Dear Editor:
There is increasing data supporting the use of fractional picosecond laser (FXL-PS) application to achieve skin rejuvenation.1,2 Intra-epidermal cavities resulting from areas of laser-induced optical breakdown (LIOB) have been reported to be associated with the deposition of new dermal collagen, elastic tissue, and mucin.1 Habbema et al3 induced dermal injury using LIOB by Nd:YAG laser with sub-nanosecond light pulses, while leaving an intact epidermis, which led to new collagen growth identified with the Herovici staining technique. Here, we present a small study of three Asian patients who demonstrated LIOB in the epidermis and the dermis following FXL-PS.
Three patients with Skin Types III and IV participated in this study. We used a 1,064-nm picosecond laser (PICOCARE; WonTech, Daejeon, South Korea) with a microlens array (MLA) with a spot size of 4mm and fluence range of 2.2 to 2.8 J/cm2 applied on the malar area of the face for two non-overlapping passes. Biopsies with a 2-mm punch were performed immediately after treatment. The biopsies were stained with hematoxylin and eosin, and immunohistochemical staining for cluster of differentiation (CD)31 and D240 was performed. Histology revealed a few variably sized intra-epidermal microcavities by coagulation degeneration and necrosis, evidenced by pyknotic nucleus and the shrinkage of cytoplasm of keratinocytes lining the inner sides of microcavities. Extravasation of erythrocytes was found in the papillary dermis (Figure 1). A focal reddish appearance of collagen fibers in the upper dermis with mild edematous changes was noted. Dilated venules and lymphatics were observed in upper dermal microspaces (Figure 2).
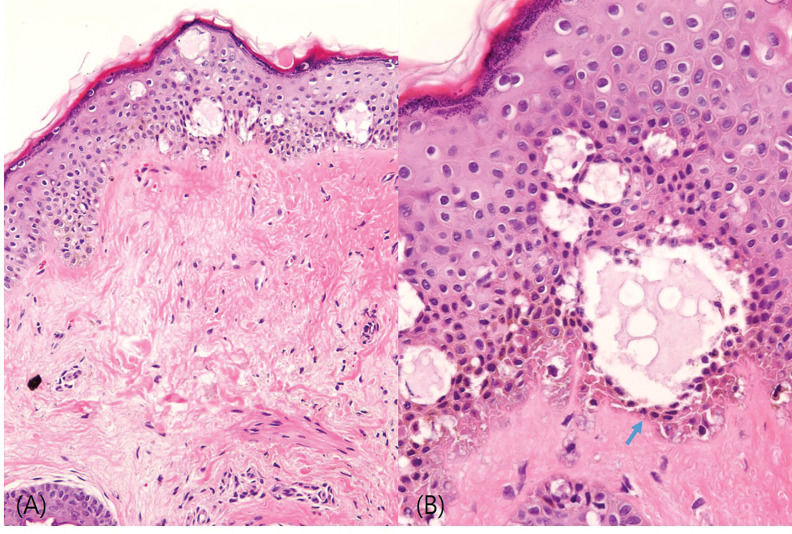
FIGURE 1.
(A) Hematoxylin and eosin (H&E) stain, 200×. (B) H&E stain, 400×. The histology revealed a few variably sized microcavities (i.e., vesicles) in the epidermis and pyknotic nuclei of keratinocytes lining the inner sides of the cavities, indicated by arrows. Dermal edematous changes in the upper dermis were noted, but no definite laser-induced microspaces were observed.
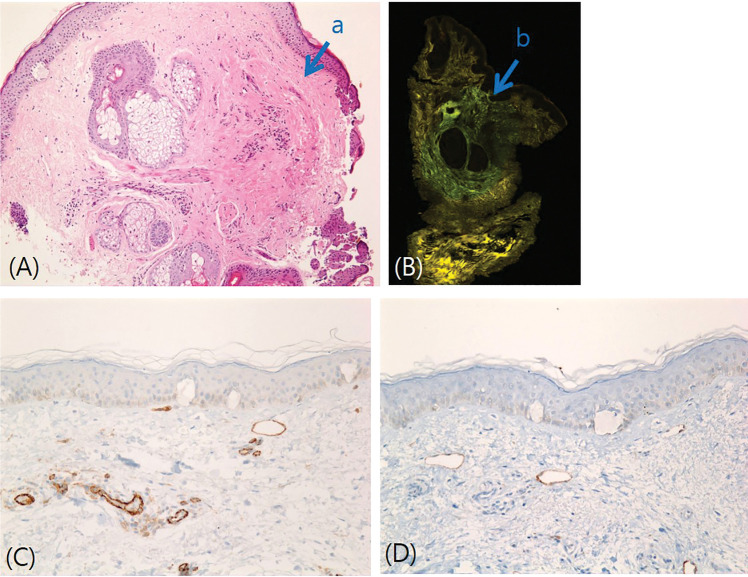
FIGURE 2.
A) Hematoxylin and eosin (H&E) stain, 200×; in the dermis, focused thermal denaturation was observed as indicated by the reddish appearance of the dermal collagen fibers; B) Fluorescence microscopy; there was a denatured collagen area in the mid-reticular dermis; C) Cluster of differentiation (CD)31, 200×; D) D2-40 200×. In the dermis, the microspaces correlated with the dilated venules and lymphatics, which were confirmed by positive stains for both CD31 and D2-40.
The initiation mechanism of LIOB begins from the production of free seed electrons using a laser pulse through multiphoton absorption or thermionic emission.4 Subsequently, electron density increases to form plasma at the focal area. This results in cavitation bubbles in the skin, which have been suggested to expand, disrupt tissue, and generate shockwaves to further disrupt the colocated tissues to generate microscopic vacuolar tissue reactions.2
Locations of LIOB have been reported differently,2–5 and these differences might be explained by the engineering characteristics of the optical element used to generate the laser micro-injury pattern.5 In the study by Habbema et al,3 dermal LIOB was achieved through two-dimensional scanning of a focused laser beam, but a MLA was used in the study by Tanghetti2 that demonstrated epidermal LIOB. In an ex-vivo study by Chun et al,6 a 1,064-nm MLA with a 4-mm spot size and a laser fluence of 2.8 J/cm2 generated patterns of cavitation and vacuolization formation, Melan-A-positive, CD31-negative cystic cavitation in the epidermis, and noticeable CD31-positive, Melan-A-negative cystic lesions in the dermis. This study demonstrated that cystic cavitation lesions in the dermis can arise in microvascular components, collagen bundles, and perivascular loose connective tissue, and dermal cystic cavitation lesions could be generated independent of the absorption properties of chromophores.6 According to Tanghetti et al,2 as the melanin content of the epidermis is reduced, the size, location, and prevalence of vacuoles decrease. The location, size, and density of the areas of cystic cavitation seem to inversely correlate with the depth of pigments and vascular components as a result of a loss of laser energy due to tissue scattering.6 Hemorrhagic vacuoles within the epidermis or superficial papillary dermis suggest that LIOB can either disrupt the neighboring blood vessels or be initiated by hemoglobin.3 Previous studies have described several factors that affect dermal remodeling, despite the primary damage to the epidermis. Fibroblast activation, which is stimulated by an epidermal injury-induced cytokine release, leads to dermal remodeling.7 Dermal remodeling, including fibroblast proliferation and angiogenesis, could also be stimulated by the traveling shockwave that is initiated in the epidermis.8,9
The results of our small study suggests that epidermal LIOB was attained, but the outcome of dermal LIOB seemed to be nonsignificant. However, we hypothesized that dermal LIOB disrupted neighboring blood vessels and lymphatics. In addition to epidermal injury-induced cytokines, changes around the vessels might have had a dermal-remodeling effect. Our results and conclusion are limited by the very small patient number. A randomized, controlled trial with a much larger patient sample is needed before any firm conclusions can be made.
REFERENCES
- Brauer JA, Kazlouskaya V, Alabdulrazzaq H et al. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015;151(3):278–284. doi: 10.1001/jamadermatol.2014.3045. [DOI] [PubMed] [Google Scholar]
- Tanghetti EA. The histology of skin treated with a picosecond alexandrite laser and a fractional lens array. Lasers Surg Med. 2016;48(7):646–652. doi: 10.1002/lsm.22540. [DOI] [PubMed] [Google Scholar]
- Habbema L, Verhagen R, Van Hal R et al. Minimally invasive non-thermal laser technology using laser-induced optical breakdown for skin rejuvenation. J Biophotonics. 2012;5(2):194–199. doi: 10.1002/jbio.201100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese B, Bonito V, Jurna M et al. Influence of absorption induced thermal initiation pathway on irradiance threshold for laser induced breakdown. Biomed Opt Express. 2015;6(4):1234–1240. doi: 10.1364/BOE.6.001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu M, Lentsch G, Korta DZ et al. In vivo multiphoton-microscopy of picosecond-laser-induced optical breakdown in human skin. Lasers Surg Med. 2017;49(6):555–562. doi: 10.1002/lsm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Lee HC, Park J et al. Pattern analysis of 532- and 1064-nm microlens array-type, picosecond-domain laser-induced tissue reactions in ex vivo human skin. Lasers Med Sci. 2019;34(6):1207–1215. doi: 10.1007/s10103-018-02711-2. [DOI] [PubMed] [Google Scholar]
- Lugar TA, Schwarz T. Evidence for an epidermal cytokine network. J Invest Dermatol. 1990;95(6 Suppl):100S–104S. doi: 10.1111/1523-1747.ep12874944. [DOI] [PubMed] [Google Scholar]
- Berta L, Farrari A, Ficco AM et al. Extracorporeal shock waves enhance normal fibroblast proliferation in vitro and activate mRNA expression for TGF-ß1 and collagen types I and III. Acta Orthop. 2009;80(5):612–617. doi: 10.3109/17453670903316793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic A, Elster EA, Anam K et al. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis. 2009;11(4):369–380. doi: 10.1007/s10456-008-9120-6. [DOI] [PubMed] [Google Scholar]