Abstract
Immunoglobulin replacement therapy (IGRT) can protect against lung function decline in CVID. We tested whether increasing IgG dosage was beneficial in patients who exhibited a decline in forced expiratory flow at 25–75% (FEF25–75%) even though they were receiving IgG doses within the therapeutic range. Of 189 CVID patients seen over 12 years; 38 patients met inclusion criteria, were seen on ≥3 visits, and demonstrated a ≥10% decrease in FEF25–75% from visits 1 to 2. FEF25%–75%, forced expiratory flow at one second (FEV1), and FEV1/FVC at visit 3 were compared among those with non-dose adjustment (non-DA) versus additional IgG dose adjustment (DA). Three FEF25–75% tiers were identified: top (>80% predicted), middle (50%−80%), and bottom (<50%). DA and non-DA groups did not differ in clinical infections or bronchodilator use, although the non-DA group tended to use more antibiotics. In the top, normal tier, FEF25%−75% increased in DA, but the change did not achieve statistical significance. In the middle moderate obstruction tier, visit 3 FEF25–75% increased among DA but not non-DA sets (11.8 ± 12.4%, p=0.003 vs. 0.3 ± 9.9%, p=0.94). Improvement in FEV1/FVC at visit 3 was also significant among DA vs. non-DA (7.2 ± 12.4%, p=0.04 vs. −0.2 ± 2.7%, p=0.85). In the bottom, severe tier, FEF25–75% was unchanged in DA (−0.5 ± 5.2%, p=0.79), but increased in non-DA (5.1 ± 5.2%, p=0.02). Among IGRT CVID patients with moderate but not severe obstruction as assessed by spirometry, increasing IgG dosage led to an increase in FEF25–75% and FEV1/FVC.
Keywords: Common variable immunodeficiency (CVID), Spirometry, FEF25-75%, Immunoglobulin replacement therapy, IgG dose adjustment, Prophylactic antibiotics
Introduction
Common variable immunodeficiency (CVID) is the most prominent primary immunodeficiency under the care of clinical immunologists [1]. CVID is a heterogeneous disorder characterized by low serum levels of most or all immunoglobulin classes and the inability to effectively produce new antibodies protective against encapsulated organisms. With an estimated prevalence of 1 in 25,000–50,000; it is the most prevalent human primary immunodeficiency among adult patients requiring medical attention and treatment.
Among our clinic population in the Southeastern United States, most CVID patients are diagnosed as adults [2]. Recurrent sinopulmonary infections, including otitis media, sinusitis, bronchitis, pneumonia, and eventually bronchiectasis, plague these patients and can result in frequent health care utilizations for treatment, with all the attendant costs in terms of medical resources as well as a diminished quality of life.
In 1952, Colonel Ogden Bruton was the first to identify a patient with a primary antibody deficiency and the first to treat with immunoglobulin replacement therapy [3]. Although his patient received gammaglobulin subcutaneously, most patients for the next thirty years were given IgG by intramuscular injection. In the United States, treatment with intravenous gammaglobulin (IVIG) was approved in 1980 and subcutaneous gammaglobulin (SCIG) was approved in 2006. In 2019, immunoglobulin (IgG) replacement therapy (IGRT) is typically administered either as an intravenous monthly infusion or as a subcutaneous weekly, bimonthly, or monthly infusion.
The use of IGRT has led to a decrease in the severity and frequency of infections such as bronchitis, pneumonia, and bronchiectasis, and thus an improved quality of life [4, 5]. Although the need for IGRT therapy has been established, how to best optimize dosing remains an open question [6]. Under dosing can lead to decreased efficacy of treatment with resulting morbidity, especially from chronic lung disease [7]; whereas overdosing can increase the risk of adverse events such as thrombotic events and headaches from aseptic meningitis [8]. Both under and over dosing also have monetary consequences for the individual patient and for society in general. Increased morbidity from under dosing can lead to increased long-term medical and quality of life costs, but over dosing can lead to short term increased costs due to the use of more immunoglobulin product.
In common clinical practice, the IGRT dose is based on body weight, with initial monthly dosing starting at 0.4 to 0.5 gm/kg and increasing to 0.6 gm/kg or higher. A universal optimal approach to IgG dosing has yet to be defined. For example, patients with bronchiectasis at initial diagnosis of CVID tend to receive higher doses of IgG therapy and may receive as much as 0.6 gm/kg or more with the hope that clinically apparent lung infections and the risk of complications will decrease [9–11]. Conversely, it has been suggested that obese patients should be dosed on the basis of body weight index rather than weight alone [6].
Trough IgG levels are often used to monitor therapy. However, individual patients often present with unique factors (i.e. enteropathy, cytopenias, autoimmunity, etc) that can require individualized dosing [9, 6]. As a result, assessment of the proper dose and changes to therapy tends to depend on the expertise, prior experience, and judgement of the clinician. Factors that may prompt an increase in immunoglobulin dosage include a history of repeated or increased infections or antibiotic usage between visits, an increased frequency of health care utilizations, and other complications such as autoimmunity, lymphoid interstitial pneumonitis, and bronchiectasis. These features underscore the need for an objective measurement of treatment efficacy that is simple, economical and amenable to routine use in a clinic setting.
In our adult immunodeficiency clinic, spirometry has been a part of routine follow-up care for each patient for decades. We present a retrospective analysis of 38 CVID patients seen over a decade who exhibited a decline in spirometric values. We compare the subsequent spirometric measurements in patients whose immunoglobulin dosage was increased to those in which it was not, and also review serum IgG levels and the range of infections, bronchodilator usage, and antibiotic usage reported by the patients in each category.
Methods
Patients and Clinical Data
All patients seen in the specialty adult immunology clinic at the University of Alabama at Birmingham between January 1, 2005 and December 31, 2016 were invited to take part in a long-term observational study of their conditions. All the study subjects were recruited under the guidelines of the UAB Institutional Review Board. Informed consent was obtained from all of the study participants. All the patients were seen by the same clinical immunologist. Patients with a diagnosis of CVID following the definitions of the Pan-American Group for Immunodeficiency and European Society for Immunodeficiencies [12] either by review of past medical records from external health care providers or by initial evaluation in our own clinic were the focus of this study. Clinical and laboratory data were collected from all the patients. A detailed medical history, including the patient-reported history of infections and medication, was taken; and a physical exam was performed.
Spirometry was conducted by the same attending physician using a WinDX spirometer (Creative BioMedics, Inc. International, San Clemente, CA). Spirometry measures the maximal volume of air forcibly exhaled from the point of maximal inhalation (FVC) and the volume of air exhaled during the first second of this maneuver (FEV1) [13]. These values are typically expressed as a percent of the predicted value (e.g. %FEV1), or as the ratio of the FEV1 to the FVC (e.g. FEV1/FVC). For example, improvement in FEV1 and FVC was used in 1987 to document the benefits of high dose IGRT [14]. Other values, such as the forced expiratory flow between 25% and 75% of the FVC (FEF25–75%) are also commonly reported. FEV1, FVC, FEV1/FVC, and FEF25–75% were measured at each visit to assess lung function. ATS/ERS criteria were used in the interpretation of lung function [15]. If determined to be medically indicated, CVID patients were placed on IGRT either through intravenous infusion (IVIG) or subcutaneous infusion (SCIG). The dose, frequency, and route of IgG treatment was adjusted at subsequent visits, as needed.
Inclusion and Exclusion Criteria
For this retrospective study, we began by screening for patients who had been seen on at least three separate visits, who were being treated with IGRT, and who had a confirmed diagnosis of CVID (Fig. S1). Of a total of 2,830 clinic visits reviewed over the 11-year period, 189 patients (with 857 visits) met criteria for the diagnosis of CVID: a marked decrease of IgG at least 2 standard deviations below the mean for age, a marked decrease in either IgM or IgA or both, onset of immunodeficiency at > 4 years of age, and had no evidence of any defined cause of hypogammaglobulinemia (e.g. patients with Hyper IgM syndrome or X-linked agammaglobulinemia were excluded). For the primary screen, we excluded patients with less than three clinic visits (n=81), a smoking history greater than 15-pack years (n=18), irregular intervals between IgG infusions (n=16), an active cancer diagnosis and treatment during study period (n=2), or no pretreatment baseline (1). We excluded individual visits if the patient was pregnant during that visit, which eliminated two patients entirely (n=2). Finally, one patient who refused gammaglobulin replacement was excluded (n=1). This left 68 patients to be reviewed in the secondary screen.
Data Extraction
The following data was extracted from the clinical chart for the remaining 68 patients if available: age, sex, weight, IgG formulation pre-treatment IgG level, pneumococcal protective titers, IgG level at each visit, date of last IgG infusion, total number of clinic visits, antibiotic use, bronchodilator use, episodes of pneumonia, minor infections, hospitalizations, %FEV1, %FVC, FEV1/FVC, and %FEF25–75%. Total dose in 4 weeks were calculated for those patients on SCIG and effective dose was calculated using total dose in 4 weeks divided by the weight in kilograms and adjusted per the manufacturer’s recommendations.
Grouping into Individual Three Visit Sets
We identified the sets of three consecutive visits of the 450 total visits from the 68 patients which exhibited a greater than or equal to 10% decrease in FEF25–75% from “visit1” (could be any visit from the patient) to “visit2”, and IgG total dose increased or did not change at “visit2” (Fig. S1). Each patient could have multiple sets of visits if they subsequently experienced another ≥ 10% decrease in FEF25–75%. In this step, 21 patients were excluded because they did not exhibit a decrease in FEF25–75% of 10% or greater, 6 patients were excluded if the increase in dosage was due to weight gain alone, two patients were excluded due to missing spirometric data, and one was excluded because the total dose of gammaglobulin was decreased at “visit2”. For the remaining 38 patients, the sets of three visits were separated into two sets that were based on whether the IgG total dose was increased at “visit2”. If the IgG dose was increased by 5 grams or more, then that data set was placed in the dose adjustment (DA) sets. If the dose of IgG was left unchanged or increased by less than 5 grams, that data set was placed into the non-dose adjustment (non-DA) sets. Of these, there were seven patients in the DA group and twelve patients in the non-DA that had only one, singleton, “visit2” where there had been a decrease in FEF25–75%. The remaining 19 patients had multiple (two or more) three visit series where there had been a decline in FEF25–75% at “visit2”. Of these, we identified 21 visit series that fell into the DA group and 26 visit series that fell into the non-DA group (Fig. S1 and Table S1).
Statistical Analysis
Chi-Squared Test or Fisher’s Exact Test was applied for the comparison of proportions. Linear mixed model was used to model the change in FEF25–75% from “visit2” to “visit3”. The outcome in the linear mixed model is the change in FEF25–75% from “visit2” to “visit3”. Visits indicating “visit2” or “visit3” was treated as fixed effect and the unique patient number was treated as random effect (random intercept only) to account for the correlation introduced by multiple assessments of the same patient. The likelihood ratio test was used to test if visits had a significant effect on the outcome. Paired t-test was also performed to be compared with linear mixed model. All tests were performed at a 5% significance level. Analyses were conducted using SAS 9.4 and RStudio 1.0.136.
Results
Patient characteristics
Of the patients screened through the database from 2005–2016, 189 were diagnosed with CVID. While sixty-eight patients met the primary inclusion criteria, thirty were excluded from the analysis because they did not meet the secondary inclusion criteria, which required a decrease in FEF25–75% of 10% or greater, no interruptions in their therapy, a change of weight less than or equal to five pounds, and no decrease in the total dose of gammaglobulin at “visit2.” This left thirty-eight unique subjects with confirmed CVID who had exhibited a decrease in pulmonary function at “visit2”. Of these 38 patients, 27 were females and 11 were males. All were of European descent (Table 1).
Table 1.
Demographics of patients (N=38).
| Mean (SD) | Median (IQR) | |
|
| ||
| Age at initial visit (years) | 50.2 (13.7) | 50.3 (41.8, 61.8) |
| Nadir IgG level (mg/dl) | 440.7 (179.9) | 453 (390, 568) |
| %Streptococcal titers protective | 0.34 (0.28) | 0.29 (0.14, 0.64) |
| Tobacco use (pack years) | 2.2 (4.4) | 0 (0, 1.5) |
| Total number of visits | 8 (3) | 8 (6, 11) |
|
| ||
| N (%) | ||
|
| ||
| Gender | ||
| Male | 11 (28.95) | |
| Female | 27 (71.05) | |
| Race | ||
| White | 38 (100) | |
|
| ||
| Known Respiratory Problems | ||
| allergic rhinitis | 10 (26.3) | |
| asthma | 9 (23.7) | |
| bronchiectasis | 7 (18.4) | |
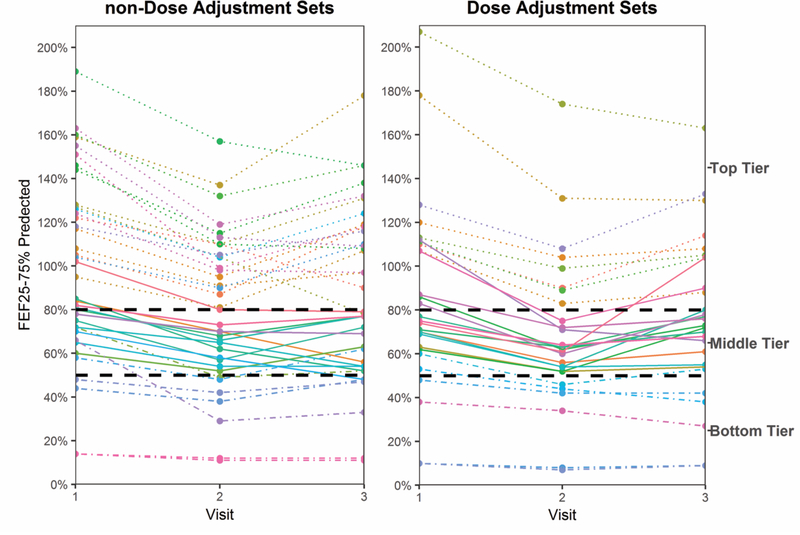
During 2005–2016 inclusive, these 38 unique CVID patients made 290 clinic visits. Their visits were grouped into 37 non-dose adjustment (non-DA) sets of three consecutive visits and 27 dose adjustment (DA) sets of three consecutive visits (Table S1). Data from nineteen of the patients with multiple visits provided data that in some cases fell into the dose adjustment sets and in other cases fit into the non-dose adjustment sets. As previously defined, assignment of the three visit set depended on whether there was a significant (>5 gm) change in the dose of immunoglobulin at visit 2 (DA set) or not (non-DA set). For example, a patient might visit the clinic on four occasions, forming two sets of three (visits 1, 2 and 3; and 2, 3, and 4). If at both visit 2 and visit 3 there was a > 10% decrease in FEF 25%−75%, but the IGRT dose was increased only at visit 3, then the first set (visits 1, 2 and 3) would fall into the non-DA set, and the second set (visits 2, 3 and 4) would fall into the DA set. The DA and non-DA sets were then further sub-divided by FEF25–75% using the following tiers: FEF25–75% ≥80% of predicted as top tier, FEF25–75% between 50–80% of predicted as middle tier, and FEF25–75% ≤50% of predicted as bottom tier (Fig. 1 and Table S1).
Fig. 1.
FEF25–75% at visits 1, 2, and 3 for the non-Dose Adjustment (non-DA) and the Dose Adjustment three visit sets (DA). Top tier: dotted line. Middle tier: solid line. Bottom tier: dotted-dashed line.
IgG dosing, Antibiotic Use, Infections, Interval Visits, and Pharmacologic Interventions
Serum IgG levels at the time of diagnosis were at least two standard deviations below the reference mean for the laboratory in which the IgG level was measured. These are shown in Fig. S2a. Also shown are the spot serum IgG levels obtained at visits 1, 2, and 3. Trough levels were not recorded because most of the patients received their gammaglobulin through either home health agencies or in infusion facilities outside our institution. IgG levels for patients receiving SCIG and IVIG are shown separately. The amount of gammaglobulin that the patients were receiving at visit 1 was sufficient to raise the patient’s IgG by more than 400 mg/dL above the baseline, untreated value. Spot IgG levels from among both the SCIG and IVIG treated patients were typically greater than 1000 mg/dL, documenting receipt of high dose IGRT. The effective prescribed dose of serum immunoglobulin in grams per kilogram of body weight at visits 1, 2, and 3 are shown in Fig. S2b. The majority of patients were receiving between 0.4 and 0.6 grams of IgG per kilogram of body weight. Doses tended to be inversely related to lung function, higher in bottom tier patients, and lower in top tier patients. For both the non-DA and the DA sets, the number of visit sets in the bottom tier was small (Table S1, 7 non-DA and 6 DA).
When the medical histories of patients were compared, there was a tendency for an increased use of prophylactic antibiotics in the non-DA sets (Table S2a). However, no significant differences in the reports of minor and serious infections between the DA and non-DA sets (Table S2b and S2c); or in the use of asthma-related medications (bronchodilators and steroids, Table S3) were recorded.
When the time interval between visits was assessed, a tendency for longer periods between visits for the non-DA sets was observed when compared to the DA sets both between visit 1 and visit 2 (431 ± 41 days [SEM] versus 370 ± 43; respectively), and between visit 2 and visit 3 (398 ± 19 days [SEM] versus 293 ± 20; respectively), (Table S4). The time interval between visit 2 and 3 also tended to be shorter than visit 1 and 2 for all of the groups. This reflected the clinical judgement of the immunologist who considered that patients undergoing changes in immunoglobulin dosing would benefit from closer observation. The intervals between visits for the non-DA and the DA groups as a function of their lung tier groups was also examined. The time interval between visits 2 and 3 tended to be shorter than the time interval between visits 1 and 2 for each of the group sets and lung tier sets except for the non-DA middle tier lung sets.
Lung Function
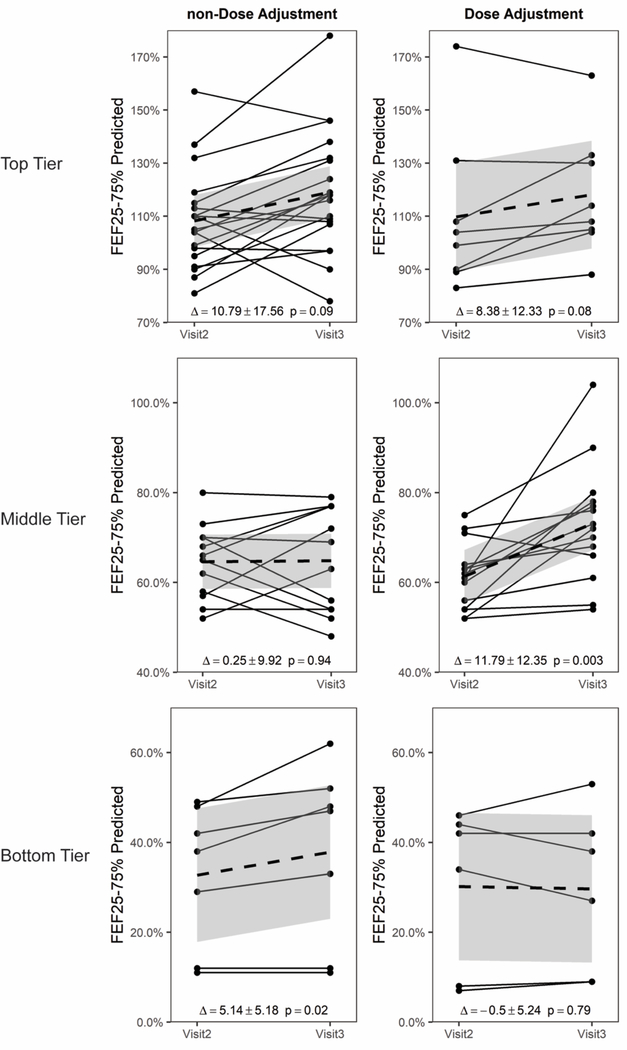
In accordance with the inclusion parameters, all 38 non-DA and 27 DA three visit sets exhibited a ≥ 10% decline in FEF25–75% between Visit 1 and Visit 2 (Fig. 1). As noted above, we separated the treatment sets into three lung function tiers representing essentially normal lung function (top), mild to moderate obstruction (middle), and severe obstruction (bottom). A significant benefit to increasing the dose of IGRT among patients in the middle tier of lung function proved statistically significant (DA Δ = 11.79 ± 12.35, p = 0.003 versus non-DA Δ = 0.25 ± 9.92, p = 0.94; Fig. 2, middle).
Fig. 2.
For changes in FEF25–75%, the Dose Adjustment sets in the middle tier section showed a statistically significant increase in FEF25–75% after dose increase; whereas the non- Dose Adjustment sets did not. The FEF25–75% at visits 2 and 3 are shown. The solid dashed line indicates the mean line with the 95% confidence interval shown in gray.
Among both the patients with top tier (normal) lung function and bottom tier (most severely degraded) lung function, no benefit from increasing the dose of IGRT was observed (Fig. 2, top and bottom). Indeed, among the bottom tier, the patients with no dose adjustment appeared to do slightly better than those with a dose adjustment (DA Δ = −0.5 ± 5.24, p =0.79 versus non-DA Δ =5.14 ± 5.18, p =0.02, Fig. 2, bottom). Again, however, the number of visit sets in the bottom tier group was small (7 non-DA and 6 DA).
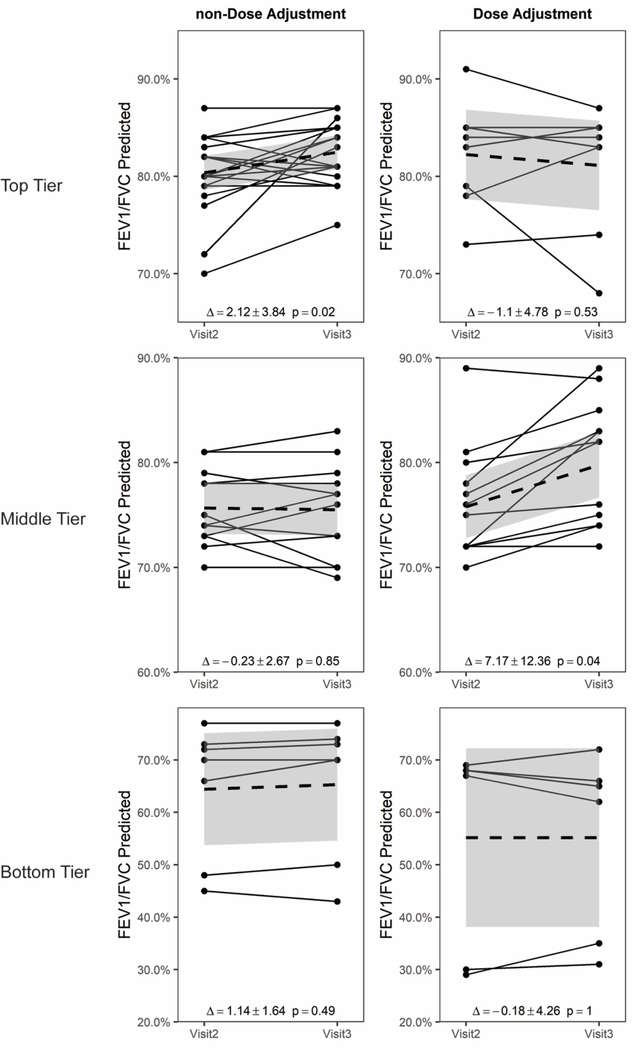
We next evaluated changes in %FEV1 (Fig. S3), FEV1/FVC (Fig. S4) and %FVC (Fig. S5). The magnitude of the changes between visits was smaller than those for FEF25–75% (Fig. 1). When the treatment sets were separated into the three lung function tiers based on FEF25–75% (top, middle and bottom), a significant benefit to increasing the dose of IGRT among patients in the middle tier of lung function was again statistically significant for FEV1/FVC (DA Δ = 7.17 ± 12.36, p = 0.04 versus non-DA Δ = −0.23 ± 2.67, p = 0.85; Fig. 3, middle). We observed no significant differences between DA and non-DA for FEV1 or FVC alone (data not shown).
Fig. 3.
For changes in FEV1/FVC, the Dose Adjustment sets in the middle tier section showed a statistically significant increase in FEV1/FVC after dose increase; whereas the non-Dose Adjustment sets did not. The FEV1/FVC at visits 2 and 3 are shown. The solid dashed line indicates the mean line with the 95% confidence interval shown in gray.
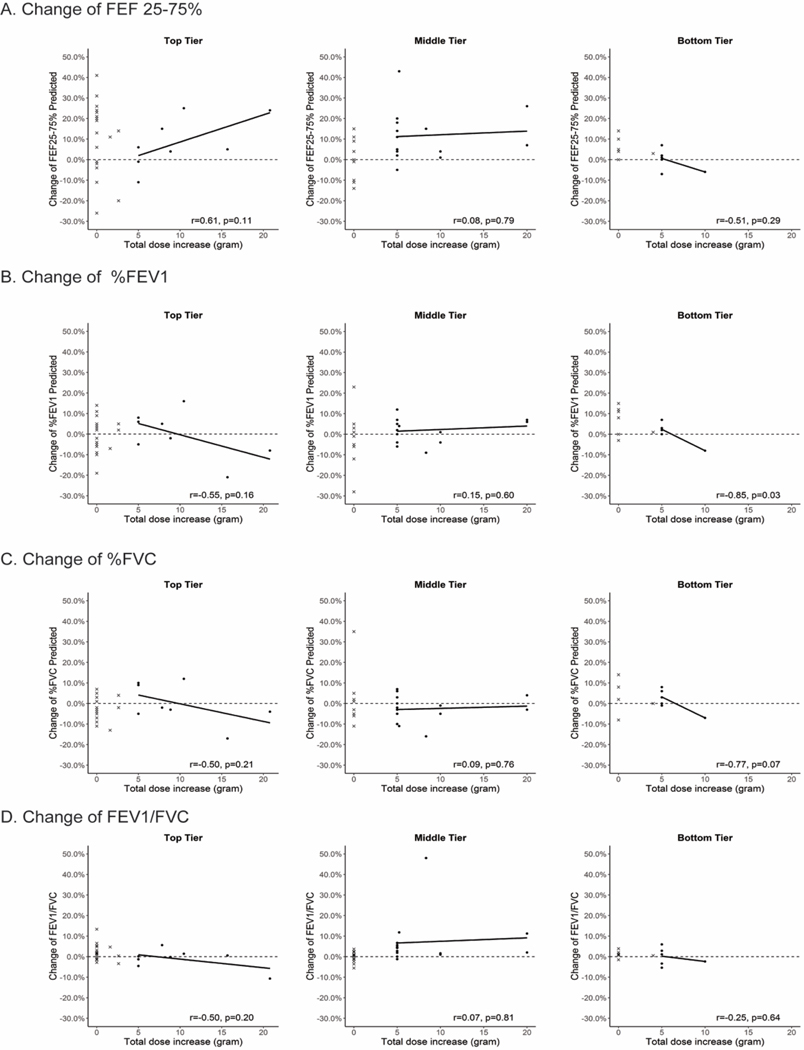
We then tested whether there was a relationship between the absolute increase in IgG in grams (Fig. 4) or in grams per kilogram (Fig. S6) per four-week period and changes between visits 2 and 3 in %FEF25–75%, %FEV1, %FVC, and %FEV1/FVC. For both %FEF25–75% and %FEV1/FVC, but not for %FEV1 or %FVC alone, in all but two patient visit sets there was an improvement in lung function among the DA patient visit sets in the middle tier for patients who had received at least either a 5 gram increase in absolute dosage or a 0.5 gram per kilogram per four week period. The lack of a correlation between the absolute increase in IgG dose and the percent increase in the relevant spirometric value suggests that there was no additional benefit to increasing the dose of IGRT by more than 5 grams or 0.5 grams per kilogram.
Fig. 4.
Changes in spirometric values in percent of predicted from visit 2 to visit 3 as a function of the absolute increase in IGRT therapy in grams per four week intervals. As the absolute dose increases, there is trend for an increase in the FEF25–75% only in the top tier group. In the middle group, there was an increase in FEF25–75% and FEV1/FVC in almost all of the study subjects receiving at least a 5 gram increase in dosage per 4 week period, although there was no obvious benefit to receiving more than 5 grams.
X axis: Absolute change in dose per four weeks in grams. Y axis: Change in percent of (A) FEF25–75%, (B) %FEV1, (C) %FVC and (D) FEV1/FVC. Solid dots represent non-Dose Adjustment, x represents Dose-Adjustment group.
Discussion
Preservation of lung function is a key feature of the management of patients with primary antibody deficiency. We found that among IGRT CVID patients with moderate but not severe obstruction as assessed by spirometry, increasing IgG dosage led to an increase in FEF25–75% and FEV1/FVC.
Although CT screening for pulmonary pathology is more sensitive measure of pulmonary pathology[16], spirometry is a pragmatic means by which the individual clinician can identify and follow changes in lung function. It is non-invasive, can be performed with a portable instrument inexpensively in the office, and the results are immediately available. Spirometry and other pulmonary function testing (PFT) have long been used to assess therapy in CVID patients since clinical symptoms may not show the subclinical effects of the disease on the lungs [17–20]. A web-based survey by the Clinical Immunology Society and the European Society of Immunodeficiencies published recently showed 98% of the respondents would perform PFT for their CVID patients with lung disease, showing the importance of spirometric studies in assessing lung disease [21]. Obstructive flow-volume curves noted in PFTs were common in over fifty percent of 587 patients [22]; airway obstruction, ventilatory restriction, and impaired gas diffused were found in 21–40% of 65 patients [23]. Chen et al [20] found that increased IVIG dose per kilogram was inversely associated with lung function decline, indicating that higher dose per kilogram appeared to be protective of lung function. Chen et al also found that trough IgG level did not have a statistically significant association with declines in either FEV1 or FVC. This finding is in keeping with our clinical observations that past a certain point trough IgG levels are less helpful in guiding adjustments to IGRT therapy in patients who are receiving adequate amounts of IgG on a gram per kilogram basis.
While FEV1 and FEV1/FVC are the most commonly used spirometric measurements to identify both the presence and degree of airflow obstruction [13], FEF25–75% is also commonly reported [13, 24]. Variation in FEF25–75% was initially thought to reflect changes exclusively in the small airways (< 2mm) [25–27]. However, subsequent studies have shown that changes in FEF may also reflect distal airflows that involve airways that have greater diameters [24]. In heterogeneous populations, FEF25–75% is seldom discordant from FEV1 and FEV1/FVC [28].
A reduction in the FEF25–75% has also been associated with other respiratory disorders. In children with asthma, reduced FEF25–75% has been shown to be associated with increased asthma severity, the need for systemic steroid use and more frequent exacerbations in the setting of normal FEV1 [29]. In adults with asthma, a reduced FEF25–75% is also independently associated with previous ICU admission, persistent symptoms, nocturnal symptoms, blood eosinophilia, bronchial hyperreactivity, and, by extension, airway inflammation [24]. The combination of rhinovirus infection in patients with particulate matter in their lungs also tends to lower FEF25–75% and FEV1 [30]. And, patients with allergic rhinitis may also demonstrate reductions in FEF25–75% [31].
For patients with a recent increase in mild to moderate obstruction who received ≥ 5 additional grams (or an additional 0.5 grams per kilogram) of gammaglobulin replacement per month, we found that two of three spirometric measures of obstruction (FEF25–75% and FEV1/FVC) demonstrated significant improvement when compared to those whose dosage was unchanged. This suggests that the decrease in FEF25–75% that led us to identify these patients might reflect reversible inflammation in smaller airways due to subclinical viral or bacterial infections that could then be ameliorated by the increased protection offered by a greater dose of gammaglobulin.
Although improvement was noted for patients in the middle tier who received an increase in gammaglobulin dosage, no statistically significant evidence of improvement related to the increase in dose was observed in patients in the top or bottom tiers. The former is consistent with the view that patients with normal lung function require less gammaglobulin than patients with evidence of obstruction [32]. The latter would suggest that patients with severe obstruction have sustained damage to the lungs that cannot be corrected or alleviated by increasing the protection offered by exogenous antibodies and the benefits of herd immunity.
In initial studies of the benefits of high-dose gammaglobulin replacement, Roifman and colleagues performed a randomized cross-over study of antibody deficient patients who received either 0.6 or 0.2 gm/kg of intravenous immunoglobulin for six months and were then switched to the alternative dose for a further six months [14]. Although the incidence of infections did not differ greatly in the high-dose and low-dose phases, the frequency of acute infection was substantially reduced in those periods when serum IgG was 500 mg/dl or more. Patients switched from high-dose to low-dose IGRT suffered deterioration of both FVC and FEV1. In contrast, patients switched from low-dose to high-dose IGRT demonstrated consistent improvement. In 10 of 12 patients, the improvement or deterioration was greater than 10%. Here, starting at much higher serum IgG levels, we see further evidence that even a small increase in the dose of gammaglobulin among patients exhibiting a recent decline in lung function can help reverse the decline.
The development of lung disease in CVID patients appears to reflect not only recurrent bacterial infection or bronchiectasis, but a generalized immune dysregulation that can occur in the absence of a history of pneumonia or bronchiectasis [33]. Although a continued high frequency or an increase in the frequency of sinopulmonary infections and antibiotics usage can be used to justify an increase in the dose of immunoglobulin G replacement therapy, subclinical function changes may occur without overt symptoms. In our patients, the improvement in spirometry occurred in the context of no significance changes in the reported frequency or severity of infections, or in the use of antibiotics. This suggests that spirometry can be a more sensitive measure of changing lung function in CVID patients than the history provided by the patients.
Three key strengths of our study are the relatively homogeneity of our Southeastern US clinic population, which is primarily of Scots Irish descent, the use of the same instrument by the same clinical immunologist, and a long-term follow-up period stretching for more than a decade.
However, our study also had limitations. First, it was a retrospective study where changes in medication were neither random nor blinded. The decision of whether to increase the dose of IGRT, or not, was based on clinical judgement alone. Thus, there is the possibility of a hidden bias in deciding whether or not to increase the dose.
Second, trough serum immunoglobulin levels for patients on intravenous gammaglobulin infusions were not available, making a direct comparison between the spirometric results and a common measure of dose sufficiency problematic. However, the average level of serum IgG measured in patients receiving subcutaneous gammaglobulin was similar to the average spot level of serum IgG in patients receiving gammaglobulin intravenously, suggesting that immunoglobulin dosage was on target.
Third, the patients at visit 1 were already receiving gammaglobulin therapy well within the recommended range and exhibited relatively well controlled symptoms. Thus, the beneficial effects of increasing the dose of gammaglobulin based on spirometry could be greater in populations where use of gammaglobulin is less aggressive.
Finally, FEF25–75% is known to exhibit wide variability. However, a positive effect on FEV1/FVC was also noted in patients who exhibited an increase in FEF25–75%, which provides internal support for the validity of the measurement.
In spite of these limitations, our study supports the value of spirometry as an objective measurement for determining whether an increase in IGRT is necessary. Our studies suggest that patients with FEF25–75% values of between 50% and 80% of predicted who have shown a decrease in this value appear to be most likely to respond to an increase in gammaglobulin dosage. A prospective study of immunoglobulin dosing is needed to further prove this contention. Such a prospective study should examine immunoglobulin dosing, spirometry, the value of a history of persistent, recurring sinopulmonary infection and prophylactic antibiotic usage in adjusting dosage. A prospective study of patients with stable, but depressed, spirometric values of obstruction within this range would benefit from an increase in immunoglobulin dosage is also warranted.
As a final point, a key complaint of many patients is fatigue at varying levels. A prospective study of immunoglobulin dose, spirometry and fatigue, as measured by a validated instrument, might shed new light on the role of subclinical sinopulmonary obstruction and immunoglobulin dosage in influencing the quality of life experienced by the patient.
Conclusions
Determination of an optimal dosage level for IGRT in primary antibody deficiencies has long been problematic. We find that increases in dosage of as little as five grams per four-week period can have beneficial effects on the lungs in patients with moderate lung dysfunction. Our findings suggest that office spirometry, a simple non-invasive technique, can be used to evaluate subclinical changes in lung function and guide IGRT dosing in these patients.
Supplementary Material
Acknowledgements
We would like to thank Dr. Ada Elgavish for helpful comments.
This work was supported by a research grant from CSL Behring, King of Prussia, PA; and utilized a database previously supported by the NIH (AI090902).
Abbreviations
- CVID
Common Variable Immunodeficiency
- FEF25–75%
Forced Expiratory Flow 25% – 75%
- FEV1
Forced Expiratory Volume one second
- FVC
Forced Vital Capacity
- IgG
Immunoglobulin G
- IGRT
Immunoglobulin replacement therapy
- PFT
Pulmonary Function Test
Footnotes
Compliance with Ethical Standards
Informed Consent:
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder HW Jr. , Schroeder HW, III, Sheikh SM. The complex genetics of common variable immunodeficiency. Journal of Investigative Medicine. 2004;52(2):90–103. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Wu EY, Tarrant TK. Immune Gamma Globulin Therapeutic Indications in Immune Deficiency and Autoimmunity. Curr Allergy Asthma Rep. 2016;16(8):55. doi: 10.1007/s11882-016-0632-7. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham-Rundles C, Siegal FP, Smithwick EM, Lion-Boule A, Cunningham-Rundles S, O’Malley J et al. Efficacy of intravenous immunoglobulin in primary humoral immunodeficiency disease. Annals of internal medicine. 1984;101(4):435–9. [DOI] [PubMed] [Google Scholar]
- 5.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109(6):1001–4. [DOI] [PubMed] [Google Scholar]
- 6.Hodkinson JP. Considerations for dosing immunoglobulin in obese patients. Clin Exp Immunol. 2017;188(3):353–62. doi: 10.1111/cei.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gathmann B, Mahlaoui N, Ceredih, Gerard L, Oksenhendler E, Warnatz K et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134(1):116–26. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Tian X, Wang X, Xiao Z. Adverse Effects of Immunoglobulin Therapy. Front Immunol. 2018;9:1299. doi: 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125(6):1354–60 e4. doi: 10.1016/j.jaci.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 10.Pourpak Z, Aghamohammadi A, Sedighipour L, Farhoudi A, Movahedi M, Gharagozlou M et al. Effect of regular intravenous immunoglobulin therapy on prevention of pneumonia in patients with common variable immunodeficiency. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2006;39(2):114–20. [PubMed] [Google Scholar]
- 11.Eijkhout HW, van Der Meer JW, Kallenberg CG, Weening RS, van Dissel JT, Sanders LA et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Annals of internal medicine. 2001;135(3):165–74. [DOI] [PubMed] [Google Scholar]
- 12.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clinical Immunology. 1999;93(3):190–7. [DOI] [PubMed] [Google Scholar]
- 13.NAEaP P. Expert Panel Report 3. National Heart, Lung and Blood Institute Produced Publications: National Institute of Health. 2007. [Google Scholar]
- 14.Roifman CM, Levison H, Gelfand EW. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet. 1987;1(8541):1075–7. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A et al. Standardisation of spirometry. The European respiratory journal. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Maarschalk-Ellerbroek LJ, de Jong PA, van Montfrans JM, Lammers JW, Bloem AC, Hoepelman AI et al. CT Screening for Pulmonary Pathology in Common Variable Immunodeficiency Disorders and the Correlation with Clinical and Immunological Parameters. J Clin Immunol. 2014;34(6):642–54. doi: 10.1007/s10875-014-0068-6. [DOI] [PubMed] [Google Scholar]
- 17.Thickett KM, Kumararatne DS, Banerjee AK, Dudley R, Stableforth DE. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. QJM. 2002;95(10):655–62. [DOI] [PubMed] [Google Scholar]
- 18.Busse PJ, Farzan S, Cunningham-Rundles C. Pulmonary complications of common variable immunodeficiency. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2007;98(1):1–8; quiz −11, 43. doi: 10.1016/S1081-1206(10)60853-8. [DOI] [PubMed] [Google Scholar]
- 19.Maglione PJ, Overbey JR, Radigan L, Bagiella E, Cunningham-Rundles C. Pulmonary radiologic findings in common variable immunodeficiency: clinical and immunological correlations. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2014;113(4):452–9. doi: 10.1016/j.anai.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Stirling RG, Paul E, Hore-Lacy F, Thompson BR, Douglass JA. Longitudinal decline in lung function in patients with primary immunoglobulin deficiencies. J Allergy Clin Immunol. 2011;127(6):1414–7. doi: 10.1016/j.jaci.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 21.Akhter J, Lefaiver CA, Scalchunes C, DiGirolamo M, Warnatz K. Immunologist’s Perspectives on Assessment and Management of Lung Disease in CVID: a Survey of the Membership of the Clinical Immunology Society and the European Society for Immunodeficiencies. J Clin Immunol. 2018;38(3):237–46. doi: 10.1007/s10875-018-0488-9. [DOI] [PubMed] [Google Scholar]
- 22.Touw CM, van de Ven AA, de Jong PA, Terheggen-Lagro S, Beek E, Sanders EA et al. Detection of pulmonary complications in common variable immunodeficiency. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2010;21(5):793–805. doi: 10.1111/j.1399-3038.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 23.Gregersen S, Aalokken TM, Mynarek G, Kongerud J, Aukrust P, Froland SS et al. High resolution computed tomography and pulmonary function in common variable immunodeficiency. Respiratory medicine. 2009;103(6):873–80. doi: 10.1016/j.rmed.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Riley CM, Wenzel SE, Castro M, Erzurum SC, Chung KF, Fitzpatrick AM et al. Clinical Implications of Having Reduced Mid Forced Expiratory Flow Rates (FEF25–75), Independently of FEV1, in Adult Patients with Asthma. PLoS One. 2015;10(12):e0145476. doi: 10.1371/journal.pone.0145476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terra Filho M, Vargas FS, Cukier A, Fiss E, Romeiro Neto M, Croce J. [Forced mid-expiratory flow rate (FEF 25–75%): a critical analysis of its value in recognizing diseases of the small airways]. Allergologia et immunopathologia. 1986;14(3):199–203. [PubMed] [Google Scholar]
- 26.Perez T, Chanez P, Dusser D, Devillier P. Small airway impairment in moderate to severe asthmatics without significant proximal airway obstruction. Respiratory medicine. 2013;107(11):1667–74. doi: 10.1016/j.rmed.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 27.McFadden ER Jr, Linden DA. A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am J Med. 1972;52(6):725–37. [DOI] [PubMed] [Google Scholar]
- 28.Quanjer PH, Weiner DJ, Pretto JJ, Brazzale DJ, Boros PW. Measurement of FEF25–75% and FEF75% does not contribute to clinical decision making. The European respiratory journal. 2014;43(4):1051–8. doi: 10.1183/09031936.00128113. [DOI] [PubMed] [Google Scholar]
- 29.Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul W. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. The Journal of asthma : official journal of the Association for the Care of Asthma. 2012;49(6):586–92. doi: 10.3109/02770903.2012.690481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vempilly J, Abejie B, Diep V, Gushiken M, Rawat M, Tyner TR. The synergetic effect of ambient PM2.5 exposure and rhinovirus infection in airway dysfunction in asthma: a pilot observational study from the Central Valley of California. Exp Lung Res. 2013;39(10):434–40. doi: 10.3109/01902148.2013.840693. [DOI] [PubMed] [Google Scholar]
- 31.Gungor D, Nadaud P, LaPergola CC, Dreibelbis C, Wong YP, Terry N et al. Infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span: a systematic review. Am J Clin Nutr. 2019;109(Supplement_7):772S–99S. doi: 10.1093/ajcn/nqy283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Gracia J, Vendrell M, Alvarez A, Pallisa E, Rodrigo MJ, de la Rosa D et al. Immunoglobulin therapy to control lung damage in patients with common variable immunodeficiency. International immunopharmacology. 2004;4(6):745–53. doi: 10.1016/j.intimp.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Schussler E, Beasley MB, Maglione PJ. Lung Disease in Primary Antibody Deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039–52. doi: 10.1016/j.jaip.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.