Abstract
胆固醇是哺乳动物体内重要的脂质,是必不可少的膜结构成分。胆固醇稳态平衡对维持细胞和机体的生命活动至关重要,机体主要通过内源胆固醇的生物合成和外源胆固醇的摄取来获得胆固醇以维持自身稳态平衡。肿瘤细胞中胆固醇代谢异常活跃,导致其增殖、存活、侵袭、转移以及对肿瘤微环境的适应能力增强。靶向胆固醇合成、降低血浆胆固醇水平和胆固醇酯化的治疗策略将为肿瘤患者的治疗带来新的希望。本文总结了胆固醇代谢调节及其在肿瘤发生发展中的研究进展,并讨论了目前干扰胆固醇代谢的肿瘤治疗新方法。
Abstract
Cholesterol is an important lipid in the body of mammals and an essential component of membrane structures. Cholesterol homeostasis is critical for the maintenance of cellular and body activities, and is mainly regulated by the balance of de novo cholesterol biosynthesis and the exogenous cholesterol uptake. Aberrantly regulated cholesterol metabolism promotes tumor cell proliferation,survival,invasion and metastasis,and their adaptability into the tumor microenvironment. Therefore,targeting cholesterol biosynthesis and reduction of plasma cholesterol levels and cholesterol esterification will provide new strategies for cancer treatment. This review summarizes the current understanding in cholesterol homeostasis regulation and its function in the occurence and development of cancer,as well as current metabolism-targeted cancer treatments.
Keywords: Cholesterol, Metabolism, Tumor, Therapy, Review
3-羟基-3-甲基戊二酸单酰辅酶A还原酶(3-hydroxy-3-methylglutaryl coenzyme A reductase,HMGCR);C1型尼曼-匹克样蛋白1(Niemann-Pick type C1 like 1,NPC1L1);低密度脂蛋白(low-density lipoprotein,LDL);低密度脂蛋白-胆固醇(LDL-cholesterol,LDL-C);高密度脂蛋白(high-density lipoprotein,HDL);高密度脂蛋白-胆固醇(HDL-cholesterol,HDL-C);B类Ⅰ型清道夫受体(scavenger receptor class B type 1,SR-B1);固醇调节元件结合蛋白2(sterol-regulatory element binding protein 2,SREBP2);核SREBP2(nuclear SREBP2,nSREBP2);酰基辅酶A:胆固醇酰基转移酶1(acyl-coenzyme A:cholesterolacyltransferase 1,ACAT-1);表皮生长因子受体(epidermal growth factor receptor,EGFR);磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K);蛋白激酶B(protein kinase B,Akt);肝脏X 受体(liver X receptor,LXR);前蛋白转化酶枯草杆菌蛋白酶/kexin 9型(proprotein convertase subtilisin/kexin type 9,PCSK9);胆固醇酯转运蛋白(cholesteryl ester transfer protein,CETP);CCAAT/增强子结合蛋白同源蛋白(CCAAT/enhancer-binding protein homologous protein,CHOP);丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK);
胆固醇是真核生物细胞膜必不可少的结构成分,决定着细胞膜的生理特性。胆固醇可以由机体内源合成,也可通过外源摄取获得,其代谢紊乱与心脑血管疾病、糖尿病等多种疾病相关 [ 1- 2] 。研究表明,肿瘤发生发展过程中存在胆固醇代谢改变,主要表现为细胞内胆固醇水平上调,代谢产物异常堆积 [3] 。寻找靶向胆固醇代谢途径的药物可能为肿瘤患者的治疗带来新的希望。本文总结了胆固醇代谢调节及其在肿瘤发生发展中的研究进展,并讨论了干扰胆固醇代谢的肿瘤治疗新方法。
1胆固醇代谢及稳态平衡
体内获取胆固醇以维持稳态平衡的方式主要有两种,一种是内源胆固醇的生物合成,另一种是外源胆固醇的摄取。
1.1内源胆固醇生物合成
胆固醇的生物合成主要发生在肝脏,细胞内的内质网是其主要场所。该过程通过近30步酶促反应(包括甲羟戊酸中的途径)将乙酰辅酶A转化为胆固醇分子,内质网跨膜蛋白HMGCR和角鲨烯单加氧酶为该过程的限速酶。HMGCR是定位于内质网的糖蛋白,可将β-羟基-β-甲戊二酸单酰辅酶A还原为甲羟戊酸 [4] 。HMGCR在转录、转录后、翻译和翻译后水平均受到调控。角鲨烯单加氧酶(由 SQLE编码)是HMGCR下游的另一种胆固醇生物合成的限速酶,可将非甾醇中间角鲨烯转化为2,3-氧化角鲨烯。
1.2外源胆固醇摄取
膳食胆固醇由肠上皮细胞膜上的NPC1L1吸收获得,并进一步被ACAT(也称固醇O-酰基转移酶)酯化,以乳糜微粒的形式被肝脏吸收 [5] 。肝脏作为主要的胆固醇合成器官,可将内源性合成和外源性摄取的胆固醇以极低密度脂蛋白的形式输送到血液中,并进一步加工成LDL供外周细胞上的LDL受体摄取。同时,外周组织中多余的胆固醇也会以HDL-C的形式被逆转运到肝脏进行再循环或以胆汁酸的形式被排泄 [6] 。
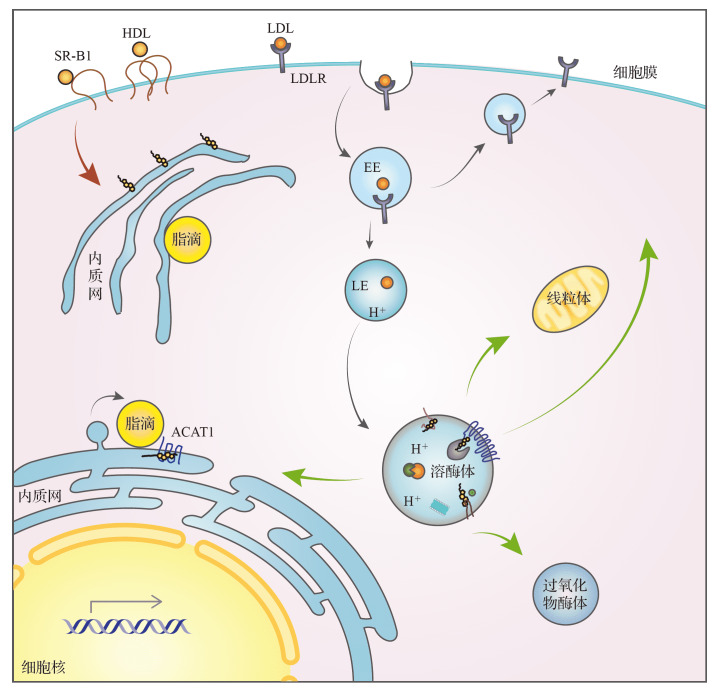
对于细胞而言,除少部分合成于内质网的胆固醇外,细胞内所需的大部分胆固醇主要来源于受体介导的外源胆固醇的摄取( 图2) [7] ,包括胞吞途径 [8] 得到的LDL-C和选择性摄取途径 [9] 得到的HDL-C,这两种途径共同调控了细胞内胆固醇的稳态平衡。其中,胞吞途径由LDL受体介导,是经典的胆固醇摄取途径。LDL颗粒与LDL受体在细胞膜结合后,通过内化最终到达溶酶体,经酸性脂肪酶水解释放游离胆固醇 [10] 。随后,游离胆固醇被转运至溶酶体膜,并进一步到达内质网进行传感、转运或酯化 [ 11- 12] ,同时也会被转运到线粒体、过氧化物酶体以及细胞膜等其他细胞器 [7] 。选择性胆固醇摄取途径主要是HDL来源胆固醇的摄取,该途径不依赖于胞吞的发生。目前对选择性胆固醇摄取的研究不多,其功能也很容易被忽略,肝脏、肾上腺和卵巢等组织可优先通过HDL受体SR-B1从HDL中摄取胆固醇 [9] 。HDL颗粒与细胞膜上的SR-B1结合后,不需要像LDL一样通过胞吞途径到达溶酶体被降解 [ 13- 14] ,HDL来源的胆固醇和胆固醇酯通过SR-B1的疏水通道被摄取到细胞膜 [15] ,并进一步转运到内质网等细胞器,该过程的具体机制还有待进一步阐明。
1.3胆固醇的稳态平衡
胆固醇的稳态平衡受到精密调控,以避免游离胆固醇含量过高引起细胞毒性 [16] 。胆固醇的合成和摄取相关基因主要由SREBP2转录调控 [17] 。当内质网膜胆固醇水平降低时,位于内质网的转录因子SREBP2会迅速响应,从内质网释放到达高尔基体,经历加工剪切形成活性的nSREBP2进入细胞核,进而上调胆固醇合成的限速酶 HMGCR和胆固醇摄取相关LDL受体表达,从而促进胆固醇的生物合成和外源摄取;而当细胞内胆固醇累积时则不会激活SREBP2通路,过量的胆固醇可在ACAT-1的作用下生成胆固醇酯,储存于脂滴;也可在胆固醇羟化酶的作用下生成羟胆固醇或环氧化酶的催化下生成环氧胆固醇,还可转化为维生素D、类固醇激素等物质 [ 18- 19] 。
2胆固醇代谢与肿瘤的发生发展
2.1肿瘤细胞胆固醇代谢异常
胆固醇可促进细胞增殖、迁移和侵袭,在肿瘤的发生发展中发挥重要作用。肿瘤细胞中胆固醇代谢调控异常主要表现为胆固醇合成水平上调、胆固醇摄取增加,大量代谢产物异常堆积,导致肿瘤细胞增殖、存活、侵袭、转移以及对肿瘤微环境的适应能力增强,从而促进肿瘤的发生发展 [3] 。
研究表明,多种与胆固醇合成相关的基因在肿瘤组织中活性增加,如胆固醇生物合成限速酶HMGCR和角鲨烯单加氧酶。HMGCR的表达在多种肿瘤中上调,包括胃癌、胶质瘤和前列腺癌 [ 20- 22] 。HMGCR过表达促进了这些肿瘤细胞的生长和迁移,而抑制HMGCR则可抑制肿瘤的发生发展,靶向抑制HMGCR的表达已被用于治疗实体癌、血液癌和具有耐药性的肿瘤 [ 23- 25] 。编码角鲨烯单加氧酶的 SQLE基因座也在多种肿瘤中拷贝数增加,其在非酒精性脂肪肝引起的肝细胞癌中过量表达,并且与胰腺癌的放射线耐受以及乳腺癌、前列腺癌、结直肠癌和鳞状上皮癌的发展或患者预后不良相关 [ 26- 28] 。
除了胆固醇合成相关蛋白高表达外,介导胆固醇摄取的LDL受体表达在多种肿瘤细胞中也呈上调趋势。研究表明,LDL受体表达与小细胞肺癌患者的不良临床预后正相关 [29] 。LDL受体高表达与乳腺癌患者无复发存活率降低相关,而在人表皮生长因子受体2过表达的乳腺癌细胞中,沉默LDL受体则可以延缓高脂血症小鼠肿瘤的生长 [30] 。同样,胰腺导管腺癌患者LDL受体表达升高与复发率增加相关,而胰腺肿瘤细胞中LDL受体缺失可显著减少胆固醇摄取,抑制肿瘤细胞增殖 [31] 。此外,在胶质瘤细胞中,LDL受体表达高于邻近的正常脑组织,并且与EGFR激活和nSREBP1表达相关 [32] 。前列腺癌细胞中肿瘤抑制因子磷酸酯酶与张力蛋白同源物缺失和PI3K/AKT通路激活可显著增加肿瘤生长所需的外源LDL的摄取 [33] 。除了LDL受体表达水平升高外,一些肿瘤细胞中HDL受体SR-B1的表达也会升高,如胶质母细胞瘤中过表达的SR-B1可促进肿瘤侵袭 [34] 。这些研究表明,异常表达的LDL受体和SR-B1对胆固醇的摄取在某些类型肿瘤细胞的增殖和侵袭中扮演着重要角色。
2.2肿瘤细胞胆固醇代谢调控机制
2.2.1调节SREBP活性对胆固醇代谢的影响
SREBP激活在肿瘤细胞胆固醇代谢异常中起核心作用。通常情况下,SREBP由INSIG蛋白、SREBP剪切激活蛋白和内质网胆固醇抑制。在肿瘤细胞中,过度活化的PI3K/AKT信号通路以及p53介导的信号通路可调控SREBP的活性。PI3K/AKT信号通路通过哺乳动物雷帕霉素靶蛋白复合物1依赖的方式激活SREBP,从而促进胆固醇的生物合成和摄取 [32] 。研究表明,在胶质瘤细胞中,EGFR Ⅷ突变体/PI3K可激活SREBP1,从而上调肿瘤细胞中LDL受体表达,促进外源胆固醇的摄取;而 p53则通过在mRNA水平抑制SREBP,调节其活性,从而抑制胆固醇的合成和摄取 [35] 。除此之外,最新研究表明,在肝癌细胞中,激活的AKT可磷酸化细胞质中的糖异生限速酶磷酸烯醇丙酮酸羧化激酶1,使其易位到内质网并作为蛋白激酶磷酸化INSIG蛋白。这种磷酸化作用减少了固醇与INSIG的结合,并破坏了INSIG蛋白与SREBP剪切激活蛋白之间的相互作用,从而激活SREBP以及下游脂质合成和摄取相关基因的转录表达,促进肿瘤的发生发展 [36] 。
2.2.2ACAT-1对胆固醇代谢的影响
胆固醇酯的形成是防止游离胆固醇积累的重要方式。内质网跨膜蛋白ACAT-1可将过量的胆固醇酯化生成胆固醇酯并储存于脂滴中。研究表明,乳腺癌、胰腺癌、胶质母细胞瘤等肿瘤细胞中ACAT-1的表达和活性均升高,胆固醇酯的含量上调 [37] 。过量胆固醇酯的生成会激活SREBP1,从而促进肿瘤转移;而抑制ACAT-1可通过阻断SREBP1调控的基因表达,抑制胶质母细胞瘤的生长和前列腺癌细胞的侵袭性 [33] 。
2.2.3LXR对胆固醇代谢的影响
LXR被胆固醇激活后可与SREBP拮抗参与体内胆固醇稳态的调控 [38] 。LXR在肿瘤细胞脂质代谢中的作用因其调控多个下游基因的表达而变得复杂。LXR可通过增加胆固醇外排和减少胆固醇摄取降低细胞内胆固醇水平。前者由LXR通过调节载脂蛋白E及其转运蛋白三磷酸腺苷结合盒转运体A1和G1的表达引起,后者通过促进LDL受体降解介导 [ 39- 40] 。研究表明,LXR激动剂或合成配体可抑制不同类型肿瘤(如胶质瘤和乳腺癌)的增殖和生长 [ 32, 41] 。此外,抑制LXR信号通路可以通过阻断糖酵解和脂肪酸生物合成酶的表达来诱导多种肿瘤细胞凋亡 [42] 。LXR的激活还可抑制免疫系统 [ 43- 44] ,通过降低细胞内胆固醇的含量来抑制淋巴细胞的增殖,从而影响体内免疫系统的稳态和免疫应答。鉴于LXR激活也可诱导细胞周期调节因子和参与免疫细胞功能相关基因的表达,因此在完整的肿瘤免疫微环境中探究LXR激动剂或拮抗剂在抑制肿瘤中的作用具有重要意义。
3靶向胆固醇代谢在肿瘤治疗中的应用
目前,在不同水平上干预胆固醇代谢的治疗药物的相关研究已经取得了很大的进展。靶向胆固醇合成相关代谢酶、脂蛋白介导的胆固醇摄取以及胆固醇储存途径可能成为潜在的预防或延缓相关肿瘤进展的有效途径。
3.1靶向胆固醇合成的治疗
肿瘤细胞胆固醇合成途径有多种潜在的抑制性靶点 [45] ,针对胆固醇合成相关蛋白的治疗药物已开展临床研究。其中,抑制甲羟戊酸途径限速酶HMGCR的他汀类药物可通过破坏细胞膜的完整性以及细胞稳态的维持、影响细胞存活相关蛋白的活性等多种途径发挥抗肿瘤作用 [ 46- 49] 。他汀类药物是最常见的降胆固醇药物,同时也是肿瘤尤其是乳腺癌临床研究中使用最广泛的胆固醇代谢靶向药物。
不同他汀类药物的特殊化学性质决定了其机制特性。普伐他汀是一种亲水性他汀类药物,被一种仅在肝脏中表达的不依赖钠的有机阴离子转运多肽1B1选择性吸收。而亲脂性HMGCR抑制剂辛伐他汀是一种通过其他机制进入细胞的疏水性他汀类药物。辛伐他汀治疗可降低甲羟戊酸途径产生的香叶基香叶基焦磷酸(蛋白异戊二烯化所必需)以及抗原呈递细胞中小GTP酶Rab5的异戊二烯化水平,从而阻滞胞内体成熟,延长抗原保留时间,增强抗原提呈和T细胞激活,最终增强抗肿瘤免疫力。此外,辛伐他汀可强有力地增强肿瘤疫苗效力并与抗程序性死亡受体1抗体协同作用进行肿瘤治疗 [50] 。
回顾性研究表明,他汀类药物治疗可延长多发性骨髓瘤、结直肠癌和转移性胰腺癌患者接受一线化疗药物联合治疗的存活期 [ 51- 53] 。值得注意的是,Ⅱ期临床试验结果显示,辛伐他汀与EGFR抑制剂吉非替尼(gefitinib)联合使用比单纯使用EGFR抑制剂治疗非小细胞肺癌抗肿瘤效果更优 [ 54- 55] 。有研究表明,亲脂性他汀类药物更容易进入肝外细胞,而亲水性他汀类药物对肝脏的选择性更高 [56] 。此外,临床数据也表明他汀类药物的抗肿瘤作用是剂量和时间依赖的 [57] 。因此,寻找用于患者分层的预测性生物标志物将为他汀类药物辅助治疗提供更准确的评估。
3.2靶向血浆胆固醇水平的治疗
研究表明,依折麦布(Ezetimibe)可作用于NPC- 1L1,显著降低肠道对胆固醇的吸收,使血浆胆固醇水平下降或增强血浆清除胆固醇的能力 [58] 。此外,LDL受体表达受到PCSK9的调控,抑制PCSK9的药物可导致肝脏LDL受体丰度增加,有效降低血浆中LDL-C水平 [59] ,这是一种通过限制外源性脂质的量以抑制肿瘤增殖的策略。近期研究表明,提高LDL-C水平的 PCSK9基因突变与乳腺癌的高风险相关,而降低LDL-C水平的 PCSK9基因突变则可显著降低雌激素受体阳性乳腺癌的风险 [60] 。近年研究也逐渐出现了一些基于改变HDL水平的治疗策略,以预防肿瘤的发展。目前,已有实验模型和大型临床试验对CETP抑制剂和其他药物(如贝特类和烟酸)提高血浆HDL-C水平的效果进行评估(例如对内分泌相关癌症的研究) [61] 。其中CETP抑制剂升高HDL-C水平的作用最强。CETP是一种由肝脏合成的血浆糖蛋白,在血液循环中与HDL结合,介导胆固醇从HDL转运至极低密度脂蛋白和LDL中,而 CETP抑制剂可阻断这一转移过程,理论上具有抗动脉粥样硬化和降低心血管风险的作用。研究表明,尽管CEPT抑制剂提高了血浆HDL-C水平,但大多数CETP抑制剂(如托彻普、达塞曲匹、韦特拉比)治疗无效或出现心血管不良反应 [ 62- 64] ,药物开发因此停止。但在一项针对动脉粥样硬化性血管疾病患者的大型试验中,另一种CETP抑制剂安塞曲匹在他汀类药物治疗的基础上可减少心血管事件的发生,但是并未改变乳腺癌等癌症的发病率 [65] 。目前关于贝特类和HDL-C与癌症风险的研究结果仍存在争议,尚不能用于临床抗癌治疗。另一种药物烟酸可使HDL-C升高20%~25%,同时降低三酰甘油和LDL-C水平 [66] ,但相关研究结果并未显示出其对肿瘤具有显著的抑制作用。综上,基于HDL的降血脂药物对人类肿瘤预防和治疗的影响仍存在争议,需要进一步的临床前和临床试验。
3.3靶向胆固醇酯化的治疗
阿伐麦布可靶向ACAT-1,抑制胆固醇酯化,从而使细胞内游离胆固醇水平升高。在肿瘤细胞中,过量游离胆固醇可通过UPR-CHOP-MAPK或p38-MAPK通路激活未折叠蛋白反应,引起内质网应激,使细胞内多种代谢稳态发生紊乱,导致细胞凋亡。研究表明,使用阿伐麦布抑制肿瘤细胞胆固醇酯化可有效抑制胰腺癌和前列腺癌等肿瘤的增殖、转移和侵袭 [67] 。因此,阿伐麦布有望成为一种抗肿瘤的候选药。
4展望
在肿瘤微环境中,肿瘤细胞和免疫细胞、脂肪细胞、内皮细胞和基质细胞等的脂质代谢受到动态调控并且相互关联,从而促进肿瘤细胞的生长、存活、增殖、迁移、侵袭和转移。基于胆固醇及其代谢产物对肿瘤发生、发展和侵袭的重要影响,人们对肿瘤细胞中胆固醇代谢的研究越来越深入。研究表明, 胆固醇的代谢不仅可通过细胞内致癌信号进行调节,还可通过肿瘤微环境的输入进行调节。反过来,胆固醇代谢紊乱也会改变肿瘤细胞中的致癌信号通路,并通过分泌成分影响邻近的正常细胞群。这种复杂性提示我们不仅需要研究肿瘤细胞中的代谢网络,还需要研究肿瘤微环境中细胞间的相互联系对肿瘤细胞代谢的影响,同时研究干扰肿瘤微环境中细胞的胆固醇代谢对抗肿瘤治疗的作用,尤其是抗肿瘤免疫和抗血管生成反应。此外,对可被致癌信号诱导和翻译后修饰的胆固醇相关酶以及胆固醇代谢调节酶结构的阐明,将有助于确定针对代谢异常调节的特定干预措施。深入了解肿瘤胆固醇代谢的特异性调节将提供一种安全性更好的抗肿瘤治疗新策略。
Funding Statement
科技部重点研发计划(2020YFA0803300); 浙江省引进培育领军型创新创业团队(2019R01001)
References
- 1.BROWN M S, RADHAKRISHNAN A, GOLDSTEIN J L. Retrospective on cholesterol homeostasis:the central role of scap[J] Annu Rev Biochem. . 2018;87(1):783–807. doi: 10.1146/annurev-biochem-062917-011852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IKONEN E. Mechanisms for cellular cholesterol transport:defects and human disease[J] Physiol Rev. . 2006;86(4):1237–1261. doi: 10.1152/physrev.00022.2005. [DOI] [PubMed] [Google Scholar]
- 3.YOSHIOKA Y,SASAKI J,YAMAMOTO M,et al.Quantitation by (1)H-NMR of dolichol,cholesterol and choline-containing lipids in extracts of normal and phathological thyroid tissue[J]. NMR Biomed,2000,13(7):377–383.DOI:10.1002/1099-1492(200011)13:7<377::aid-nbm658>3.0.co;2-e . [DOI] [PubMed]
- 4.LISCUM L, FINER-MOORE J, STROUD R M, et al. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase,a glycoprotein of the endop- lasmic reticulum[J] J Biol Chem. . 1985;260(1):522–530. doi: 10.1016/S0021-9258(18)89764-2. [DOI] [PubMed] [Google Scholar]
- 5.KO C W, QU J, BLACK D D, et al. Regulation of intestinal lipid metabolism:current concepts and relevance to disease[J] Nat Rev Gastroenterol Hepatol. . 2020;17(3):169–183. doi: 10.1038/s41575-019-0250-7. [DOI] [PubMed] [Google Scholar]
- 6.NELSON J K, KOENIS D S, SCHEIJ S, et al. EEPD1 Is a novel LXR target gene in macrophages which regulates ABCA1 abundance and cholesterol efflux[J] Arterioscler Thromb Vasc Biol. . 2017;37(3):423–432. doi: 10.1161/ATVBAHA.116.308434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MENG Y, HEYBROCK S, NECULAI D, et al. Cholesterol handling in lysosomes and beyond[J] Trends Cell Biol. . 2020;30(6):452–466. doi: 10.1016/j.tcb.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 8.BROWN M S, GOLDSTEIN J L. A receptor-mediated pathway for cholesterol homeostasis[J] Science. . 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 9.MEYER J M, GRAF G A, VAN DER WESTHUYZEN D R. New developments in selective cholesteryl ester uptake[J] Curr Opin Lipidology. . 2013;24(5):386–392. doi: 10.1097/MOL.0b013e3283638042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DAS A, BROWN M S, ANDERSON D D, et al. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis [J/OL] eLife. . 2014;3:e02882. doi: 10.7554/eLife.02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GOLDSTEIN J L, BROWN M S. The LDL receptor[J] ATVB. . 2009;29(4):431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LUO J, YANG H, SONG B L. Mechanisms and regulation of cholesterol homeostasis[J] Nat Rev Mol Cell Biol. . 2020;21(4):225–245. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 13.GLASS C, PITTMAN R C, CIVEN M, et al. Uptake of high-density lipoprotein-associated apoprotein A-I and cholesterol esters by 16 tissues of the rat in vivo and by adrenal cells and hepatocytes in vitro[J] . J Biol Chem. . 1985;260(2):744–750. doi: 10.1016/S0021-9258(20)71160-9. [DOI] [PubMed] [Google Scholar]
- 14.GLASS C, PITTMAN R C, WEINSTEIN D B, et al. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein:selective delivery of cholesterol ester to liver,adrenal,and gonad[J] Proc Natl Acad Sci USA. . 1983;80(17):5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NECULAI D, SCHWAKE M, RAVICHANDRAN M, et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36[J] Nature. . 2013;504(7478):172–176. doi: 10.1038/nature12684. [DOI] [PubMed] [Google Scholar]
- 16.HAMPTON R Y. A cholesterol toggle switch[J] Cell Metab. . 2008;8(6):451–453. doi: 10.1016/j.cmet.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 17.HORTON J D, SHAH N A, WARRINGTON J A, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes[J] Proc Natl Acad Sci USA. . 2003;100(21):12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GOLDSTEIN J L, DEBOSE-BOYD R A, BROWN M S. Protein sensors for membrane sterols[J] Cell. . 2006;124(1):35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 19.BOVENGA F, SABBÀ C, MOSCHETTA A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer[J] Cell Metab. . 2015;21(4):517–526. doi: 10.1016/j.cmet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 20.LI C S, WU W, XIE K K, et al. HMGCR is up-regulated in gastric cancer and promotes the growth and migration of the cancer cells[J] Gene. . 2016;587(1):42–47. doi: 10.1016/j.gene.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 21.QIU Z, YUAN W, CHEN T, et al. HMGCR positively regulated the growth and migration of glioblastoma cells[J] Gene. . 2016;576(1):22–27. doi: 10.1016/j.gene.2015.09.067. [DOI] [PubMed] [Google Scholar]
- 22.ASHIDA S, KAWADA C, INOUE K. Stromal regulation of prostate cancer cell growth by mevalonate pathway enzymes HMGCS1 and HMGCR[J] Oncol Lett. . 2017;14(6):6533–6542. doi: 10.3892/ol.2017.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KONG Y, CHENG L, MAO F, et al. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC)[J] J Biol Chem. . 2018;293(37):14328–14341. doi: 10.1074/jbc.RA118.004442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LEE J S, ROBERTS A, JUAREZ D, et al. Statins enhance efficacy of venetoclax in blood cancers[J] Sci Transl Med. . 2018;10(445):eaaq1240. doi: 10.1126/scitranslmed.aaq1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.YANG J, WANG L, JIA R. Role of de novo cholesterol synthesis enzymes in cancer[J] . J Cancer. . 2020;11(7):1761–1767. doi: 10.7150/jca.38598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BROWN D N, CAFFA I, CIRMENA G, et al. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer[J] Sci Rep. . 2016;6(1):19435. doi: 10.1038/srep19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CIRMENA G, FRANCESCHELLI P, ISNALDI E, et al. Squalene epoxidase as a promising metabolic target in cancer treatment[J] Cancer Lett. . 2018;425:13–20. doi: 10.1016/j.canlet.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 28.LIU D, WONG C C, FU L, et al. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target [J/OL] Sci Transl Med. . 2018;10(437):eaap9840. doi: 10.1126/scitranslmed.aap9840. [DOI] [PubMed] [Google Scholar]
- 29.ZHOU T, ZHAN J, FANG W, et al. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC)[J] BMC Cancer. . 2017;17(1):269. doi: 10.1186/s12885-017-3239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GALLAGHER E J, ZELENKO Z, NEEL B A, et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia[J] Oncogene. . 2017;36(46):6462–6471. doi: 10.1038/onc.2017.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GUILLAUMOND F, BIDAUT G, OUAISSI M, et al. Cholesterol uptake disruption,in association with chemotherapy,is a promising combined metabolic therapy for pancreatic adenocarcinoma[J] Proc Natl Acad Sci USA. . 2015;112(8):2473–2478. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GUO D, REINITZ F, YOUSSEF M, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway[J] Cancer Discov. . 2011;1(5):442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.YUE S, LI J, LEE S Y, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressi- veness[J] Cell Metab. . 2014;19(3):393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MENARD J A, CHRISTIANSON H C, KUCHARZEWSKA P, et al. Metastasis stimulation by hypoxia and acidosis-induced extracellular lipid uptake is mediated by proteoglycan-dependent endocytosis[J] Cancer Res. . 2016;76(16):4828–4840. doi: 10.1158/0008-5472.CAN-15-2831. [DOI] [PubMed] [Google Scholar]
- 35.ZELCER N, HONG C, BOYADJIAN R, et al. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor[J] Science. . 2009;325(5936):100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AYLON Y, OREN M. The Hippo pathway,p53 and cholesterol[J] Cell Cycle. . 2016;15(17):2248–2255. doi: 10.1080/15384101.2016.1207840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.XU D, WANG Z, XIA Y, et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis[J] Nature. . 2020;580(7804):530–535. doi: 10.1038/s41586-020-2183-2. [DOI] [PubMed] [Google Scholar]
- 38.OHTAKI S, WANIBUCHI M, KATAOKA-SASAKI Y, et al. ACTC1 as an invasion and prognosis marker in glioma[J] JNS. . 2017;126(2):467–475. doi: 10.3171/2016.1.JNS152075. [DOI] [PubMed] [Google Scholar]
- 39.IKONEN E. Cellular cholesterol trafficking and compartmentalization[J] Nat Rev Mol Cell Biol. . 2008;9(2):125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 40.LIN C Y, GUSTAFSSON J Å. Targeting liver X receptors in cancer therapeutics[J] Nat Rev Cancer. . 2015;15(4):216–224. doi: 10.1038/nrc3912. [DOI] [PubMed] [Google Scholar]
- 41.TAO R, XIONG X, DEPINHO R A, et al. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6[J] J Lipid Res. . 2013;54(10):2745–2753. doi: 10.1194/jlr.M039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FLAVENY C A, GRIFFETT K, EL-GENDY B E D M, et al. Broad anti-tumor activity of a small molecule that selectively targets the warburg effect and lipogenesis[J] Cancer Cell. . 2015;28(1):42–56. doi: 10.1016/j.ccell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.RIOLS F,BERTRAND-MICHEL J. Analysis of oxysterols[J]. Methods Mol Biol,2018,1730:267-275.DOI:10.1007/978-1-4939-7592-1_19 . [DOI] [PubMed]
- 44.CHEN J, YE Y, LIU P, et al. Suppression of T cells by myeloid-derived suppressor cells in cancer[J] Human Immunol. . 2017;78(2):113–119. doi: 10.1016/j.humimm.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 45.SAXENA K, SHIPLEY G G. Structural studies of detergent-solubilized and vesicle-reconstituted low-density lipoprotein (LDL) receptor[J] Biochemi- stry. . 1997;36(50):15940–15948. doi: 10.1021/bi971579p. [DOI] [PubMed] [Google Scholar]
- 46.SIVAPRASAD U, ABBAS T, DUTTA A. Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells[J] Mol Cancer Ther. . 2006;5(9):2310–2316. doi: 10.1158/1535-7163.MCT-06-0175. [DOI] [PubMed] [Google Scholar]
- 47.SONG X, LIU B C, LU X Y, et al. Lovastatin inhibits human B lymphoma cell proliferation by reducing intracellular ROS and TRPC6 expression[J] BBA- Mol Cell Res. . 2014;1843(5):894–901. doi: 10.1016/j.bbamcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.TU Y S, KANG X L, ZHOU J G, et al. Involvement of Chk1–Cdc25A-cyclin A/CDk2 pathway in simvastatin induced S-phase cell cycle arrest and apoptosis in multiple myeloma cells[J] Eur J Pharmacol. . 2011;670(2-3):356–364. doi: 10.1016/j.ejphar.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 49.MENTER D G, RAMSAUER V P, HARIRFOROOSH S, et al. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites [J/OL] PLoS One. . 2011;6(12):e28813. doi: 10.1371/journal.pone.0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.XIA Y, XIE Y, YU Z, et al. The Mevalonate pathway is a druggable target for vaccine adjuvant discovery [J/OL] Cell. . 2018;175(4):1059–1073.e21. doi: 10.1016/j.cell.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 51.ABDEL-RAHMAN O. Statin treatment and outcomes of metastatic pancreatic cancer:a pooled analysis of two phase Ⅲ studies[J] Clin Transl Oncol. . 2019;21(6):810–816. doi: 10.1007/s12094-018-1992-3. [DOI] [PubMed] [Google Scholar]
- 52.BRÅNVALL E, EKBERG S, ELORANTA S, et al. Statin use is associated with improved survival in multiple myeloma:A Swedish population‐based study of 4315 patients[J] Am J Hematol. . 2020;95(6):652–661. doi: 10.1002/ajh.25778. [DOI] [PubMed] [Google Scholar]
- 53.CARDWELL C R, HICKS B M, HUGHES C, et al. Statin use after colorectal cancer diagnosis and survival:a population-based cohort study[J] JCO. . 2014;32(28):3177–3183. doi: 10.1200/JCO.2013.54.4569. [DOI] [PubMed] [Google Scholar]
- 54.HAN J Y, LEE S H, YOO N J, et al. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer[J] Clin Cancer Res. . 2011;17(6):1553–1560. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- 55.LEE Y, LEE K H, LEE G K, et al. Randomized phase Ⅱ study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer[J] Cancer Res Treat. . 2017;49(4):1001–1011. doi: 10.4143/crt.2016.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DUNCAN R E, EL-SOHEMY A, ARCHER M C. Statins and the risk of cancer[J] JAMA. . 2006;295(23):2720. doi: 10.1001/jama.295.23.2720-a. [DOI] [PubMed] [Google Scholar]
- 57.KIM S T, KANG J H, LEE J, et al. Simvastatin plus capecitabine–cisplatin versus placebo plus capecita- bine–cisplatin in patients with previously untreated advanced gastric cancer:A double-blind randomised phase 3 study[J] Eur J Cancer. . 2014;50(16):2822–2830. doi: 10.1016/j.ejca.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 58.FARMER J A. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis (the SEAS trial)[J]. Curr Atheroscler Rep,2009,11(2):82–83 . [PubMed]
- 59.JOSEPH L, ROBINSON J G. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition and the future of lipid lowering therapy[J] Prog Cardio- vascular Dis. . 2015;58(1):19–31. doi: 10.1016/j.pcad.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 60.NOWAK C, ÄRNLÖV J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk[J] Nat Commun. . 2018;9(1):3957. doi: 10.1038/s41467-018-06467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.REVILLA G, CEDÓ L, TONDO M, et al. LDL,HDL and endocrine-related cancer:From pathogenic mechanisms to therapies[J/OL] Seminars Cancer Biol. . 2020 doi: 10.1016/j.semcancer.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 62.LINCOFF A M, NICHOLLS S J, RIESMEYER J S, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease[J] N Engl J Med. . 2017;376(20):1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 63.SCHWARTZ G G, OLSSON A G, ABT M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome[J] N Engl J Med. . 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 64.BARTER P J, CAULFIELD M, ERIKSSON M, et al. Effects of torcetrapib in patients at high risk for coronary events[J] N Engl J Med. . 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 65.BOWMAN L, HOPEWELL J C, CHEN F, et al. HPS3/TIMI55–REVEAL collaborative group. effects of anacetrapib in patients with atherosclerotic vascular disease[J] N Engl J Med. . 2017;377(13):1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 66.Kamanna S V, Kashyap L M. Mechanism of action of niacin[J] Am J Cardiology. . 2008;101(8):S20–S26. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 67.LI J, GU D, LEE S S Y, et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer[J] Oncogene. . 2016;35(50):6378–6388. doi: 10.1038/onc.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]