Abstract

The stability of dispersions containing charged particles may obviously be regulated by salt. In some systems, the effective charge, as measured by the potential some small distance away from the particles, can have a sign opposite to the bare surface charge. If charge reversal takes place, there is typically a salt concentration regime within which colloidal stability increases with added salt. These experimental findings on dispersions have been corroborated by atomic force microscopy investigations, where an attraction is found at short separations. This attraction is stronger than expected from standard DLVO theory, and there has been considerable debate concerning its origin. In this work, we use simple coarse-grained models of these systems, where the bare surfaces carry a uniform charge density, and ion-specific adsorption is absent. Our hypothesis is that these experimental observations can be explained by such a simplistic pure Coulomb based model. Our approach entails grand canonical Metropolis Monte Carlo (MC) simulations as well as correlation-corrected Poisson-Boltzmann (cPB) calculations. In the former case, all ions have a common size, while the cPB utilizes a point-like model. We devote significant attention on apparent surface charge densities and interactions between large flat model surfaces immersed in either a 2:1 salt or a 3:1 salt. In contrast to most of the previous theoretical efforts in this area, we mainly focus on the weak long-ranged repulsion and its connection to an effective surface charge. We find a charge reversal and a concomitant development of a free energy barrier for both salts. The experimentally observed nonmonotonic dependence of colloidal stability on the salt concentration is reproduced using MC simulations as well as cPB calculations. A strong attraction is observed at short range for all investigated cases. We argue that in our model, all non-DLVO aspects can be traced to ion–ion correlations.
Introduction
The intricate behavior of colloid and surface interactions in multivalent electrolyte solutions has been studied using both experimental1−9 and theoretical.10−25 approaches. These interactions are often of practical relevance, such as the condensation of DNA,26 but they are also of more fundamental interest. At weak electrostatic coupling strengths, that is, moderate surface charge densities, low ion valencies and high dielectric screening, such systems behave as one would expect from Poisson–Boltzmann and DLVO27,28 theory. At large separations, the electric double layers that develop near the particle surfaces generate repulsive forces. For low concentrations of monovalent salt, screening of the particle charge is inefficient, that is, the outer counterion layer is very diffusive (in an aqueous solution). This generates a long-ranged free energy barrier, which is often strong enough to prevent flocculation. As the salt concentration is increased, ionic screening becomes more efficient, thereby generating more short-ranged double layer interactions. This leads to weaker barriers, which destabilize the dispersion.
When ionic correlations become important, which in an aqueous solution usually require the presence of multivalent counterions, more interesting behaviors emerge. A well-known example is a short-ranged correlation attraction between like-charged colloidal particles, or surfaces.11 Another correlation-driven phenomenon is overcharging, or charge inversion, where the counterions overcompensate the bare surface charge. Charge inversion also means that the electrophoretic mobility is reversed. Overcharging has been found and studied in a range of different systems.20−25,29−31 In an experimental study by Van Der Heyden et al.,32 charge inversion was shown to occur in biologically relevant mixtures of salt. It should be noted that ion-specific adsorption can also generate charge reversal.29−31,33
Charge inversion also has a strong impact on colloidal stability. Sinha et al.34 and Ruiz-Cabello et al.35 used a combination of atomic force microscopy (AFM) and colloidal stability studies to demonstrate charge inversion in the presence of counterions with a valency exceeding two. Moreover, they showed that, in salt solutions containing ions with a high valency, colloidal stability displays a minimum at the isoelectric point, where the multivalent ions are able to perfectly neutralize the charged particles. At higher concentrations, charge inversion takes place, which has a stabilizing effect. These observations were corroborated by AFM measurements, with a repulsion at low and high concentrations of a multivalent salt, but a monotonic attraction at intermediate concentrations. This overall response was also found when the counterions are oligomeric9 or polymeric.5 We note that in the stability studies, the systems are canonical, that is, there is no infinitely large “bulk” solution, from (or to) which additional charge can be added. The AFM (or the surface force apparatus, SFA) setup, on the other hand, is grand canonical, in the sense that two macroscopically large surfaces are immersed in a solution large enough to ensure that the overall system contains more than enough counterion charge to neutralize the surfaces, even at minute salt concentrations. Nevertheless, at low enough concentrations, entropy will prevent this to occur.
There have also been theoretical studies on the relation between overcharging and free energy barriers. Turesson et al.36 utilized a grand canonical approach to study the interaction between two charged flat surfaces, immersed in a polyelectrolyte solution, where the polymers (or oligomers) were counterions to the surfaces. They found that even for moderately charged surfaces, overcharging occurs, with a concomitant double-layer repulsion between (effective) charge-inverted surfaces. In a subsequent work,37 they established that similar barriers are found if the polyions are replaced by spherical macroions with the same valency. One possible way to regulate the degree of overcharging, and thus the free energy barrier, is to insert a neutral midsection of the polyions.38
Overcharging will not be observed in a pure mean-field treatment, such as the Poisson–Boltzmann (PB) theory, but emerges when spatial correlation between the counterions is taken into account.20−25 The magnitude of the overcharging depends on the surface charge density. Hence, for two colloidal particles with a different magnitude of their charges, but the same charge sign, there is a possibility that the more highly charged particle is overcharged, while the other is not. This in turn would result in a long-range attraction, despite the ions having the same charge. A similar argument applies to oppositely charged particles, or surfaces, which might facilitate a long-ranged repulsion.3,39,40
In this work, we use a modified PB theory as well as grand canonical Monte Carlo (MC) simulations to calculate the interaction between charged surfaces in the presence of a multivalent salt at various concentrations. Using correlation-corrected PB (cPB) theory,41 we approximate spatial correlations between the counterions via a simple modification to the interaction potential between the multivalent cations. A great advantage of the cPB is that it gives direct access to the interaction free energy between the surfaces. It is also very simple and amounts to a tiny modification of the corresponding PB version, when formulated as a classical density functional theory (DFT).13,42 In contrast to most of the previous works on ion correlations and surface forces, we do not focus on the attraction at short range, but instead on how these correlations influence the interactions at long range. We calculate apparent surface charge densities (see below) and provide an explicit link between overcharging and the interaction between charged surfaces, at long range. We also investigate the range of the correlation attraction and discuss possible connections to surface heterogeneity. Our results are compared with experimental AFM data, on similar systems.
It should be noted that ion-specific behaviours,29−31 as well as effects from the molecular nature of the solvent,43 are outside the scope of this work.
Models and Methods
Our model surfaces consists of two planar and infinitely large walls. These are separated by a distance h and carry a smeared-out charge, represented by a surface charge density σs. A model aqueous salt solution fills the gap between the surfaces. Water is represented as a dielectric continuum via a dielectric constant ϵr = 78.3. In the simulations, the ion charges are centered in softly repulsive spheres, as described below. In the cPB treatment, however, ions are represented as point charges. The ions are in equilibrium with a bulk solution of a specified concentration.
The Coulomb interaction (free) energy between ions α and λ, separated by a distance r, is
| 1 |
where β is the inverse thermal energy, whereas the valency of ion α is denoted by zα. We define the Bjerrum length as lB = βe2/(4πϵ0ϵr), where e is the elementary charge and ϵ0 is the permittivity of vacuum.
In the simulations, all ions also repel each other by a short-ranged softly repulsive core potential, ϕs
| 2 |
where d = 4 Å. The reason to choose a soft, rather than hard, core is technical, as it will reduce noise in the pressure evaluations.
In the original PB theory, interactions between like charges are overestimated. This is because the mean-field treatment assumes a uniform charge distribution, and neglects the fact that ions of like charge will avoid close proximity. To correct for this overestimation, a modified interaction potential, ϕeffαα, is used between like-charged ions, which effectively accounts for the “Coulomb hole” that like-charge ion correlations generate.41 Specifically, the repulsion between them is weaker than the Coulomb interaction, at separations below some value Rc
| 3 |
where ϕcorrαα(r) is described as a tangent continuation of ϕCoul(r) at Rcα
| 4 |
There are in principle other alternatives that also would improve the original formulation (PB).
Rcα should reflect
the average nearest-neighbour distance
between ions of like charge. If ion α is a counterion to the
oppositely charged surfaces, then Rc will be determined by “crowding”
at the surfaces. In this work, we only include correlation-corrections
between (usually multivalent) counterions, whereas correlations between
the monovalent coions are neglected. In the original cPB formulation,41Rcα was defined as: 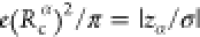 , which is physically reasonable (see ref (41) for further motivation),
but it is still an ad hoc quantification. Using this value, we arrive
at cPB predictions that are in qualitative agreement with the simulation
results. However, we noted that the simulated concentrations below
which overcharging disappears are better reproduced (quantitatively)
if Rc is increased by 50%. Hence, in the main paper, we have
used an adjusted value
, which is physically reasonable (see ref (41) for further motivation),
but it is still an ad hoc quantification. Using this value, we arrive
at cPB predictions that are in qualitative agreement with the simulation
results. However, we noted that the simulated concentrations below
which overcharging disappears are better reproduced (quantitatively)
if Rc is increased by 50%. Hence, in the main paper, we have
used an adjusted value
| 5 |
The results obtained with the original41 definition of Rcα are presented in the Supporting Information.
We shall by x denote the direction normal to the surfaces. The two flat walls are located at x = 0 and x = h and extend indefinitely in the (y, z) plane. They impose a hard-wall exclusion, ensuring that the x coordinate, xi, of all ions (center of mass) is located between these values, that is, 0 < xi < h. In principle, there is also an external wall potential, Vex(x, h), originating from the smeared-out surface charge density, but cancellation effects remove the dependence on x. This is because an y, z integration, in combination with an electroneutrality constraint, gives us βVextα(x,h) = −2πlBzα(σsx + σs(h – x)) = −2πlBzασsh, as the surfaces are equally charged. This appears to generate a separation-dependent potential, to be accounted for in grand canonical steps. However, because we always insert and remove electroneutral groups of ions, the surface charge potential needs not be considered in these processes and thus not in any MC steps (neither canonical nor grand canonical). In cPB, and PB, calculations, electroneutrality is maintained via a Donnan potential as has been previously described.41
Of central interest in this work is the interaction free energy per unit area, as the distance between the surfaces is varied. An advantage of the cPB (and PB) is that the free energy is directly available. In the simulations, periodic boundary conditions were applied along the directions (y, z) parallel to the surfaces. An external potential field, which was calculated from previously simulated ion density profiles, was used to account for the long-ranged interactions.44,45 In the grand canonical addition and deletion moves, an overall electroneutral group of ions was attempted to be added to, or removed from, the simulation cell. Thus, when performing an addition move of, for example, a 3:1 salt, one cation and three anions were attempted to be inserted at random positions. In the MC simulations, the free energies were calculated from the normal pressure. The pressure component normal to the surfaces, Pn, was obtained by calculating the average x-projection of all interaction forces per unit area, acting across a chosen plane at xp, and then adding the corresponding ideal pressure, Pid, where βPid = ∑αnα(xp). The position of the plane, xp, can in principle be arbitrarily chosen, but the system symmetry does suggest either the location of a wall or the midplane between the surfaces as “natural” choices. The latter turns out to give superior statistical properties because the density usually is modest and slowly varying near the midplane. Hence, we have in almost all cases evaluated Pn at the midplane of the slit, but we do include a single comparison with the corresponding results at a surface. The normal pressure is then calculated for a range of surface separations. A cubic spline fit to these data results in an approximation of Pn as a function of separation, Pn(h). By integrating this function, we arrive at the net interaction free energy per unit area, Δgs
| 6 |
where Pb is the bulk pressure, which in practice was estimated by the normal pressure at the largest investigated separation. Adopting the Derjaguin approximation,46 these interaction free energies are proportional to the force per radius measured in AFM, or SFA, experiments.
Our investigation will cover 2:1 and 3:1 salt solutions, at various concentrations. Some simulated and calculated data for a 1:1 salt solution are reported in the Supporting Information. Using the equilibrated ionic density profiles, we calculated an “apparent” surface charge density, σapp(x), defined as
| 7 |
where n+(x) and z+ denote the cation concentration and valency, respectively, with an analogous notation for the anions. The temperature was set to 298 K in all simulations and cPB (or PB) calculations.
Results and Discussion
We shall focus our attention on two different systems: surfaces with σs = −0.01e/Å2, immersed in 2:1 salt solutions, and surfaces with −0.005e/Å2, immersed in 3:1 salt solutions. The results obtained for these systems are presented in separate sections below.
3:1 Salt, σs = −0.005e/Å2
The cPB calculations produce interaction free energies directly as they are based on a free energy (classical) DFT. The simulation approach, however, generates normal pressures, Pn, which need to be integrated in order to arrive at interaction free energies. In principle, one can calculate the normal pressure across any plane parallel with the confining surfaces, but two choices are arguably more natural than others: the midplane or one of the walls. The latter choice has two disadvantages compared to the former: the density at contact requires extrapolation, and the overall noise is quite substantial, as compared with midplane evaluations. Normal pressure data from both approaches are given Figure 1. The comparison is not completely fair because the wall pressures were calculated via density samplings from (roughly) the last 25% of the production simulations, but it is nevertheless clear that the midplane pressure calculations display considerably less noise.
Figure 1.
Simulated normal pressure curves. Model surfaces, with σs, −0.005e/Å2 were immersed in a 23 mM 3:1 salt solution. Displayed are results from midplane (black plus signs) and wall (red crosses) evaluations. The line is a guide to the eye. Fewer data are reported for the normal pressure across a confining wall.
Simulated and calculated net interaction free energy curves, at various concentrations of 3:1 salt, are presented in Figure 2. In order to highlight the importance of correlations, we have also included a graph (Figure 2c) with data from standard PB calculations, that is, where the effective potential is equal to the Coulomb potential (Rc = 0). Simulations and cPB calculations display the same qualitative behaviour, with a substantial free energy barrier at low (0.23 mM) and high (23 mM) concentrations. At an intermediate concentration of about 4 mM, however, the barrier has vanished, resulting in monotonically attractive interactions (at least down to very short separations). The attraction results from well-established correlation effects, but curiously enough, so does the barrier that is gradually built up at concentrations above this threshold value. All of these effects are, as expected, lost with a pure PB treatment, as demonstrated in Figure 2c. In that case, the interaction is always monotonically repulsive, with a range that drops monotonically with salt concentration—a well-known DLVO result.
Figure 2.

Interaction free energies per unit area with surface separation, as obtained from MC simulations [graph (a)], cPB calculations [graph (b)], and standard PB calculations [graph (c)]. Model surfaces, with σs = −0.005e/Å2, were immersed in a 3:1 salt solution. The dashed curves are numerical two-parameter fits, of the long-range regimes, to the expression βΔgs = a0e–a1h.
For a given surface charge density, there is obviously a threshold concentration of 3:1 salt (around 4 mM, when σs = −0.005e/Å2), above which overcharging occurs, and a free energy barrier is restored. It should be noted that this barrier has a shorter range than the barriers that are obtained at “undercharging” concentrations. This is a simple screening effect, resulting from an increased ionic strength. In fact, at very high salt concentrations, we expect the barrier to diminish as a result of ionic screening, although we have not investigated this regime.
The dashed curves in Figure 2 are numerical fits, of the long-range tails, to the expression βΔgs = a0e–a1h. We will discuss these fits in more detail below.
We have already hinted that the free energy barriers are directly related to the surfaces being either under- or overcharged, that is, there is a remaining effective surface charge, when the charge contribution from counter- and coions in the vicinity surfaces have been accounted for. This is illustrated in Figure 3, where we plot how the apparent charge density varies with surface distance, at some large separation. We see how the bare surface charge is gradually being counteracted by a very diffuse layer at a low salt concentration, 0.23 mM, only achieving full neutralization at the midplane—which of course is demanded by electroneutrality. At a high concentration (23 mM), the bare negative surface charge is overcompensated by the trivalent counterions, resulting in an effective positive charge outside the primary (“Stern”) ion layer, that is, σapp displays a local extremum near each surface. Further away from this extremum, the apparent surface charge density decays slowly toward a zero value at the midplane, in a manner similar to that at low concentrations. Finally, at an intermediate concentration, 4 mM, the σapp(x) profile is essentially flat, outside a primary counterion layer that just barely neutralizes the surface charge. In the two former cases, there is a long-ranged repulsion (see Figure 2), which is absent at 4 mM. Hence, we can relate a long-ranged slope of σapp(x) to a long-ranged double-layer repulsion. Moreover, the range of the slope itself is related to range of the repulsion, and at low ionic strengths, σapp(x) displays a very gradual drop, which is reflected in a correspondingly “stretched” free energy barrier. On the other hand, with a flat σapp(x) profile, resulting from perfect neutralization by the primary counterion layer, there is no such long-ranged repulsion and thus no free energy barrier.
Figure 3.

Apparent surface charge density plotted against a separation-normalized surface distance at various 3:1 salt concentrations. The separations were h = 500 Å (0.23 mM), 200 Å (4 mM), and 100 Å (23 mM), where the values were chosen large enough to achieve near bulk-like conditions at the midplane. The results from cPB calculations as well as MC simulations are shown. Conditions as in Figure 2.
The quantitative performance of cPB is far from perfect, but still satisfactory, given its simplicity. Predicted free energy barriers do vary somewhat too strongly with salt concentration. This is possibly related to the fact that the ions have no hard core (“size”) in the cPB treatment.
These curves are in qualitative agreement with AFM measurements,9,34,35 although tetravalent counterions seemed to be required in order to establish a pronounced “overcharging barrier”. However, in Figure 4c in the work reported by Ruiz-Cabello and co-workers,35 there is an indication of a weak barrier building up at the highest investigated concentration, for trivalent counterions. We can also make crude quantitative comparisons between simulated and measured interactions. Applying the Derjaguin approximation, Δgs ≈ F/R, where F is the forces between curved surfaces of radius R, we can convert the maximum value, βΔgs ≈ 2.2e–5/Å2, at 23 mM (MC) to F/R ≈ 0.03 mN/m. Comparing with AFM data,9,34,35 we note that this is quite a reasonable value.
Figure 4.

Cation density profiles at various concentrations of 3:1 salt. Conditions as in Figure 2.
The occurrence of overcharging, or “charge reversal”, can actually be seen directly from the counterion density profile. Specifically, upon overcharging, this profile will display a local minimum near the surface. This is illustrated in Figure 4, where we again note a very nice agreement between the simple cPB theory and MC simulations.
At long range, we expect to recover the linearized PB predictions of an exponentially decreasing interaction free energy, decaying as e–κh, where κ = [∑i(βnie2zi2/(ϵrϵ0)]1/2. However, in the simulations, the formation of ion pairs, and higher-order clusters, may in principle lead to an increased effective Debye length, κ–1. We recall that the dashed lines in Figure 2 are numerical fits to the expression βΔgs = a0e–a1h. Such fits were performed at 0.23 mM (fitting range: 100–500 Å) and 23 mM (fitting range: 40–70 Å). These fits agree reasonably well with cPB as well as MC data, even though there is a small “intrinsic” error arising from the fact that we have set Δgs to zero at the largest separation, which of course is an impossible limit in the fitted expression, for any finite value of a0. The fitted decay lengths are 1/a1 ≈ 69 Å (MC) and 73 Å (cPB) at 0.23 mM and 1/a1 ≈ 8.1 Å (MC) and 7.2 Å (cPB) at 23 mM. These are in reasonable agreement with the expected Debye lengths, 1/κ ≈ 82 and 8.2 Å, respectively. The fact that the simulated estimate of the decay length actually is smaller than 1/κ suggests that ion clustering is rare under these conditions. We can proceed with attempts to connect the amplitude factor a0 to linearized PB approximations. In the latter case, a constant surface charge ansatz implies47 that βΔgs ≈ 2 * σeffe–κh/(κϵrϵ0), where σeff is some “effective” surface charge density, which classically would agree with a value of σapp(x) at a position (x) just outside the “Stern layer”. If we relate the fitted a0 values to σeff in this manner, we end up with the following values at a low concentration (0.23 mM), where no overcharging occurs: σeff ≈ −0.000064e/Å2 (MC) and σeff ≈ −0.00011e/Å2 (cPB). Compared with the corresponding apparent surface charge density profiles in Figure 3, these values seem quite reasonable because they roughly coincide with a value of σapp(x) outside the steep regime near the surface (the “Stern layer”). However, applying the same procedure for our fits at a high concentration (23 mM), where there is a pronounced overcharging, generates quite high values: σeff ≈ 0.00095e/Å2 (MC) and σeff ≈ 0.0022e/Å2 (cPB). There may be several reasons for this, one of which probably is that the local maximum of σapp(x) varies with separation. There is of course also the aspect that the mean-field-based idea of a inner “Stern layer”, outside of which there is a “diffuse layer”, is not really compatible with correlation-induced overcharging.
Let us now turn our attention to the attractive part of the curves. Experimentally, it is common to fit this part to an exponential expression, the rationale being that it originates from attraction between charged patches on heterogeneous surfaces.48−50 In Figure 5, we have attempted such a fit for the 4 mM concentration, where the interaction is monotonically attractive. Now, if we apply the idea of a surface composed of correlating charged species, aligned on a lattice and separated a distance a, we expect an inverse decay length of [κ2 + (2π/a)2]1/2.49 Equating this to the fitted inverse decay length, 0.2095/Å, we arrive at a ≈ 21 Å. This is actually close to the average separation per trivalent ion, 24.5 Å, if we assume a perfect surface charge neutralization by these ions.
Figure 5.
Fitting the simulated interaction curve for the 4 mM 3:1 salt (monotonically attractive) to an exponential expression, as indicated in the legend (dashed line).
In principle, this lends support to the picture of an attraction resulting from correlations between two approaching surfaces carrying an adsorbed lattice-like monolayer of trivalent ions. However, we would argue that this amounts to an oversimplified view. In reality, the simulated distribution of trivalent ions on our model surfaces is far from lattice-like.
In Figure 6, we present a configurational simulation snapshot, where we have isolated one of the surfaces together with its primary layer of adsorbed trivalent ions (4 mM 3:1 salt). The distribution is obviously more “liquid like” than “lattice like”. Note, however, that this does not invalidate the notion of correlations between ions at opposing surfaces, but it does demonstrate that these surfaces (with adsorbed counterions) are not strongly heterogeneous, that is, there is no long-range order. We would argue that intersurface ion correlations form a subclass of the broader description “ion correlations”, that is, they are not competing theories, in our view. It should also be noted that the main ideas underlying the cPB formulation are based on correlations between adsorbed ions at a surface.
Figure 6.

A simulation snapshot focusing on the distribution of trivalent ions (circles) very near one of the surfaces (4 mM 3:1 salt).
2:1 Salt, σs = −0.01e/Å2
We now proceed to corresponding investigations for a system with more highly charged surfaces, but with a reduced valency (+2) of the counterions. We expect qualitatively similar behaviours. It is nevertheless of interest to obtain quantified measures, which furthermore will allow us to test the cPB performance under quite different conditions.
Profiles of the surface charge density are provided, for a range of different salt concentrations, in Figure 7. We note that even though the surfaces are more highly charged, a higher salt concentration is required to produce overcharging, as compared with the 3:1 salt solutions discussed above. The threshold concentration, at which the primary counterion layer is able to perfectly neutralize the surfaces, is in this case about 60–70 mM. An even higher surface charge density would see this value drop. As before, we observe overcharging at concentrations exceeding this value, and “undercharging” at lower concentrations. Weak ionic screening leads to a slow decay of σapp(x) toward the vanishing midplane value when the salt concentration is low.
Figure 7.

Apparent surface charge density, plotted against a separation-normalized surface distance, at various 3:1 salt concentrations. The separations were h = 100 Å (14, 66 mM) and 50 Å (229 mM), where the values were chosen large enough to achieve near bulk-like conditions at the midplane. The results from cPB calculations as well as MC simulations are shown. Model surfaces, with σs = −0.01e/Å2, were immersed in a 2:1 salt solution.
Finally, we turn our attention to the corresponding interaction free energies, displayed in Figure 8. We note the expected non-monotonic behaviour, where a long-ranged barrier at low concentrations is removed as the salt concentration is increased to the threshold value that corresponds to “perfect neutralization” (Figure 7). We have seen that a further increase generates overcharging and this manifests itself by the re-establishment of a free energy barrier. This barrier has a relatively short range, on account of an increased ionic screening, but can nevertheless reach high values. Experimentally, overcharging and the concomitant re-establishment of a repulsive barrier at high salt seems like a rare observation, that is, generally a more highly valent counterion is required. There are a number of possible reasons for this, such as: our use of rather small model ions, ion-specific adsorption (presumable of anions), a rare occurrence (in practice) of such highly charged surfaces, or the fact that we do not include any van der Waals attraction in our treatment.
Figure 8.

Variations of free energies per unit area with surface separation, as obtained from MC simulations and cPB calculations. Conditions as in Figure 7.
It is gratifying to note that the cPB calculations are quite accurate also for this system, which deviates considerably from the 3:1 salt solution previously investigated. The overall tendency of cPB to overestimate how much the barrier height, as well as the apparent surface charge density, varies with concentration is found here too. This may possibly be related to the absence of any excluded volume interactions between the ions in the cPB treatment. However, the theory is extremely simple, and the improvement over standard PB is quite dramatic. The latter will not even qualitatively predict overcharging, or an attractive correlation regime, under any circumstances. It should be noted that transforming a PB code, using a DFT formulation, into its cPB correspondence only requires a few (less than 5) lines of code.
Conclusions
This work demonstrates that experimental observations of overcharging, and its direct relation to the reformation of free energy barriers, can be captured by a very simple primitive model description, free of any bare surface heterogeneity or ion-specific adsorption. This does not mean that such aspects always are irrelevant, only that they are not required to explain the observed behaviours. We argue that the simple extension of PB to form cPB, whereby correlation effects are approximately managed, is useful and pedagogically appealing. Moreover, the mentioned experimental observations are qualitatively captured not only by simulations but also by the cPB calculations. We hope that this work can help to merge the seemingly disparate views of “ion correlation attraction” and “patch attraction” (or “heterogeneous surface attraction”).
Acknowledgments
J.F. acknowledges financial support from the Swedish Research Council. M. Borkovec and G. Trefalt are acknowledged for fruitful discussions and constructive criticism. We also thank the LU cluster organization LUNARC for providing computational resources.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.langmuir.1c02268.
The authors declare no competing financial interest.
Supplementary Material
References
- Dahlgren M. A. G.; Waltermo A.; Blomberg E.; Claesson P. M.; Sjoestroem L.; Aakesson T.; Joensson B. Salt Effects on the Interaction between Adsorbed Cationic Polyelectrolyte Layers – Theory and Experiment. J. Phys. Chem. 1993, 97, 11769. 10.1021/j100147a033. [DOI] [Google Scholar]
- Dahlgren M. A. G.; Hollenberg H. C. M.; Claesson P. M. The Order of Adding Polyelectrolyte and Salt Affects Surface Forces and Layer Structures. Langmuir 1995, 11, 4480. 10.1021/la00011a049. [DOI] [Google Scholar]
- Besteman K.; Zevenbergen M. A. G.; Heering H. A.; Lemay S. G. Direct observation of charge inversion by multivalent ions as a universal electrostatic phenomenon. Phys. Rev. Lett. 2004, 93, 170802. 10.1103/physrevlett.93.170802. [DOI] [PubMed] [Google Scholar]
- Lin W.; Galletto P.; Borkovec M. Charging and Aggregation of Latex Particles by Oppositely Charged Dendrimers. Langmuir 2004, 20, 7465–7473. PMID: 15323490 10.1021/la049006i. [DOI] [PubMed] [Google Scholar]
- Gillies G.; Lin W.; Borkovec M. Charging and Aggregation of Positively Charged Latex Particles in the Presence of Anionic Polyelectrolyte. J. Phys. Chem. B 2007, 111, 8626. 10.1021/jp069009z. [DOI] [PubMed] [Google Scholar]
- Popa I.; Gillies G.; Papastavrou G.; Borkovec M. Attractive Electrostatic Forces between Identical Colloidal Particles Induced by Adsorbed Polyelectrolytes. J. Phys. Chem. B 2009, 113, 8458. 10.1021/jp904041k. [DOI] [PubMed] [Google Scholar]
- Gebbie M. A.; Valtiner M.; Banquy X.; Fox E. T.; Henderson W. A.; Israelachvili J. N. Ionic liquids behave as dilute electrolyte solutions. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 9674–9679. 10.1073/pnas.1307871110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbie M. A.; Dobbs H. A.; Valtiner M.; Israelachvili J. N. Long-range electrostatic screening in ionic liquids. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 7432–7437. 10.1073/pnas.1508366112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazzami Gudarzi M.; Trefalt G.; Szilagyi I.; Maroni P.; Borkovec M. Forces between negatively charged interfaces in the presence of cationic multivalent oligoamines measured with the atomic force microscope. J. Phys. Chem. C 2015, 119, 15482–15490. 10.1021/acs.jpcc.5b04426. [DOI] [Google Scholar]
- Oosawa F.Polyelectrolytes; Marcel Dekker: New York, 1971. [Google Scholar]
- Guldbrand L.; Jönsson B.; Wennerström H.; Linse P. Electrical Double Layer Forces, A Monte Carlo Study. J. Chem. Phys. 1984, 80, 2221. 10.1063/1.446912. [DOI] [Google Scholar]
- Kjellander R.; Marčelja S. Interaction of charged surfaces in electrolyte solutions. Chem. Phys. Lett. 1986, 127, 402–407. 10.1016/0009-2614(86)80304-9. [DOI] [Google Scholar]
- Nordholm S. Generalized van der Waals theory. XII. Application to ionic solutions. Aust. J. Chem. 1984, 37, 1. 10.1071/ch9840001. [DOI] [Google Scholar]
- Nordholm S. Simple analysis of the thermodynamic properties of the one-component plasma. Chem. Phys. Lett. 1984, 105, 302. 10.1016/0009-2614(84)85035-6. [DOI] [Google Scholar]
- Mier-y-Teran L.; Suh S.; White H. S.; Davis H. T. A nonlocal free energy density functional approximation for the electrical double layer. J. Chem. Phys. 1990, 92, 5087. 10.1063/1.458542. [DOI] [Google Scholar]
- Valleau J. P.; Ivkov R.; Torrie G. M. Colloid stability: The forces between charged surfaces in an electrolyte. J. Chem. Phys. 1991, 95, 520. 10.1063/1.461452. [DOI] [Google Scholar]
- Tang Z.; Scriven L. E.; Davis H. T. Effects of solvent exclusion on the force between charged surfaces in electrolyte solution. J. Chem. Phys. 1994, 100, 4527. 10.1063/1.466283. [DOI] [Google Scholar]
- Lamperski S.; Outhwaite C. W.; Bhuiyan L. B. A modified Poisson-Boltzmann analysis of the solvent primitive model electrical double layer. Mol. Phys. 1996, 87, 1049. 10.1080/00268979600100721. [DOI] [Google Scholar]
- Greberg H.; Kjellander R. Charge inversion in electric double layers and effects of different sizes for counterions and coions. J. Chem. Phys. 1998, 108, 2940–2953. 10.1063/1.475681. [DOI] [Google Scholar]
- Netz R. R. Electrostatistics of counter-ions at and between planar charged walls: From Poisson-Boltzmann to the strong-coupling theory. Eur. Phys. J. E: Soft Matter Biol. Phys. 2001, 5, 557. 10.1007/s101890170039. [DOI] [Google Scholar]
- Moreira A. G.; Netz R. R. Binding of Similarly Charged Plates with Counterions Only. Phys. Rev. Lett. 2001, 87, 078301. 10.1103/PhysRevLett.87.078301. [DOI] [PubMed] [Google Scholar]
- Quesada-Pérez M.; González-Tovar E.; Martín-Molina A.; Lozada-Cassou M.; Hidalgo-Álvarez R. Overcharging in Colloids: Beyond the Poisson–Boltzmann Approach. ChemPhysChem 2003, 4, 234–248. 10.1002/cphc.200390040. [DOI] [PubMed] [Google Scholar]
- Aubouy M.; Trizac E.; Bocquet L. r. Effective charge versus bare charge: an analytical estimate for colloids in the infinite dilution limit. J. Phys. A: Math. Gen. 2003, 36, 5835–5840. 10.1088/0305-4470/36/22/302. [DOI] [Google Scholar]
- Boroudjerdi H.; Kim Y.; Naji A.; Netz R.; Schlagberger X.; Serr A. Statics and dynamics of strongly charged soft matter. Phys. Rep. 2005, 416, 129–199. 10.1016/j.physrep.2005.06.006. [DOI] [Google Scholar]
- Naji A.; Kanduč M.; Forsman J.; Podgornik R. Perspective: Coulomb fluids - Weak coupling, strong coupling, in between and beyond. J. Chem. Phys. 2013, 139, 150901. 10.1063/1.4824681. [DOI] [PubMed] [Google Scholar]
- Bloomfield V. A. DNA condensation by multivalent cations. Biopolymers 1997, 44, 269–282. . [DOI] [PubMed] [Google Scholar]
- Derjaguin B.; Landau L. Theory of the Stability of Strongly Charged Lyophobic Sols and of the Adhesion of Strongly Charged Particles in Solutions of Electrolytes. Acta Physicochim. URSS 1941, 14, 633–662. [Google Scholar]
- Verwey E. J. W.; Overbeek J. T. G.. Theory of the Stability of Lyophobic Colloids; Elsevier Publishing Company Inc.: Amsterdam, 1948. [Google Scholar]
- Pavlovic M.; Huber R.; Adok-Sipiczki M.; Nardin C.; Szilagyi I. Ion specific effects on the stability of layered double hydroxide colloids. Soft Matter 2016, 12, 4024–4033. 10.1039/c5sm03023d. [DOI] [PubMed] [Google Scholar]
- Rouster P.; Pavlovic M.; Szilagyi I. Destabilization of titania nanosheet suspensions by inorganic salts: Hofmeister series and Schulze-Hardy rule. J. Phys. Chem. B 2017, 121, 6749–6758. 10.1021/acs.jpcb.7b04286. [DOI] [PubMed] [Google Scholar]
- Katana B.; Takács D.; Csapó E.; Szabó T.; Jamnik A.; Szilagyi I. Ion Specific Effects on the Stability of Halloysite Nanotube Colloids—Inorganic Salts versus Ionic Liquids. J. Phys. Chem. B 2020, 124, 9757–9765. 10.1021/acs.jpcb.0c07885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heyden F. H. J.; Stein D.; Besteman K.; Lemay S. G.; Dekker C. Charge inversion at high ionic strength studied by streaming currents. Phys. Rev. Lett. 2006, 96, 224502. 10.1103/physrevlett.96.224502. [DOI] [PubMed] [Google Scholar]
- de Vos W. M.; Lindhoud S. Overcharging and charge inversion: Finding the correct explanation(s). Adv. Colloid Interface Sci. 2019, 274, 102040. 10.1016/j.cis.2019.102040. [DOI] [PubMed] [Google Scholar]
- Sinha P.; Szilagyi I.; Montes Ruiz-Cabello F. J.; Maroni P.; Borkovec M. Attractive Forces between Charged Colloidal Particles Induced by Multivalent Ions Revealed by Confronting Aggregation and Direct Force Measurements. J. Phys. Chem. Lett. 2013, 4, 648–652. PMID: 26281881 10.1021/jz4000609. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabello F. J. M.; Trefalt G.; Csendes Z.; Sinha P.; Oncsik T.; Szilagyi I.; Maroni P.; Borkovec M. Predicting Aggregation Rates of Colloidal Particles from Direct Force Measurements. J. Phys. Chem. B 2013, 117, 11853–11862. PMID :24 015897 10.1021/jp406061f. [DOI] [PubMed] [Google Scholar]
- Turesson M.; Åkesson T.; Forsman J. Interactions between charged surfaces immersed in a polyelectrolyte solution. Langmuir 2007, 23, 9555–9558. 10.1021/la7017852. [DOI] [PubMed] [Google Scholar]
- Turesson M.; Woodward C. E.; Åkesson T.; Forsman J. Simulations of Surface Forces in Polyelectrolyte Solutions. J. Phys. Chem. B 2008, 112, 5116. 10.1021/jp800632e. [DOI] [PubMed] [Google Scholar]
- Turesson M.; Åkesson T.; Forsman J. Block Polyelectrolytes and Colloidal Stability. J. Colloid Interface Sci. 2009, 329, 67. 10.1016/j.jcis.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Trulsson M.; Jönsson B.; Akesson T.; Forsman J.; Labbez C. Repulsion between Oppositely Charged Surfaces in Multivalent Electrolytes. Phys. Rev. Lett. 2006, 97, 068302. 10.1103/PhysRevLett.97.068302. [DOI] [PubMed] [Google Scholar]
- Forsman J. Density functional theories of surface interactions in salt solutions. J. Chem. Phys. 2009, 130, 064901. 10.1063/1.3071195. [DOI] [PubMed] [Google Scholar]
- Forsman J. A simple correlation-corrected Poisson-Boltzmann theory. J. Phys. Chem. B 2004, 108, 9236–9245. 10.1021/jp049571u. [DOI] [Google Scholar]
- Nordholm S.; Johnson M.; Freasier B. Generalized van der Waals theory. III. The prediction of hard sphere structure. Aust. J. Chem. 1980, 33, 2139. 10.1071/ch9802139. [DOI] [Google Scholar]
- Schlaich A.; dos Santos A. P.; Netz R. R. Simulations of Nanoseparated Charged Surfaces Reveal Charge-Induced Water Reorientation and Nonadditivity of Hydration and Mean-Field Electrostatic Repulsion. Langmuir 2019, 35, 551–560. 10.1021/acs.langmuir.8b03474. [DOI] [PubMed] [Google Scholar]
- Torrie G. M.; Valleau J. P. Electrical double layers. I. Monte Carlo study of a uniformly charged surface. J. Chem. Phys. 1980, 73, 5807–5816. 10.1063/1.440065. [DOI] [Google Scholar]
- Jönsson B.; Wennerström H.; Halle B. Ion distributions in lamellar liquid crystals. A comparison between results from Monte Carlo simulations and solutions of the Poisson-Boltzmann equation. J. Phys. Chem. 1980, 84, 2179–2185. 10.1021/j100454a014. [DOI] [Google Scholar]
- Derjaguin B. Untersuchungen über die Reibung und Adhäsion, IV. Kolloid-Z. 1934, 69, 155. 10.1007/bf01433225. [DOI] [Google Scholar]
- Israelachvili J. N.Intermolecular and Surface Forces, 2nd ed.; Academic Press: London, 1991. [Google Scholar]
- Tsao Y.-H.; Evans D. F.; Wennerström H. Long-range attractive force between hydrophobic surfaces observed by atomic force microscopy. Science 1993, 262, 547–550. 10.1126/science.8211182. [DOI] [PubMed] [Google Scholar]
- Miklavic S. J.; Chan D. Y. C.; White L. R.; Healy T. W. Double Layer Forces between Heterogeneous Charged Surfaces. J. Phys. Chem. 1994, 98, 9022–9032. 10.1021/j100087a034. [DOI] [Google Scholar]
- Forsman J.; Jönsson B.; Åkesson T. Long-Ranged Attractive Forces between Neutral Surfaces Due to Amphiphile Aggregation. J. Phys. Chem. B 1998, 102, 5082–5087. 10.1021/jp980022w. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




