Abstract
支气管哮喘是由多种细胞包括气道的炎性细胞和结构细胞及细胞组分参与的气道慢性炎症性疾病,其特征为气道炎症、气道高反应性、可逆性气道阻塞和气道重塑。以往哮喘被认为主要由2型炎症因子驱动,Th2细胞分泌IL-4、IL-5和IL-13,引起气道嗜酸性粒细胞炎症。随着研究的深入,发现中性粒细胞与哮喘炎症过程也存在密切关系。哮喘患者气道中的中性粒细胞在趋化增加的同时凋亡下降,从而导致中性粒细胞数量增多。中性粒细胞比嗜酸性粒细胞更早到达,通过产生弹性蛋白酶、髓过氧化物酶、中性粒细胞胞外诱捕网、趋化因子和细胞因子等,参与哮喘的发生及发展过程。针对这些效应分子的拮抗剂如抗IL-8受体抗体、抗IL-17抗体,DNA酶显示出对中性粒细胞哮喘的治疗作用,但应用于临床还需要更多的实验数据支持。本文主要就中性粒细胞在哮喘中的地位和作用作一综述。
Abstract
Bronchial asthma is a chronic respiratory disease,characterized by airway inflammation,airway hyperresponsiveness,reversible airway obstruction and airway remodeling,in which a variety of cells including airway inflammatory cells and structural cells are involved. Previous studies have shown that asthma is mainly driven by Th2 cytokines IL-4,IL-5,and IL-13,leading to airway eosinophil inflammation. With further research,however,it has been found that neutrophils are also closely related to asthma. Numbers of neutrophils are elevated in airway through increased chemotaxis and decreased apoptosis,which is earlier than eosinophils,leading to airway neutrophilic inflammation. Neutrophils can produce elastase,myeloperoxidase,neutrophil extra- cellular traps,chemokines and cytokines,participating in the occurrence and development of asthma. The antagonists against these molecules,such as anti-IL-8 receptor antibody,anti-IL-17 antibody,and DNase,have shown positive effects on neutrophilic asthma,but further studies are needed to support their clinical application. This article mainly reviews the role of neutrophils in asthma and related mechanisms.
Keywords: Neutrophils, Asthma, Neutrophilic asthma, Therapy; Review
白细胞介素(interleukin,IL);粒细胞-巨噬细胞集落刺激因子(granulocyte-macrophage colony-stimulating factor,GM-CSF);肿瘤坏死因子(tumor necrosis factor,TNF);白三烯B4(leukotriene B4,LTB4);中性粒细胞胞外诱捕网(neutrophil extra- cellular traps,NET);基质金属蛋白酶(matrix metalloproteinase,MMP);髓过氧化物酶(myeloperoxidase,MPO);嗜酸性粒细胞阳离子蛋白(eosinophil cationic protein,ECP);基质金属蛋白酶组织抑制因子1(tissue inhibitor of matrix metalloproteinase 1,TIMP-1);脱氧核糖核酸酶I (deoxyribonuclease I,DNase I);趋化因子CXC亚家族受体(CXC subfamily receptor,CXCR);
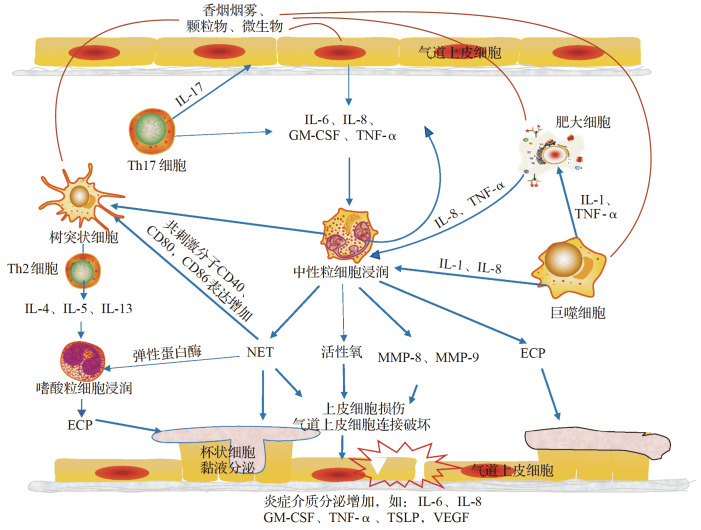
支气管哮喘(以下简称哮喘)是由多种细胞(包括气道的炎性细胞和结构细胞)以及细胞组分参与的气道慢性炎症性疾病,临床表现为喘息、气急、咳嗽等 [1] 。以往认为哮喘主要由2型炎症因子驱动,Th2细胞分泌IL-4、IL-5和IL-13,引起气道嗜酸性粒细胞炎症 [1] 。随着研究的深入,发现50%以上的哮喘存在以非嗜酸性粒细胞浸润为主的气道炎症,多以中性粒细胞性浸润为主 [ 2- 3] 。中性粒细胞哮喘与持续性哮喘、重症哮喘、致死性哮喘关系密切,已逐渐引起临床医生和科研工作者的关注和重视。中性粒细胞在骨髓中发育,在外周血中完全成熟,其作为先天免疫防御的第一道防线,通过吞噬、脱颗粒、免疫应激发挥抗病原体作用 [4] 。然而目前的研究表明中性粒细胞的功能远不止于此,其还能分泌广泛的细胞因子、细胞胞外诱捕网等效应分子,参与先天和适应性免疫反应,其作用机制示意见 图1。因此,中性粒细胞在多种疾病的发病机制中起着至关重要的作用,包括自身免疫性疾病、慢性炎症性疾病(如哮喘)等 [5] 。本文就近年对中性粒细胞在哮喘中的作用和地位研究进展作一综述。
图 1 .
中性粒细胞在哮喘中的作用机制示意图香烟烟雾、颗粒物、微生物等刺激气道上皮细胞、树突状细胞、巨噬细胞等产生IL-6、IL-8、GM-CSF等细胞因子,引起中性粒细胞趋化增加及凋亡下降;浸润的中性粒细胞活化可产生活性氧、MMP、NET等,引起气道上皮损伤,损伤的气道上皮可进一步分泌趋化因子及炎症介质等,加重气道炎症反应.IL:白细胞介素;GM-CSF:粒细胞-巨噬细胞集落刺激因子;TNF:肿瘤坏死因子;NET:中性粒细胞胞外诱捕网;MMP:基质金属蛋白酶;ECP:嗜酸性粒细胞阳离子蛋白;TSLP:胸腺基质淋巴细胞生成素;VEGF:血管内皮生长因子.
1中性粒细胞哮喘的认识
哮喘是一种异质性疾病,可根据临床症状、特异性诱因、治疗反应或炎症表型进行分类 [6] 。由过敏原刺激导致的以气道嗜酸性粒细胞炎症为基础的哮喘可通过糖皮质激素或相应的IL-4、IL-5抗体治疗,但还有一部分哮喘患者并未显示出对这些药物的治疗效应,其气道炎症细胞浸润并非以嗜酸性粒细胞为主,目前将这一类型哮喘归为非嗜酸性粒细胞哮喘。根据浸润细胞的比例,非嗜酸性粒细胞哮喘可进一步分为中性粒细胞哮喘、混合粒细胞哮喘和寡细胞哮喘 [ 7- 8] 。中性粒细胞哮喘目前还没有明确的定义。Liu等 [7] 将中性粒细胞哮喘描述为痰嗜酸性粒细胞小于2.5%,中性粒细胞大于65%;而Taylor等 [9] 则认为中性粒细胞哮喘表型为痰液中存在61%或更多的中性粒细胞;还有研究认为,中性粒细胞哮喘的诊断存在年龄差异,但其中性粒细胞均大于61% [10] 。除了诊断标准,中性粒细胞哮喘炎症细胞占比的稳定性也存在争议。研究发现,在两年的时间里,只有8%的中性粒细胞哮喘患者炎症细胞占比保持了稳定,而非嗜酸性粒细胞哮喘(包括中性粒细胞哮喘)不管在短期(4周)还是长期(5.3年)表型均稳定 [ 8, 11] 。
目前认为,多种因素如烟草暴露 [12] 、激素使用 [13] 、各种微生物感染 [ 14- 16] 、颗粒吸入 [ 17- 18] 、臭氧暴露 [19] 均可使哮喘模型气道中性粒细胞增多。
2哮喘患者气道中性粒细胞增多的机制
2.1中性粒细胞趋化增加
中性粒细胞趋化需要趋化因子与受体结合,并通过与血管识别、黏附、滚动、迁移等过程到达趋化部位。哮喘患者的痰液和鼻腔分泌物中均可检测到趋化因子IL-8水平升高 [20] ,中性粒细胞在IL-8的趋化下利用选择素和整合素结合穿过内皮细胞,分泌蛋白酶穿透基底膜,到达炎症部位,参与气道炎症反应 [21] 。除了IL-8,哮喘患者痰液中IL-17A水平显著高于健康人群,并且IL-17A水平与痰液中性粒细胞计数呈正相关 [22] 。动物实验也发现,经卵清蛋白及脂多糖刺激的小鼠Th17细胞进入气道并释放IL-17,气道中性粒细胞随之增加 [23] 。IL-17A可促进上皮细胞和内皮细胞分泌细胞因子(如IL-6、GM-CSF、TNF-α等)、趋化因子(IL-1、IL-8)及炎症介质(如一氧化氮和MMP等),促进中性粒细胞活化和募集。另外,Th17还可直接产生并分泌趋化因子IL-8,促进中性粒细胞活化和募集,从而介导中性粒细胞引起的炎症 [ 24- 25] 。
2.2中性粒细胞凋亡减少
多种机制共同导致哮喘中性粒细胞寿命延长,气道炎症加重。哮喘患者血浆、支气管肺泡灌洗液或痰液中可检测到IL-8、IL-17A、LTB4、GM-CSF和TNF-α等炎症介质浓度增加 [ 26- 28] 。GM-CSF可通过细胞外调节蛋白激酶1/2途径抑制中性粒细胞凋亡 [29] 。LTB4通过与其相应的LTB4受体结合,下调环磷酸腺苷介导的中性粒细胞凋亡 [30] 。除此之外,LTB4还可通过活性氧依赖的促凋亡蛋白Bad降解来抑制中性粒细胞凋亡,其机制有赖于核因子κB信号通路的氧化还原调节 [ 31- 32] 。S100A8和S100A9是钙结合S100蛋白家族的重要成员,主要由中性粒细胞和单核细胞表达。哮喘小鼠或患者支气管肺泡灌洗液中S100A8和S100A9浓度较正常支气管肺泡灌洗液升高 [ 33- 34] 。经 S100A8和S100A9刺激后的人支气管上皮细胞分泌单核细胞趋化蛋白1、IL-6和IL-8,产生的细胞因子抑制半胱天冬酶9和半胱天冬酶3的激活,使哮喘的中性粒细胞凋亡减少 [35] 。
3中性粒细胞在哮喘起始阶段的作用
哮喘小鼠气道中不仅存在中性粒细胞,而且其出现时间更早于嗜酸性粒细胞,也就是说中性粒细胞可能在哮喘起始阶段发挥作用。笔者团队在以往研究中发现,第一次卵清蛋白刺激之后,中性粒细胞从第3个小时开始增多,而嗜酸性粒细胞直到第24小时才开始增多 [36] 。Toussaint等 [37] 也发现,小鼠哮喘模型给予鼻病毒刺激后第1天可见中性粒细胞明显增加,而嗜酸性粒细胞无明显变化。在低剂量脂多糖和屋尘螨诱导的小鼠哮喘模型中,中性粒细胞从第6小时开始增加,且该群中性粒细胞还可分泌NET诱导哮喘炎症 [16] 。以上研究均提示,中性粒细胞作为血液中最丰富的白细胞,是先天免疫的第一道防线,可能比嗜酸性粒细胞更早地参与哮喘的发生。
机制研究还进一步证实了中性粒细胞在后续哮喘炎症中的作用。Radermecker等 [16] 发现,低剂量脂多糖刺激后募集到气道的中性粒细胞可特异性表达CXCR4,该群细胞可分泌NET,诱导哮喘炎症和Th2反应。这可能与NET募集并激活树突状细胞,表面共刺激分子CD40、CD80和CD86表达增加,促进树突状细胞提呈抗原有关 [38] 。在鼻病毒诱导的小鼠哮喘模型中,研究者发现来源于NET的双链DNA介导鼻病毒诱导的过敏性哮喘加重,表现为气道炎症细胞浸润,细胞因子分泌增加,黏液分泌增多 [37] 。后续研究也表明,通过抑制中性粒细胞及其产生的物质,可以减轻气道炎症及哮喘的症状。如由脂多糖和屋尘螨诱导的小鼠哮喘模型中,中性粒细胞趋化因子受体CXCR2抗体、DNaseI、弹性蛋白酶抑制剂等可减轻小鼠气道炎症细胞浸润,使气道炎症评分及黏液分泌下降,细胞因子合成减少,从而气道炎症减轻 [16] 。另一项研究也显示了类似的结果,在由屋尘螨及鼻病毒构建的哮喘急性加重模型中,通过DNase I降解NET可以有效缓解哮喘急性加重小鼠的气道炎症 [37] 。
上述研究均提示中性粒细胞可能比嗜酸性粒细胞更早到达气道,通过产生NET,分泌各种酶、炎症介质、细胞因子等介导哮喘Th2炎症,引起气道高反应性。中性粒细胞在哮喘早期炎症的具体作用、机制和途径值得重视和进一步探索。
4中性粒细胞在哮喘效应阶段的作用
中性粒细胞在哮喘的不同阶段分泌的物质不同 [39] 。早期主要分泌MMP-9、弹性蛋白酶、氧自由基和MPO等,晚期主要分泌ECP和IL-8。
MMP-9可由不同的细胞产生,但主要来源于中性粒细胞 [ 39- 40] ,可降解细胞外基质。中性粒细胞哮喘患者体内总MMP-9水平非常高,且与患者肺功能呈负相关,但99%以上的MMP-9不活跃,存在高水平的MMP-9/TIMP-1复合物,MMP/TIMP-1失衡参与了哮喘气道重塑 [ 40- 41] 。弹性蛋白酶参与的哮喘病理生理过程包括上皮细胞损伤、支气管黏液腺化生和气道高反应性。除此之外,弹性蛋白酶还可诱导IL-8分泌,促进中性粒细胞向肺募集,并诱导嗜酸细胞产生ECP [39] 。中性粒细胞还能释放氧自由基,通过直接与周边细胞分子发生反应,或间接抑制抗蛋白酶活性等导致组织损伤 [39] 。除此之外,活性氧是一种关键的信号分子,刺激炎症因子产生,参与过敏性气道炎症,从而引起气道高反应性 [ 42- 43] 。Kim等 [44] 也指出活性氧可用于评估中性粒细胞在严重哮喘中的功能状态。研究表明,哮喘患者与健康人群相比MPO水平更高 [45] 。分泌的MPO可以与呼吸爆发时产生的过氧化氢反应产生次氯酸,导致周边组织损伤及炎症反应 [46] 。MPO还与中性粒细胞弹性蛋白酶、双链DNA等参与构成NET,导致气道上皮及血管内皮受损 [ 47- 48] 。IL-8是中性粒细胞强有力的趋化因子,气道上皮细胞、嗜酸性粒细胞及中性粒细胞等多种细胞均可产生IL-8 [39] ,早期分泌的MMP-9和弹性蛋白酶可以促进气道上皮细胞分泌IL-8,导致气道持续的中性粒细胞趋化的恶性循环 [13] 。IL-8除了引起气道炎症外,还可通过促进气道平滑肌细胞的增殖及延长其寿命来增加气道平滑肌细胞的数量,从而加重气道重塑及气道高反应性 [ 49- 50] 。
5以中性粒细胞为靶点的哮喘治疗方法
IL-17细胞因子通过与IL-17受体结合发挥趋化中性粒细胞的作用 [51] 。IL-17抗体可减轻小鼠哮喘模型中的气道炎症浸润和气道高反应性 [52] 。哮喘合并感染患者进行抗IL-17单克隆抗体治疗后,支气管肺泡灌洗液中性粒细胞数量显著减少 [24] 。但另一项临床试验研究显示,中重度哮喘患者使用抗IL-17A受体抗体并未显示出期望的治疗效果 [53] 。一项IL-13和IL-17的双特异性抗体BITS7201A临床试验结果显示,该药物的安全性和耐受性可,但其抗体形成率高,可能影响治疗效果,并且可能增加过敏反应 [54] 。因此,对于IL-17相关信号通路在哮喘治疗中的应用还需要进一步研究。
CXCR2是IL-8的高亲和力受体,SCH527123是一种新型的小分子,可同时阻断CXCR1和CXCR2,降低哮喘受试患者气道中性粒细胞的水平,但其治疗效果仍未明确 [55] 。另一项研究结果也表明,使用SCH527123可减少哮喘患者痰液中约37%的中性粒细胞,但并未体现更好的哮喘控制及肺功能改善等作用 [56] 。其他CXCR2拮抗剂如AZD5069也可以减少痰液中90%的中性粒细胞数量,但另一项ⅡB期临床试验并未提示这一药物可以改善临床结果 [ 57- 58] 。
大环内酯类药物不仅具有抗菌功能,还可以发挥抗炎作用 [59] 。大环内酯类药物通过抑制气道上皮细胞核因子κB受体抑制IL-8释放,以及抑制磷脂酰肌醇3-激酶等途径发挥作用,抑制气道炎症 [ 10, 60] 。在小鼠哮喘模型中,克拉霉素协同地塞米松能更有效地下调气道阻力,减轻气道炎症细胞浸润,缓解哮喘症状 [59] 。在严重难治性非嗜酸性粒细胞哮喘患者中,克拉霉素治疗可降低主要中性粒细胞炎症标志物IL-8水平 [61] 。患有持续症状性哮喘的受试者在口服阿奇霉素治疗48周后哮喘发作较少,生活质量改善,但这种疗法的长期影响还需要进一步评估 [62] 。总体来说,大环内酯类药物对哮喘有改善作用,但是否可纳入中性粒细胞哮喘的常规治疗,及其用药指征和用量等还需要更多的临床证据支持。
NET具有抗菌功能,但其过量存在可导致肺组织不同程度损伤。动物实验表明,通过降解NET的成分及结构可以减轻哮喘小鼠的气道炎症和细胞因子分泌,同时减轻哮喘小鼠急性加重的炎症表现 [ 16, 37] 。未来NET有望用于哮喘的预防及哮喘加重的治疗中。
6结语
中性粒细胞已引起临床医生和科研工作者的重视,但中性粒细胞在哮喘中的作用尚存在许多疑问,其发病机制尚未完全清楚。未来期待更多阐述中性粒细胞在哮喘中的研究面世,尤其是中性粒细胞哮喘的诊断标准、浸润到肺组织的中性粒细胞亚型、中性粒细胞在哮喘早期炎症的介导作用等。这些将有助于获得准确的流行病学资料、推动高质量的中性粒细胞哮喘临床研究,并为中性粒细胞哮喘的治疗提供更多思路和精准化治疗的方向。
Funding Statement
国家重点研发计划(2017YFC131060); 浙江省自然科学基金(LQ18H010002)
References
- 1.PETERS M C, WENZEL S E. Intersection of biology and therapeutics:type 2 targeted therapeutics for adult asthma[J] Lancet. . 2020;395(10221):371–383. doi: 10.1016/S0140-6736(19)33005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ZHANG J, ZHU Z, ZUO X, et al. The role of NTHi colonization and infection in the pathogenesis of neutrophilic asthma[J] Respir Res. . 2020;21(1):170. doi: 10.1186/s12931-020-01438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GRUNWELL J R, STEPHENSON S T, TIROUVAN- ZIAM R, et al. Children with neutrophil-predominant severe asthma have proinflammatory neutrophils with enhanced survival and impaired clearance[J] J Allergy Clin Immunol-Practice. . 2019;7(2):516–525.e6. doi: 10.1016/j.jaip.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KHAN M A, ALI Z S, SWEEZEY N, et al. Progression of cystic fibrosis lung disease from childhood to adulthood:neutrophils,neutrophil extracellular trap (NET) formation,and net degradation[J] Genes. . 2019;10(3):183. doi: 10.3390/genes10030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MANTOVANI A, CASSATELLA M A, COSTANTINI C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity[J] Nat Rev Immunol. . 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 6.RADERMECKER C,LOUIS R,BUREAU F,et al. Role of neutrophils in allergic asthma[J]. Curr Opin Immunol,2018,54:28–34.DOl:10.1016/j.coi. 2018.05.006 . [DOI] [PubMed]
- 7.LIU W, CHEN H, ZHANG D, et al. A retrospective study of clinical features of cough variant asthma in Chinese adults[J] Allergy Asthma Clin Immunol. . 2019;15(1):3. doi: 10.1186/s13223-019-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SIMPSON J L, SCOTT R, BOYLE M J, et al. Inflamma- tory subtypes in asthma:Assessment and identification using induced sputum[J] Respirology. . 2006;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 9.TAYLOR S L,LEONG L E X,CHOO J M,et al. Inflam- matory phenotypes in patients with severe asthma are associated with distinct airway microbiology[J]. J Allergy Clin Immunol,2018,141(1):94–103.e115.DOl:10.1016/j.jaci.2017.03.044 . [DOI] [PubMed]
- 10.NAIR P, PRABHAVALKAR K S. Neutrophilic asthma and potentially related target therapies[J] Curr Drug Targets. . 2020;21(4):374–388. doi: 10.2174/1389450120666191011162526. [DOI] [PubMed] [Google Scholar]
- 11.NAIR P, AZIZ-UR-REHMAN A, RADFORD K. Therapeutic implications of ‘neutrophilic asthma’ [J] Curr Opin Pulmonary Med. . 2015;21(1):33–38. doi: 10.1097/MCP.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 12.PANETTIERI JR. R A. Neutrophilic and pauci-immune phenotypes in severe asthma[J] Immunol Allergy Clinics North Am. . 2016;36(3):569–579. doi: 10.1016/j.iac.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 13.SEYS S F, LOKWANI R, SIMPSON J L, et al. New insights in neutrophilic asthma[J] Curr Opin Pulmonary Med. . 2019;25(1):113–120. doi: 10.1097/MCP.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 14.PATEL K K, WEBLEY W C. Respiratory chlamydia infection induce release of hepoxilin A3 and histamine production by airway neutrophils[J] Front Immunol. . 2018;9 doi: 10.3389/fimmu.2018.02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WANG G, PANG Z, CHEN-YU HSU A, et al. Combined treatment with SB203580 and dexamethasone suppresses non-typeable Haemophilus influenzae-induced Th17 inflammation response in murine allergic asthma[J] Eur J Pharmacol. . 2019;862:172623. doi: 10.1016/j.ejphar.2019.172623. [DOI] [PubMed] [Google Scholar]
- 16.RADERMECKER C, SABATEL C, VANWINGE C, et al. Locally instructed CXCR4hi neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps[J] Nat Immunol. . 2019;20(11):1444–1455. doi: 10.1038/s41590-019-0496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GENG X, WANG X, LUO M, et al. Induction of neutrophil apoptosis by a Bcl-2 inhibitor reduces particulate matter-induced lung inflammation[J] Aging. . 2018;10(6):1415–1423. doi: 10.18632/aging.101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PANG L,ZOU S,SHI Y,et al. Apigenin attenuates PM2.5-induced airway hyperresponsiveness and inflammation by down-regulating NF-κB in murine model of asthma[J]. Int J Clin Exp Pathol,2019,12(10):3700–3709 . [PMC free article] [PubMed]
- 19.SHORE S A. Mechanistic basis for obesity-related increases in ozone-induced airway hyperresponsi- veness in mice[J] Ann ATS. . 2017;14(Supplement_5):S357–S362. doi: 10.1513/AnnalsATS.201702-140AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RAY A, KOLLS J K. Neutrophilic inflammation in asthma and association with disease severity[J] Trends Immunol. . 2017;38(12):942–954. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MATSUSHIMA H, GENG S, LU R, et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells[J] Blood. . 2013;121(10):1677–1689. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ZHAN C, XU R, LIU J, et al. Increased sputum IL-17A level in non-asthmatic eosinophilic bronchitis[J] Lung. . 2018;196(6):699–705. doi: 10.1007/s00408-018-0166-y. [DOI] [PubMed] [Google Scholar]
- 23.WHITEHEAD G S,KANG H S,THOMAS S Y,et al. Therapeutic suppression of pulmonary neutrophilia and allergic airway hyperresponsiveness by a RORγt inverse agonist[J/OL]. JCI Insight,2019,5(14):e125528.DOl:10.1172/jci.insight.125528 . [DOI] [PMC free article] [PubMed]
- 24.WITOWSKI J, KSIĄŻEK K, JÖRRES A. Interleukin-17:a mediator of inflammatory responses[J] Cell Mol Life Sci. . 2004;61(5):567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LUCHERINI O M, LOPALCO G, CANTARINI L, et al. Critical regulation of Th17 cell differentiation by serum amyloid-A signalling in Behcet’s disease[J] Immunol Lett. . 2018;201:38–44. doi: 10.1016/j.imlet.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 26.DIMITROVA D, YOUROUKOVA V, IVANOVA-TODOROVA E, et al. Serum levels of IL-5,IL-6,IL-8,IL-13 and IL-17A in pre-defined groups of adult patients with moderate and severe bronchial asthma[J] Respiratory Med. . 2019;154:144–154. doi: 10.1016/j.rmed.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 27.PAL K, FENG X, STEINKE J W, et al. Leukotriene A4 hydrolase activation and leukotriene B4 production by eosinophils in severe asthma[J] Am J Respir Cell Mol Biol. . 2019;60(4):413–419. doi: 10.1165/rcmb.2018-0175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ELLER M C N, VERGANI K P, SARAIVA-ROMANHOLO B M, et al. Can inflammatory markers in induced sputum be used to detect phenotypes and endotypes of pediatric severe therapy-resistant asthma?[J] Pediatr Pulmonol. . 2018;53(9):1208–1217. doi: 10.1002/ppul.24075. [DOI] [PubMed] [Google Scholar]
- 29.PINTARD C, BEN KHEMIS M, LIU D, et al. Apocynin prevents GM-CSF-induced-ERK1/2 activation and -neutrophil survival independently of its inhibitory effect on the phagocyte NADPH oxidase NOX2[J] Biochem Pharmacol. . 2020;177:113950. doi: 10.1016/j.bcp.2020.113950. [DOI] [PubMed] [Google Scholar]
- 30.HILLIARD K A, BLAHO V A, JACKSON C D, et al. Leukotriene B4 receptor BLT1 signaling is critical for neutrophil apoptosis and resolution of experimental Lyme arthritis[J] FASEB J. . 2020;34(2):2840–2852. doi: 10.1096/fj.201902014R. [DOI] [PubMed] [Google Scholar]
- 31.MCCRACKEN J M, ALLEN L A H. Regulation of human neutrophil apoptosis and lifespan in health and disease[J] J Cell Death. . 2014;7:JCD.S11038. doi: 10.4137/JCD.S11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BARCELLOS-DE-SOUZA P,CANETTI C,BARJA-FIDALGO C,et al. Leukotriene B(4) inhibits neutrophil apoptosis via NADPH oxidase activity: redox control of NF-κB pathway and mitochondrial stability[J]. Biochim Biophys Acta,2012,1823(10):1990–1997.DOl:10.1016/j.bbamcr.2012. 07.012 . [DOI] [PubMed]
- 33.顾晓菲,陈鑫淼,陈慧君,等. S100A8/RAGE、Caveolin-1在中性粒细胞性支气管哮喘大鼠中的作用及罗红霉素对其表达的影响[J]. 中华结核和呼吸杂志,2019,42(11):845–851. DOI: 10.3760/cma.j.issn.1001-0939.2019.11.012 ; GU Xiaofei,CHEN Xinmiao,CHEN Huijun,et al. The role of S100A8/RAGE and Caveolin-1 and the effect of roxithromycin on their expression in a rat model of neutrophilic asthma[J]. Chinese Journal of Tuberculosis and Respiratory Diseases,2019,42(11):845–851. DOI: 10.3760/cma.j.issn.1001-0939.2019.11.012. (in Chinese) . [DOI] [PubMed]
- 34.KIM D H, CHOI E, LEE J S, et al. House dust mite allergen regulates constitutive apoptosis of normal and asthmatic neutrophils via toll-like receptor 4 [J/OL] PLoS One. . 2015;10(5):e0125983. doi: 10.1371/journal.pone.0125983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KIM D H, GU A, LEE J S, et al. Suppressive effects of S100A8 and S100A9 on neutrophil apoptosis by cytokine release of human bronchial epithelial cells in asthma[J] Int J Med Sci. . 2020;17(4):498–509. doi: 10.7150/ijms.37833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WENG Q, ZHU C, ZHENG K, et al. Early recruited neutrophils promote asthmatic inflammation exacerbation by release of neutrophil elastase[J] Cellular Immunol. . 2020;352:104101. doi: 10.1016/j.cellimm.2020.104101. [DOI] [PubMed] [Google Scholar]
- 37.TOUSSAINT M,JACKSON D J,SWIEBODA D,et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation[J]. Nat Med,2017,23(6):681–691.DOl:10.1038/nm. 4332 . [DOI] [PMC free article] [PubMed]
- 38.WEERAPPULI P D, LOUTTIT C, KOJIMA T, et al. Extracellular trap-mimicking DNA‐histone mesostructures synergistically activate dendritic cells[J/OL] Adv Healthcare Mater. . 2019;8(22):1900926. doi: 10.1002/adhm.201900926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MONTESEIRÍN J. Neutrophils and asthma[J]. J Investig Allergol Clin Immunol,2009,19(5):340–354 . [PubMed]
- 40.GRZELA K, LITWINIUK M, ZAGORSKA W, et al. Airway remodeling in chronic obstructive pulmonary disease and asthma:the role of matrix metallopro- teinase-9[J] Arch Immunol Ther Exp. . 2016;64(1):47–55. doi: 10.1007/s00005-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SIMPSON J L, SCOTT R J, BOYLE M J, et al. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma[J] Am J Respir Crit Care Med. . 2005;172(5):559–565. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 42.CUI H, HUANG J, LU M, et al. Antagonistic effect of vitamin E on nAl2O3-induced exacerbation of Th2 and Th17-mediated allergic asthma via oxidative stress[J] Environ Pollut. . 2019;252:1519–1531. doi: 10.1016/j.envpol.2019.06.092. [DOI] [PubMed] [Google Scholar]
- 43.LI Y, ZHANG L, WANG X, et al. Effect of Syringic acid on antioxidant biomarkers and associated inflam- matory markers in mice model of asthma[J] Drug Dev Res. . 2019;80(2):253–261. doi: 10.1002/ddr.21487. [DOI] [PubMed] [Google Scholar]
- 44.KIM S H, UUGANBAYAR U, TRINH H K T, et al. Evaluation of neutrophil activation status according to the phenotypes of adult asthma[J] Allergy Asthma Immunol Res. . 2019;11(3):381. doi: 10.4168/aair.2019.11.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.KEATINGS V M, BARNES P J. Granulocyte activation markers in induced sputum:comparison between chronic obstructive pulmonary disease,asthma,and normal subjects[J] Am J Respir Crit Care Med. . 1997;155(2):449–453. doi: 10.1164/ajrccm.155.2.9032177. [DOI] [PubMed] [Google Scholar]
- 46.ALI I, KHAN S N, CHATZICHARALAMPOUS C, et al. Catalase prevents myeloperoxidase self-destruction in response to oxidative stress[J] J Inorg Biochem. . 2019;197:110706. doi: 10.1016/j.jinorgbio.2019.110706. [DOI] [PubMed] [Google Scholar]
- 47.MOSCHONAS I C, TSELEPIS A D. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis[J] Atherosclerosis. . 2019;288:9–16. doi: 10.1016/j.atherosclerosis.2019.06.919. [DOI] [PubMed] [Google Scholar]
- 48.LACHOWICZ-SCROGGINS M E, DUNICAN E M, CHARBIT A R, et al. Extracellular DNA,neutrophil extracellular traps,and inflammasome activation in severe asthma[J] Am J Respir Crit Care Med. . 2019;199(9):1076–1085. doi: 10.1164/rccm.201810-1869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MURCIA R Y, VARGAS A, LAVOIE J P. The interleukin-17 induced activation and increased survival of equine neutrophils is insensitive to glucocorticoids [J/OL] PLoS One. . 2016;11(5):e0154755. doi: 10.1371/journal.pone.0154755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.张星慧,常晓悦. 中性粒细胞性哮喘与其相关细胞因子[J]. 国际呼吸杂志,2017,37(23):1815–1818. DOI:10.3760/cma.j.issn.1673-436X.2017.23.011 ; ZHANG Xinghui,CHANG Xiaoyue. Neutrophilic asthma and other cytokines[J]. International Journal of Respiration,2017,37(23): 1815–1818. DOI: 10.3760/cma.j.issn.1673-436X.2017.23. 011. (in Chinese)
- 51.RODRÍGUEZ-CERDEIRA C,GONZÁLEZ-CESPÓN J L,MARTÍNEZ-HERRERA E,et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management[J]. G Ital Dermatol Venereol,2020. DOI: 10.23736/S0392-0488.20. 06580-3 . [DOI] [PubMed]
- 52.LIANG L, HUR J, KANG J Y, et al. Effect of the anti-IL-17 antibody on allergic inflammation in an obesity-related asthma model[J] Korean J Intern Med. . 2018;33(6):1210–1223. doi: 10.3904/kjim.2017.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.BUSSE W W, HOLGATE S, KERWIN E, et al. Randomized,double-blind,placebo-controlled study of brodalumab,a human anti–IL-17 receptor monoclonal antibody,in moderate to severe asthma[J] Am J Respir Crit Care Med. . 2013;188(11):1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 54.STATON T L, PENG K, OWEN R, et al. A phase I,randomized,observer-blinded,single and multiple ascending-dose study to investigate the safety,phar- macokinetics,and immunogenicity of BITS7201A,a bispecific antibody targeting IL-13 and IL-17,in healthy volunteers[J] BMC Pulm Med. . 2019;19(1):5. doi: 10.1186/s12890-018-0763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.TODD C M, SALTER B M, MURPHY D M, et al. The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects[J] Pulm Pharmacol Ther. . 2016;41:34–39. doi: 10.1016/j.pupt.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 56.NAIR P,GAGA M,ZERVAS E,et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized,placebo-controlled clinical trial[J]. Clin Exp Allergy,2012,42(7):1097–1103. DOl:10.1111/j.1365-2222.2012.04014.x . [DOI] [PubMed]
- 57.WATZ H,UDDIN M,PEDERSEN F,et al. Effects of the CXCR2 antagonist AZD5069 on lung neutrophil recruitment in asthma[J]. Pulm Pharmacol Ther,2017,45:121–123. DOl:10.1016/j.pupt.2017.05.012 . [DOI] [PubMed]
- 58.O’BYRNE P M, METEV H, PUU M, et al. Efficacy and safety of a CXCR2 antagonist,AZD5069,in patients with uncontrolled persistent asthma:a randomised,double-blind,placebo-controlled trial[J] Lancet Respiratory Med. . 2016;4(10):797–806. doi: 10.1016/S2213-2600(16)30227-2. [DOI] [PubMed] [Google Scholar]
- 59.AN T J, RHEE C K, KIM J H, et al. Effects of macrolide and corticosteroid in neutrophilic asthma mouse model[J] Tuberc Respir Dis. . 2018;81(1):80. doi: 10.4046/trd.2017.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.PANETTIERI R A JR. The role of neutrophils in asthma[J]. Immunol Allergy Clin North Am,2018,38(4):629–638. DOl:10.1016/j.iac.2018.06.005 . [DOI] [PubMed]
- 61.LOVERDOS K, BELLOS G, KOKOLATOU L, et al. Lung microbiome in asthma:current perspectives[J] JCM. . 2019;8(11):1967. doi: 10.3390/jcm8111967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.GIBSON P G, YANG I A, UPHAM J W, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES):a randomised,double-blind,placebo-controlled trial[J] Lancet. . 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]