Abstract
Background:
Acute exacerbations of chronic rhinosinusitis (AECRS) are associated with significant morbidity and decreased quality of life. There are sparse data assessing the real-world impact of biologics on AECRS.
Objectives:
We sought to determine the impact of type 2–targeting biologics on the frequency of medication use for AECRS episodes.
Methods:
Antibiotic and/or systemic corticosteroid courses for AECRS were identified in a retrospective study from November 2015 to February 2020, at a single academic health system. The estimated yearly rates for antibiotic and corticosteroid courses were evaluated before and after initiation of type 2 biologics.
Results:
One-hundred and sixty-five patients with chronic rhinosinusitis (CRS) had received either omalizumab (n = 12), mepolizumab (n = 42), benralizumab (n = 44), dupilumab (n = 61), or reslizumab (n = 6). Seventy percent had CRS with nasal polyps, and 30% had CRS without nasal polyps. All the patients had asthma. When all the biologics were combined, the estimated yearly rate for antibiotics for AECRS decreased from 1.34 (95% confidence interval [CI], 1.12–1.59) to 0.68 (95% CI, 0.52–0.88) with biologic use (49% reduction, p < 0.001). Those with frequent AECRS (three or more courses of antibiotics in the 1 year before biologic use) had a larger degree of reduction, with an estimated yearly rate of 4.15 (95% CI, 3.79–4.55) to 1.58 (95% CI, 1.06–2.35) with biologic use (n = 27; 62% reduction; p < 0.001). Within the total cohort, the estimated yearly rate for systemic corticosteroids for AECRS decreased from 1.69 (95% CI, 1.42–2.02) to 0.68 (95% CI, 0.53–0.88) with biologic use (60% reduction; p < 0.001).
Conclusion:
Type 2–targeting biologics reduced medication use for AECRS. This suggested that biologics may be a therapeutic option for patients with frequent AECRS.
Keywords: type 2 inflammation, chronic rhinosinusitis (CRS), nasal polyps, acute exacerbations of chronic rhinosinusitis (AECRS), antibiotics, corticosteroids, omalizumab, benralizumab, mepolizumab, dupilumab
Chronic rhinosinusitis (CRS) is an inflammatory disease of the nose and paranasal sinuses that affects ∼6–12% of the general population.1–4 Acute exacerbation of CRS (AECRS) is defined as acute worsening of CRS intensity, with a return to baseline frequently with interventions, including antibiotics and/or systemic corticosteroid use.5,6 CRS-related antibiotics and oral corticosteroids are both associated with reduced CRS-specific and general health-related quality of life.7 CRS is one of the top five diagnoses associated with antibiotic prescriptions in the ambulatory care setting.8 It is estimated that 19% of patients with CRS have frequent AECRS that leads to four or more antibiotic courses per year.9 Moreover, short-term oral corticosteroids are associated with an increased risk of adverse events, including fractures and venous thromboembolism.10,11 Frequent AECRS is associated with type 2 inflammation.9
Monoclonal biologics that target type 2 inflammation have improved management options in patients with predominant type 2 airway disease. The impact of biologics (specifically, omalizumab) on antibiotics in CRS is limited to two small retrospective studies that showed reduced episodes of acute rhinosinusitis.12,13 In addition, one small study observed reduced antibiotics with biologic use in patients with CRS but did not report if it was specifically for AECRS.14 The impact of biologics on reducing systemic corticosteroids is based on clinical trials for assessment of dupilumab and omalizumab in CRS with nasal polyps (CRSwNP).15,16 Investigating how biologics impact antibiotic and systemic corticosteroid use for AECRS may better inform treatment strategies for patients with CRS. We hypothesize that biologics decrease antibiotic and corticosteroid use for AECRS. To examine this, we determined the effect of type 2–targeting biologics on the frequency of antibiotic and corticosteroid prescriptions for AECRS.
METHODS
Study Design and Identification of Patients with AECRS
This retrospective study was conducted across 10 hospitals affiliated with Northwestern Medicine, one of the largest health systems in metropolitan Chicago, and was approved by the Northwestern University Feinberg School of Medicine's institutional review board. Patients ≥ 18 years of age prescribed biologics for the treatment of a type 2 respiratory disease, including CRS, asthma, or nasal polyps, were identified by an automated chart review that used Northwestern Medicine's Enterprise Data Warehouse (EDW), an electronic repository of health records of >6.6 million distinct patients.
The EDW was searched between November 2015 and February 2020. Patients were included if they (1) had a upper or lower respiratory disease as listed in the International Classification of Diseases, Ninth Revision, or the International Classification of Diseases, Tenth Revision (https://www.who.int/standards/classifications/classification-of-diseases) diagnosis codes of CRS (473.x, J32.x), nasal polyposis (471.x, J33.x), or asthma (493.x, J45.x); and (2) their records documented a type 2–targeting biologic. Type 2–targeting biologics were searched via full-text keyword search for the following: (1) omalizumab (Xolair; Novartis AG, Switzerland), (2) mepolizumab (Nucala; GlaxoSmithKline, UK), (3) reslizumab (Cinqair; Teva, Israel), (4) benralizumab (Fasenra; AstraZeneca, UK), and (5) dupilumab (Dupixent; Sanofi and Regeneron, MA). Next, by manual chart review, we included patients evaluated in the otolaryngology or allergy and immunology clinics, and diagnosed with CRS by either a sinus computed tomography or endoscopy. All patients met the diagnosis of CRSwNP or CRS without nasal polyps (CRSsNP) as defined by the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis.17 The decision for the type of biologic chosen was based on shared decision-making between the managing physician and the patient. We excluded patients for whom there was no evidence of initiation of the prescribed biologic, incomplete or unavailable documentation before the biologic was initiated, age < 18 years, a biologic initiated for chronic urticaria, or if a biologic was initiated during a clinical trial. If the biologic was started before 2015, then these patients were excluded in an effort to uniformly assess biologics actively used since 2015.
Quantitation of Antibiotic and Corticosteroid Courses for AECRS
AECRS is defined as worsening of sinonasal symptoms that results in a prescription of a systemic antibiotic.6 A manual chart review of individual records was performed to determine the number of antibiotics for AECRS during the 12 months before biologic initiation and during biologic use. Data extraction included only antibiotics specifically prescribed for AECRS. Antibiotic courses provided for worsening lower respiratory symptoms were included if the investigators were able to confirm that the patients also reported worsening sinonasal symptoms. Once a biologic was initiated, the number of antibiotics was analyzed as an estimated yearly rate. In addition to analyzing the entire cohort, we assessed antibiotic use in those with three or more courses of antibiotics in the 12 months before initiating the biologic. Antibiotics administered within the immediate 3-week period after any sinus surgery were excluded from the analysis. Medical records were reviewed with the same approach for systemic corticosteroid courses prescribed for AECRS.
Statistical Analysis
Descriptive statistics were calculated for all variables of interest. Categorical variables were summarized by using counts and percentages. Continuous variables were measured by using median (interquartile range) or mean ± standard deviation (SD), as appropriate. To examine the relationship between primary predictor of interest (before versus after) and outcome (systemic antibiotic or corticosteroid courses), a generalized estimating equation (GEE) was performed with the variable of interest as the outcome and time (before versus after) as the predictor one at a time. A random participant effect was included in the generalized estimating equation to distinguish between-within versus between-participant variances. We used the Poisson distribution, log link, and time offset for count outcomes for total antibiotic courses and, separately, total corticosteroid courses for AECRS. Subgroup analyses (CRSwNP, CRSsNP, by biologics) with similar statistical strategies as mentioned above were also performed. A p value of <0.05 was viewed as significant.
RESULTS
Study Population Characteristics
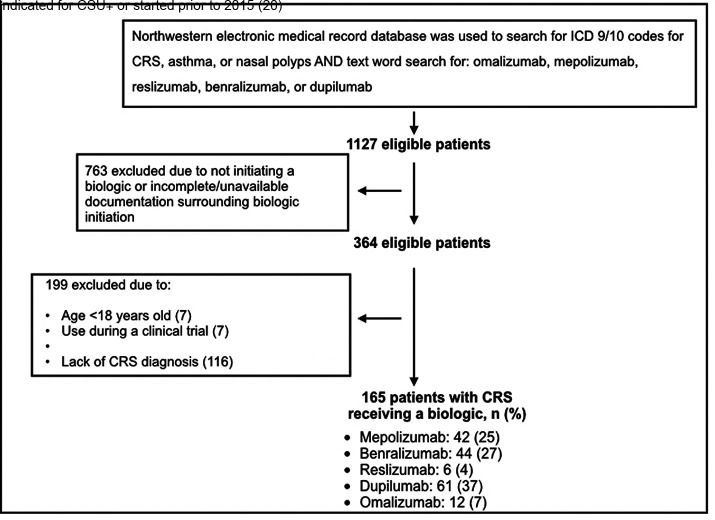
The EDW search yielded 165 patients with CRS who received a type 2–targeting biologic (Fig. 1). The mean ± SD duration of therapy with biologics was 16 ± 13 months (range, 1–58 months). The mean ± SD duration of therapy in months was longer for omalizumab (30.9 ± 19.7 months), mepolizumab (23.1 ± 15.1 months), and reslizumab (21.3 ± 9.4 months) than for benralizumab (13.1 ± 7 months) and dupilumab (10.7 ± 6.9 months) as a result of the respective dates of approval and prescribing patterns. Both asthma and CRSwNP were indications for biologic in 48 of 165 patients, whereas asthma alone was the indication in 117 of 165 patients. Cohort characteristics are summarized in Table 1. The mean ± SD age was 55 ± 13 years, and the patients were predominantly women (60%). Our cohort's primary race and/or ethnicity was non-Hispanic white (65%), followed by non-Hispanic African American (19%). CRS phenotypes included 115 of 165 patients with CRSwNP (70%) and 50 of 165 patients with CRSsNP (30%). All the patients had asthma (100%).
Figure 1.
Algorithm for inclusion of study participants. ∔Chronic spontaneous urticaria.
Table 1.
Demographics and clinical characteristics of the study participants
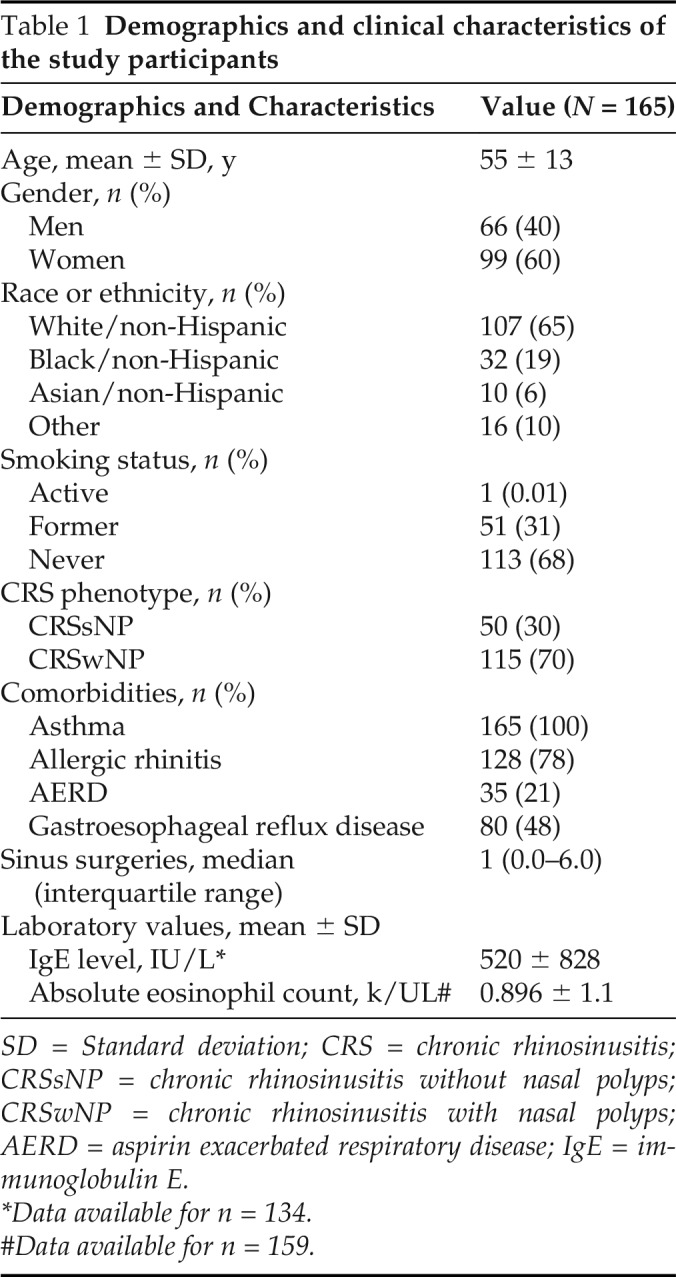
| Demographics and Characteristics | Value (N = 165) |
|---|---|
| Age, mean ± SD, y | 55 ± 13 |
| Gender, n (%) | |
| Men | 66 (40) |
| Women | 99 (60) |
| Race or ethnicity, n (%) | |
| White/non-Hispanic | 107 (65) |
| Black/non-Hispanic | 32 (19) |
| Asian/non-Hispanic | 10 (6) |
| Other | 16 (10) |
| Smoking status, n (%) | |
| Active | 1 (0.01) |
| Former | 51 (31) |
| Never | 113 (68) |
| CRS phenotype, n (%) | |
| CRSsNP | 50 (30) |
| CRSwNP | 115 (70) |
| Comorbidities, n (%) | |
| Asthma | 165 (100) |
| Allergic rhinitis | 128 (78) |
| AERD | 35 (21) |
| Gastroesophageal reflux disease | 80 (48) |
| Sinus surgeries, median (interquartile range) | 1 (0.0–6.0) |
| Laboratory values, mean ± SD | |
| IgE level, IU/L* | 520 ± 828 |
| Absolute eosinophil count, k/UL# | 0.896 ± 1.1 |
SD = Standard deviation; CRS = chronic rhinosinusitis; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; AERD = aspirin exacerbated respiratory disease; IgE = immunoglobulin E.
*Data available for n = 134.
#Data available for n = 159.
Clinical Outcomes
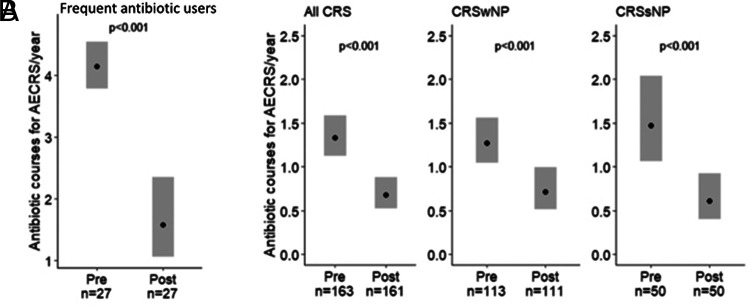
We first analyzed those with frequent AECRS, defined as requiring three or more courses of antibiotics in the year before biologic use (n = 27). The estimated yearly rate of antibiotic courses with biologic use improved by 62% from baseline of 4.15 (95% CI, 3.79–4.55) to 1.58 (95% CI, 1.06–2.35) (p < 0.001) (Fig. 2A). We also assessed antibiotic courses prescribed for AECRS before and during biologic use for the entire cohort. The estimated yearly rate of antibiotic courses decreased from 1.34 (95% CI, 1.12–1.59) to 0.68 (95% CI, 0.52–0.88) with biologic initiation (49% reduction; p < 0.001) (Fig. 2B). When separated by phenotype, the estimated yearly rate of antibiotic courses in patients with CRSwNP reduced from 1.27 (95% CI, 1.04–1.56) to 0.71 (95% CI, 0.51–0.99) with biologic use (44% reduction; p < 0.001) (Fig. 2B). In CRSsNP, the estimated yearly rate of antibiotic courses decreased from 1.47 (95% CI, 1.07–2.04) to 0.61 (95% CI, 0.40–0.92) with biologic use (59% reduction; p < 0.001) (Fig. 2B).
Figure 2.
Biologics were associated with decreased antibiotic courses for AECRS in frequent users of antibiotics and the overall cohort. (A) Biologics were associated with an estimated 62% decrease in antibiotic courses per year in patients with CRS and frequent antibiotic use (requiring three or more antibiotic courses in the year before biologic use). (B) Biologics were associated with decreased antibiotic courses per year in all patients with CRS, including CRSwNP and CRSsNP. Statistical analyses were performed by using the generalized estimating equation, and significance was determined by using the two-tailed test with p < 0.05. The black circle represents estimated yearly rate. AECRS = Acute exacerbation of chronic rhinosinusitis; CRS = chronic rhinosinusitis; CRSwNP = chronic rhinosinusitis with nasal polyps; CRSsNP = chronic rhinosinusitis without nasal polyps.
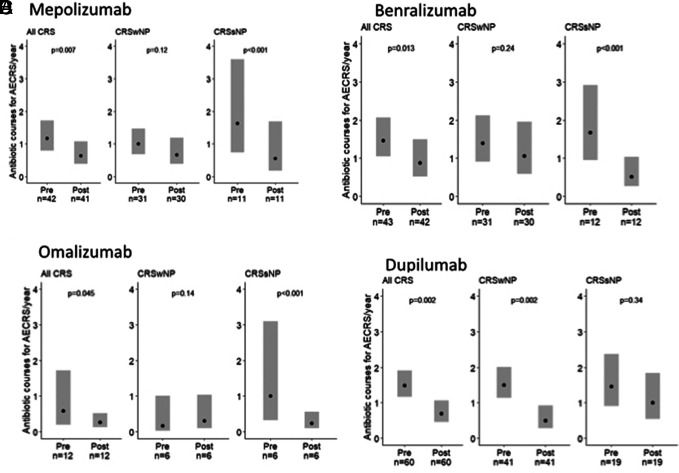
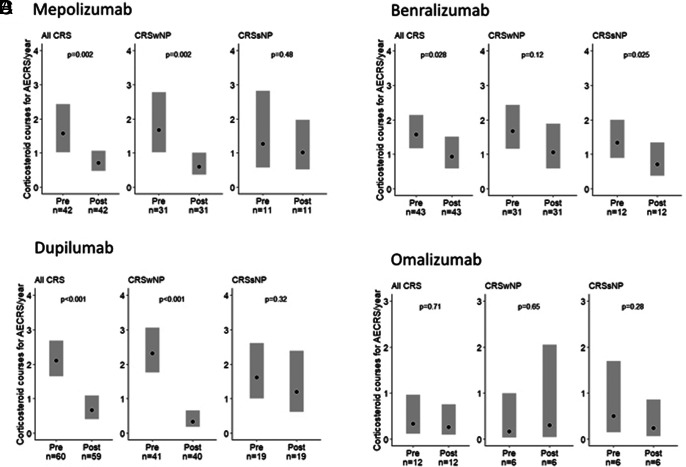
The impact of individual biologics on antibiotic courses for AECRS is shown in Fig. 3. Specifically, mepolizumab reduced estimated yearly antibiotic use in CRSsNP by 66% (p < 0.001), and there was a trend toward a decrease in CRSwNP (33% reduction; p = 0.12). Benralizumab reduced yearly antibiotic use in CRSsNP by 69% (p < 0.001), and there was a trend toward a decrease in CRSwNP (23% reduction; p = 0.24). Omalizumab reduced yearly antibiotic use in CRSsNP by 76% (p < 0.001), but there was no difference in patients with CRSwNP (p = 0.14). In contrast, dupilumab reduced antibiotic use in CRSwNP by 67% (p = 0.002), and there was a trend toward a decrease in CRSsNP (31% reduction; p = 0.34). There was no association with the use of reslizumab with antibiotic reduction in the combined CRS group (n = 6) (data not shown). The estimated yearly rate of corticosteroid courses for AECRS when combining all biologics reduced from 1.69 (95% CI, 1.42–2.02) to 0.68 (95% CI, 0.53–0.88) with biologic initiation (60% reduction; p < 0.001) (Fig. 4). The impact of individual biologics on corticosteroid use for AECRS was also assessed. Mepolizumab, benralizumab, and dupilumab were associated with reduced corticosteroids (Fig. 5). A reduction in corticosteroids in those who were taking omalizumab or reslizumab was not seen, likely due to an insufficient sample size.
Figure 3.
Effect of individual biologics on antibiotic courses for AECRS. (A) Mepolizumab reduced antibiotics in total CRS and CRSsNP but not CRSwNP, (B) benralizumab reduced antibiotics in total CRS and CRSsNP but not CRSwNP, (C) omalizumab reduced antibiotics in total CRS and CRSsNP but not CRSwNP, and (D) dupilumab reduced antibiotics in total CRS and CRSwNP but not CRSsNP. Statistical significance was determined by using the two-tailed test with p < 0.05. AECRS = Acute exacerbation of chronic rhinosinusitis; CRS = chronic rhinosinusitis; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps.
Figure 4.
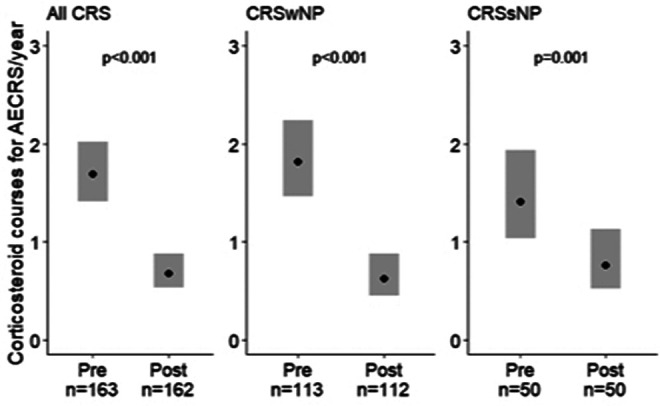
Biologics were associated with decreased corticosteroid courses per year for AECRS. Biologics were associated with decreased corticosteroid courses per year in all the patients with CRS, including CRSwNP and CRSsNP. Statistical analyses were performed by using the generalized estimating equation, and significance was determined by using the two-tailed test with p < 0.05. The black circle represents the estimated yearly rate. AECRS = Acute exacerbation of chronic rhinosinusitis; CRS = chronic rhinosinusitis; CRSwNP = chronic rhinosinusitis with nasal polyps; CRSsNP = chronic rhinosinusitis without nasal polyps.
Figure 5.
The effect of individual biologics on yearly corticosteroid courses for AECRS. (A) Mepolizumab reduced corticosteroids in total CRS and CRSwNP but not CRSsNP, (B) benralizumab reduced corticosteroids in total CRS and CRSsNP but not CRSwNP, (C) dupilumab reduced corticosteroids in total CRS and CRSwNP but not CRSsNP, and (D) omalizumab was not associated with a reduction in corticosteroids. Statistical analyses were performed by using the generalized estimating equation, and significance was determined by using the two-tailed test with p < 0.05. The black circle represents the estimated yearly rate. AECRS = Acute exacerbation of chronic rhinosinusitis; CRS = chronic rhinosinusitis; CRSwNP = chronic rhinosinusitis with nasal polyps; CRSsNP = chronic rhinosinusitis without nasal polyps.
DISCUSSION
We reported a decrease in AECRS when using type 2–targeting biologics. Our study provided evidence that biologic use may curtail the number of antibiotics and corticosteroids prescribed for AECRS, a diagnosis associated with frequent antibiotic and/or corticosteroid prescriptions.7,8,18 Antibiotic overuse contributes to antibiotic resistance and Clostridium difficile infection, which is why the U.S. Centers for Disease Control and Prevention continues to prioritize the implementation of antibiotic stewardship programs. In addition, the use of short-term corticosteroids, often for respiratory conditions, is associated with an increased risk of serious adverse events, including sepsis, heart failure, gastrointestinal bleeding, venous thromboembolism, and fractures.10,11,19 The cumulative use of short-term systemic corticosteroids showed a dose-dependent increase in developing an adverse event.19 Although a large proportion of our cohort had a low absolute baseline estimated yearly rate for antibiotics and corticosteroids for AECRS, the potential cumulative reduction in the use of these medications over time may be considered an important benefit given the lifetime chronicity of CRS.
Moreover, a 62% reduction in antibiotic use in patients who required three or more antibiotic courses in the 12 months before biologic initiation suggests that those with frequent acute exacerbations could be a primary group that benefits from biologic therapy, with a reduction in the multiple untoward consequences of frequent antibiotic and corticosteroid use. Although we observed decreased use of antibiotics for AECRS with mepolizumab, benralizumab, dupilumab, and omalizumab, and decreased corticosteroid courses with mepolizumab, benralizumab, and dupilumab, we could not directly compare the biologics because of the study design. Based on an assessment of the impact of individual biologics, there was variability in antibiotic reduction based on CRS phenotypes. Specifically, dupilumab was associated with a significant reduction in antibiotic use in CRSwNP. In CRSsNP, mepolizumab, benralizumab, and omalizumab were associated with decreased antibiotic use for AECRS.
The mechanism by which biologics decreased AECRS is not well established. Several studies have identified interleukin (IL) 4, IL-13, and possibly IL-5 as notable cytokines responsible for an impaired epithelial barrier in airway diseases.20–25 Results of these studies suggest that type 2 cytokines may weaken the mechanical barrier and increase the permeability of molecules, including unwanted pathogens, which can lead to susceptibility to colonization and infection. It is thus possible that restoration of the barrier with type 2–targeting biologics may be one of the main mechanisms that leads to clinical benefit. Analysis of emerging data suggests that type 2 inflammation characterizes up to 50% of the patients with CRSsNP, which may explain the positive effect of these biologics, although no biologics are currently approved for patients with CRSsNP.26,27
In addition, biologics may positively modify the functional innate host defense against pathogens and reduce the propensity to acquire bacterial infections. Previous studies show decreased expression of antimicrobial proteins in the presence of type 2 inflammation.28–30 Thus, the type 2 cytokine microenvironment may predispose to infection, particularly with Staphylococcus aureus, as seen in patients with atopic dermatitis.29 As such, dupilumab has shown reduced colonization of S. aureus and the need for concomitant antibiotics in patients with atopic dermatitis.31–34 It is possible that biologics may improve the innate antimicrobial immune response in patients with CRS. Prospective studies that investigate nasal and sinus antimicrobial properties and S. aureus colonization by using type 2–targeting biologics can provide more insight into this. Antibiotic and corticosteroid prescriptions for worsening sinonasal symptoms were chosen as surrogate markers for AECRS because these are usually used for AECRS treatment.5,6 In addition, use of antibiotics and/or corticosteroids has been reported as an indirect metric when evaluating CRS exacerbations.35–37 That said, the role of antibiotics as treatment for CRS exacerbations is unclear because viral infections may be the cause for acute infectious exacerbations.6
We used a rigorous algorithm in the study by first searching the EDW and then verifying the use of a biologic and a CRS diagnosis by manual chart review. All the patients had a computed tomography of the sinus or an endoscopy, which confirmed the diagnosis of CRS. Our CRS population had a predominance of nasal polyps, which may be partly because this phenotype is highly associated with moderate-to-severe asthma, which was the main indication for biologic use when this study was conducted.38 This also explains the 100% asthma comorbidity observed in our patients with CRS. Our center practices antibiotic stewardship, so providers are less likely to administer antibiotics unless patients have persistent symptoms with evidence of infection, such as purulent discharge or positive cultures. This may explain our small baseline number of antibiotics per year. However, the cumulative reduction in antibiotics may be a secondary benefit from biologic therapy, especially in individuals with frequent AECRS because this phenomenon has been associated with type 2 inflammation.9 Longer-term studies are needed to evaluate this.
Limitations
Limitations of this work included the retrospective study design and lack of a control group treated without biologics. The study relied on detailed physician documentation, which is variable, and limited our ability to make causal inferences from the data. The available details on exacerbations may have been affected by the frequency of clinic visits and limited documentation of medication adherence. Although treatment of episodes at facilities outside of the medical record may have occurred, physician documentation often included assessment of interval antibiotic and corticosteroid use at each follow-up visit, and were counted if the definition of AECRS had been met. In many cases, antibiotic prescriptions were administered based on the treating physician's clinical judgment, which reflects the real-world practice and often is not associated with obtaining bacterial cultures. To minimize the inclusion of antibiotics prescribed for other indications, we confirmed through the manual chart review that all antibiotic prescriptions were for AECRS. Some of the exacerbations may have been triggered by viral and not bacterial etiology.
A small portion of patients (10.8%) had sinus surgery after biologic initiation or started a biologic within 8 weeks of surgery. We did not exclude these patients as part of the analyses because this was a real-world observational study. Due to the study's retrospective nature, there may have been some bias in patient selection. The patients in our cohort had variable levels of CRS control. As indications for type 2 biologic use in refractory CRS grows, those patients being initiated reflect a more severe CRS group; this warrants evaluation as part of a future study. Our study did not directly compare biologics due to inherent challenges and confounders, including differences in patient clinical characteristics, different periods on treatment, and varying sample sizes. Our smaller sample size for omalizumab and reslizumab likely reflected differences in physician and patient preferences for treatment choice of airway disease. Also, analysis was from a single medical organization in a large metropolitan region, so generalizability is limited.
CONCLUSION
We reported that type 2–targeting biologics in patients with CRS may reduce AECRS as judged by a decrease in antibiotic and corticosteroid use. Patients who experience frequent antibiotic use for AECRS may thus have a primary benefit from biologic therapy. Those with a lower rate of antibiotic or corticosteroid use may see a cumulative reduction with time, which may be an important secondary benefit of biologic therapy. Analysis of our data potentially highlights an unrecognized clinical benefit of type 2–targeting biologics in reducing antibiotic use for AECRS. If confirmed, this property of these drugs can be used as part of decision-making when selecting biologic treatment that is appropriate for individual patients.
ACKNOWLEDGMENTS
We thank Anna Pawlowski and Quan Mai for assistance with the Northwestern Medicine Enterprise Data Warehouse.
Footnotes
This work was supported by National Institutes of Health grants: Chronic Rhinosinusitis Integrative Studies Program P01AI145818, T32AI083216, R01AI137174; Division of Allergy and Immunology, Department of Medicine, Northwestern University Feinberg School of Medicine; and by the Ernest Bazley Foundation
L.C. Grammer III serves as a consultant for Astellas Pharmaceuticals. R. Kalhan reports grants and personal fees from Boehringer Ingelheim, PneumRx, Spiration, AstraZeneca; also received personal fees from CVS Caremark, Aptus Health, GlaxoSmithKline (GSK), Boston Scientific, Boston Consulting Group, outside the submitted work. R.C. Kern is a consultant of Lyra Therapeutics, Sanofi/Regeneron, GSK, and Genentech. B.K. Tan has served on advisory boards for Sanofi/Genzyme and Optinose. R.P. Schleimer is a consultant for Intersect ENT, GSK, Merck, Sanofi, AstraZeneca/Medimmune, Genentech, Otsuka, Actobio Therapeutics, Lyra Therapeutics, Astellas Pharm Inc, Genzyme/Sanofi Corp, and Celgene Corp; is also a consultant with stock/stock options that are not valued yet with Allakos, Aurasense, BioMarck, Exicure, and Aqualung Therapeutics Corp. W.W. Stevens has served on an advisory board for GSK. A.T. Peters reports personal fees from Sanofi/Regeneron, grants and fees from AstraZeneca and Optinose, outside the submitted work. The remaining authors have no conflicts of interest pertaining to this article
REFERENCES
- 1. Dietz de Loos D, Lourijsen ES, Wildeman MAM, et al. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J Allergy Clin Immunol. 2019; 143:1207–1214. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch AG, Stewart WF, Sundaresan AS, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017; 72:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Center for Health Statistics CDC. National Health Interview Survey 2018. 2021; Summary Health Statistic Tables for U.S. Adults. Available online at https://www.cdc.gov/nchs/fastats/sinuses.htm; accessed on March 28, 2020.
- 4. Hirsch AG, Nordberg C, Bandeen-Roche K, et al. Radiologic sinus inflammation and symptoms of chronic rhinosinusitis in a population-based sample. Allergy. 2020; 75:911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on rhinology and allergy: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021; 11:213–739. [DOI] [PubMed] [Google Scholar]
- 6. Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020; 58(suppl S29):1–464. [DOI] [PubMed] [Google Scholar]
- 7. Yamasaki A, Hoehle LP, Phillips KM, et al. Association between systemic antibiotic and corticosteroid use for chronic rhinosinusitis and quality of life. Laryngoscope. 2018; 128:37–42. [DOI] [PubMed] [Google Scholar]
- 8. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013; 132:1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwah JH, Somani SN, Stevens WW, et al. Clinical factors associated with acute exacerbations of chronic rhinosinusitis. J Allergy Clin Immunol. 2020; 145:1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017; 357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao T-C, Huang Y-W, Chang S-M, et al. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020; 173:325–330. [DOI] [PubMed] [Google Scholar]
- 12. Tiotiu A, Oster JP, Roux PR, et al. Effectiveness of omalizumab in severe allergic asthma and nasal polyposis: a real-life study. J Investig Allergol Clin Immunol. 2020; 30:49–57. [DOI] [PubMed] [Google Scholar]
- 13. Chandra RK, Clavenna M, Samuelson M, et al. Impact of omalizumab therapy on medication requirements for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016; 6:472–477. [DOI] [PubMed] [Google Scholar]
- 14. Kotisalmi E, Hakulinen A, Mäkelä M, et al. A comparison of biologicals in the treatment of adults with severe asthma — real-life experiences. Asthma Res Pract. 2020; 6;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019; 2;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1. Epub 2019 Sep 19. Erratum in: Lancet. 2019 Nov 2;394(10209):1618. PMID: 31543428. [DOI] [PubMed]
- 16. Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020; 146:595–605. [DOI] [PubMed] [Google Scholar]
- 17. Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016; 6(suppl 1):S22–S209. [DOI] [PubMed] [Google Scholar]
- 18. Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018; 11:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seibold MA. Interleukin-13 stimulation reveals the cellular and functional plasticity of the airway epithelium. Ann Am Thorac Soc. 2018; 15(suppl 2):S98–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013; 1:e24333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020; 75:54–62. [DOI] [PubMed] [Google Scholar]
- 23. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012; 130:1087–1096.e10. [DOI] [PubMed] [Google Scholar]
- 24. Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017; 139:93–103. [DOI] [PubMed] [Google Scholar]
- 25. Ramanathan M, Jr, Lee W-K, Spannhake EW, et al. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008; 22:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delemarre T, Holtappels G, De Ruyck N, et al. Type 2 inflammation in chronic rhinosinusitis without nasal polyps: another relevant endotype. J Allergy Clin Immunol. 2020; 146:337–343.e6. [DOI] [PubMed] [Google Scholar]
- 27. Tan BK, Klingler AI, Poposki JA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017; 139:699–703.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei Y, Xia W, Ye X, et al. The antimicrobial protein short palate, lung, and nasal epithelium clone 1 (SPLUNC1) is differentially modulated in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2014; 133:420–428. [DOI] [PubMed] [Google Scholar]
- 29. Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002; 347:1151–1160. [DOI] [PubMed] [Google Scholar]
- 30. Schroeder S, Robinson ZD, Masterson JC, et al. Esophageal human β-defensin expression in eosinophilic esophagitis. Pediatr Res. 2013; 73:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Callewaert C, Nakatsuji T, Knight R, et al. IL-4Rα Blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol. 2020; 140:191–202.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eichenfield LF, Bieber T, Beck LA, et al. Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol. 2019; 20:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ou J, Wang J, Xu Y, et al. Staphylococcus aureus superantigens are associated with chronic rhinosinusitis with nasal polyps: a meta-analysis. Eur Arch Otorhinolaryngol. 2014; 271:2729–2736. [DOI] [PubMed] [Google Scholar]
- 34. Tripathi A, Conley DB, Grammer LC, et al. Immunoglobulin E to staphylococcal and streptococcal toxins in patients with chronic sinusitis/nasal polyposis. Laryngoscope. 2004; 114:1822–1826. [DOI] [PubMed] [Google Scholar]
- 35. Phillips KM, Hoehle LP, Bergmark RW, et al. Acute exacerbations mediate quality of life impairment in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2017; 5:422–426. [DOI] [PubMed] [Google Scholar]
- 36. Banoub RG, Phillips KM, Hoehle LP, et al. Relationship between chronic rhinosinusitis exacerbation frequency and asthma control. Laryngoscope. 2018; 128:1033–1038. [DOI] [PubMed] [Google Scholar]
- 37. Rank MA, Wollan P, Kita H, et al. Acute exacerbations of chronic rhinosinusitis occur in a distinct seasonal pattern. J Allergy Clin Immunol. 2010; 126:168–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017; 195:302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]