Abstract
We showcase efficient synthetic protocols of cyclopentadienyl metal dicarbonyl, CpM(CO)2 (M = Rh and Ir). Reflecting the relativistic effect, the 1H and 13C signals of the Cp ring in CpIr(CO)2 were upfield shifted when compared with the Rh analogue. A missing dinuclear complex, (CpIr)2(μ-CO)(CO)2, was spontaneously generated together with [CpIr(CO)]3 by the loss of CO. The crystallographic analyses unambiguously determined their unique structures with one and three Ir–Ir bonds, respectively.
Introduction
Among group 9 metal complexes, compounds bearing an η5-cyclopentadienyl ligand (η5-Cp) are the classical ones, which have been extensively utilized in a number of catalytic systems.1 The representative complexes are formulated as CpM(CO)2, i.e., cyclopentadienyl metal(I) dicarbonyl. As such, CpCo(CO)2 has been employed for catalytic cycloaddition to generate aromatic compounds from corresponding acetylene derivatives.2 While CpCo(CO)2 is readily available from commercial suppliers, rhodium and iridium analogues are required to be synthesized in laboratories. Early studies on group 9 metal complexes bearing an η5-Cp ligand have demonstrated their lability despite their 18-electron configuration,3 and researchers, therefore, paid their attention to the use of thermodynamically stable complexes having bulkier ligands such as an η5-Cp* (η5-pentamethylcyclopentadienyl)3 or η5-Ind (η5-indenyl) groups.4 As a consequence, reliable spectroscopic data of the simplest group 9 metal complexes (CpM(CO)2) are still invisible in this research area, placing an obstacle for the use of these compounds as potential precursors to generate other complexes with 18 valence electrons by ligand exchange5 as well as catalysts in C–H activation1a (Figure 1).
Figure 1.
Group 9 metal complexes CpM(CO)2 utilized as catalysts in C–H activation and precursors of other complexes with 18 valence electrons.
More critically, chlorotricarbonyliridium (IrCl(CO)3),6 an iridium source for the synthesis of CpIr(CO)2,7 can be purchased from commercial suppliers, albeit being expensive. Otherwise, it can only be prepared through a tedious and lengthy carbonylation process of iridium salts using a specially equipped apparatus.7,8 The alternative synthetic route for CpIr(CO)2 and its derivatives was, afterward, developed in 1995 even though the yields of the final step are unstable (8–92%) for the ring-substituted derivatives.9 Of particular interest is to gain structural insights into a missing dinuclear complex, i.e., saturated CpIr(μ-CO)(CO)2,7 whereas unsaturated [Cp*Ir(CO)]2 has been well-characterized.10 Herein, we report efficient synthetic protocols and complete characterization data for CpRh(CO)2 and CpIr(CO)2 as well as structures of their unique oligomerization products.
Results and Discussion
Synthesis
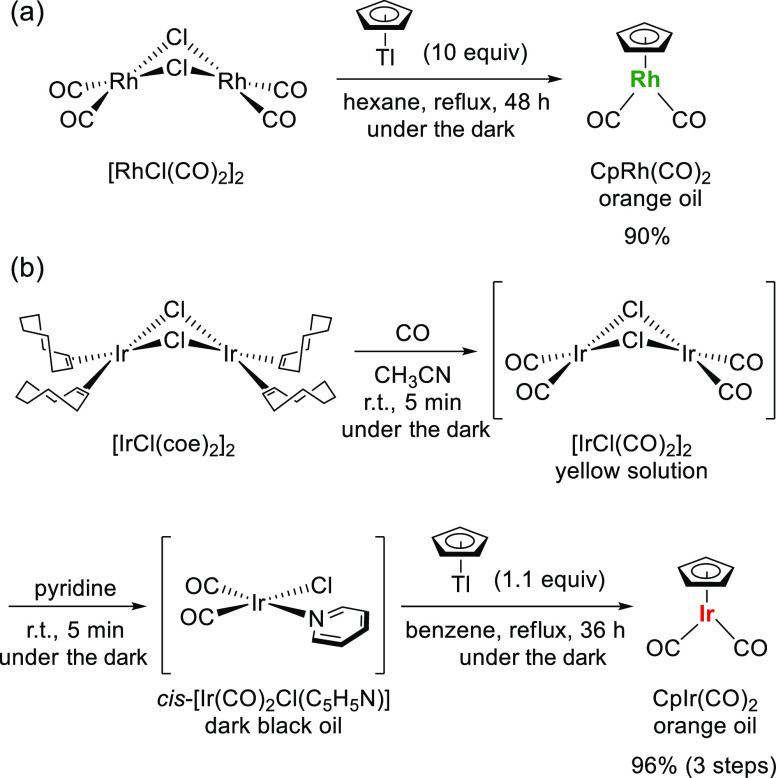
Cyclopentadienyl rhodium(I) dicarbonyl CpRh(CO)2 was synthesized by a modified Lees and Drolet’s method (Figure 2a).11 The rhodium(I) dicarbonyl chloride dimer [RhCl(CO)2]2 was dissolved in dehydrated hexane to gain a clear yellow solution. After addition of thallium cyclopentadienide (CpTl), the resulting suspension was heated at the reflux temperature for 48 h in the dark under an argon flow. The reaction mixture was then passed through a Celite pad to obtain a yellow solution, which was evaporated under a reduced pressure at temperatures below 15 °C to give the desired CpRh(CO)2 in a 90% isolated yield as an orange oil.
Figure 2.
Synthesis of (a) CpRh(CO)2 and (b) CpIr(CO)2.
Classically, cyclopentadienyl iridium(I) dicarbonyl CpIr(CO)2 has been synthesized by the reaction of IrCl(CO)3, which is less commonly available, with either sodium (CpNa)7 or thallium (CpTl)12 cyclopentadienide in high yields (95 and 80%, respectively). Unfortunately, the method analogous to that for CpRh(CO)2 is not applicable since the iridium(I) dicarbonyl chloride dimer [IrCl(CO)2]2 cannot exist in a monomeric form under dry conditions or in apolar solvents such as pentane and toluene due to polymerization generating [IrCl(CO)2]n as dark-black solids.13 Alternatively, Blais and Rausch reported the four-step synthesis of CpIr(CO)29 in which a square planar 16e complex, cis-chlorodicarbonyl(pyridine)iridium (cis-[Ir(CO)2Cl(C5H5N)]), was prepared from IrCl3·3H2O via three steps14 and subsequently reacted with CpNa. In 1994, Roberto and co-workers found that IrCl(CO)2 exists as its dimer only in acetonitrile and could be transformed into easily handled cis-[Ir(CO)2Cl(C5H5N)] in the presence of pyridine.13 Building on these findings, we prepared CpIr(CO)2 via shorter steps as shown in Figure 2b. To a suspension of the chlorobis(cyclooctene)iridium(I) dimer [IrCl(coe)2]2 in dehydrated acetonitrile, CO gas was introduced by bubbling for 5 min at room temperature. An excessive amount of pyridine was added to the thus-obtained clear yellow solution containing [IrCl(CO)2]2 and further stirred for 5 min. After removal of the solvent as well as residual pyridine under a reduced pressure, cis-[Ir(CO)2Cl(C5H5N)] was obtained as a dark-black oil, which was subsequently refluxed with CpTl in benzene for 36 h under an argon atmosphere. In order to remove unreacted [IrCl(CO)2]2 and CpTl, hexane was added, and the resulting mixture was passed through a Celite pad to obtain a yellow solution, which was then evaporated under a reduced pressure at temperatures below 15 °C to give the desired CpIr(CO)2 in a 96% overall yield as an orange oil.
IR Vibrational Modes
These reaction protocols were well-reproducible and scalable (confirmed up to 0.5 g). The structures of CpRh(CO)2 and CpIr(CO)2 were determined by comparing IR vibrational frequencies with reported ones (neat).7 The dilute solutions (5 vol % in hexane) were used for IR spectroscopy (KBr). The observed bands corresponding to asymmetric and symmetric CO stretching modes matched well within an error of ±8 cm–1 (Table 1).
Table 1. Characteristic IR Vibrational Frequencies of 5 vol % CpM(CO)2 in Hexane (KBr).
| metal | CpM(CO)2 (cm–1) | ref7 (cm–1) |
|---|---|---|
| Co | 1966, 2029 | 1965, 2037 |
| Rh | 1981, 2045 | 1987, 2051 |
| Ir | 1955, 2032 | 1957, 2037 |
NMR Spectroscopy
The 1H and 13C NMR spectra of CpM(CO)2 in C6D6 are depicted in Figure 3. A little is known about 1H chemical shifts of the title compounds: Rh, 5.48 ppm (C6D6);15 Ir, 4.19 (C6D6)7 and 5.46 ppm (CDCl3).9 Our observation, however, largely differs from reported ones: Rh, 4.86 ppm; Ir, 4.71 ppm. Note that all complexes did not show concentration dependence for the 1H and 13C chemical shifts in our study. The trend of 1H signals (δ(Cp): Rh > Ir > Co) was explainable by the strength of the Pauling’s electronegativity: Rh (2.28) > Ir (2.20) > Co (1.88),16 reflecting a relativistic effect.17 In contrast, the observed carbonyl signals follow a descending order of Co (205.54 ppm) > Rh (192.36 ppm) > Ir (173.62 ppm). This correlates with calculated M–CO binding energies: Co (25.9 kcal/mol) < Rh (29.8 kcal/mol) < Ir (52.3 kcal/mol) at the M06-2X level of theory with basis sets of LanL2DZ for metals and 6-31G(d,p) for the rest. The carbonyl ligands in CpCo(CO)2 were observed as a broadened signal in the 13C NMR spectrum due to strong quadrupolar interactions with the adjacent 59Co atom, while those in CpRh(CO)2 were detected as a doublet signal weakly coupled with 103Rh (I = −1/2, JC–Rh = 84.0 Hz).
Figure 3.
(a) 1H (500 MHz) and (b) 13C (126 MHz) NMR spectra of CpM(CO)2 in C6D6.
Crystallization of CpTl
For the synthesis of CpM(CO)2 (Figure 2), CpTl purchased from commercial suppliers are readily available without further purification even though the powdery sample is usually colored with gray. To avoid contamination, it can be purified with a hot hexane solution to obtain colorless needle crystals. The crystals prepared by sublimation had been initially considered to adopt a space group of Cc in terms of similar cell parameters to an indium analogue,18 which was later corrected as C2/c.19 Upon comparing the thermal ellipsoids between the crystal obtained from a tetrahydrofuran solution20 and that from the hot hexane solution, the latter one was less disordered with an R1 factor of 1.58%. As was reported previously, the Cp anions and Tl cations were confirmed to be alternately arranged as a zigzag chain with a Cp–Tl distance of 2.762 Å and a Cp–Tl–Cp angle of 129.10° (Figure S10).
Oligomerization
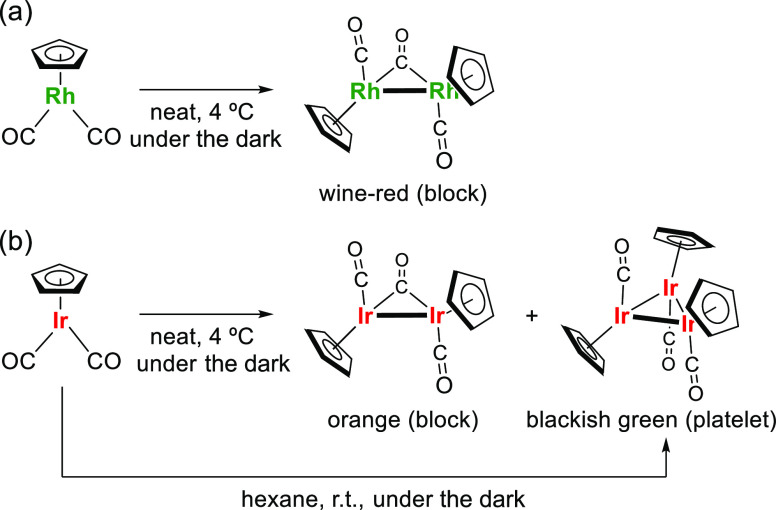
The synthesized complexes, CpM(CO)2, can be stored under an inert atmosphere of N2 at 4 °C over two years despite forming precipitates in a tiny amount during storage. The supernatant, however, worked well for the common ligand exchange reactions with olefins21 and did not show significant change in 1H NMR and IR spectra. Upon seeing the precipitates, we noticed the presence of single crystals, revealing their structures as oligomers by X-ray crystallography (vide infra). Such dimerization was previously known to occur by light irradiation to CpRh(CO)2.22 The structure of the product, i.e., (CpRh)2(μ-CO)(CO)2, is the same as that obtained as wine-red block crystals by spontaneous dimerization (Figure 4a). This reaction is probably promoted by the inevitable contamination of oxygen.23 Since the statement, by Fischer and Brenner in 1962, that CpIr(CO)2 is unable to be dimerized upon exposure to light or air as opposed to CpRh(CO)2,7 a saturated CpIr(I) dimer, i.e., (CpIr)2(μ-CO)(CO)2, has long been a missing complex. Under harsh and specifically tuned conditions, Cp*Ir(CO)2 is known to undergo dimerization to give unsaturated dimer [Cp*Ir(CO)]2.10 Only a few papers have predicted a saturated dimeric structure without definitive evidence.24 Our observation undoubtedly confirmed that CpIr(CO)2 could be dimerized to generate (CpIr)2(μ-CO)(CO)2 as orange block crystals by aging over time (Figure 4b). Herein, we fit the last piece of a class of dinuclear group 9 metal complexes bearing η5-Cp ligands ((CpM)2(μ-CO)(CO)2). To our surprise, a [CpM(CO)]3-type trimer was also formed as blackish-green platelet crystals from the same mother liquor of CpIr(CO)2. This complex had been prepared only from CpIr(CO)H2 via trimerization of the 16e complex CpIr(CO) generated in situ by the thermal elimination of H2.24a
Figure 4.
Oligomerization of (a) CpRh(CO)2 and (b) CpIr(CO)2.
Crystallography
The crystal structures of (CpRh)2(μ-CO)(CO)2 and (CpIr)2(μ-CO)(CO)2 confirmed a trans-disposition of the CO ligands (Figure 5a,b). The bond angles and M–CO bond lengths are comparable between Rh (4d) and Ir(5d) complexes due to relativistic contraction of inner-core s orbitals for the latter, thus falling into the same space group of P1̅ with similar cell parameters. Upon counting the cluster valence electrons (CVE) for (CpM)2(μ-CO)(CO)2 (34e), the metals should obey the linear orientation with an M–M bond according to the Wade–Mingos–Lauher rule,25 which agreed well with the crystal structures showing Rh–Rh (2.6798(9) Å) and Ir–Ir (2.6980(11) Å) bonds. In a similar manner, [CpIr(CO)]3 with CVE of 48e adopts a form close to an equilateral triangle constituted of Ir3 with intermetallic distances of 2.6920(10), 2.6694(9), and 2.6760(10) Å, which are slightly shortened when compared with those in (CpIr)2(μ-CO)(CO)2. In this structure, one of the Cp rings faces to an opposite side across the triangular plane, rendering it Cs symmetry.
Figure 5.

Single-crystal X-ray structures of (a) (CpRh)2(μ-CO)(CO)2, (b) (CpIr)2(μ-CO)(CO)2, and (c) [CpIr(CO)]3. Thermal ellipsoids are shown in 50% probability.
Different from oligomers being in a solid state under ambient conditions (Figure 4), mononuclear complexes CpM(CO)2 exist as a liquid (Figure 2). The solid-state structures of the pristine complexes have been, therefore, unavailable. By carefully testing the melting point, CpIr(CO)2 was found to be frozen at less than 11–14 °C. Thus, a hexane solution of CpIr(CO)2 was prepared, and single crystals were grown by slow evaporation at 4 °C. As the result, colorless platelet crystals were obtained. Since these crystals underwent a phase transition at −173 °C, X-ray diffraction analysis was performed at −10 °C. The structure of CpIr(CO)2 was then unambiguously determined as shown in Figure 6. Note that Co and Rh analogues were not crystallized under similar conditions presumably due to rather lower melting points. The M–CO bond lengths of CpIr(CO)2 (1.845(9) and 1.851(8) Å) are comparable to those of M–(η2-CO) in (CpIr)2(μ-CO)(CO)2 (1.853(12) and 1.848(12) Å). Upon seeing the packing structure, CH/π interactions between the Cp rings were found in addition to short contacts between the carbonyl oxygens and the adjacent Cp protons.
Figure 6.

Single-crystal X-ray structure of CpIr(CO)2: (a) side and (b) top views. Thermal ellipsoids are shown in 50% probability.
Conclusions
In summary, we demonstrated the reproducible and scalable synthesis of CpRh(CO)2 and CpIr(CO)2, the latter of which was, for the first time, characterized by single-crystal X-ray diffraction analysis. By comparing NMR spectra, both 1H and 13C signals corresponding to the Cp ring in CpIr(CO)2 showed a relativistic effect on chemical shifts, which are explainable by the Pauling’s electronegativity. The spontaneous oligomerization of CpRh(CO)2 and CpIr(CO)2 was also investigated crystallographically. Even though CpIr(CO)2 has been known to be thermodynamically stable as opposed to the Rh analogue, we found the formation of metal clusters including dimers and trimers, in which the former one was isostructural to the Rh analogue owing to the relativistic contraction of inner-core s orbitals.
Experimental Section
General
The 1H and 13C NMR measurements were carried out at room temperature with a JEOL JNM ECA500 instrument. The NMR chemical shifts were reported in ppm with reference to residual protons and carbons of C6D6 (δ 7.15 ppm in 1H NMR and δ 128.00 ppm in 13C NMR). APCI (atmospheric pressure chemical ionization) mass spectra were measured on a Bruker micrOTOF-Q II. IR spectra were taken with a Shimadzu IR-Affinity 1S.
Hexane was purchased from Kanto Chemical Co., Inc. Acetonitrile, pyridine, and benzene were purchased from FUJIFILM Wako Pure Chemical Corporation. [RhCl(CO)2]2 and [IrCl(coe)2]2 (coe: cyclooctene) were purchased from Sigma-Aldrich Co. LLC. Thallium cyclopentadienide (CpTl) was purchased from Tokyo Chemical Industry Co., Ltd.
All reactions were carried out under an Ar atmosphere. Unless otherwise noted, materials purchased from commercial suppliers were used without further purification.
Computational Methods
All calculations were conducted using the Gaussian 09 program. All structures at the stationary states were optimized at the M06-2X level of theory with basis sets of LanL2DZ for metals and 6-31G(d,p) for the rest without any symmetry assumptions and confirmed by the frequency analyses at the same level of theory.
Synthesis of CpRh(CO)2
The rhodium(I) dicarbonyl chloride dimer [RhCl(CO)2]2 (250 mg, 0.643 mmol) was placed into a two-neck 300 mL flask and degassed through three vacuum-Ar cycles. Dehydrated hexane (150 mL, 4.29 mM) was added to gain a clear yellow solution. After addition of CpTl (1.73 g, 6.44 mmol, and 10.0 equiv), the resulting suspension was heated at the reflux temperature for 48 h in the dark under an argon flow (oil bath). The reaction mixture was then passed through a Celite pad to obtain a yellow solution, which was evaporated under a reduced pressure at temperatures below 15 °C to give the desired CpRh(CO)2 (258 mg, 1.15 mmol) in a 90% isolated yield as an orange oil.
For CpRh(CO)2, IR (KBr) ν: 1981, 2045 cm–1; 1H NMR (500 MHz, C6D6): δ 4.86 (s, 5H); 13C NMR (126 MHz, C6D6): δ 192.36 (d, JC–Rh = 84.0 Hz), 87.85 (d, JC–Rh = 3.6 Hz); HRMS (APCI) m/z: [2 M–2CO]•– calcd for C12H10O2Rh2, 391.8791; found, 391.8810.
Synthesis of CpIr(CO)2
The chlorobis(cyclooctene)iridium(I) dimer [IrCl(coe)2]2 (500 mg, 0.558 mmol) was placed into a two-neck 100 mL flask and degassed through three vacuum-Ar cycles. Dehydrated acetonitrile (90.0 mL, degassed by Ar bubbling for 30 min prior to use) was added to the suspension. CO gas was then introduced by bubbling for 5 min at room temperature. An excessive amount of pyridine (3.00 mL) was added to the thus-obtained clear yellow solution containing [IrCl(CO)2]2 and further stirred for 5 min. After removal of the solvent as well as residual pyridine under a reduced pressure, cis-[Ir(CO)2Cl(C5H5N)] was obtained as a dark-black oil (442 mg), which was transferred into a two-neck 50 mL flask containing CpTl (331 mg, 1.23 mmol, and 1.10 equiv) and degassed through three vacuum-Ar cycles. Dehydrated benzene (10.0 mL, degassed by Ar bubbling for 20 min prior to use) was added and refluxed for 36 h under an argon atmosphere (oil bath). In order to remove unreacted [IrCl(CO)2]2 and CpTl, hexane was added, and the resulting mixture was passed through a Celite pad to obtain a yellow solution, which was then evaporated under a reduced pressure at temperatures below 15 °C to give the desired CpIr(CO)2 (336 mg, 1.07 mmol) in a 96% overall yield as an orange oil.
For CpIr(CO)2, IR (KBr) ν: 1955, 2032 cm–1; 1H NMR (500 MHz, C6D6): δ 4.71 (s, 5H); 13C NMR (126 MHz, C6D6): δ 173.62, 83.73; HRMS (APCI) m/z: [M + H]+ calcd for C7H6IrO2, 314.9997; found, 314.9990.
Acknowledgments
Financial support was partially provided by the JSPS KAKENHI Grant Numbers JP17H06119, JP20K15260, and JP20H05218. The NMR measurements were partly supported by the Joint Usage/Research Center (JURC) at the ICR, Kyoto University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05739.
Detailed experimental procedures, characterization data, and computational results (PDF)
Crystallographic data for CpTl (CIF)
Crystallographic data for [CpIr(CO)]3 (CIF)
Crystallographic data for CpIr(CO)(CO)2 (CIF)
Crystallographic data for CpRh(CO)(CO)2 (CIF)
Crystallographic data for CpIr(CO)2 (CIF)
Accession Codes
CCDC 2099705 ([CpIr(CO)]3), 2099704 ((CpIr)2(μ-CO)(CO)2), 2099707 (CpTl), and 2099706 ((CpRh)2(μ-CO)(CO)2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
The authors declare no competing financial interest.
Supplementary Material
References
- a Jones W. D. Activation of C–H Bonds: Stoichiometric Reactions. Top Organomet. Chem. 1999, 3, 9–46. 10.1007/3-540-68525-1_2. [DOI] [Google Scholar]; b Thiel I.; Hapke M. The broad diversity of CpCo(I) complexes. Rev. Inorg. Chem. 2014, 34, 217–245. 10.1515/revic-2014-0001. [DOI] [Google Scholar]
- Lautens M.; Klute W.; Tam W. Transition Metal-Mediated Cycloaddition Reactions. Chem. Rev. 1996, 96, 49–92. 10.1021/cr950016l. [DOI] [PubMed] [Google Scholar]
- Blaser H.-U.; Federsel H.-J.. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions, 2nd Ed.; Wiley-VCH: Weinheim, 2010. [Google Scholar]
- Bochmann M.Organometallics and Catalysis: An Introduction; Oxford University Press: Oxford, 2015. [Google Scholar]
- O’Connor J. M.; Casey C. P. Ring-Slippage Chemistry of Transition-Metal Cyclopentadienyl and Indenyl Complexes. Chem. Rev. 1987, 87, 307–318. 10.1021/cr00078a002. [DOI] [Google Scholar]
- a Krogmann K.; Binder W.; Hausen H. D. Crystal Structure of ″Ir(CO)3Cl″ = Ir(CO)2.93Cl1.07. Angew. Chem., Int. Ed. 1968, 7, 812. 10.1002/anie.196808121. [DOI] [Google Scholar]; b Tsuji Y.; Hoffmann R.; Miller J. S. Revisiting Ir(CO)3Cl. Polyhedron 2016, 103, 141–149. 10.1016/j.poly.2015.09.050. [DOI] [Google Scholar]
- Fischer E. O.; Brenner K. S. Über Aromatenkomplexe von Metallen LXVII. Cyclopentadienyl-iridium-di-carbonyl. Z. Naturforschg 1962, 17, 774–775. 10.1515/znb-1962-1116. [DOI] [Google Scholar]
- Hieber W.; Lagally H.; Mayr A. Über Metallcarbonyle. XXXVI. Kohlenoxydverbindungen von Iridiumhalogeniden. Z. Anorg. Allgem. Chem. 1941, 246, 138–148. 10.1002/zaac.19412460203. [DOI] [Google Scholar]
- Blais M. S.; Rausch M. D. A new synthetic route to functionally substituted (η5-cyclopentadienyl) dicarbonyliridium compounds. J. Organomet. Chem. 1995, 502, 1–8. 10.1016/0022-328X(95)05627-2. [DOI] [Google Scholar]
- Ball R. G.; Graham W. A. G.; Heinekey D. M.; Hoyano J. K.; McMaster A. D.; Mattson B. M.; Michel S. T. Synthesis and Structure of [(η-C5Me5)Ir(CO)]2. Inorg. Chem. 1990, 29, 2023–2025. 10.1021/ic00335a051. [DOI] [Google Scholar]
- Drolet D. P.; Lees A. J. Solution Photochemistry of (η5-C5R5)Rh(CO)2 (R = H, Me) Complexes: Pathways for Photosubstitution and C–H/Si–H Bond Activation Reactions. J. Am. Chem. Soc. 1992, 114, 4186–4194. 10.1021/ja00037a022. [DOI] [Google Scholar]
- Gardner S. A.; Andrews P. S.; Rausch M. D. Reactions of π-Cydopentadienyldicarbonylrhodium and π-Cyclopentadienyldicarbonyíiridium with Disubstituted Acetylenes. Inorg. Chem. 1973, 12, 2396–2402. 10.1021/ic50128a036. [DOI] [Google Scholar]
- Roberto D.; Cariati E.; Psaro R.; Ugo R. Formation of [Ir(CO)2Cl]x (x = 2, n) Species by Mild Carbonylation of [Ir(cyclooctene)2Cl]2 Supported on Silica or in Solution: A New Convenient Material for the Synthesis of Iridium(I) Carbonyl Complexes. Organometallics 1994, 13, 4227–4231. 10.1021/om00023a027. [DOI] [Google Scholar]
- Klabunde U. Dicarbonylchloro(p-toluidine)iridium(I). Inorg. Synth. 1974, 15, 82–84. [Google Scholar]
- Dunwoody N.; Sun S.-S.; Lees A. J. Quantitative Photochemistry and Mechanisms for a Series of Rhodium Dicarbonyl Derivatives. Inorg. Chem. 2000, 39, 4442–4451. 10.1021/ic000395n. [DOI] [Google Scholar]
- Allred A. L. Electronegativity Values from Thermochemical Data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. 10.1016/0022-1902(61)80142-5. [DOI] [Google Scholar]
- a Pitzer K. S. Relativistic Effects on Chemical Properties. Acc. Chem. Res. 1979, 12, 271–276. 10.1021/ar50140a001. [DOI] [Google Scholar]; b Pyykkö P. Relativistic Effects in Structural Chemistry. Chem. Rev. 1988, 88, 563–594. 10.1021/cr00085a006. [DOI] [Google Scholar]
- Frasson E.; Menegus F.; Panattoni C. Chain Structure of the Cyclopentadienils of Monovalent Indium and Thallium. Nature 1963, 199, 1087–1089. 10.1038/1991087a0. [DOI] [Google Scholar]
- Olbrich F.; Behrens U. Crystal structure of catena-cyclopentadienyl thallium, [Tl(C5H5)]. Z. Kristallogr. - New Cryst. Struct. 1997, 212, 47. [Google Scholar]
- Dashti-Mommertz A.; Neumüller B.; Melle S.; Haase D.; Uhl W. [Tl3Cp2][CpMo(CO)3] – a Salt with the New Cation [Tl3Cp2]+. Structural Reinvestigation of MCp (M = In, Tl). Z. Anorg. Allg. Chem. 1999, 625, 1828–1832. . [DOI] [Google Scholar]
- Hashikawa Y.; Murata M.; Wakamiya A.; Murata Y. Co(I)-Mediated Removal of Addends on the C60 Cage and Formation of the Monovalent Cobalt Complex CpCo(CO)(η2-C60). Org. Lett. 2016, 18, 6348–6351. 10.1021/acs.orglett.6b03238. [DOI] [PubMed] [Google Scholar]
- Mills O. S.; Nice J. P. Carbon Compounds of the Transition Metals IX. Evidence for and Structure of Dicyclopentadienyltricarbonyldirhodium. J. Organomet. Chem. 1967, 10, 337–342. 10.1016/S0022-328X(00)93096-1. [DOI] [Google Scholar]
- a Fischer E. O.; Bittler K. Über Aromatenkomplexe von Metallen LIII. Dimeres Cyclopentadienyl-rhodium-di-carbonyl [C5H5Rh(CO)2]2. Z. Naturforschg 1961, 16, 835–836. 10.1515/znb-1961-1214. [DOI] [Google Scholar]; b Cotton F. A.; Hunter D. L. Conditions for the Occurrence of Bridging Carbonyl Groups. Inorg. Chem. 1974, 13, 2044. 10.1021/ic50138a055. [DOI] [Google Scholar]
- a Shapley J. R.; Adair P. C.; Lawson R. J.; Pierpont C. G. Thermal Elimination of Dihydrogen from (η5-C5H5)Ir(CO)H2. Formation, Structure, and Reactivity of Cs-(η5-C5H5)3lr3(CO)3. Inorg. Chem. 1982, 21, 1701–1702. 10.1021/ic00134a096. [DOI] [Google Scholar]; b Abad J. A. Preparation of Mono- or Di-metal Indenyl-Iridium Derivatives and of Tri-metal Iridium-Platinum Complexes. Inorg. Chim. Acta 1986, 121, 213–217. 10.1016/S0020-1693(00)84522-3. [DOI] [Google Scholar]
- a Wade K. The Structural Significance of the Number of Skeletal Bonding Electron-pairs in Carboranes, the Higher Boranes and Borane Anions, and Various Transition-metal Carbonyl Cluster Compounds. J. Chem. Soc. D 1971, 792–793. 10.1039/c29710000792. [DOI] [Google Scholar]; b Mingos D. M. P. A General Theory for Cluster and Ring Compounds of the Main Group and Transition Elements. Nature (London), Phys. Sci. 1972, 236, 99–102. 10.1038/physci236099a0. [DOI] [Google Scholar]; c Lauher J. W. The Bonding Capabilities of Transition Metal Clusters. J. Am. Chem. Soc. 1978, 100, 5305–5315. 10.1021/ja00485a011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.