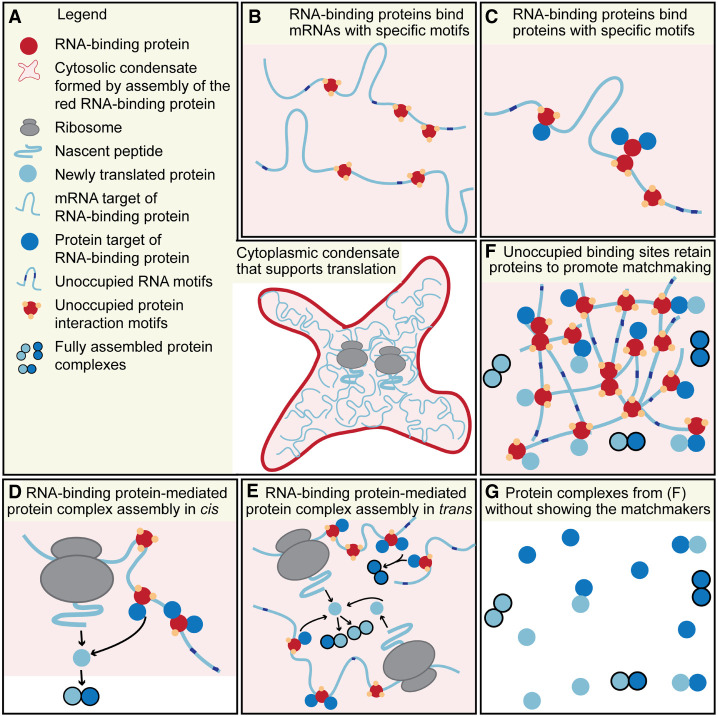
FIGURE 1.
Model for condensate RNA-binding proteins to serve as protein matchmakers for complex assembly. (A) An RNA-binding protein (red) assembles into a cytoplasmic condensate that supports translation. (B) The RNA-binding protein (red) binds to specific mRNAs (mRNA target of the RNA-binding protein, light blue) which results in mRNA enrichment in the cytoplasmic condensate. (C) The RNA-binding protein (red) uses specific protein domains (yellow dots) to bind to specific proteins (protein target of the RNA-binding protein, blue dots) which results in their recruitment into the condensate. The protein interaction domains are used for homotypic interaction to assemble the condensate and for heterotypic interaction to recruit and retain protein targets in the condensate. The multivalent protein interaction domains provide specificity for the enriched proteins in the condensate which serve as potential subunits of complexes. (D) Translation of the mRNA target allows the newly translated protein to form protein–protein interactions with a protein target in cis. A fully formed protein complex is indicated by the black outline of the proteins and signifies that all potential retention sites are masked. This allows the fully formed complex to leave the condensate. (E) As in D, but the proximity of other mRNA and protein targets also allows interactions in trans between protein targets recruited by neighboring mRNAs and among newly translated mRNA targets. (F) Unoccupied multivalent interaction domains of the RNA-binding protein allow recruited proteins and newly translated proteins to be transiently retained within the condensate network. Repeated binding allows them to scan the condensate to increase the chance of encounter of specific interactors. After complex assembly, they leave the condensate through masking of the retention sites. (G) Without visualizing the protein matchmakers that consist of the condensate RNA-binding protein and the bound mRNAs, protein complex assembly appears to occur through diffusion and random encounter.