Abstract
Platelet-rich plasma (PRP) has been integrated into numerous treatment regimens for medical and aesthetic dermatology. While some of these approaches are well-established, many uses are underreported in the literature. We sought to identify and summarize the emerging dermatologic applications for PRP by conducting a comprehensive PubMed search of studies published between 2000 and 2020. These studies were reviewed to synthesize collection methods, treatment schedule, adverse effects, and the impact of therapy for new and emerging uses for PRP. In general, we identified positive treatment outcomes for skin rejuvenation, scar revision, alopecia, pigmentary disorders, lichen sclerosus, leprosy-induced peripheral neuropathy, plaque psoriasis, and nail disorders. Widely, therapy was well-tolerated and suitable for all reported phototypes. The variations in collection and application sequences make concrete recommendations difficult to discern, underscoring the need for a standardized approach to preparation and treatment methods. We hope this review serves as an outline for new and interesting uses for PRP and will help readers familiarize themselves with this exciting technology for comfortable integration into their practices.
Keywords: Platelet-rich plasmas, PRP, emerging treatments, aesthetic dermatology, medical dermatology
In recent years, platelet-rich plasma (PRP) has gained popularity in the field of dermatology. As its name suggests, PRP is composed of concentrated platelets suspended in a solution of plasma. It is produced via centrifugation of whole blood, and its autologous nature suggests an excellent safety profile. Platelets have a well-known biological role in wound repair, and PRP harnesses this innate healing potential in a controlled manner by delivering a supraphysiologic concentration of platelets to target tissues. The concept originated in hematology in the 1970s and has since been safely employed in many medical, surgical, and dental applications.1 The healing and regenerative effects of PRP make it an attractive treatment modality for a number of dermatologic conditions. In this review, we identify, synthesize, and discuss the current evidence on the applications in medical and aesthetic dermatology for which PRP might be beneficial.
Biologic background and collection summary. Platelets are anucleate cytoplasmic fragments of megakaryocytes. Physiologically, they play a crucial role in primary hemostasis, serving as the “first responders” in wound healing. Upon tissue damage and subendothelial collagen exposure, platelets adhere to the damaged vessel wall, activate, and aggregate, forming a platelet plug that sets the framework for subsequent thrombus formation and primes the area for healing and repair.
Platelet alpha granules contain bioactive proteins, termed growth factors (GFs), which are exocytosed upon activation and are largely responsible for the regenerative properties of PRP. The principal GFs include vascular endothelial GF, platelet-derived GF, transforming GF (TGF), epidermal GF, fibroblast GF (FGF), and insulin-like GF 1. Together, they exert a wide range of physiologic effects, many of which are attractive for both aesthetic and medical dermatology. For instance, platelet releasate stimulates fibroblast proliferation, migration, collagen synthesis, elastin synthesis, and differentiation into myofibroblasts.2–11 These GFs also promote follicular growth, lipogenesis, angiogenesis, and extracellular matrix (ECM) remodeling.2,10,12 Therefore, PRP is biologically suited for many dermatologic applications, such as skin rejuvenation, hair restoration, scar revision, and wound healing, among others. This review attempts to clarify whether these perceived benefits on a cellular and molecular level translate into clinical improvement.
A typical PRP preparation consists of the collection of whole blood into a vial prefilled with a citrate-containing anticoagulant, 1- to 2-step centrifugation, the addition of an (often calcium-based) activator, and the application of the product (Figure 1). Centrifugation separates whole blood into layers based on the relative densities of its components. A single spin cycle produces three layers: a lower red blood cell layer, a middle buffy coat layer, and an upper plasma layer. The buffy coat layer contains the leukocytes, so inclusion of this layer in the final PRP product provides a rich leukocyte concentration.13–16 Occasionally, the upper plasma layer (with or without the buffy coat) is transferred to a new vial and centrifuged a second time. A portion of the upper platelet-poor plasma is then removed, and the platelet pellet is mechanically resuspended in the remaining solution.15 Many permutations have been applied to this algorithm, particularly with respect to the addition and type of anticoagulant or activator. These protocol alterations afford a great diversity of PRP products, and some of these varied harvesting methods yield PRP-derived products that have been assigned different titles, such as platelet-rich fibrin (PRF), PRF matrix, and plasma rich in GFs.
FIGURE 1.
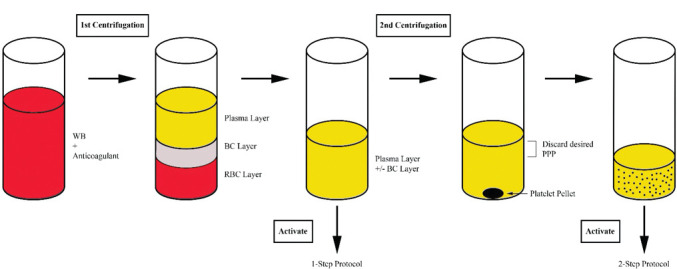
The typical process for a one- or two-step PRP preparation protocol. Abbreviations: WB, whole blood; BC, buffy coat; RBC, red blood cell; PPP, platelet-poor plasma
METHODS
In order to provide a comprehensive perspective of emerging applications in aesthetic and medical dermatology for which PRP may be useful, we conducted a PubMed search in April 2020 to identify these reports. We utilized a broad review of PRP literature to generate our search terms in order to maximize topics of interest. Our algorithmic approach and exclusion criteria can be found in Figure 2. Controlled and non-controlled studies on PRP monotherapy were included since pre- and post-treatment changes can indicate the efficacy of PRP; however, combination therapy research was only included if the study in question was comparatively controlled and designed so that clinical outcomes could be attributed to the addition of PRP. In an effort to focus more on emerging uses of PRP in dermatology, we identified applications with preliminary clinical literature, which we defined as less than 30 clinical studies. Applications with more than 30 clinical studies, as was the case for androgenetic alopecia (AGA) and wound healing, were excluded from our comprehensive analysis as their utility is well-substantiated by the literature. These topics will be briefly summarized utilizing recent, comprehensive reviews. For all other applications, we evaluated and compared the available clinical reports ourselves, which included 73 studies. Data acquisition focused on the following details: study design, participant characteristics, PRP preparation parameters, delivery method, treatment schedule, comparisons, and clinical outcomes pertaining to PRP.
FIGURE 2.
Study data-collection method
Given inherent individual differences and the multiple steps involved in PRP isolation, the final product is prone to high variability. To add complexity, there are inconsistencies in study caliber, reported parameters, treatment schedules and modalities used, follow-up duration, and measured outcome(s). These factors culminate in immense heterogeneity between studies, so meaningful statistical comparison could not be performed; therefore, this review serves primarily as a qualitative content analysis that compiles, organizes, and consolidates the current evidence for intriguing, novel dermatologic applications of PRP.
RESULTS
Seventy-three studies were included in our review, encompassing treatment modalities for skin rejuvenation, scar revision, alopecia, pigmentary disorders, genital lichen sclerosus, leprosy-induced peripheral neuropathy, plaque psoriasis, and nail disorders. Delivery methods ranged from topical application to intradermal (ID), deep dermal, subdermal, subfollicular, subcutaneous, perineural, and intramatricial injections. Forty-five studies had a treatment group with PRP monotherapy, while 35 studies had a treatment group with PRP in combination with either microneedling (n=0 studies), fractional laser resurfacing (n=17 studies), subcision (n=3 studies), microdermabrasion (n=1 study), excimer laser (n=1 study), NV-UVB (n=1 study), methotrexate (n=1 study), or intralesional corticosteroids (n=1 study).
Collection and application. The volume of harvested whole blood ranged from 8 to 60 mL, with 65.8% of reports specifying the type of anticoagulant utilized and 1.4% clearly indicating the absence of an anticoagulant. Among the 48 studies that reported an anticoagulant, sodium citrate (including trisodium citrate) and acid citrate dextrose with or without solution A (ACD and ACD-A) were most commonly reported, composing 56.3% and 29.2% of the reported total, respectively. Other anticoagulants reported included ethylenediaminetetraacetic acid, heparin calcium, citrate phosphate dextrose with or without adenine, and citrate dextrose; there was also a single study in which blood was collected into vials prefilled with calcium gluconate and ethanol.17
Centrifugation speed was reported in 80.8% of studies. Among them, 30.5% performed one centrifugation, while 69.5% completed two cycles. Centrifugation parameters were only compared in studies that reported speeds as multiples of gravity (g), i.e., 61% of all studies. For one-cycle protocols, centrifugation settings ranged from 70 to 1,200 × g for five to eight minutes, with an average of 637.1 × g for seven minutes. For two-cycle protocols, the first cycle ranged from 110 to 2,000 × g for three to 15 minutes with an average of 585 × g for 8.8 minutes, and the second cycle ranged from 400 to 5,000 × g for 5 to 20 minutes with an average of 1,711 × g for 9.3 minutes. Centrifugation temperature was reported by 8.2% of studies and included 4°C,18 16°C,19 18°C,20 and 20°C,17 and some studies reported a room temperature of 22°C,21,22 Of the 36 studies that noted the volume of collected whole blood and resultant PRP volume, the whole blood to PRP yield ranged from 20:1 to 2:1, with an average of 1mL of PRP produced per roughly 6mL of whole blood.
The buffy layer was clearly identified in 34.2% of studies, of which 56% included the buffy layer in their final product, while 44% discarded it. An exogenous activator was specified by 58.9% of reports, and 2.7% specified the use of no activator. The most common activators were calcium-based (93% of reported activators), including calcium chloride, calcium gluconate; a single study used calcium bicarbonate.17 The only identified non-calcium activator was plasma rich in GFs, used in three studies.
Analgesia was utilized in 63% of studies. Among them, topical preparations were employed by 84.8% of providers, with or without occlusion, for durations ranging from 15 minutes to two hours. Other analgesia methods included local anesthesia, nerve block, cold compress, forced-air cooling, distractive devices, and the addition of sodium bicarbonate and lidocaine to the PRP product.
Skin rejuvenation. Twenty-four studies reported PRP use in skin rejuvenation. PRP was used as monotherapy in 19 studies, with fractional laser resurfacing completed in four studies, and with microneedling performed in one study. Of the 19 studies on PRP monotherapy,18,23–40 two were randomized controlled trials (RCTs)23,40, four were comparative studies,24–27 and 13 were prospective case series.18,28–39 PRP monotherapy was employed for soft tissue augmentation or to rejuvenate aged skin. Interventions included topical or ID, deep dermal, subdermal, or subcutaneous injection to the tear troughs, marionette lines, forehead, periocular rhytids, nasolabial folds, midface, neck, or hands. Excluding one-time procedures, the treatment schedule ranged from two to six sessions with two- to four-week intervals (average of 3.2 sessions with 2.8-week intervals). The sole study on topical PRP monotherapy was applied twice daily for eight weeks.40 In all 19 studies, PRP monotherapy showed improvement compared to baseline in at least one clinical outcome, with 13 studies listing significant P-values.18,23–26,28-29,31,33,35,37-38,40 Biometric measurements revealed improvements in skin color homogeneity, redness, texture, firmness, wrinkles, pores, elasticity, barrier function, and capacitance compared to baseline.25,28,31,34,37 Of 13 studies that evaluated patient-reported outcomes, all noted satisfaction and cosmetic improvements.18,23,24,28–32,34,36,37,39–40 Per blinded raters in comparative studies, there was no significant difference in aesthetic outcomes compared to a saline control23 and ready-made GF mesotherapy,24 PRP was inferior to GF concentrate,26 and PRP was slightly inferior to amnion allograft in subjective improvement of the ogee curve27; however, there were fewer adverse effects noted with the PRP monotherapy treatment compared to either readymade GF mesotherapy or amnion allograft.24,27
Of the five studies on PRP with fractional laser resurfacing, four were RCTs with intrapatient controls,41–44 and one was a non-randomized comparative study.45 Fractional CO₂ laser (FCL) was used in four studies, while one study used fractional erbium laser.43 Three studies applied topical PRP following laser administration, while single studies performed subcutaneous PRP post-laser42 and pre-laser ID PRP injection followed by post-laser topical application.41 Three studies treated facial skin, while two others evaluated how PRP modulates the healing process when applied to a 1-cm2 area on the arm. For two studies with successive treatment sessions, treatment schedules consisted of three sessions with four-week to three-month intervals, and one study performed one FCL treatment session followed by topical application of PRP twice daily for 12 weeks. Compared to controls, the studies reported statistically significant benefits in skin texture, elasticity, wrinkles, or post-procedural erythema and edema. No impact was noted on post-procedural reepithelialization in one study.42 In studies noting patient-reported outcomes, all favored the PRP treatment group.41–43,45
El-Domyati et al46 conducted a split-face study of 24 patients evaluating PRP combined with microneedling for skin rejuvenation compared to microneedling alone or with trichloroacetic acid (TCA). The treatment schedule consisted of six sessions separated by two-week intervals. At conclusion, blinded and independent raters assessed photographs with a five-point scale and noted significant improvement in skin wrinkles, texture, and overall satisfaction with the combined PRP and microneedling treatment compared to either microneedling alone or with TCA.
Scar revision. We included 21 studies on PRP for atrophic facial scars. All examined PRP in combination with minimally invasive therapy, stratified as 10 studies considering fractional laser therapy,47–56 eight studies considering microneedling,21,22,57–62 one study considering microneedling and subcision,63 and two studies considering subcision alone.64,65 Most studies targeted atrophic acne scars, apart from two that included post-traumatic and varicelliform scars.52,58 Of 17 studies noting participant phototypes, 13 studies reported patient phototypes of III and/or VI.
Of the 10 studies on PRP combined with fractional laser resurfacing, seven were RCTs47–51,54,55 and three were comparative studies.52,53,56 Apart from one study that evaluated a fractional erbium-doped yttrium aluminum garnet laser,47 all studies used an FCL. The combination treatment was completed at the same visit in all studies except one in which PRP and laser therapies were completed on alternating visits.47 Application techniques included FCL to the atrophic facial scars, then either percutaneous or topical PRP; 2 to 3 treatments were performed in 3- to 4-week intervals (average of 2.3 sessions with 3.9-week intervals). Eight studies reported statistically significant improvements in scar appearance in the PRP group compared to control,47–49,51–55 with seven noting diminished duration and/or severity of post-laser adverse effects (erythema, edema, crusting, oozing).48,49,51–54,56 One study revealed significantly worse erythema and edema in the PRP-treated group a few days after therapy, though no significant difference in the duration of these effects was identified.50 Gawdat et al54 examined FCL in combination with either ID or topical PRP and found both applications significantly enhanced scar appearance compared to FCL alone.54 There was no significant difference between the two combinations in regard to the degree of scar improvement, but the topical PRP group did have a significantly diminished pain score.
Eight studies evaluated the combination of PRP with microneedling, encompassing one RCT57 and seven comparative studies.21,22,58–62 One study performed percutaneous PRP and microneedling treatments on alternating visits,58 and the rest completed the combination treatments at the same visit, performing three to six sessions with two- to four-week intervals (average of 3.9 sessions with 3.3-week intervals). All three studies on percutaneous PRP combined with microneedling showed significant clinical improvement and patient satisfaction with combined PRP and microneedling compared to controls.57–59 In one study, there was more severe pain and erythema in the microneedling group than in the ID PRP group.58 Topical PRP with microneedling led to mixed results: two studies showed this combination to produce greater clinical improvement than microneedling alone,60,61 while one study showed no significant difference between therapies.22 Topical PRP with microneedling produced modest benefits over topical vitamin C with microneedling21 and produced no significant difference compared to TCA CROSS (100%).62 One study showed significantly less post-procedure erythema and edema with microneedling and topical PRP compared to microneedling alone.22
Bhargava et al63 RCT evaluated the combination of microneedling with subcision and topical PRP compared to microneedling with subcision alone. Participants received three sessions of their respective treatments in three-week intervals. Upon completion, the PRP group had greater clinical improvement and a shorter duration of post-procedure erythema and edema.
Two studies evaluated the combination of subcision and percutaneous PRP for the treatment of atrophic acne scars.64,65 Participants received 3 to 4 monthly sessions of subcision followed by intralesional PRP. Collectively, ID and subcutaneous PRP monotherapy showed significantly greater improvement and fewer adverse effects than subcision combined with PRP. Subcision alone showed the least clinical improvement.
Striae distensae, also known as stretchmarks, are produced via stretching of the skin. They can be erythematous or white, termed striae rubra or striae alba, respectively. Four studies on striae distensae fulfilled our criteria, including two RCTs,66,67 and two comparative studies.68,69 These studies evaluated the efficacy of PRP compared to carboxytherapy,66,69 tripolar radiofrequency,66 tretinoin,68 or microdermabrasion67 for both striae alba and rubra or striae alba only.69 All participants had skin phototype III or VI. The treatment schedule ranged from 3 to 6 sessions of 1- to 4-week intervals (average of 4.5 sessions with 2.6-week intervals). Commonly reported application parameters included 0.1mL per injection, 1- to 2-cm spacing between injections, and with PRP injected either intradermally or subcutaneously. ID PRP was superior to tretinoin68 and microdermabrasion alone.67 Regarding the degree of clinical improvement, ID and subcutaneous PRP led to no significant difference compared to carboxytherapy.66,69 Regarding patient satisfaction ratings, ID PRP was inferior to carboxytherapy and tripolar radiofrequency.66 Erythematous, truncal lesions responded the best to PRP.66 All four studies included a treatment group that received PRP monotherapy, and improvement from baseline in all studies was recorded.
One clinical study on the use on percutaneous PRP for keloid scars fulfilled our criteria.70 This RCT injected all 40 patients with four sessions of ILC, and, after the last session, also injected the keloids of 20 patients with ID PRP. The PRP group had significant improvement in clinical appearance and diminished post-steroid adverse effects.
Alopecia. We assessed five studies on PRP injections for alopecia areata (AA), including three RCTs,71–73 one comparative study,17 and one case series.74 One study focused on patients with alopecia totalis17; one study included patients with AA, alopecia totalis, and alopecia universalis72; and the rest of the studies examined patients exclusively with AA. Excluding solitary studies, the treatment schedule ranged from 3 to 6 sessions with 2- to 4-week intervals (average of 4 sessions with 3.5-week intervals). Injection details, specified in two studies, included 0.1mL per injection into the dermis or subfollicular plane with 1- to 2-cm spacing between injections. PRP showed improvement compared to baseline, controls, and standard therapies. Compared to ILC, two studies reported greater hair regrowth and a lower relapse rate with PRP, while there was no significant difference in dystrophic hairs and dysesthesia between the groups.71,73 Compared to minoxidil, PRP showed quicker regrowth and decreased dystrophy and short vellus hairs.72
One article shared two case reports on PRP successfully used to treat cicatricial alopecia.75 One patient had central centrifugal cicatricial alopecia with a component of AGA, and the other patient had lichen planopilaris; both received three sessions of 4 to 5 mL of PRP with three-week intervals. Injection depth was not specified. The patients showed global improvement, yet there was regression of improvement noted at six months, suggesting a need for maintenance therapy.
Pigmentary disorders. We reviewed four studies on PRP for non-segmental vitiligo, including three RCTs,76–78 and one comparative study.79 Patients received 4 to 8 sessions of PRP with 2- to 3-week intervals (average of 6 sessions with 2.5-week intervals). All studies used ID PRP as an adjunct to either FCL,76,77 excimer laser,78 or narrowband ultraviolet B (NB-UVB).79 The excimer and NB-UVB therapy were twice weekly and the FCL was either concurrent with the PRP treatment or every two weeks. PRP injections were placed 0.5 to 2 cm apart, intradermally, with 0.1 mL applied per site. Overall, the combination of PRP with FCL, excimer, or NB-UVB was superior to laser or phototherapy alone. Two studies administered ID PRP alone to a treatment group, and one study found it to be superior to FCL alone,76 while the other found it to be inferior to FCL alone.77 The face or trunk had the best response to PRP-enhanced treatment,76–79 while it was less effective on acral sites.
One RCT on melasma met our inclusion criteria.80 This study included 10 female patients with bilateral mixed-type melasma. Patients received four sessions of ID PRP to one half of face and normal saline to the other half with two-week intervals. Biometric measurements demonstrated conflicting results regarding improvements in melanin concentration, although blinded rater review and patient satisfaction was significantly greater on the PRP side. We reviewed three studies on periorbital hyperpigmentation, including one RCT,20 one comparative study,81 and one case series.37 Patients had 4 to 7 treatment sessions of ID PRP with two-week intervals. Two studies showed significant improvements compared to baseline at 3 to 6 months.37,81 Similar to the study on PRP compared to carboxytherapy for striae distensae, PRP did not show significant clinical improvement compared to carboxytherapy.81 PRP was inferior to a combined TCA 3.75%/lactic acid 15% peel in terms of degree of improvement and patient satisfaction.20
Lichen sclerosus (LS). Six studies on PRP for LS met our criteria, including one RCT82 and five case series.83–87 Four studies focused on vulvar lesions,82,83,86,87 one focused on penile lesions,85 and one considered both female and male patients.84 Patients received 1 to 6 mL of intralesional (ID, subdermal, or unspecified) PRP for two to 10 sessions with 2- to 12-week intervals (average of 3.2 sessions with 5.5-week intervals), with one study including an additional treatment performed at one year.87 For vulvar LS, Goldstein et al initially documented clinical improvement in a case series,86 but their subsequent RCT found no improvement compared to a saline control group.82 Tedesco et al83 and Behnia-Willison et al87 reported decreased vulvar LS lesion count, symptoms, and steroid use in patients treated with PRP. A case series on both vulvar and penile lesions showed improvement compared to baseline in 62% of patients.84 For penile LS, one study showed improvement in quality of life scores among all patients, and clinically mitigated resumption of steroids.85
Limited applications. There is anecdotal evidence that PRP might be beneficial in a variety of other dermatologic conditions. One RCT displayed that a single perineural injection of PRP significantly improved leprosy peripheral neuropathy.88 A prospective comparative trial showed that the combination of intralesional PRP with methotrexate substantially improved patient PASI scores.89 Two cases of nail dystrophy (lichen striatus and idiopathic trachyonychia) refractory to topical and/or intramatricial corticosteroids showed improvement after intramatricial PRP injections.19
Table 1 provides summarized data from reviewed studies showing benefit with PRP therapy. While structured treatment guidelines would be helpful, these are, at this junction, premature, and this chart merely offers a loose framework for those interested in implementing such therapeutic options in their practices.
TABLE 1.
Summary of platelet-rich plasma applications which showed benefit, including type of therapy, method of analgesia (if reported), delivery and treatment methods, and level of evidence in accordance with the Evidence-based Medicine modified Grading of Recommendations, Assessment, and Development evidence rating scalea
| APPLICATION | ANALGESIA | DELIVERY METHODS | TREATMENT SCHEDULE | EVIDENCE |
|---|---|---|---|---|
| Skin rejuvenation | ||||
| PRP Monotherapy | Anesthetic cream × 15–60 min, cooling compress, or nerve block |
|
|
|
| PRP Combined with Fractional Laser Resurfacing | Anesthetic cream × 30–60 min |
|
|
|
| PRP Combined with Microneedling | Anesthetic cream × 45–60 min |
|
|
|
| Scar revision | ||||
| Atrophic facial scars treated wth PRP combined with fractional laser resurfacing | Anesthetic cream × 30–60 min, cooling compress, or forced-air cooling |
|
|
|
| Atrophic facial scars treated with PRP combined with microneedling and/or subcision | Anesthetic cream × 30–120 min, cooling compress, or local anesthetic |
|
|
|
| Striae distensae | Anesthetic cream × 45–60 min |
|
|
|
| Keloids | NR |
|
|
|
| Alopecia | ||||
| Alopecia areata | Local anesthetic |
|
|
|
| Cicatricial alopecia | NR |
|
|
|
| Pigmentary disorders | ||||
| Vitiligo | Anesthetic cream × 30–60 min |
|
|
|
| Melasma | Anesthetic cream × 45 min |
|
|
|
| Periorbital hyperpigmentation | Anesthetic cream × 60 min |
|
|
|
| Lichen sclerosus | ||||
| Lichen sclerosus | Anesthetic cream × 30 min or local anesthetic |
|
|
|
| Other | ||||
| Leprosy peripheral neuropathy | NR |
|
|
|
| Psoriasis | NR |
|
|
|
| Nail disorders | Anesthetic cream or vibratory device |
|
|
|
Abbreviations: ID, intradermal; DD, deep dermal; SD, subdermal; SQ, subcutaneous; CF, crow’s feet; NLF, nasolabial fold; G, gauge; Q, every; BID, twice per day; FCL, fractional CO2 laser; PPP, platelet-poor plasma; FEL, fractional erbium laser; UO, under occlusion; FEYL, fractional erbium-YAG laser; MN, microneedling; MD, microdermabrasion; NR, not reported; ILC, intralesional corticosteroids; AA, alopecia areata; CCCA, central centrifugal cicatricial alopecia; AGA, androgenetic alopecia; LPP, lichen planopilaris; NB-UVB, narrow-band ultraviolet-B; TCA, trichloroacetic acid; LA, lactic acid; IL, intralesional; PNF, proximal nail fold; LNF, lateral nail fold
aThe GRADE evidence rating scale we used can be found here: https://www.essentialevidenceplus.com/product/ebm_loe.cfm?show=grade#accept
DISCUSSION
Collection sequence. Given the abundance of steps involved in producing PRP, it is understandable that there is wide variance in the final products. This is further complicated by the inconsistent reporting of PRP harvesting steps. Our review underscores the need for unified data. Of the 73 studies we reviewed, 67.1% provided details on anticoagulation, 80.8% reported centrifugation speed(s), 34.2% reported buffy content, 61.6% provided details on activation, and 49.3% reported how much venous blood was collected and how much PRP was produced. This ambiguity makes comparative assessment of results challenging, thereby discrediting the quality of evidence for PRP. Additionally, many seemingly insignificant factors have been suggested to influence final yield, such as tube geometry and venipuncture duration.90,91
Venipuncture methods and anticoagulant use were largely reproduced among studies. While no anticoagulant was clearly superior, our review suggests that ACD or ACD-A or sodium citrate buffers seem to serve as the favored anticoagulants, as they may translate into the highest active platelet concentration. Both of these are citrate-based, which act by chelating calcium. Ethylenediaminetetraacetic acid was historically preferred, but fell out of favor when it was shown to damage platelet membranes.15,92–94
Centrifugation can be represented as multiples of earth’s gravitational field (g) or as revolutions per minute (rpm), with the centrifuge rotor radius required to convert between these units.15 The use of both terms without documenting rotor radius hinders comparison between studies, which is why we are hopeful future studies will consistently report speeds as a measure of “g.” Centrifuge speed significantly influences final PRP composition, with high speeds linked to weakened platelet integrity and the risk of pushing platelets into the buffy coat layer resulting in diminished concentration; low speeds may inadequately separate platelets from the cellular layers.95–98 The majority of studies utilized two-step centrifugation, composed of an initial “soft spin” to separate the mixture into the red blood cell, buffy coat, and plasma layers; this is followed by a “hard spin,” which improves platelet yield by decreasing the plasma volume in which the platelets are suspended.96,99,100 The literature reports a very broad range of centrifugation speeds. Research suggests that platelet damage begins to ensue at speeds of greater than 800 g.95–97 If this is indeed the case, then it would mean that 28.6% of studies with one-spin protocols and 75.9% of studies with two-step protocols were vulnerable to a loss of platelet integrity. Platelet health is requisite in the final PRP product, as GFs need to exocytose from platelets to complete their tertiary structure and become bioactive.101 GFs inadvertently released through cell damage impair the product quality and yield underwhelming results.
Including the buffy coat layer (leukocytes) has been actively explored and debated. Leukocyte-rich PRP (L-PRP) might be desired for specific scenarios, such as skin rejuvenation, since it has been shown to produce greater volumization and wrinkle reduction.102 The proposed positives for L-PRP include synergy between leukocytes and platelets4; antimicrobial activity103–107; improved wound healing106; and the production of MMPs that help modulate angiogenesis, ECM remodeling, and hair cycling.108 Opponents advocate that L-PRP has catabolic effects via proteases and reactive oxygen species that hinder tissue healing.109–111 Further research is needed to help clarify the optimal leukocyte content for specific applications.
It is unclear whether the addition of exogenous activators to PRP benefits application15,112 because platelets activate in the presence of endogenous collagen or thrombin.93,113,114 Upon initiation of the activation cascade, PRP is most bioactive immediately, with 70% of platelet degranulation occurring within the first 10 minutes and around 95% within the first hour.101,115 After this initial release, platelets secrete additional proteins throughout their lifespan (around 5–9 days).101,115 Therefore, if using an activator, it is recommended to apply the product within 10 minutes of activation. A dual-syringe mixing system can be used to mix the activator and PRP at the time of application.116 Alternatively, relying on endogenous activation appears to produce a more gradual, sustained release of GFs, which might be beneficial in some clinical settings.12,117,118 The most common exogenous activators used in these studies were calcium-based and act by replenishing the calcium chelated during anticoagulation within the collection vial. Some studies have reported the use of thrombin as a common activator.
Other than the study by Doghaim et al,18 in which their topical PRP product was refrigerated for 90 days, no other studies mentioned storage of PRP. If storage can be successfully employed, this therapy might become even more attractive to patients, as it would hasten the procedure and negate the need for repeated venipuncture. A few studies found that frozen and even lyophilized samples had comparable levels of GFs when compared to fresh PRP, though the longest freeze time was only three weeks.119 Studies on lyophilized PRP determined that PRP could either be activated before or after freeze-drying120 or not activated at all.121 Further studies on PRP storage are needed.
In 2018, to help formulate consensus recommendations for the research and use of PRP, a working group of 10 experts from the Platelet Physiology Subcommittee of the Scientific Standardization Committee of the International Society on Thrombosis and Haemostasis devised a classification system. Their system distinguishes between PRP and PRF and acknowledges the erythrocyte content (“red” if > 10% erythrocytes), leukocyte content (“L” if > 1% leukocytes), activation status (I, without activation; II, with activation; or III, frozen-thawed preparation), platelet concentration (A, < 900,000/uL; B, 900,000–1,700,000/uL; or C, > 1,700,000/uL), and method of platelet retrieval (1, gravitational centrifugation; 2, standard cell separator; or 3, selective filtration).93 For example, Red-L-PRP IB1 means that the PRP contains greater than 10% erythrocytes, greater than 1% leukocytes, no activation, a platelet concentration of between 900 and 1,700 × 103, and it was prepared using gravitational centrifugation. If adopted, this nomenclature will make future study methods more transparent. Additionally, precise methodology should be provided in the manuscript to ensure reproducibility. Implementing these details into future literature will afford meaningful comparisons and robust conclusions. Once the reporting of PRP harvesting is standardized, it can then be optimized for specific indications.
Skin rejuvenation. The pathogenesis of skin aging is characterized by the loss of skin thickness, namely due to a reduction in collagen. Histologically, there is thinning of collagen and elastin with reduced density of the ECM, translating into clinical rhytids and visualization of underlying vascular structures.122 Fibroblasts are a main target of rejuvenation techniques because they produce collagen, elastin, and other ECM components. Thus, PRP’s stimulatory effect on fibroblast proliferation is an enticing modality for skin rejuvenation.
Skin rejuvenation using PRP monotherapy has reproducibly correlated to an improved appearance of the aged face. Yet, it is inconclusive whether PRP is superior to control therapy or other treatment modalities. ID injections themselves likely account for a portion of the improvement since skin piercing has a biostimulatory effect, though differences were reported in texture, wrinkles, and skin thickness by patient or biometric measurements when comparing ID PRP to ID saline controls, suggesting that PRP offers a benefit over the injection alone.23,25 The skin-rejuvenating effects appear to be subtle, with some studies showing equivocal improvement by objective ratings, yet patients more uniformly reported significant improvement.23,24,29 One study on topical PRP monotherapy reported no significant difference compared to control, but the retrieved serum was stored at 4°C for 90 days, potentially diminishing the application effect.40
The results for PRP combined with fractional laser therapy strongly suggests that percutaneous or topical PRP might produce synergistic clinical outcomes and reduce post-procedural erythema and edema. Topical PRP also enhanced the clinical efficacy of microneedling. Combination therapy may assist with transepidermal movement of the PRP, though the true explanation is likely more complex as studies investigating microneedling effects have shown conflicting efficacy in promoting molecular penetration.123,124 Using fluorescein-stained platelets, one study determined that platelets can navigate almost the entire depth of the microneedle channel when applied at an optimal time of 5 to 30 minutes after microneedling and followed by skin massaging.123 Contrarily, using tattoo dye pigment as an analog for PRP, another study found minimal dermal penetration when applied with microneedling.124
In the realm of skin rejuvenation, PRP shows promise as a monotherapy when applied percutaneously, has synergistic effects and diminished downtime when combined with fractional laser resurfacing, and enhances the aesthetic outcomes of microneedling.
Scar revision. PRP’s stimulatory effects on ECM remodeling could account for its potential benefit in scar revision.2,3,6,7,125 For the purpose of treating atrophic facial scars, the results strongly favor improved outcomes when PRP is added as an adjunct to laser treatment, microneedling, and subcision. Fewer adverse effects were noted when PRP was combined with all three modalities. For PRP combined with fractional laser therapy, it is debatable whether topical or percutaneous application is more efficacious. Gawdat et al54 noted topical PRP had the same efficacy as ID PRP with a lower pain score, while Faghihi et al50 found that ID PRP worsened the severity of post-laser adverse effects but not terminal clinical appearance. Kar et al56 found that topical PRP did not improve scar quality compared to laser application alone. Therefore, percutaneous PRP appears to more consistently enhance scar quality but might result in added post-procedural downtime, so the delivery method should be a joint decision between physician and patient. In terms of injection discomfort, a study by Ibrahim et al58 reported that ID PRP was significantly less painful than microneedling. ID PRP combined with microneedling showed significant improvement compared to control therapy. The results for topical PRP with microneedling were not as convincing but still favored improvement over microneedling alone and microneedling combined with vitamin C. Three studies evaluating percutaneous PRP monotherapy suggested a positive effect,58,62,64 with one study reporting ID PRP to be superior to combination ID PRP and subcision in terms of adverse effects and tolerability.64 Collectively, there was a minimal risk of inducing PIH with PRP, supporting its safety in skin of color.48,54,62,68,69
Considering the relatively poor treatment options for striae distensae, percutaneous PRP monotherapy showed improvement compared to baseline and might offer a useful adjunct or alternative for patients who cannot receive or are refractory to standard therapeutic options. ID PRP was superior to other modalities in treating striae rubra, whereas it was equivalent to carboxytherapy or tripolar radiofrequency for managing striae alba.66
PRP also benefitted atrophic and keloidal scarring. In-vitro and animal models have shown that FGF, released from stimulated platelets, can decrease the scar elevation index by recycling collagen.126 Therefore, PRP shows potential as a therapy for hypertrophic and keloidal scars. One study reported that the addition of PRP to ILC treatment of keloids produced significant improvement in keloid characteristics and mollified unwanted steroid effects. Other studies showed benefit with the use of PRP at the surgical margins of excised keloidal tissue, with or without concomitant use of cryosurgery or superficial radiation.127–130
Alopecia. Relative to other dermatologic uses, PRP use for alopecia has a robust body of evidence, though standardized application guidelines remain limited due to formulary variation in PRP products. In AGA, follicles miniaturize into vellus hairs and prematurely transition to the telogen phase.131 PRP is believed to promote hair growth by inducing perifollicular angiogenesis, activating anti-apoptotic pathways, prolonging the anagen phase, promoting proliferation of dermal papilla cells, and stimulating differentiation of hair follicle stem cells.132–134
Recent reviews have proposed 3 to 4 monthly injections of PRP followed by maintenance therapy every 2 to 3 months as a treatment schedule for AGA.135–138 More frequent treatments are utilized in stubborn disease. While most studies utilize ID delivery,107 some advocate for subdermal application, which is speculated to improve product diffusion and require fewer injections.136,140
Due to the benefits seen in AGA, many studies have evaluated PRP’s role in alopecia areata. AA has a variable disease course, so non-controlled studies require careful interpretation. Some studies have tried to account for this by recruiting patients with recalcitrant AA. PRP showed benefit over baseline, control therapy, minoxidil 5%, and ILC in a variety of outcomes and appears to be a reasonable alternative to ILC.
Cicatricial alopecias eventuate in hair follicle destruction. PRP’s ability to stimulate hair growth combined with its remodeling of scarred tissue suggests it might be a helpful therapeutic adjunct for cicatricial alopecias. While the evidence on PRP for cicatricial alopecia is clearly limited by small sample size and lack of objective measurements, it has shown promise in treating lichen planopilaris/frontal fibrosing alopecia and central centrifugal cicatricial alopecia.141–144 Additional research is needed to corroborate or challenge these findings.
Pigmentary disorders. Preliminary studies have evaluated the use of PRP in treating disorders, including vitiligo, melasma, and periorbital hyperpigmentation. The theorized molecular mechanism for PRP’s utility in both depigmented and hyperpigmented conditions is based on GF melanocyte regulation. TGF-β has been suggested to inhibit melanin synthesis,145,146 while FGF promotes melanocyte proliferation and melanogenesis.147,148 It is likely that these processes serve to balance melanocyte activity, since PRP has proven useful for conditions with seemingly opposite pathogenic mechanisms. Nonetheless, clinical research has reported a benefit in both applications. ID PRP shows promise as an adjunct to laser and phototherapies for vitiligo, as a monotherapy for melasma, and as a monotherapy for periorbital hyperpigmentation. The use in vitiligo requires careful follow-up to ensure patients do not Koebnerize.149
LS. LS is characterized by chronic inflammation, scarring, and atrophy, primarily in the anogenital region. Patients can experience debilitating pruritus, pain, secondary erosions, and adhesions. The gold standard for therapy remain high-potency topical corticosteroids, but that poses an obvious risk when utilized in the genital area. Therefore, alternate effective treatment options would be of great utility. Intralesional PRP has been shown to alleviate symptoms, diminish lesions, reduce steroid use, and improve quality of life in patients with genital LS in five case series. This evidence is encouraging, but a RCT showed no significant difference between ID PRP and ID saline. Additional studies are needed to clarify the efficacy of PRP for LS.
Wound healing. The use of PRP for wound care is intuitive given platelets’ physiologic role in initiating post-traumatic healing. Animal and clinical studies have corroborated these theories.150 PRP has been successfully employed for various wound healing applications, including pressure ulcers,151,152 diabetic ulcers,153–155 venous leg ulcers,156 radiation dermatitis,157 ulcerations of leprosy,158,159 refractory lipodermatosclerosis,160 necrobiosis lipoidica,161 scleroderma ulcers,162–164 resistant oral pemphigus vulgaris erosions,165,166 graft-versus-host disease oral ulcers,167 erosive oral lichen planus,168 pyoderma gangrenosum,169–171 ecthyma gangrenosum,172 ulcerated tophaceous gout,173 acquired immunodeficiency syndrome-related crural ulcers,174 livedoid vasculopathy,175 and burns.176
A Cochrane meta-analysis from 2016 evaluated the efficacy of PRP in chronic wound healing and concluded that PRP might improve healing in diabetic foot ulcers, but the evidence for other chronic wounds was unclear.177 A more recent systematic review on PRP and chronic wounds by Hesseler et al16 found utility for PRP use in a variety of wounds, suggesting the following treatment regimen for chronic wound healing: activated PRP or leukocyte-rich PRF topically applied 1 to 2 times weekly for 3 to 6 weeks.16
Adverse effects. Although PRP’s autologous nature implies an excellent safety profile, it is not without potential complications. The most notable adverse events included injection site pain in 11 patients23,81 and PIH in one patient following microneedling.21 Pain was most notable in patients not receiving pre-procedural analgesia,81 suggesting pain-reducing methods substantially improve tolerability. Otherwise, PRP was well-tolerated, with no severe adverse effects reported. The most commonly reported events included erythema, edema, injection site pain, and bruising. Additional adverse effects included scalp pruritus,178 transient hair shedding,178,179 cervical lymphadenopathy,178,180 serum sickness,181 allergic reaction to calcium citrate preparation,182 cutaneous sarcoidal lesions,183,184 and one case of irreversible monocular blindness after glabellar injection.185 Due to its potential to nourish neoplastic growth, it is advocated to avoid PRP treatment in areas of previous malignancy.1,186,187 The efficacy of PRP in patients with active autoimmune disease is equivocal, with some reports cautioning against its use, while others report benefits in this population.181 Interestingly, PRP seems to reduce the effectiveness of neurotoxins.188
Injection site pain can be minimized with addition of lidocaine,27,189,190 bicarbonate,27,191,192 or through use of a smaller-gauge needle.27 The type of both anticoagulant and activator may also alter the final solution’s pH, with ACD-A and calcium chloride associated with burning due to their low pH.193–196 Preparations that use these solutions would benefit from the addition of a bicarbonate buffer to decrease injection-site pain; protocols can also omit anticoagulation or activation altogether.31 Tolerability of treatment appears to improve with subsequent sessions.71
Limitations. Our review has several limitations. To compile a comprehensive overview of current literature for aesthetic and medical dermatologic applications for PRP, we included certain non-controlled and non-randomized studies. Of the 73 total studies included in our review, 29 were RCTs; therefore, 44 studies are inherently prone to selection, reporting, and publication biases. The methods of our review may be subject to selection bias in that we searched a single database and our search terms could have failed to capture some pertinent studies. The reviewed studies had small sample sizes, short follow-up periods, and subjective evaluations; though this was our methodology for selecting new and emerging applications, it also diminishes the power of this review. The results are not generalizable to all potential candidates of PRP therapy because many reports identified diverse exclusionary criteria. Other major limitations include the formulary variations, inconsistent reporting, and the heterogeneity in clinical outcomes measured, making it difficult to fully evaluate literature.
CONCLUSION
We sought to provide a summarized review of the current evidence regarding the utility of PRP in medical and aesthetic dermatology, focusing on preliminary applications. Most studies reported favorable outcomes. PRP offers a convenient, biocompatible therapeutic option that has not only produced clinical improvement in multiple dermatologic diseases but has improved or mitigated many postprocedural adverse effects. This review underscores the need for robust, randomized, controlled studies to better elucidate the utility of this treatment. We hope this review enforces the need for standardization in future research so that emerging uses for PRP in dermatology can be shared and improved through the collective review of high-quality clinical applications.
REFERENCES
- Acebes-Huerta A, Arias-Fernández T, Bernardo Á et al. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfus Apher Sci. 2020;59(1):102716. doi: 10.1016/j.transci.2019.102716. [DOI] [PubMed] [Google Scholar]
- Cho JW, Kim SA, Lee KS. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med. 2012;29(1):32–36. doi: 10.3892/ijmm.2011.803. [DOI] [PubMed] [Google Scholar]
- Cho EB, Park GS, Park SS et al. Effect of platelet-rich plasma on proliferation and migration in human dermal fibroblasts. J Cosmet Dermatol. 2019;18(4):1105–1112. doi: 10.1111/jocd.12780. [DOI] [PubMed] [Google Scholar]
- Devereaux J, Nurgali K, Kiatos D et al. Effects of platelet-rich plasma and platelet-poor plasma on human dermal fibroblasts. Maturitas. 2018;117:34–44. doi: 10.1016/j.maturitas.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Wirohadidjojo YW, Budiyanto A, Soebono H. Platelet-rich fibrin lysate can ameliorate dysfunction of chronically UVA-irradiated human dermal fibroblasts. Yonsei Med J. 2016;57(5):1282–1285. doi: 10.3349/ymj.2016.57.5.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farghali HA, AbdElKader NA, Khattab MS et al. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci Rep. 2017;37(2):BSR20160503. doi: 10.1042/BSR20160503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian LJ, Chowdhury SR, Bin Saim A et al. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy. 2015;17(3):293–300. doi: 10.1016/j.jcyt.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Suh DH, Lee SJ, Lee JH et al. Treatment of striae distensae combined enhanced penetration platelet-rich plasma and ultrasound after plasma fractional radiofrequency. J Cosmet Laser Ther. 2012;14(6):272–276. doi: 10.3109/14764172.2012.738916. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Tobalem M, Vigato E et al. Nonactivated versus thrombin-activated platelets on wound healing and fibroblast-to-myofibroblast differentiation in vivo and in vitro. Plast Reconstr Surg. 2012;129(1):46e–54e. doi: 10.1097/PRS.0b013e3182362010. [DOI] [PubMed] [Google Scholar]
- Sclafani AP, McCormick SA. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Arch Facial Plast Surg. 2012;14(2):132–136. doi: 10.1001/archfacial.2011.784. [DOI] [PubMed] [Google Scholar]
- Kushida S, Kakudo N, Suzuki K. et al. Effects of platelet-rich plasma on proliferation and myofibroblastic differentiation in human dermal fibroblasts. Ann Plast Surg. 2013. 71 2 219-224 [DOI] [PubMed] [Google Scholar]
- Gentile P, Cole JP, Cole MA et al. Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18(2):408. doi: 10.3390/ijms18020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer K, Kankaya Y, Çolak Ö. An important and overlooked parameter in platelet rich plasma preparation: the mean platelet volume. J Cosmet Dermatol. 2019;18(2):474–482. doi: 10.1111/jocd.12682. [DOI] [PubMed] [Google Scholar]
- Oudelaar BW, Peerbooms JC, Huis In 't Veld R et al. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. 2019;47(2):479–487. doi: 10.1177/0363546517746112. [DOI] [PubMed] [Google Scholar]
- Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthet Surg. 2014;7(4):189–197. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in medical dermatology: a systematic review. J Am Acad Dermatol. 2019;81(3):834–846. doi: 10.1016/j.jaad.2019.04.037. [DOI] [PubMed] [Google Scholar]
- Khademi F, Tehranchinia Z, Abdollahimajd F et al. The effect of platelet rich plasma on hair regrowth in patients with alopecia areata totalis: a clinical pilot study. Dermatol Ther. 2019;32(4):e12989. doi: 10.1111/dth.12989. [DOI] [PubMed] [Google Scholar]
- Doghaim NN, El-Tatawy RA, Neinaa YME. Assessment of the efficacy and safety of platelet poor plasma gel as autologous dermal filler for facial rejuvenation [published online ahead of print, 2019 Feb 26]. J Cosmet Dermatol. 2019. 10.1111/jocd.12876. [DOI] [PubMed]
- Kaur I, Jakhar D. Intramatricial platelet-rich plasma therapy: a novel treatment modality in refractory nail disorders. Dermatol Ther. 2019;32(2):e12831. doi: 10.1111/dth.12831. [DOI] [PubMed] [Google Scholar]
- Ellabban NF, Eyada M, Nada H et al. Efficacy and tolerability of using platelet-rich plasma versus chemical peeling in periorbital hyperpigmentation. J Cosmet Dermatol. 2019;18(6):1680–1685. doi: 10.1111/jocd.12964. [DOI] [PubMed] [Google Scholar]
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars [published correction appears in J Cutan Aesthet Surg. 2015;8(1):75]. J Cutan Aesthet Surg. 2014;7(4):209–212. doi: 10.4103/0974-2077.150742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29(3):281–286. doi: 10.1080/09546634.2017.1365111. [DOI] [PubMed] [Google Scholar]
- Alam M, Hughart R, Champlain A et al. Effect of platelet-rich plasma injection for rejuvenation of photoaged facial skin: a randomized clinical trial. JAMA Dermatol. 2018;154(12):1447–1452. doi: 10.1001/jamadermatol.2018.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawdat HI, Tawdy AM, Hegazy RA et al. Autologous platelet-rich plasma versus readymade growth factors in skin rejuvenation: a split face study. J Cosmet Dermatol. 2017;16(2):258–264. doi: 10.1111/jocd.12341. [DOI] [PubMed] [Google Scholar]
- Du R, Lei T. Effects of autologous platelet-rich plasma injections on facial skin rejuvenation. Exp Ther Med. 2020;19(4):3024–3030. doi: 10.3892/etm.2020.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla GP, Dhurat RS, Shetty G et al. Safety and efficacy of growth factor concentrate in the treatment of nasolabial fold correction: split face pilot study. Indian J Dermatol. 2015;60(5):520. doi: 10.4103/0019-5154.159628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A, Augenstein A. Amniotic allograft implantation for midface aging correction: a retrospective comparative study with platelet-rich plasma. Aesthetic Plast Surg. 2019;43(5):1345–1352. doi: 10.1007/s00266-019-01422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts PA, Pinto PC, Girão L. Autologous pure platelet-rich plasma injections for facial skin rejuvenation: Biometric instrumental evaluations and patient-reported outcomes to support antiaging effects. J Cosmet Dermatol. 2019;18(4):985–995. doi: 10.1111/jocd.12802. [DOI] [PubMed] [Google Scholar]
- Lee ZH, Sinno S, Poudrier G et al. Platelet rich plasma for photodamaged skin: a pilot study. J Cosmet Dermatol. 2019;18(1):77–83. doi: 10.1111/jocd.12676. [DOI] [PubMed] [Google Scholar]
- Fedyakova E, Pino A, Kogan L et al. An autologous protein gel for soft tissue augmentation: in vitro characterization and clinical evaluation. J Cosmet Dermatol. 2019;18(3):762–772. doi: 10.1111/jocd.12771. [DOI] [PubMed] [Google Scholar]
- Aust M, Pototschnig H, Jamchi S et al. Platelet-rich plasma for skin rejuvenation and treatment of actinic elastosis in the lower eyelid area. Cureus. 2018;10(7):e2999. doi: 10.7759/cureus.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez Gómez N, Pino Castresana A, Segurado Miravalles G Autologous platelet-rich gel for facial rejuvenation and wrinkle amelioration: a pilot study [published online ahead of print, 2018 Nov 18]. J Cosmet Dermatol. 2018. 10.1111/jocd.12823. [DOI] [PubMed]
- Elnehrawy NY, Ibrahim ZA, Eltoukhy AM et al. Assessment of the efficacy and safety of single platelet-rich plasma injection on different types and grades of facial wrinkles. J Cosmet Dermatol. 2017;16(1):103–111. doi: 10.1111/jocd.12258. [DOI] [PubMed] [Google Scholar]
- Cameli N, Mariano M, Cordone I et al. Autologous pure platelet-rich plasma dermal injections for facial skin rejuvenation: clinical, instrumental, and flow cytometry assessment. Dermatol Surg. 2017;43(6):826–835. doi: 10.1097/DSS.0000000000001083. [DOI] [PubMed] [Google Scholar]
- Cabrera-Ramírez JO, Puebla-Mora AG, González-Ojeda A et al. Platelet-rich plasma for the treatment of photodamage of the skin of the hands. Actas Dermosifiliogr. 2017;108(8):746–751. doi: 10.1016/j.ad.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Díaz-Ley B, Cuevast J, Alonso-Castro L et al. Benefits of plasma rich in growth factors (PRGF) in skin photodamage: clinical response and histological assessment. Dermatol Ther. 2015;28(4):258–263. doi: 10.1111/dth.12228. [DOI] [PubMed] [Google Scholar]
- Mehryan P, Zartab H, Rajabi A et al. Assessment of efficacy of platelet-rich plasma (PRP) on infraorbital dark circles and crow's feet wrinkles. J Cosmet Dermatol. 2014;13(1):72–78. doi: 10.1111/jocd.12072. [DOI] [PubMed] [Google Scholar]
- Sclafani AP. Platelet-rich fibrin matrix for improvement of deep nasolabial folds. J Cosmet Dermatol. 2010;9(1):66–71. doi: 10.1111/j.1473-2165.2010.00486.x. [DOI] [PubMed] [Google Scholar]
- Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010;9(5):466–472. [PubMed] [Google Scholar]
- Draelos ZD, Rheins LA, Wootten S et al. Pilot study: Autologous platelet-rich plasma used in a topical cream for facial rejuvenation [published online ahead of print, 2019 Jul 26]. J Cosmet Dermatol. 2019;18(5):1348–1352. doi: 10.1111/jocd.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Q, Chang P, Guo B et al. The clinical efficacy of autologous platelet-rich plasma combined with ultra-pulsed fractional CO2 laser therapy for facial rejuvenation. Rejuvenation Res. 2017;20(1):25–31. doi: 10.1089/rej.2016.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Gallo J. Evaluation of the effect of platelet-rich plasma on recovery after ablative fractional photothermolysis [published correction appears in JAMA Facial Plast Surg. [2015; 17:(2)155]. JAMA Facial Plast Surg. 2015;17(2):97–102. doi: 10.1001/jamafacial.2014.1085. [DOI] [PubMed] [Google Scholar]
- Shin MK, Lee JH, Lee SJ et al. Platelet-rich plasma combined with fractional laser therapy for skin rejuvenation. Dermatol Surg. 2012;38(4):623–630. doi: 10.1111/j.1524-4725.2011.02280.x. [DOI] [PubMed] [Google Scholar]
- Na JI, Choi JW, Choi HR et al. Rapid healing and reduced erythema after ablative fractional carbon dioxide laser resurfacing combined with the application of autologous platelet-rich plasma. Dermatol Surg. 2011;37(4):463–468. doi: 10.1111/j.1524-4725.2011.01916.x. [DOI] [PubMed] [Google Scholar]
- Araco A. A prospective study comparing topic platelet-rich plasma vs. placebo on reducing superficial perioral wrinkles and restore dermal matrix. J Cosmet Laser Ther. 2019;21(6):309–315. doi: 10.1080/14764172.2019.1605448. [DOI] [PubMed] [Google Scholar]
- El-Domyati M, Abdel-Wahab H, Hossam A. Combining microneedling with other minimally invasive procedures for facial rejuvenation: a split-face comparative study. Int J Dermatol. 2018;57(11):1324–1334. doi: 10.1111/ijd.14172. [DOI] [PubMed] [Google Scholar]
- El-Taieb MA, Ibrahim HM, Hegazy EM et al. Fractional erbium-YAG laser and platelet-rich plasma as single or combined treatment for atrophic acne scars: a randomized clinical trial. Dermatol Ther (Heidelb). 2019;9(4):707–717. doi: 10.1007/s13555-019-00318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal O, Tawfik AA, Abdalla N et al. Fractional CO2 laser versus combined platelet-rich plasma and fractional CO2 laser in treatment of acne scars: image analysis system evaluation. J Cosmet Dermatol. 2019;18(6):1665–1671. doi: 10.1111/jocd.12909. [DOI] [PubMed] [Google Scholar]
- Min S, Yoon JY, Park SY et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50(4):302–310. doi: 10.1002/lsm.22776. [DOI] [PubMed] [Google Scholar]
- Faghihi G, Keyvan S, Asilian A et al. Efficacy of autologous platelet-rich plasma combined with fractional ablative carbon dioxide resurfacing laser in treatment of facial atrophic acne scars: a split-face randomized clinical trial. Indian J Dermatol Venereol Leprol. 2016;82(2):162–168. doi: 10.4103/0378-6323.174378. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kim BJ, Kim MN et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37(7):931–938. doi: 10.1111/j.1524-4725.2011.01999.x. [DOI] [PubMed] [Google Scholar]
- Makki M, Younes AEKH, Fathy A et al. Efficacy of platelet-rich plasma plus fractional carbon dioxide laser in treating posttraumatic scars. Dermatol Ther. 2019;32(5):e13031. doi: 10.1111/dth.13031. [DOI] [PubMed] [Google Scholar]
- Abdel Aal AM, Ibrahim IM, Sami NA et al. Evaluation of autologous platelet-rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20(2):106–113. doi: 10.1080/14764172.2017.1368667. [DOI] [PubMed] [Google Scholar]
- Gawdat HI, Hegazy RA, Fawzy MM et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars [published correction appears in Dermatol Surg. 2014;40(5):601]. Dermatol Surg. 2014;40(2):152–161. doi: 10.1111/dsu.12392. [DOI] [PubMed] [Google Scholar]
- Abdel-Maguid EM, Awad SM, Hassan YS Efficacy of stem cell-conditioned medium vs. platelet-rich plasma as an adjuvant to ablative fractional CO2 laser resurfacing for atrophic post-acne scars: a split-face clinical trial [published online ahead of print, 2019 Jul 5]. J Dermatolog Treat. 2019. pp. 1–8. [DOI] [PubMed]
- Kar BR, Raj C. Fractional CO2 laser vs fractional CO2 with topical platelet-rich plasma in the treatment of acne scars: a split-face comparison trial. J Cutan Aesthet Surg. 2017;10(3):136–144. doi: 10.4103/JCAS.JCAS_99_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porwal S, Chahar YS, Singh PK. A comparative study of combined dermaroller and platelet-rich plasma versus dermaroller alone in acne scars and assessment of quality of life before and after treatment. Indian J Dermatol. 2018;63(5):403–408. doi: 10.4103/ijd.IJD_118_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim ZA, El-Ashmawy AA, Shora OA. Therapeutic effect of microneedling and autologous platelet-rich plasma in the treatment of atrophic scars: a randomized study. J Cosmet Dermatol. 2017;16(3):388–399. doi: 10.1111/jocd.12356. [DOI] [PubMed] [Google Scholar]
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15(4):434–443. doi: 10.1111/jocd.12207. [DOI] [PubMed] [Google Scholar]
- Darmawan H, Kurniawati Y. Split-face comparative study of microneedling with platelet-rich plasma versus microneedling alone in treating acne scars. Skinmed. 2019;17(3):207–209. [PubMed] [Google Scholar]
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison. J Cosmet Dermatol. 2018;17(1):73–83. doi: 10.1111/jocd.12459. [DOI] [PubMed] [Google Scholar]
- Nofal E, Helmy A, Nofal A et al. Platelet-rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: a comparative study. Dermatol Surg. 2014;40(8):864–873. doi: 10.1111/dsu.0000000000000091. [DOI] [PubMed] [Google Scholar]
- Bhargava S, Kroumpouzos G, Varma K et al. Combination therapy using subcision, needling, and platelet-rich plasma in the management of grade 4 atrophic acne scars: a pilot study. J Cosmet Dermatol. 2019;18(4):1092–1097. doi: 10.1111/jocd.12935. [DOI] [PubMed] [Google Scholar]
- Hassan AS, El-Hawary MS, Abdel Raheem HM et al. Treatment of atrophic acne scars using autologous platelet-rich plasma vs combined subcision and autologous platelet-rich plasma: A split-face comparative study. J Cosmet Dermatol. 2020;19(2):456–461. doi: 10.1111/jocd.13048. [DOI] [PubMed] [Google Scholar]
- Deshmukh NS, Belgaumkar VA. Platelet-rich plasma augments subcision in atrophic acne scars: a split-face comparative study. Dermatol Surg. 2019;45(1):90–98. doi: 10.1097/DSS.0000000000001614. [DOI] [PubMed] [Google Scholar]
- Ahmed NA, Mostafa OM. Comparative study between: Carboxytherapy, platelet-rich plasma, and tripolar radiofrequency, their efficacy and tolerability in striae distensae. J Cosmet Dermatol. 2019;18(3):788–797. doi: 10.1111/jocd.12685. [DOI] [PubMed] [Google Scholar]
- Ibrahim ZA, El-Tatawy RA, El-Samongy MA et al. Comparison between the efficacy and safety of platelet-rich plasma vs. microdermabrasion in the treatment of striae distensae: clinical and histopathological study. J Cosmet Dermatol. 2015;14(4):336–346. doi: 10.1111/jocd.12160. [DOI] [PubMed] [Google Scholar]
- Gamil HD, Ibrahim SA, Ebrahim HM et al. Platelet-rich plasma versus tretinoin in treatment of striae distensae: a comparative study. Dermatol Surg. 2018;44(5):697–704. doi: 10.1097/DSS.0000000000001408. [DOI] [PubMed] [Google Scholar]
- Hodeib AA, Hassan GFR, Ragab MNM et al. Clinical and immunohistochemical comparative study of the efficacy of carboxytherapy vs platelet-rich plasma in treatment of stretch marks. J Cosmet Dermatol. 2018;17(6):1008–1015. doi: 10.1111/jocd.12481. [DOI] [PubMed] [Google Scholar]
- Hewedy ES, Sabaa BEI, Mohamed WS Combined intralesional triamcinolone acetonide and platelet rich plasma versus intralesional triamcinolone acetonide alone in treatment of keloids [published online ahead of print, 2020 Mar 4]. J Dermatolog Treat. 2020. pp. 1–7. [DOI] [PubMed]
- Albalat W, Ebrahim HM. Evaluation of platelet-rich plasma vs intralesional steroid in treatment of alopecia areata [published online ahead of print, 2019 May 10]. J Cosmet Dermatol. 2019. 10.1111/jocd.12858. [DOI] [PubMed]
- El Taieb MA, Ibrahim H, Nada EA et al. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther. 2017;30(1):e12437. doi: 10.1111/dth.12437. [DOI] [PubMed] [Google Scholar]
- Trink A, Sorbellini E, Bezzola P et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169(3):690–694. doi: 10.1111/bjd.12397. [DOI] [PubMed] [Google Scholar]
- Singh S. Role of platelet-rich plasma in chronic alopecia areata: our centre experience. Indian J Plast Surg. 2015;48(1):57–59. doi: 10.4103/0970-0358.155271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina Y, Aguh C. Use of platelet-rich plasma in cicatricial alopecia. Dermatol Surg. 2019;45(7):979–981. doi: 10.1097/DSS.0000000000001635. [DOI] [PubMed] [Google Scholar]
- Kadry M, Tawfik A, Abdallah N et al. Platelet-rich plasma versus combined fractional carbon dioxide laser with platelet-rich plasma in the treatment of vitiligo: a comparative study. Clin Cosmet Investig Dermatol. 2018;11:551–559. doi: 10.2147/CCID.S178817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelghani R, Ahmed NA, Darwish HM. Combined treatment with fractional carbon dioxide laser, autologous platelet-rich plasma, and narrow band ultraviolet B for vitiligo in different body sites: a prospective, randomized comparative trial. J Cosmet Dermatol. 2018;17(3):365–372. doi: 10.1111/jocd.12397. [DOI] [PubMed] [Google Scholar]
- Khattab FM, Abdelbary E, Fawzi M. Evaluation of combined excimer laser and platelet-rich plasma for the treatment of nonsegmental vitiligo: a prospective comparative study. J Cosmet Dermatol. 2020;19(4):869–877. doi: 10.1111/jocd.13103. [DOI] [PubMed] [Google Scholar]
- Ibrahim ZA, El-Ashmawy AA, El-Tatawy RA et al. The effect of platelet-rich plasma on the outcome of short-term narrowband-ultraviolet B phototherapy in the treatment of vitiligo: a pilot study. J Cosmet Dermatol. 2016;15(2):108–116. doi: 10.1111/jocd.12194. [DOI] [PubMed] [Google Scholar]
- Sirithanabadeekul P, Dannarongchai A, Suwanchinda A. Platelet-rich plasma treatment for melasma: a pilot study. J Cosmet Dermatol. 2020;19(6):1321–1327. doi: 10.1111/jocd.13157. [DOI] [PubMed] [Google Scholar]
- Nofal E, Elkot R, Nofal A et al. Evaluation of carboxytherapy and platelet-rich plasma in treatment of periorbital hyperpigmentation: a comparative clinical trial. J Cosmet Dermatol. 2018;17(6):1000–1007. doi: 10.1111/jocd.12478. [DOI] [PubMed] [Google Scholar]
- Goldstein AT, Mitchell L, Govind V et al. A randomized double-blind placebo-controlled trial of autologous platelet-rich plasma intradermal injections for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2019;80(6):1788–1789. doi: 10.1016/j.jaad.2018.12.060. [DOI] [PubMed] [Google Scholar]
- Tedesco M, Garelli V, Elia F et al. Usefulness of video thermography in the evaluation of platelet-rich plasma effectiveness in vulvar lichen sclerosus: preliminary study. J Dermatolog Treat. 2021;32(5):568–571. doi: 10.1080/09546634.2019.1685644. [DOI] [PubMed] [Google Scholar]
- Tedesco M, Pranteda G, Chichierchia G et al. The use of PRP (platelet-rich plasma) in patients affected by genital lichen sclerosus: clinical analysis and results. J Eur Acad Dermatol Venereol. 2019;33(2):e58–e59. doi: 10.1111/jdv.15190. [DOI] [PubMed] [Google Scholar]
- Casabona F, Gambelli I, Casabona F et al. Autologous platelet-rich plasma (PRP) in chronic penile lichen sclerosus: the impact on tissue repair and patient quality of life. Int Urol Nephrol. 2017;49(4):573–580. doi: 10.1007/s11255-017-1523-0. [DOI] [PubMed] [Google Scholar]
- Goldstein AT, King M, Runels C et al. Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2017;76(1):158–160. doi: 10.1016/j.jaad.2016.07.037. [DOI] [PubMed] [Google Scholar]
- Behnia-Willison F, Pour NR, Mohamadi B et al. Use of platelet-rich plasma for vulvovaginal autoimmune conditions like lichen sclerosus. Plast Reconstr Surg Glob Open. 2016;4(11):e1124. doi: 10.1097/GOX.0000000000001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjayani S, Wirohadidjojo YW, Adam AM et al. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet-rich plasma. Int J Dermatol. 2014;53(1):109–113. doi: 10.1111/ijd.12162. [DOI] [PubMed] [Google Scholar]
- Chakravdhanula U, Anbarasu K, Verma VK et al. Clinical efficacy of platelet rich plasma in combination with methotrexate in chronic plaque psoriatic patients. Dermatol Ther. 2016;29(6):446–450. doi: 10.1111/dth.12388. [DOI] [PubMed] [Google Scholar]
- Piao L, Park H, Jo CH. Theoretical prediction and validation of cell recovery rates in preparing platelet-rich plasma through a centrifugation. PLoS One. 2017;12(11):e0187509. doi: 10.1371/journal.pone.0187509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JH, Roberts KC. Database review of possible factors influencing point-of-care platelet gel manufacture. J Extra Corpor Technol. 2004;36(3):250–254. [PubMed] [Google Scholar]
- Alsousou J, Thompson M, Hulley P et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8):987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- Harrison P. Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1895–1900. doi: 10.1111/jth.14223. [DOI] [PubMed] [Google Scholar]
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297–301. doi: 10.1016/s0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118(6):147e–159e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- Perez AG, Lana JF, Rodrigues AA et al. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014;2014:176060. doi: 10.1155/2014/176060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugrillon A, Eichler H, Kern S et al. Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg. 2002;31(6):615–619. doi: 10.1054/ijom.2002.0322. [DOI] [PubMed] [Google Scholar]
- Yin W, Xu H, Sheng J et al. Optimization of pure platelet-rich plasma preparation: a comparative study of pure platelet-rich plasma obtained using different centrifugal conditions in a single-donor model. Exp Ther Med. 2017;14(3):2060–2070. doi: 10.3892/etm.2017.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JH, Kim W, Park KU et al. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med. 2015;43(12):3062–3070. doi: 10.1177/0363546515608481. [DOI] [PubMed] [Google Scholar]
- Nagata MJ, Messora MR, Furlaneto FA et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: an experimental study in rabbits. Eur J Dent. 2010;4(4):395–402. [PMC free article] [PubMed] [Google Scholar]
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Kawazoe T, Kim HH. Tissue augmentation by white blood cell-containing platelet-rich plasma. Cell Transplant. 2012;21:2–3. 601–607. doi: 10.3727/096368911X605538. [DOI] [PubMed] [Google Scholar]
- Saad Setta H, Elshahat A, Elsherbiny K et al. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8(3):307–312. doi: 10.1111/j.1742-481X.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykberg RG, Driver VR, Carman D et al. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: a prospective case series. Ostomy Wound Manage. 2010;56(6):36–44. [PubMed] [Google Scholar]
- Rappl LM. Effect of platelet rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord injury. Int Wound J. 2011;8(2):187–195. doi: 10.1111/j.1742-481X.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki T, Dohan Ehrenfest DM, Everts PA et al. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol. 2012;13(7):1153–1162. doi: 10.2174/138920112800624373. [DOI] [PubMed] [Google Scholar]
- Edelblute CM, Donate AL, Hargrave BY et al. Human platelet gel supernatant inactivates opportunistic wound pathogens on skin. Platelets. 2015;26(1):13–16. doi: 10.3109/09537104.2013.863859. [DOI] [PubMed] [Google Scholar]
- Hou C, Miao Y, Wang X et al. Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in the hair cycle. Exp Ther Med. 2016;12(1):231–237. doi: 10.3892/etm.2016.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifer MA, Maerz T, Baker KC et al. Matrix metalloproteinase content and activity in low-platelet, low-leukocyte and high-platelet, high-leukocyte platelet rich plasma (PRP) and the biologic response to PRP by human ligament fibroblasts. Am J Sports Med. 2014;42(5):1211–1218. doi: 10.1177/0363546514524710. [DOI] [PubMed] [Google Scholar]
- Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang J, Wu H et al. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells—implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6(1):173. doi: 10.1186/s13287-015-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschi RS, Hashem AM, Halasa B et al. Current evidence for clinical efficacy of platelet rich plasma in aesthetic surgery: a systematic review. Aesthet Surg J. 2017;37(3):353–362. doi: 10.1093/asj/sjw178. [DOI] [PubMed] [Google Scholar]
- Harrison S, Vavken P, Kevy S et al. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med. 2011;39(4):729–734. doi: 10.1177/0363546511401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufa D, Shealy B, Jacobson M et al. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66(4):684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008;22(6):432–438. doi: 10.1097/BOT.0b013e31817e793f. [DOI] [PubMed] [Google Scholar]
- Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107(1):229–239. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- Hausauer AK, Humphrey S. The physician's guide to platelet-rich plasma in dermatologic surgery part I: definitions, mechanisms of action, and technical specifications. Dermatol Surg. 2020;46(3):348–357. doi: 10.1097/DSS.0000000000002147. [DOI] [PubMed] [Google Scholar]
- Gentile P, Garcovich S. Autologous activated platelet-rich plasma (AA-PRP) and non-activated (A-PRP) in hair growth: a retrospective, blinded, randomized evaluation in androgenetic alopecia. Expert Opin Biol Ther. 2020;20(3):327–337. doi: 10.1080/14712598.2020.1724951. [DOI] [PubMed] [Google Scholar]
- Hosny N, Goubran F, Badr-Eldin Hasan B et al. Assessment of vascular endothelial growth factor in fresh versus frozen platelet rich plasma. J Blood Transfus. 2015;2015:706903. doi: 10.1155/2015/706903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Yong Z, Yuk KS et al. Growth factor release from lyophilized porcine platelet-rich plasma: quantitative analysis and implications for clinical applications. Aesthetic Plast Surg. 2016;40(1):157–163. doi: 10.1007/s00266-015-0580-y. [DOI] [PubMed] [Google Scholar]
- da Silva LQ, Huber SC, De Lima Montalvão SA et al. Platelet activation Is not crucial for platelet-rich plasma (PRP), when used as autologous therapeutic product, and could be lyophilized without any growth factor loss. Blood. 2016;128(22):2639. [Google Scholar]
- Abuaf OK, Yildiz H, Baloglu H et al. Histologic evidence of new collagen formulation using platelet rich plasma in skin rejuvenation: a prospective controlled clinical study. Ann Dermatol. 2016;28(6):718–724. doi: 10.5021/ad.2016.28.6.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki GH. Micro-needling depth penetration, presence of pigment particles, and fluorescein-stained platelets: clinical usage for aesthetic concerns. Aesthet Surg J. 2017;37(1):71–83. doi: 10.1093/asj/sjw120. [DOI] [PubMed] [Google Scholar]
- Dhurat R, Sharma A, Goren A et al. Mission impossible: dermal delivery of growth factors via microneedling. Dermatol Ther. 2019;32(3):e12897. doi: 10.1111/dth.12897. [DOI] [PubMed] [Google Scholar]
- Kim DH, Je YJ, Kim CD et al. Can platelet-rich plasma be used for skin rejuvenation? evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23(4):424–431. doi: 10.5021/ad.2011.23.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HX, Lin C, Lin BB et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS One. 2013;8(4):e59966. doi: 10.1371/journal.pone.0059966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersant B, Sid-Ahmed-Mezi M, Picard F et al. Efficacy of autologous platelet concentrates as adjuvant therapy to surgical excision in the treatment of keloid scars refractory to conventional treatments: a pilot prospective study. Ann Plast Surg. 2018;81(2):170–175. doi: 10.1097/SAP.0000000000001448. [DOI] [PubMed] [Google Scholar]
- Azzam EZ, Omar SS. Treatment of auricular keloids by triple combination therapy: surgical excision, platelet-rich plasma, and cryosurgery. J Cosmet Dermatol. 2018;17(3):502–510. doi: 10.1111/jocd.12552. [DOI] [PubMed] [Google Scholar]
- Jones ME, McLane J, Adenegan R et al. Advancing keloid treatment: a novel multimodal approach to Ear Keloids. Dermatol Surg. 2017;43(9):1164–1169. doi: 10.1097/DSS.0000000000001145. [DOI] [PubMed] [Google Scholar]
- Jones ME, Hardy C, Ridgway J. Keloid management: a retrospective case review on a new approach using surgical excision, platelet-rich plasma, and in-office superficial photon X-ray radiation therapy. Adv Skin Wound Care. 2016;29(7):303–307. doi: 10.1097/01.ASW.0000482993.64811.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumeyer A, Tosti A, Messenger A Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011. pp. S1–S57. [DOI] [PubMed]
- Gupta AK, Carviel J. A mechanistic model of platelet-rich plasma treatment for androgenetic alopecia. Dermatol Surg. 2016;42(12):1335–1339. doi: 10.1097/DSS.0000000000000901. [DOI] [PubMed] [Google Scholar]
- Cervelli V, Garcovich S, Bielli A The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014. 2014. p. 760709. [DOI] [PMC free article] [PubMed]
- Li ZJ, Choi HI, Choi DK Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012. pp. 1040–1046. 38(7 Pt 1) [DOI] [PubMed]
- Hesseler MJ, Shyam N. Platelet-rich plasma and its utilities in alopecia: a systematic review. Dermatol Surg. 2020;46(1):93–102. doi: 10.1097/DSS.0000000000001965. [DOI] [PubMed] [Google Scholar]
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44(9):1191–1200. doi: 10.1097/DSS.0000000000001567. [DOI] [PubMed] [Google Scholar]
- Ferrando J, García-García SC, González-de-Cossío AC et al. A proposal of an effective platelet-rich plasma protocol for the treatment of androgenetic alopecia. Int J Trichology. 2017;9(4):165–170. doi: 10.4103/ijt.ijt_27_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emer J. Platelet-rich plasma (PRP): current applications in dermatology. Skin Therapy Lett. 2019;24(5):1–6. [PubMed] [Google Scholar]
- Hausauer AK, Humphrey S. The physician's guide to platelet-rich plasma in dermatologic surgery part II: clinical evidence. Dermatol Surg. 2020;46(4):447–456. doi: 10.1097/DSS.0000000000002148. [DOI] [PubMed] [Google Scholar]
- Stevens J, Khetarpal S. Platelet-rich plasma for androgenetic alopecia: a review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2018;5(1):46–51. doi: 10.1016/j.ijwd.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanča Ž, Goren A, Getaldić-Švarc B et al. Platelet-rich plasma as a novel treatment for lichen planopillaris. Dermatol Ther. 2016;29(4):233–235. doi: 10.1111/dth.12343. [DOI] [PubMed] [Google Scholar]
- Jha AK. Platelet-rich plasma as an adjunctive treatment in lichen planopilaris. J Am Acad Dermatol. 2019;80(5):e109–e110. doi: 10.1016/j.jaad.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Jha AK. Platelet-rich plasma for the treatment of lichen planopilaris. J Am Acad Dermatol. 2018. 79 5 e95-e96 [DOI] [PubMed] [Google Scholar]
- Özcan D, Tunçer Vural A, Özen Ö. Platelet-rich plasma for treatment resistant frontal fibrosing alopecia: a case report. Dermatol Ther. 2019;32(5):e13072. doi: 10.1111/dth.13072. [DOI] [PubMed] [Google Scholar]
- Martínez-Esparza M, Jiménez-Cervantes C, Beermann F et al. Transforming growth factor-beta1 inhibits basal melanogenesis in B16/F10 mouse melanoma cells by increasing the rate of degradation of tyrosinase and tyrosinase-related protein-1. J Biol Chem. 1997;272(7):3967–3972. doi: 10.1074/jbc.272.7.3967. [DOI] [PubMed] [Google Scholar]
- Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol. 2004;36(8):1482–1491. doi: 10.1016/j.biocel.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Wang Y, Viennet C, Robin S et al. Precise role of dermal fibroblasts on melanocyte pigmentation. J Dermatol Sci. 2017;88(2):159–166. doi: 10.1016/j.jdermsci.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35(2):193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejjiyar M, Sahibi M, El Gueouatri M et al. Vitiligo et phénomène de Koebner suite à des injections de plasma riche en plaquettes [Vitiligo and Koebner phenomenon following platelet-rich plasma injections]. Pan Afr Med J. 2019;32:58. doi: 10.11604/pamj.2019.32.58.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JX, Chowdhury SR, Saim AB et al. Platelet-rich plasma with keratinocytes and fibroblasts enhance healing of full-thickness wounds. J Tissue Viability. 2017;26(3):208–215. doi: 10.1016/j.jtv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Volakakis E, Papadakis M, Manios A et al. Platelet-rich plasma improves healing of pressure ulcers as objectively assessed by digital planimetry. Wounds. 2019;31(10):252–256. [PubMed] [Google Scholar]
- Ramos-Torrecillas J, García-Martínez O, De Luna-Bertos E et al. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol Res Nurs. 2015;17(2):152–158. doi: 10.1177/1099800414535840. [DOI] [PubMed] [Google Scholar]
- Li T, Ma Y, Wang M et al. Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds. Infect Drug Resist. 2019;12:297–309. doi: 10.2147/IDR.S186651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver VR, Hanft J, Fylling CP et al. Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):68–87. [PubMed] [Google Scholar]
- Akingboye AA, Giddins S, Gamston P et al. Application of autologous derived-platelet rich plasma gel in the treatment of chronic wound ulcer: diabetic foot ulcer. J Extra Corpor Technol. 2010;42(1):20–29. [PMC free article] [PubMed] [Google Scholar]
- Miłek T, Nagraba Ł, Mitek T et al. Autologous platelet-rich plasma reduces healing time of chronic venous leg ulcers: a prospective observational study. Adv Exp Med Biol. 2019;1176:109–117. doi: 10.1007/5584_2019_388. [DOI] [PubMed] [Google Scholar]
- Iervolino V, Di Costanzo G, Azzaro R et al. Platelet gel in cutaneous radiation dermatitis. Support Care Cancer. 2013;21(1):287–293. doi: 10.1007/s00520-012-1635-0. [DOI] [PubMed] [Google Scholar]
- Saha S, Patra AC, Gowda SP et al. Effectiveness and safety of autologous platelet-rich plasma therapy with total contact casting versus total contact casting alone in treatment of trophic ulcer in leprosy: An observer-blind, randomized controlled trial. Indian J Dermatol Venereol Leprol. 2020;86(3):262–271. doi: 10.4103/ijdvl.IJDVL_571_18. [DOI] [PubMed] [Google Scholar]
- Conde-Montero E, Horcajada-Reales C, Clavo P et al. Neuropathic ulcers in leprosy treated with intralesional platelet-rich plasma. Int Wound J. 2016;13(5):726–728. doi: 10.1111/iwj.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KH, Shin MK, Kim NI. Refractory lipodermatosclerosis treated with intralesional platelet-rich plasma. J Am Acad Dermatol. 2011;65(5):e157–e158. doi: 10.1016/j.jaad.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Motolese A, Vignati F, Antelmi A et al. Effectiveness of platelet-rich plasma in healing necrobiosis lipoidica diabeticorum ulcers. Clin Exp Dermatol. 2015;40(1):39–41. doi: 10.1111/ced.12474. [DOI] [PubMed] [Google Scholar]
- Giuggioli D, Colaci M, Manfredi A et al. Platelet gel in the treatment of severe scleroderma skin ulcers. Rheumatol Int. 2012;32(9):2929–2932. doi: 10.1007/s00296-011-2038-0. [DOI] [PubMed] [Google Scholar]
- Shetty S, Shenoi SD. Autologous platelet-rich fibrin in treatment of scleroderma ulcer. Int Wound J. 2016;13(5):1065–1066. doi: 10.1111/iwj.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru H, Kajihara I, Yamanaka K et al. Platelet-rich plasma therapy is effective for the treatment of refractory skin ulcers in patients with systemic sclerosis. Mod Rheumatol. 2015;25(4):660–661. doi: 10.3109/14397595.2014.924204. [DOI] [PubMed] [Google Scholar]
- El-Komy MHM, Saleh NA, Saleh MA. Autologous platelet-rich plasma and triamcinolone acetonide intralesional injection in the treatment of oral erosions of pemphigus vulgaris: a pilot study. Arch Dermatol Res. 2018;310(4):375–381. doi: 10.1007/s00403-018-1824-x. [DOI] [PubMed] [Google Scholar]
- El-Komy MH, Hassan AS, Abdel Raheem HM et al. Platelet-rich plasma for resistant oral erosions of pemphigus vulgaris: a pilot study. Wound Repair Regen. 2015;23(6):953–955. doi: 10.1111/wrr.12363. [DOI] [PubMed] [Google Scholar]
- Picardi A, Ferraro AS, Miranda M et al. Therapeutic efficiency of platelet gel for the treatment of oral ulcers related to chronic graft versus host disease after allogeneic haematopoietic stem cell transplantation. Oral Implantol (Rome). 2017;10(4):398–405. doi: 10.11138/orl/2017.10.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loré B, Saraceno R, Poladas G et al. Oral lichen planus: therapy and phenotype. G Ital Dermatol Venereol. 2018;153(4):459–463. doi: 10.23736/S0392-0488.16.05399-2. [DOI] [PubMed] [Google Scholar]
- Budamakuntla L, Suryanarayan S, Sarvajnamurthy SS et al. Autologous platelet rich plasma in pyoderma gangrenosum—two case reports. Indian J Dermatol. 2015;60(2):204–205. doi: 10.4103/0019-5154.152539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Lopez MA, Burón-Alvarez I, Villegas-Fernández C. Refractory pyoderma gangrenosum treated with platelet-rich plasma. J Eur Acad Dermatol Venereol. 2016;30(8):1423–1424. doi: 10.1111/jdv.13265. [DOI] [PubMed] [Google Scholar]
- Fortunato L, Barone S, Bennardo F, Giudice A. Management of facial pyoderma gangrenosum using platelet-rich fibrin: A Technical Report. J Oral Maxillofac Surg. 2018;76(7):1460–1463. doi: 10.1016/j.joms.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Rajan M B, Singh S. Utility of platelet rich fibrin gel therapy in nonhealing ulcer secondary to ecthyma gangrenosum. Dermatol Ther. 2019;32(3):e12887. doi: 10.1111/dth.12887. [DOI] [PubMed] [Google Scholar]
- Chen D, Wang C, Cui L, Ran X. Autologous Platelet-Rich Gel Treatment of Chronic Nonhealing Ulcerated Tophaceous Gout. Indian J Dermatol. 2020;65(2):141–144. doi: 10.4103/ijd.IJD_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik-Bielecka A, Skowroński R, Jędrusik-Pawłowska M, Pierchała M. The application of L-PRP in AIDS patients with crural chronic ulcers: a pilot study. Adv Med Sci. 2018;63(1):140–146. doi: 10.1016/j.advms.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Takeuchi S, Okano T et al. Therapeutic effect of autologous platelet-rich plasma (PRP) on recalcitrant cutaneous ulcers in livedoid vasculopathy. JAAD Case Rep. 2015;1(5):310–311. doi: 10.1016/j.jdcr.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao DL, Zhao YQ, Li ZY. Effectiveness of platelet rich plasma in burn wound healing: a systematic review and meta-analysis [published online ahead of print, 2020 Feb 21]. J Dermatolog Treat. 2020. pp. 1–7. [DOI] [PubMed]
- Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;5:CD006899. doi: 10.1002/14651858.CD006899.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic O, Morrison B, Tosti A. Adverse effects of platelet-rich plasma and microneedling. J Am Acad Dermatol. 2020;82(2):501–502. doi: 10.1016/j.jaad.2019.07.109. [DOI] [PubMed] [Google Scholar]
- Ayatollahi A, Hosseini H, Shahdi M et al. Platelet-rich plasma by single spin process in male pattern androgenetic alopecia: Is it an effective treatment?. Indian Dermatol Online J. 2017;8(6):460–464. doi: 10.4103/idoj.IDOJ_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geizhals S, Grunfeld J, Kwon H et al. Cervical lymphadenopathy from PRP treatment with microneedling therapy. J Cosmet Dermatol. 2019;18(3):910–911. doi: 10.1111/jocd.12772. [DOI] [PubMed] [Google Scholar]
- Owczarczyk-Saczonek A, Wygonowska E, Budkiewicz M et al. Serum sickness disease in a patient with alopecia areata and Meniere' disease after PRP procedure. Dermatol Ther. 2019;32(2):e12798. doi: 10.1111/dth.12798. [DOI] [PubMed] [Google Scholar]
- Latalski M, Walczyk A, Fatyga M et al. Allergic reaction to platelet-rich plasma (PRP): case report. Medicine (Baltimore). 2019;98(10):e14702. doi: 10.1097/MD.0000000000014702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhakoff N, Ojong O, Ilyas M et al. Platelet-rich plasma injections and the development of cutaneous sarcoid lesions: a case report. JAAD Case Rep. 2020;6(4):348–350. doi: 10.1016/j.jdcr.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa N, Funasaka Y, Goto H et al. Platelet-Rich Plasma Injection and Cutaneous Sarcoidal Granulomas. Ann Dermatol. 2017;29(2):239–241. doi: 10.5021/ad.2017.29.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyam K, Kavoussi SC, Ehrlich M et al. Irreversible blindness following periocular autologous platelet-rich plasma skin rejuvenation treatment. Ophthalmic Plast Reconstr Surg. 2017;33(3S Suppl 1):S12–S16. doi: 10.1097/IOP.0000000000000680. [DOI] [PubMed] [Google Scholar]
- Foster TE, Puskas BL, Mandelbaum BR et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- Dauendorffer JN, Fraitag S, Dupuy A. Carcinome basocellulaire après réjuvénation par injection de plasma autologue riche en plaquettes [Basal cell carcinoma following platelet-rich plasma injection for skin rejuvenation]. Ann Dermatol Venereol. 2013;140(11):723–724. doi: 10.1016/j.annder.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Bulam H, Ayhan S, Sezgin B et al. The Inhibitory Effect of Platelet-Rich Plasma on Botulinum Toxin Type-A: An Experimental Study in Rabbits. Aesthetic Plast Surg. 2015;39(1):134–140. doi: 10.1007/s00266-014-0418-z. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Azma K, Tavana B et al. Autologous blood versus corticosteroid local injection in the short-term treatment of lateral elbow tendinopathy: a randomized clinical trial of efficacy. Am J Phys Med Rehabil. 2010;89(8):660–667. doi: 10.1097/PHM.0b013e3181ddcb31. [DOI] [PubMed] [Google Scholar]
- Krogh TP, Fredberg U, Stengaard-Pedersen K et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625–635. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- Gosens T, Peerbooms JC, van Laar W et al. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- de Vos RJ, Weir A, van Schie HT et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Ito A. A new economic method for preparing platelet-rich plasma. Plast Reconstr Surg Glob Open. 2014;2(6):e162. doi: 10.1097/GOX.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan MB, Shofer FS, Catalfamo JL. Effects of anticoagulant on pH, ionized calcium concentration, and agonist-induced platelet aggregation in canine platelet-rich plasma. Am J Vet Res. 2009;70(4):472–477. doi: 10.2460/ajvr.70.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo C, Roffi A, Grigolo B et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016;2016:6591717. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]