Abstract
We show that the yeast TFIID (yTFIID) component yTAFII47 contains a histone fold domain (HFD) with homology to that previously described for hTAFII135. Complementation in vivo indicates that the yTAFII47 HFD is necessary and sufficient for vegetative growth. Mutation of highly conserved residues in the α1 helix of the yTAFII47 HFD results in a temperature-sensitive phenotype which can be suppressed by overexpression of yTAFII25, as well as by yTAFII40, yTAFII19, and yTAFII60. In yeast two-hybrid and bacterial coexpression assays, the yTAFII47 HFD selectively heterodimerizes with yTAFII25, which we show contains an HFD with homology to the hTAFII28 family We additionally demonstrate that yTAFII65 contains a functional HFD which also selectively heterodimerizes with yTAFII25. These results reveal the existence of two novel histone-like pairs in yTFIID. The physical and genetic interactions described here show that the histone-like yTAFIIs are organized in at least two substructures within TFIID rather than in a single octamer-like structure as previously suggested. Furthermore, our results indicate that ySPT7 has an HFD homologous to that of yTAFII47 which selectively heterodimerizes with yTAFII25, defining a novel histone-like pair in the SAGA complex.
Transcription factor TFIID, one of the general factors required for accurate and regulated initiation by RNA polymerase II, comprises the TATA binding protein and TATA binding protein-associated factors (TAFIIs) (4, 15). The cDNAs encoding many human TAFIIs (hTAFIIs) have been isolated, revealing a striking sequence conservation with yeast TAFIIs (yTAFIIs) and Drosophila TAFIIs (dTAFIIs). A subset of TAFIIs are present not only in TFIID but also in the SAGA, PCAF, STAGA, and TFTC complexes (7, 13, 23, 27, 36).
Genetic studies with yeast have shown that TAFIIs play an important role in transcriptional regulation of many genes (14). Temperature-sensitive mutations in yTAFII145 and yTAFII90 result in cell cycle arrest and lethality, but the expression of only a small number of genes is affected (3, 35). In contrast, tight temperature-sensitive mutations in yTAFII17, yTAFII25, yTAFII60, and yTAFII61/68, which are present in the TFIID and SAGA complexes, or in the TFIID-specific yTAFII40 have a more dramatic effect, the transcription of the majority of yeast genes being affected (2, 21, 24–26, 29).
Initial sequence alignments indicated that hTAFII80 (corresponding to dTAFII60 and yTAFII60), hTAFII31 (dTAFII40 and yTAFII17), and hTAFII20 (dTAFII30α and yTAFII61/68) presented obvious sequence similarity to histones H4, H3, and H2B, respectively (17, 20). Structural studies show that dTAFII60 and dTAFII40 interact via a histone fold and form an H3-H4-like heterotetramer (37). These findings, together with biochemical experiments and genetic interaction data obtained with yeasts, led to the proposal that TFIID and the other TAFII-containing complexes contain a histone octamer-like substructure composed of an hTAFII80-hTAFII31 heterotetramer and two hTAFII20 homodimers (8, 16).
Subsequent data show this model to be an oversimplification. hTAFII28 and hTAFII18 are also histone-like, since they interact via a histone fold domain (HFD) to form a heterodimer (5). The SAGA, PCAF, TFTC, and STAGA component SPT3 shows extensive sequence homology to the HFDs of both hTAFII18 and hTAFII28 in its N- and C-terminal regions, respectively, and could potentially form a histone-like pair by intramolecular interactions. Contrary to what was first suggested, hTAFII20 does not homodimerize but rather heterodimerizes with hTAFII135 (10). In yeasts, the hTAFII20 homologue yTAFII68 heterodimerizes with the SAGA component yADA1, and it has been suggested that yTAFII68 may also heterodimerize with yTAFII48, a potential homologue of hTAFII135, in yTFIID (28, 30). These results indicate that there are many more histone-like pairs in TFIID and SAGA than originally suspected. Recent electron microscopy studies show that TFIID comprises three or four lobes arranged in a horseshoe fashion around a central groove (1, 6). Within the present limits of resolution it appears that no single lobe of TFIID would be big enough to harbor all the known histone fold TAFIIs, suggesting that they are shared among two or more of the lobes.
We previously reported that hTAFII135 contained an HFD with significant sequence homology to the SAGA component yADA1 (10). Now we show that the hTAFII135 HFD also shares significant sequence homology with an HFD in yTAFII47 (34). In complementation experiments, the yTAFII47 HFD is necessary and sufficient for vegetative yeast growth. The temperature-sensitive phenotype of a mutation in the yTAFII47 HFD can be rescued by overexpression of yTAFII25 and, at less restrictive temperatures, by yTAFII60, yTAFII40, and yTAFII19. In yeast two-hybrid and bacterial coexpression experiments, the yTAFII47 HFD mediates selective heterodimerization with the conserved core domain of yTAFII25. There are therefore both genetic and physical interactions between these two yTAFIIs, suggesting that they form an additional histone-like pair in yTFIID. Consistent with this idea, we show the conserved core domain of yTAFII25 to be an HFD which shares homology with the HFD of the hTAFII28 family.
Recently, yTAFII65 was identified as a novel component of yTFIID (30). Now we demonstrate that yTAFII65 contains an HFD with homology to that of yTAFII17, dTAFII40, and hTAFII31. In contrast to the HFD of yTAFII47, the yTAFII65 HFD is not sufficient for growth. Nevertheless, deletion or mutation of this domain results in temperature sensitivity, showing that it is important for yTAFII65 function. Surprisingly, the yTAFII65 HFD also selectively heterodimerizes with yTAFII25, which thus has two heterodimerization partners in TFIID.
Finally, as yTAFII47 and yTAFII65 are not present in SAGA, we sought a heterodimerization partner for yTAFII25 in this complex. Our results indicate that ySPT7 contains an HFD with homology to that of yTAFII47. This domain mediates selective heterodimerization with yTAFII25. Together our results reveal the existence of novel histone-like pairs in the TFIID and SAGA complexes. They highlight the important functional and structural role played by this motif in these complexes and provide evidence that the histone-like yTAFIIs assemble into at least two distinct substructures within TFIID.
MATERIALS AND METHODS
Yeast strains.
The yeast strains used in this study are YSLS67 (Mata ura3-1 leu2-3,112 trp1-1 his3-11,15 ade2-11 can1-100 taf47Δhisg-hisg [pRS416-TAF47]), YSLS67/47 (MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 ade2-11 can1-100 taf47Δhisg-hisg [pAS3-TAF47)], YSLS67/47HFD (MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 ade2-11 can1-100 taf47Δhisg-hisg [pAS3-TAF47(1–81)]), YSLS67/47m1 (MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 ade2-11 can1-100 taf47Δhisg-hisg [pAS3-TAF47(R13D, I14E)]), YSLS67/VP16AD47HFD (MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 ade2-11 can1-100 taf47Δhisg-hisg [pASV3-TAF47(1–81)]), YSLS58 (MATa ura3Δ0 leu2Δ0 his3Δ1 lys2Δ0 MET15 KAN Δtaf65 [pRS416-TAF65]), YSLS58/65 (MATa ura3Δ0 leu2Δ0 his3Δ1 lys2Δ0 MET15 KAN Δtaf65 [pAS3-TAF65]), YSLS58/65ΔHFD (MATa ura3Δ0 leu2Δ0 his3Δ1 lys2Δ0 MET15 KAN Δtaf65 [pAS3-TAF65(103–510)]), YSLS58/65m1 (MATa ura3Δ0 leu2Δ0 his3Δ1 lys2Δ0 MET15 KAN Δtaf65 [pAS3-TAF65(L64P, L67P)]), YSLS58/VP16AD65 (MATa ura3Δ0 leu2Δ0 his3Δ1 lys2Δ0 MET15 KAN Δtaf65 [pASV3-TAF65]), and L40 [MATa trp1-901 leu2-3,112 his3-Δ200 ade2 LYS2::(LexAop)4-HIS3 URA3::(LexAop)8-Lac].
Construction of recombinant plasmids.
All yeast and bacterial expression vectors were constructed by PCR using primers with the appropriate restriction sites, and constructs were verified by automated DNA sequencing. Details of constructions are available on request. LexA fusions were constructed in the multicopy vector pBTM116 containing the TRP1 marker, and the VP16 fusions were constructed in the multicopy vector pASV3 containing the LEU2 marker (10). For complementation, wild-type or mutated yTAFIIs were cloned in the multicopy pAS3 plasmid with a LEU− marker.
Two-hybrid, complementation, and high-copy-number temperature-sensitive suppression assays.
All yeast strains were transformed by the lithium acetate technique. For two-hybrid assays, transformants were selected on Trp− Leu− plates. Quantitative β-galactosidase assays on individual L40 transformants were determined as previously described (10). Reproducible results were obtained in several independent experiments, and the results of a typical experiment are shown in the figures. Yeast strains YSLS67 and YSLS58, used for plasmid shuffling of TAF47 and TAF65, were derived from YJR10 (34) and YSLS41 (30) by sporulation and tetrad dissection. For complementation assays, the rescue plasmids indicated in the relevant figures were transformed and the wild-type TAF/URA3 plasmid was shuffled out by two passes on media containing 5-fluoroorotic acid. For suppression of the yTAFII47(R13D, I14E) mutant strain, cells were transformed with high-copy-number plasmids with a URA3 marker expressing the indicated yTAFIIs and serial dilutions of the transformants were spotted at 30, 34, and 36°C. Plates at the restrictive temperatures were photographed after 3 days of growth. In all experiments cultures were grown in yeast extract-peptone-dextrose unless selection was necessary, in which case all cultures were grown in the appropriate selective synthetic dextrose (SD) medium.
Coexpression in Escherichia coli.
Coexpression in E. coli was performed as previously described (9a, 10). All plasmids were constructed by PCR, and details are available on request. The yTAFII47, yTAFII65, and SPT7 histone fold regions were expressed as glutathione S-transferase (GST) fusion proteins in pGEX2T. Native untagged, yTAFII25, hTAFII30, and yTAFII68 HFDs were expressed from a modified version of the vector pACYC184 (New England Biolabs). Plasmids pairs were introduced into E. coli strain BL21(DE3), and double transformants were selected on plates containing ampicillin and chloramphenicol. Bacteria were amplified to an optical density at 600 nm of 0.45 and induced for 4 h at 25°C with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cell were lysed by sonication in buffer (25 mM Tris-HCl [pH 6.0] and 0.4 M NaCl), and the soluble fraction was collected after centrifugation at 14,000 rpm for 20 min at 4°C in an Eppendorf centrifuge. Aliquots of the soluble fraction from a 10-ml bacterial culture were then incubated with glutathione-Sepharose (Pharmacia). Binding and washing were done essentially as described previously (10). Bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and staining with Coomassie brilliant blue.
RESULTS
yTAFII47 contains an HFD which suffices for vegetative growth.
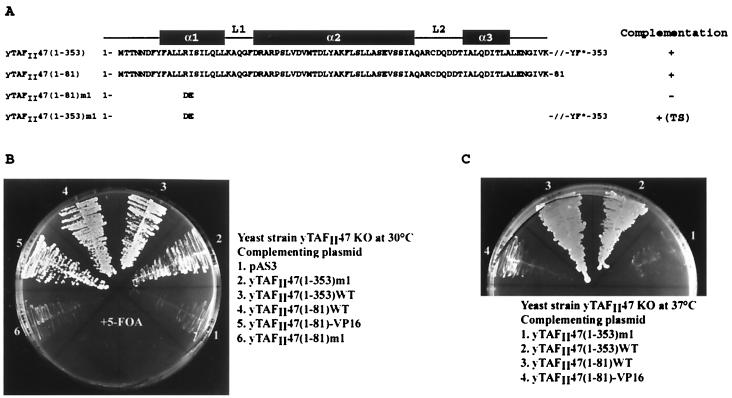
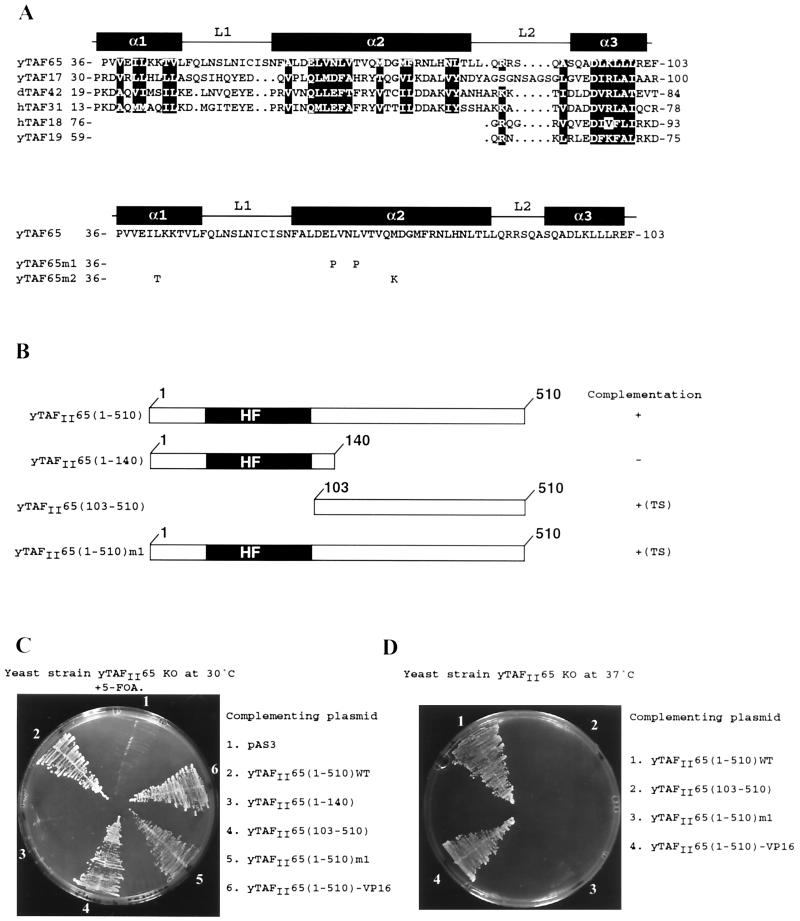
Previously we reported that hTAFII135 amino acids 870 to 944 showed significant sequence homology to H2A, NC2α and yADA1 (10) (Fig. 1). The hTAFII135 HFD also shows significant homology to yTAFII48, the hTAFII135 homologue in yTFIID (28, 30) (Fig. 1). Database searches also revealed a significant similarity between the HFD of the TAFII135 family and the N-terminal region of yTAFII47 (Fig. 1). In the yTAFII47 HFD, the conserved RI(V, M) residues are found in the α1 helix, followed by an amphipathic α2 helix with numerous conserved hydrophobic residues. The α3 helix is characterized by the presence of D(V, I, L) residues. This pattern of sequence conservation is analogous to that seen amongst other histone fold proteins (5, 10, 32). Several metazoan sequences encoding potential proteins with homology to the yTAFII47 HFD were also detected in these searches (Fig. 1) (32). This observation indicates that yTAFII47 is a histone-like protein belonging to an evolutionarily conserved family. In addition to the α1, α2, and α3 helices, which comprise the minimal HFD, there is a potential additional αC helix in this family.
FIG. 1.
Alignment of the HFD sequences of the hTAFII135 family with those of ADA1 and the yTAFII47 family. h, human; y, Saccharomyces cerevisiae; d, Drosophila melanogaster; S.pombe, Schizosaccharomyces pombe; Anopheles, Anopheles gambiae; mouse, Mus musculus; Candida, Candida albicans; zebra fish, Danio rerio. The positions of the predicted α helices and loops are indicated above the sequence based on homology with H2A (10, 22). Positions with conserved, mainly hydrophobic, amino acids are in white on a black background. Other residues conserved within the yTAFII47 family are boxed in gray. Amino acids were classified as follows: small residues, P, A, G, S, and T; hydrophobic residues, L, I, V, A, F, M, C, Y, and W; polar and/or acidic residues, D, E, Q, and N; basic residues, R, K, and H. Threonine residues are occasionally present in otherwise hydrophobic positions. The amino acids sequences shown without numbers are predicted from genomic, expressed sequence tag, or sequence tagged site sequences. The accession numbers for the indicated sequences are as follows: S. pombe, SPT:CAB90151; Candida, 396380B03; Anopheles, GB CN501GI9 AL143170; zebra fish, GB AW343321fi76b06.y1; mouse, GB AA692266ur52c07.
To determine whether the yTAFII47 HFD is important for function, we performed complementation experiments by plasmid shuffle in a yTAFII47 null strain. Expression vectors for wild-type or mutated yTAFII47 proteins were constructed (Fig. 2A), and their ability to rescue growth of the null strain was evaluated. As previously described, yTAFII47 is essential for vegetative yeast growth (34). Expression of full-length yTAFII47 efficiently restored the growth of the null strain at 30°C, whereas no growth was seen with the expression vector alone (1 and 3 in Fig. 2B). Growth was also rescued by yTAFII47(1–81) containing only the minimal HFD (construct 4 in Fig. 2B). These two strains showed comparable growth rates in liquid culture (data not shown). This 1–81 domain rescued growth both when expressed as a native protein and when expressed as a fusion with the VP16 activating domain from a two-hybrid expression vector (construct 5 in Fig. 2B). Mutation of the highly conserved amino acids R13 and I14 in the α1 helix (construct m1 in Fig. 2A) abolished the ability of yTAFII47(1–81) to rescue growth (construct 6 in Fig. 2B). In contrast, this mutation did not abolish yTAFII47 function in the context of the full-length protein (construct 2 in Fig. 2B). The (1–353)m1 mutant did, however, show a temperature-sensitive phenotype, as it did not rescue growth at 37°C while both the wild-type protein and the 1–81 deletion rescued growth at this temperature (compare constructs 1 and 3 in Fig. 2C). The VP16-TAFII47(1–81) fusion also showed a temperature-sensitive phenotype (construct 4 in Fig. 2C). These results indicate that the yTAFII47 HFD is an essential functional domain necessary and sufficient for vegetative yeast growth.
FIG. 2.
The yTAFII47 HFD is sufficient for growth. (A) The sequence of the yTAFII47 HFD is shown along with that of mutant m1. The locations of the potential α helices and loops are indicated above the sequence. The ability of each mutant to complement the null strain is indicated on the right. TS, temperature sensitive. (B and C) Growth of yeasts plated at the indicated temperatures. 5-FOA, 5-fluoroorotic acid.
Genetic interaction between yTAFII47 and other histone-like yTAFIIs.
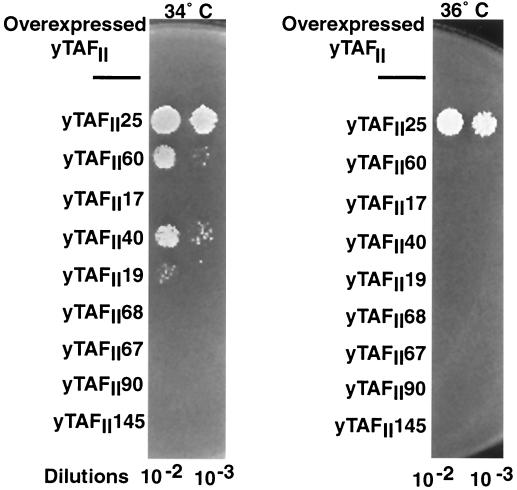
Possible genetic interactions between yTAFII47 and other yTAFIIs were examined. To look for such interactions, we tested the ability of other yTAFIIs to rescue the temperature-sensitive phenotype of the yTAFII47(1–353)m1 allele when overexpressed at the nonpermissive temperature.
The yTAFII47(1–353)m1 strain did not grow at 34°C (Fig. 3). At 34°C, the temperature-sensitive phenotype was efficiently suppressed by overexpression of yTAFII25 and partially suppressed by overexpression of yTAFII60, yTAFII40, and yTAFII19 (Fig. 3). At 36°C, however, only yTAFII25 could suppress the temperature-sensitive phenotype (Fig. 3). No significant growth was seen when any of the other yTAFIIs were overexpressed at either temperature (Fig. 3), while all strains showed equivalent growth at 30°C (data not shown). Analogous results were obtained with the yTAFII47(1–81)-VP16 allele (data not shown). These results show a genetic interaction between yTAFII47 and three other known histone-like yTAFIIs, yet the strongest interaction was observed with yTAFII25, which has not previously been described as histone-like.
FIG. 3.
Genetic interactions among histone-like yTAFIIs. The growth of serial dilutions of strains with the yTAFII47(1–353)m1 allele at 34 and 36°C is shown. The overexpressed yTAFIIs used to rescue the growth at the nonpermissive temperatures are shown on the left.
The HFD of yTAFII47 mediates heterodimerization with yTAFII25.
The strong genetic interaction between yTAFII47 and yTAFII25 prompted us to determine whether they also interact physically. The yTAFII47 HFD was fused to the VP16 activation domain or the LexA DNA binding domain and tested for interactions with LexA or VP16 fusions of the core domain of yTAFII25 and the HFDs of other yTAFIIs in a series of yeast two-hybrid experiments. Interactions were assessed by measuring β-galactosidase activity in strain L40, which harbors a LexA-responsive LacZ gene (10, 33).
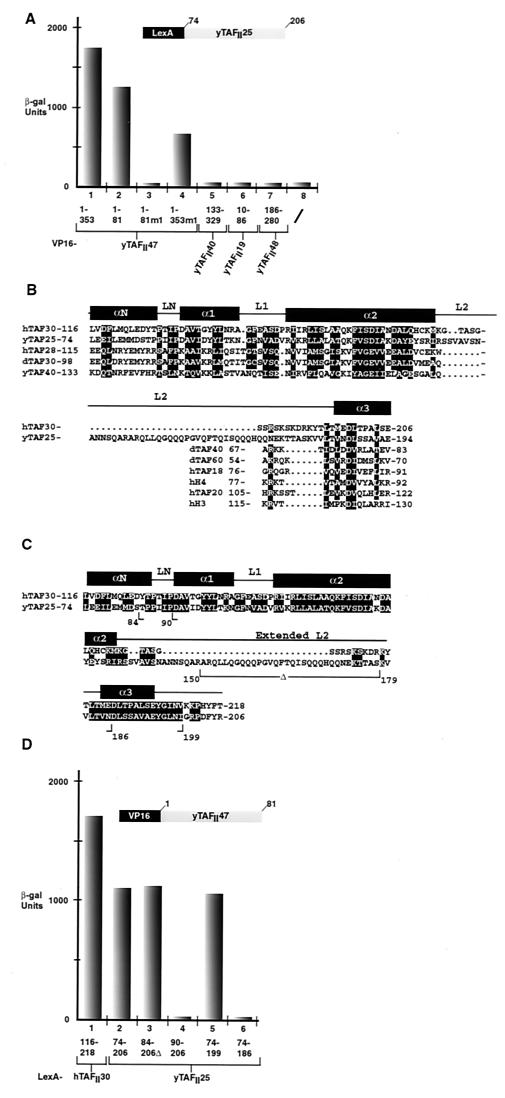
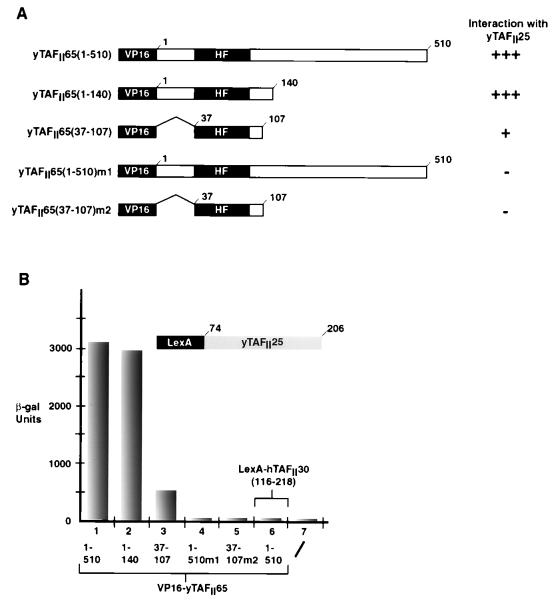
In two-hybrid assays, a strong interaction between yTAFII47 and yTAFII25(74–206) was observed (Fig. 4A, column 1). This interaction required only the HFD of yTAFII47(1–81) and was abolished by mutation m1 in the α1 helix (Fig. 4A, columns 2 and 3). In the context of the full-length yTAFII47, the m1 mutation reduced but did not abolish the interaction (Fig. 4A, column 4). In contrast, we detected no interactions between the HFD of yTAFII47 and the HFDs of yTAFII40 or yTAFII19 (which themselves strongly interact in two-hybrid assays [data not shown]), yTAFII60, yTAFII68, yTAFII48, and yTAFII65 (summarized in Table 1). Moreover, we did not observe homodimerization of yTAFII47. Similarly, with the exception of yTAFII65 (see below), yTAFII25 did not interact with the HFDs of the other yTAFIIs tested (Fig. 4A, columns 5 to 7, and Table 1), although a possible homodimerization was seen (Table 1; also, see Discussion). These results indicate that yTAFII47 selectively heterodimerizes with yTAFII25.
FIG. 4.
Selective heterodimerization between yTAFII47 and yTAFII25 in two-hybrid assays. (A) Quantification of β-galactosidase activity in two-hybrid assays. The VP16-yTAFII47, VP16-yTAFII40, VP16-yTAFII19, and yTAFII48 fusions shown below each column were assayed in a LexA-yTAFII25(74–206) background as indicated above the graph. β-gal, β -galactosidase. (B) Alignment of yTAFII25 and hTAFII30 with members of the hTAFII28 family from yeast and D. melanogaster. Conserved positions are in white against a black background. The positions of the α helices and loops of hTAFII28 are indicated. Alignment of the α3 helix of yTAFII25 and hTAFII30 with that of the other indicated histone fold proteins is also shown. (C) The sequences of the conserved region of yTAFII25 and hTAFII30 are shown. Conserved amino acids are white on a black background. The end points of the deletions tested in two-hybrid assays are indicated by the arrows below the sequence. Δ, internal deletion. (D) Mapping of the yTAFII25 region required for interaction with yTAFII47. The LexA-yTAFII25 (LexA-hTAFII30) deletions indicated below the graph were assayed in the VP16-yTAFII47(1–81) strain.
TABLE 1.
Interactions between TFIID components and various HFDs
| Complex | Histone fold | Interaction with HFDa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TFIID
|
TFIID + SAGA
|
||||||||
| yTAFII47 | yTAFII48 | yTAFII65 | yTAFII40 | yTAFII19 | yTAFII25 | yTAFII68 | yTAFII60 | ||
| TFIID | yTAFII47 | − | − | − | − | − | +++ | − | − |
| yTAFII65 | − | − | − | − | − | +++ | − | − | |
| TFIID + SAGA | yTAFII25 | +++ | − | +++ | − | − | +? | − | ND |
−, no interaction; +?, possible homodimerization; +++, strong interaction; ND, not determined.
Sequence alignments have shown that hTAFII30, yTAFII25, and their homologues from other species have a bipartite structure with a highly conserved C-terminal domain and an unconserved N-terminal region (12) (Fig. 4C). In yTAFII25, it is the conserved C-terminal region (amino acids 74 to 206) which mediates interaction with the yTAFII47 HFD (Fig. 4A and D, column 2). We therefore compared this region of yTAFII25 to the other known histone-like TAFIIs. In doing this, we noted a significant similarity between the sequences of yTAFII25/hTAFII30 and the HFD of the hTAFII28 family of proteins (Fig. 4B). This similarity predicted the existence of potential αN, α1, and α2 helices within yTAFII25 and hTAFII30, the nonconserved sequence corresponding to an insertion in the L2 loop of yTAFII25 (Fig. 4B). In contrast, the proposed α3 helix of yTAFII25 contains the conserved D(V, I, L) pair and shows better homology to several other known histone-like proteins than to hTAFII28.
We next tested the effect of deleting various regions of the yTAFII25 HFD on heterodimerization with yTAFII47 (Fig. 4C). Deletion of the αN and a large part of the extended L2 loop [yTAFII25(84–206)Δ] had no effect on interaction (Fig. 4D, column 3). However, deletion of the LN led to a loss of interaction despite the fact that the proposed α1 remained in this construct [yTAFII25(90–206)] (Fig. 4D, column 4). At the C terminus, interaction with yTAFII47 was not affected by deletion up to amino acid 199, leaving intact the proposed α3 helix [yTAFII25(74–199)] (Fig. 4D, column 5), whereas interaction was abolished when the α3 was truncated (74–186) (column 6). The conserved C-terminal domain of hTAFII30 interacted with yTAFII47 as efficiently as yTAFII25 [hTAFII30(116–218) and yTAFII25(74–206)] (Fig. 4D, columns 1 and 2). Together, these results indicate that the conserved C-terminal domain of the yTAFII25 family contains an HFD which heterodimerizes with yTAFII47.
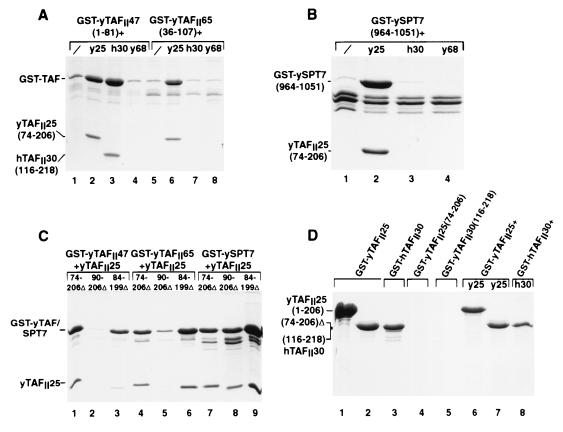
To observe direct heterodimerization of yTAFII47 and yTAFII25, these proteins were coexpressed in E. coli. A native version of yTAFII25(74–206) or hTAFII30(116–218) was coexpressed with a GST fusion of the yTAFII47 HFD. When expressed alone, the GST-yTAFII47 fusion is largely insoluble and little soluble protein is recovered on the glutathione-Sepharose beads (Fig. 5A, lane 1). In contrast, coexpression with the yTAFII25 or hTAFII30 HFD solubilizes GST-yTAFII47, which is retained in the form of a complex with each of these proteins on the beads (Fig. 5A, lanes 2 and 3). At first, the GST-yTAFII47 chimera appears to be more abundant than the untagged yTAFII25 or hTAFII30 protein. However, the GST moiety of the chimera stains strongly with Coomassie brilliant blue. When this disproportionate staining is taken into account, the yTAFII47-yTAFII25 ratio would be closer to the 1:1 ratio expected for a heterodimeric complex. This is a selective heterodimerization, since neither solubilization nor complex formation was seen when GST-yTAFII47(1–81) was coexpressed with the HFD of yTAFII68 (lane 4), previously shown to heterodimerize with yADA1 in this assay (10), yTAFII40, or yTAFII19 (data not shown). In control experiments, the native yTAFII25(74–206) and hTAFII30(116–218) proteins were insoluble when expressed alone and were not retained on glutathione beads (Fig. 5D, lanes 4 and 5). These results confirm the direct heterodimerization of the TFIID components yTAFII47 and yTAFII25, indicating that they form a novel histone-like pair.
FIG. 5.
Coexpression of yTAFII47 and yTAFII65 with yTAFII25 in E. coli. (A) Bacteria were transformed to express the proteins shown above each lane. Following extract preparation, the soluble protein retained on glutathione-Sepharose beads was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and staining with Coomassie brilliant blue. The locations of the GST-yTAFII fusions and the retained yTAFII25 and hTAFII30 proteins are indicated. (B) yTAFII25-ySPT7 heterodimerization in E. coli. The locations of the GST-ySPT7 fusion and yTAFII25 are shown. (C) Heterodimerization with yTAFII25 deletion mutants. The GST-yTAFII47, GST-yTAFII65, and GST-SPT7 proteins were coexpressed with the untagged yTAFII25 deletion mutants as indicated. The soluble proteins retained on glutathione-Sepharose beads are shown. (D) Lack of evidence for yTAFII25 and hTAFII30 homodimerization. The GST fusions of yTAFII25 and hTAFII30 were expressed alone or in combination with the native HFDs indicated above each lane as in panel A.
Solubilization and complex formation were also observed when GST-yTAFII47(1–81) was expressed with yTAFII25(74–206)Δ in which the extended L2 loop has been deleted (Fig. 5C, lane 1). A similar result was seen with yTAFII25(84–199)Δ, whereas almost no soluble complex was observed with yTAFII25(90–206)Δ (Fig. 5C, lanes 2 and 3). Thus, as observed in the two-hybrid experiments, yTAFII47 and yTAFII25 heterodimerization does not require the extended L2 loop but does require the LN region.
yTAFII65 contains a functional HFD which mediates selective heterodimerization with yTAFII25.
The above results reveal the presence of considerably more histone fold proteins in TFIID than originally suspected. This prompted us to examined the sequences of other yTAFIIs for the presence of potential HFDs. Analysis of the novel yTFIID subunit yTAFII65 (30) indicates the presence of a potential HFD with similarity to yTAFII17/dTAFII40/hTAFII31 between amino acids 37 and 103 at the N terminus of the protein (Fig. 6A). To determine whether this is a functional domain of the protein, we tested the ability of yTAFII65 mutants to complement the growth of the yTAFII65 null strain.
FIG. 6.
yTAFII65 contains a histone fold motif. The sequence of yTAFII65 is aligned with the sequences of members of the yTAFII17 family. The conserved positions are white against a black background, and the positions of the α helices and loops are indicated. The sequences of the m1 and m2 mutants are indicated below the wild-type sequence. (B) The structures of the yTAFII65 mutants used in complementation experiments are schematized. The HFD is depicted as a black box. TS, temperature sensitive. (C and D) Growth of yeasts plated at the indicated temperatures. 5-FOA, 5-fluoroorotic acid.
Deletions or mutations in the yTAFII65 HFD were generated (Fig. 6A and B) and used to complement the yTAFII65 null strain. At 30°C, growth was seen with the full-length protein, whereas no growth was seen when the null strain was complemented with yTAFII65(1–140) containing only the HFD (Fig. 6C, constructs 2 and 3). Surprisingly, growth at 30°C was also seen using the deletion 103–510, in which the HFD is deleted, and with mutant (1–510)m1, which contains a double amino acid substitution in the α2 helix (Fig. 6C, constructs 4 and 5). These two strains were, however, temperature sensitive, since they did not grow at 37°C, while growth was seen with the wild-type protein (Fig. 6D, constructs 1 to 4). Therefore, in contrast to yTAFII47, the yTAFII65 HFD is not sufficient for vegetative growth. The HFD is nevertheless an important functional domain at 37°C, since its deletion or mutation generates a temperature-sensitive phenotype.
We next tested the ability of yTAFII65(1–140) to heterodimerize with the HFDs of other yTAFIIs in the two-hybrid assay. Surprisingly, a selective interaction was seen only with yTAFII25 (Table 1 and Fig. 7B, column 2). Interaction with yTAFII25 was seen with full-length yTAFII65 (Fig. 7B, column 1), and this interaction was abolished by mutation m1, which also generated a temperature-sensitive phenotype (Fig. 7A, column 4). Interaction with the yTAFII65 HFD alone was reduced compared to yTAFII65(1–510) or (1–140) [yTAFII65(37–107) (Fig. 7A, column 3). Nevertheless, interaction with yTAFII25 was totally abolished when two amino acid changes were introduced into the α1 and α2 helices of the yTAFII65 HFD [yTAFII65(37–103)m2 in Fig. 6A and 7B, column 5]. Interaction with yTAFII65 required the conserved C-terminal domain of yTAFII25 that was required for interaction with yTAFII47 (data not shown). However, in contrast to yTAFII47, yTAFII65 does not interact with hTAFII30 (Fig. 7B, column 6).
FIG. 7.
The histone fold of yTAFII65 is required for heterodimerization with yTAFII25. (A) Structures of the yTAFII65 deletion mutants used in the two-hybrid assays. (B) The VP16-yTAFII65 deletions indicated below the graph were assayed in the LexA-yTAFII25(74–206) strain. For column 6, yTAFII65 was assayed in the LexA-hTAFII30(116–218) strain. β-gal, β-galactosidase.
As described above for yTAFII47, direct yTAFII65-yTAFII25 heterodimerization was verified by coexpression in E. coli. The yTAFII65(36–107) HFD was fused to GST and expressed either alone or in combination with the HFD of yTAFII25, hTAFII30, or yTAFII68. As observed for yTAFII47, the GST-yTAFII65 protein was essentially insoluble when expressed alone, while solubilization and complex formation were observed when GST-yTAFII65 was coexpressed with yTAFII25(74–206) (Fig. 5A, lanes 5 and 6). In agreement with the two-hybrid assay data, heterodimerization was also observed with yTAFII25(74–206)Δ and yTAFII25(84–199)Δ but was strongly reduced with (90–206)Δ (Fig. 5C, lanes 4 to 6). Similarly, no complex was formed with the hTAFII30 core domain (Fig. 5A, lane 7), and in an additional control no heterodimerization was seen with the yTAFII68 HFD (Fig. 5A, lane 8). Therefore, while yTAFII47 can heterodimerize with yTAFII25 or hTAFII30, heterodimerization with yTAFII65 is selective for yTAFII25 and is not seen with the closely related hTAFII30. These results indicate that the TFIID component yTAFII65 selectively and directly heterodimerizes with yTAFII25 to form an additional histone-like pair.
yTAFII25 heterodimerizes with the SAGA component ySPT7.
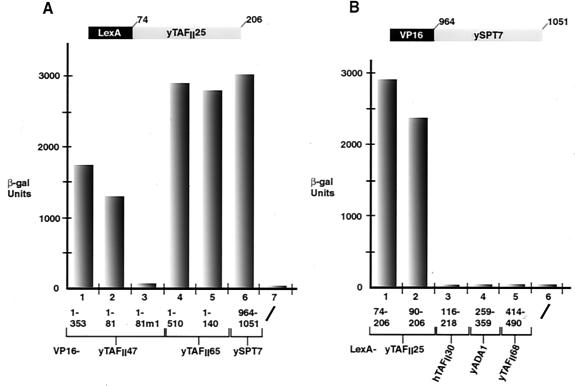
The above results indicate that yTAFII25 heterodimerizes with yTAFII47 and yTAFII65. yTAFII25 is present in TFIID and SAGA, yet both yTAFII47 and yTAFII65 are TFIID specific and are not present in SAGA (29, 30). We therefore sought a potential heterodimerization partner for yTAFII25 in the SAGA complex. Database searches with the yTAFII47 HFD showed that ySPT7 contains a potential HFD between amino acids 975 and 1051 with similarity to that of yTAFII47 (Fig. 1) (32). This observation prompted us to test the ability of the ySPT7 HFD to interact with that of yTAFII25. In two-hybrid assays, a strong interaction between ySPT7(964–1051) and yTAFII25(74–206) was observed (Fig. 8A, column 6, and B, column 1). The yTAFII25-ySPT7 two-hybrid interaction was comparable to that seen with both yTAFII47 and yTAFII65 (Fig. 8A, columns 1, 4, 5, and 6).
FIG. 8.
Heterodimerization of the SAGA components yTAFII25 and ySPT7. (A) The VP16 fusions shown below each column were transformed into the LexA-yTAFII25(74–206) strain. (B) The LexA fusions shown below each column were transformed into the VP16-SPT7(964–1051) strain. β-gal, β-galactosidase.
Interestingly, the ySPT7 HFD interacted not only with yTAFII25(74–206) but also with yTAFII25(90–206), which did not interact with either yTAFII47 or yTAFII65 (Fig. 8B, columns 1 and 2). This indicates that the determinants of yTAFII25 required for interaction with ySPT7 are not exactly the same as those required for interaction with yTAFII47 and yTAFII65. In contrast, ySPT7 did not heterodimerize with hTAFII30(116–218) or with the other SAGA components yTAFII68 and ADA1, showing that heterodimerization with yTAFII25 is highly specific (Fig. 8B, columns 3 to 5).
Heterodimerization between yTAFII25 and ySPT7 was verified directly by coexpression in E. coli. The GST-ySPT7(964–1051) fusion protein was insoluble when expressed alone, whereas in the presence of yTAFII25(74–206) a soluble heterodimeric complex was formed (Fig. 5B, lanes 1 and 2). Therefore, the SAGA components yTAFII25 and ySPT7 directly heterodimerize to form a histone-like pair. Consistent with the two-hybrid assay results, no complex was formed with hTAFII30(116–218) or with yTAFII68 (lanes 3 and 4). In contrast to what was observed with yTAFII47 and yTAFII65, complex formation with yTAFII25(90–206)Δ was as efficient as that with yTAFII25(74–206)Δ and yTAFII25(84–199)Δ (Fig. 5C, lanes 7 to 9). This is in good agreement with the two-hybrid assay data and confirms that there is a differential requirement for the LN loop in heterodimerization with ySPT7 compared with yTAFII47 and yTAFII65.
Potential homodimerization of yTAFII25.
It has previously been reported that both yTAFII25 and hTAFII30 can interact with themselves (18, 19). Indeed, in our two-hybrid experiments, we observed an interaction between LexA and VP16 fusions of the yTAFII25 HFD (Fig. 8A, column 7, and Table 1). We therefore addressed the potential yTAFII25 homodimerization in the coexpression assay. In contrast to GST-yTAFII47, GST-yTAFII65, and GST-ySPT7, both full-length yTAFII25 and the yTAFII25 (hTAFII30) HFD GST fusions were soluble when expressed alone (Fig. 5D, lanes 1 to 3). However, when these GST fusions are coexpressed with the corresponding native HFDs, the untagged HFDs are not retained on the GST-Sepharose beads (Fig. 5D, lanes 6 to 8) as they are when expressed with GST-yTAFII47, GST-yTAFII65, or GST-ySPT7. Thus, while we readily observe heterodimerization using this assay, we fail to detect homodimerization of yTAFII25 and hTAFII30 via their HFDs.
DISCUSSION
Novel histone-like components in TFIID and SAGA.
We previously reported that hTAFII135 and yADA1 contained HFDs (10). Here we show that yTAFII47, yTAFII25, and yTAFII65 have significant sequence similarities with other TAFIIs shown experimentally to contain histone folds. These similarities are comparable to those described for other histone-like proteins (9). Although definitive proof that these are bone fide HFDs will require that their structures be determined, the sequence similarities and the results of our coexpression studies suggest that yTAFII47, yTAFII65, yTAFII25, and ySPT7 are potential histone-like proteins which heterodimerize to form novel pairs in the TFIID and SAGA complexes. Thus, in total there are nine histone-like yTAFIIs, and the known genetic and physical interactions suggest that they are organized in at least two substructures within TFIID.
Our database searches using the hTAFII135 HFD showed the presence of a potential HFD in yTAFII47. Further support for this comes from Sullivan et al., who also identified yTAFII47 as a histone-like protein using an alternative algorithm (32). The positions of the α helices, notably the α1 helix, proposed by these authors differ significantly from that shown in Fig. 1. This is due mainly to the absence of gaps in the loops in their alignments. Previous alignments of the hTAFII135 sequence with that of H2A, whose structure has been determined, favor the alignment shown in Fig. 1A. Furthermore, mutation of the conserved RI pair in yTAFII47 abolishes interaction with yTAFII25 and the same mutation in hTAFII135 abolishes interaction with hTAFII20 (our unpublished data). Both observations point to these amino acids being located in the α1 helix, as indicated in our alignments. A definitive assignment of the precise α1 helix boundaries will require that the structure of this molecule be determined. The presence of the highly conserved D(I, V, L) pair allows the position of the α3 helix in each of the proteins described here to be determined based on the alignment with histone fold proteins of known structure.
Interestingly, these database searches reveal the existence of potential metazoan homologues of yTAFII47. As the metazoan homologues of yTAFII25 (hTAFII30 and dTAFII24/16) (12, 18) are known TFIID components, it is only to be expected that metazoan TFIID will contain a homologue of their heterodimerization partner, yTAFII47. It will be interesting to determine whether the human and mouse proteins revealed in these database searches are TFIID components. These observations reinforce the idea that the structure and function of TFIID have been strongly conserved throughout evolution. Hence, it is likely that metazoan TFIID also contains a homologue of yTAFII65 and, as yTAFII25 and hTAFII30 are components of SAGA, TFTC, and PCAF, we would expect these complexes to contain a homologue of ySPT7.
yTAFII47-yTAFII25, a novel histone-like pair in yTFIID.
Our results show that the yTAFII47 HFD is necessary and sufficient for the function of this protein in vivo. The growth of the yTAFII47 null strain can be complemented by expression of the HFD alone, and its function is abolished by mutation of the well-conserved RI pair in the α1 helix. Interestingly, this same mutation is not lethal in the context of the full-length protein but rather results in a temperature-sensitive phenotype. One interpretation of this result is that other regions of yTAFII47 missing in the 1–81 mutant act to stabilize the interactions of the mutant protein at permissive temperatures. This is also suggested by the observation that heterodimerization with yTAFII25 is abolished by mutation m1 in yTAFII47 in the context of the minimal HFD, yet in the context of the full-length yTAFII47 mutation m1 does not abolish heterodimerization. It is therefore likely that heterodimerization is necessary for function in vivo. In addition to the three α helices which constitute the minimal HFD, αC and αN helices can often be found. In the yTAFII47 family, a short region downstream of the α3 helix is conserved, and computer algorithms predict that this conserved sequence may form an α helix. Therefore, this αC helix or another, as-yet-undefined domain in yTAFII47 may act to stabilize interactions with yTAFII25 or with other components of yTFIID, but their function becomes evident only when the HFD is mutated.
Our results show that the temperature-sensitive phenotype of the yTAFII47(1–353)m1 allele can be suppressed by overexpression of several other histone-like yTAFIIs. At 34°C, suppression is most efficient with the direct heterodimerization partner yTAFII25. A weaker suppression is seen with yTAFII40 and yTAFII19, suggesting that this pair makes close contact with the yTAFII47-yTAFII25 pair in native yTFIID. Overexpression of yTAFII60, but not its heterodimeric partner yTAFII17, can suppress the temperature-sensitive phenotype of this allele, suggesting that this pair may also in some way contact the yTAFII47-yTAFII25 pair. At higher temperatures, however, only the direct heterodimerization partner yTAFII25 can suppress the temperature-sensitive phenotype of the yTAFII47(1–353)m1 allele.
We also tested the ability of overexpressed yTAFII65 to suppress the TAFII47 temperature-sensitive mutation. Interestingly, we found that even at permissive temperatures, yTAFII65 overexpression was toxic in the yTAFII47 temperature-sensitive background but not in the yTAFII47 wild-type or other backgrounds. One interpretation of this result is that at 30°C, the TAFII47-TAFII25 interaction is already sufficiently weakened that when yTAFII65 is overexpressed it competes with yTAFII47(1–353)m1 and titrates yTAFII25. Consequently, there is no longer enough of the TAFII47-TAFII25 heterodimer for the yeast to survive, providing evidence that formation of the yTAFII47-yTAFII25 heterodimer is essential for viability.
In addition to a genetic interaction, our results show that the yTAFII47 HFD interacts physically with the conserved C-terminal domain of yTAFII25 (and hTAFII30) both in yeast two-hybrid assays and by coexpression in E. coli. The minimum yTAFII25 region necessary for interaction with yTAFII47 is located between amino acids 84 and 199. Comparison with the sequences of other families of histone-like TAFIIs revealed a similarity with the hTAFII28 family in the α1-L1-α2 region, but the α3 helix of yTAFII25 shows higher homology to those of other known histone fold proteins. Like yTAFII40 and ySPT3, yTAFII25 contains a large insertion in the L2 loop.
This alignment predicts the existence of a possible αN helix in yTAFII25 and hTAFII30. The presence of an α helix at this position is also predicted by secondary structure computer algorithms (our unpublished data). Deletion of this helix, however, does not lead to a loss of interaction with yTAFII47 or yTAFII65. Interestingly, the putative LN loop is highly conserved in the yTAFII25/hTAFII30 family (12), and its deletion results in a loss of interaction with yTAFII47 and yTAFII65, but not with ySPT7. This strongly suggests that the LN region plays a critical role in heterodimerization by making direct contacts with yTAFII47 and yTAFII65 as is seen in the hTAFII28-hTAFII18 pair (5), while in the yTAFII25-ySPT7 heterodimer this interaction either does not take place or is not essential for complex formation.
Introduction of stop codons truncating the α2 helix abolishes yTAFII25 function. Moreover, in a screen for yTAFII25 temperature-sensitive mutants, several alleles with substitutions at G101 were found (29). This position corresponds to the G residue in the L1 loop, highly conserved in both the yTAFII25 and hTAFII28 families. A more detailed functional analysis of yTAFII25 reveals a tight correlation between its ability to interact with its heterodimerization partners and its function (D. Kirschner and L. Tora, unpublished data).
It has previously been suggested that yTAFII25 and hTAFII30 may homodimerize (18, 19). Klebanow et al. reported two-hybrid interactions using full-length yTAFII25 fusions. Our two-hybrid experiments show that this potential homodimerization requires only the HFD of yTAFII25. As it is possible that the two-hybrid interaction is indirect, we wished to visualize homodimerization directly. However, while the GST-yTAFII25(74–206) fusion was relatively soluble in E. coli when expressed alone, no homodimerization was seen when it was coexpressed with native yTAFII25(74–206). Therefore, under the conditions used to observe heterodimerization via the HFD, we detected no yTAFII25 homodimerization. Consequently, it is unlikely that the observed yTAFII25 oligomerization represents the formation of a histone-like homodimer. This does not, however, exclude the possibility that oligomerization of yTAFII25 may occur in some other way, involving for example loop-loop interactions or via the exposed hydrophilic faces of the α helices.
yTAFII65-yTAFII25, a novel histone-like pair in yTFIID.
Here we have identified an HFD in the N terminus of yTAFII65. This HFD shows high homology to the yTAFII17/hTAFII31 family. Both two-hybrid analysis and coexpression in E. coli indicate that the yTAFII65 HFD mediates a selective heterodimerization with the same yTAFII25 domain that is required for interaction with yTAFII47. The yTAFII65 HFD alone is sufficient to allow interaction with yTAFII25, and interaction is abolished by mutations within this domain. These results strongly suggest that yTAFII25-yTAFII65 form an additional histone-like pair in TFIID. Unlike yTAFII47, yTAFII65 does not interact with the hTAFII30 HFD despite their high homology. We have previously reported that hTAFII20, but not yTAFII68, interacts with hTAFII135. In this case, an important determinant for specificity was mapped to the L2-α3 region of the hTAFII20/yTAFII68 HFD (10). This observation together with those reported here indicate that there is a strict specificity code which determines the choice of a heterodimerization partner.
The yTAFII65 HFD is not sufficient for growth and in fact is not essential at 30°C. Nevertheless, the yTAFII65 HFD contributes to function, since its deletion or mutation results in a temperature-sensitive phenotype. It has previously been shown that introduction of proline residues in the α2 helix of yTAFII60 and yTAFII17 results in a temperature-sensitive phenotype (24). This is also true for yTAFII65, since the m1 mutation which introduces two prolines generates a temperature-sensitive phenotype. This same mutant abolishes interaction with yTAFII25. In yTAFII47 and yTAFII68 the HFD is necessary and sufficient for growth. In contrast, however, yTAFII65 must contain another essential domain(s) located between amino acids 103 and 510. Further complementation analysis reveals that this essential domain(s) is located between amino acids 161 and 406 (our unpublished data). In this context, it is interesting that, as with yTAFII47, we attempted to suppress the temperature-sensitive phenotype of this mutation by overexpression of other yTAFIIs. In this case, however, the only genetic interaction detected was with yTAFII68, whose overexpression suppressed the temperature-sensitive phenotype not only of yTAFII65(1–510)m1 but also of yTAFII65(103–510), in which the HFD is deleted (our unpublished data). There is therefore a genetic interaction between yTAFII68 and yTAFII65, but this involves a functional domain(s) of yTAFII65 other than the HFD.
yTAFII25-ySPT7 a novel histone-like pair in ySAGA.
We have shown here that yTAFII25 can heterodimerize with yTAFII47 and yTAFII65. Both of these heterodimerization partners are TFIID specific and are not present in SAGA. yTAFII25 is, however, a SAGA subunit, and therefore, an additional heterodimerization partner for yTAFII25 must exist in this complex. Our results, and those of Sullivan et al. (32), identified an HFD in ySPT7, and we show that this HFD heterodimerizes with the yTAFII25 HFD in both the two-hybrid and bacterial coexpression assays. This HFD is found in the C-terminal half of the protein, which has been reported to partially rescue the phenotype of the SPT7 null strain (11). Genetic studies have also shown that mutations in SPT7 have the same severe phenotype as mutations in ADA1 and SPT20. Mutation of each of these proteins completely disrupts the SAGA complex, showing that they are critical for its structural integrity (31). We have previously reported a direct interaction between the TAFII and ADA families of proteins through the heterodimerization of yTAFII68 with yADA1 (10). Here we show that the histone fold is also the interface between the TAFII and the SPT families in SAGA. Together with the genetic studies, this suggests that the yTAFII68-yADA1 and yTAFII25-SPT7 pairs are both key structural elements of SAGA.
Implications for TFIID structure.
In summary, our present results show yTFIID to comprise at least nine histone-like yTAFIIs rather than the three originally described. These yTAFIIs assemble into five heterodimeric pairs (yTAFII60-yTAFII17, yTAFII40-yTAFII19, yTAFII68-yTAFII48, yTAFII25-yTAFII47, and yTAFII25-yTAFII65). Amongst these, yTAFII25 appears to be a key player which can form two distinct heterodimers in TFIID. Previous results have indicated that yTAFII47 can be coimmunoprecipitated with yTAFII65 in extracts from yeast strains harboring an epitope-tagged yTAFII65 (30). This excludes the possibility that the yTAFII25-yTAFII47 and yTAFII25-yTAFII65 pairs are present in distinct populations of yTFIID complexes; instead, it indicates that the two pairs coexist in the same population of yTFIID.
It has previously been shown that a temperature-sensitive allele of yTAFII17 can be suppressed by overexpression of yTAFII60 or yTAFII68 and that a temperature-sensitive allele of yTAFII60 can be suppressed by overexpressed yTAFII17 and yTAFII68 (24). Furthermore, yTAFII48 overexpression can suppress a temperature-sensitive mutant of yTAFII68 (28). These genetic interactions were interpreted as providing evidence of an octamer-like substructure in TFIID (24). Our present results showing genetic interactions between yTAFII47 and yTAFII25, yTAFII40, yTAFII19, and yTAFII60 suggest a more complex picture which is difficult to interpret in the context of a single octamer-like substructure. Altogether, the existing data suggest that there may be at least two potential substructures, one composed of yTAFII68, yTAFII60, yTAFII48, and yTAFII17 as described by Michel et al. and Reese et al. (24, 28) and the other composed minimally of yTAFII47, yTAFII40, yTAFII25, and yTAFII19 as reported here. The suppression of the yTAFII47 temperature-sensitive allele by overexpressed yTAFII60 further implies interplay between the substructures via yTAFII60. The stoichiometry of the yTAFIIs present within each substructure and whether they interact in a way analogous to the core histones in the nucleosome octamer remain to be determined. Similarly, it is not clear whether the yTAFII25-yTAFII65 pair associates with the yTAFII25-yTAFII47 substructure via the yTAFII25-yTAFII25 interactions discussed above or whether it is located elsewhere within TFIID.
ACKNOWLEDGMENTS
We thank S. Hollenberg for the generous gift of yeast strain L40, L. Carré for technical assistance, S. Thuault for gifts of material and critical comments, S. Werten for gifts of materials, S. Vicaire and D. Stephane for DNA sequencing, the staff of cell culture and oligonucleotide facilities, and B. Boulay and J. M. Lafontaine for illustrations.
C.R. was supported by EMBO fellowships. This work was supported by grants from the CNRS, the INSERM, the Hôpital Universitaire de Strasbourg, the Ministère de la Recherche et de la Technologie, the Association pour la Recherche contre le Cancer, the Ligue Nationale contre le Cancer, the Human Frontier Science Programme, and NIH grant GM52461.
REFERENCES
- 1.Andel F, III, Ladurner A G, Inouye C, Tjian R, Nogales E. Three-dimensional structure of the human TFIID-IIA-IIB complex. Science. 1999;286:2153–2156. doi: 10.1126/science.286.5447.2153. [DOI] [PubMed] [Google Scholar]
- 2.Apone L M, Virbasius C A, Holstege F C, Wang J, Young R A, Green M R. Broad, but not universal, transcriptional requirement for yTAFII17, a histone H3-like TAFII present in TFIID and SAGA. Mol Cell. 1998;2:653–661. doi: 10.1016/s1097-2765(00)80163-x. [DOI] [PubMed] [Google Scholar]
- 3.Apone L M, Virbasius C M, Reese J C, Green M R. Yeast TAFII90 is required for cell-cycle progression through G2/M but not for general transcription activation. Genes Dev. 1996;10:2368–2380. doi: 10.1101/gad.10.18.2368. [DOI] [PubMed] [Google Scholar]
- 4.Bell B, Tora L. Regulation of gene expression by multiple forms of TFIID and other novel TAFII-containing complexes. Exp Cell Res. 1999;246:11–19. doi: 10.1006/excr.1998.4294. [DOI] [PubMed] [Google Scholar]
- 5.Birck C, Poch O, Romier C, Ruff M, Mengus G, Lavigne A C, Davidson I, Moras D. Human TAFII28 and TAFII18 interact through a histone fold encoded by atypical evolutionary conserved motifs also found in the SPT3 family. Cell. 1998;94:239–249. doi: 10.1016/s0092-8674(00)81423-3. [DOI] [PubMed] [Google Scholar]
- 6.Brand M, Leurent C, Mallouh V, Tora L, Schultz P. Three-dimensional structures of the TAFII-containing complexes TFIID and TFTC. Science. 1999;286:2151–2153. doi: 10.1126/science.286.5447.2151. [DOI] [PubMed] [Google Scholar]
- 7.Brand M, Yamamoto K, Staub A, Tora L. Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction. J Biol Chem. 1999;274:18285–18289. doi: 10.1074/jbc.274.26.18285. [DOI] [PubMed] [Google Scholar]
- 8.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 9.Burley S K, Xie X, Clark K L, Shu F. Histone-like transcription factors in eukaryotes. Curr Opin Struct Biol. 1997;7:94–102. doi: 10.1016/s0959-440x(97)80012-7. [DOI] [PubMed] [Google Scholar]
- 9a.Fribourg, S., C. Romier, S. Werten, Y. G. Gangloff, A. Poterszman, and D. Moras. Dissecting the interaction network of multiprotein complexes by pairwise coexpression of subunits in E. coli. J. Mol. Biol., in press. [DOI] [PubMed]
- 10.Gangloff Y G, Werten S, Romier C, Carre L, Poch O, Moras D, Davidson I. The human TFIID components TAFII135 and TAFII20 and the yeast SAGA components ADA1 and TAFII68 heterodimerize to form histone-like pairs. Mol Cell Biol. 2000;20:340–351. doi: 10.1128/mcb.20.1.340-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gansheroff L J, Dollard C, Tan P, Winston F. The Saccharomyces cerevisiae SPT7 gene encodes a very acidic protein important for transcription in vivo. Genetics. 1995;139:523–536. doi: 10.1093/genetics/139.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgieva S, Kirschner D B, Jagla T, Nabirochkina E, Hanke S, Schenkel H, de Lorenzo C, Sinha P, Jagla K, Mechler B, Tora L. Two novel Drosophila TAFIIs have homology with human TAFII30 and are differentially regulated during development. Mol Cell Biol. 2000;20:1639–1648. doi: 10.1128/mcb.20.5.1639-1648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates J R, Workman J L. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 14.Green M R. TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes. Trends Biochem Sci. 2000;25:59–63. doi: 10.1016/s0968-0004(99)01527-3. [DOI] [PubMed] [Google Scholar]
- 15.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann A, Oelgeschlager T, Roeder R G. Considerations of transcriptional control mechanisms: do TFIID-core promoter complexes recapitulate nucleosome-like functions? Proc Natl Acad Sci USA. 1997;94:8928–8935. doi: 10.1073/pnas.94.17.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann A, Roeder R G. Cloning and characterization of human TAF20/15. Multiple interactions suggest a central role in TFIID complex formation. J Biol Chem. 1996;271:18194–18202. doi: 10.1074/jbc.271.30.18194. [DOI] [PubMed] [Google Scholar]
- 18.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 19.Klebanow E R, Poon D, Zhou S, Weil P A. Isolation and characterization of TAF25, an essential yeast gene that encodes an RNA polymerase II-specific TATA-binding protein-associated factor. J Biol Chem. 1996;271:13706–13715. doi: 10.1074/jbc.271.23.13706. [DOI] [PubMed] [Google Scholar]
- 20.Kokubo T, Gong D W, Wootton J C, Horikoshi M, Roeder R G, Nakatani Y. Molecular cloning of Drosophila TFIID subunits. Nature. 1994;367:484–487. doi: 10.1038/367484a0. [DOI] [PubMed] [Google Scholar]
- 21.Komarnitsky P B, Michel B, Buratowski S. TFIID-specific yeast TAF40 is essential for the majority of RNA polymerase II-mediated transcription in vivo. Genes Dev. 1999;13:2484–2489. doi: 10.1101/gad.13.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luger K, Mader A W, Richmond R K, Sargent D F, Richmond T J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 23.Martinez E, Kundu T K, Fu J, Roeder R G. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem. 1998;273:23781–23785. doi: 10.1074/jbc.273.37.23781. [DOI] [PubMed] [Google Scholar]
- 24.Michel B, Komarnitsky P, Buratowski S. Histone-like TAFs are essential for transcription in vivo. Mol Cell. 1998;2:663–673. doi: 10.1016/s1097-2765(00)80164-1. [DOI] [PubMed] [Google Scholar]
- 25.Moqtaderi Z, Keaveney M, Struhl K. The histone H3-like TAF is broadly required for transcription in yeast. Mol Cell. 1998;2:675–682. doi: 10.1016/s1097-2765(00)80165-3. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan K, Jackson B M, Rhee E, Hinnebusch A G. yTAFII61 has a general role in RNA polymerase II transcription and is required by Gcn4p to recruit the SAGA coactivator complex. Mol Cell. 1998;2:683–692. doi: 10.1016/s1097-2765(00)80166-5. [DOI] [PubMed] [Google Scholar]
- 27.Ogryzko V V, Kotani T, Zhang X, Schlitz R L, Howard T, Yang X J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 28.Reese J C, Zhang Z, Kurpad H. Identification of a yeast transcription factor IID subunit, TSG2/TAF48. J Biol Chem. 2000;275:17391–17398. doi: 10.1074/jbc.M001635200. [DOI] [PubMed] [Google Scholar]
- 29.Sanders S L, Klebanow E R, Weil P A. TAF25p, a non-histone-like subunit of TFIID and SAGA complexes, is essential for total mRNA gene transcription in vivo. J Biol Chem. 1999;274:18847–18850. doi: 10.1074/jbc.274.27.18847. [DOI] [PubMed] [Google Scholar]
- 30.Sanders S L, Weil P A. Identification of two novel TAF subunits of the yeast Saccharomyces cerevisiae TFIID complex. J Biol Chem. 2000;275:13895–13900. doi: 10.1074/jbc.275.18.13895. [DOI] [PubMed] [Google Scholar]
- 31.Sterner D E, Grant P A, Roberts S M, Duggan L J, Belotserkovskaya R, Pacella L A, Winston F, Workman J L, Berger S L. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol Cell Biol. 1999;19:86–98. doi: 10.1128/mcb.19.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan S A, Aravind L, Makalowska I, Baxevanis A D, Landsman D. The histone database: a comprehensive WWW resource for histones and histone fold-containing proteins. Nucleic Acids Res. 2000;28:320–322. doi: 10.1093/nar/28.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.vom Baur E, Zechel C, Heery D, Heine M J, Garnier J M, Vivat V, Le Douarin B, Gronemeyer H, Chambon P, Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- 34.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIS. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 35.Walker S S, Shen W C, Reese J C, Apone L M, Green M R. Yeast TAFII145 required for transcription of G1/S cyclin genes and regulated by the cellular growth state. Cell. 1997;90:607–614. doi: 10.1016/s0092-8674(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 36.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAFII-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 37.Xie X, Kokubo T, Cohen S L, Mirza U A, Hoffmann A, Chait B T, Roeder R G, Nakatani Y, Burley S K. Structural similarity between TAFs and the heterotetrameric core of the histone octamer. Nature. 1996;380:316–322. doi: 10.1038/380316a0. [DOI] [PubMed] [Google Scholar]