Abstract
Background
Human papilloma virus infects and proliferates in skin or mucosal cells to cause warts. Most of the current therapeutic modalities are ablative, act only on treated lesions, and lack a well-defined treatment endpoint. These being blind procedures, recurrence rates are high, owing to the remnant virus. Intralesional immunotherapy plays a significant role, as it potentially acts on treated and distant lesions.
Objectives
We sought to study and compare the efficacy, safety profile, and recurrence rates of intralesional immunotherapy modalities (vitamin D3; measles, mumps, and rubella [MMR] vaccine; and tuberculin purified protein derivative [PPD]) in treating viral warts.
Methods
An open-label interventional study of 60 cases of cutaneous viral warts was performed in a tertiary care center attached to a medical college after obtaining approval from the institutional ethics committee. Each patient was consecutively assigned into Group 1 (vitamin D3: 0.2mL of 15mg/mL), Group 2 (MMR: 0.5mL), or Group 3 (tuberculin PPD: 0.1mL of 10TU). One or two warts were injected per session every two weeks. Response was assessed. Adverse effects were noted. Cases were followed up monthly for three months.
Results
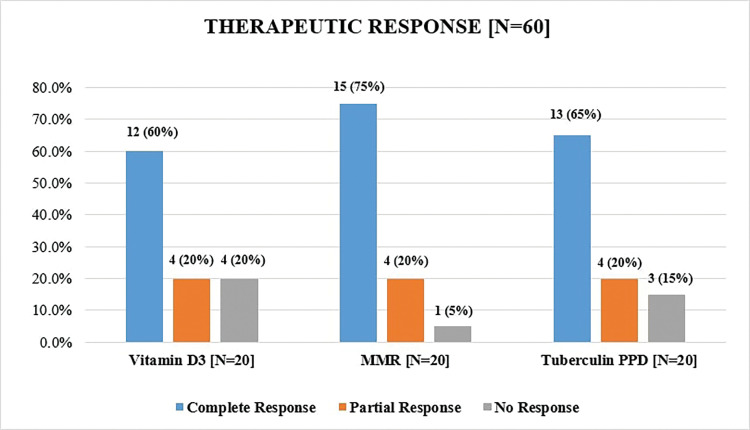
The MMR group had the maximum patients with complete response (15 of 20, 75%) followed by tuberculin PPD group (13 of 20, 65%) and vitamin D3 group (12 of 20, 60%). No major adverse drug reactions were reported in any of the groups.
Conclusion
Immunotherapy offers a safe and promising approach in patients with extensive cutaneous viral warts in difficult to treat sites.
Keywords: Immunotherapy, warts, vitamin D3, MMR, tuberculin PPD
Human papilloma virus (HPV) infects and proliferates in both keratinizing and nonkeratinizing epithelium producing cutaneous, genital, oral, and laryngeal warts. HPV mainly infects the basal cell layer through defects in the epithelium. Trauma and maceration are important predisposing factors. According to a population survey in adults, the highest prevalence of cutaneous warts was in social class three (manual and non-manual workers).1
The immune system in otherwise healthy individuals apparently fails to clear warts for months or years. Langerhans cell numbers are reduced within warts, and T lymphocytes are rare within the epidermal compartment, suggesting a subdued local immune response. In cases with recurrent or recalcitrant warts, it is possible that there may be inability by the immune system to target certain HPV proteins, possibly due to poor antigen presentation, poor effector response, or virally induced local immunosuppression resulting in the development of tolerance.2
Treatment of viral warts is variable. No single treatment is 100-percent effective, with failures and recurrences being common. Oral retinoids, such as isotretinoin and acitretin, have been used in the doses 0.5mg/kg/day, due to their effects on keratinocyte differentiation and proliferation and their immunomodulatory effects. Response rates of 71 percent and 39 percent have been reported with isotretinoin and acitretin, respectively, but recurrence is common (17%). Adverse effects include cheilitis, xerosis, and photosensitivity.3 Retinoids may be used as combination therapy with other topical treatments in extensive warts. Topical therapy, physical therapy, and systemic treatments have all been utilized with variable results and adverse effects. Most of the current therapeutic modalities for viral warts are ablative in nature and are limited by high rates of recurrence. Also, they are not suitable for numerous lesions (Tables 1–3).4
TABLE 1.
Topical therapies for viral warts
| TREATMENT MODALITY | DESCRIPTION | MECHANISM OF ACTION | RESPONSE | ADVERSE EFFECTS |
|---|---|---|---|---|
| Salicylic acid6,7,8 | Organic acid (available as 16.7% paint and 40% in corn caps for plantar warts) | Ablates HPV infected epidermal cells and softens hyperkeratotic epidermis associated with warts. | Cure rates of up to 75% have been reported. | Potential for an irritant reaction |
| Imiquimod6,9 | Toll-like receptor 7 (TLR-7) agonist (available as 5% cream) | Significant role in induction of innate immune response | Complete clearance in 30–89% cases according to a few non-controlled trials | Erythema, flu-like symptoms, fatigue, diarrhea, fever, skin blistering, erosion, excoriation, flaking, edema, paresthesia, pruritus, burning, tenderness, stinging, crusting, rash, superficial ulcer |
| Diphencyprone6 | Sensitizing agent | Induces a local type intravenous hypersensitivity reaction. | Overall response rates of 60% have been reported. | Severe blistering or generalized eczema at the contact site |
| 5-Fluorouracil (5-FU)7 | Chemotherapeutic agent (available as 5% cream) | Interferes with the synthesis of DNA and RNA in infected cells. | 20% showed complete clearance. | Erythema, ulceration, crusting, and occasional hypopigmentation |
| Tretinoin3,7,8 | Retinoids (vitamin A analogues) (available as 0.025% and 0.05% cream) | Disrupt epidermal growth and differentiation, thereby reducing the bulk of the wart. They may also have an immunomodulatory inhibitory effect on HPV replication. | 85% clearance rate as compared to 32% in controls; used in verruca plana. | Retinoid dermatitis |
| Trichloroacetic acid (TCA)10,11 | Available in concentrations of 10–90% | Causes destruction of tissue by hydrolysis of cellular proteins leading to cell death. | 20% showed complete clearance with 40% TCA weekly after 4 weeks. Longer treatment duration or higher concentrations yield higher cure rates. Particularly used in verruca plana and plantar warts. | Itching, pain, erythema, hyperpigmentation. Rare adverse effects include infection, ulceration and scarring. |
HPV: human papilloma virus
TABLE 3.
Intralesional therapies for viral warts
| TREATMENT MODALITY | DESCRIPTION | MECHANISM OF ACTION | RESPONSE | ADVERSE EFFECTS |
|---|---|---|---|---|
| Bleomycin6,14,15,16 | Antimitotic chemotherapeutic agent | Inhibits DNA synthesis by preventing thymidine incorporation. Ablates the virus by causing intense inflammation and stimulating an immune response to HPV. | The response rates range from 16–94% owing to variations in protocol and study designs in different trials. Recurrence in 15% of cases. | Hemorrhagic eschar (ecchymosis), hyperpigmentation, hypopigmentation, scarring |
| 5-Fluorouracil (5-FU)7,16 | Antimitotic chemotherapeutic agent | Interferes with the synthesis of DNA and RNA in infected cells. | 45–65% showed complete resolution in 4 weeks. Recurrence in 20% cases. | Pain during and within a day of injection, hyperpigmentation, hypopigmentation, itching |
| Candida antigen16,17,18 | Immunotherapeutic agent | Stimulates a cell-mediated immune response (CMI) leading to recruitment and proliferation of various immune cells and release of cytokines. High prevalence of immunity to Candida in the general population. | 24–71% response rate | Pruritus, burning, blistering, peeling, and local erythema and edema, rash, constitutional symptoms |
| Bacillus Calmette-Guerin (BCG)19 | Immunotherapeutic agent | Acquisition of a widespread CMI against HPV as a response to BCG injection. | 70% showed complete clearance at the end of 3 months. | Pain, edema, ulceration, scarring, flu-like symptoms |
HPV: human papilloma virus; CMI: cell-mediated immunity
TABLE 2.
Energy based therapies for viral warts
| TREATMENT MODALITY | MECHANISM OF ACTION | RESPONSE | ADVERSE EFFECTS |
|---|---|---|---|
| Radiofrequency ablation12 | Tissue destruction with various waveforms of alternating electric current whose frequencies fall within the range of radiofrequency (500–4000khz) | The overall cure rate ranges between 33% and 80% depending on the number of sessions and the type of warts. Recurrence rate of 9% reported. | Pain, hypopigmentation, secondary infection, scarring |
| Electrosurgery12 | Utilizes galvanic or direct current for generation of heat and destruction of tissue. | Overall success rate of 56–80%. Recurrence rate of 41% reported. | Pain, hypopigmentation, secondary infection, scarring |
| Cryotherapy6,7,12 (Liquid nitrogen at -196°C by open spray method and -20°C by dipstick method) | Necrotic destruction of HPV infected keratinocytes besides inducing local inflammation that triggers an effective cell mediated immune response | Cure rate ranges from 44–47%. Recurrence rate of 58% reported as treatment end-point cannot be clearly determined. | Pain, blistering, hypopigmentation or hyperpigmentation, scarring. |
| Carbon dioxide lasers6,8 | Aim at blood vessels that are rampant in briskly growing warts. The destruction of microvasculature is mediated by selective photothermolysis of oxyhemoglobin. | Remission rates of 49.5%, 63%, and 64% have been reported | Intraoperative and post-treatment pain, crust formation, scarring, pigmentary change. |
| Photodynamic therapy 6,7 | Uses aminolevulinic acid (ALA) as the photosensitizing agent. Upon stimulation by light, accumulated | Cure rates of 56% and 75% have been reported. Used in few controlled trials, results are variable. | Minor pain, itching, mild hypopigmentation. |
HPV: human papilloma virus
The cell-mediated immunity (CMI) has a role in the HPV proliferation through stimulation of cytokines. Intralesional immunotherapy works on the basis that the immune system is able to recognize the injected antigens, which could induce a delayed type of hypersensitivity reaction to the antigen as well as against the wart virus, making the immune system more capable of recognizing and clearing HPV at both the treated and distant warts and preventing recurrences.5
Through this study, an attempt was made to evaluate the role of intralesional immunotherapy in cutaneous warts and to make a head-to-head comparison of three modalities, namely vitamin D3; measles, mumps, and rubella (MMR) vaccine; and tuberculin purified protein derivative (PPD).
Our objective was to study and compare the response in terms of efficacy, safety profile, and recurrence to three modalities of intralesional immunotherapy (vitamin D3, MMR vaccine, and tuberculin PPD) in the treatment of viral warts.
METHODS
An open-label interventional study was carried out at a dermatology outpatient department (OPD) in a tertiary care center attached to a medical college. After receiving approval from the institutional ethics committee, the study was conducted for a period of 18 months, from January 2018 to July 2019. Sample size was calculated as 57 using one-way ANOVA Test Pairwise. A total of 68 patients were enrolled, of which 60 completed the study. Data was entered and analyzed using the SPSS (Statistical Package for Social Science) Version 20 and presented as number and percentage in the form of tables and bar charts. Chi square test was applied for analysis. P-value less than 0.05 was considered as significant.
Inclusion criteria were patients 12 to 65 years of age with cutaneous viral warts who had not taken any treatment for viral warts in the past four weeks. Exclusion criteria included having oral and anogenital warts, being younger than 12 years or older than 65 years of age, being immunosuppressed, being pregnant or lactating, having active bacterial and viral infections, having previous history of hypersensitivity to the intralesional agent being used, and being not willing to participate in the study.
After receiving written informed consent from participants, investigators performed complete blood count, random blood sugar, urine routine and microscopic examination, and serum HIV testing to rule out immunosuppressed status.
Patients were divided into three groups. Each patient with viral warts was consecutively assigned into Group 1 (vitamin D3), Group 2 (MMR vaccine), and Group 3 (tuberculin PPD) (n=20 per group). In all the groups, injection was given intralesionally into the largest one or two warts with a 27g insulin syringe. For Group 1, the selected wart(s) was injected first with 0.2mL of lignocaine (20mg/mL); after a few minutes, 0.2mL of 15mg/mL vitamin D3 was injected slowly into the base of the wart(s). For Group 2, MMR vaccine 0.5cc (after reconstitution with sterile water) and for Group 3, tuberculin PPD at a dose of 10TU (0.1mL) was injected. Subsequently the same lesion was injected every two weeks for a maximum of four injections or until all lesions disappeared, whichever came first. Patients were followed monthly for three months.
Evaluation of response. Clinical photographs were taken at the baseline visit and then at every follow up visit. Response was recorded at the final visit as “complete response” if all the warts disappeared completely (injected as well as distant warts); “partial response” for reduction in the number or size of warts (but not complete resolution); “no response” if there was no effect on the existing lesions and/or occurrence of new lesions while on immunotherapy; and “recurrence” in cases of new warts appearing following complete clearance during or after the follow-up period.
Adverse effects (local or constitutional symptoms, if any) were noted. Patients having complete response were then followed every month for a period of three months to note any residual skin changes or recurrence. Patients having partial or no response were treated with either cryotherapy or electrocautery.
RESULTS
The mean age in all three treatment groups were comparable and were in the range of 21 to 30 years (Table 4). Male patients outnumbered female patients in all three treatment groups (Table 5). The highest efficacy was seen in the MMR group, with complete response in 15 (75%) patients, followed by tuberculin PPD group and vitamin D3 group with complete response in 13 (65%) and 12 (60%) patients, respectively (Figure 1).
TABLE 4.
Mean age of patients in each treatment group
| GROUP | N | MEAN AGE |
|---|---|---|
| Vitamin D3 | 20 | 24.10 |
| MMR | 20 | 26.40 |
| Tuberculin PPD | 20 | 29.25 |
| Total | 60 | 26.58 |
MMR: measles, mumps, and rubella; PPD: purified protein derivative
TABLE 5.
Sex distribution of cases in each treatment group
| GROUP | MALE | FEMALE | TOTAL |
|---|---|---|---|
| Vitamin D3 | 15 (75%) | 5 (25%) | 20 |
| MMR | 16 (80%) | 4 (20%) | 20 |
| Tuberculin PPD | 16 (80%) | 4 (20%) | 20 |
| Total | 47 (78.33%) | 13 (21.67%) | 60 |
MMR: measles, mumps, and rubella; PPD: purified protein derivative
FIGURE 1.
Therapeutic response in each treatment group (p=0.878)
MMR: measles, mumps, and rubella; PPD: purified protein derivative
Of the patients showing complete response, 17 of 40 (42.5%) required three doses of either of the intralesional immunotherapies. However, the highest number of patients who showed complete response in a single dose belonged to the MMR group (5 of 7, 71.4%). Response in injected warts was greater than in distant warts in all the groups (Table 6).
TABLE 6.
Response in injected and distant warts in each treatment group
| RESPONSE | GROUP | |||
|---|---|---|---|---|
| VITAMIN D3 (N=20) | MMR (N=20) | TUBERCULIN PPD (N=20) | P-VALUE | |
| Injected warts | 16 (80.00%) | 18 (90.00%) | 17 (85.00%) | 0.676* |
| Distant warts | 14 (70.00%) | 17 (85.00%) | 15 (75.00%) | 0.496* |
*p-value >0.05 (not significant)
MMR: measles, mumps, and rubella; PPD: purified protein derivative
All 12 patients (100%) with palmoplantar, palmar, plantar, and periungual warts showed complete response to immunotherapy. Twenty-two of 33 patients (66.67%) with verruca vulgaris and 3 of 4 patients (75%) with verruca vulgaris and periungual warts showed complete response. Only 2 of 8 (25%) patients with verruca plana showed complete response (Figures 2–7).
FIGURE 2.
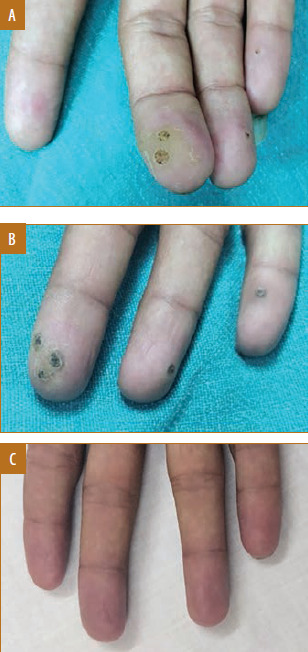
Male patient, 30 years of age, with verruca vulgaris treated with vitamin D3 showed complete response in four doses with mild post-inflammatory hypopigmentation; A) Baseline visit; B) after two doses; and C) after four doses
FIGURE 7.
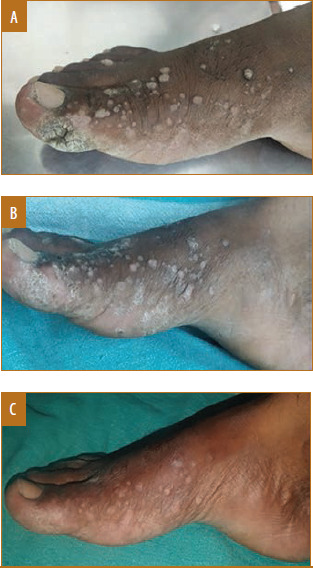
Male patient, 17 years of age, with palmar warts treated with tuberculin purified protein derivative showed complete response in two doses; A) Baseline visit; B) after one dose; C) after two doses
FIGURE 3.
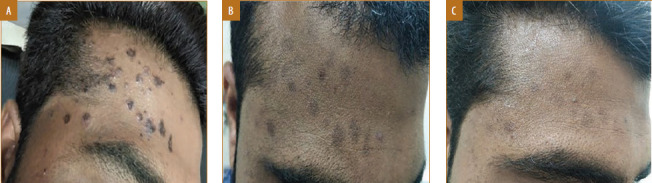
Male patient, 22 years of age, with verruca plana treated with vitamin D3 showed complete response in four doses with mild post-inflammatory hyperpigmentation; A) Baseline visit; B) after two doses; and C) after four doses
FIGURE 4.
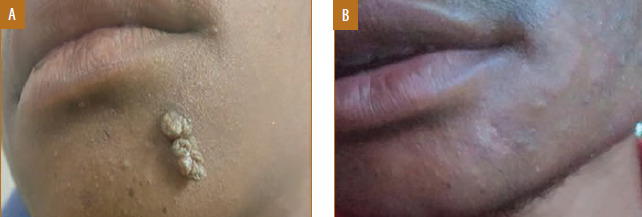
Male patient, 17 years of age, with verruca vulgaris treated with measles, mumps, and rubella vaccine showed complete response in two doses with mild post-inflammatory hypopigmentation; A) Baseline visit; B) after two doses
FIGURE 5.
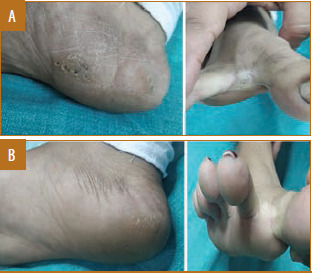
Female patient, 19 years of age, with plantar warts treated with measles, mumps, and rubella vaccine showed complete response in three doses; A) Baseline visit; B) after three doses
FIGURE 6.
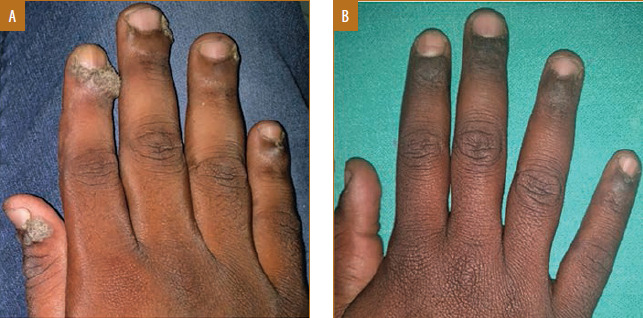
Male patient, 19 years of age, with periungual warts treated with measles, mumps, and rubella (MMR) vaccine showed complete response in one dose; A) Baseline visit; B) after one dose
Palmoplantar and periungual warts showed complete response maximum with MMR, while verruca plana showed complete response maximum with tuberculin PPD. Subungual warts showed no response to either of the immunotherapy modalities.
Maximum adverse effects were seen in the vitamin D3 group, followed by tuberculin PPD, and least in the MMR group (Figure 8). Painful swelling was seen most commonly in the tuberculin PPD group followed by the vitamin D3 group (Figures 9 and 10), which may have occurred due to accidental intradermal injection, and it subsided in 3 to 4 days with oral analgesics and anti-inflammatory drugs.
FIGURE 8.
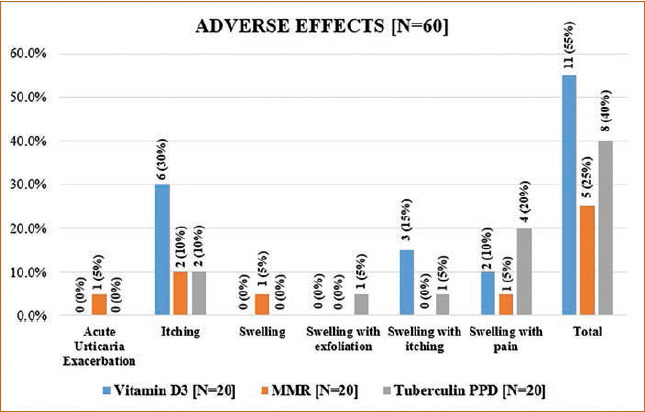
Adverse effects in each treatment group
FIGURE 9.
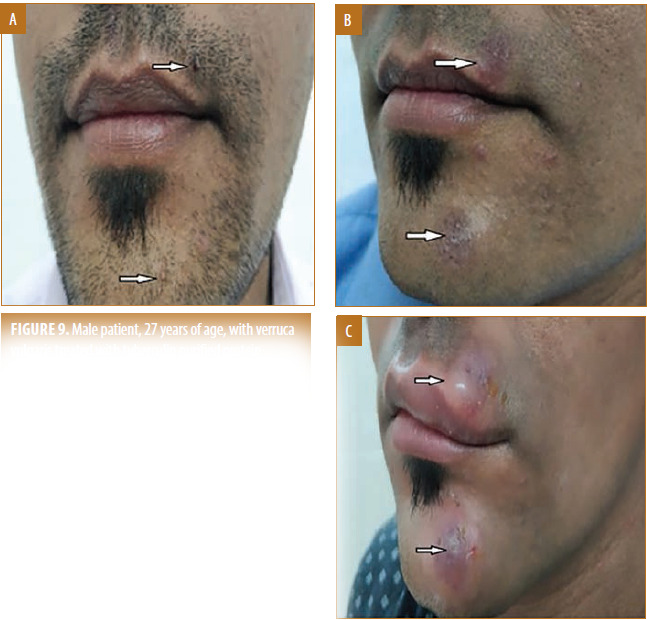
Male patient, 27 years of age, with verruca vulgaris treated with tuberculin purified protein derivative (PPD) showed local pain and inflammation at the site of accidental intradermal injection; A) Baseline visit; B) after one dose; C) after two doses
FIGURE 10.
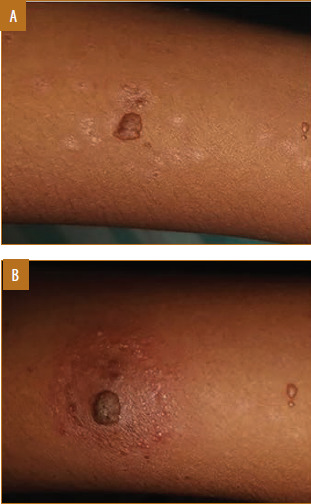
Female patient, 17 years of age, with verruca vulgaris treated with vitamin D3 developed pain and swelling at injection site; A) Baseline visit; B) after one dose
Post-inflammatory dyspigmentation was the most common residual skin change observed at three months follow-up in the tuberculin PPD group, MMR group, and vitamin D3 group in decreasing order. Mild scarring was observed in a single patient in the MMR group.
Of the 12 patients showing complete response to vitamin D3, only one case of verruca vulgaris (8.3%) showed recurrence of lesions at three-month follow up. The MMR and tuberculin PPD groups did not show any recurrence in cases of complete response. However, two patients in the tuberculin PPD group developed new lesions during the course of treatment.
DISCUSSION
Vitamin D3. Vitamin D3 potentially regulates proliferation and differentiation of the epidermal cells and modulates production of cytokines. It mediates its effects via the vitamin D receptor (VDR). The keratinocytes, melanocytes, fibroblasts, and immune system cells of the skin have VDR.20 The expression of VDR and vitamin D1 hydroxylase genes is upregulated by toll-like receptor (TLR) activation of human macrophages, which in turn leads to induction of the antimicrobial peptide.21 By mediation through VDR-dependent pathway, vitamin D3 inhibits the expression of interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor (TNF)-alpha, and TNF-gamma, and hence exerting immunomodulatory effects.22
Complete response with vitamin D3 was seen in a higher number of cases in other studies as compared to the present study (Table 7).22,23,24
TABLE 7.
Comparison of overall response to vitamin D3 with other studies
| RESPONSE TO VITAMIN D3 | PRESENT STUDY* (N=20) | KAVYA ET AL*22 (N=42) | SINGH ET AL**23 (N=40) | RAGHUKUMAR ET AL***24 (N=60) |
| Complete | 12 (60%) | 33 (78.57%) | 29 (72.5%) | 54 (90%) |
| Partial | 4 (20%) | 6 (14.28%) | 8 (20%) | 4 (6.66%) |
| No | 4 (20%) | 3 (7.14%) | 3 (7.5%) | 2 (3.33%) |
*Dose 0.2mL
**Dose 0.5mL
***Dose 0.2 to 0.5mL
In the present study, complete response was achieved with one and two doses in one (8.33%) patient each, and with three and four doses in five (41.67%) patients each. Similar results were reported by Raghukumar et al24 where on an average 3.66 injections were required to achieve a complete response, and all cases showed complete clearance of the distant noninjected warts as well. However, in a study by Akula et al,25 more than six sessions were needed for complete clearance of the warts.
Recurrence occurred in one patient with palmoplantar warts in a study by Kavya et al22 while in our study recurrence occurred in one patient with verruca vulgaris and plantar wart.
In the present study, mild itching was observed as the most frequent adverse effect in six out of 20 (30%) patients, followed by mild swelling with itching, mild swelling with pain, and hyperpigmentation in three (15%), two (10%), and two (10%) patients, respectively. The most common adverse effect was reported as swelling at injection site in 33 (78.6%) patients by Kavya et al22 and transient mild-to-moderate pain in 60 (100%) patients by Raghukumar et al.24
MMR. MMR vaccine induces cellular and humoral immune systems, and hence, destruction of HPV and infected host cells is accelerated. Mononuclear cell proliferation occurs by intralesional injection of antigens which promotes Th1 cytokine responses. Eventually, the cells infected with HPV are eradicated by activation of the cytotoxic T cells and the natural killer cells.26
Nofal et al26 had complete response in a higher number of patients compared to the present study (Table 8). Mahajan et al29 reported complete response in two of 27 (7.4%) patients eight weeks after the fifth dose (Table 9).29
TABLE 8.
Comparison of overall response to MMR vaccine with other studies
| RESPONSE TO MMR | PRESENT STUDY* (N=20) | NOFAL ET AL*26 (N=85) | DHOPE ET AL*27 (N=20) | AGRAWAL ET AL**28 (N=30) |
|---|---|---|---|---|
| Complete | 15 (75%) | 57 (81.4%) | 13 (65%) | 18 (60%) |
| Partial | 4 (15%) | 7 (10%) | 7 (10%) | 6 (20%) |
| No | 1 (10%) | 6 (8.6%) | 1 (5%) | 6 (20%) |
*Dose 0.5mL
**Dose 0.3mL
MMR: measles, mumps, and rubella
TABLE 9.
Comparison of number of doses of MMR vaccine required for complete response with other studies
| NO. OF DOSES | NO. (%) OF CASES | |
|---|---|---|
| PRESENT STUDY* (N=15) (AGED 12–65 YEARS) | MAHAJAN ET AL**29 (N=27) (AGED 5–18 YEARS) | |
| 1 | 5 (33.3%) | 1 (3.7%) |
| 2 | 1 (6.67%) | 2 (7.4%) |
| 3 | 6 (40%) | 2 (7.4%) |
| 4 | 3 (20%) | 7 (25.9%) |
| 5 | Not assessed | 15 (55.6%) |
*Dose 0.5mL
**Dose 0.25mL
MMR: measles, mumps, and rubella
In the present study, all patients in the MMR group with palmar, plantar, and periungual warts showed complete response, whereas seven of 10 (70%) patients with verruca vulgaris had complete response. However, in a study done in Korea, verruca vulgaris showed significantly greater therapeutic response to intralesional MMR than other varieties of warts.30
Pain during injection was a common adverse effect in studies by Nofal et al26 and Dhope et al27 but not in the present study (Table 10). No recurrence was observed in the present study and in studies by Nofal et al and Mahajan et al.26,29 However, Agrawal et al28 reported recurrence in three (16.6%) patients.
TABLE 10.
Comparison of overall response to MMR vaccine with other studies
| ADVERSE EFFECTS | PRESENT STUDY* (N=20) | NOFAL ET AL*26 (N=70) | DHOPE ET AL*27 (N=20) |
|---|---|---|---|
| Swelling at injection site | 1 (5%) | N/A | 4 (20%) |
| Itching | 2 (10%) | N/A | N/A |
| Swelling with pain | 1 (5%) | N/A | N/A |
| Pain during injection | N/A | 60 (85.7%) | 17 (85%) |
| Flu-like symptoms | N/A | 6 (8.6%) | 2 (10%) |
| Erythema | N/A | N/A | 5 (25%) |
| Acute urticaria exacerbation | 1 (5%) | N/A | N/A |
MMR: measles, mumps, and rubella; N/A: not applicable
Tuberculin PPD. Injecting tuberculin PPD into the HPV infected tissue may generate strong proinflammatory signals and attract antigen presenting cells. Association with the production of Th1 cytokines like interleukin-4 (IL-4), interleukin-5 (IL-5), IL-8, interferon (IFN)-gamma, and TNF-alpha has been found.31,32 The immunostimulation is generalized, which helps in clearing distant warts as well.33 PPD increases serum interleukin-12 (IL-12) levels, hence boosting the immunity to combat the HPV.34 Because of the high prevalence of tuberculosis infection in developing countries such as India, it is easy to induce a positive CMI response with PPD.35 PPD is devoid of any viable organisms; therefore, its use is considered as safe in children and in pregnant women.35
Complete response rate in the present study was comparable to study by Nimbalkar et al31 but less than other studies (Table 11).23,33 In the present study, 6 of 13 patients (46.15%) required three doses of tuberculin PPD for complete clearance while two and four doses were required by 3 of 13 (23.08%) cases each. Only one patient (7.69%) showed complete response in a single dose. The distant warts also responded to immunotherapy in 15 of 20 (75%) patients.
TABLE 11.
Comparison of response to tuberculin PPD with other studies
| RESPONSE TO VITAMIN D3 | PRESENT STUDY* (N=20) | KERURE ET AL*33 (N=89) | SINGH ET AL**23 (N=40) | NIMBALKAR ET AL*31 (N=45) |
|---|---|---|---|---|
| Complete | 13 (65%) | 84 (94.4)% | 32 (80%) | 28 (62.2%) |
| Partial | 4 (20%) | 0 (0)% | 6 (15%) | 8 (17.8%) |
| No | 3 (15%) | 5 (5.6)% | 2 (5%) | 9 (20%) |
*Dose 0.1mL (10TU)
PPD: purified protein derivative
In a study conducted in Thailand, a 67-percent cure rate was achieved with three sessions, while 14 percent of the patients had complete clearance in a single session. In 87 percent of the patients, the distant warts disappeared along with the injected ones. Only one patient had recurrence during the six-month period of follow-up.36 One of the possible reasons could be that half of the patients in this study were under the age of 15 years (21 of 42), as the younger age group is known to respond better to immunotherapy.8,36,37,38,39
The response according to type of wart varied in different studies (Table 12).
TABLE 12.
Complete response with tuberculin PPD according to type of wart
| TYPE OF WART | PRESENT STUDY (N=20) | SAOJI ET AL35 (N=55) | RAJASHEKAR ET AL40 (N=17) | JAISWAL ET AL41 (N=51) |
|---|---|---|---|---|
| Palmar | 1 (100%) | N/A | 4 (44.4%) | 2 (100%) |
| Plantar | 1 (100%) | 10 (83%) | 0 (0%) | 11 (78.5%) |
| Periungual | 1 (100%) | N/A | 5 (83.3%) | 7 (100%) |
| Verruca vulgaris | 7 (63.6%) | 20 (80%) | 6 (60%) | 8 (47%) |
| Verruca plana | 1 (33.3%) | 12 (67%) | N/A | 6 (60%) |
| Subungual + periungual wart | 0 (0%) | N/A | N/A | N/A |
| Verruca plana + palmar wart | 1 (100%) | N/A | N/A | N/A |
| Verruca vulgaris + periungual wart | 1 (100%) | N/A | N/A | N/A |
PPD: purified protein derivative; N/A: not applicable
The most common adverse effect seen with tuberculin PPD in the present study was mild swelling with pain in four of 20 (20%) patients. Saoji et al35 reported mild swelling and redness in 13 of 55 (23.6%) patients, eyelid edema (injection at eyebrow lesion), eczematous lesion at injection site, low grade fever, and body ache (dose 25TU) were observed in one of 55 (1.8%) patients each. Painful nodule was the only adverse effect reported by Rajashekar et al40 in one of 17 (5.9%) patients. Nimbalkar et al31 observed alopecia areata in scalp at injection site in two of 45 (4.4%) patients, pain at injection site, abscess at injection site, and immediate hypersensitivity (urticarial) in one of 45 (2.2%) patients each. Post-inflammatory dyspigmentation was observed in six of 20 (30%) patients in the present study and eight of 45 (17.8%) patients in the study by Nimbalkar et al.31
No recurrence was observed in the present study and in studies by Nimbalkar et al and Chandra et al.31,42 However, Saoji et al reported recurrence in one (2.4%) patient.35
Limitations. The sample size was small and posttreatment follow-up period was only three months, therefore precise statistical evaluation and validation was not feasible. Studies with a larger sample size with site specific evaluation of each treatment modality and a longer follow-up period can shed more light on comparative efficacy of each modality of immunotherapy.
CONCLUSION
As per the present study, the maximum number of patients showing complete response belonged to the MMR group (15 of 20, 75%), followed by the tuberculin PPD group (13 of 20, 65%) and vitamin D3 group (12 of 20, 60%).
Adverse effects were reported by the majority of patients in the vitamin D3 group (11 of 20, 55%), followed by the tuberculin PPD group (8 of 20, 40%) and MMR group (5 of 20, 25%). No major adverse drug reactions were reported in any of the groups.
Immunotherapy appears to offer a safe and promising treatment for cases having extensive cutaneous viral warts, especially in difficult to treat sites.
REFERENCES
- Seetharam KA. Mumbai: Bhalani Publishing house; 2015. Viral Infections. In: Sacchidanand S, Oberoi C, Inamadar AC, eds. IADVL Textbook of Dermatology. 4th ed. pp. 596–600. [Google Scholar]
- Sterling JC. Viral Infections. Rook’s Textbook of Dermatology. 9th ed. In: Griffiths CEM, Barker J, Bleiker T, editors. West Sussex: Blackwell Publishing; 2016. pp. 25.43–25.56. [Google Scholar]
- Oren-Shabtai M, Snast I, Noyman Y Topical and systemic retinoids for the treatment of cutaneous viral warts: a systematic review and meta-analysis. Dermatol Ther. 2020. p. e14637. [DOI] [PubMed]
- Podder I, Bhattacharya S, Mishra V et al. Immunotherapy in viral warts with intradermal Bacillus Calmette-Guerin vaccine versus intradermal tuberculin purified protein derivative: a double-blind, randomized controlled trial comparing effectiveness and safety in a tertiary care center in Eastern India. Indian J Dermatol Venereol Leprol. 2017;83(3):411. doi: 10.4103/0378-6323.193623. [DOI] [PubMed] [Google Scholar]
- Daulatabad D, Pandhi D, Singal A. BCG vaccine for immunotherapy in warts: is it really safe in a tuberculosis endemic area? Dermatol Ther. 2016;29(3):168–172. doi: 10.1111/dth.12336. [DOI] [PubMed] [Google Scholar]
- Lynch MD, Cliffe J, Morris-Jones R. Management of cutaneous viral warts. BMJ. 2014;348:g3339. doi: 10.1136/bmj.g3339. [DOI] [PubMed] [Google Scholar]
- Boull C, Groth D. Update: treatment of cutaneous viral warts in children. Pediatr Dermatol. 2011;28(3):217–229. doi: 10.1111/j.1525-1470.2010.01378.x. [DOI] [PubMed] [Google Scholar]
- Sterling JC, Handfield-Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144(1):4–11. doi: 10.1046/j.1365-2133.2001.04066.x. [DOI] [PubMed] [Google Scholar]
- Jobanputra KS, Rajpal AV, Nagpur NG. Imiquimod. Indian J Dermatol Venereol Leprol. 2006. 72 466-469 [DOI] [PubMed] [Google Scholar]
- Cengiz FP, Emiroglu N, Su O, Onsun N. Effectiveness and safety profile of 40% trichloroacetic acid and cryotherapy for plantar warts. J Dermatol. 2016;43(9):1059–1061. doi: 10.1111/1346-8138.13370. [DOI] [PubMed] [Google Scholar]
- Jayaprasad S, Subramaniyan R, Devgan S. Comparative evaluation of topical 10% potassium hydroxide and 30% trichloroacetic acid in the treatment of plane warts. Indian J Dermatol. 2016;61(6):634. doi: 10.4103/0019-5154.193670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal P, Dhali TK, D'Souza P. Comparative study of efficacy of radiofrequency ablation, electrodesiccation, and cryosurgery in the treatment of cutaneous warts. J Dermatol and Dermatologic Surgery. 2019;23(1):24–29. [Google Scholar]
- Park HS, Choi WS. Pulsed dye laser treatment for viral warts: a study of 120 patients. J Dermatol. 2008;35(8):491–498. doi: 10.1111/j.1346-8138.2008.00509.x. [DOI] [PubMed] [Google Scholar]
- Ockenfels HM. Therapeutic management of cutaneous and genital warts. JDDG: J Dtsch Dermatol Ges. 2016;14(9):892–899. doi: 10.1111/ddg.12838. [DOI] [PubMed] [Google Scholar]
- Unni M, Tapare V. Intralesional bleomycin in the treatment of common warts. Indian J Drugs Dermatol. 2017;3(2):73–76. [Google Scholar]
- Hodeib AA, Al-Sharkawy BG, Hegab DS, Talaat RA. A comparative study of intralesional injection of Candida albicans antigen, bleomycin and 5-fluorouracil for treatment of plane warts. J Dermatol Treat. 2021;32(6):663–668. doi: 10.1080/09546634.2019.1688236. [DOI] [PubMed] [Google Scholar]
- Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32(6):797–801. doi: 10.1111/pde.12667. [DOI] [PubMed] [Google Scholar]
- Nofal A, Soliman M, Hamdy F, Alakad R. Intralesional Candida antigen versus intralesional tuberculin in the treatment of recalcitrant genital warts: a comparative study. J Am Acad Dermatol. 2020;82(6):1512–1514. doi: 10.1016/j.jaad.2019.12.050. [DOI] [PubMed] [Google Scholar]
- Rao AG, Haqqani R. Study of BCG immunotherapy in the management of multiple, extensive non-genital cutaneous common warts. Indian Dermatol Online J. 2020;11:784–788. doi: 10.4103/idoj.IDOJ_461_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas H, Ergin C, Demir B, Ekiz O. Intralesional vitamin D injection may be an effective treatment option for warts. J Cutan Med Surg. 2016;20(2):118–122. doi: 10.1177/1203475415602841. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Kavya M, Shashikumar BM, Harish MR, Shweta BP. Safety and efficacy of intralesional vitamin D3 in cutaneous warts: an open uncontrolled trial. J Cutan Aesthet Surg. 2017;10(2):90–94. doi: 10.4103/JCAS.JCAS_82_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Mohan A, Gupta AK, Pandey AK. A comparative study between intralesional PPD and vitamin D3 in treatment of viral warts. Int J Res Dermatol. 2018;4(2):197–201. [Google Scholar]
- Raghukumar S, Ravikumar BC, Vinay KN et al. Intralesional vitamin D3 injection in the treatment of recalcitrant warts: a novel proposition. J Cutan Med Surg. 2017;21(4):320–324. doi: 10.1177/1203475417704180. [DOI] [PubMed] [Google Scholar]
- Akula ML, Shetty M, Shetty V et al. Comparative study of therapeutic efficacy of intralesional vitamin D3 versus intralesional purified protein derivative in the treatment of warts. Indian J Clin Exp Dermatol. 2018;4(3):226–231. [Google Scholar]
- Nofal A, Nofal E. Intralesional immunotherapy of common warts: successful treatment with mumps, measles and rubella vaccine. J Euro Acad Dermatol Venereol. 2010;24(10):1166–1170. doi: 10.1111/j.1468-3083.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- Dhope A, Madke B, Singh AL. Effect of measles mumps rubella vaccine in treatment of common warts. Indian J Drugs Dermatol. 2017;3(1):14–19. [Google Scholar]
- Agrawal C, Vyas K, Mittal A et al. A randomized double blind controlled study comparing the efficacy of intralesional MMR vaccine with normal saline in the treatment of cutaneous warts. Indian Dermatol Online J. 2018;9(6):389–393. doi: 10.4103/idoj.IDOJ_111_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan VK, Chauhan PS, Sharma A et al. Evaluation of efficacy and safety of intralesional Measles-Mumps-Rubella virus vaccine for the treatment of common warts in children and adolescents. Indian J Paediatr Dermatol. 2019;20(3):231–235. doi: 10.4103/idoj.IDOJ_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na CH, Choi H, Song SH et al. Two-year experience of using the measles, mumps and rubella vaccine as intralesional immunotherapy for warts. Clin Exp Dermatol. 2014;39(5):583–589. doi: 10.1111/ced.12369. [DOI] [PubMed] [Google Scholar]
- Nimbalkar A, Pande S, Sharma R, Borkar M. Tuberculin purified protein derivative immunotherapy in the treatment of viral warts. Indian J Drugs Dermatol. 2016;2(1):19–23. [Google Scholar]
- Pande S, Sontakke A, Tayade BO. Purified protein derivative immunotherapy for viral warts and interpretation of tuberculin skin tests and interferon gamma release assay for diagnosis of tuberculosis in India. Indian J Drugs Dermatol. 2016;2(2):73–74. [Google Scholar]
- Kerure AS, Nath AK, Oudeacoumar P. Intralesional immunotherapy with tuberculin purified protein derivative for verruca: a study from a teaching hospital in South India. Indian J Dermatol Venereol Leprol. 2016;82(4):420–422. doi: 10.4103/0378-6323.175910. [DOI] [PubMed] [Google Scholar]
- Abd-Elazeim FM, Mohammed GF, Fathy A, Mohamed RW. Evaluation of IL-12 serum level in patients with recalcitrant multiple common warts, treated by intralesional tuberculin antigen. J Dermatol Treat. 2014;25(3):264–267. doi: 10.3109/09546634.2013.768760. [DOI] [PubMed] [Google Scholar]
- Saoji V, Lade NR, Gadegone R, Bhat A. Immunotherapy using purified protein derivative in the treatment of warts: an open uncontrolled trial. Indian J Dermatol Venereol Leprol. 2016. 82 1 42-6 [DOI] [PubMed] [Google Scholar]
- Wananukul S, Chatproedprai S, Kittiratsacha P. Intralesional immunotherapy using tuberculin PPD in the treatment of palmoplantar and periungual warts. Asian Biomed. 2010;3(6):739–743. [Google Scholar]
- Chandrashekar L. Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol. 2011;77(3):261–263. doi: 10.4103/0378-6323.79694. [DOI] [PubMed] [Google Scholar]
- Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141(5):589–594. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- El-Taweel AE, Salem RM, Allam AH. Cigarette smoking reduces the efficacy of intralesional vitamin D in the treatment of warts. Dermatol Ther. 2019;32(2):e12816. doi: 10.1111/dth.12816. [DOI] [PubMed] [Google Scholar]
- Rajashekar TS, Amulya R, Sathish S, Kumar S. Comparative study of intralesional BCG and PPD in the treatment of multiple cutaneous warts. Indian J Clin Exp Dermatol. 2018;4(1):1–6. [Google Scholar]
- Jaiswal A, Gupta K, Sharma RP, Bedi G. Immunotherapy with PPD in treatment of warts: an open labelled study from western Uttar Pradesh. Indian J Clin Exp Dermatol. 2019;5(1):41–45. [Google Scholar]
- Chandra S, Sil A, Datta A et al. A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with Mycobacterium w vaccine in the treatment of multiple viral warts. Indian J Dermatol Venereol Leprol. 2019;85(4):355–366. doi: 10.4103/ijdvl.IJDVL_549_18. [DOI] [PubMed] [Google Scholar]