Abstract
Background:
Treatment studies of children and adolescents with internalizing disorders suggest that the combination of a selective serotonin reuptake inhibitor (SSRI) and cognitive behavioral therapy (CBT) consistently produces greater improvement than either treatment alone. We sought to determine how response to combined treatment varies across disorders (anxiety versus depression), and by specific patient characteristics.
Methods:
Three large National Institutes of Health-funded trials of children and adolescents with major depression (n=2) and anxiety disorders (n=1) were evaluated, each comparing CBT + SSRI to SSRI only, Bayesian Hierarchical Models (BHMs) were used, for endpoint response, time course of response and predictors of response in participants who received SSRI or SSRI+CBT.
Results:
SSRI+CBT significantly decreased symptoms by week 4 (p<0.001) across disorders. This improvement continued at week 8 and 12 (p<0.001); however, the additive benefit of CBT over SSRI monotherapy was not statistically significant until week 12 (p<0.001). The fastest response to SSRI+CBT was for patients who were younger, with milder baseline anxiety/depression symptoms and depressive disorders. The slowest response for SSRI+CBT was for boys, adolescents, minoritized children, those with severe symptoms and externalizing disorders.
Limitations:
Limitations included inconsistent moderators, variation in the number of observations over time and a lack of genetic or pharmacokinetic variables related to SSRI exposure across studies.
Conclusions:
The superiority of SSRI+CBT for youth with depression and anxiety is further supported. For purposes of rapid and greater relief, combination treatment is the superior approach across anxiety and depression and is robust to a range of participant characteristics. However, the added value of CBT (with an SSRI) occurs late in treatment. These findings represent a step towards understanding heterogeneity of treatment response and raise the possibility that interventions could be better tailored or adapted based on patient characteristics.
Keywords: anxiety, depression, major depressive disorder, SSRI, paroxetine, sertraline, fluoxetine, clinical trial
Background
Anxiety and depression are among the most common psychiatric conditions of childhood and adolescence, and they carry a risk for devastating long-term sequelae (e.g., heightened risk of suicide, educational underachievement, impaired parent and peer relationships, and secondary mental health disorders such as substance use). Selective serotonin reuptake inhibitors (SSRIs) (Asarnow et al., 2009; Emslie et al., 2009, 2002; March et al., 2009) offer a well-supported, evidence-based intervention strategy that can meaningfully improve symptoms and functioning. Ample evidence documents their safety and tolerability in children and adolescents, and they offer the added advantage of being readily available in most communities. And while SSRIs are useful as a monotherapy, when combined with cognitive behavior therapy (CBT, COMB) they consistently produce the largest positive effects for youth anxiety (Walkup et al., 2008) and depression (Brent et al., 2008a; March et al., 2009). What is less understood, however, is which patients are likely to benefit most from combination treatment versus SSRI monotherapy, and what kinds of differences to expect in magnitude or rate of response when choosing this approach. Such information is crucial given the context of limited resources and the investment of time and money that comes with initiating a course of CBT.
Prior multi-site trials of treatments for youth anxiety and depressive disorders provide a valuable platform for examining which treatments work best for which youth. The following NIH-funded, studies have compared SSRI, CBT, and COMB: the Child/Adolescent Anxiety Multimodal Study (CAMS) (N=488), the Treatment of SSRI-Resistant Depression Study (TORDIA) (N=334) and the Treatment of Adolescents with Depression Study (TADS) (N=438). These were sophisticated studies with systematic, evidence-based assessment and well-characterized samples. In each, combination treatment outperformed medication monotherapy for average endpoint response (CAMS: 81% for combination therapy vs. 55% for SSRI monotherapy; TADS: 71% for combination therapy vs. 61% for SSRI monotherapy; TORDIA: 55% for combination therapy vs. 40% for SSRI monotherapy) (Brent et al., 2008a; March et al., 2009; Walkup et al., 2008). Despite this average endpoint response across treatments, however, many youth failed to significantly improve. The reasons for this remain poorly understood but are directly relevant to practice (Brent et al., 2008a; March et al., 2009; Walkup et al., 2008). Additionally, several international trials have attempted to understand the contribution of this heterogeneity using a more pragmatic design, including the Adolescent Depression Antidepressant and Psychotherapy Trial (ADAPT) (Goodyer et al., 2007) and the Youth Depression Alleviation-Combined Treatment (YoDA-C) (Davey et al., 2019), although these studies introduced additional heterogeneity with the inclusion of young adults (age 18-25 years), partial blinding, assessment of outcomes by non-clinicians, and relatively few acute outcomes being measured.
TADS, TORDIA, and CAMS laid the foundation for understanding the potentially synergistic effects of CBT and SSRIs and provided a glimpse into individual predictors of treatment response. Across all three studies, lower levels of baseline symptom severity predicted better treatment response (Asarnow et al., 2009; Compton et al., 2014; Curry et al., 2006). Additionally, being younger in TADS (Curry et al., 2006), having less family conflict and no non-suicidal self-injurious behavior in TORDIA (Asarnow et al., 2009), and having lower caregiver strain and a primary diagnosis other than social anxiety disorder in CAMS (Compton et al., 2014) predicted better responses across treatments. Although this information is helpful for understanding endpoint outcomes, clinicians selecting from both treatment strategies when treating youth with depressive and anxiety disorders currently lack data on individual predictors of differences in the trajectory and magnitude of response and the differences in this response. This is important given that treatment decisions are made in a fluid manner throughout the course of treatment; few providers will wait 12 weeks to consider a shift in strategy. Thus, there is a need not only to understand who responds to which treatments, but also how quickly improvement occurs in different groups. Understanding this heterogeneity of treatment response could allow us to refine treatment approaches and identify patients who would benefit most from specific interventions, particularly as different patients may benefit from monotherapy with an SSRI or CBT versus combined treatment with an SSRI and CBT.
As would be expected, response to SSRI monotherapy and SSRI+CBT varies considerably across patients. This variation results from differences in treatment delivery, timing of enrollment, prior treatment, expectations of treatment success and, importantly, unobserved differences between patients (e.g., differences in SSRI metabolism, pharmacodynamic differences). These features also vary across CAMS, TADS and TORDIA. While studies have examined individual response in subsets of patients and in individual studies, these examinations lack power or have yielded conflicting findings. Understanding this variation across patients and across these studies is better accomplished with Bayesian hierarchical models (BHMs). BHMs integrate multi-level information (e.g., individual patient, treatment, comorbidity) to estimate the change in symptoms or probability of improvement at each level of a hierarchy, with sub-models within the hierarchy combined with the observed data to account for uncertainty (Figure 1) (McGlothlin and Viele, 2018). BHMs allow observed variability to be separated so that random differences vs. true differences in treatment outcomes can be identified (McGlothlin and Viele, 2018). For example, applying a BHM to studies of depressive and anxiety disorders that use different SSRIs, allows us to examine the influence of disorder (e.g., MDD or anxiety disorder) and different SSRIs on response while controlling for the influence of other factors. Thus, the BHM allows us to determine treatment effects “in relation to the totality of information available in all the studies” (McGlothlin and Viele, 2018). Finally, while subgroup analyses have been conducted in individual trials, BHMs that evaluate multiple studies can estimate treatment effects at each level of the hierarchy (e.g., study, sex, age, disorder, type of SSRI). By including more information and using a multi-level approach, BHMs provide more accurate estimates of treatment effects compared to separately examining multiple subgroups, which boosts statistical power (McGlothlin and Viele, 2018).
Figure 1. Bayesian Hierarchical Modeling of response, across trials, in patients treated with selective serotonin reuptake inhibitors (SSRIs) or SSRIs+cognitive behavioral therapy (CBT).
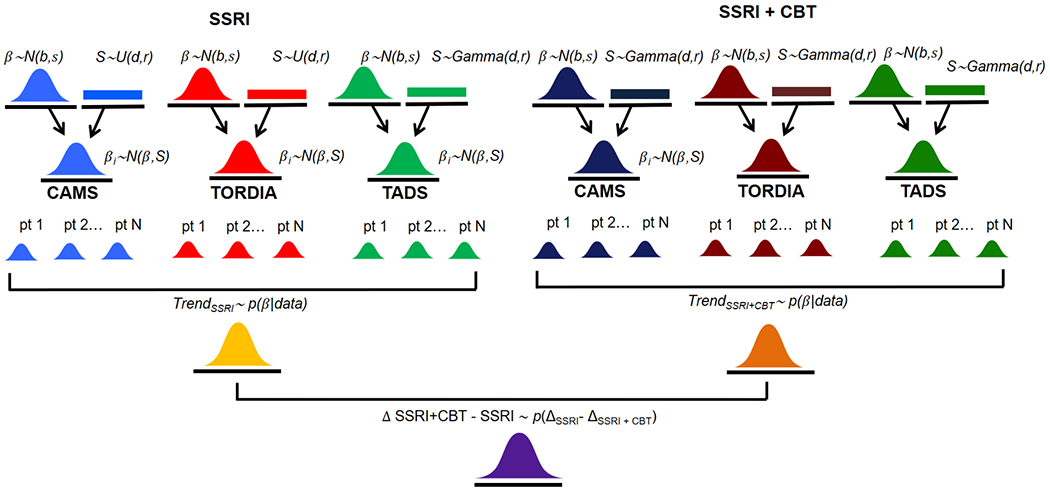
N(μ, σ) represents the normal distribution with mean, μ and standard deviation, σ. U(a, b) represents the uniform distribution in the interval a to b. p(δ|data) represents the numerically computed MCMC posterior distributions. CAMS, Child/Adolescent Anxiety Multimodal Study; TORDIA, Treatment of SSRI-Resistant Depression Study; TADS, Treatment of Adolescent Depression Study
Aggregating response data from large NIH-funded trials of SSRIs and CBT in pediatric anxiety and depressive disorders allows not only the primary comparison but also the impact of specific variables to be evaluated with greater statistical power than can be accomplished in individual trials. With these considerations in mind, this study aimed to examine the magnitude and trajectory of response to SSRI or combined SSRI+CBT using BHMs and posterior updating from the three large, NIH-funded randomized controlled trials of SSRIs and SSRI+CBT in children and adolescents with anxiety and/or depressive disorders. We hypothesized that SSRI+CBT would be associated with greater improvement in symptoms (and response) compared to SSRI monotherapy and that the additional benefit of CBT would emerge later than the improvement associated with SSRI treatment, given general response patterns in the individual component studies. We further hypothesized that improvement would vary greatly among individuals (compared to the average treatment effect) and that individual response (and improvement in symptoms) for all treatments would have the greatest effect in younger patients, patients without externalizing disorders, and those with milder baseline symptoms. For patients who receive SSRI monotherapy, we hypothesized that patients with MDD would have attenuated responses compared to those without MDD given expert opinions and commentaries in the field (Mohat and Walkup) and that younger patients would have greater responses secondary to increased medication exposure. For the SSRI monotherapy and SSRI+CBT treatment models, we examined additional covariates; however, these were exploratory and were included to capture observable heterogeneity. Finally, we anticipated that additional benefits of CBT would be greatest in younger patients and in patients with anxiety disorders rather than major depressive disorder.
Methods
Source of data
The study team accessed the complete, de-identified, original TORDIA, TADS and CAMS data sets from the NIMH (Brent et al., 2008b; March, 2004; Walkup et al., 2008). The analyses focused on the acute phase of each trial in which symptom severity (based on Clinical Global Impression-Severity [CGI-S]) was determined at baseline, week 4, week 8 and week 12. While some studies included CGI-Improvement (CGI-I) at post-baseline visits and some included other dimensional measures of symptom severity, CGI-S was selected as the primary outcome as it was the consistent measure across all time points in all studies. Clinical and demographic data were extracted as previously described (Suresh et al., 2020).
Study details
TORDIA was a prospective, randomized controlled trial of outpatients (N=334) aged 12 to 18 years with MDD. Patients who failed at least 8 weeks of SSRI trial were randomized, using a 2×2 balanced factorial design, to a switch to a different SSRI (paroxetine, citalopram, or fluoxetine, 20-40 mg daily) or venlafaxine ER (150-225 mg) or to switch to a different SSRI plus CBT or to a switch to venlafaxine plus CBT. Additional study details and its results have been described elsewhere (Brent et al., 2008b, 2009; Kennard et al., 2009). Medication switches that were combined with CBT yielded higher response rates compared to a medication switch (without addition of CBT).
TADS was a prospective, randomized trial involving adolescents (aged 12-17) with MDD (N=439) who were randomized to (1) fluoxetine monotherapy (10-40 mg/d), (2) CBT monotherapy, (3) CBT + fluoxetine (10-40 mg/d), or (4) placebo. Following 12 weeks of treatment, patients who received fluoxetine + CBT were significantly more improved compared to patients who had received either CBT or fluoxetine monotherapy. Additionally, fluoxetine was superior to CBT and all active treatments were superior to placebo.
CAMS randomized children and adolescents, aged 7-17 years (N=488) to sertraline monotherapy, sertraline + CBT, CBT or placebo. Sertraline monotherapy was well tolerated and was superior to placebo, but did not statistically differ from CBT, while the combination treatment was superior to both monotherapy conditions in terms of clinical global improvement scores.
Institutional review boards at each site approved and monitored the protocol in addition to external monitoring by a data safety and monitoring board. Patients and at least one parent per participant provided informed assent and consent. For this study, no additional ethics approval or informed consent was required; this secondary analysis used publicly available data.
Symptom Severity and Response Trajectory Modeling
Individual trajectories of symptom severity and response were modeled using log-linear trend and individual log-linear trend “random effects” coefficients BHMs. Compared to a standard mixed model with repeated measures (MMRM), these models have several advantages (Suresh et al., 2020; Walker and Shostak, 2010). Logarithmic and logistic trend BHMs explicitly model a patient’s correlation in symptoms over time rather than adjusting for estimation bias, and the BHM does not rely on asymptotic theory (Gelman et al., 2004). Further, BHMs facilitate examination of the sensitivity of the results to varying degrees of heterogeneity (Suresh et al., 2020). Specifically, the BHM for individual trajectory of symptom severity was specified as follows:
| Hyperprior: | δ ~ N(μ0, σ0),. |
| Model standard error prior: | σ ~ U(a, b). |
| Individual trends prior: | δi ~ N(δ, ω0). |
| Patient-level characteristics prior (e.g., sex, age): | β ~ N(β0, v0) |
| Likelihood: | yit ~ N(μit, σ), |
where μit = α + δitrendt + (Xi × trendt)β, trendt = log(t), t = 1, 2, 3, 4, so that the δis capture unobserved heterogeneity in individual patient improvement trajectories, and the interaction terms (Xi × trendt) capture variations in trajectories across individuals due to observed individual characteristics, with Xi containing individual covariates (i.e., a treatment type indicator for CBT, age, sex, race, presence of MDD, baseline symptom severity, presence of externalizing disorder). The same models were used to analyze improvement related to specific SSRIs.
Improvement in symptoms (i.e., CGI-S score) was defined as: yit = CGISit – CGISi1 = change in symptom severity from baseline. Prior parameter values used for all BHM estimation were: α ~ N(0,1) μ0 = −0.5, σ0 = 1, a = 0.01, b = 30, β0 = 0, v0 = 1, ω0 = 0.3. Prior and posterior densities were compared to ensure the priors were relatively uninformative (i.e., reasonably flat over the domain of the posterior with non-negligible probability, Supplemental Figure 1).
For categorical response (CGI-S ≤ 2), the BHM was specified as a Bernoulli distribution with a logistic link function, yit ~ Bern(zit), zit = 1/(1 + e−μit) with hierarchical priors, α ~ N(0,1), δi ~ N(0,1), μ ~ N(0,1), β ~ N(μ, σ), σ ~ U[0.01,30].
Heterogeneity
Multi-level BHMs which specify a study level between the patient level and overall effect level distributions allows for both heterogeneity across studies and heterogeneity across individuals within each study. In a BHM, changes in heterogeneity assumptions affect the sensitivity of the model. Reducing the number of levels substantially reduces the number of model parameters, improving estimation precision at the cost of assuming greater homogeneity. Thus, models with and without study level distributions were estimated to evaluate across-study heterogeneity, and individual heterogeneity with and without across-study heterogeneity (i.e., with and without the second row of distributions in Figure 1). Finally, models with and without individual heterogeneity were also examined to evaluate the robustness of the results to changes in the heterogeneity assumption (i.e., with and without the third row of distributions in Figure 1).
Sensitivity Analyses
Additionally, to evaluate the robustness of the results, a variety of trajectory model specifications were estimated, including a time indicators specification, and the sensitivity of the results to variations in the degree of unobserved heterogeneity across individuals was examined. Individual covariates (i.e., a treatment type indicator for SSRI+CBT, age, sex, race, indicator for MDD or anxiety, presence of externalizing disorder, baseline symptom severity) were included in all model specifications. Linear, log-linear, linear-log, and time indicator dummy variables (fixed and random effects) specifications were estimated to evaluate robustness of results across reasonable model specifications. The logarithmic trend individual “fixed effects” model was specified using a difference in differences approach, which cancels out the individual fixed effects from the model, yit = Xitβ + δitrendt + εit, εit = (uit − ui1) ~ N(0, σ2). The same logarithmic trajectory modeling strategy was utilized to examine and compare improvement related to individual SSRIs.
For the end-point analyses, change from baseline to week 12 was examined using two approaches. First, adopting the standard distributional assumptions of a Normal distribution for mean change in symptom severity and Bernoulli/Binomial for categorical response leads to Student-t and Beta posterior distributions respectively, from which a Monte Carlo pseudo-sample can be directly obtained (Strawn et al., 2018; Suresh et al., 2020). Means, SDs, CrIs and p-values were obtained by posterior simulation assuming uninformative priors. Second, longitudinal models with time indicators (fixed and random effects models) were estimated to incorporate patient characteristics.
Models were estimated using the Hamiltonian Monte Carlo No U-Turn Sampler in the package Turing.jl, in Julia (version 1.6) (Bezanson et al., 2014; Ge et al., 2018). This approach—Markov Chain Monte Carlo (MCMC)—utilizes repeated random sampling to numerically estimate the probability distribution for change in symptom severity (e.g., CGI-S) and response (CGI-S ≤2). From the BHM posterior simulation samples, differences in response were computed along with 95% credible intervals (CrIs) and other summary statistics. Means are expressed ± their posterior standard deviation (SD). For all analyses, Bayesian posterior p-values ≤0.05 were considered statistically significant (Suresh et al., 2020). Further details of this approach and the MCMC estimation procedure for the BHM, including examples and executable code, is available at https://github.com/tszanalytics/Juliacon2019 (Kruschke, 2014; McGlothlin and Viele, 2018; Mills and Strawn, 2019).
Results
Linear, log-linear, linear-log, time indicator dummy variables (fixed and random effects) specifications were estimated and the results were consistent across all specifications and matched the end-point analyses. Additionally, we specified multi-level BHMs with study level specification to identify heterogeneity within studies and in the total sample (Supplemental Table 1).
Time course of CBT+SSRI benefit relative to SSRI benefit
Patients who received SSRI+CBT had statistically significant decreases in symptoms (reflected by CGI-S score) by week 4 (p<0.001) and improvement continued at week 8 and 12 (p<0.001). The additive benefit of CBT was not statistically significant at week 4 (p=0.287) or 8 (p=0.890) but became statistically significant by week 12 (p<0.001). Additionally, alternative models (e.g., linear and log-linear trend models) also demonstrated this delayed additive benefit from CBT. At week 8, patients who received SSRI+CBT did not enjoy significant improvement relative to those who received SSRI monotherapy (p=0.667); however, by week 12, SSRI+CBT was associated with a statistically significant advantage (βweek 12 SSRI = −0.557±0.028, p<0.001, (βweek 12 additive effect of CBT=−15±0.032, p<0.001). In other words, adding CBT to SSRI treatment was associated with 27% additional improvement compared to simply providing an SSRI. For response (CGI-S ≤2), youth who received SSRI+CBT were significantly more likely to respond by week 8 (p<0.001) and this improvement continued at week 12 (p<0.001). The additive benefit of CBT in terms of the probability of response was not statistically significant at week 4 (p=0.839) or 8 (p=0.153) but became statistically significant by week 12 (p<0.001, Figure 2A).
Figure 2: Improvement in youth with depressive and anxiety disorders who received treatment with SSRIs or SSRIs+CBT.
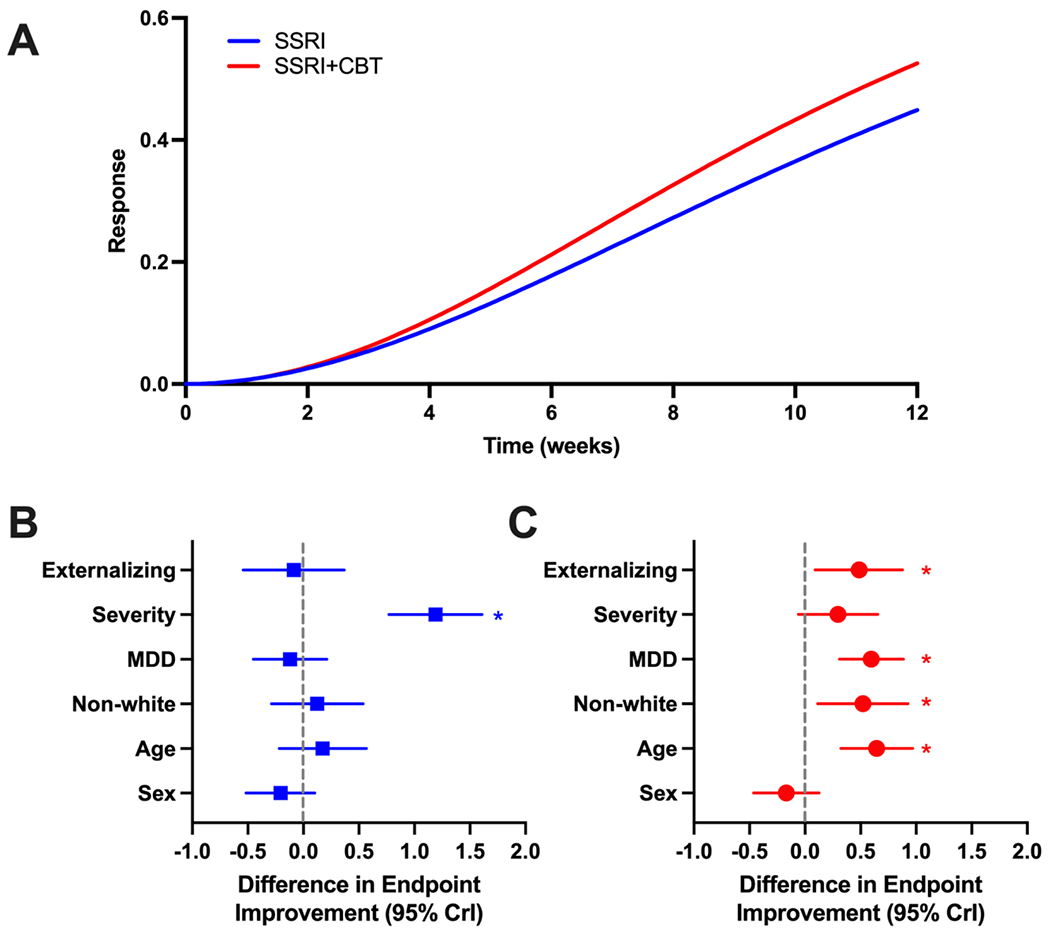
Individual trajectories of improvement are shown for patients who receive SSRIs (blue line) or SSRI+CBT (red line) in panel A. Endpoint improvement for patients who received SSRI monotherapy or SSRI+CBT are shown in B and C. Note: Positive values reflect less improvement. For example, in 2B, a large and significant value corresponds with patients who had greater severity improving significantly less, matching the trajectories for symptom severity comparisons shown in Figure 3F
Predictors of SSRI-related improvement trajectory
In patients treated with SSRIs, younger patients (β=−0.227, CrI: −0.342 to −0.112, p<0.001, Figure 3) had greater improvement over time. Having MDD as the primary disorder was also associated with a greater improvement over time in treatment response trajectory relative to anxiety as the primary disorder (β=−0.249, CrI: −0.357 to −0.141, p<0.001, Figure 3). For patients who received SSRIs, girls (β=−0.064, CrI: −0.138 to 0.009, p=0.088) and boys had similar responses over time. Patients who had more severe symptoms (β=0.498, CrI: 0.403 to 0.593, p<0.001, Figure 3), had significantly less improvement over time. Patients who were not white (β=0.187, CrI: −0.013 to 0.171, p=0.092, Figure 3) did not differ from patients who were white. Finally, patients with externalizing disorder (β=−0.032, CrI: −0.131 to 0.066, p=0.520, Figure 3) did not differ from those without an externalizing disorder over time.
Figure 3: Improvement Trajectories in Youth Treated with Selective Serotonin Reuptake Inhibitors (SSRIs) or SSRI+cognitive behavioral therapy (CBT).
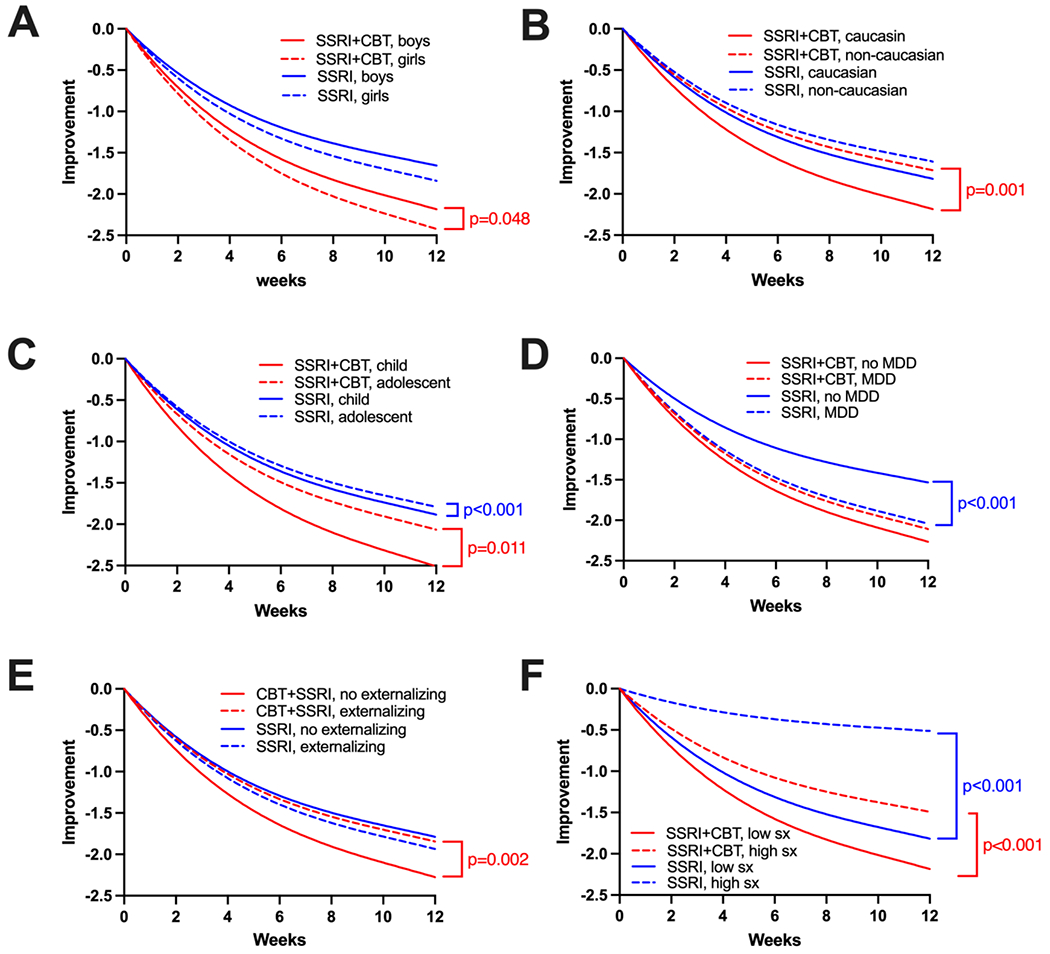
P-values are shown for significant differences in trajectory. Differences in endpoint response are shown in Figure 2. MDD, major depressive disorder; sx, symptoms.
SSRI-related improvement at endpoint
Patients with more severe baseline anxiety or depressive symptoms were less likely to improve with SSRI treatment at endpoint compared to patients with milder symptoms at baseline (Cohen’s d: 0.865; mean difference: 1.19 [positive value reflects less improvement]; CrI: 0.769 to 1.609; p<0.001). Sex (Cohen’s d: −0.147; p=0.193), age (Cohen’s d: 0.115, p=0.389), MDD (Cohen’s d: −0.083; p=0.481), being white (Cohen’s d: 0.086; p=0.560) and having an externalizing disorder (Cohen’s d: −0.059; p=0.715) were not associated with the endpoint response (Figure 2B).
Predictors of CBT+SSRI-related improvement trajectory
For patients who received CBT and SSRI, girls (β=−0.078, CI: −0.001 to −0.155, p=0.048) and younger patients (β=−0.141, CI: −0.250 to −0.033, p=0.011) had greater improvement over time. By contrast, patients who had more severe symptoms (β=0.276, CrI: 0.185 to 0.367, p<0.001), were not white (β=0.187, CrI: 0.081 to 0.294, p=0.001) and those who had an externalizing disorder (β=0.166, CrI: 0.064 to 0.269, p=0.002) had less improvement over time. The presence of MDD was not associated with a difference in treatment response trajectory (β=0.066, CrI: −0.038 to 0.170, p=0.216, Figure 3).
CBT+SSRI-related improvement at endpoint
In patients who received both CBT and SSRIs, being older (Cohen’s d: 0.489; mean difference: 0.645; CrI: 0.321 to 0.969; p<0.001), having MDD (Cohen’s d: 0.455; mean difference: 0.596; CrI: 0.309 to 0.883; p<0.001), having an externalizing disorder (Cohen’s d: 0.387; mean difference: 0.487; CrI: 0.093 to 0.881; p=0.016) and being non-white (Cohen’s d: 0.392; mean difference: 0.0520; CrI: 0.114 to 0.926; p=0.012) were associated with a lower likelihood of response at endpoint. The severity of baseline anxiety or depressive symptoms (Cohen’s d: 0.223, p=0.101) nor sex (Cohen’s d: −0.125, p=0.267) were associated with endpoint response (Figure 2C).
Differences in endpoint improvement for individual SSRIs in patients receiving CBT
For sertraline-treated patients, there was a statistically significant additive benefit of CBT (Cohen’s d=−0.452; mean difference: −0.688, CrI: −1.05 to −0.327; p<0.001). Similar effects were observed for paroxetine-treated youth (Cohen’s d=−0.618; mean difference: −0.727; CrI: −1.384 to −0.068; p=0.030). Patients who received citalopram and those who received citalopram + CBT had similar responses (Cohen’s d=0.574; mean difference: 0.639, CrI: −0.113 to 1.385; p=0.096). Patients who received fluoxetine or fluoxetine + CBT had similar responses (Cohen’s d=−0.012; mean difference: −0.015; CrI: −0.335 to 0.038; p=0.929).
Improvement for individual SSRIs
Sertraline and paroxetine were associated with a statistically significant advantage in symptom reduction over time when combined with CBT, but this difference was not statistically significant for citalopram (mean difference: 0.639±0.383; p=0.096) and fluoxetine (mean difference: −0.015±0.164, p=0.929, Table 1). Statistically significant advantages were observed, at endpoint, for sertraline (p<0.001) and at a trend level for citalopram (p=0.065), but not for paroxetine (p=0.233 or fluoxetine (p=0.448, Table 1).
TABLE 1: Improvement for Specific Selective Serotonin Reuptake Inhibitors (SSRIs) with and without Cognitive Behavioral Therapy (CBT).
Negative coefficients indicate that the addition of CBT results in greater improvement over time compared to SSRI monotherapy.
| Endpoint Improvement for Specific SSRIs with and without CBT | ||||
|---|---|---|---|---|
|
| ||||
| Intervention | Mean | Standard error | 95% CrI | p-value |
| citalopram + CBT | 3.25 | 0.296 | 2.674 to 3.828 | <0.001 |
| citalopram | 2.611 | 0.244 | 2.135 to 3.088 | <0.001 |
| difference | 0.639 | 0.383 | −0.113 to 1.385 | 0.096 |
|
| ||||
| sertraline + CBT | 2.493 | 0.113 | 2.271 to 2.715 | <0.001 |
| sertraline | 3.181 | 0.146 | 2.894 to 3.466 | <0.001 |
| difference | −0.688 | 0.185 | −1.05 to −0.327 | <0.001 |
|
| ||||
| fluoxetine + CBT | 3.074 | 0.126 | 2.828 to 3.32 | <0.001 |
| fluoxetine | 3.089 | 0.105 | 2.883 to 3.295 | <0.001 |
| difference | −0.015 | 0.164 | −0.335 to 0.308 | 0.929 |
|
| ||||
| paroxetine + CBT | 2.873 | 0.243 | 2.397 to 3.349 | <0.001 |
| paroxetine | 3.6 | 0.231 | 3.147 to 4.052 | <0.001 |
| difference | −0.727 | 0.335 | −1.384 to −0.068 | 0.030 |
|
| ||||
| Additive benefit of CBT on the trajectory of improvement | ||||
|
| ||||
| CBT Effect | Coefficient | Standard error | 95% CrI | p-value |
| fluoxetine | 0.034 | 0.045 | −0.054 to 0.122 | 0.448 |
| citalopram | 0.262 | 0.139 | −0.011 to 0.534 | 0.065 |
| paroxetine | −0.111 | 0.093 | −0.292 to 0.070 | 0.233 |
| sertraline | −0.166 | 0.042 | −0.083 to −0.249 | <0.001 |
Among the SSRIs, citalopram was associated with the greatest improvement over time (β=−0.795, p=0.001) followed by sertraline, fluoxetine and paroxetine (Table 1).
Assessment of heterogeneity and sensitivity analyses
The mean trend coefficient estimates for change in symptoms were similar in the models with individual trends (βtrend = −0.742, p<0.001; βSSRI+CBT = −0.074, p=0.034) and those that assume a homogeneous trend (βtrend = −0.739, p=0.04; βSSRI+CBT =−0.074, p=0.027). That said, there was greater heteroegeneity of improvement in patients receiving SSRI monotherapy compared to patients receiving SSRI+CBT (Figure 4, Supplemental Figure 2). All models were estimated with covariates (e.g., age, sex, race, presence of externalizing disorders) and the results were robust across specifications. For CAMS, TADS and TORDIA the response trajectory coefficients were: −0.665 (CrI: −0612 to −0.717), −0.584 (CrI: =0.522 to −0.645), and −0.607 (CrI: −0.554 to −0.659). Further, the average treatment effects across studies did not significantly differ (CAMS vs. TADS, p=0.280; CAMS vs. TORDIA, p=0.420; TADS vs. TORDIA, p=0.710). Similarly, treatment effects in patients who received CBT+SSRI did not significantly differ across trials nor did they differ in patients who received SSRI monotherapy (Supplemental Table 1).
Figure 4:
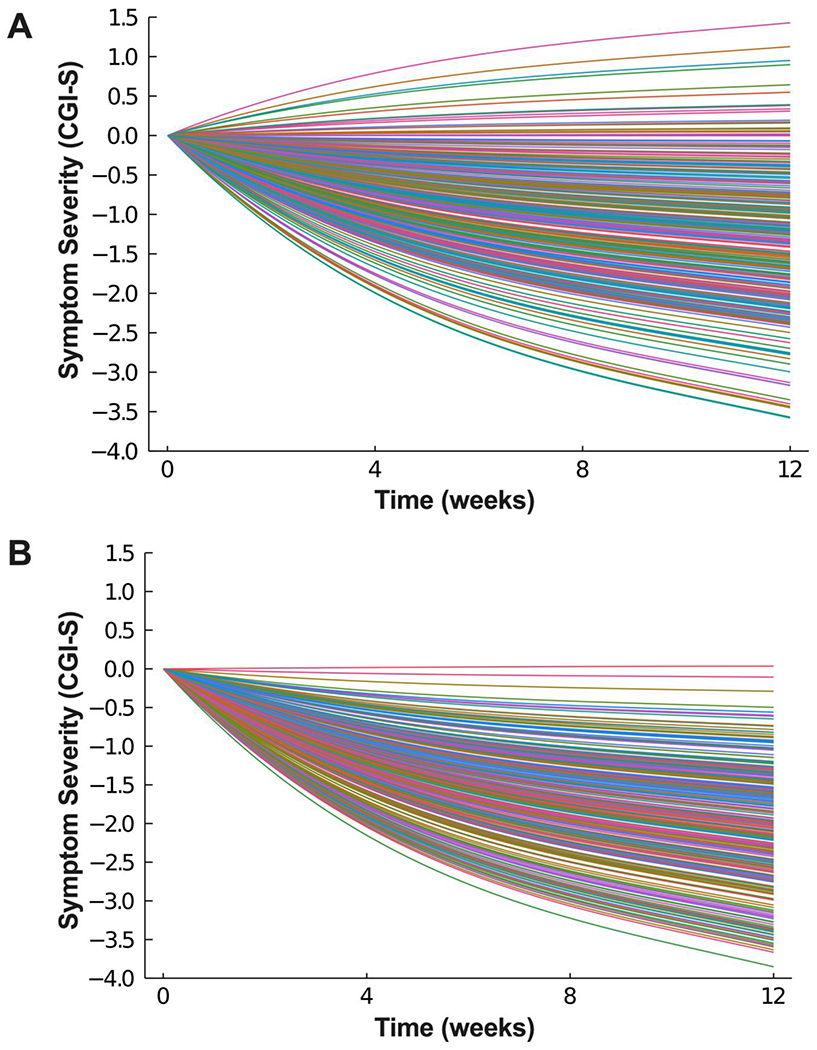
Heterogeneity in Improvement Trajectory among Patients Treated with Selective Serotonin Reuptake Inhibitors (SSRIs) (A) or SSRI+Cognitive Behavioral Therapy (CBT) (B).
Limitations
While this is one of very few applications of BHMs in psychiatry and sheds new light on treatment selection in youth with depressive and anxiety disorders, there are several important limitations that warrant additional discussion. First, there may have been differences in exposure, with some SSRIs, related to pharmacokinetic genes (Strawn et al. 2019) and other factors, that contributed to variability in response (Sakolsky et al. 2011). Second, several of these findings are based on an assumption of a deterministic logarithmic trend model to gain degrees of freedom, which imposes a more restrictive functional form for the trajectory of response but leads to greater precision in evaluating response, though we also examined a nonparametric time indicator specification and these results confirmed our findings. Third, prior treatment experience (e.g., failed SSRI trial) may have influenced treatment response and this would have been most pronounced in TORDIA wherein individuals were required to fail one prior SSRI trial. However, response trajectory was statistically similar across all three studies. Fourth, we were limited in our ability to examine all moderators of treatment response and, despite the impressive sample size of TORDIA, TADS, and CAMS, had a limited number of observations over time, which precluded a deeper analysis examining differential trajectories of improvement within putative subgroups that included combined characteristics (e.g., sex + age + ADHD comorbidity). Similarly, because of small sample sizes, we could not explore specific predictors of treatment response in patients with anxious depression. Fifth, several international trials of depression (Adolescent Depression Antidepressant and Psychotherapy Trial (ADAPT) (Goodyer et al., 2007) or depression and anxiety (Youth Depression Alleviation-Combined Treatment (YoDA-C)) (Davey et al., 2019) were not included in this analysis. These studies included heterogeneous samples, with young adults (age 18-25 years), had partial blinding, outcomes assessed by non-clinicians, and only measured outcomes at weeks 6 and 12 (which limits trajectory modeling). Sixth, regarding differences in response and response trajectory for individual SSRIs across studies, these differences could relate to multiple factors which generally represent unobserved factors, including pharmacodynamic and pharmacokinetic variation that are linked with differences in response and tolerability in children and adolescents. However, we cannot exclude the possibility that some SSRIs may be more effective in MDD vs anxiety disorders or vice versa. Finally, though quality of life and tolerability measures were collected, heterogeneity and inconsistency (with regard to side effect ratings, duration, severity and temporal stability) precluded their incorporation in our BHM.
Discussion
This study—the largest examination of SSRIs and CBT in youth with depressive and anxiety disorders to date—examined predictors of COMB and SSRI monotherapy response, finding evidence that combination treatment is robust across disorders. Younger youth and those with milder baseline symptoms respond best to COMB, whereas males, adolescents, minority participants, and those with more severe baseline symptoms and externalizing disorders may take longer to respond. Importantly, while the addition of CBT significantly improved the overall magnitude of response, on average increasing response relative to SSRI monotherapy, it did not accelerate the speed of response.
Given that, across studies, the response to SSRI monotherapy leaves considerable room for improvement, it is encouraging that COMB offers substantially better and less heterogeneous outcomes and that it does so for youth across depressive and anxiety disorders. At the same time, the present findings highlight key considerations for both parents and providers. First, the advantages of including CBT in treatment take time to appear. Although the endpoint outcome may be clearly superior, in this study the additional benefit of CBT was not evident until week 12. This is to be expected, given that for both anxiety and depression, CBT is a skills-based approach comprising a range of different techniques. These techniques build on each other sequentially over time, and in some cases (e.g., anxiety), key ingredients (i.e., exposure) are not introduced until well into treatment. For families deciding between SSRI monotherapy and COMB, setting expectations regarding treatment course will be key. Moreover, as the evidence base for brief and single-session CBT interventions grows (Schleider et al., 2020; Schleider and Weisz, 2017), it will be important to examine how these treatments work in concert with SSRIs and whether it is possible to achieve significant treatment effects more quickly.
Separate from this issue, several patient characteristics meaningfully influenced response to COMB, but not SSRIs. Boys, older and minority participants, and participants with more severe baseline symptoms and externalizing disorders had a slower response when treated with CBT+SSRI. Younger participants and those with depressive disorders and milder baseline symptoms improved fastest when treated with SSRI monotherapy. Regarding externalizing disorders, and specifically ADHD, it is worth noting that when stimulants were used in the component studies, they consisted primarily of shorter-acting medications. It is commonly observed in clinical practice that breakthrough ADHD symptoms often emerge in the afternoon—roughly the time when these youth with ADHD were beginning psychotherapy. Thus, it is possible that optimizing treatment of the externalizing disorder could restore the benefit associated with CBT. Additionally, that older youth did not do as well with CBT+SSRI treatment may relate to developmental factors. For example, older patients with depression and anxiety disorders may report impairment or specific symptoms (e.g., psychic, somatic, avoidance/withdrawal) differently than younger patients. For example, in older adolescents, the interpersonal context of symptoms may be more amenable to therapies that include an interpersonal focus, such as IPT-A. Of note, these factors may be less represented in the traditional CBT included in CAMS, TADS, and TORDIA. Ultimately, strategies to tailor CBT interventions to specific patient characteristics are urgently needed to realize the value of combination treatment over SSRI alone.
For patients with more severe symptoms, both COMB and SSRI monotherapy had significantly slower improvement over time while the endpoint response was also significantly attenuated in patients treated with SSRI monotherapy. This finding is consistent with prior analyses suggesting that anxious patients with severe (compared to moderate) symptoms do best with COMB (Taylor et al., 2018). The reasons why patients with more severe illness fare worse with SSRI monotherapy is complex and may relate to COMB addressing non-biological contributors to high symptom severity. For example, in patients with more severe symptoms, caregiver strain is increased (Compton et al., 2014) and caregiver strain may be addressed indirectly in COMB by the psychosocial component. Additionally, more family accommodation contributes to more severe symptoms and ostensibly perpetuates psychopathology in youth with more severe symptoms (Walkup et al., 2021). Also, such family factors, including accommodation and caregiver strain are uniquely addressed in COMB.
In our analysis, girls did better with COMB compared to boys. While the reasons for this difference are speculative, there are a number of potential mechanisms that could subtend this difference. First, cortical, and subcortical maturation is sexually dimorphic (Shaw et al., 2014; Raznahan et al., 2010). Secondary to earlier prefrontal maturation, girls may have a prefrontal advantage that could relate to age-specific superiority in cognitive functions that are required for maximal benefit from CBT and are based in prefrontal regions. Additionally, subcortical development is sexually dimorphic and ventral striatal systems that subserve inhibitory control and reward processing develop later in boys compared to girls (Shaw et al., 2014). Given the development of skills in CBT that rely on inhibitory control and reward processing, this delayed development raises the possibility that boys—at certain developmental stages—may lack the neurobiologic substrate to maximally benefit from CBT-related interventions that target these processes. Finally, given that many of the therapists participating in these trials were female, the pairing of female-female may have contributed to differences in the therapeutic alliance which is known to affect outcomes in some children and adolescents (Cummings et al., 2013).
While individual differences among SSRIs should be interpreted with caution secondary to the sample sizes, several of the SSRI monotherapy findings warrant additional discussion. Citalopram monotherapy produced greater improvement over time, and, at endpoint, sertraline (at a 7% threshold) and citalopram monotherapy produced greater improvement compared to the other SSRIs. Both sertraline and citalopram are metabolized by the polymorphic enzyme CYP2C19 which produces substantial variability in plasma concentrations in pediatric patients (Strawn et al, 2021). However, in the trials included in this analysis, both citalopram and sertraline were aggressively dosed compared to routine clinical practice; this may have enhanced their apparent efficacy, particularly in rapid and ultrarapid metabolizers. Additionally, these SSRIs are among the most serotonergically selective and, in meta-analyses of SSRIs in pediatric patients, greater serotonergic selectivity predicts greater response (Strawn et al, 2015). Both paroxetine and fluoxetine have greater pharmacokinetic complexity and, unlike sertraline and citalopram, have non-linear kinetics which may contribute to difficult to predict exposure associated with dose titration. Finally, paroxetine is poorly tolerated in multiple meta-analyses of SSRIs in pediatric patients, potentially secondary to its short half-life, anticholinergic properties, and mechanism-based CYP2D6 inhibition (Cipriani et al, 2018; Dobson et al, 2019). These factors could contribute to its relative inferiority to other SSRIs in some of our comparisons.
It is important to note that minority youth did not respond as well to COMB, particularly in light of the growing recognition that psychosocial treatments are not always developed using diverse and representative sets of youth and that they are not always administered in a culturally sensitive manner. Concerningly, these findings dovetail with data suggesting that minority youth are much less likely to receive quality, standard of care psychotherapy and pharmacotherapy (Cummings et al., 2019). The finding that minority participants did not benefit as much from COMB as their white counterparts underscores the need to develop and test culturally tailored CBT adaptations, and to carefully consider the role of variables such as alliance, engagement, and family involvement in mediating outcome. Interestingly, in a recent prospective trial of CBT-based interventions in youth with OCD, minority youth had better outcomes with enhanced family therapy. This finding and others suggest that specific aspects of CBT may be particularly important for minority youth but are underemphasized in current protocols. For example, family involvement varies considerably across CBTs, and greater family involvement in psychotherapy predicts greater improvement in non-whites (Pina et al., 2009). Further, family involvement in these cases may relate to cultural factors; some minority families may see family involvement as particularly important, especially in families that see individuals as interdependent on one another. Further, given that the understanding of symptoms and psychopathology varies across races and groups, the relative importance of attribution of symptoms and autonomy may complicate treatment of depression and anxiety within some families. Most importantly, members of minority groups, in particular Blacks, have suffered institutionalized discrimination within the white-dominated healthcare system (Nong et al. 2020) for centuries, and this discrimination has enduring consequences in terms of distrust in the healthcare system (Armstrong et al., 2013; Boulware et al., 2003). If this discrimination and the resulting distrust is the primary factor that attenuates the effectiveness of CBT across disorders in pediatric patients, the solution may require much more than adaptation of a CBT protocol.
Given the time course of improvement associated with the addition of CBT in SSRI-treated patients, research should focus on individualized approaches to psychotherapy as opposed to contemporary one-size-fits-all approaches. Sequential or staged approaches introducing psychotherapy later in treatment also warrants further study. For example, one small study of depressed patients treated with interpersonal psychotherapy for adolescents (IPT-A) found that adapting the intervention (frequency of IPTA-A or addition of fluoxetine) earlier (week 4) produced greater improvement compared to the same “augmentation” changes just 4 weeks later (Gunlicks-Stoessel et al., 2019). The heterogeneity in response associated with the one-size-fits-all approach to CBT suggests that tailoring interventions could be warranted in some individuals with slow responses to combined CBT+SSRI treatment.
Supplementary Material
Highlights.
In youths with depression and anxiety, SSRIs +CBT consistently produce greater improvement than monotherapy.
How SSRI+CBT vs SSRI response varies across disorders and patients is unknown.
SSRI+CBT significantly decreased symptoms by week 4 across disorders.
The additive benefit of CBT over SSRI monotherapy is not statistically significant until week 12.
The fastest response to SSRI+CBT was for patients who were younger, with milder baseline anxiety/depression symptoms and depressive disorders.
ACKNOWLEDGMENTS
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) through Grants R01HD098757 (JRS) and R01HD099775 (JRS) and by the Yung Family Foundation. The authors thank the original study investigators and the patients and their families who participated in CAMS, TADS and TORDIA.
CONFLICTS OF INTEREST
Dr. Strawn has received research support from Edgemont, Shire, Forest Research Institute, Otsuka, the Yung Family Foundation and the National Institutes of Health (NICHD, NIMH and NIEHS). He receives royalties from Springer Publishing for two texts, UpToDate and has received material support from Myriad genetics and honoraria from CMEology, the American Academy of Child & Adolescent Psychiatry, Genomind and Neuroscience Educational Institute. He provides consultation to the U.S. Food and Drug Administration as a Special Government Employee. Mr. Suresh, Dr. Mills and Dr. Strawn receive research support from the Yung Family Foundation. Dr. Croarkin has received research grant support Pfizer, Inc, Neuronetics, Inc, and NeoSync, Inc. He has received equipment support from Neuronetics, Inc. and MagVenture Inc,. He has received supplies and genotyping services from Assurex Health, Inc. for an investigator-initiated study. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc. He has served as a paid consultant for Procter & Gamble Company and Myriad Neuroscience. Dr. Walkup serves on the advisory boards of the Anxiety and Depression Association of America, the Tourette Association of America and the Trichotillomania Learning Center. He receives royalties from Wolters-Klewer, Oxford University Press and Guilford Press. Finally, he has received honoraria from the American Academy of Child & Adolescent Psychiatry, American Academy of Pediatrics and the American Psychiatric Association
ROLE OF FUNDING SOURCE
These original studies (CAMS, TADS and TORDIA) were supported by the National Institutes of Mental Health. The current analyses were supported by the Yung Family Foundation and The Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD; Grant R01HD098757). None of the study funders had any role in the analysis, writing or content of this piece and had no role in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong K, Putt M, Halbert CH, Grande D, Schwartz JS, Liao K, Marcus N, Demeter MB, Shea JA, 2013. Prior experiences of racial discrimination and racial differences in health care system distrust. Med. Care doi: 10.1097/MLR.0b013e31827310a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow JR, Emslie G, Clarke G, Wagner KD, Spirito A, Vitiello B, Iyengar S, Shamseddeen W, Ritz L, Mccracken J, Strober M, Suddath R, Leonard H, Porta G, Keller M, Brent D, 2009. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: predictors and moderators of treatment response. J. Am. Acad. Child Adolesc. Psychiatry 48, 330–9. doi: 10.1097/CHI.0b013e3181977476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanson J, Edelman A, Karpinski S, Shah VB, 2014. Julia: A Fresh Approach to Numerical Computing 59, 65–98. [Google Scholar]
- Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR, 2003. Race and trust in the health care system. Public Health Rep. doi: 10.1016/S0033-3549(04)50262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, Vitiello B, Iyengar S, Birmaher B, Ryan ND, Zelazny J, Onorato M, Kennard B, Mayes TL, DeBar LL, McCracken JT, Strober M, Suddath R, Leonard H, Porta G, Keller MB, 2009. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am. J. Psychiatry 166, 418–426. doi: 10.1176/appi.ajp.2008.08070976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J, 2008a. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA - J. Am. Med. Assoc 299, 901–913. doi: 10.1001/jama.299.8.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J, 2008b. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA 299, 901–13. doi: 10.1001/jama.299.8.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton SN, Peris TS, Almirall D, Birmaher B, Sherrill J, Kendall PC, March JS, Gosch E. a, Ginsburg GS, Rynn M. a, Piacentini JC, McCracken JT, Keeton CP, Suveg CM, Aschenbrand SG, Sakolsky D, Iyengar S, Walkup JT, Albano AM, 2014. Predictors and moderators of treatment response in childhood anxiety disorders: results from the CAMS trial. J. Consult. Clin. Psychol 82, 212–24. doi: 10.1037/a0035458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Settipani CA, Read KL, Compton SN, March J, Sherrill J, Piacentini J, McCracken J, Walkup JT, Ginsburg G, Albano AM, Rynn M, Birmaher B, Sakolsky D, Gosch E, Keeton C, Kendall PC, 2013. The therapeutic relationship in cognitive-behavioral therapy and pharmacotherapy for anxious youth. J. Consult. Clin. Psychol 81, 859–864. doi: 10.1037/a0033294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JR, Ji X, Lally C, Druss BG, 2019. Racial and Ethnic Differences in Minimally Adequate Depression Care Among Medicaid-Enrolled Youth. J. Am. Acad. Child Adolesc. Psychiatry doi: 10.1016/j.jaac.2018.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry J, Rohde P, Simons A, Silva S, Vitiello B, Kratochvil C, Reinecke M, Feeny N, Wells K, Pathak S, Weller E, Rosenberg D, Kennard B, Robins M, Ginsburg G, March J, 2006. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). J. Am. Acad. Child Adolesc. Psychiatry doi: 10.1097/01.chi.0000240838.78984.e2 [DOI] [PubMed] [Google Scholar]
- D.J. S, J.M. P, G.J. E, G.N. C, K.D. W, B. V, M.B. K, B. B, J.R. A, N.D. R, J.T. M, M.J. S, S. I, G. P, 2011. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J. Clin. Psychopharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Chanen AM, Hetrick SE, Cotton SM, Ratheesh A, Amminger GP, Koutsogiannis J, Phelan M, Mullen E, Harrison BJ, Rice S, Parker AG, Dean OM, Weller A, Kerr M, Quinn AL, Catania L, Kazantzis N, McGorry PD, Berk M, 2019. The addition of fluoxetine to cognitive behavioural therapy for youth depression (YoDA-C): a randomised, double-blind, placebo-controlled, multicentre clinical trial. The Lancet Psychiatry. doi: 10.1016/S2215-0366(19)30215-9 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG, 2002. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J. Am. Acad. Child Adolesc. Psychiatry 41, 1205–1215. doi: 10.1097/00004583-200210000-00010 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S, 2009. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial. J. Am. Acad. Child Adolesc. Psychiatry 48, 721–729. doi: 10.1097/CHI.0b013e3181a2b304 [DOI] [PubMed] [Google Scholar]
- Ge H, Xu K, Ghahramani Z, 2018. Turing: A Language for Flexible Probabilistic Inference. Proc. Twenty-First Int. Conf. Artif. Intell. Stat 84, 1682–1690. [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Rubin DB, 2004. Bayesian Data Analysis, Chapman Texts in Statistical Science Series, doi: 10.1007/s13398-014-0173-7.2 [DOI]
- Goodyer I, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, Breen S, Ford C, Barrett B, Leech A, Rothwell J, White L, Harrington R, 2007. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: Randomised controlled trial. Br. Med. J doi: 10.1136/bmj.39224.494340.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunlicks-Stoessel M, Mufson L, Bernstein G, Westervelt A, Reigstad K, Klimes-Dougan B, Cullen K, Murray A, Vock D, 2019. Critical Decision Points for Augmenting Interpersonal Psychotherapy for Depressed Adolescents: A Pilot Sequential Multiple Assignment Randomized Trial. J. Am. Acad. Child Adolesc. Psychiatry doi: 10.1016/j.jaac.2018.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard BD, Clarke GN, Weersing VR, Asarnow JR, Shamseddeen W, Porta G, Berk M, Hughes JL, Spirito A, Emslie GJ, Keller MB, Wagner KD, Brent DA, 2009. Effective Components of TORDIA Cognitive-Behavioral Therapy for Adolescent Depression: Preliminary Findings. J. Consult. Clin. Psychol doi: 10.1037/a0017411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK, 2014. Doing Bayesian data analysis: A tutorial with R, JAGS, and Stan, second edition, Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan, Second Edition. doi: 10.1016/B978-0-12-405888-0.09999-2 [DOI] [Google Scholar]
- March J, Silva S, Curry J, Wells K, Fairbank J, Burns B, Domino M, Vitiello B, Severe J, Riedal K, Goldman M, Feeny N, Findling R, Stull S, Baab S, Weller EB, Robbins M, Weller RA, Jessani N, Waslick B, Sweeney M, Dublin R, Walkup J, Ginsburg G, Kastelic E, Koo H, Kratochvil C, May D, LaGrone R, Vaughan B, Albano AM, Hirsch GS, Podniesinki E, Chu A, Reincecke M, Leventhal B, Rogers G, Jacobs R, Pathak S, Wells J, Lavanier SA, Danielyan A, Rohde P, Simons A, Grimm J, Frank S, Emslie G, Kennard B, Hughes C, Mayes TL, Rosenberg D, Benazon N, Butkus M, Bartoi M, 2009. The Treatment for Adolescents With Depression Study (TADS): outcomes over 1 year of naturalistic follow-up. Am. J. Psychiatry 166, 1141–9. doi: 10.1176/appi.ajp.2009.08111620 [DOI] [PubMed] [Google Scholar]
- March JS, 2004. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. J. Am. Med. Assoc doi: 10.1001/jama.292.7.807 [DOI] [PubMed] [Google Scholar]
- McGlothlin AE, Viele K, 2018. Bayesian Hierarchical Models. JAMA - J. Am. Med. Assoc doi: 10.1001/jama.2018.17977 [DOI] [PubMed] [Google Scholar]
- Mills Jeffrey A; Strawn JR, 2019. Probabilistic Biostatistics with Julia, in: JuliaCon Proceedings, pp. 1–6. [Google Scholar]
- Pina AA, Villalta IK, Zerr AA, 2009. Exposure-based cognitive behavioral treatment of anxiety in youth: An emerging culturally-prescriptive framework. Behav. Psychol. Psicol. Conduct [Google Scholar]
- Schleider JL, Dobias M, Fassler J, Shroff A, Pati S, 2020. Promoting Treatment Access Following Pediatric Primary Care Depression Screening: Randomized Trial of Web-Based, Single-Session Interventions for Parents and Youths. J. Am. Acad. Child Adolesc. Psychiatry 59, 770–773. doi: 10.1016/j.jaac.2020.01.025 [DOI] [PubMed] [Google Scholar]
- Schleider JL, Weisz JR, 2017. Little Treatments, Promising Effects? Meta-Analysis of Single-Session Interventions for Youth Psychiatric Problems. J. Am. Acad. Child Adolesc. Psychiatry doi: 10.1016/j.jaac.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, Sauley BA, Welge JA, 2018. The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 57, 235–244.e2. doi: 10.1016/j.jaac.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Poweleit EA, Ramsey LB, 2019. CYP2C19-Guided Escitalopram and Sertraline Dosing in Pediatric Patients: A Pharmacokinetic Modeling Study. J. Child Adolesc. Psychopharmacol doi: 10.1089/cap.2018.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh V, Mills JA, Croarkin PE, Strawn JR, 2020. What next? A Bayesian hierarchical modeling re-examination of treatments for adolescents with selective serotonin reuptake inhibitor-resistant depression. Depress. Anxiety doi: 10.1002/da.23064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Lebowitz ER, Jakubovski E, Coughlin CG, Silverman WK, Bloch MH, 2018. Monotherapy Insufficient in Severe Anxiety? Predictors and Moderators in the Child/Adolescent Anxiety Multimodal Study. J. Clin. Child Adolesc. Psychol doi: 10.1080/15374416.2017.1371028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G, Shostak J, 2010. Common Statistical Methods for Clinical Research with SAS Examples, 3rd ed. SAS Institute. [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC, 2008. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N. Engl. J. Med 359, 2753–2766. doi: 10.1056/NEJMoa0804633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Friedland SJ, Peris TS, Strawn JR, 2021. Dysregulation, Catastrophic Reactions, and the Anxiety Disorders. Child Adolesc. Psychiatr. Clin. N. Am doi: 10.1016/j.chc.2020.10.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


