Abstract
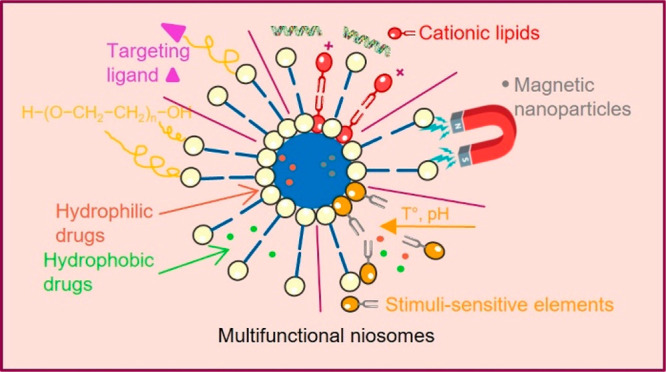
Niosomes are a type of vesicular nanocarrier exploited for enhancing the therapeutic efficacy of various drugs in clinical practice. Niosomes comprise a bilayer hydrophobic membrane enclosing a central cavity filled with an aqueous phase, and therefore, they can encapsulate and deliver both hydrophobic and hydrophilic substances. Niosomal nanocarriers are preferred over other bilayer structures such as liposomes due to their chemical stability, biodegradability, biocompatibility, low production cost, low toxicity, and easy storage and handling. In addition, the niosomal membrane can be easy modified by the inclusion of ligands or stimulus-sensitive segments for achieving targeted delivery and triggered release of the encapsulated cargo. This mini-review outlines the current advances in designing functional niosomes and their use as platforms for developing advanced drug and gene delivery systems.
1. Introduction
In the past years, research efforts have been focused on the elaboration of various drug delivery systems, aiming to overcome the limitations of conventional dosage forms and respectively to ensure an improved bioavailability, reduced side effects, controlled drug release, and targeted delivery. In this context, vesicular systems such as liposomes have successfully been implemented in clinical practice as an advantageous technological approach to achieve the requested demands. The latter boosted the elaboration of different types of vesicular carriers such as niosomes, transferosomes, ethosomes, pharmacosomes, etc., which retain the characteristic lamellar structure, but differ in the type of structural components (Figure 1).1
Figure 1.
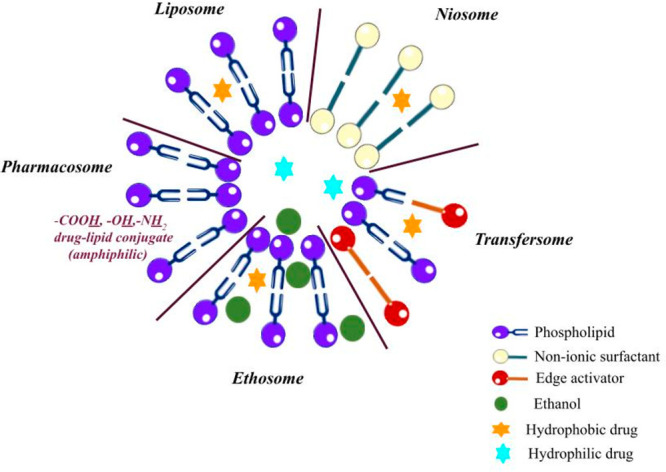
Sketch of different types of vesicular nanocarriers.
Niosomes are vesicular systems formed by nonionic surfactants via self-assembly in aqueous solution assisted by physical agitation or elevated temperature.2 The use of nonionic surfactants as membrane forming constituents instead of phospholipids overcomes many of the disadvantages associated with liposomes, such as insufficient chemical stability, predisposition of phospholipids to oxidation, high production cost, necessity of special handling, and storage conditions.3 Their specific structure—an inner aqueous compartment surrounded by a hydrophobic membrane—allows incorporation (and codelivery, respectively) of hydrophobic and hydrophilic drug molecules.1 Furthermore, niosomes are osmotically active, nontoxic, non-immunogenic, biocompatible, and biodegradable. Initially reported in the 1970s as a feasible approach in the cosmetic industry, niosomes were patented by L’Oreal in the 1980s as a cosmetic product.2 Their favorable characteristics determine the increased research interest, as well the wide exploitation beyond the scope of cosmetic industry. Over the years, niosomes have been investigated as a promising drug delivery platform for various routes of administration—oral, parenteral, dermal/transdermal, ocular, and pulmonary (Figure 2).1,3 Other important areas of application include the use of niosomes in gene and vaccine delivery.4 In particular, cationic niosomes can successfully replace viral vectors as gene carriers and transfer genetic material to the target cell without risk of occurrence of immunogenic, mutagenic, or cytotoxic reactions. Regarding vaccine delivery, niosomes are estimated to act as adjuvants, improving the immunogenicity of the subunit vaccines and their targeting to the corresponding cells.5 The utilization of niosomes as drug delivery systems in such diverse areas of application is feasible due to the possibility to modify their physicochemical properties during the elaboration process via a proper selection of composition elements and the method of preparation. The functionalization of niosomes is of great interest in targeted drug delivery, especially in the field of cancer therapy.6 Thus, different technological strategies have been explored to achieve target delivery, distinguishing two main mechanisms: passive targeting (elaboration of long circulation “stealth” nanosized vesicles by the inclusion of polyethylene glycol (PEG)) and active targeting (surface functionalization via specific ligands, binding to corresponding receptor/antigen expressed on cancer cells).7 However, in certain circumstances, the objectives set for implementation cannot be fully met, which further determines the development of novel niosomal carriers. As the current tendency, one may outline strategies elaborating multifunctional vesicles with the potential to serve as an “ideal” nanocarrier in the therapeutic areas where the conventional drugs are characterized with narrow therapeutic windows and suffer from severe dose-dependent side effects. On this basis, besides the well-established niosomal classification built on the size or number of bilayers (e.g., small (<100 nm) and large (≥100 nm); unilamellar and multilamellar), niosomes may be further categorized depending on their functionality as conventional and structurally modified vesicles (i.e., “stealth”, stimuli sensitive, “smart”, magnetic, multifunctional, etc.).
Figure 2.
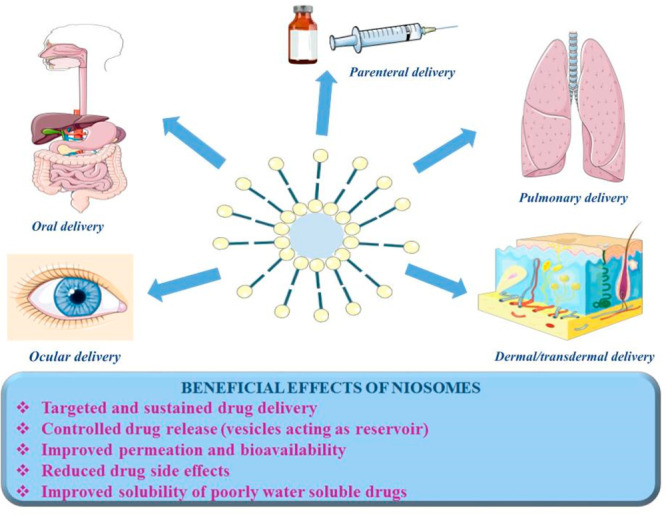
Beneficial effects of niosomes in accordance with the most commonly used delivery routes.
This review discusses the salient characteristics of niosomes, including the main structural components, factors affecting their physicochemical properties, as well as the latest strategies for structural modification and development of multifunctional niosomes. Special emphasis is put on their versatile applications, highlighting the recent findings in the field of advanced drug delivery.
2. Formulation Aspects
The main components involved in the preparation of niosomes are nonionic surfactants and lipids. The formation of niosomes is also influenced by the nature of the encapsulated drug, temperature/pH of hydration medium, as well by the inclusion of different additives, used to improve vesicles properties or to impart specific characteristics depending on delivery targets.
2.1. Nonionic Surfactants
The essential amphiphilic components building niosomes are nonionic surfactants. They are preferred over the other surface active agents (positively/negatively charged or amphoteric) due to their higher stability, biocompatibility, and low toxicity.5 The most frequently utilized nonionic surfactants for niosome production are as follows:
Alkyl ethers – alkyl glycerol ethers and polyoxyethylene alkyl ethers (Brij)
Alkyl amides – alkyl galactosydes/glucosides containing in their structure amino acid moieties
Alkyl esters – sorbitan fatty acid esters (Span) and polyoxyethylene sorbitan fatty acid esters (Tween)
Block copolymer – poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO–PPO–PEO) triblock copolymers (Poloxamers, Pluronics)
The main characteristics of nonionic surfactants, influencing the formation of niosomes are the hydrophilic–lipophilic balance (HLB) value, critical packing parameter (CPP) value, as well as their chemical structure and phase transition temperature (Tc; the temperature at which reversible changes occur from a gel to a liquid phase).6
The HLB value of nonionic surfactants describes the degree to which a given molecule is hydrophilic or lipophilic and is an important parameter often used as an indicator for niosome forming capability. Generally, it is considered that the lipophilic surfactants, with HLB values between 4 and 8, are suitable for preparation of niosomes; however, under certain conditions hydrophilic surfactants (HLB > 14) may also act as bilayer forming components.3,8 A crucial factor in this process is the inclusion of other element(s), participating in the membrane bilayer arrangement, such as cholesterol at optimal concentration (most often), or even the encapsulated lipophilic drug (e.g., curcumin).8
CPP value of nonionic surfactants is another important parameter used to prognosticate the shape of the formed nanostructures. It is expressed as the ratio between the volume of hydrophobic group (v), divided by the product of critical hydrophobic group length (lc), and the area of polar headgroup (a0).3 CPP values between 1/2 and 1 correspond to self-assembly of surfactants into bilayer structure, while outside this range spherical or nonspherical micelles are formed.5
The chemical structure of surfactants and their phase transition temperature are other factors to be considered during development of niosomes. Usually, surfactants with chain length between 12 and 18 carbons are suitable for preparation of niosomes,9 although some high molar mass amphiphiles such as PEO–PPO–PEO copolymers are also used as structural components of the vesicles. Regarding the effect of phase transition temperature, surfactants with high Tc, such as Span 60 (Tc 56–58 °C) provide the possibility to achieve high entrapment efficiency values.5
2.2. Cholesterol
Cholesterol is the most frequently used lipid for improving the mechanical strength and rigidity of niosomal membrane, as well as for reducing water permeability.1 By interaction with nonionic surfactants, cholesterol alters gel/liquid phase transition temperature of the system and affects membrane fluidity.6 The amount of cholesterol to be included depends primarily on the HLB values of nonionic surfactants and needs optimization during the development process, since it has an impact not only on the membrane properties or arrangement (in case of surfactants with HLB values > 10) but also on the physicochemical characteristics of the vesicles, for instance, size, entrapment efficiency, and physical stability.5
2.3. Additives
One of the favorable features of niosomes is the ability to modify their structure/physicochemical parameters via the inclusion of different additives. Thereby, the drug delivery process and pharmacokinetics may be further improved, as they are primarily determined by vesicle characteristics, rather than drug physicochemical properties. Different additives may be included to niosomal composition to impart desired features.
2.3.1. Charge Inducers
Generally, charge inducing agents are included in niosomes in order to increase their physical stability, respectively, to hinder vesicle agglomeration, by electrostatic repulsive forces.5 Most frequently used charge inducers are dicetyl phosphate and phosphatidic acid (both negatively charged), and stearyl amine, stearylpyridinium chloride, and cetylpyridinium chloride bearing a positive charge.3 The surface charge of vesicles contributes to improving the technological or biopharmaceutical characteristics of niosomes. Negatively charged inducers like dicetyl phosphate may also lead to higher entrapment efficiency, superior colloid stability, and homogeneity of the system compared to classic (uncharged) niosomes.3,6
2.3.2. Cationic/Helper Lipids—Elaboration of Niosomes as Gene Delivery Platform
Cationic lipids are the fundamentals for gene delivery purposes, due to their electrostatic interaction with the negatively charged [PO4]3– groups of DNA.4 Usually, a cationic lipid is composed of four functional domains: a hydrophilic headgroup (in charge for the interaction with DNA); a hydrophobic domain (saturated/unsaturated aliphatic chains responsible for interaction with cell membranes); a linker (amide, ester, ether bonds connecting the two functional parts, affecting the stability of the lipid); a backbone domain (serinol, glycerol groups, separating the headgroup from the hydrophobic segment).10 Among the most commonly used cationic lipids are 2,3-di(tetradecyloxy)propan-1-amine hydrochloride salt (DTPA), N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl sulfate salt (DOTAP), and dimethyl didodecyl ammonium bromide (DDAB).4 Helper lipids are generally included in the formulation process to improve the physicochemical properties of lipid emulsion, vesicle colloidal stability, or gene delivery, since they can alter niosomal morphology, permeability, and nucleic acid release.10 Squalene, cholesterol, and squalane are among the most frequently utilized helper lipids.4
2.3.3. Polyethylene Glycol—Elaboration of “Stealth” Niosomes
PEG is one of the most exploited polymers for vesicle surface coating, due to its favorable characteristics such as biocompatibility, solubility in both polar and nonpolar solvents, low toxicity in vivo, and weak immunogenicity.7,11 The hydrophilic nature of PEO facilitates the formation of hydration shell onto vesicle surfaces (a process referred as PEGylation), which imparts “stealth” properties to the nanocarriers and contributes to their steric stabilization. Thereby, vesicles recognition from the mononuclear phagocyte system (MPS), respectively, opsonization and phagocytosis, are prevented, and longer blood circulation time and the possibility to reach the target site are ensured.12 However, the application of “stealth” niosomes is usually associated with low vesicle uptake into the targeted cells.13 This fact determines the necessity to improve their selectivity, for instance, by conjugation of various biorecognition molecules to vesicular surface (active targeting strategy), by the inclusion of versatile functional moieties or via the current advantageous approach—elaboration of multifunctional niosomes. The latter are characterized with improved therapeutic efficacy, because of the synergic benefits of combining more than one targeting mechanism. Some of the recent strategies for elaboration of multifunctional niosomes and the obtained results therewith are described below.
3. Recent Strategies for Developing Multifunctional Niosomes
As discussed in the previous sections, niosomes are nanosized vesicular aggregates developed for sustained, controlled, and/or targeted delivery of both hydrophilic and hydrophobic drugs and therapeutic macromolecules (proteins and genes). Although the “classical” niosomal formulations can solve the problems associated with the low aqueous solubility and stability of drugs in biological milieu, some issues regarding drug leakage and poor control over the release rate, the rapid clearance of the carrier from the bloodstream by MPS, and the ability to achieved high concentration of encapsulated cargo at the targeted site require further improvement of the systems.6 The incorporation of additional functionalities in niosomes is a step toward overcoming the biological complexity and therapeutic challenges during treatment. Various approaches have been employed to modify niosome properties and to achieve a better performance and enhanced therapeutic effects (Table 1.).
Table 1. Approaches for Elaboration of Functionalized Niosomal Carriers for Efficient Drug Delivery.
| type of niosomes/approach | biological prerequisites | therapeutic advantage |
|---|---|---|
| Long circulating niosomes/surface modification with hydrophilic polymers such as PEG | Enhanced permeability and retention (ERP) effect | Passive targeting to tissues with loose vasculature and, thus, high concentration of the encapsulated drug at the targeted site |
| Targeted niosomes/surface decoration with ligands, aptamers, antibodies, and other targeting moieties | Overexpression of various receptors (folate, transferring, etc.) in a wide range of tumor cells | Active targeting by ligand–receptor interaction once the niosomes reached the target site via the systemic circulation |
| Smart or stimuli-sensitive niosomes/membrane modification with additives that undergo structural changes in response of change in internal or external stimuli | Differences in the pH, enzyme composition, temperature in tumors, or inflamed tissues as compared to normal tissues. External stimuli such as magnetic field, temperature, and ultrasound | Triggered drug release at the target site, thus decreasing exposure to normal cells |
As mentioned above, PEGylaton of niosomes can enhance the colloid stability and protect drugs from enzyme actions. Noisome surface can also be decorated with ligand molecules for specific interaction and increased internalization in particular cell populations. Sensitive to various stimuli, niosomes can be fabricated by embedding or integrating specific structural elements (e.g., functional groups, segments, nanoparticles) into the vesicle thus providing targeted drug release properties. Despite the indisputable effectiveness of the described functionalized niosome carriers, the use of only one approach does not allow achievement of the full potential of the carriers.13 Next step in realizing the full therapeutic potential of niosomes is the design of multifunctional niosomes, using a combination of two or more modification strategies. The most promising and advantageous multifunctional niosomes in the field of cancer and brain targeting and gene therapy are described below.
3.1. Multifunctional Niosomes in Cancer Therapy
Targeting properties of niosomes are especially advantageous in cancer therapy, as classic chemotherapeutics are associated with poor therapeutic efficacy, nonspecific localization in the organism, systemic toxicity, and side effects.14 Different strategies were used for efficient niosomal delivery of anticancer drugs.
3.1.1. Long Circulating Stimuli-Responsive Niosomes
Ensuring a long plasma half-life is a necessary prerequisite for passive accumulation of niosomes in the target compartment, but in order to achieve optimal bioavailability of their cargo, additional functionalization of the carriers is required, allowing active accumulation or targeted drug release. To improve the efficiency of niosomal drug delivery, Davarpanah et al. elaborated PEGylated niosomes which have been additionally magnetized using Fe3O4/SiO2 magnetic nanoparticles (MNPs).15 In that study, PEGylation was intended for increasing the bioavailability of niosomes, and the magnetization was used to make them able to target specific tissues under an external magnetic field (Figure 3). In vitro assessment of niosomal formulations loaded with the antitumor agent Carboplatin toward the MCF-7 breast cancer cell line, revealed that PEGylated magnetic niosomes have an increased cytotoxicity toward these cells in the presence of an external magnetic field. In addition, PEGylation improved drug entrapment and resulted in a sustained release of Carboplatin. Magneto-niosome formulations, suitable for a magnetic parenteral delivery of Doxorubicin, have been prepared from Tween 60 and Pluronic L64 surfactants without adding cholesterol.16 Due to the hydrophobicity and flexibility of PPO segments, the copolymers formed more compact aggregates with lower surface area. The PEO blocks of copolymer contributed to reducing contact with blood components and extending the half-life of nanodrugs in the bloodstream. The developed magneto-niosome formulations exhibited good stability for long period and a controlled drug release profile.
Figure 3.
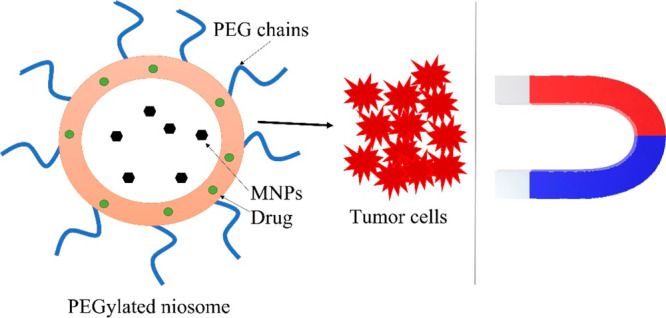
Schematic representation of the concept of magnetic field triggered niosomal delivery to target tumor site.
Tavano and coauthors studied different multifunctional niosomes possessing spontaneous stealth and thermosensitive properties, due to the presence of PluronicL64 and its derivative in the bilayer.17 Calcein and 5-FU were used as model drugs. It has been demonstrated that Pluronic L64-based niosomes possess spontaneous thermosensitive properties: drug releases were found to be more pronounced at 42 °C (Figure 4). Thus, a mild hyperthermia can trigger a controlled drug release at desired location and time.
Figure 4.
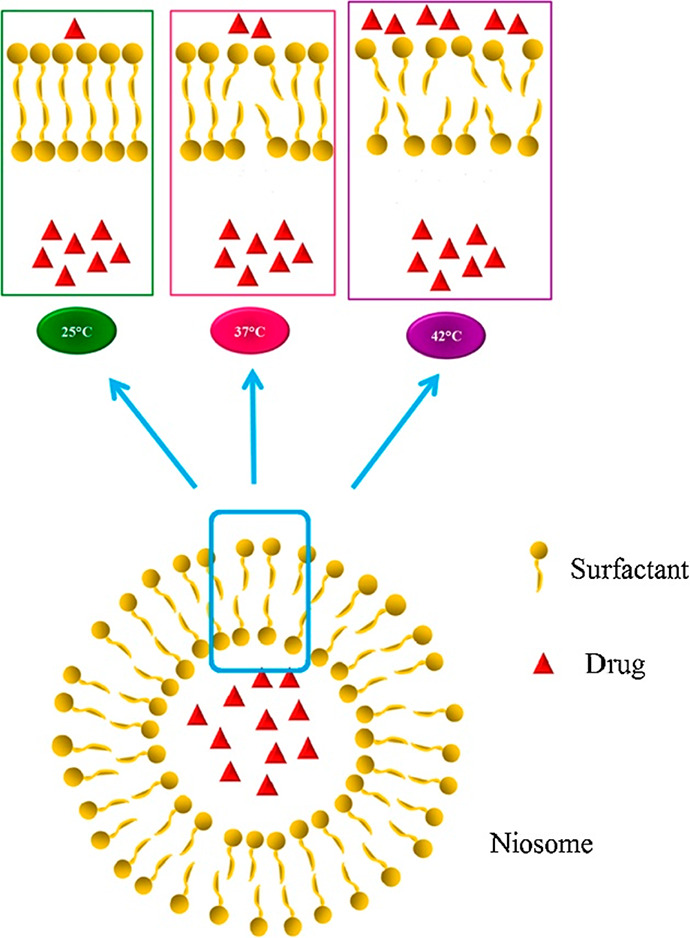
Schematic representation of drug release from thermosensitive niosomes. Reprinted with permission from ref (17). Copyright 2016 Elsevier.
Considering the low pH in solid tumors, an attractive strategy for tumor-selective delivery is based on pH-sensitive niosomes. Pereira et al. have developed a nanosized pH-sensitive niosomes composed of Span20, cholesterol, and 5 mol % of pH (low) insertion peptide (pHLIP) conjugated with DSPE lipids (DSPE-pHLIP) or hydrophobic fluorescent dye, pyrene, (Pyr-pHLIP).18 The pHLIP fragment was employed to impart acidity-driven destabilization of the formulation. The pharmacokinetic evaluation was carried out with fluorescently labeled (R18) pHLIP-coated niosomes after intravenous treatment of BALB/c mice, bearing 4T1 breast cancer. The pharmacokinetics and tissue distribution study showed long circulation and selective accumulation in tumors with minimal exposure of normal tissues, namely, kidney, liver, and muscles. Moreover, the pHLIP coated niosomes attained 2–3 times higher tumor uptake vs the control PEGylated nontargeted system.
3.1.2. Stimuli-Responsive Niosomes Decorated with Targeting Ligands
Hu et al. have designed a niosomal targeted system via galactose homing moieties to hepatoma cells. The systems were also rendered pH-sensitive via introducing cholesteryl hemisuccinate (CHEMS) in the niosomal membranes to allow optimized cellular delivery of their cargo.19 The vesicles were glycosylated via grafting with galactosylated stearate to impart active targeting to hepatoma cells. These niosomes, loaded with the anticancer natural product tanshinone IIA, demonstrated acidity-triggered release patterns. In vitro cytotoxicity study using a spectrum of tumor cell lines of different origin, namely, ovarian cancer (A2780), colon cancer (HCT8), and hepatoma (Huh7, HepG2) proved the concept for specific tropism and activity in hepatoma cells as compared to the free drug. The in vivo evaluation showed that the niosomal encapsulation drastically prolonged the circulation time and the total exposure (AUC) of the entrapped tanshinone IIA. Moreover, the biodistribution study confirmed the preferential liver accumulation of the system, due to the employed targeting strategy.
3.1.3. Long-Circulating Niosomes Decorated with Targeting Ligands
Tavano et al. prepared a tumor-targeted niosomal system for delivery of Doxorubicin by mixing an opportunely modified Pluronic L64 copolymer and cholesterol, and subsequent attachment of Transferrin (Tf) moiety to the polymer chain end.20 Tf-conjugate niosomes (L64/Chol-R-Tf) demonstrated much higher cellular uptake to MCF-7 and MDA-MB-231 cells than the unmodified niosomes (Figure 5). Moreover, Doxorubicin-loaded Tf-niosomes achieved a significant reduction of cell viability in a dose- and time-related manner.
Figure 5.
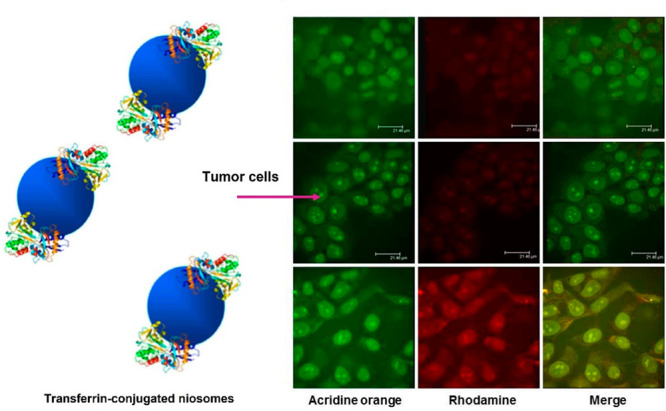
Confocal microscopic analysis of intracellular localization of unmodified (L64/Chol (top), L64/Chol-R (middle)) and Tf-conjugated niosomes (L64/Chol-R-Tf (bottom)) in MCF-7 human breast cancer cells. Fluorescence of rhodamine is excited at 555 nm and detected at a wavelength of 580 nm; for acridine orange, the fluorescence is excited at 460 nm and detected at a wavelength of 650 nm. Scale bars represent 21 μm. Adapted with permission from ref (20). Copyright 2013 American Chemical Society.
Further on, niosomes obtained from modified Pluronic L64, coupled with transferrin (Tf) and folic acid (FA), have been studies as multifunctional systems for controlled release of doxorubicin and curcumin.21 These systems possessed coordinated action of stealth, active targeting, and internalizing in tumor cells to achieve intracellular drug delivery. In vitro evaluations of the anticancer activity demonstrated the strong potential of dual-loaded niosomes with doxorubicin and curcumin with respect to the formulations containing only doxorubicin, thereby confirming the synergistic effects of this combination. FA-functionalized niosomes were formulated and loaded with Letrozole and Curcumin for chemotherapy of breast cancer.22 Span80, cholesterol, and DSPE-PEG2000-folate were used as membrane building components. The Curcumin/Letrozole coloaded niosomes showed good biocompatibility with HEK-293 normal cells and considerable cytotoxicity against MCF-7 cells and MD-MB-231 breast cancer cells. The in vitro studies also revealed that the Curcumin/Letrozole coloaded niosomes enhanced the apoptosis rate in both cell lines as compared to the mixture of free drugs, which was due to higher cellular uptake of the niosomal formulation through folate receptor-mediated endocytosis. Liu et al. have developed a targeted niosomal delivery of daunorubicin (DNR) against acute myeloid leukemia (AML), using anti-CD123 antibodies as homing moieties.23 The antibodies were conjugated to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol)2000] (Mal-PEG2000-DSPE), and the latter was grafted to the membranes of niosomes at varying densities. The antibody-targeted niosomes showed superior uptake in AML cells, versus the pain niosomes, in a ligand-density dependent fashion. The mechanistic studies corroborated a receptor-mediated endocytosis as the dominant internalization process. The in vitro cytotoxicity bioassay in AML cell lines showed that daunorubicin-loaded targeted formulation exhibited ca. 2.5- and 3-fold lower IC50 values cytotoxicity vs the plain niosomal drug in NB4 and THP-1 cells, respectively. The in vitro findings were corroborated by an in vivo anti-neoplastic activity study in THP-1 bearing NOD/SCID mice whereby the CD123-targeted platform significantly outclassed the free drug, the nontargeted formulation, and the saline-negative control in terms of median survival times. Seleci et al. have developed a niosome-based drug delivery platform for doxorubicin with combined long-circulation, cell-penetrating properties and active tumor targeting.24 The presented formulation was based on PEGylated niosomes, modified with cell-penetrating peptide (CysTAT) and decorated with a specific aptamer (S2.2), targeting the MUC-1 glycoprotein which is overexpressed in many solid tumors such as lung, ovarian, breast, and prostate carcinomas, among others. The vesicles were prepared from Span60, cholesterol, and Mal-PEG2000-DSPE and loaded with doxorubicin. Thereafter, the loaded niosomes were surface decorated with a CysTAT–MUC1 aptamer conjugate by thioether bonding. The cellular uptake and subcellular trafficking of the vesicles were evaluated by fluorescence microscopy and flow cytometry analysis which showed that in MUC-1 positive cells HeLa the doxorubicin levels were highest after treatment with the CysTAT–MUC1 aptamer-modified pegylated niosomes, followed by the free drug and the nontargeted niosomes, whereas in the MUC-1-negative U87 cells free drug showed superior uptake. These findings were corroborated by the in vitro cytotoxicity bioassay whereby the MUC1 targeted formulation exhibited superior cytotoxicity to free drug in HeLa but not in U87 cells, which confirms the rationale for this platform as a targeted doxorubicin formulation.
3.2. Multifunctional Niosomes for Brain Targeting
A different intriguing strategy for tissue-specific targeting using niosomes is based on decoration with homing moieties imparting superior penetration of the blood brain barrier. To address this objective, PEGylated Span/CHOL niosomes loaded with Temozolomide (an oral DNA-alkylating agent) were conjugated with chlorotoxin (CTX), a 36-amino-acid peptide as a brain-targeting moiety.25 The in vitro cytotoxicity of the CTX-decorated platform vs the nonmodified niosomal formulation or free drug was assessed in U-373 MG human glioblastoma cell line and showed that the encapsulation of the drug is not detrimental for its anti-neoplastic activity. The pharmacokinetics and tissue distribution study in mice showed that targeted niosomal preparation attained ca. 3-fold increase in Temozolomide levels in the brain, with a concomitant 2-fold and 1.5-fold decrease in the accumulation liver and kidney. An alternative approach for successful BBB transfer and delivery of therapeutics to the brain is based on carbohydrate decorated niosomes. The glucose moiety is an attractive candidate for brain delivery due to the overabundant expression of GLUT-1 transporters on BBB to facilitate glucose transport and ensure the high energetic demands of the tissue. On this basis, Dufes et al. elaborated a niosomal preparation containing N-palmitoyl glucoseamine as GLUT-1 targeting fragment as a CNS delivery system for vasoactive intestinal peptide (VIP).26125I-labeled VIP was loaded in the niosomes to allow comparative brain delivery and distribution of the cargo after intravenous application of VIP in solution or encapsulated in the targeted niosomes or in control niosomes, not bearing glucose moieties. The pharmacokinetic study showed that the niosomal preparations allowed delivery of intact VIP to the CNS, unlike the solution of the nonencapsulated hormone. The glucose-decorated niosomes attained superior brain delivery of the cargo, as compared to the plain niosomes. Niosomes, comprising glucopyranose (GP), alanine (A), or/and glutathione (GSH) ligands, have been designed as single or dual BBB targeting nanovesicles of large biomolecules such as serum albumin. Span 60, Solulan C24 (cholesterylpoly-24-oyxyethyleneether), cholesterol, N-dodecyl-β-d-glucopyranose, dodecanoyl-alanine, and PEGylated-GSH were used to prepare niosomes of various compositions (Figure 6).27 Ligands targeting brain endothelial transporters elevated the permeability of the albumin cargo across the BBB in the culture model and in mice. Moreover, dual-ligand decoration of niosomes was more effective than single ligand labeling.
Figure 6.
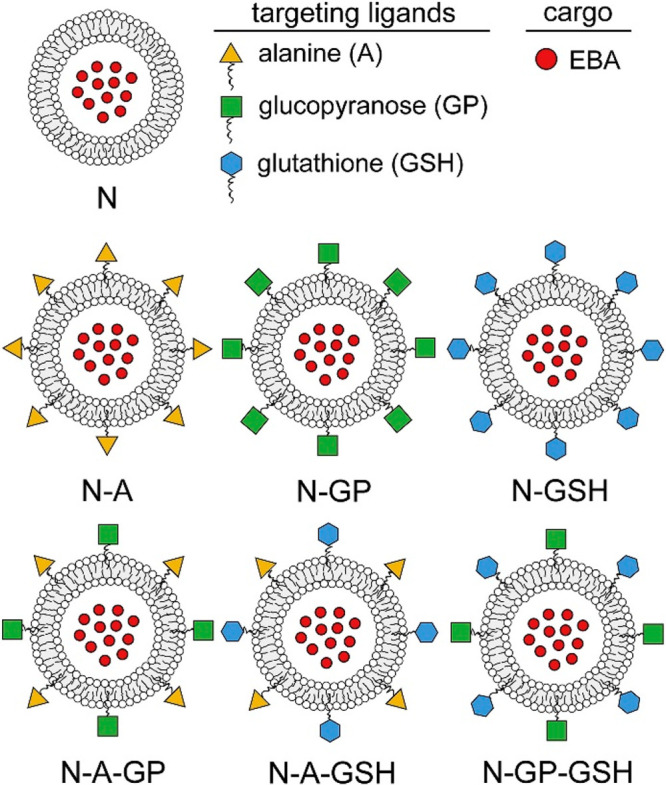
Schematic drawing of nontargeted (N), single ligand targeted (N-A: alanine-, N-GP: glucopyranose-, N-GSH: glutathione-targeted) and dual-targeted niosomes (N-A-GP: glucose-alanine-, N-A-GSH: alanine-glutathione-, NGP-GSH: glucose-glutathione-targeted). Reproduced with permission from ref (27). Copyright 2018 Elsevier.
3.3. Multifunctional Niosomes as Gene Delivery Systems
Multifunctional niosomes have been used to improve the transfection efficiency and overcome limitations in gene therapy. In a recent paper, magnetic cationic niosomes were reported as a delivery platform for Lifeguard (LFG)-specific siRNA inside the hydrophilic niosomal core.11 During the preparation process, superparamagnetic iron oxide nanoparticles (FexOy-NPs) were incorporated within the niosomal bilayer structure to allow increased cellular uptake via an external magnetic field, and thereafter the siRNA loading was carried out. The hybrid niosomal carriers were tested for apoptogenic activity against BT-474 cells in a combination with either erlotinib or trastuzumab. The niosome-mediated downregulation of the anti-apoptotic LFG gene in BT-474 cells proved to increase the activity of the targeted anti-neoplastic agents more profoundly as compared to their combination with non-entrapped siRNA. Multifunctional niosomal carrier based on Tween-60, cholesterol, DOTAP, and DSPE-PEG2000 with optimal size and surface charge has been employed as transfection carrier for miRNAs targeting the anti-apoptotic Bcl-2 protein mRNA.28 The niosome-mediated transfection of PC3 prostate cancer cells using two miRNAs (miR-15a and miR-16-1) proved to significantly downregulate the Bcl-2 gene with a concomitant increased cell death rate indicating the synergistic effects of this codelivery and the plausibility of the niosomal carrier as transfection enhancers.
3.4. Multifunctional Niosomes for Theranostics
Advanced niosomal formulations, intended for combined therapy and diagnosis (theranostics) have been developed by Demir et al.29 Gold nanoparticles (AuNPs) and a photosensitizer, protoporphyrine IX (PpIX), were spontaneously encapsulated in FA-modified targeted Tween 80/Chol niosomes, and these systems were applied to cancer cells via passive targeting process. The presence of AuNPs and PpIX makes these nanovesicles promising candidates for radiotherapy (RT) and photodynamic therapy (PDT), respectively, as well as for combined therapy (PDT+RT) and cell imaging applications. Experiments with HeLa and A549 cell lines assessed the therapeutic efficiencies of AuNP-PpIX-FA niosomes by RT and PDT. Due to the encapsulant materials’ therapeutic properties, it was proven that theranostic niosomes can be successfully used for the RT-PDT combined therapy modality for HeLa cells thanks to active targeting capability of vesicles. The combination of gene delivery and cell labeling capacity into a single system is considered a very attractive theranostic platform. Yang and co-workers developed a theranostic niosomal formulation for efficient delivery of small RNAs [small interfering RNA (siRNA)/microRNA (miRNA)] to human mesenchymal stem cells (hMSCs) to promote differentiation and to specifically label the transfected cells for the in vivo tracking purpose.30 Indocyanine green (ICG) was encapsulated in a nonionic surfactant vesicle composed of sorbitan monooleate (Span 80), a cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and a PEGylated lipid (TPGS). The encapsulation of amphiphilic ICG molecules in the niosomes resulted in dye self-quenching because of their proximity. Next, siRNA or miRNA were complexed through electrostatic interactions with the positive headgroup of DOTAP on the surface of niosomes (Figure 7). The theranostic niosome/siRNA complex (iSPN) mediated efficient intracellular delivery, resulting in specific gene silencing in hMSCs. In addition, following inhibition of miR-138 by iSPN/anti-miR-138, enhanced osteogenic differentiation of hMSCs was achieved. Furthermore, the developed niosomes exhibited OFF/ON activatable fluorescence upon decomposition or cellular internalization, resulting in efficient NIR labeling of stem cells and live tracking in animals.
Figure 7.
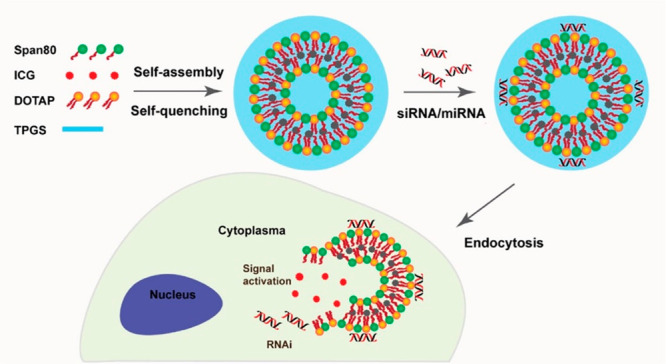
Illustration showing the design of an ICG-containing theranostic niosome system (iSPN) for intracellular delivery of siRNA/miRNA and activatable labeling of cells upon dequenching. Reproduced with permission from ref (30). Copyright 2018 American Chemical Society.
4. Conclusions
Multifunctional niosomes are outstanding candidates for sustained, controlled, and targeted delivery and codelivery of both hydrophilic and hydrophobic drugs and therapeutic macromolecules (proteins and genes). They have been developed by modifying conventional niosomes by inserting specific structural elements such as functional groups, segments, and nanoparticles. The incorporation of more than one functionality in the niosomal carrier is a step toward overcoming the biological complexity and therapeutic challenges associated with the classical niosomes during treatment. Generally, the design of multifunctional niosomes involves a combination of two or more modification strategies for incorporating polyethylene glycol, active targeting moieties, and stimuli-sensitive segments/nanoparticles. The PEGylaton of niosomes provides enhanced colloid stability and long circulation in the bloodstream and protects drugs from enzymatic degradation. The surface decoration of niosomal membrane with ligands, aptamers, antibodies, and other targeting moieties helps to achieve selective interactions with specific receptors and increased internalization in particular cell populations. Modification of niosomes with additives, which can induce changes of the physicochemical properties of the system in response to internal (differences in the pH, enzyme composition, temperature in tumors or inflamed tissues) or external stimuli (magnetic field, temperature, ultrasound, etc.), makes possible drug release at the target site.
Multifunctional niosomes are characterized with improved therapeutic efficacy, because of the synergic benefits of combining more than one targeting mechanism. The use of such carriers is especially advantageous in cancer therapy, as the classic chemotherapeutics are associated with poor therapeutic efficacy, nonspecific localization in the organism, systemic toxicity, and side effects. Multifunctional niosomes have great potential for successful delivery of therapeutics to the brain as well as for improved transfection efficiency in gene therapy. Advanced niosomal formulations developed for theranostics can be used to gain information that assists with diagnostics, treatment, and monitoring of treatment response of patients. It can be expected that in the near future the extensive research focused on innovative niosome formulations will lead to the development of new products for the pharmaceutical industry and will boost the “personalized medicine” approach.
Acknowledgments
This work was supported by a grant from the Bulgarian National Science Fund (KP-06-H43/3-2020).
Biographies
Denitsa Momekova is an expert on the design and application of functionalized nanosized drug delivery systems and drug targeting. She obtained her Ph.D. degree in 2008 at the Faculty of Pharmacy, Medical University of Sofia with a thesis on sterically stabilized and stimuli-sensitive liposomes. In 2016 she was appointed as Professor at the Department of Pharmaceutical Technology and Biopharmaceutics, Faculty of Pharmacy of the Medical University of Sofia.
Viliana Gugleva obtained her Ph.D. in Pharmaceutical Technology and Biopharmacy in 2021 from the Medical University of Sofia, Bulgaria. Currently, she works as an Assistant Professor at the Department of “Pharmaceutical technologies”, Medical University - Varna “Prof. Dr. Paraskev Stoyanov”, Bulgaria. Her research interests include nanotechnology, vesicular systems, stimuli-sensitive carries, in situ gels, ocular, and topical drug delivery.
Petar Dimitrov Petrov, D.Sc., is Professor and head of laboratory “Functional and nanostructured polymers” at the Institute of Polymers of the Bulgarian Academy of Sciences. He graduated from the University of Chemical Technology and Metallurgy, Bulgaria, with M.Sc. degree in chemical technology (1996) and Ph.D. in macromolecular chemistry (2001). His research activities have recently focused on the synthesis and coassembly of amphiphilic block copolymers, photochemical cross-linking of natural and synthetic polymers, fabrication of supermacroporous cryogels, polymeric complexes, nanoparticles, and nanocomposites for applications in medicine, pharmacy, and biotechnology.
Author Contributions
This paper was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Masjedi M.; Montahaei T. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications. J. Drug Delivery Sci. Technol. 2021, 61, 102234. 10.1016/j.jddst.2020.102234. [DOI] [Google Scholar]
- Singh T. G.; Sharma N.. Nanobiomaterials in cosmetics: current status and future prospects. In Nanobiomaterials in Galenic Formulations and Cosmetics; William Andrew Publishing, 2016; pp 149–174. [Google Scholar]
- Ag Seleci D.; Seleci M.; Walter J.-G.; Stahl F.; Scheper T. Niosomes as nanoparticular drug carriers: fundamentals and recent applications. J. Nanomater. 2016, 2016, 7372306. 10.1155/2016/7372306. [DOI] [Google Scholar]
- Grijalvo S.; Puras G.; Zárate J.; Sainz-Ramos M.; Qtaish N. A. L.; López T.; Mashal M.; Attia N.; Díaz Díaz D.; Pons R.; Fernández E.; Pedraz J. L.; Eritja R. Cationic niosomes as non-viral vehicles for nucleic acids: Challenges and opportunities in gene delivery. Pharmaceutics 2019, 11, 50. 10.3390/pharmaceutics11020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G. P.; Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery - an overview. Acta Pharm. Sin. B 2011, 1 (4), 208–19. 10.1016/j.apsb.2011.09.002. [DOI] [Google Scholar]
- Bhardwaj P.; Tripathi P.; Gupta R.; Pandey S. Niosomes: A review on niosomal research in the last decade. J. Drug Delivery Sci. Technol. 2020, 56, 101581. 10.1016/j.jddst.2020.101581. [DOI] [Google Scholar]
- Attia M. F.; Anton N.; Wallyn J.; Omran Z.; Vandamme T. F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71 (8), 1185–98. 10.1111/jphp.13098. [DOI] [PubMed] [Google Scholar]
- Sahu A. K.; Mishra J.; Mishra A. K. Introducing Tween-curcumin niosomes: preparation, characterization and microenvironment study. Soft Matter 2020, 16 (7), 1779–1791. 10.1039/C9SM02416F. [DOI] [PubMed] [Google Scholar]
- Asthana S. G.; Sharma P. K.; Asthana A. In vitro and in vivo evaluation of niosomal formulation for controlled delivery of Clarithromycin. Scientifica 2016, 2016, 6492953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda E.; Puras G.; Agirre M.; Zárate J.; Grijalvo S.; Pons R.; Eritja R.; Martinez-Navarrete G.; Soto-Sanchez C.; Fernández E.; Pedraz J. L. Niosomes based on synthetic cationic lipids for gene delivery: the influence of polar head-groups on the transfection efficiency in HEK-293, ARPE-19 and MSC-D1 cells. Org. Biomol. Chem. 2015, 13 (4), 1068–81. 10.1039/C4OB02087A. [DOI] [PubMed] [Google Scholar]
- Maurer V.; Altin S.; Ag Seleci D.; Zarinwall A.; Temel B.; Vogt P. M.; Strauß S.; Stahl F.; Scheper T.; Bucan V.; Garnweitner G. In-vitro application of magnetic hybrid niosomes: Targeted siRNA-delivery for enhanced breast cancer therapy. Pharmaceutics 2021, 13, 394. 10.3390/pharmaceutics13030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk J. S.; Xu Q.; Kim N.; Hanes J.; Ensign L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Delivery Rev. 2016, 99, 28–51. 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano L.; Muzzalupo R. Multi-functional vesicles for cancer therapy: The ultimate magic bullet. Colloids Surf., B 2016, 147, 161–171. 10.1016/j.colsurfb.2016.07.060. [DOI] [PubMed] [Google Scholar]
- Olusanya T. O. B.; Haj Ahmad R. R.; Ibegbu D. M.; Smith J. R.; Elkordy A. A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. 10.3390/molecules23040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davarpanah F.; Khalili Yazdi A.; Barani M.; Mirzaei M.; Torkzadeh-Mahani M. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. Daru, J. Pharm. Sci. 2018, 26, 57–64. 10.1007/s40199-018-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano L.; Vivacqua M.; Carito V.; Muzzalupo R.; Caroleo M. C.; Nicoletta F. Doxorubicin loaded magneto-niosomes for targeted drug delivery. Colloids Surf., B 2013, 102, 803–807. 10.1016/j.colsurfb.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Tavano L.; Rossi C. O.; Picci N.; Muzzalupo R. Spontaneous temperature-sensitive Pluronic based niosomes: Triggered drug release using mild hyperthermia. Int. J. Pharm. 2016, 511 (2), 703–708. 10.1016/j.ijpharm.2016.07.064. [DOI] [PubMed] [Google Scholar]
- Pereira M. C.; Pianella M.; Wei D.; Moshnikova A.; Marianecci C.; Carafa M.; Andreev O. A.; Reshetnyak Y. K. pH-sensitive pHLIP coated niosomes. Mol. Membr. Biol. 2016, 33 (3–5), 51–63. 10.1080/09687688.2017.1342969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Zhang J.; Deng L.; Hu H.; Hu J.; Zheng G. Galactose-modified pH-sensitive niosomes for controlled release and hepatocellular carcinoma target delivery of Tanshinone IIA. AAPS PharmSciTech 2021, 22, 96. 10.1208/s12249-021-01973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano L.; Muzzalupo R.; Mauro L.; Pellegrino M.; Andò S.; Picci N. Transferrin-conjugated Pluronic niosomes as a new drug delivery system for anticancer therapy. Langmuir 2013, 29 (41), 12638–12646. 10.1021/la4021383. [DOI] [PubMed] [Google Scholar]
- Tavano L.; Mauro L.; Naimo G. D.; Bruno L.; Picci N.; Andò S.; Muzzalupo R. Further Evolution of multifunctional niosomes based on Pluronic surfactant: Dual active targeting and drug combination properties. Langmuir 2016, 32 (35), 8926–8933. 10.1021/acs.langmuir.6b02063. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh I.; Yaraki M. T.; Ahmadi S.; Chiani M.; Nourouzian D. Folic acid-functionalized niosomal nanoparticles for selective dual-drug delivery into breast cancer cells: An in-vitro investigation. Adv. Powder Technol. 2020, 31 (9), 4064–4071. 10.1016/j.apt.2020.08.011. [DOI] [Google Scholar]
- Liu F. R.; Jin H.; Wang Y.; Chen C.; Li M.; Mao S. J.; Wang Q.; Li H. Anti-CD123 antibody-modified niosomes for targeted delivery of daunorubicin against acute myeloid leukemia. Drug Delivery 2017, 24 (1), 882–890. 10.1080/10717544.2017.1333170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleci D. A.; Seleci M.; Jochums A.; Walter J.-G.; Stahl F.; Scheper T. Aptamer mediated niosomal drug delivery. RSC Adv. 2016, 6 (91), 87910–87918. 10.1039/C6RA19525C. [DOI] [Google Scholar]
- De A.; Venkatesh N.; Senthil M.; Sanapalli B. K. R.; Shanmugham R.; Karri V. V. S. R. Smart niosomes of Temozolomide for enhancement of brain targeting. Nanobiomedicine 2018, 11 (5), 1849543518805355. 10.1177/1849543518805355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufes C.; Gaillard F.; Uchegbu I. F.; Schätzlein A. G.; Olivier J. C.; Muller J. M. Glucose-targeted niosomes deliver vasoactive intestinal peptide (VIP) to the brain. Int. J. Pharm. 2004, 285 (1–2), 77–85. 10.1016/j.ijpharm.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Mészáros M.; Porkoláb G.; Kiss L.; Pilbat A.-M.; Kóta Z.; Kupihár Z.; et al. Niosomes decorated with dual ligands targeting brain endothelial transporters increase cargo penetration across the blood-brain barrier. Eur. J. Pharm. Sci. 2018, 123, 228–240. 10.1016/j.ejps.2018.07.042. [DOI] [PubMed] [Google Scholar]
- Ghaffari M.; Kalantar S. M.; Hemati M.; Dehghani Firoozabadi A.; Asri A.; Shams A.; Jafari Ghalekohneh S.; Haghiralsadat F. Co-delivery of miRNA-15a and miRNA-16–1 using cationic PEGylated niosomes downregulates Bcl-2 and induces apoptosis in prostate cancer cells. Biotechnol. Lett. 2021, 43 (5), 981–994. 10.1007/s10529-021-03085-2. [DOI] [PubMed] [Google Scholar]
- Demir B.; Barlas F.; Gumus Z. P.; Ünak P.; Timur S. Theranostic niosomes as a promising tool for combined therapy and diagnosis: “All-in-One” Approach. ACS Appl. Nano Mater. 2018, 1 (6), 2827–2835. 10.1021/acsanm.8b00468. [DOI] [Google Scholar]
- Yang C.; Gao S.; Song P.; Dagnæs-Hansen F.; Jakobsen M.; Kjems J. Theranostic niosomes for efficient siRNA/MicroRNA delivery and activatable near-infrared fluorescent tracking of stem cells. ACS Appl. Mater. Interfaces 2018, 10 (23), 19494–19503. 10.1021/acsami.8b05513. [DOI] [PubMed] [Google Scholar]


