Abstract
Background
Vitiligo is a common pigmentary disease that affects 0.5% to 1% of the global population. The main manifestation of vitiligo is skin depigmentation, which significantly influences appearance and brings enormous psychological stress for patients. C-X-C motif chemokine ligand 9 (CXCL9), CXCL10 , CXCL11 and CXCL12 are linked to the Th1 pattern and have been suggested as one of the most relevant chemokine axes that promote T cell migration in different autoimmune and inflammatory process . These were suggested to promote melanocyte-specific cytotoxic T lymphocyte (CTLs) to infiltrate into the basal layer of the epidermis to attack melanocytes, resulting in the deficiency of melanin.
Objective
The aim of this study was to evaluate the role of CXCL10 and CXCL12 in the pathogenesis of vitiligo and to detect its relationship to disease activity.
Methods
Forty patients with non-segmental vitiligo (NSV; 20 patients with active disease and 20 stable patients). This group included 20 male patients and 20 female patients, with ages ranging from 10 to 65 years. Twenty healthy age- and sex-matched controls were included. The control group included 10 males and 10 females with ages ranging from 10 to 65 years. We measured the serum level of CXCL10 and CXCL12 in the patients and controls using the enzyme-linked immunosorbent assay (ELISA) method.
Results
Serum CXCL10 level was highly significantly increased in patients with vitiligo compared to controls. There was a high statistically significant difference between patients with active disease and those with stable disease regarding serum level of CXCL10 with higher level of CXCL10 in active type.
Conclusion
Our results suggest that vitiligo might be associated with increased serum levels of CXCL10 and CXCL12. There is a positive relationship to disease activity, indicating that CXCL10 and CXCL12 may play a significant role in the pathogenesis of vitiligo.
Keywords: Vitiligo, chemokines, CXCL10, CXCL12
Multiple pathogenetic factors have been proposed to clarify the etiology of vitiligo, including neural theory, genetic predisposition, and impaired antioxidative defense. A complex interaction between genetic, environmental, biochemical, and immunological events is likely to generate a permissive milieu. Therefore, loss of melanocytes in vitiligo appears to occur through a combination of several mechanisms that act in concert.1
Many kinds of immune cells as well as cytokines participate in vitiligo, the most important of which are melanocyte-specific cluster of differentiation 8 (CD8)+ T-cells (cytotoxic T lymphocytes; CTLs). In the peripheral blood of vitiligo patients, there is an increased proportion of autoreactive T-cells. Similarly, in skin lesions, CTLs remarkably infiltrate into the epidermis and accumulate around the dying melanocytes. Generally, CTLs migrate into the skin following various chemokine signals. Chemokines are important inflammatory factors that participate in many autoimmune responses.2
C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, and CXCL11 are linked to the Th1 pattern and have been suggested as one of the most relevant chemokine axes that promote T-cell migration in different autoimmune and inflammatory processes.3
In vitiligo, CXCL9 and CXCL10 have been suggested to promote melanocyte-specific CTLs to infiltrate into the basal layer of the epidermis to attack melanocytes, resulting in the deficiency of melanin.2
C-X-C motif chemokine ligand 12 (CXCL12) is a key chemokine that regulates tissue homeostasis and inflammatory responses. Its tissue expression directs lymphocytes to the site of inflammation. CXCL12 is crucial for the migration of Langerhans cells from the epidermis to the dermis after antigen exposure. This allows its further transport to the draining lymph node and the generation of a targeted T-cell–mediated immune response.4
In light of this evidence, we designed a study to test for the presence of CXCL10 and CXCL12 circulating serum levels in patients with vitiligo to identify whether they play a role in the pathogenesis of vitiligo.
METHODS
This case-control study included 40 patients with clinically diagnosed vitiligo. Eligible patients were those attending the dermatology, venereology, and andrology outpatient clinics at Zagazig University Hospital during the period from May to October 2018. Approval from the institutional review board was secured and informed consent was collected from patients and controls before starting the work.
Study population. Study participants were divided into two groups. The first group included 40 patients with nonsegmental vitiligo (NSV), including 20 patients with active disease and 20 stable patients. This group included 20 men and 20 women with ages ranging from 10 to 65 years. Separately, the control group included 20 healthy subjects who were matched to vitiligo patients according to age and sex. This group included 10 men and 10 women with ages ranging from 10 to 65 years.
The following patients were excluded from this study: patients receiving systemic treatment in the last two months; patients with any autoimmune disease, such as rheumatoid arthritis, inflammatory bowel disease, psoriasis, or systemic lupus erythematosus; patients with type 1 diabetes mellitus; and patients with any thyroid and/or neoplastic diseases.
All included patients were subjected to the following:
Careful history-taking, including personal history (age, sex); present history of vitiligo (age of onset, course, duration of the disease); past history of previous diseases or previous treatments; and family history of vitiligo or other autoimmune diseases.
Clinical examination, including a general examination to exclude diseases that alter the levels of CXCL10 and CXCL12 and other associated diseases, such as diabetes mellitus, hyperthyroidism, and autoimmune diseases as well as a local examination for the diagnosis of vitiligo type and extent of the disease.
An assessment of disease activity wherein a six-point vitiligo disease activity (VIDA) score was used to grade the disease activity per anatomic site according to the patient’s own opinion. In this context, disease activity was indicated as follows: (+4) active in past six weeks, (+3) active the in past three months, (+2) active in the past six months, (+1) active in the past year, (0) stable for at least one year, and (−1) stable for at least one year with spontaneous repigmentation.5
Laboratory examinations. Venous blood samples (5 mL) were collected from each patient and control subjects, then dispensed in tubes and left to clot for 30 minutes at room temperature and centrifuged at 1,000 rpm for 15 minutes. Subsequently, the collected sera were stored at −70°C until analysis, and repeated freeze and thaw cycles were avoided. Separately, serum levels of CXCL10 and CXCL12 were measured in the patients and controls using the enzyme-linked immunosorbent assay (ELISA) method by (STAT FAX 303, USA) and correlated with age, sex, duration of disease, type, activity of vitiligo and family history.
ELISA method principle. The ELISA kits employed a double-antibody sandwich ELISA approach to assay the levels of human CXCL10 and CXCL12 in samples. CXCL10 or CXCL12 was added to monoclonal antibody enzyme, which was precoated with CXCL10 or CXCL12 monoclonal antibody and incubated. Then, CXCL10 or CXCL12 antibodies were added with biotin and combined with horseradish peroxidase-conjugated streptavidin to form an immune complex. Incubation was carried out, and the cells were washed again. We added chromogen solutions A and B. The color of the liquid changed to blue. Then, under the effect of acid, the colors finally became yellow. The chrome color and the concentration of CXCL10 or CXCL12 of the sample were positively correlated.
Assay procedure. The assay procedure was as follows:
During standard dilution, all reagents were allowed to reach room temperature. For dilution of the standard, 120 μL of standard diluent was placed in each tube. Then, 120 μL of standard was added in the first tube, and 120 μL was moved from the first tube into the second. Next, 120 μL was pipetted from the second tube to the third tube, and the dilution series was produced. Each of the concentrations was repeated to obtain the mean value of each well.
-
Sample injection
Blank well: Samples were not added, and CXCL10-antibody or CXCL12 antibody labeled with biotin, horseradish peroxidase-conjugated streptavidin, only chromogen solution A or B, and stop solution were allowed; the other operations were the same.
Standard well: Standard (50 μL) and horseradish peroxidase-conjugated streptavidin (50 μL) were added (since the standard already has the combined biotin antibody, it was not necessary to add the antibody).
To-be-tested wells: Sample (40 μL) was added, together with both (CXCL10 antibody (10 μL) or CXCL12 antibody (10 μL) and horseradish peroxidase–conjugated streptavidin (50 μL). Then, the sealing membrane was sealed, gently shaken, and incubated for 60 minutes at 37°C.
Configuration of liquid: Wash solution was diluted 30-fold with distilled water.
Washing: The membrane was removed carefully, the liquid was drained, and the remaining water was shaken.
Chromogen solution: First, 50 μL of chromogen A, followed by 50 μL of chromogen solution B, was added to each well. This was gently mixed and incubated for 10 minutes at 37°C away from the light.
Stop: Stop solution (50 μL) was added to each well to stop the reaction (the blue changed to yellow immediately).
Final measurement: The blank well was taken as 0; we measured the optical density (OD) under 450-nm wavelengths, which should be carried out within 15 minutes after adding the stop solution.
According to standard concentrations and the corresponding OD values, we calculated the standard curve line regression equation to determine the corresponding sample’s concentration.
Calculation. The standard density was taken as the horizontal, the OD value was taken as the vertical, and the standard curve was drawn on graph paper. To determine the corresponding density according to the sample, OD values were calculated by the sample curve (i.e., the result is the sample density).
RESULTS
Regarding the sociodemographic characteristics of the studied vitiligo group, ages ranged from 10 to 65 years, with a mean of 27.83±16.47 years (median: 23 years), and half of the study population was male (50%). Meanwhile, ages of the control group ranged from 10 to 65 years, with a mean of 33.8±13.19 years (median: 35 years), and half were also male (50.0%); there was no statistically significant difference between the two groups regarding age or sex.
The mean duration of vitiligo in the studied group was 5.44±7.3 years, ranging from one month to 36 years. Most of the studied group had a negative family history of vitiligo (80%), and half (50%) received topical treatment as shown in Table 1.
TABLE 1.
Present history and clinical picture among vitiligo patients
| PATIENT HISTORY | STUDIED VITILIGO CASES (N = 40) | |
|---|---|---|
| N | % | |
| Duration of disease (years) | ||
| Mean ± standard deviation | 5.44 ± 7.3 | |
| Median (range) | 3 (0.08–36) | |
| Family history of vitiligo | ||
| Positive | 8 | 20.0 |
| Negative | 32 | 80.0 |
| Previous topical treatment | ||
| Positive | 20 | 50.0 |
| Negative | 20 | 50.0 |
Regarding the clinical types of vitiligo, more than half of the studied group (52.5%) had vitiligo vulgaris, more than one-third had acrofacial vitiligo (37.5%), and only 10% suffered from universal vitiligo.
Regarding VIDA scores among vitiligo patients, the mean VIDA score among the studied vitiligo population was 2.03±1.62 points; 30% of the studied group had activity for six weeks or less period, 15% had activity from six weeks to three months, 30% had activity lasting from six to 12 months, and only 2.5% were stable for at least for one year with spontaneous repigmentation, respectively, as shown in Table 2.
TABLE 2.
VIDA score among vitiligo patients
| SCORE | STUDIED VITILIGO CASES (N = 40) | |
|---|---|---|
| N | % | |
| VIDA score | ||
| Mean ± standard deviation | 2.03±1.62 | |
| Median (range) | 1.5 (−1 to 4) | |
| VIDA scale | ||
| +4: Activity of 6 weeks or less | 12 | 30.0 |
| +3: Activity of 6 weeks to 3 months | 6 | 15.0 |
| +2: Activity of 3 to 6 months | 2 | 5.0 |
| +1: Stable of 6 to 12 months | 12 | 30.0 |
| 0: Stable at least for 1 year | 7 | 17.5 |
| −1: Stable at least for 1 year with spontaneous repigmentation | 1 | 2.5 |
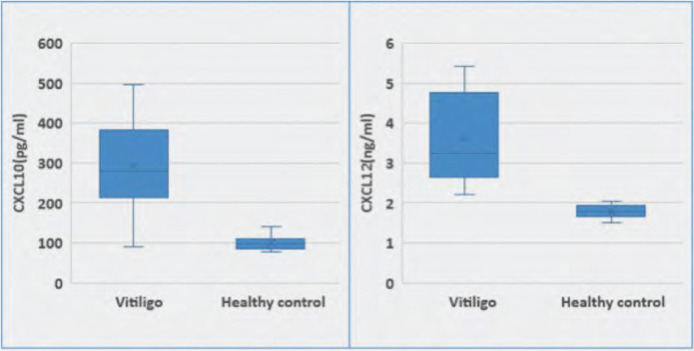
The mean serum level of CXCL10 in the studied vitiligo group was statistically higher than the mean serum level of CXCL10 in the healthy control group (292.6±109.8 pg/mL vs. 99.25±18.31 pg/mL, respectively). The mean serum level of CXCL12 in the studied vitiligo group was statistically higher than that of the mean serum level of CXCL12 in the healthy control group (3.62±1.08 ng/mL vs. 1.78±0.16 ng/mL) (Figure 1).
FIGURE 1.
Boxplots for comparison of chemokines CXCL10 and CXCL12 among the studied groups.
Serum levels of CXCL10 ranged from 91 to 496pg/mL in vitiligo patients and from 76 to 141pg/mL in healthy controls, respectively, while serum levels of CXCL12 ranged from 2.21 to 5.42ng/mL in vitiligo patients and from 1.51 to 2.03ng/mL in healthy controls. Serum levels of both chemokines CXCL10 and CXCL12 were higher in vitiligo patients than healthy controls (Figure 1).
There was a significant positive correlation between patient age and the duration of vitiligo, while there was a significant negative correlation between age and VIDA score among vitiligo patients (r = −0.359; p<0.05), respectively. Also, there was a significantly negative correlation between the duration of vitiligo, VIDA score, and chemokines CXCL10 and CXCL12 (r=−0.735, r=−0.709, and r=−0.716; p<0.05), respectively, as shown in Table 3.
TABLE 3.
Correlation between chemokines CXCL10 and CXCL12, duration of disease, age of patients, and VIDA score among vitiligo patients
| CORRELATION COEFFICIENT | AGE | DURATION | VIDA score | CXCL10 (pg/ml) | |
|---|---|---|---|---|---|
| Duration | (r) | 0.330 | |||
| p-value | 0.038* (S) | ||||
| VIDA score | (r) | −0. 359 | −0. 735 | ||
| p-value | 0.023* (S) | 0.000** (HS) | |||
| CXCL10 (pg/mL) | (r) | −0. 250 | −0. 709 | 0.962 | |
| p-value | 0.060 (NS) | 0.000** (HS) | 0.000** | ||
| CXCL12 (ng/mL) | (r) | −0.125 | −0.716 | 0.963 | 0.931 |
| p-value | 0.652 (NS) | 0.000** (HS) | 0.000** (HS) | 0.000** (HS) | |
*Correlation coefficient (r) is significant at the 0.05 level (S)
**Correlation coefficient (r) is significant at the 0.01 level (HS)
Regarding VIDA score and chemokines CXCL10 and CXCL12, there was a significant positive correlation between them, where, the greater the vitiligo activity, the higher the levels of chemokines CXCL10 and CXCL12 (p=0.000).
Regarding disease activity among the studied vitiligo patients, the mean serum level of CXCL10 in the studied vitiligo group was statistically higher in patients with active vitiligo than those with stable disease (385.2±58.44 pg/mL vs. 200.0±57.25 pg/mL). Also, the mean serum level of CXCL12 in the studied vitiligo group was statistically higher in patients with active vitiligo than those with stable disease (4.58±0.61 ng/mL vs. 2.66±0.31ng/mL), as shown in Table 4.
TABLE 4.
Chemokines CXCL10 and CXCL12 in relation to disease activity among the studied vitiligo patients (N=40)
| VARIABLE | ACTIVITY STATE ACCORDING TO VIDA SCORE (N = 40) | TEST* | P-VALUE | |
|---|---|---|---|---|
| Stable (n = 20) | Active (n = 20) | |||
| Serum level of CXCL10 (pg/mL) | ||||
| Mean ± SD | 200.0 ± 57.25 | 385.2 ± 58.44 | 0.000 | 0.000* (HS) |
| Median (range) | 215(91–275) | 382(283–496) | ||
| Serum level of CXCL12 (ng/mL) | ||||
| Mean ± SD | 2.66 ± 0.31 | 4.58 ± 0.61 | 1.000 | 0.000* (HS) |
| Median (range) | 2.63 (2.21–3.32) | 4.77(3.17–5.42) | ||
| Mean ± SD | 200.0 ± 57.25 | 385.2 ± 58.44 | 0.000 | 0.000* (HS) |
| Median (range) | 215(91–275) | 382(283–496) | ||
*Mann–Whitney U test
**NS: not significant (p > 0.05)
Serum levels of CXCL10 ranged from 283 to 496 pg/mL in patients with active vitiligo and from 91 to 275 pg/mL in stable patients, while serum levels of CXCL12 ranged from 3.17 to 5.42 ng/mL in patients with active vitiligo and from 2.21 to 3.32 ng/mL in stable patients. Thus, serum levels of chemokines CXCL10 and CXCL12 were higher in patients with active vitiligo than those with stable disease.
Regarding the receiver operating characteristic curve for cutoff points, given an area under the curve of 1.000, a CXCL10 level of greater than 291 pg/mL had a sensitivity of 95% and specificity of 100%, with high statistical significance in predicting the activity state of vitiligo. Also, considering an area under the curve of 0.998, a CXCL12 level of greater than 3.24 (ng/mL) had a sensitivity of 95% and specificity of 99.5%, with high statistical significance in predicting the activity state of vitiligo.
Regarding sex, previous topical treatment, and family history, the mean level of chemokines CXCL10 among the studied male vitiligo patients was 310 ± 101.2 pg/mL, while, in female vitiligo patients, it was 275.2 ± 117.7 pg/dL, with no significant difference according to sex. Also, there was no significant difference in the level of CXCL10 when considering family history and previous topical treatment.
Separately, the mean level of CXCL12 among the studied male vitiligo patients was 3.89±1.06 ng/mL, while, in female vitiligo patients, it was 3.36±1.06 ng/mL, with no statistically significant difference. Also, there was no significant difference in the level of CXCL12 with respect to family history and previous topical treatment, as shown in Table 5.
TABLE 5.
Chemokines CXCL12 among vitiligo patients according to sex and clinical history
| VARIABLE | CHEMOKINE CXCL12 (NG/ML) | TEST* | P-VALUE | |
|---|---|---|---|---|
| Sex | Male (n = 20) | Female (n = 20) | ||
| Mean ± SD | 3.89 ± 1.06 | 3.36 ± 1.06 | 140.50 | 0.108** (NS) |
| Median (range) | 4.07(2.21–5.42) | 2.88(2.21–5.12) | ||
| Family history | Negative (n = 32) | Positive (n = 8) | ||
| Mean ± SD | 3.51 ±1.24 | 3.65 ±1.05 | #116.00 | 0.703** (NS) |
| Median (range) | 2.86(2.27–5.23) | 3.42(2.21–5.42) | ||
| Previous topical treatment | Negative (n = 20) | Positive (n = 20) | ||
| Mean ± SD | 3.37± 1.05 | 3.88 ± 1.08 | #143.00 | 0.127** (NS) |
| Median (range) | 2.93(2.21–5.42) | 4.04(2.21–5.39) | ||
*Mann–Whitney U test
**NS: not significant (p>0.05)
Regarding vitiligo type, the mean serum level of CXCL10 in the studied vitiligo group was statistically higher in patients with acrofacial vitiligo than among either patients with vitiligo vulgaris and the universal type (338.8±92.5 pg/mL vs. 286.2±107.05 and 152.5±56.74 pg/mL, respectively). Also the mean serum level of CXCL12 in the studied vitiligo group was statistically higher in patients with acrofacial vitiligo than those with vitiligo vulgaris or the universal type (4.02±1.02 ng/mL vs. 3.58±1.05 and 2.36±0.18 ng/mL, respectively), as shown in (Figures 2) and (3).
FIGURE 2.
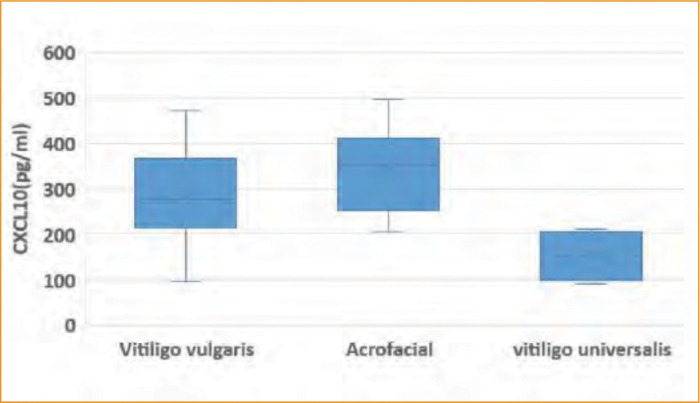
Boxplot representing chemokines CXCL10 in relation to the extent of disease among the studied vitiligo patients (n=40).
FIGURE 3.
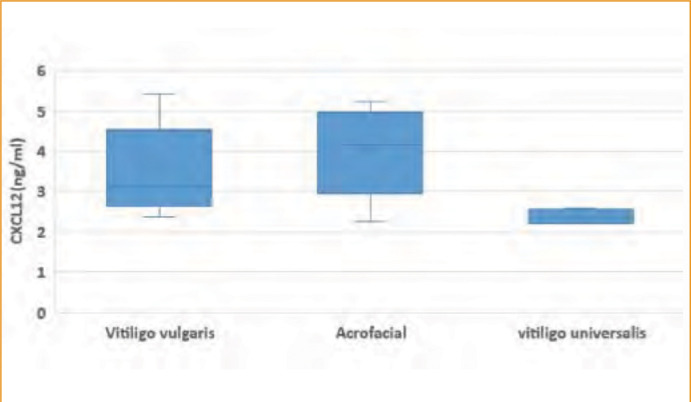
Boxplot representing CXCL12 in relation to the extent of disease among the studied vitiligo patients (n=40).
DISCUSSION
Vitiligo is an acquired disease with a variable course. It is characterized clinically by well-defined, depigmented macules or patches thought to occur secondary to melanocyte dysfunction and loss. It is the most common depigmentation disorder, affecting approximately 0.5% to 2.0% of the population, and has no predilection for sex or race. Vitiligo is categorized into nonsegmental and segmental subtypes, with the latter occurring in a minority (5%–16%) of patients.6
The etiology of vitiligo is poorly understood. Numerous factors have been implicated in the development of vitiligo, including stress, trauma, exposure to sunlight, infections, malignancies, neural abnormalities, melatonin receptor dysfunction, impaired melanocyte migration, some drugs, endocrine diseases, and cytotoxic compounds, and these causal factors may act independently.7
CTLs are the key factors attacking melanocytes in vitiligo. In patients with vitiligo, both in the peripheral blood and in skin lesions, the number of CTLs is significantly increased.8 In inflamed tissues, recruited Th1 lymphocytes are responsible for enhanced interferon gamma and tumor necrosis factor alpha production, which, in turn, stimulate the secretion of CXCL10 from inflammatory cells.9
CXCL10 binds to its specific receptor, chemokine CXCR3, and regulates immune responses by the recruitment and activation of T-cells and monocytes. It has been shown that the tissue expression levels of CXCR3 and CXCL10 are increased in various autoimmune diseases and that they play fundamental parts in leukocyte homing into the inflamed tissues and contribute to the process of tissue damage.10
However, whether CXCL10 and CXCL12 are related to the pathogenesis of vitiligo remains unknown. We estimated serum CXCL10 and CXCL12 levels in vitiligo patients and evaluated whether there is a possible relationship between serum CXCL10 and CXCL12 levels and the disease activity in 60 subjects divided into two groups (patients and control subjects). In this arrangement, 40 patients were matched with 20 healthy controls according to age and sex.
The present study showed that serum CXCL10 levels were highly significantly increased in patients with vitiligo compared to controls (p=0.000). These findings are in agreement with those of Ferrari et al,11 who examined serum CXCL10 in nonsegmental vitiligo. These authors studied a population of 50 patients with vitiligo and 50 healthy controls, 50% of whom were female. In their study, serum levels of CXCL10 were statistical significantly different between patients and controls (p=0.0001), with higher levels recorded in vitiligo patients.
In this study, there was a highly statistically significant difference between patients with active disease and those with stable disease with respect to serum levels of CXCL10, with higher levels of CXCL10 present in those with active disease (p=0.000). These findings are in agreement with those of Wang et al,12 who examined CXCL10 as a potential clinical marker for vitiligo by flow cytometry. These authors found that expression levels of serum CXCL10 were significantly elevated in patients with both progressive and stable vitiligo compared to healthy controls, with higher levels apparent in patients with progressive disease than in those in with stable disease (p<0.01). They also found that the expression level of serum CXCL9 was significantly elevated in patients with both progressive and stable vitiligo compared to healthy controls.
The present study revealed a highly statistically significant difference in serum CCXL10 level between the different grades of disease activity using the VIDA score, with a significant increase in CCXL10 level in concert with greater disease activity (p=0.000).
Zhang et al13 examined circulating chemokines CXCL10 and CCL20 as potential biomarkers for vitiligo activity and reported that serum levels of CXCL10 and CCL20 were significantly increased in vitiligo patients compared to healthy controls. Further, the concentration levels of both serum CXCL10 and CCL20 in the active stage were significantly higher than those in the stable stage.
Abdalla et al14 evaluated serum CXCL10 as an activity marker for vitiligo using ELISA in 55 patients with nonsegmental vitiligo (including 30 with active disease and 25 with stable disease) and 30 healthy controls. These authors reported that serum levels of CXCL10 were elevated in all vitiligo patients compared to controls and were higher in the active versus stable vitiligo subgroup.
Mouia et al3 reported that CXCL10 and CXCL9 levels in the serum of patients with vitiligo and alopecia areata were significantly elevated compared with healthy controls. Levels were also increased in patients with progressive vitiligo lesions compared to patients with stable disease. The expression of CXCL10 messenger RNA was significantly greater in nonlesional and perilesional vitiligo skin compared with the skin of healthy controls.
In the present study, there was no statistically significant difference in serum CCXL10 between cases with and without previous topical treatment. These findings were in agreement with those of Yang et al,2 who reported that serum levels of CXCL10 were not affected by previous topical treatments and were significantly elevated in patients with active vitiligo relative to those in stable patients (p<0.05). These authors also reported that the levels of chemokines CXCL8 and CCL5 were significantly elevated in the serum of vitiligo patients.
Regazzetti et al15 detected that CXCL10 is elevated in perilesional skin in vitiligo patients when compared to healthy control skin. On the contrary, however, they did not observe any increase in CXCL10 expression in stable depigmented lesions that lack melanocytes.
CXCL12 can be upregulated by pro-inflammatory cytokines, oxidative stress, and hypoxia, and it is likely that increased tumor necrosis factor alpha and interleukin 1-alpha levels in the lesional skin of patients with nonsegmental vitiligo contribute to the deregulation of these chemokines in melanocytes, leading to the recruitment of leukocytes and the establishment of melanocyte-specific adaptive immunity.16
In the present study, serum CXCL12 levels were significantly increased in patients with vitiligo compared to controls (p=0.000). There was a highly statistically significant difference between patients with active disease and those with stable disease regarding serum levels of CXCL12, with higher levels of CXCL12 in the active type (p=0.000). In addition, there was no statistically significant difference in serum CCXL12 levels between cases with and without previous topical treatment.
The above findings are in agreement with those of Speeckaert et al,4 who examined alterations in CXCL12 in the serum of vitiligo patients and analyzed the association between serum CXCL12, prognosis and previous topical treatment. These authors found that serum CXCL12 levels were significantly increased in patients when compared to controls (p=0.041). Also, there were significantly increased CXCL12 levels in patients with active disease in compared to all other vitiligo patients (p=0.012).
Rezk et al17 examined chemotactic signatures in cultured vitiligo melanocytes. Their analysis revealed that melanocytes in early lesions show altered expression levels of several chemotaxis-associated molecules, including elevated secretions of CXCL12 and CCL5. Higher levels of these chemokines coincided with prominent infiltration of antigen-presenting cells and T-cells in the skin.
The present study revealed there is a highly statistically significant difference in serum CXCL12 levels between the different grades of disease activity using the VIDA score, with a significant increase in CXCL12 levels in concert with greater disease activity (p=0.000). This result has not been discussed in any paper before.
In the present study, there was a statistically significant difference between patients with vitiligo vulgaris, acrofacial vitiligo, and the universal type of vitiligo with respect to serum levels, with low levels of CXCL10 and CXCL12 present in patients with the universal type (p<0.05). The reason for this may be that vitiligo universalis patients are stable; this result has not been discussed in any paper before.
The present study recorded a highly significant negative correlation between the duration of vitiligo and chemokines CXCL10 and CXCL12 (p<0.05), which may be explained by the presence of other factors that affect these levels in serum such as disease activity. This result was not discussed in any paper.
The present study showed no statistically significant difference in serum CCXL10 and CXCL12 levels between cases with and without a family history of vitiligo. Also, there was no statistically significant difference in serum CCXL10 and CXCL12 levels in relation to age in the studied group.
In summary, the present study showed that serum CXCL10 and CXCL12 levels were highly significantly increased in patients with vitiligo compared to controls. There was a highly statistically significant difference between patients with active disease and those with stable disease regarding serum levels of CXCL10 and CXCL12, with higher serum levels present in the active group. There was a statistically significant difference between patients with vitiligo vulgaris, acrofacial vitiligo, and those with the universal type of vitiligo regarding serum levels, with low levels of CXCL10 and CXCL12 in patients with the universal type. There was a highly statistically significant difference in serum CCXL10 and CXCL12 between the different grades of disease activity according to VIDA score. There was no statistically significant difference in serum CCXL10 and CXCL12 levels between cases with and without a family history of vitiligo. There was no statistically significant difference in serum CCXL10 and CXCL12 levels between cases with and without previous topical treatment.
CONCLUSION
From this study, we can conclude that vitiligo is associated with increased serum levels of CXCL10 and CXCL12. There is a positive relationship with disease activity, indicating that CXCL10 and CXCL12 may play a significant role in the pathogenesis of vitiligo.
Recommendations. Given the present study, we suggest future studies be conducted on a greater number of vitiligo patients to confirm the role of both CXCL10 and CXCL12 in the pathogenesis of vitiligo. Future studies must be conducted involving different types of vitiligo patients to determine whether a relationship exists between CXCL10 andCXCL12 and the type of vitiligo. Future studies must be conducted involving patients with different durations of vitiligo to detect the relationship between CXCL10 and CXCL12 and the duration of disease. Neutralization of the actions of CXCL10 and CXCL12 may provide new options in the treatment of vitiligo.
REFERENCES
- Mario V, Francesca C, Carmen M et al. IL-33 circulating serum levels are increased in patients with non-segmental generalized vitiligo. Arch Dermatol Res. 2016;308(7):527–530. doi: 10.1007/s00403-016-1675-2. [DOI] [PubMed] [Google Scholar]
- Yang L, Yang S, Lei J et al. Role of chemokines and the corresponding receptors in vitiligo: a pilot study. J Dermatol. 2018;45(1):31–38. doi: 10.1111/1346-8138.14004. [DOI] [PubMed] [Google Scholar]
- Mouia A, Sormani L, Youssef M et al. Study of comparative expression of CXCL9, CXCL10, INF in vitiligo and alopecia areata patients. Pigment Cell Melanoma Res. 2016;30(2):259–261. doi: 10.1111/pcmr.12559. [DOI] [PubMed] [Google Scholar]
- Speeckaert R, Ongenae K, Geel N. Alternation of CXCL12 in serum vitiligo patient. J Invest Dermatol. 2017;137(7):1586–1588. doi: 10.1016/j.jid.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Njoo MD, Vodegel RM, Westerhof W. Depigmentation therapy in vitiligo universalis with topical 4-metho-xypnenol and the Q-switched ruby laser. J Am Acad Dermatol. 2000. 42(5 Pt 1):760 769 [DOI] [PubMed]
- Ezzedine K, Eleftheriadou V, Whitton M et al. Vitiligo. Lancet. 2015;386(9988):74–84. doi: 10.1016/S0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- Cruz M, Carrasco M, Porras R et al. Immunopathogenesis of vitiligo. Autoimmune Rev. 2011;10(12):762–765. doi: 10.1016/j.autrev.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Ferrari SM, Corrado A et al. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14(2):174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Ferrari SM, Giuggioli D et al. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014;13(3):272–280. doi: 10.1016/j.autrev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Groom JR, Luster AD. CXCR3 ligands redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SM, Fallahi P, Santaguida G et al. Circulating CXCL10 is increased in non-segmental vitiligo, in presence or absence of autoimmune thyroiditis. Autoimmun Rev. 2017;16(9):946–950. doi: 10.1016/j.autrev.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Wang XX, Wang QQ, Wu JQ et al. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol. 2016;174(6):1318–1326. doi: 10.1111/bjd.14416. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kang Y, Chen S et al. Circulating CCL 20: A potential biomarker for active vitiligo together with the number of Th1/17 cells. J Dermatol Sci. 2019;93(2):92–100. doi: 10.1016/j.jdermsci.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Abdallah M, El-Mofty M, Anbar T et al. CXCL-10 and Interleukin-6 are reliable serum markers for vitiligo activity. pigment. Cell Melanoma Res. 2018;31(2):330–336. doi: 10.1111/pcmr.12667. [DOI] [PubMed] [Google Scholar]
- Regazzetti C, Joly F, Marty C et al. Add transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol. 2015;135(12):3105–3114. doi: 10.1038/jid.2015.335. [DOI] [PubMed] [Google Scholar]
- Alexeev V. CXCL12 as a predictor of vitiligo activity and disease progression. J Invest Dermatol. 2016;137(7):1588–1590. doi: 10.1016/j.jid.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Rezk AF, Kemp DM, El-Domyati M et al. Misbalanced CXCL12and CCL5 chemotactic signals in vitiligo onset and progression. J Invest Dermatol. 2017;37(5):1126–1134. doi: 10.1016/j.jid.2016.12.028. [DOI] [PubMed] [Google Scholar]