Abstract
Background:
Diabetes is associated with worse asthma morbidity. Metformin, which treats diabetes, may have a role among patients with asthma and glycemic dysfunction.
Objective:
To determine the association between metformin use and asthma exacerbations among patients with diabetes.
Methods:
We queried the Johns Hopkins EHR from April 1, 2013 to May 31, 2018. Adults with asthma and diabetes were followed from first hemoglobin A1c (HbA1c) test to an asthma-related systemic corticosteroid prescription, emergency department (ED) visit, or hospitalization. Multivariable Cox models estimated time to each outcome associated with metformin use, modeled as either time-invariant (status at HbA1c testing) or time-dependent (based on fill data). Mediation of results by HbA1c was assessed. Sensitivity analysis was performed by propensity score matching.
Results:
The cohort comprised of 1,749 adults with asthma and diabetes. Metformin use at entry was associated with a lower hazard of asthma-related ED visits (adjusted hazard ratio [aHR] 0.40, 95% confidence interval [CI] 0.22–0.75) but not steroid prescription (aHR 0.89; 95% CI 0.70–1.13) or hospitalization (aHR 0.38, 95% CI 0.13–1.12). HbA1c did not mediate the association with ED visits. With metformin exposure modeled as time-dependent, metformin use was additionally associated with lower hazard of asthma-related hospitalization (aHR 0.30, 95% CI 0.09–0.93). Results were consistent within a sub-cohort of 698 metformin users matched 1:1 to non-users by propensity score.
Conclusion:
Metformin use, independent of glycemic control and obesity, was associated with lower hazard of asthma-related ED visits and hospitalizations. Metformin may have benefit in patients with asthma and glycemic dysfunction.
Keywords: asthma, diabetes, metabolic disease, glycemic dysfunction, obesity, metformin
INTRODUCTION
Asthma can be precipitated and exacerbated by both allergic and non-allergic factors. Glycemic dysfunction, encompassing insulin resistance, prediabetes, and diabetes, has been suggested as one such non-allergic factor1. Multiple clinical and epidemiological cohorts of asthma have consistently reported positive associations of glycemic dysfunction and asthma morbidity independent of the presence of obesity2–5. Glycemic dysfunction may worsen asthma control through effects of insulin on airway smooth muscle remodeling and contractility and increased susceptibility to infection. Thus, it is possible that medications which improve glycemic dysfunction could improve asthma in some individuals.
Metformin is an oral medication that is indicated for the initial treatment of type 2 diabetes mellitus6. It improves insulin resistance and glycemic control and has direct anti-inflammatory effects. Translational studies have reported benefits of metformin administration in asthma models, including inhibition of airway smooth muscle remodeling and amelioration of eosinophilic infiltration and allergic inflammation7,8. Yet, evidence of clinical benefit in human subjects is limited. An inverse relationship between metformin use and adverse asthma-related outcomes has been reported in studies within administrative databases from the United States and Taiwan9,10. However, these studies have been limited by an inability to measure critical confounders, chiefly concurrent glycemic control, body mass index (BMI), smoking, and race.
To address these limitations, we sought to determine the association of metformin use and asthma exacerbation in an electronic health record (EHR)-based cohort of individuals with asthma and diabetes. We hypothesized that metformin use would be associated with lower risks of asthma-related steroid prescription, emergency department visit, and hospitalization.
METHODS
Cohort derivation
We queried the EHR of the Johns Hopkins Health System from April 1, 2013 to May 31, 2018. This source population represents patients seen in Johns Hopkins community outpatient clinics and all hospitals within the Baltimore-Washington, D.C. area (Johns Hopkin Hospital, Johns Hopkins Bayview Medical Center, Suburban Hospital, Sibley Memorial Hospital, and Howard County General Hospital).
We isolated a cohort of patients with asthma and diabetes. First, we identified adult (age>18 years) patients with asthma on the basis of two separate outpatient or one inpatient International Classification of Diseases (ICD) diagnostic code for asthma recorded in any encounter position during the sampling period11. From this group, we identified patients who had HbA1c testing, requiring that this test occurred after identification of asthma. Patients with an HbA1c ≥6.5 or were taking diabetes medications on the date of measurement were identified as having diabetes12. Patients with pre-diabetes were not included as metformin treatment is controversial for this group.
The index date for both metformin and non-metformin users was set to the time of HbA1c testing. Included patients were required to have at least one active outpatient medication at the index date, to ensure some intensity of outpatient surveillance, at least one additional contact with the healthcare system afterwards, to ensure that they were still eligible to observe outcomes, and a body mass index recorded within the prior year, as this was considered a critical confounder and also reflected a recent physical examination. We excluded patients with chronic kidney disease and type 1 diabetes which are both contraindications to metformin treatment. We also excluded individuals with chronic lung disease other than asthma (chronic obstructive pulmonary disease, interstitial lung disease, and bronchiectasis) or an active order for a systemic corticosteroid during their index date. A diagram of cohort derivation (Figure E2) and ICD codes utilized in this study (Table E1) are included in the online supplement.
This study was approved by the Institutional Review Board of the Johns Hopkins School of Medicine and the Research and Projects Committee of Johns Hopkins Community Physicians. Data handling and chart validation activities were performed in compliance with institutional guidelines.
Exposure and covariate assessment
We assessed metformin exposure as time-independent or time-dependent (Figure E1). In the time-independent approach, individuals were classified as metformin users or non-users if they were taking this medication on their index date, thus assuming persistent use or non-use through the follow-up period. This approach requires less assumptions regarding the timing or duration of possible benefit of metformin at the expense of greater exposure misclassification13.
In the time-dependent approach, individuals were free to switch between use and non-use. Exposure periods were constructed based on medication lists which were updated at each clinical encounter. We allowed a 30-day grace period (treating gaps of up to this length as continuously exposed) to accommodate late refills, which may occur if a patient has extra doses from an earlier refill or skipped doses14. In the absence of contrary data, we assumed no carry-over effects.
Age, sex, race, smoking status, and comorbidity information were extracted at the index date. Comorbidity burden was represented by the Charlson Comorbidity Index (CCI), modified to exclude criteria for diabetes, collected over the year prior to the index date15.
Three asthma-related outcomes were assessed: hospitalization with a principal diagnosis of asthma, emergency department visit with a diagnosis of asthma in the first or second diagnostic position, and a new outpatient order for a systemic corticosteroid within fourteen days of an encounter where asthma was a listed diagnosis. These outcomes mirror clinical trial definitions for moderate or severe asthma exacerbation16 and have been previously applied in database-driven studies of asthma17,18. Use of the first or second diagnostic position for asthma-related emergency department visits was due to the high frequency of symptom-based diagnoses in the first position and is a validated approach for identifying asthma-related ED events19. We also performed an internal validation of study outcomes.
Analytic approach
Baseline covariate distributions between metformin users and non-users were compared by standardized differences. The standardized difference is more appropriate than p-values when comparing groups with large numbers. Larger values reflect greater imbalance, with a standardized difference of >0.1 approximately corresponding to a p-value <0.0520.
The association of metformin use and time to first occurrence of each asthma outcome was estimated by a multivariable Cox proportional hazards model. Because individuals can move outside of the catchment area or otherwise become ineligible to experience the outcome, follow-up was censored at the date of their last contact with the health system within the sampling frame. We also performed a sensitivity analysis whereby individuals were censored on May 31, 2018.
Based on an analysis of assumed relationships between metformin use, study outcomes, and other variables (depicted in the directed acyclic graph included as Figure E3), we selected a set of covariates to estimate the independent metformin and asthma relationship21. We adjusted for age, sex, race (Black, White, other), smoking status (current, former, never), BMI, CCI, insurance type as a proxy for access to care and socioeconomic status (Medicaid, Medicare, Tricare, other), and use of thiazolidinedione (TZD) or glucagon-like peptide-1 (GLP-1) medications. Adjustment for TZD and GLP-1 was done based on biologically plausible reports that these medications may modulate asthma severity independent of glycemic control22,23, but it was omitted from the hospitalization outcome due to insufficient variation across events.
We next did a traditional mediation analysis of HbA1c on the observed metformin—asthma association24. Because such analysis requires additional control for exposure—mediator confounding, among others, the number of distinct diabetes medications was added as a covariate as treatment intensity would affect both HbA1c and likelihood of metformin use. The indirect effect of metformin mediated by HbA1c was calculated as the difference in the metformin coefficient (natural logarithm of the hazard ratio) without and with HbA1c adjustment. Confidence intervals were derived through bootstrap estimation25. Mediation analysis was only done for time-independent estimates because there was insufficient data to model HbA1c as time-varying and only for outcomes that had an association with metformin use.
Finally, as an exploratory analysis, we tested for effect measure modification by sex, obesity, race, and inhaled corticosteroid use. Statistical significance was accepted at a two-sided p-value of <0.05. Medication use data was extracted using SAS 9.4 (Cary, NC). All analyses were performed in Stata 15 (College Station, TX).
Propensity score-matched sub-cohort
As a sensitivity analysis, we ran parallel models in a sub-cohort of metformin users and non-users matched by propensity score. This score, representing likelihood of receiving metformin treatment, was estimated by a logistic regression model that included all baseline covariates including indicator variables for individual diabetes and asthma medications26. Users were matched to non-users in a 1:1 ratio without replacement by the nearest neighbor method. Distributions were trimmed to areas of common support. The caliper distance was initially set to 0.2 times the standard deviation of the propensity score logit and then incrementally narrowed to produce balanced groups as determined by estimation of standardized differences and visual inspection of propensity score distributions27. Analyses within this cohort included only metformin as the predictor with standard errors adjusted for clustering by match pair. We also analyzed the full cohort stratified by propensity score quintiles and weighted on the inverse probability of treatment, both modified to estimate the average treatment effect on treated (ATT)26.
Internal validation
For each outcome, we randomly selected 60 charts for validation, stratified by whether the outcome occurred (detect chart) or not (non-detect chart) and by baseline metformin use. Two pulmonologists (MCM, AF) independently reviewed 10% of charts and confirmed high concordance (kappa >0.9), and the remaining charts were singly reviewed (AF). Reviewers determined whether a detected event represented an asthma exacerbation or, for non-detect charts, whether an asthma event of that type occurred at any time during follow-up. Positive and negative predictive values with 95% confidence intervals were estimated. Difference in accuracy by metformin use was assessed by a Fisher’s exact test. We examined 151 unique charts due to overlap between asthma-related hospitalizations and ED visits.
RESULTS
Cohort description
A total of 1,749 individuals with concurrent asthma and diabetes were identified (Figure E2). Patients were under observation within the EHR for a median (interquartile range) of 3.2 (1.4–4.4) years. Approximately half were of Black or African American race and three-fourths were female. Mean age (standard deviation) was 54 (14) years. Mean BMI was 37 (9) kg/m2, with 78% of participants being classified as obese. With respect to between-group differences, metformin users were younger, had less comorbidity burden, and were more likely to also use sulfonylureas (Table 1). Other characteristics were similar between these two groups.
Table 1.
Baseline characteristics (n=1749)
| Characteristic | Metformin non-users | Metformin users | Standardized difference§ |
|---|---|---|---|
| Number | 888 | 861 | |
|
| |||
| Age, mean (SD) | 55.1 (14.3) | 53.3 (13.6) | 0.13 |
|
| |||
| Female sex, n (%) | 650 (73) | 638 (74) | 0.02 |
|
| |||
| Race, n (%) | 0.07 | ||
| Black | 456 (51) | 413 (48) | |
| White | 349 (39) | 355 (41) | |
| Other | 83 (9) | 93 (11) | |
|
| |||
| Smoking status | 0.04 | ||
| Current | 90 (10) | 97 (11) | |
| Former | 272 (31) | 254 (30) | |
| Never | 526 (59) | 510 (59) | |
|
| |||
| Insurance type | 0.17 | ||
| Medicaid | 123 (14) | 106 (12) | |
| Medicare | 287 (32) | 229 (27) | |
| Tricare | 79 (9) | 107 (12) | |
| Private/other | 399 (45) | 419 (49) | |
|
| |||
| Body mass index, mean (SD) | 37.1 (9.1) | 37.4 (8.8) | 0.03 |
|
| |||
| Asthma medications* | |||
| Inhaled corticosteroid | 286 (32) | 298 (35) | 0.05 |
| Long-acting beta agonist | 167 (19) | 167 (19) | 0.02 |
| Leukotriene modifier | 126 (14) | 127 (15) | 0.02 |
|
| |||
| Diabetes medications* | |||
| Sulfonylurea | 106 (12) | 208 (24) | 0.32 |
| Thiazolidinedione | 13 (2) | 22 (3) | 0.08 |
| Glucagon-like peptide-1 agonist | 15 (2) | 24 (3) | 0.07 |
| Dipeptidyl peptidase 4 inhibitor | 36 (4) | 87 (10) | 0.24 |
| Insulin | 172 (19) | 160 (19) | 0.02 |
|
| |||
| Hemoglobin A1c, %, mean (SD) | 7.7 (1.7) | 7.6 (2.0) | 0.05 |
|
| |||
| Charlson Comorbidity Index | 0.36 (0.73) | 0.22 (0.49) | 0.21 |
medication classes with less than 1% prevalence in all groups are not listed
a larger standardized difference reflects a greater difference between groups, values above 0.1 are considered statistically significant
SD: standard deviation
Metformin use and asthma exacerbations in the primary cohort
There were 278 oral corticosteroid prescriptions over 4,583 person-years, 46 emergency department visits for asthma over 5,026 person-years, and 16 hospitalizations for asthma over 5,064 person-years of follow-up.
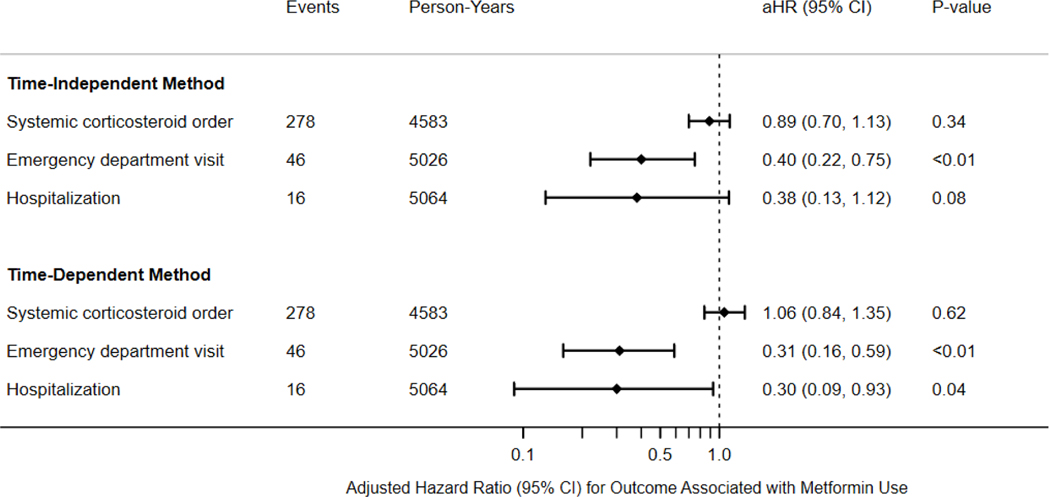
With metformin exposure modeled as time-independent, 861 (49%) patients had an active prescription for metformin at the index date. In adjusted analysis, metformin use was associated with lower hazard of ED visit for asthma (aHR 0.40, 95% CI 0.22, 0.75) but not with hospitalization for asthma (aHR 0.38, 95% CI 0.13, 1.12) or corticosteroids for asthma (aHR 0.89; 95% CI 0.70–1.13) (Figure 1).
Figure 1.
Association of metformin use and study outcomes. Metformin use is assessed at baseline (time-independent) or varying over follow-up (time-dependent). Models are adjusted for age, sex, race, smoking status, body mass index, Charlson Comorbidity Index, insurance type, and use of thiazolidinedione or glucagon-like peptide-1 medications (omitted from hospitalization outcome); aHR: adjusted hazard ratio; CI: confidence interval.
After the index date, a substantial proportion of metformin non-users and users started or stopped metformin, respectively. For example, for asthma-related hospitalizations, 314/888 (35%) metformin non-users subsequently started at least one prescription for metformin, and 377/861 (44%) metformin users discontinued use at least once prior to event occurrence or censoring. Among those who changed their metformin use during follow-up, most switched once. In the time-dependent analysis, metformin use was additionally associated with a lower hazard of asthma-related hospitalization (aHR 0.30; 95% CI 0.09, 0.93) and continued to be associated with lower hazard of ED visits for asthma (aHR 0.31; 95% CI 0.16, 0.59).
These results were consistent in a sensitivity analysis extending censoring time to the end of the sampling frame. We also did not identify effect measure modification for any outcome by sex, obesity, race, or inhaled corticosteroid use (not shown).
Mediation of metformin and asthma-related ED visits by HbA1c
After confirmation that metformin use was associated with asthma-related ED visits, we next confirmed that metformin use was associated with a difference in HbA1c (adjusted mean difference −0.23%; 95% CI −0.40%, −0.06%). After additional adjustment for HbA1c, however, there was minimal change in the coefficient associated with metformin use in models estimating its relationship with asthma-related ED visits. Consequently, we found no statistical evidence that HbA1c was a mediator of this association (Table E2).
Metformin use and asthma exacerbations in the propensity score-matched sub-cohort
From the primary cohort, 1,396 (80%) of individuals were successfully matched on similar likelihood of baseline metformin treatment as estimated by the propensity score. The differences in characteristics within the primary cohort were not apparent after propensity score matching, especially with regard to comorbidity burden (Table E3). Similar to the primary analysis, in the propensity score-matched cohort metformin use was associated with lower hazard of asthma-related ED visits in the time-independent method and with both asthma-related ED visits and hospitalizations in the time-dependent method (Table E4). Results with alternative methods of applying the propensity score are also included in Table E4.
Internal validation
Chart validation was performed. The positive predictive values for asthma-related corticosteroids, ED visits, and hospitalizations were high and ranged from 77 to 93% (Table E5). Interestingly, despite requiring that a steroid order be accompanied by an asthma diagnostic code for the steroid order to be considered asthma-related, there were numerous false positives where steroids were prescribed for other indications, such as musculoskeletal pain, nausea, and rash, within clinical encounters where asthma was a listed diagnosis. However, there was no evidence that accuracy was different between metformin users and non-users for any outcome (p-value >0.05 for all comparisons).
DISCUSSION
In this retrospective observational study of adults with asthma and diabetes, we report an inverse relationship between metformin use and asthma-related ED visits and hospitalizations. These results suggest that metformin may have a role in individuals with asthma and concurrent glycemic dysfunction. Prospective investigation is necessary to evaluate these findings.
An inverse association between metformin use and adverse asthma-related outcomes has been previously reported by our group and others9,10. These studies utilized cohorts derived from administrative databases which were vulnerable to uncontrolled confounding from important covariates such as BMI, glycemic control, race, and smoking. These limitations are better addressed in this study, where the use of clinical data permitted their ascertainment. The population is additionally drawn from a large academic health system encompassing individuals from a broad range of socioeconomic status and enriched for minorities, which is complementary to these previous studies. Most critically, this study is the first to distinguish possible actions of metformin independent of its impact on glycemic control, finding that HbA1c was not a mediator of the identified relationships.
Metformin is an oral anti-hyperglycemic medication that is indicated for the initial treatment of type 2 diabetes mellitus6. Its effects, which remain incompletely understood, extend beyond improvements in glycemic control28. Several mechanisms whereby metformin may improve asthma are possible. Insulin resistance, the metabolic derangement underlying diabetes and of which is improved by metformin, has been associated with worse airway smooth muscle hyper-reactivity and contractility in translational studies29,30. In an animal model of allergic obesity-associated asthma, metformin attenuated the degree of eosinophilic airway infiltration with improvements in TNF-a inflammatory signaling and inducible NOS expression7. In vitro, metformin also impaired TGF-β1-induced airway smooth muscle proliferation, a pathway active in airway remodeling in asthma8. Importantly, the finding that the metformin—asthma exacerbation association is not mediated by HbA1c suggests that potential mechanisms are not dependent on hyperglycemia per se. However, further insight is limited due to the absence of detectable effect measure modification in subgroup analyses.
Metformin use was associated with a lower hazard of ED visits and hospitalizations for asthma but not with differences in hazard of prescription for oral corticosteroids. This pattern is similar to that reported by a previous study within claims data and suggests that metformin may be more relevant to severe forms of asthma exacerbation10. Such heterogeneity can reflect differential impacts on pathways that lead to worsening of asthma control and emphasize the need for a comprehensive prospective study of metformin among individuals with asthma and glycemic dysfunction.
An internal validation confirmed acceptable positive and negative predictive values for our outcomes. However, we note that efforts to further improve accuracy of asthma-related corticosteroids beyond the observed 77% in this study will likely require integration of additional data sources to better infer clinical intent, as false positives were often due to asthma being addressed in the same visit as another condition which necessitated steroids. We further note that the performance of these definitions is expected to vary from cohort to cohort31. Overall, incidence rates for events were low and consistent with estimates from other real-world populations.
Our results should not be interpreted as conclusive. These are three key limitations which prompt the need for confirmatory investigation. Due to sample size limitations, metformin users were prevalent rather than incident users, preventing the ability to disentangle potential time-dependent and dose-dependent effects of metformin use. Prevalent users by virtue of maintaining a chronic medication may also be more likely to engage in healthy behaviors such as adherence to asthma medications, as depicted by our causal diagram. However, all participants were required to have had their HbA1c measured with continued contact with the health system, used at least one outpatient medication, and underwent at least one examination, suggesting that these factors may be less influential. Second, our comparator group were individuals with an apparent indication for metformin but were not receiving it, which may suggest the presence of unmeasured comorbidities and health factors that can also worsen asthma. Because metformin is the only first-line agent for diabetes, we were unable to compare metformin users to users of other diabetes medications (an active comparator) as those using second-line diabetes medications would also be more likely to have worse health status13. Consistent results in time-dependent analysis and the propensity score-matched sub-cohort are reassuring but cannot fully discount the potential for such confounding especially by lifestyle factors such as physical activity and diet. Finally, because covariates were measured during routine clinical activity, study generalizability is limited by selection biases related to the seeking of healthcare attention. Indeed, our population was older and obese which does not reflect the overall population of individuals with asthma. Such biases, many of which are intrinsic to EHR studies, are reviewed in greater detail elsewhere32.
In conclusion, we report an inverse association between metformin use and asthma-related ED visits and hospitalizations in an electronic health record-derived cohort of adults with asthma and diabetes. This suggests that metformin may have a role in improving asthma morbidity among patients with glycemic dysfunction. Prospective studies are warranted to define potential mechanisms and to better identify target populations which may derive particular benefit.
Supplementary Material
HIGHLIGHTS.
What is already known about this topic: Metformin use has been associated with lower risk of asthma exacerbation in claims-based studies. Results are limited by unmeasured confounding, particularly by glycemic control and body mass index.
What does this article add to our knowledge: In a retrospective cohort of adults with asthma and diabetes derived from an electronic health record, metformin use was associated with lower risk of asthma-related ED visits and hospitalizations in a manner that was independent of glycemic control and obesity.
How does this study impact current management guidelines: Treatment of metabolic dysfunction with metformin may improve asthma control. Prospective investigation with measurement of metabolic intermediaries is necessary to understand potential mechanisms and sub-types of asthma which may benefit.
ACKNOWLEDGEMENTS
We thank Bama Padmanaban and the Johns Hopkins Center for Clinical Data Analysis for their invaluable assistance in cohort extraction.
Funding Statement
Research reported in this manuscript was supported by a grant from the COPD Foundation (TDW) and in part by resources from the National Institutes of Health (K23HL151669) and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institutes of Health, Department of Veterans Affairs, or the United States government.
Disclosures
TDW and AA have no disclosures. CK discloses research funding from the National Institutes of Health, associate editorship at the Journal of Allergy and Clinical Immunology, board membership at the American Board of Allergy and Immunology, and royalties from Up-to-Date, outside of submitted work. NNH discloses research funding from the National Institutes of Health and speakership and advisory fees from Mylan, Theravance, AstraZeneca, and GlaxoSmithKline, outside of submitted work. MCM discloses research funding from the National Institutes of Health, consultancies for GlaxoSmithKline and Celegene, and royalties from Up-to-Date, outside of submitted work.
ABBREVIATIONS
- BMI
Body mass index
- CCI
Charlson Comorbidity Index
- EHR
Electronic health record
- GLP-1
Glucagon-like peptide-1
- ICD
International Classification of Diseases
- HbA1c
Hemoglobin A1c
- TZD
Thiazolidinedione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pite H, Aguiar L, Morello J, Monteiro EC, Alves AC, Bourbon M, et al. Metabolic Dysfunction and Asthma: Current Perspectives. J Asthma Allergy. 2020. July 27;13:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet JC, et al. Evidence for Exacerbation-Prone Asthma and Predictive Biomarkers of Exacerbation Frequency. Am J Respir Crit Care Med. 2020. June 1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price D, Wilson AM, Chisholm A, Rigazio A, Burden A, Thomas M, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016. January 7;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu TD, Brigham EP, Keet CA, Brown TT, Hansel NN, McCormack MC. Association Between Pre-Diabetes/Diabetes and Asthma Exacerbations in a Claims-Based Obese Asthma Cohort. J Allergy Clin Immunol Pract [Internet]. 2019. March 8 [cited 2019 Mar 26];0(0). Available from: https://www.jaci-inpractice.org/article/S2213-2198(19)30244-2/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Han Y-Y, Forno E, Yan Q, Rosser F, Chen W, et al. Glycated hemoglobin A1c, lung function, and hospitalizations among adults with asthma. J Allergy Clin Immunol Pract. 2020. June 19; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association AD. Introduction: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019. January 1;42(Supplement 1):S1–2. [DOI] [PubMed] [Google Scholar]
- 7.Calixto MC, Lintomen L, André DM, Leiria LO, Ferreira D, Lellis-Santos C, et al. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PloS One. 2013;8(10):e76786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y, Liu L, Li S, Wang K, Ke R, Shi W, et al. Activation of AMPK inhibits TGF-β1-induced airway smooth muscle cells proliferation and its potential mechanisms. Sci Rep. 2018. Feb 26;8(1):3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C-Y, Erickson SR, Wu C-H. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirol Carlton Vic. 2016;21(7):1210–8. [DOI] [PubMed] [Google Scholar]
- 10.Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of Metformin Initiation and Risk of Asthma Exacerbation: A Claims-Based Cohort Study. Ann Am Thorac Soc. 2019. August 15; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nissen F, Quint JK, Wilkinson S, Mullerova H, Smeeth L, Douglas IJ. Validation of asthma recording in electronic health records: a systematic review. Clin Epidemiol. 2017. December 1;9:643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019. January 1;42(Supplement 1):S29–33. [DOI] [PubMed] [Google Scholar]
- 13.Salvo F, Faillie J-L. Interest and challenges of pharmacoepidemiology for the study of drugs used in diabetes. Therapie. 2019. April;74(2):255–60. [DOI] [PubMed] [Google Scholar]
- 14.Weisman A, King LK, Mamdani M. Reporting and variability of constructing medication treatment episodes in pharmacoepidemiology studies: A methodologic systematic review using the case study of DPP-4 inhibitors and cardiovascular outcomes. Pharmacoepidemiol Drug Saf. 2020. August;29(8):939–50. [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005. November;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 16.Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations: Standardizing Endpoints for Clinical Asthma Trials and Clinical Practice. Am J Respir Crit Care Med. 2009. July;180(1):59–99. [DOI] [PubMed] [Google Scholar]
- 17.Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, et al. High Blood Eosinophil Count Is a Risk Factor for Future Asthma Exacerbations in Adult Persistent Asthma. J Allergy Clin Immunol Pract. 2014. November 1;2(6):741–750.e4. [DOI] [PubMed] [Google Scholar]
- 18.Schatz M, Meckley LM, Kim M, Stockwell BT, Castro M. Asthma Exacerbation Rates in Adults Are Unchanged Over a 5-Year Period Despite High-Intensity Therapy. J Allergy Clin Immunol Pract. 2014. September 1;2(5):570–574.e1. [DOI] [PubMed] [Google Scholar]
- 19.Travers D, Lich KH, Lippmann SJ, Weinberger M, Yeatts KB, Liao W, et al. Defining emergency department asthma visits for public health surveillance, North Carolina, 2008–2009. Prev Chronic Dis. 2014. June 12;11:E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Commun Stat - Simul Comput. 2009. May 14;38(6):1228–34. [Google Scholar]
- 21.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc. 2018. September 19;16(1):22–8. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen D-V, Linderholm A, Haczku A, Kenyon N. Glucagon-like peptide 1: A potential anti-inflammatory pathway in obesity-related asthma. Pharmacol Ther. 2017. December;180:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinne ST, Feemster LC, Collins BF, Au DH, Perkins M, Bryson CL, et al. Thiazolidinediones and the risk of asthma exacerbation among patients with diabetes: a cohort study. Allergy Asthma Clin Immunol. 2014. July 3;10(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 25.Burgos Ochoa L, Rijnhart JJ, Penninx BW, Wardenaar KJ, Twisk JW, Heymans MW. Performance of methods to conduct mediation analysis with time-to-event outcomes. Stat Neerlandica. 2020;74(1):72–91. [Google Scholar]
- 26.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar Behav Res. 2011. May;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011. April;10(2):150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saisho Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr Metab Immune Disord - Drug TargetsFormerly Curr Drug Targets - Immune Endocr Metab Disord. 2015. September 1;15(3):196–205. [DOI] [PubMed] [Google Scholar]
- 29.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014. August;51(2):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dekkers BGJ, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009. October;41(4):494–504. [DOI] [PubMed] [Google Scholar]
- 31.Sallakh MAA, Vasileiou E, Rodgers SE, Lyons RA, Sheikh A, Davies GA. Defining asthma and assessing asthma outcomes using electronic health record data: a systematic scoping review. Eur Respir J. 2017. June 1;49(6):1700204. [DOI] [PubMed] [Google Scholar]
- 32.Weber GM, Adams WG, Bernstam EV, Bickel JP, Fox KP, Marsolo K, et al. Biases introduced by filtering electronic health records for patients with “complete data.” J Am Med Inform Assoc JAMIA. 2017. November 1;24(6):1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.