Abstract

Helminths have always been studied as one of the critically annoying pathogens of parasite classes due to their adverse effects on the ecosystem of human life. They have the potency to negatively affect their hosts as points of disease, infection, cancer, and death, but in this study, we found interesting electronic properties in Fasciola hepatica, Parascaris equorum (with and without larvae), Dicrocoelium dendriticum, Taenia multiceps, and Moniezia expansa eggs. This claim is attributed to some surprising characteristics such as significant diode behavior [forward bias, 5.36–11.17 (±0.01) V, versus the ground, GND] and backward bias (−45.0 to −125.0 (±7.0) V, versus the GND) and highly active negative resistance (−2.59 to −7.11) × 1015 (±1.5) Ω in the AC mode. These traits were measured by the “blind patch-clamp, single-unit recording” methodology using a three-microelectrode system, implanted onto each tested egg under giga ohm sealed conditions (6.28 ± 0.02 GΩ cm–1 and n = 4). All the characteristic parameters were simultaneously attributed to the helminth egg structure by acceptable reproducibility (percentage of relative standard deviation: > 5%) and high enough rectitude with enough differentiation in their magnitudes, relatively. The reliability of these results was further confirmed using multiple calibrated techniques such as alternative/direct current voltammetry. Also, the significant role of water molecules as the key medium in creating these properties is evaluated qualitatively. In addition, the study aims at introducing these interesting parameters as a new approach to the fabrication of bio-based electronic elements, which are considered as a novel class of helminth egg-detection and -identification probes.
1. Introduction
The word bioelectronic is used to emphasize that every biomaterial could interact and combine with other electrical elements to make a signal or device.1 These materials (molecules) can be the goal of the interaction or the heart of the bioelectronic devices.2 Many efforts of manufacturing, recognizing, determining, and sensing have been carried out in this field by using/sensing several types of the biomolecules such as enzymes,3 proteins,4 lipoproteins,5 carbohydrates,6 DNA,6 and so on.3−6 The aims of this branch of science are miniaturization,7 amplification,8 and modification9 in the sensing and recognition of targeting molecules.10 Therefore, scientists have researched and developed bioelectronic devices by semi-synthesizing or exploring new biomaterials.11
On the other hand, a diode (rectifier) is an electronic device allowing electrical current to move through it in one direction with far greater ease than in the other.12 By the invention of the diode, many types of this device, like Gunn diodes, Schottky diodes, photodiodes, and light-emitting diodes (LED), with several applications have been introduced to the universe of technology.13 In addition, negative electrical resistance is an unusual property of some electronic circuits and devices. An increase in electrical voltage across the device’s terminals often results in a decrease in electric current flowing through it.14 However, negative resistance is an uncommon property. Still, many types of diode such as tunnel diodes, Gunn diodes, active filters, and gas-discharge tubes have shown some parts of these unique features.15 Today, many scientists are focused on the electrical/optical properties by synthesis and modeling of biomolecules to use them as a bio-based diode like nucleic acid bases in the OLEDs (organic light-emitting diodes),16 fluorescence protein in the bio-hybrid LEDs,17 and modeling of microtubule tubulin as a diode.18
Parasitic helminths are usually known as undesired pathogens, causing various diseases in both human and animal species. They have a negative impact on human health and animal productions, often by inhabiting some parts of its life cycle in the body of another larger animal as the host.19,20 Helminths can choose different hosts to pollute, such as soil, water, plants, animals, and, most importantly, humans. Helminth contamination for humans would also have a devastating effect(s) such as disease, infection, and even dangerous side effects like cancer and even death.21,22
Serious attention to the life cycle of the helminth eggs sometimes shows the living modes of these pathogens, even under tough conditions like deficiency of oxygen, nutrient media, light, and so on.23 Although they are often inactive under these stiff conditions,24,25 the inactive metabolite reveals their sophisticated cell physiology.26 In this article, active negative resistance and the diode behavior of the helminth eggs are evaluated in detail.
There are very few research studies about the biomolecules for their use in the field of electronics, and production, extraction, manipulation, and fabrication of electronic devices from them also require cost, time, and proficiency. In this work, we introduce helminth eggs as available, inexpensive, and almost safe sources of diode unit biomaterials. These interesting electronic features of helminth eggs introduce them as inspiring creatures for the next generation of electronics and sensors. In addition, this would provide novel pathways to detect and identify the helminth eggs based on their negative resistance and diode behaviors. Consequently, these characteristics are comparable with the electrical current detection probes such as enzyme-linked immunosorbent assay, immune-assay system, and so forth.27−29 To the best of our knowledge, up to now, there have not been any introduced materials with completely mentioned diode behaviors and negative resistance characteristics.
2. Results and Discussion
2.1. Electrochemical Impedance Spectroscopy
As mentioned above, electrochemical impedance spectroscopy is utilized to access a general perspective about each helminth egg. The Nyquist plots and the equivalent circuits are shown in Figure 1. As shown, elements including electrical capacitance, inductance, resistance, and negative resistance are approved for all the tested helminth eggs. It should be noted that, to simplify the interpretation processes, only the electrochemical impedance spectrum of the Fasciola hepatica is presented in Figure 1; however, this condition can be generalized and extended to other eggs, without observing a significant difference, and the results do not differ significantly. This phenomenon pointed to the dependency of the equivalent circuit on the structure of the helminth eggs; however, the value of each element changed for both detection and recognition of the helminth egg.
Figure 1.
(a) Nyquist plot in the absence of helminth eggs, (b) two-dimensional Nyquist plot for F. hepatica, (c) three-dimensional Nyquist plot for a sample of F. hepatica, (d) equivalent circuit as an example for the absence of helminth eggs, and (e) equivalent circuit as an example for the presence of F. hepatica. Conditions: All conditions and procedures are expressed in detail as mentioned above during implanting two microelectrodes with 0.0124 ± 0.0008 mm inter-electrode distance, error bar ± standard deviation.
As shown in Figure 1a, in the absence of any connection to the helminth eggs, the result
shows that the RC equivalent circuit has the behavior of an external
dummy cell, as proved in the equivalent circuit in Figure 1d,33 whereas after setting blind-patch clamp (single-unit recording)
connections under the giga ohm sealed condition, the imaginary part
of the Nyquist plot (Figure 1b) showed negative values between −22.0 to 0.0 Ω.
Due to the electrochemical impedance spectroscopy (EIS) spectrum and
equivalent circuit in Figure 1e, this unusual interaction of the helminth eggs with the
AC perturbation can be interpreted as each of them behaving like a
large capacitor owing to the very low capacitance impedance, based
on Xc = 1/(j·C·ω),
where ω = 2πυ and j =  .34 Also, this
phenomenon can be attributed to the induction of helminth eggs, which
is related to enormous inductance impedance based on XL = j.L.ω, where j =
.34 Also, this
phenomenon can be attributed to the induction of helminth eggs, which
is related to enormous inductance impedance based on XL = j.L.ω, where j =  .34
.34
According to the fitted equivalent circuit (Figure 1e), besides the presence of a common resistance, the existence of a vast negative resistance was also proved by analyzing helminth eggs (F. hepatica as the selected sample). In addition, due to the similarity of output results for helminth eggs, the existence of fantastic electrical elements is proved. To prevent the confusion of the contents and due to the importance and its exciting features of negative resistance, we have continued to more precisely focus on this parameter in the following sections of this paper. In addition, the diode behaviors of helminth eggs are studied because of their relationships to the negative resistance.
2.2. Diode Characteristics
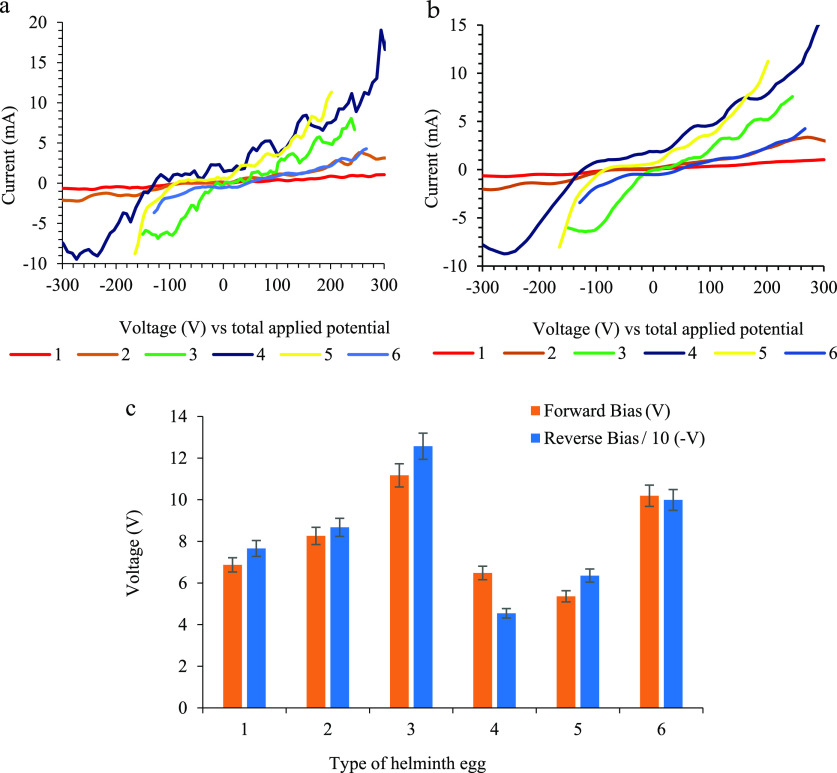
The diode behavior of each egg was evaluated via applying and sweeping a linear sweep DC potential. To access this aim, voltage was applied between 0.0 to ± 300.0 V (DC vs total applied potential) with a 100.0 ± 0.1 mV/s scan rate using a programmable potentiostat/galvanostat function generator. This potential range is considered as the potential window of the helminth eggs, without dealing with any electrical damage and shock when the electrical current flows through them. The potential is applied to the implanted three-microelectrode system, including working, pseudo reference, and counter probes, implanted onto each helminth egg as a natural bio-diode.
By controlling the Ohmic potential of the system, the helminth egg was conditioned via the formation of giga ohm sealed conditions between the working and pseudo reference microelectrode. This process led to minimization of the uncompensated resistance (Ru)35 possibly, besides resulting in having an ideally pseudoreference behavior, accompanied by a simultaneous electrical current of the system at both normal and reverse primary input and output systems (bias). The I–V curve is selected to evaluate the barrier potential (Vb) and the break-down potential (Vbreakdown) of each tested helminth egg. Also, results and the extended data are illustrated in Figure 2.
Figure 2.
(a) Extended data of diode behavior, (b) smoothed extended data of diode behavior, and (c) results of diode behavior of the helminth eggs. Note that in order to prevent clutter, error bars are eliminated. Condition: Each set of reported data is the average of three replicate data under similar conditions during implanting three microelectrodes with 0.0124 ± 0.0008 mm inter-electrode distance, error bar ± standard deviation.
According to Figure 2b, some of the tested helminth eggs exhibited a relatively ideal diode behavior (electric current flux in a range of applied voltages) like F. hepatica, Parascaris equorum without/with larvae, and Moniezia expansa. On the other hand, other helminth eggs showed a non-ideal diode behavior because of the appearance of some significant background electrical currents under the potential condition lower than the barrier potential at forward bias. This phenomenon is probably attributed to the structure. To better explain this, special consideration is given to the results for the presentation of confident diode information such as ideality, the voltage range of ideality, and forward/backward voltage bias and exposure in Table 1. It seems that the existence of a unique structure and material in the helminth eggs play a very critical role in making them a diode element during sweeping the electrical voltage and measuring the electrical currents.
Table 1. Forward and Backward Bias Voltage and Ideality Behaviors of Helminth Eggsa.
| type of helminth egg | forward bias (V) ± SD (n = 4) | backward bias (−V) ± SD (n = 4) | ideality | voltage range of ideality |
|---|---|---|---|---|
| F. hepatica | 6.87 ± 0.38 | 76.59 ± 6.00 | ideal | Vb = −79.9 to Vf = 20.0 V |
| P. equorum without larvae | 8.26 ± 0.28 | 86.73 ± 6.49 | ideal | Vb = −76 to Vf = −22.0 V |
| P. equorum with larvae | 11.17 ± 0.39 | 125.7 ± 7.71 | ideal | Vb = −11 to Vf = 14.0 V |
| Dicrocoelium dendriticum | 6.48 ± 0.34 | 45.45 ± 8.07 | nonideal | |
| M. expansa | 5.36 ± 0.45 | 63.56 ± 3.24 | ideal | Vb = −16 to Vf = 13.0 V |
| T. multiceps | 10.19 ± 0.54 | 99.88 ± 8.88 | nonideal |
Each set of reported data is the average of three replicate data under similar conditions during implanting three microelectrodes with 0.0124 ± 0.0008 mm inter-electrode distance, error bar ± standard deviation.
As shown, each helminth egg processes a particular potential range that can be considered as a selective fingerprint probe for both detection and identification of the helminth egg with enough reliability and accuracy.
2.3. Negative Resistance Related to the Helminth Eggs
The negative electrical resistance of each helminth egg was measured by two independent procedures:
-
(i)
measuring the resistance value via analyzing the resistor element in the equivalent circuit using EIS and
-
(ii)
via estimation of the slope of the current–potential (I–V) curve by applying an AC potential (vs total applied potential), by AC voltammetry, based on Ohm’s law (Z = V/j.I, where j =
 ).
).
2.3.1. Effect of Electrical Current on the Negative Resistance Behavior of the Helminth Egg
Besides the AC mode of the applied electrical potential on the negative resistance behavior of each helminth egg, the maximum electrical current capacity of each helminth egg, in other words, “maximum allowed Electrical current flow” at the AC mode in a fixed frequency range such as 50.0 Hz, was also important. The negative electrical resistance was observed in the “controlled current blind-patch clamp, single-unit recording” method (Figure 3). This technique was achieved by controlling (buffering) the electrical current using an electrical current divider circuit via paralleling or feedbacking each helminth egg. This was achieved using an external high-impedance electronic element such as an operational amplifier (op-amp). However, to have maximum sensitivity and the highest negative resistance, a fixed possible electrical current density as large as 1.14 (±0.09) × 10–3 (n = 5) A cm–2 as the “threshold and critical” electrical current density must be flown along with each tested helminth egg.
Figure 3.
Resistance determination from (a) negative resistance measurement by EIS and scanning AC voltage and (b) negative AC resistance of helminth’s eggs by voltage scanning. Note that to prevent clutter, error bars are eliminated. (For five repetitions during implanting three microelectrodes with 0.0124 ± 0.0008 mm inter-electrode distance, error bar ± standard deviation).
2.4. Semi-Qualitative Proposed Interpretation
Up to now, the existence of different types of biological units such as proteins,36 lipids,37 phenolic compounds,38 carbohydrates,39 water,40 and oxygen41 has been proved in the structure of the helminth eggs by several reports. However, exact amounts, sequence, and configuration of these biomaterials in the construction of the helminth egg are still not reported, and only few little-glance studies about the helminth egg formation are reported.42,43
To find out the unique role of the biological component in the mentioned parameters, the helminth eggs were carefully weighed before and after heating up to 60 ± 1.0 °C in a vacuum oven at 720.0 ± 0.3 Torr pressure along a 5.0 h time interval. Amazingly, the weight ratio between the water removed and the remaining material was an exact match. To understand the efficacy of the removed materials on the diode and negative resistance behavior, experiments were performed again to measure the parameters mentioned above. These pieces of evidence revealed that the helminth eggs efficiently reacted as a non-repeatable electrical resistor, and consequently, all parameters were experimentally determined (All results are illustrated in Table 2).
Table 2. Effect of Weight Loss on the Diode Behavior and Negative Resistancea.
| type of helminth egg | fresh average weight ± SD (n = 10) (g) | dried average weight ± SD (n = 10) (g) | removed average weight/fresh average weight ± SD (n = 10) | forward/backward bias voltage after drying (V) ± SD (n = 10) | negative resistance after drying (Ω) ± SD (n = 10) |
|---|---|---|---|---|---|
| F. hepatica | 1.0.0034 ± 0.0028 | 0.0018 ± 0.0015 | 0.44 ± 0.00 | random data with RSD > 140% (n = 15) | not detected (totally disappeared) |
| P. equorum without larvae | 0.0054 ± 0.0027 | 0.0049 ± 0.0024 | 0.09 ± 0.00 | ||
| P. equorum with larvae | 0.0038 ± 0.0025 | 0.0027 ± 0.0018 | 0.28 ± 0.00 | ||
| D. dendriticum | 0.0031 ± 0.0023 | 0.0014 ± 0.0004 | 0.52 ± 0.00 | ||
| M. expansa | 0.0058 ± 0.0011 | 0.0024 ± 0.0004 | 0.53 ± 0.00 | ||
| T. multiceps | 0.0054 ± 0.0029 | 0.0023 ± 0.012 | 0.56 ± 0.00 |
The results were reported based on four repetitions during implanting three-microelectrodes with 0.0124 ± 0.0008 mm inter-electrode distance, error bar ± standard deviation.
Apparently, according to the experiments, it is concluded that the removed materials (especially water) from the system played a vital role during the creation of these parameters. It seems that the removed materials contain plenty of water, some dissolved oxygen, and little CO2 produced from the helminth egg metabolism. Biomaterials in the parasite’s egg structure can absorb different amounts of water molecules depending on their hydrophilicity because of the presence of polar functional groups. Among them, proteins make up a more significant percentage of these substances, which, according to their orientation and configuration, are distributed in the 3-D space of the helminth eggs as separate vitelline cells. Each of these vitelline cells can play a role of a separate diode based on the amount of water absorbed in its structure. In addition to the mentioned parameters, the diode property also emerges again, possibly due to the presence of water medium in the construction and surface of alive tissues such as proteins, although this phenomenon was observed in previous refs (44) and (45).
Each vitelline cell can act in series or parallel to its neighboring vitelline cell, which ultimately results in the electrical diode behavior throughout system of each tested helminth egg. It is also important to mention that the form of the shell of the helminth egg, due to its structure (composition) and its lipid layer, can in turn play a role in the behavior of the diode element. Therefore, very high forward and backward voltage exhibited by the helminth egg is attributed to these features.
From the results of the diode behavior, when the weight ratio between water molecules to remained materials is relatively high, helminth eggs showed a non-ideal diode behavior like D. dendriticum and T. multiceps. On the other hand, when this ratio is relatively low and water in the system has a regular arrangement, helminth eggs show a normal (i.e., fairly ideal condition) diode behavior like F. hepatica, P. equorum (with and without larvae), and M. expansa. It seems that by increasing water in the structure, water molecules can move between the vitelline cells.
Based on the literature,46,47 a noticeable negative dielectric constant would appear because of water layers in contact with the biological component. On the other hand, this phenomenon is simulated like a capacitor parallel to negative resistance. In this manner and with consideration to the equivalent circuit of the EIS, which exhibited directly in the helminths eggs, the active negative resistance is probable due to the placement of a layer of water medium in contact with the biological structure.48 Therefore, the measured active negative electrical resistance in the helminth egg directly correlated to the equivalent elements of the active negative electrical resistance of different tissue layers of the egg’s surface matrix to the water medium.
To further investigate the effect of water on the development of these parameters in the helminth egg, instructions have been implemented. The first case to examine the reversibility of the electronic behavior of the helminth eggs is based on immersion of the heated parasite eggs in 100.0 mL of water medium in light and dark for 10.0 h. In this case, sodium chloride solution concentration is 0.1 mol L–1. The second method is based on placing the helminth eggs in a saturated-moisture environment with relative humidity (RH < 60%) up to 10.0 h. Despite all these efforts, no diode behavior or negative resistance was observed in the structure of these parasite eggs, which is a reason for the irreversibility of the behavior. In addition to the heating mentioned above, ethanol is also used to inactivate the parasite eggs. The experiments were repeated, with the result being the same as in the heating mode.
3. Conclusions
According to the results presented above and the explanations given in this article, in addition to introducing helminth eggs as an available, low-cost, robust diode under almost ideal conditions, they can be an inspiring platform to produce inexpensive and affordable micro- and nanodiode structures. On the other hand, one of the crucial aspects of the electronic world is negative resistance. In this paper, for the first time, this exciting parameter is observed in the biomaterials, which can be the basis for energy production and a better understanding of other phenomena through simulations resulting from the inspiration of this event. In Table 3, the comparison between some publications and diode behaviors of the helminth eggs is shown. However, some agitation has existed in the diode behavior, but it is not uncommon to claim that every parasite egg is a new world in electronics. Employing this attitude, these intrinsic parameters can be a valuable probe for identification and differentiation between several types of helminth eggs.
Table 3. Comparison between This Work and Some Publicationsa.
| materials used in bio-based diode | forward bias (V) | backward bias (−V) ± SD (n = 4) | ideality | refs |
|---|---|---|---|---|
| this work | 5.36–11.17 | 45.45–125.7 | relatively ideal | |
| DNA bases thymine and adenine | 8.0 | (16) | ||
| fluorescent proteins | (17) | |||
| firefly (Pyrocoelia rufa) | not reported | not reported | ideal | (49) |
| micro tubule (modeling) | –0.05 | (18) |
The potential and voltages reported vs the total applied potentials, bias: basic input and output system.
4. Experimental Section
All required reagents and solutions are reported entirely in the Supporting Information (see section: Reagents and Materials). All parts of the essential parameters of each tested egg were determined based on proper designs, which completely are described in the Supporting Information (see section: Instruments). These parts completely covered all types of methods and instruments. These designs are based on the blind patch-clamp, single unit recording methodology,30 under the Giga ohm sealed condition31 using Implanted Microelectrodes methodology,32 whose procedures were comprehensively explained in the Supporting Information (see section: Procedure).
About these descriptions, it is necessary to note that notations 1, 2, 3, 4, 5, and 6 refer to different types of the helminth eggs, including F. hepatica, P. equorum (in the absence or presence of any larvae), D. dendriticum, M. expansa, and T. multiceps, respectively. The related procedures were performed in three parts, which are mentioned in the following section.
4.1. Collection of Helminth Eggs
F. hepatica and D. dendriticum eggs were collected from the adult worms that were obtained from the livers of naturally infected sheep slaughtered at the Shiraz slaughterhouse (Zarghan, Fars, Iran). In addition, P. equorum eggs were obtained from the specimens, referred to the animal clinic, “School of Veterinary Medicine”, Shiraz University, Shiraz, Iran. T. multiceps eggs were collected from the gravid proglottids of adult worms collected from a naturally infected dog referred to the small animal clinic of “School of Veterinary Medicine”, Shiraz University (Shiraz, Iran). The M. expansa eggs were obtained from the gravid proglottids of adult worms collected from the small intestine of naturally infected sheep slaughtered at the “Shiraz Slaughterhouse.”
The uterus of adult female P. equorum and the uterine area of adult F. hepatica and D. dendriticum and gravid proglottids of T. multiceps and M. expansa were separately crushed using a mortar and pestle, dissolved in unchlorinated water, and passed through a 500 μm mesh sieve to separate coarse tissue residues from the helminth eggs. Subsequently, the passed materials, containing the eggs, were washed several times with unchlorinated water in 50.0, 25.0, and finally, 10.0 mL calibrated cylinders for 20.0, 15.0, and 15.0 min, respectively. After the total removal of the supernatant, the sedimented eggs were transferred into 2.0 mL microtubes, containing phosphate buffer solution [PBS, (1×), 0.1 mol L–1], and stored at 4.0 ± 0.5 °C until use.
4.2. Electrochemical Impedance Spectroscopy Test
Confident electrical information about each helminth egg is accessed by using EIS via a scanning frequency between 0.1 Hz to 1.0 MHz (±0.1) at a fixed amplitude (25.00 ± 0.01 mV and n = 3, vs total applied potential). To reliably carry out this experiment, an electrochemical electroanalyzer as a programmable electrical waveform (function) generator and a data acquisition system were used, and the frequency range was divided to 50 points in the logarithmic mode.
4.3. Diode and Negative Resistance Tests
The following procedures are applied to access reliable results about the diode and negative resistance behavior of helminth eggs.
4.3.1. Diode Measurement
The I–E curve as a criterion of the diode behavior for helminth eggs was obtained by scanning of voltages between different intervals (based on the type of helminth eggs) with a 100.0 ± 0.1 mV/s scan rate by using a potentiostat/galvanostat for voltage application and data acquisition.
4.3.2. Negative Resistance Measurement
Negative resistance of helminth eggs was evaluated based on the I–E curve by scanning AC voltage from −300.0 to 300.0 V and reading the output current and equivalent circuit of EIS measurement.
Acknowledgments
The authors wish to acknowledge the support of this work by the Shiraz University Research Council.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04954.
Materials and reagents, procedure, setup of electrochemical impedance spectroscopy, active surface area of the helminth eggs, single unit recording, giga ohm sealed conditions (PDF)
Author Contributions
M.M.D. directed the research group, supported the necessary methods, and edited the article and R.S. consulted the project, analyzed the data, and edited the article. He also performed all the electrical experiments, analyzed the data, and wrote the article and M.M. conceptualized the study, collected the helminth eggs, and edited the manuscript.
This study was financially supported by Shiraz University.
The authors declare no competing financial interest.
Notes
Ethics committee: This study was admitted and approved by the ethics committee of the Shiraz University Council.
Supplementary Material
References
- Willner I.; Katz E.. Bioelectronics: From Theory to Applications; John Wiley & Sons, 2006. [Google Scholar]
- Rasooly A.; Herold K. E.; Herold K. E.. Biosensors and Biodetection; Springer, 2009. [DOI] [PubMed] [Google Scholar]
- Schneider E.; Clark D. S. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron. 2013, 39, 1–13. 10.1016/j.bios.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Leca-Bouvier B.; Blum L. J. Biosensors for protein detection: a review. Anal. Lett. 2005, 38, 1491–1517. 10.1081/AL-200065780. [DOI] [Google Scholar]
- Lu S.; Yu T.; Wang Y.; Liang L.; Chen Y.; Xu F.; Wang S. Nanomaterial-based biosensors for measurement of lipids and lipoproteins towards point-of-care of cardiovascular disease. Analyst 2017, 142, 3309–3321. 10.1039/c7an00847c. [DOI] [PubMed] [Google Scholar]
- Zhang G.-J.; Huang M. J.; Ang J. A. J.; Yao Q.; Ning Y. Label-free detection of carbohydrate–protein interactions using nanoscale field-effect transistor biosensors. Anal. Chem. 2013, 85, 4392–4397. 10.1021/ac3036525. [DOI] [PubMed] [Google Scholar]
- Soleymani L.; Li F. Mechanistic challenges and advantages of biosensor miniaturization into the nanoscale. ACS Sens. 2017, 2, 458–467. 10.1021/acssensors.7b00069. [DOI] [PubMed] [Google Scholar]
- Mittal S.; Kaur H.; Gautam N.; Mantha A. K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. 10.1016/j.bios.2016.08.028. [DOI] [PubMed] [Google Scholar]
- Mowbray S.; Amiri A. A brief overview of medical fiber optic biosensors and techniques in the modification for enhanced sensing ability. Diagnostics 2019, 9, 23. 10.3390/diagnostics9010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighbayan D.; Sadighbayan K.; Tohid-Kia M. R.; Khosroushahi A. Y.; Hasanzadeh M. Development of electrochemical biosensors for tumor marker determination towards cancer diagnosis: Recent progress. TrAC, Trends Anal. Chem. 2019, 118, 73–88. 10.1016/j.trac.2019.05.014. [DOI] [Google Scholar]
- Ali J.; Najeeb J.; Ali M. A.; Aslam M. F.; Raza A. Biosensors: their fundamentals, designs, types and most recent impactful applications: a review. J. Biosens. Bioelectron. 2017, 8, 235. 10.4172/2155-6210.1000235. [DOI] [Google Scholar]
- Tooley M.Electronic Circuits: Fundamentals and Applications; Routledge, 2019. [Google Scholar]
- Hayes T. C.; Horowitz P.. Student Manual for the Art of Electronics; Cambridge University Press, 1989. [Google Scholar]
- Amos S. W.; Amos R.. Newnes Dictionary of Electronics; Elsevier, 2002. [Google Scholar]
- Graf R. F.Modern Dictionary of Electronics; Newnes, 1999. [Google Scholar]
- Gomez E. F.; Venkatraman V.; Grote J. G.; Steckl A. J. DNA bases thymine and adenine in bio-organic light emitting diodes. Sci. Rep. 2014, 4, 7105. 10.1038/srep07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espasa A.; Lang M.; Aguiño C. F.; Sanchez-deAlcazar D.; Fernández-Blázquez J. P.; Sonnewald U.; Cortajarena A. L.; Coto P. B.; Costa R. D. Long-living and highly efficient bio-hybrid light-emitting diodes with zero-thermal-quenching biophosphors. Nat. Commun. 2020, 11, 879. 10.1038/s41467-020-14559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R.; Durai K.; Net S.; Balraj A.; Priya W. Modeling a micro tubule as a diode. J. Biosens. Bioelectron. 2011, 2, 106. 10.4172/2155-6210.1000106. [DOI] [Google Scholar]
- Bogitsh B. J.; Carter C. E.; Oeltmann T. N.. Human Parasitology; Academic Press, 2018. [Google Scholar]
- Mehlhorn H.; Mehlhorn H.. Human Parasites; Springer, 2016. [Google Scholar]
- Daszak P.; Cunningham A. A.; Hyatt A. D. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science 2000, 287, 443–449. 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Chappell L. H.Physiology of Parasites; Springer Science & Business Media, 2013. [Google Scholar]
- Barigozzi C.In Vito Volterra Symposium on Mathematical Models in Biology: Proceedings of a Conference, Held at the Centro Linceo Interdisciplinare, Accademia Nazionale Dei Lincei, Rome December 17–21, 1979; Springer Science & Business Media, 2013; Vol. 39.
- Tielens A. G. M. Energy generation in parasitic helminths. Parasitol. Today 1994, 10, 346–352. 10.1016/0169-4758(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Bundy D. A.; Golden M. H. The impact of host nutrition on gastrointestinal helminth populations. Parasitology 1987, 95, 623–635. 10.1017/s0031182000058042. [DOI] [PubMed] [Google Scholar]
- Richards W. H. G. Active immunization of chicks against Plasmodium gallinaceum by inactivated homologous sporozoites and erythrocytic parasites. Nature 1966, 212, 1492–1494. 10.1038/2121492a0. [DOI] [PubMed] [Google Scholar]
- Mettler M.; Grimm F.; Capelli G.; Camp H.; Deplazes P. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J. Clin. Microbiol. 2005, 43, 5515–5519. 10.1128/jcm.43.11.5515-5519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liance M.; Janin V.; Bresson-Hadni S.; Vuitton D.-A.; Houin R.; Piarroux R. Immunodiagnosis of EchinococcusInfections: Confirmatory Testing and Species Differentiation by a New Commercial Western Blot. J. Clin. Microbiol. 2000, 38, 3718–3721. 10.1128/jcm.38.10.3718-3721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. S.; Park D. K.; Kim H. C.; Choi M.-H.; Chai J.-Y. Automatic identification of human helminth eggs on microscopic fecal specimens using digital image processing and an artificial neural network. IEEE Trans. Biomed. Eng. 2001, 48, 718–730. 10.1109/10.923789. [DOI] [PubMed] [Google Scholar]
- Castañeda-Castellanos D. R.; Flint A. C.; Kriegstein A. R. Blind patch clamp recordings in embryonic and adult mammalian brain slices. Nat. Protoc. 2006, 1, 532. 10.1038/nprot.2006.75. [DOI] [PubMed] [Google Scholar]
- Kornreich B. G. The patch clamp technique: principles and technical considerations. J. Vet. Cardiol. 2007, 9, 25–37. 10.1016/j.jvc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Simeral J. D.; Kim S.-P.; Black M. J.; Donoghue J. P.; Hochberg L. R. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J. Neural. Eng. 2011, 8, 025027. 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouzari M. S.; Berkemeier F.; Schmitz G.; Wilmer D. On the physical interpretation of constant phase elements. Solid State Ionics 2009, 180, 922–927. 10.1016/j.ssi.2009.04.002. [DOI] [Google Scholar]
- Blatt F. J.; Blatt F. J.. Principles of Physics; Allyn and Bacon Boston: London, 1989. [Google Scholar]
- Bard A. J.; Faulkner L. R.. Electrochemical Methods Fundamentals and Applications; Wiley, 2001; Vol. 2 ( (482), ), pp 580–632. [Google Scholar]
- Velásquez M. T. O. d.; Martínez J. L.; Monje–Ramírez I.; Rojas-Valencia M. N. Destruction of helminth (Ascaris suum) eggs by ozone. Ozone: Sci. Eng. 2004, 26, 359–366. 10.1080/01919510490482188. [DOI] [Google Scholar]
- Sasz H. J.; Lescure O. L. Interrelationships between the carbohydrate and lipid metabolism of Ascaris lumbricoides egg and adult stages. Comp. Biochem. Physiol. 1966, 18, 845–857. 10.1016/0010-406x(66)90217-9. [DOI] [PubMed] [Google Scholar]
- Schiller E. L.; Bueding E.; Turner V. M.; Fisher J. Aerobic and anaerobic carbohydrate metabolism and egg production of Schistosoma mansoni in vitro. J. Parasitol. 1975, 61, 385–389. 10.2307/3279308. [DOI] [PubMed] [Google Scholar]
- Passey R. F.; Fairbairn D. The conversion of fat to carbohydrate during embryonation of Ascaris eggs. Can. J. Biochem. Physiol. 1957, 35, 511–525. 10.1139/o57-061. [DOI] [PubMed] [Google Scholar]
- Maule A. G.; Marks N. J.. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology; CABI, 2006. [Google Scholar]
- Barrett J.Biochemistry of Parasitic Helminths; MacMillan Publishers Ltd., 1981. [Google Scholar]
- Wharton D. Nematode egg-shells. Parasitology 1980, 81, 447–463. 10.1017/s003118200005616x. [DOI] [PubMed] [Google Scholar]
- Smyth J. D.; Clegg J. A. Egg-shell formation in trematodes and cestodes. Exp. Parasitol. 1959, 8, 286–323. 10.1016/0014-4894(59)90027-x. [DOI] [PubMed] [Google Scholar]
- Dragoman D.; Dragoman M.. Bionanoelectronics: Bioinquiring and Bioinspired Devices; Springer Science & Business Media, 2012. [Google Scholar]
- Shoseyov O.; Levy I.. Nanobiotechnology: Bioinspired Devices and Materials of the Future; Springer Science & Business Media, 2008. [Google Scholar]
- Sugahara A.; Ando Y.; Kajiyama S.; Yazawa K.; Gotoh K.; Otani M.; Okubo M.; Yamada A. Negative dielectric constant of water confined in nanosheets. Nat. Commun. 2019, 10, 850. 10.1038/s41467-019-08789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli L.; Esfandiar A.; Fabregas R.; Hu S.; Ares P.; Janardanan A.; Yang Q.; Radha B.; Taniguchi T.; Watanabe K.; Gomila G.; Novoselov K. S.; Geim A. K. Anomalously low dielectric constant of confined water. Science 2018, 360, 1339–1342. 10.1126/science.aat4191. [DOI] [PubMed] [Google Scholar]
- Tredgold R. H. A possible mechanism for the negative resistance characteristic of axon membranes. Nature (London), New Biol. 1973, 242, 209–210. 10.1038/newbio242209a0. [DOI] [PubMed] [Google Scholar]
- Kim J.-J.; Lee J.; Yang S.-P.; Kim H. G.; Kweon H.-S.; Yoo S.; Jeong K.-H. Biologically inspired organic light-emitting diodes. Nano Lett. 2016, 16, 2994–3000. 10.1021/acs.nanolett.5b05183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.