12 (0.04-0.41) | 0.6 (0.6-0.7) | 0.61 (0.54-0.69) | ||
| Sesame, % (95% CI) | 0.01 (0-1.35) | 0.07 (0.01-0.88) | 0.2 (0.2-0.3) | 0.21 (0.17-0.25) | 0.07 (0.05-0.10) | |
| Mustard, % (95% CI) | 0.00 (0-2.29) | |||||
| Wheat, % (95% CI) | 0.09 (0.03-0.29) | 0.16 (0.09-0.29) | 0.8 (0.7-0.9) | 0.23 (0.10-0.53) | 0.14 (0.04-0.53) | 0.06 (0.02-0.23) |
| Buckwheat, % (95% CI) | 0.03 (0-0.37) | 0.16 (0.13-0.21) | ||||
| Hen’s egg, % (95% CI) | 0.08 (0.03-0.25) | 0.32 (0.17-0.60) | 0.8 (0.7-0.9) | 0.94 (0.53-1.67) | 0.30 (0.22-0.40) | 0.18 (0.04-0.80) |
| Cow’s milk, % (95% CI) | 0.16 (0.07-0.35) | 0.35 (0.20-0.63) | 1.1 (0.9-2.1)∗ | 0.79 (0.26-2.33) | 0.48 (0.38-0.61) | 0.07 (0.01-0.82) |
| Celery, % (95% CI) | 0.14 (0.04-0.45) | 0.04 (0-1.50) | ||||
| Shrimp/crustaceans, % (95% CI) | 0.41 (0.21-0.80) | 0.11 (0.04-0.29) | 1.9 (1.8-2.1) | 0.72 (0.36-1.45) | 0.27 (0-7.5) | 0.55 (0.36-0.84) |
| Fish, % (95% CI) | 0.14 (0.06-0.33) | 0.04 (0.01-0.18) | 0.9 (0.8-1.0) | 0.52 (0.24-1.12) | 0.22 (0.01-1.4) | 0.29 (0.26-0.32) |
| Soybean, % (95% CI) | 0.04 (0.01-0.19) | 0.19 (0.07-0.57) | 0.6 (0.5-0.7) | 0.20 (0.06-0.66) | 0.07 (0.06-0.09) | |
| Lentil, % (95% CI) | 0.04 (0.01-0.35) | 0.10 (0.01-0.79) | ||||
| Peach, % (95% CI) | 0.64 (0.24-1.67) | 0.40 (0.20-0.80) | ||||
| Kiwi, % (95% CI) | 0.61 (0.32-1.17) | 0.49 (0.28-0.83) | 0.02 (0-0.70) | |||
| Banana, % (95% CI) | 0.12 (0.02-0.71) | 0.33 (0.13-0.85) | ||||
| Apple, % (95% CI) | 0.67 (0.30-1.51) | 0.65 (0.31-1.36) | ||||
| Carrot, % (95% CI) | 0.49 (0.27-0.91) | 0.24 (0.11-0.51) | ||||
For further details, see this article’s Supplementary Methods.
Prevalence based on Canadian data.
References
- 1.Food and Agriculture Organization of the United Nations; World Health Organization; Codex Alimentarius Commission General standard for the labelling of prepackaged foods. 1985. http://www.fao.org/ag/humannutrition/32500-0b35b42f09191500b210bf4812e762381.pdf Available at:
- 2.Prescott S.L., Pawankar R., Allen K.J., Campbell D.E., Sinn J.K.h., Fiocchi A., et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen K.J., Turner P.J., Pawankar R., Taylor S., Sicherer S., Lack G., et al. Precautionary labelling of foods for allergen content: are we ready for a global framework? World Allergy Organ J. 2014;7:10. doi: 10.1186/1939-4551-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Allen K.J., Suaini N.H.A., McWilliam V., Peters R.L., Koplin J.J. The global incidence and prevalence of anaphylaxis in children in the general population: a systematic review. Allergy. 2019;74:1063–1080. doi: 10.1111/all.13732. [DOI] [PubMed] [Google Scholar]
- 5.Turner P.J., Campbell D.E. A food allergy syndrome by any other name? Clin Exp Allergy. 2014;44:1458–1460. doi: 10.1111/cea.12425. [DOI] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 7.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarzer G., Chemaitelly H., Abu-Raddad L.J., Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10:476–483. doi: 10.1002/jrsm.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullins R.J., Wainstein B.K., Barnes E.H., Liew W.K., Campbell D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. 2016;46:1099–1110. doi: 10.1111/cea.12748. [DOI] [PubMed] [Google Scholar]
- 11.Turner P.J., Gowland M.H., Sharma V., Ierodiakonou D., Harper N., Garcez T., et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol. 2015;135:956–963.e1. doi: 10.1016/j.jaci.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baseggio Conrado A., Ierodiakonou D., Gowland M.H., Boyle R.J., Turner P.J. Food anaphylaxis in the United Kingdom: an analysis of national data, 1998-2018. BMJ. Published online February 17, 2021. doi:10.1136/bmj.n251. Erratum in: BMJ. March 19, 2021 doi: 10.1136/bmj.n733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirot E., He F., Gould L.H., Hadler J.L. Deaths, hospitalizations, and emergency department visits from food-related anaphylaxis, New York City, 2000-2014: implications for fatality prevention. J Public Health Manag Pract. 2020;26:548–556. doi: 10.1097/PHH.0000000000001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y.S., Kastner M., Harada L., Xu A., Salter J., Waserman S. Anaphylaxis-related deaths in Ontario: a retrospective review of cases from 1986 to 2011. Allergy Asthma Clin Immunol. 2014;10:38. doi: 10.1186/1710-1492-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouessel G., Beaudouin E., Tanno L.K., Drouet M., Deschildre A., Labreuche J., et al. Food-related anaphylaxis fatalities: analysis of the Allergy Vigilance Network® database. Allergy. 2019;74:1193–1196. doi: 10.1111/all.13717. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey N.B., Guffey D., Anagnostou K., Coleman N.E., Davis C.M. Epidemiology of anaphylaxis in critically ill children in the United States and Canada. J Allergy Clin Immunol Pract. 2019;7:2241–2249. doi: 10.1016/j.jaip.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pouessel G., Chagnon F., Trochu C., Labreuche J., Lejeune S., Recher M., et al. Anaphylaxis admissions to pediatric intensive care units in France. Allergy. 2018;73:1902–1905. doi: 10.1111/all.13483. [DOI] [PubMed] [Google Scholar]
- 18.Worm M., Moneret-Vautrin A., Scherer K., Lang R., Fernandez-Rivas M., Cardona V., et al. First European data from the network of severe allergic reactions (NORA) Allergy. 2014;69:1397–1404. doi: 10.1111/all.12475. [DOI] [PubMed] [Google Scholar]
- 19.Grabenhenrich L.B., Dölle S., Moneret-Vautrin A., Köhli A., Lange L., Spindler T., et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137:1128–1137.e1. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Aurich S., Dölle-Bierke S., Francuzik W., Bilo M.B., Christoff G., Fernandez-Rivas M., et al. Anaphylaxis in elderly patients—data from the European Anaphylaxis Registry. Front Immunol. 2019;10:750. doi: 10.3389/fimmu.2019.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown S.G., Stone S.F., Fatovich D.M., Burrows S.A., Holdgate A., Celenza A., et al. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132:1141–1149.e5. doi: 10.1016/j.jaci.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 22.McWilliam V.L., Koplin J.J., Field M.J., Sasaki M., Dharmage S.C., Tang M.L.K., et al. Self-reported adverse food reactions and anaphylaxis in the SchoolNuts study: a population-based study of adolescents. J Allergy Clin Immunol. 2018;141:982–990. doi: 10.1016/j.jaci.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Andrew E., Nehme Z., Bernard S., Smith K. Pediatric anaphylaxis in the prehospital setting: incidence, characteristics, and management. Prehosp Emerg Care. 2018;22:445–451. doi: 10.1080/10903127.2017.1402110. [DOI] [PubMed] [Google Scholar]
- 24.Charatsi A., Mulier S., Aversano G., Casimir G. Food anaphylaxis experience in children in Brussels. Clin Transl Allergy. 2014;4(suppl 1):P87. [Google Scholar]
- 25.Asai Y., Yanishevsky Y., Clarke A., La Vieille S., Delaney J.S., Alizadehfar R., et al. Rate, triggers, severity and management of anaphylaxis in adults treated in a Canadian emergency department. Int Arch Allergy Immunol. 2014;164:246–252. doi: 10.1159/000365631. [DOI] [PubMed] [Google Scholar]
- 26.Gabrielli S., Clarke A., Morris J., Eisman H., Gravel J., Enarson P., et al. Evaluation of prehospital management in a Canadian emergency department anaphylaxis cohort. J Allergy Clin Immunol Pract. 2019;7:2232–2238.e3. doi: 10.1016/j.jaip.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Feuerhake T., Aguilera-Insunza R., Morales P.S., Talesnik E., Linn K., Thone N., et al. Clinical characterization of Chilean patients with IgE-mediated food allergy. Rev Chil Pediatr. 2018;89:448–453. doi: 10.4067/S0370-41062018005000403. [DOI] [PubMed] [Google Scholar]
- 28.Jiang N., Yin J., Wen L., Li H. Characteristics of anaphylaxis in 907 Chinese patients referred to a tertiary allergy center: a retrospective study of 1,952 episodes. Allergy Asthma Immunol Res. 2016;8:353–361. doi: 10.4168/aair.2016.8.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz Oropeza A., Lassen A., Halken S., Bindslev-Jensen C., Mortz C.G. Anaphylaxis in an emergency care setting: a one year prospective study in children and adults. Scand J Trauma Resusc Emerg Med. 2017;25:111. doi: 10.1186/s13049-017-0402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corriger J., Beaudouin E., Rothmann R., Penven E., Haumonte Q., Thomas H., et al. Epidemiological data on anaphylaxis in French emergency departments. J Investig Allergol Clin Immunol. 2019;29:357–364. doi: 10.18176/jiaci.0348. [DOI] [PubMed] [Google Scholar]
- 31.Renaudin J., Beaumont P., Sabouraud D., Dumond P., Liabeuf V., Tscheiller S., et al. Anaphylaxie alimentaire sévère : données recueillies par le Réseau d’Allergo-Vigilance® (2002-2017) et allergènes émergents. Rev Fr Allergologie. 2017;57:e3–e7. [Google Scholar]
- 32.Li P.H., Leung A.S.Y., Li R.M.Y., Leung T.F., Lau C.S., Wong G.W.K. Increasing incidence of anaphylaxis in Hong Kong from 2009 to 2019—discrepancies of anaphylaxis care between adult and paediatric patients. Clin Transl Allergy. 2020;10:51. doi: 10.1186/s13601-020-00355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barzegar S., Rosita A., Pourpak Z., Bemanian M.H., Shokouhi R., Mansouri M., et al. Common causes of anaphylaxis in children: the first report of anaphylaxis registry in Iran. World Allergy Organ J. 2010;3:9–13. doi: 10.1097/WOX.0b013e3181c82128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahanchian H., Behmanesh F., Azad F.J., Ansari E., Khoshkhui M., Farid R., et al. A survey of anaphylaxis etiology and treatment. Med Gas Res 2019;8:129-134. Retracted in: Med Gas Res. 2019;9:54. doi: 10.4103/2045-9912.248262. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Nabavi M., Lavavpour M., Arshi S., Bemanian M.H., Esmaeilzadeh H., Molatefi R., et al. Characteristics, etiology and treatment of pediatric and adult anaphylaxis in Iran. Iran J Allergy Asthma Immunol. 2017;16:480–487. [PubMed] [Google Scholar]
- 36.Maris I., O’Sullivan R., Hourihane J. Food induced anaphylaxis in Irish children. Clin Transl Allergy. 2015;5(suppl 3):P102. [Google Scholar]
- 37.Cohen N., Capua T., Pivko-Levy D., Ben-Shoshan M., Rimon A., Benor S. Improved diagnosis and treatment of anaphylaxis in a pediatric emergency department (2013-2018) J Allergy Clin Immunol Pract. 2019;7:2882–2884.e2. doi: 10.1016/j.jaip.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 38.Rolla G., Mietta S., Raie A., Bussolino C., Nebiolo F., Galimberti M., et al. Incidence of food anaphylaxis in Piemonte region (Italy): data from registry of Center for Severe Allergic Reactions. Intern Emerg Med. 2013;8:615–620. doi: 10.1007/s11739-013-0978-y. [DOI] [PubMed] [Google Scholar]
- 39.Muramatsu K, Imamura H, Tokutsu K, Fujimoto K, Fushimi K, Matsuda S. Epidemiological study of hospital admissions for food-induced anaphylaxis using the Japanese Diagnosis Procedure Combination Database. J Epidemiol. Published online November 28, 2020. https://doi.org/10.2188/jea.JE20200309 [DOI] [PMC free article] [PubMed]
- 40.Imamura T., Kanagawa Y., Ebisawa M. A survey of patients with self-reported severe food allergies in Japan. Pediatr Allergy Immunol. 2008;19:270–274. doi: 10.1111/j.1399-3038.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 41.Solé D., Ivancevich J.C., Borges M.S., Coelho M.A., Rosário N.A., Ardusso L., et al. Anaphylaxis in Latin American children and adolescents: the Online Latin American Survey on Anaphylaxis (OLASA) Allergol Immunopathol (Madr) 2012;40:331–335. doi: 10.1016/j.aller.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Bedolla-Barajas M., Bedolla-Pulido T.R., Camacho-Peña A.S., González-García E., Morales-Romero J. Food hypersensitivity in mexican adults at 18 to 50 years of age: a questionnaire survey. Allergy Asthma Immunol Res. 2014;6:511–516. doi: 10.4168/aair.2014.6.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ontiveros N., Valdez-Meza E.E., Vergara-Jiménez M.J., Canizalez-Román A., Borzutzky A., Cabrera-Chávez F. Parent-reported prevalence of food allergy in Mexican schoolchildren: a population-based study. Allergol Immunopathol (Madr) 2016;44:563–570. doi: 10.1016/j.aller.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Ouahidi I., Aarab L., Dutau G. The effect of thermic and acid treatment on the allergenicity of peanut proteins among the population of the region of Fes-Meknes in Morocco. Rev Francaise Allergol. 2010;50:15–21. [Google Scholar]
- 45.Kool B., Chandra D., Fitzharris P. Adult food-induced anaphylaxis hospital presentations in New Zealand. Postgrad Med J. 2016;92(1093):640–644. doi: 10.1136/postgradmedj-2015-133530. [DOI] [PubMed] [Google Scholar]
- 46.Speakman S., Kool B., Sinclair J., Fitzharris P. Paediatric food-induced anaphylaxis hospital presentations in New Zealand. J Paediatr Child Health. 2018;54:254–259. doi: 10.1111/jpc.13705. [DOI] [PubMed] [Google Scholar]
- 47.Khan N.U., Shakeel N., Makda A., Mallick A.S., Ali Memon M., Hashmi S.H., et al. Anaphylaxis: incidence, presentation, causes and outcome in patients in a tertiary-care hospital in Karachi, Pakistan. QJM. 2013;106:1095–1101. doi: 10.1093/qjmed/hct179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Vera M.J., Tagaro I.C. Anaphylaxis diagnosis and management in the emergency department of a tertiary hospital in the Philippines. Asia Pac Allergy. 2020;10:e1. doi: 10.5415/apallergy.2020.10.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poziomkowska-Gęsicka I., Kurek M. Clinical manifestations and causes of anaphylaxis. analysis of 382 cases from the anaphylaxis registry in West Pomerania province in Poland. Int J Environ Res Public Health. 2020;17:2787. doi: 10.3390/ijerph17082787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaspar A, Santos N, Faria E, Câmara R, Rodrigues-Alves R, Carrapatoso I, et al. Anaphylaxis: a decade of a nationwide allergy society registry. J Investig Allergol Clin Immunol. Published online July 30, 2020. https://doi.org/10.18176/jiaci.0515 [DOI] [PubMed]
- 51.Abunada T., Al-Nesf M.A., Thalib L., Kurdi R., Khalil S., El Kassem W., et al. Anaphylaxis triggers in a large tertiary care hospital in Qatar: a retrospective study. World Allergy Organ J. 2018;11:20. doi: 10.1186/s40413-018-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esakova N.V., Treneva M.S., Okuneva T.S., Pampura A.N. Food anaphylaxis: reported cases in Russian Federation children. Am J Public Health Res. 2015;3:187–191. [Google Scholar]
- 53.Alkanhal R., Alhoshan I., Aldakhil S., Alromaih N., Alharthy N., Salam M., et al. Prevalence triggers and clinical severity associated with anaphylaxis at a tertiary care facility in Saudi Arabia: a cross-sectional study. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000011582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goh S.H., Soh J.Y., Loh W., Lee K.P., Tan S.C., Heng W.J.K., et al. Cause and clinical presentation of anaphylaxis in Singapore: from infancy to old age. Int Arch Allergy Immunol. 2018;175:91–98. doi: 10.1159/000485127. [DOI] [PubMed] [Google Scholar]
- 55.Chippendale S., Levin M. Severe allergic reactions at a tertiary paediatric service, 2014-2016. University of Cape Town, South Africa, 2018. open.uct.ac.za/handle/11427/29566 Available at:
- 56.Lee S.Y., Ahn K., Kim J., Jang G.C., Min T.K., Yang H.J., et al. A multicenter retrospective case study of anaphylaxis triggers by age in korean children. Allergy Asthma Immunol Res. 2016;8:535–540. doi: 10.4168/aair.2016.8.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho H., Kim D., Choo Y., Park J., Choi J., Jang D., et al. Common causes of emergency department visits for anaphylaxis in Korean community hospitals: a cross-sectional study. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000014114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeong K., Ye Y.M., Kim S.H., Kim K.W., Kim J.H., Kwon J.W., et al. A multicenter anaphylaxis registry in Korea: clinical characteristics and acute treatment details from infants to older adults. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moro Moro M., Tejedor Alonso M.A., Esteban Hernández J., Múgica García M.V., Rosado Ingelmo A., Vila Albelda C. Incidence of anaphylaxis and subtypes of anaphylaxis in a general hospital emergency department. J Investig Allergol Clin Immunol. 2011;21:142–149. [PubMed] [Google Scholar]
- 60.Alvarez-Perea A., Ameiro B., Morales C., Zambrano G., Rodríguez A., Guzmán M., et al. Anaphylaxis in the pediatric emergency department: analysis of 133 cases after an allergy workup. J Allergy Clin Immunol Pract. 2017;5:1256–1263. doi: 10.1016/j.jaip.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Lacombe-Barrios J., Gómez F., Pérez N., Barrionuevo E., Doña I., Fernández Tahía D., et al. Accuracy of the diagnosis of allergic reactions in the emergency department. J Investig Allergol Clin Immunol. 2019;29:222–230. doi: 10.18176/jiaci.0313. [DOI] [PubMed] [Google Scholar]
- 62.de Silva N.R., Dasanayake W.M.D.K., Karunatilake C., Wickramasingha G.D., De Silva B.D., Malavige G.N. Aetiology of anaphylaxis in patients referred to an immunology clinic in Colombo, Sri Lanka. Allergy Asthma Clin Immunol. 2018;14:81. doi: 10.1186/s13223-018-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vetander M., Helander D., Flodström C., Ostblom E., Alfvén T., Ly D.H., et al. Anaphylaxis and reactions to foods in children—a population-based case study of emergency department visits. Clin Exp Allergy. 2012;42:568–577. doi: 10.1111/j.1365-2222.2011.03954.x. [DOI] [PubMed] [Google Scholar]
- 64.Chan C.F., Chen P.H., Huang C.F., Wu T.C. Emergency department visits for food allergy in Taiwan: a retrospective study. Pediatr Neonatol. 2014;55:275–281. doi: 10.1016/j.pedneo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Manuyakorn W., Benjaponpitak S., Kamchaisatian W., Vilaiyuk S., Sasisakulporn C., Jotikasthira W. Pediatric anaphylaxis: triggers, clinical features, and treatment in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2015;33:281–288. doi: 10.12932/AP0610.33.4.2015. [DOI] [PubMed] [Google Scholar]
- 66.Rangkakulnuwat P., Sutham K., Lao-Araya M. Anaphylaxis: ten-year retrospective study from a tertiary-care hospital in Asia. Asian Pac J Allergy Immunol. 2020;38:31–39. doi: 10.12932/AP-210318-0284. [DOI] [PubMed] [Google Scholar]
- 67.Ghazali H., Gammoudi M., Yahmadi A., Chaaebeni G., Souyah A., Souissi S. Anaphylaxis in an emergency department: epidemiology, clinical features and management. Tunis Med. 2017;95:45–52. [PubMed] [Google Scholar]
- 68.Civelek E., Erkoçoğlu M., Akan A., Özcan C., Kaya A., Vezir E., et al. The etiology and clinical features of anaphylaxis in a developing country: a nationwide survey in Turkey. Asian Pac J Allergy Immunol. 2017;35:212–219. doi: 10.12932/AP0752. [DOI] [PubMed] [Google Scholar]
- 69.Vezir E., Erkoçoğlu M., Kaya A., Toyran M., Özcan C., Akan A., et al. Characteristics of anaphylaxis in children referred to a tertiary care center. Allergy Asthma Proc. 2013;34:239–246. doi: 10.2500/aap.2013.34.3654. [DOI] [PubMed] [Google Scholar]
- 70.Noimark L., Wales J., Du Toit G., Pastacaldi C., Haddad D., Gardner J., et al. The use of adrenaline autoinjectors by children and teenagers. Clin Exp Allergy. 2012;42:284–292. doi: 10.1111/j.1365-2222.2011.03912.x. [DOI] [PubMed] [Google Scholar]
- 71.Huang F., Chawla K., Järvinen K.M., Nowak-Węgrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012;129:162–168.e1-e3. doi: 10.1016/j.jaci.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Motosue M.S., Bellolio M.F., Van Houten H.K., Shah N.D., Campbell R.L. National trends in emergency department visits and hospitalizations for food-induced anaphylaxis in US children. Pediatr Allergy Immunol. 2018;29:538–544. doi: 10.1111/pai.12908. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez-Estrada A., Silvers S.K., Klein A., Zell K., Wang X.F., Lang D.M. Epidemiology of anaphylaxis at a tertiary care center: a report of 730 cases. Ann Allergy Asthma Immunol. 2017;118:80–85. doi: 10.1016/j.anai.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 74.Dyer A.A., Lau C.H., Smith T.L., Smith B.M., Gupta R.S. Pediatric emergency department visits and hospitalizations due to food-induced anaphylaxis in Illinois. Ann Allergy Asthma Immunol. 2015;115:56–62. doi: 10.1016/j.anai.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Carlson G., Coop C. Pollen food allergy syndrome (PFAS): a review of current available literature. Ann Allergy Asthma Immunol. 2019;123:359–365. doi: 10.1016/j.anai.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 76.Lyons S.A., Burney P.G.J., Ballmer-Weber B.K., Fernandez-Rivas M., Barreales L., Clausen M., et al. Food allergy in adults: substantial variation in prevalence and causative foods across Europe. J Allergy Clin Immunol Pract. 2019;7:1920–1928.e11. doi: 10.1016/j.jaip.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 77.Lyons S.A., Clausen M., Knulst A.C., Ballmer-Weber B.K., Fernandez-Rivas M., Barreales L., et al. Prevalence of food sensitization and food allergy in children across Europe. J Allergy Clin Immunol Pract. 2020;8:2736–2746.e9. doi: 10.1016/j.jaip.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 78.Deng S., Yin J. Mugwort pollen–related food allergy: lipid transfer protein sensitization and correlation with the severity of allergic reactions in a Chinese population. Allergy Asthma Immunol Res. 2019;11:116–128. doi: 10.4168/aair.2019.11.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turner P.J., Campbell D.E. Epidemiology of severe anaphylaxis: can we use population-based data to understand anaphylaxis? Curr Opin Allergy Clin Immunol. 2016;16:441–450. doi: 10.1097/ACI.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gupta R.S., Warren C.M., Smith B.M., Jiang J., Blumenstock J.A., Davis M.M., et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2018.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G., Chemaitelly H., Abu-Raddad L.J., Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10:476–483. doi: 10.1002/jrsm.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaru B.I., Hickstein L., Panesar S.S., Roberts G., Muraro A., Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- Lyons S.A., Burney P.G.J., Ballmer-Weber B.K., Fernandez-Rivas M., Barreales L., Clausen M., et al. Food allergy in adults: substantial variation in prevalence and causative foods across Europe. J Allergy Clin Immunol Pract. 2019;7:1920–1928.e11. doi: 10.1016/j.jaip.2019.02.044. [DOI] [PubMed] [Google Scholar]
- Lyons S.A., Clausen M., Knulst A.C., Ballmer-Weber B.K., Fernandez-Rivas M., Barreales L., et al. Prevalence of food sensitization and food allergy in children across Europe. J Allergy Clin Immunol Pract. 2020;8:2736–2746.e9. doi: 10.1016/j.jaip.2020.04.020. [DOI] [PubMed] [Google Scholar]
- Gupta R.S., Warren C.M., Smith B.M., Jiang J., Blumenstock J.A., Davis M.M., et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2018.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A.E., Elliott S.J., St Pierre Y., Soller L., La Vieille S., Ben-Shoshan M. Temporal trends in prevalence of food allergy in Canada. J Allergy Clin Immunol Pract. 2020;8:1428–1430.e5. doi: 10.1016/j.jaip.2019.10.021. [DOI] [PubMed] [Google Scholar]
- Gupta R.S., Warren C.M., Smith B.M., Blumenstock J.A., Jiang J., Davis M.M., et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142 doi: 10.1542/peds.2018-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Koplin J.J., Dharmage S.C., Field M.J., Sawyer S.M., McWilliam V., et al. Prevalence of clinic-defined food allergy in early adolescence: the SchoolNuts study. J Allergy Clin Immunol. 2018;141:391–398.e4. doi: 10.1016/j.jaci.2017.05.041. [DOI] [PubMed] [Google Scholar]
- Lee A.J., Thalayasingam M., Lee B.W. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3:3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.C., Tsai T.C., Huang C.F., Chang F.Y., Lin C.C., Huang I.F., et al. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012;42:1310–1315. doi: 10.1111/j.1445-5994.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- Mahesh P.A., Wong G.W., Ogorodova L., Potts J., Leung T.F., Fedorova O., et al. Prevalence of food sensitization and probable food allergy among adults in India: the EuroPrevall INCO study. Allergy. 2016;71:1010–1019. doi: 10.1111/all.12868. [DOI] [PubMed] [Google Scholar]
- Li J., Ogorodova L.M., Mahesh P.A., Wang M.H., Fedorova O.S., Leung T.F., et al. Comparative study of food allergies in children from China, India, and Russia: the EuroPrevall-INCO surveys. J Allergy Clin Immunol Pract. 2020;8:1349–1358.e16. doi: 10.1016/j.jaip.2019.11.042. [DOI] [PubMed] [Google Scholar]
- Lao-araya M., Trakultivakorn M. Prevalence of food allergy among preschool children in northern Thailand. Pediatr Int. 2012;54:238–243. doi: 10.1111/j.1442-200X.2011.03544.x. [DOI] [PubMed] [Google Scholar]
- Santadusit S., Atthapaisalsarudee S., Vichyanond P. Prevalence of adverse food reactions and food allergy among Thai children. J Med Assoc Thai. 2005;88(suppl 8):S27–S32. [PubMed] [Google Scholar]
- Yamamoto-Hanada K., Pak K., Saito-Abe M., Yang L., Sato M., Irahara M., et al. Allergy and immunology in young children of Japan: the JECS cohort. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Lee J.Y., Jeon H.Y., Yang H.K., Lee K.J., Han Y., et al. Prevalence of immediate-type food allergy in Korean schoolchildren in 2015: a nationwide, population-based study. Allergy Asthma Immunol Res. 2017;9:410–416. doi: 10.4168/aair.2017.9.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]