Abstract

Traditional approaches to achieving dopant functionalized Si involve grafting the dopant to the Si substrates through O–Si or C–Si bonds, resulting in indirect attachment of the dopant to the Si. Recently, ultrahigh vacuum work has demonstrated that high densities of direct B–Si bonds enable unprecedented electronic behaviors in Si that make it possible for Si to be used as a next-generation electronic material. As solvothermal approaches are inherently amenable to scale-up, there is currently a push to develop solvothermal approaches for the formation of direct dopant-Si bonds. Thus far, B–Si chemistries for next-generation electronic materials have been demonstrated with boron trichloride and bis(pinacolatodiboron). In this work, we use a combination of experimental work and computational studies to examine the reactivity of a phenyl derivatized boron trichloride, namely dichlorophenylborane, with H–Si(100). We determine that despite the stability and ease for the formation of C–Si bonds, the organic component, the phenyl group remains attached to the B and does not yield competitive formation of products via a Si–C bond. This reaction proved a new solvothermal method for the formation of direct B–Si bonds that, with further work, can be leveraged in developing next-generation electronic materials.
Introduction
Developing solvothermal ways of directly attaching dopant-containing molecules to Si has recently garnered attention as a potentially scalable method for the development of heavily doped electronic devices. While attachment of dopants through tethering linkers, such as C or O species, has been well-developed, solvothermal chemistries that form a direct B–Si bond have only recently been an area of research. The interest in developing direct B–Si chemistries was motivated by the discovery using atomically precise advanced manufacturing (APAM) work that high densities of direct dopant-Si bonds enable unprecedented electronic behavior of Si.1−5 Due to the promising potential of forming direct dopant-Si bonds, recent work has focused on expanding the process from ultrahigh vacuum scanning tunneling microscopy method into solvothermal processes that are more amenable to scale-up. Unlike traditional solvothermal work, direct B–Si chemistries for next-generation electronic materials do not require monolayer formation. In fact, the formation of a full monolayer would likely have deleterious electronic effects, as large, paired clusters of boron have been found to be electrically inactive.6,7
Recent solvothermal chemistry work involving direct B–Si bond formation includes the reaction of boron trichloride, BCl3, with H–Si(100), and Cl–Si(100) to form a direct B–Si bond.8 In this work, Silva-Quinones et al. demonstrated BCl3 can be used to attach B to both Cl-Si and H-Si surfaces. The ability to react B by thermal energy alone led to a fairly clean reaction system.9 Additionally, this work showed that temperature can be leveraged to guide selectively, with temperatures below 70 °C, leading to reactions with Cl-Si and not H-Si.8 This result provided a route toward future work leveraging this selective chemistry on patterned hydrogen/halogen surfaces.9 Other recent work focused on the reaction of bis(pinacolatodiboron), B2Pin2, with H–Si(100) provided the proof-of-principle quantification that solvothermal chemistry on wet-prepared Si(100) can obtain B concentrations on the order of the levels achieved using UHV processes.10 While the B2Pin2 results were promising, catalyst involvement complicated the surface chemistry.
In this work, we report a new strategy for forming direct B–Si bonds on technologically relevant Si(100) using PhBCl2 as the doping molecule (Figure 1). The purpose of this work was to investigate whether a phenyl substituted BCl3, specifically dichlorophenylborane (PhBCl2), would behave similarly to BCl3 and allow for direct attachment of the B to the Si surface. Given the stability and ease for the formation of C–Si bonds and the BCl2’s potential to act as a leaving group, it was unclear if this reaction would result in the organic component attached to the surface via a Si–C bond or the desired direct B–Si bonds. Using X-ray photoelectron spectroscopy (XPS) to confirm the presence of B and density functional theory (DFT) to examine the energetics of potential configurations, we demonstrate that PhBCl2 preferably reacts with H–Si(100) through B–Si bonds. While this work does not provide monolayer coverages, with further optimization of surface coverage, the phenyl ring can potentially achieve patterning akin to that achieved in self-assembled monolayers on Si.11,12 Overall, this work provides a new method to achieve direct bonds between B and Si and is another tool in the set of chemistries being developed for next-generation electronic materials.
Figure 1.
Schematic of the reaction of dichlorophenylborane with H–Si(100) in this work that enables (a) direct attachment between B and Si and (b) has a phenyl group that can be further derivatized to enable guided self-assembly.
Results and Discussion
XPS was used to confirm the success of the reaction (see Figure 2). Comparing the Cl 2p region of the PhBCl2 sample against the benzene-only sample revealed that the exposure to PhBCl2 led to an obvious Cl 2p peak consisting of 2p3/2 and 2p1/2 components, which were not seen when only benzene was present. This peak could be attributed to either Cl-B or Cl-Si based on literature refs (20−22). In the B 1s region, both samples displayed a Si plasmon peak. The PhBCl2 sample displayed an additional small but statistically significant peak at 191.8 eV. As physisorbed species were removed by an extensive cleaning procedure that involved copious rinsing and sonication in benzene (described in the Experimental Section), we are confident that the B 1s peak represents chemisorption. Overall, the presence of Cl 2p and B 1s peaks in XPS indicated the reaction was successful.
Figure 2.
XPS characterization of the reaction of H-Si with a PhBCl2/benzene solution and benzene only. (a) B 1s regions demonstrated that a small amount of B was present when PhBCl2 was included in the reaction solution. (b) The Cl 2p region demonstrated that only the PhBCl2 exposed sample had Cl.
In an endeavor to improve the B coverage, we increased the concentration of PhBCl2 from 2.6 to 4.4 M (1–1.5 mL), while keeping the benzene volume constant at 2 mL. This resulted in an increase in the amount of precursor attached to the surface, as evidenced by a slight increase in signal of the B 1s and Cl 2p regions (see Figure 3). The ratio of Cl/Si (normalized with Si = 1) increased from 0.017:1 to 0.031:1 and the B/Si ratio increased from 0.010:1 to 0.014:1 with the increase in precursor concentration. The ratio of Cl/B was roughly 2:1, matching computational results of likely chemisorption configurations described later in this work. The increase in coverage indicated that the reaction rate was in a concentration-dependent regime, and the resulting coverages are sub-monolayer. Further improvement of coverage could likely be achieved by varying other parameters, such as increasing the temperature, increasing the time, or using sonication23,24 or light25 to activate the reaction on H–Si(100).
Figure 3.

XPS characterization of samples exposed to 2.6 and 4.4 M PhBCl2 in benzene. (a) XPS of the Cl 2p regions showed a slight increase in the Cl intensity with an increased precursor concentration. (b) XPS of the B 1s region showed a slight increase in the B intensity with an increased precursor concentration.
Direct B–Si Bond: Combined XPS and Computational Studies
While XPS data can confidently determine that the reaction leads to B attached to the surface, determination of the exact surface composition from XPS is limited by the overlap of B with the Si plasmon peak and similarities in binding energies of interest. While XPS results indirectly suggested B–Si bond formation, due to the inherent uncertainty of the characterization, we also performed computation evaluation to further understand the Si surface reaction of PhBCl2 and study the formation of a direct B–Si bond.
The XPS BE of B of 191.8 eV obtained in this work could be compared to the measured binding energy (BE) of B species, as the BE is expected to shift to higher values with increasing electronegativity of the substituents. As expected, the PhB(Cl)-Si BE was higher than the BE of pure B (187 ± 0.5 ev)26,27 and lower than BE of B-O species in other B–Si chemistries, such as B2Pin2 on H-Si, where the B-O species in the molecule appears at 194 ± 1 eV.10 This suggests that the B observed post-reaction is not a B-O species. We could not do a direct comparison as the literature data for B bound to Cl, Si, and a phenyl ring in the same study was not available. We further analyzed the Si 2p region to try to determine the attachment (Figure 4). Confident confirmation of B–Si peak from the Si region is difficult, as the B–Si occurs at a similar position to O–Si.10,14 The peak on the PhBCl2-exposed sample occurs at 102.1 eV, which is slightly different from the 102.8 eV peak of Si-O on the sample exposed to only benzene. The resolution is not high enough to deconvolute the sample peak into B–Si and O–Si, so the slight shift in the peak in the Si region does not add additional support for the presence of a B–Si bond. Additional support for a B–Si bond rather than a C–Si bond was provided by the lack of a shoulder in the lower binding energy region C 1s peak (C–Si should appear at ∼283 to 284 eV) (Figure 4). The lack of a C–Si peak provides experimental evidence that the reaction is not happening through the phenyl ring (with subsequent removal of BCl2) but rather directly through a B bond. Overall, the correlation of the Cl 2p peak with literature values for B-Cl, the position of the peak in the B–Si region, and the lack of C–Si in the Si 2p region are suggestive of B attachment.
Figure 4.

(a) XPS spectra of the Si 2p region of H–Si(100) exposed to PhBCl2/benzene versus H–Si(100) exposed to only benzene. With only benzene exposure, this peak ∼102 to 104 eV is attributed to SiOx species. With exposure to PhBCl2, this peak shows a slight shift to a lower BE and a lower intensity, suggesting this peak contains B–Si rather than SiOx species. (b) The XPS C 1s region of the sample lacked a peak between 283 and 284 eV, where C–Si would appear.
To supplement the XPS data, we used density functional theory (DFT) results to elucidate the exact bonding mechanism of PhBCl2 in contact with Si(100). While we initially used a clean dihydride-terminated Si(100) surface for simulation, attempts at adsorption on this surface-induced reconstruction to the monohydride-terminated Si(100)-2 × 1 surface, as can be seen in the supporting information. There is also evidence for this reconstruction occurring from literature experimental results on wet-chemically prepared Si(111)-H-1 × 1, which reconstructed to Si(111)-H-2 × 1 with desorption of H,28 and this is generally consistent with prior calculations looking at B2Pin2 on a Si(100) surface.10 The remainder of the results were therefore reported on the monohydride-terminated Si(100)-2 × 1 surface. While this is clearly an approximation of the larger surface, it is representative of the bonding trends for PhBCl2 in contact with Si(100).
PhBCl2 is not reactive in contact with a H-terminated Si surface. As shown in Figure 5a, PhBCl2 physisorbs when placed in direct contact with the H-terminated surface with an adsorption energy of −0.03 eV. If no further reaction occurred, this would result in no B attachment after cleaning the sample. We thus explore the possibility of one of the Cl atoms from the PhBCl2 pulling off a H from the Si resist and detaching to form HCl, leaving a dangling bond on the Si surface at which the remaining PhBCl could easily adsorb, as shown in Figure 5b. This configuration is unstable, however, with an adsorption energy of +0.94 eV. Thus, even at elevated temperatures, it is unlikely that PhBCl2 will strip the H resist from a Si surface. Therefore, we expect that PhBCl2 interaction with the surface will be limited to areas where the H has already been removed. It is well known that small amounts of defects are present, even on UHV prepared surfaces which produce more pristine surfaces than the wet-chemically prepared H–Si(100) surfaces prepared in this study.13,29−35 In addition to bare Si from defect sites, thermal energy over a long period in solution is also sufficient to remove hydrogen from a silicon surface, as evidenced by hydrosilylation reactions that proceed on bare Si sites introduced on H-Si via thermal energy.25,34,36,37 A combination of defects and thermal energy likely provides the bare Si sites that allow for the PhBCl2 reaction.
Figure 5.
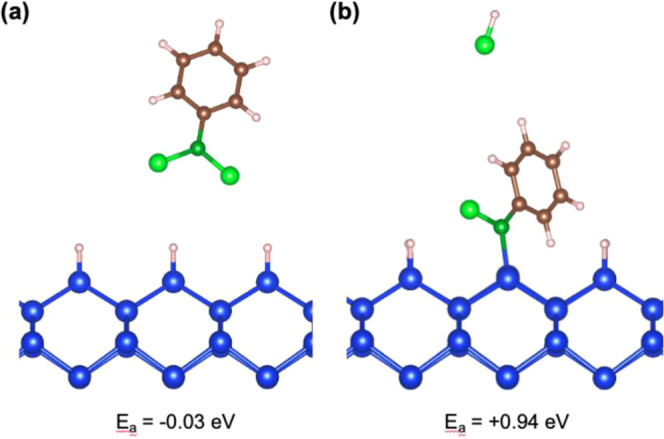
Calculated equilibrium positions for PhBCl2 (a) physisorbing on a hydrogen resist and (b) pulling off a hydrogen atom to form HCl and allowing the remaining PhBCl to directly bond to the silicon surface. This formation of HCl is highly unfavorable, making it unlikely that PhBCl2 will strip a hydrogen resist from silicon on its own.
We examined three possibilities
for the reaction of PhBCl2 with a bare Si site. Placing
the PhBCl2 molecule directly
on the silicon surface led to weak physisorption (Figure 6a). Next (Figure 6b), we examined a Cl breaking off and directly bonding to
a neighboring Si atom, allowing the remaining PhBCl to directly attach
to Si through the B atom. Finally (Figure 6c), we examined the energy for a phenyl group
detaching to the neighboring Si atom and BCl2 directly
attaching to Si through the B. Both configurations are favorable,
with the Cl removal from PhBCl2 being the more favored
option with an adsorption energy of −1.60 eV, compared to an
adsorption energy of −1.21 eV for the phenyl ring detaching
from the molecule. Both reactions are found to be barrierless, i.e.,
a PhBCl2 molecule floating above a bare silicon surface
will dissociate into either configuration spontaneously. Although
both pathways are energetically favorable, given the 0.4 eV difference
in adsorption energy, a single Cl atom coming off PhBCl2 will be almost entirely dominant. If we assume a Boltzmann distribution
of outcomes, the ratio of potentials for each is 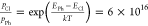 , heavily favoring
the chlorine atom leaving.
Therefore, the most favorable configuration for PhBCl2 dissociation
leaves a phenyl ring directly attached to the boron atom.
, heavily favoring
the chlorine atom leaving.
Therefore, the most favorable configuration for PhBCl2 dissociation
leaves a phenyl ring directly attached to the boron atom.
Figure 6.

The calculated equilibrium positions for PhBCl2 on a bare Si surface. (a) PhBCl2 directly approaching the surface resulting in weak physisorption. (b) A Cl atom moved off the molecule, bonding to the Si atom on the opposite end of the dimer, allowing the remaining PhBCl to bond to the Si through B. (c) A Phenyl ring broke off and bound to a nearby Si atom, allowing the remaining BCl2 to bond to the Si through B. Both dissociation configurations were favorable and did not require overcoming a kinetic barrier. The 0.4 eV difference in favor of (b) ensured that (b) was the heavily dominant configuration.
From this information, we propose a pathway to explain the reaction mechanism. First, the PhBCl2 was exposed to a H-terminated Si surface and did not largely react at H-terminated sites, at best physisorbing. However, bare Si sites present from thermally induced H-abstraction as well as defects that are always present from surface preparation presented open sites for the reaction. The concentration dependence observed in the experimental data suggests that the open sites are not the limiting “reactant” and implies that open sites are being continually generated throughout the thermal reaction. At these open sites, the PhBCl2 spontaneously dissociated on the silicon surface, forming a direct Si-B bond with the phenyl group still directly attached to the boron.
The work reported here demonstrates that PhBCl2 can react directly with the Si surface, which is a first step toward developing methods for building more advanced architectures on the surface with solvothermal chemistry. Previous research in the development of organic electronics, where organics are bound to Si through C–Si bonds or O–Si bonds, has already demonstrated that lateral control of molecular spacing can be achieved by varying substituent properties, with larger substituents leading to increased spacing between neighboring molecules.6,11,12 Future work improving the coverage of PhBCl2 and incorporating bulkier substituents on the phenyl ring may allow for control over dopant spacing using bottom-up self-assembly. This level of spacing may be useful to avoid boron atoms incorporating into silicon as dimers, which are believed to be electrically inactive.7,38 Additionally, it may enable control over ordering of dopant for tuning the electronic behavior of the materials.39 Despite the coverage limitation in this work, the achievement of a direct B–Si bond using a molecule with a substituent that has previously been leveraged for bottom-up patterning is a first step toward processes for control over dopant placement using solvothermal chemistry.
Conclusions
Using a combination of experimental and computational work, we have successfully demonstrated that PhBCl2 can be attached to Si(100) via a direct B–Si bond. XPS results demonstrate the successful attachment of B to the surface. DFT calculations demonstrate that the most favorable configuration for PhBCl2 dissociation results in a direct B–Si bond, with a phenyl ring directly attached to the boron atom at the terminating end of the molecule. While other configurations are energetically favorable, Boltzmann statistics indicate the phenyl ring remaining directly attached to the boron will be extremely dominant. This reaction establishes the proof-of-principle that B-phenyl molecules will react with H–Si(100) to produce B–Si(100) and enables next step reactions with functionalized phenyl ring to allow for control over B placement by directing the spacing of the parent molecule via phenyl substituents. While this work focuses on B, it is likely that this concept would similarly be applicable for other acceptors and donors.
Experimental Section
Surface Preparation
Experiments were run using 1 cm × 1 cm intrinsic Si(100) covered in 3 nm of thermal oxide. Substrates were prepared using an RCA clean with a buffered oxide etch (BOE) step, followed by thermal oxidation, resulting in a pristine, buried interface. The samples were immersed for 3 min in 5:1 BOE (J.T. Baker, microelectronics grade), followed by a quick (5–10 s) dip into 17+ MΩ water to remove the oxide. H-termination was achieved with a 3 min dip in 40% ammonium fluoride (aq) (J.T. Baker, microelectronics grade) using the Hines method13 to prevent bubble formation by pulling the sample through the interface every 15 s. Samples were subjected to a final rinse in 17+ MΩ water; they were dried with N2 gun and immediately used for chemical functionalization.
B–Si Reaction
PhBCl2 (97%) and benzene, C6H6 (anhydrous, 99.8%), were used as purchased from Sigma-Aldrich (USA). The H-terminated Si(100) substrates were introduced into a N2-filled glovebox and placed into individual 15 mL glass pressure vessels prefilled with a solution composed of 2 mL of benzene and 0–1.5 mL of PhBCl2, as described in the Results section. Controls were run with only C6H6. The pressure vessels were sealed, removed from the glovebox, and heated in an oil bath at 120 °C overnight (∼18 h). The pressure vessels were removed from the oil bath and returned to the glovebox for sample work-up. The samples were rinsed with ∼1 mL C6H6 and then placed in 20 mL scintillation vials filled with 2 mL of C6H6. The vials were capped and sealed with Teflon tape and parafilm before being removed from the glovebox for sonication, thereby maintaining the N2 atmosphere during sonication. After sonication, the samples were returned to the glovebox, rinsed 3× with ∼1 mL C6H6, and dried by blowing N2 from the glovebox environment over the samples with a pipette. The samples that reacted with PhBCl2 were noticeably harder to dry, i.e., they retained more C6H6 than samples exposed to only benzene. This indicated that exposure to PhBCl2 led to functionalization, while the benzene-exposed samples were not. As functionalization involved depositing a phenyl group, it was consistent that reacting the samples with PhBCl2 increased the hydrophobicity.
X-ray Photoelectron Spectroscopy (XPS)
To minimize the exposure to ambient conditions, samples were sealed in the glovebox for transport and briefly exposed during transfer to the N2 filled load lock. A Kratos Ultra DLD spectrometer with a monochromatic Al Kα source was used to obtain spectra. It was operating with 200 mW power, a base pressure of ∼10–10 Torr throughout the analysis, and a grazing incidence angle of 60° from surface normal to increase surface sensitivity. Survey scans were collected with a pass energy of 100 eV, and high-resolution scans were collected with a pass energy of 20 eV. Spectra were processed and analyzed using CasaXPS using the Si 2p peak for calibration. Baseline subtraction and peak fits were done using automated baseline and component fit options in CasaXPS, with 2p regions fit with a Shirley background and 1s regions with a Linear background. Analyzed data was then exported and replotted in Origin. The Si plasmon peaks were further smoothed with a Loess 0.05 fit. Peaks were referenced to the NIST XPS database unless otherwise specified.14
Computational Details
We predict the adsorption energy of each configuration with DFT using
where Eads is the DFT calculated adsorption energy, Eslab/molecule is the DFT energy of the slab with the molecule of interest adsorbed, Eslab is the DFT energy of the silicon slab exposed to vacuum, and Emolecule is the DFT energy of the molecule of interest, PhBCl2, in vacuum. A negative adsorption energy indicates that the configuration is thermodynamically favorable.
We performed all adsorption energy calculations on the 4×4 supercell of a seven-layer-thick silicon (100) slab with a 20 Å vacuum region. On one side of the slab, silicon is exposed to a hydrogen resist. On the other end of the slab, the dangling bonds of the silicon are tied off with a selenium atom to prevent spurious surface effects. Selenium was determined to be optimal for completing the silicon bonds with minimal strain effects. We independently measured the energy of the PhBCl2 molecule in a 15 Å3 box. Molecules were allowed to adsorb on the surface, and then the structure was relaxed until forces converged below 50 meV/ Å.
All electronic structure calculations were performed using the plane wave Quantum ESPRESSO software package.15 We used norm-conserving pseudopotentials with Perdew–Burke–Ernzerhof exchange correlation16 from the PseudoDojo repository.17 We used a kinetic energy cutoff of the Kohn–Sham orbitals and charge densities of 50 and 200 Ry, respectively, with 0.0001 Ry of Marzari–Vanderbilt electronic smearing.18 We used a 2 × 2 × 1 Monkhorst–Pack grid.19 Reaction barriers were calculated using the nudged elastic band method, as implemented within Quantum ESPRESSO, with seven images used for each reaction.
Acknowledgments
This work was supported by the Laboratory Directed Research and Development Program at Sandia National Laboratories and was performed, in part, at the Center for Integrated Nanotechnologies, a U.S. DOE, Office of Basic Energy Sciences user facility. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under Contract DE-NA0003525. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy or the United States Government.
Glossary
Abbreviations Used
- PhBCl2
dichlorophenylborane
- C6H6
benzene
- Si
silicon/silicon wafer
- H-Si
hydrogen-terminated Si
- B–Si
Boron-terminated Si or Boron–Si bond
- DFT
density functional theory
- XPS
X-ray photoelectron spectroscopy
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04619.
DFT calculations for physisorption and reconstruction to the H–Si(100)-2 × 1 surface (PDF)
Author Contributions
§ E.F. and Q.C. contributed equally to this work. The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Schofield S. R.; Curson N. J.; Simmons M. Y.; Ruess F. J.; Hallam T.; Oberbeck L.; Clark R. G. Atomically precise placement of single dopants in Si. Phys. Rev. Lett. 2003, 91, 136104 10.1103/PhysRevLett.91.136104. [DOI] [PubMed] [Google Scholar]
- Ward D. R.; Anderson E. M.; Bussmann E.; Tracy L.; Lu T.; Maurer L. N.; Baczewski A.; Campbell D. M.; Marshall M. T.; Misra S. Atomic precision advanced manufacturing for digital electronics. Electron. Device Fail. Anal. 2020, 22, 4–10. [Google Scholar]
- Mazzola F.; Chen C.-Y.; Rahman R.; Zhu X.-G.; Polley C. M.; Balasubramanian T.; King P. D. C.; Hofmann P.; Miwa J. A.; Wells J. W. The sub-band structure of atomically sharp dopant profiles in Silicon. npj Quantum Mater. 2020, 5, 34 10.1038/s41535-020-0237-1. [DOI] [Google Scholar]
- Škereň T.; Köster S. A.; Douhard B.; Fleischmann C.; Fuhrer A. Bipolar device fabrication using a scanning tunnelling microscope. Nat. Electron. 2020, 3, 524–530. 10.1038/s41928-020-0445-5. [DOI] [Google Scholar]
- Dwyer K. J.; Baek S.; Farzaneh A.; Dreyer M.; Williams J. R.; Butera R. E. B-doped δ-Layers and nanowires from area-selective deposition of BCl3 on Si(100). ACS Appl. Mater. Interfaces 2021, 13, 41275–41286. 10.1021/acsami.1c10616. [DOI] [PubMed] [Google Scholar]
- Tarnow E. Theory of two boron neutral pair defects in Silicon. J. Phys.: Condens. Matter 1992, 4, 5405–5410. 10.1088/0953-8984/4/24/010. [DOI] [Google Scholar]
- Stolk P. A.; Gossmann H. J.; Eaglesham D. J.; Poate J. M. Implantation and transient boron diffusion: The role of the Silicon self-interstitial. Nucl. Instrum. Methods Phys. Res., Sect. B 1995, 96, 187–195. 10.1016/0168-583X(94)00481-1. [DOI] [Google Scholar]
- Silva-Quinones D.; He C.; Butera R. E.; Wang G. T.; Teplyakov A. V. Reaction of BCl3 with H- and Cl-terminated Si(100) as a pathway for selective, monolayer doping through wet chemistry. Appl. Surf. Sci. 2020, 533, 146907 10.1016/j.apsusc.2020.146907Get. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick E.; Dwyer K.; Wang G. T.; Misra S.; Butera R. E. The stability of Cl-, Br-, and I-passivated Si(100)-(2 × 1) in ambient environments for atomically-precise pattern preservation. J. Phys.: Condens. Matter 2021, 33, 444001 10.1088/1361-648x/ac1aa4. [DOI] [PubMed] [Google Scholar]
- Frederick E.; Campbell Q.; Kolesnichenko I. V.; Peña L. F.; Benavidez A.; Anderson E. M.; Wheeler D. R.; Misra S. Ultradoping Boron on Si(100) via solvothermal chemistry. Chem.-Eur. J. 2021, 27, 13337 10.1002/chem.202102200. [DOI] [PubMed] [Google Scholar]
- Li F.; Basile V. M.; Pekarek R. T.; Rose M. J. Steric spacing of molecular linkers on passivated Si(111) photoelectrodes. ACS Appl. Mater. Interfaces 2014, 6, 20557–20568. 10.1021/am506244m. [DOI] [PubMed] [Google Scholar]
- Liao K.-C.; Ismail A. G.; Kreplak L.; Schwartz J.; Hill I. G. Designed organophosphonate self-assembled monolayers enhance device performance of pentacene-based organic thin-film transistors. Adv. Mater. 2010, 22, 3081–3085. 10.1002/adma.201001310. [DOI] [PubMed] [Google Scholar]
- Aldinger B. S.; Gupta A.; Clark I. T.; Hines M. A. The same etchant produces both near-atomically flat and microfaceted Si(100) surfaces: The effects of gas evolution on etch morphology. J. Appl. Phys. 2010, 107. 10.1063/1.3402580. [DOI] [Google Scholar]
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg MD, 20899, 2000. [Google Scholar]
- Giannozzi P.; Baroni S.; Bonini N.; Calandra M.; Car R.; Cavazzoni C.; Ceresoli D.; Chiarotti G. L.; Cococcioni M.; Dabo I.; Dal Corso A.; de Gironcoli S.; Fabris S.; Fratesi G.; Gebauer R.; Gerstmann U.; Gougoussis C.; Kokalj A.; Lazzeri M.; Martin-Samos L.; Marzari N.; Mauri F.; Mazzarello R.; Paolini S.; Pasquarello A.; Paulatto L.; Sbraccia C.; Scandolo S.; Sclauzero G.; Seitsonen A. P.; Smogunov A.; Umari P.; Wentzcovitch R. M. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 2009, 21, 395502 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- van Setten M. J.; Giantomassi M.; Bousquet E.; Verstraete M. J.; Hamann D. R.; Gonze X.; Rignanese G. M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. 10.1016/j.cpc.2018.01.012. [DOI] [Google Scholar]
- Marzari N.; Vanderbilt D.; Payne M. C. Ensemble density-functional theory for ab initio molecular dynamics of metals and finite-temperature insulators. Phys. Rev. Lett. 1997, 79, 1337–1340. 10.1103/PhysRevLett.79.1337. [DOI] [Google Scholar]
- Monkhorst H. J.; Pack J. D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. 10.1103/PhysRevB.13.5188. [DOI] [Google Scholar]
- Kobelev A. A.; Barsukov Y. V.; Andrianov N. A.; Smirnov A. S. Boron trichloride plasma treatment effect on ohmic contact resistance formed on GaN-based epitaxial structure. J. Phys.: Conf. Ser. 2015, 586, 012013 10.1088/1742-6596/586/1/012013. [DOI] [Google Scholar]
- Sungauer E.; Pargon E.; Mellhaoui X.; Ramos R.; Cunge G.; Vallier L.; Joubert O.; Lill T. Etching mechanisms of HfO2, SiO2, and poly-Si substrates in BCl3 plasmas. J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom. 2007, 25, 1640–1646. 10.1116/1.2781550. [DOI] [Google Scholar]
- Bansal A.; Li X.; Yi S. I.; Weinberg W. H.; Lewis N. S. Spectroscopic studies of the modification of crystalline Si(111) surfaces with covalently-attached alkyl chains using a chlorination/alkylation method. J. Phys. Chem. B 2001, 105, 10266–10277. 10.1021/jp010284p. [DOI] [Google Scholar]
- Frederick E.; Dickerson P. N.; Zhong Y. L.; Bernasek S. L. Substituent effects on the kinetics of bifunctional Styrene SAM formation on H-terminated Si. Langmuir 2014, 30, 7687–7694. 10.1021/la501417s. [DOI] [PubMed] [Google Scholar]
- Zhong Y. L.; Bernasek S. L. Mild and efficient functionalization of Hydrogen-terminated Si(111) via sonochemical activated hydrosilylation. J. Am. Chem. Soc. 2011, 133, 8118–8121. 10.1021/ja2020839. [DOI] [PubMed] [Google Scholar]
- Buriak J. M. Illuminating Silicon surface hydrosilylation: An unexpected plurality of mechanisms. Chem. Mater. 2014, 26, 763–772. 10.1021/cm402120f. [DOI] [Google Scholar]
- Caussat B.; Scheid E.; de Mauduit B.; Berjoan R. Influence of dopant concentration and type of substrate on the local organization of low-pressure chemical vapour deposition in situ Boron doped silicon films from Silane and Boron Trichloride. Thin Solid Films 2004, 446, 218–226. 10.1016/j.tsf.2003.10.010. [DOI] [Google Scholar]
- Lee J.; Kim T.; Ryu S. U.; Choi K.; Ahn G. H.; Paik J. G.; Ryu B.; Park T.; Won Y. S. Study on the aging mechanism of Boron Potassium Nitrate (BKNO3) for sustainable efficiency in pyrotechnic Mechanical Devices. Sci. Rep. 2018, 8, 11745 10.1038/s41598-018-29412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R. S.; Higashi G. S.; Chabal Y. J.; Becker A. J. Atomic-scale conversion of clean Si(111):H-1 × 1 to Si(111)-2 × 1 by electron-stimulated desorption. Phys. Rev. Lett. 1990, 65, 1917–1920. 10.1103/PhysRevLett.65.1917. [DOI] [PubMed] [Google Scholar]
- Angermann H.; Kliefoth K.; Flietner H. Preparation of H-terminated Si surfaces and their characterization by measuring the surface state density. Appl. Surf. Sci. 1996, 104–105, 107–112. 10.1016/S0169-4332(96)00128-6. [DOI] [Google Scholar]
- Clark I. T.; Aldinger B. S.; Gupta A.; Hines M. A. Aqueous etching produces Si(100) surfaces of near-atomic flatness: Strain minimization does not predict surface morphology. J. Phys. Chem. C 2010, 114, 423–428. 10.1021/jp908527e. [DOI] [Google Scholar]
- Hines M. A.; Faggin M. F.; Gupta A.; Aldinger B. S.; Bao K. Self-propagating reaction produces near-ideal functionalization of Si(100) and flat surfaces. J. Phys. Chem. C 2012, 116, 18920–18929. 10.1021/jp306477x. [DOI] [Google Scholar]
- Chabal Y. J.; Higashi G. S.; Raghavachari K.; Burrows V. A. Infrared-spectroscopy of Si(111) and Si(100) surfaces after Hf treatment - Hydrogen termination and surface morphology. J. Vac. Sci. Technol., A 1989, 7, 2104–2109. 10.1116/1.575980. [DOI] [Google Scholar]
- Chabal Y. J. Infrared-spectroscopy of semiconductor surfaces - H-terminated Silicon surfaces. J. Mol. Struct. 1993, 292, 65–80. 10.1016/0022-2860(93)80090-I. [DOI] [Google Scholar]
- Thissen P.; Seitz O.; Chabal Y. J. Wet chemical surface functionalization of oxide-free Silicon. Prog. Surf. Sci. 2012, 87, 272–290. 10.1016/j.progsurf.2012.10.003. [DOI] [Google Scholar]
- Peng W. N.; Rupich S. M.; Shafiq N.; Gartstein Y. N.; Malko A. V.; Chabal Y. J. Silicon surface modification and characterization for emergent photovoltaic applications based on energy transfer. Chem. Rev. 2015, 115, 12764–12796. 10.1021/acs.chemrev.5b00085. [DOI] [PubMed] [Google Scholar]
- Linford M. R.; Fenter P.; Eisenberger P. M.; Chidsey C. E. D. Alkyl monolayers on Silicon prepared from 1-Alkenes and Hydrogen-terminated Silicon. J. Am. Chem. Soc. 1995, 117, 3145–3155. 10.1021/ja00116a019. [DOI] [Google Scholar]
- de Smet L. C. P. M.; Zuilhof H.; Sudhölter E. J. R.; Lie L. H.; Houlton A.; Horrocks B. R. Mechanism of the hydrosilylation reaction of alkenes at porous Silicon: Experimental and computational Deuterium labeling studies. J. Phys. Chem. B 2005, 109, 12020–12031. 10.1021/jp044400a. [DOI] [PubMed] [Google Scholar]
- Zhu J.; dela Rubia T. D.; Yang L. H.; Mailhiot C.; Gilmer G. H. Ab initio pseudopotential calculations of B diffusion and pairing in Si. Phys. Rev. B 1996, 54, 4741–4747. 10.1103/PhysRevB.54.4741. [DOI] [PubMed] [Google Scholar]
- Daukiya L.; Seibel J.; De Feyter S. Chemical modification of 2D materials using molecules and assemblies of molecules. Adv. Phys.: X 2019, 4, 1625723 10.1080/23746149.2019.1625723. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




