Abstract
Alterations in regional subcortical brain volumes have been investigated as part of the efforts of an international consortium, ENIGMA, to identify reliable neural correlates of major depressive disorder (MDD). Given that subcortical structures are comprised of distinct subfields, we sought to build significantly from prior work by precisely mapping localized MDD‐related differences in subcortical regions using shape analysis. In this meta‐analysis of subcortical shape from the ENIGMA‐MDD working group, we compared 1,781 patients with MDD and 2,953 healthy controls (CTL) on individual measures of shape metrics (thickness and surface area) on the surface of seven bilateral subcortical structures: nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus. Harmonized data processing and statistical analyses were conducted locally at each site, and findings were aggregated by meta‐analysis. Relative to CTL, patients with adolescent‐onset MDD (≤ 21 years) had lower thickness and surface area of the subiculum, cornu ammonis (CA) 1 of the hippocampus and basolateral amygdala (Cohen's d = −0.164 to −0.180). Relative to first‐episode MDD, recurrent MDD patients had lower thickness and surface area in the CA1 of the hippocampus and the basolateral amygdala (Cohen's d = −0.173 to −0.184). Our results suggest that previously reported MDD‐associated volumetric differences may be localized to specific subfields of these structures that have been shown to be sensitive to the effects of stress, with important implications for mapping treatments to patients based on specific neural targets and key clinical features.
Keywords: amygdala, ENIGMA, hippocampus, major depressive disorder (MDD), nucleus accumbens, shape analysis
1. INTRODUCTION
Major depressive disorder (MDD) is one of the leading causes of disability worldwide, with relatively high rates of lifetime prevalence and recurrence (World Health Organization, 2017). MDD is often triggered by stressful experiences and is commonly associated with various affective symptoms (e.g., abnormalities in emotion regulation, reduced motivation in the face of positive incentives, sustained experiences of negative affect; Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Woody & Gibb, 2015), as well as with cognitive deficits (e.g., attention, learning, working memory, processing speed, motor functioning; McIntyre et al., 2013). Several subcortical regions—particularly the hippocampus, amygdala, and structures of the striatum—through their connections with one another and with cortical structures, are important for supporting a number of these cognitive and affective processes that are disturbed in MDD (Davidson et al., 2002). In a recent multisite effort, we examined morphological alterations at the level of subcortical gray matter volumes in MDD (Schmaal et al., 2016), and found lower total hippocampal volumes, mainly driven by patients with recurrent episodes and by patients with a relatively early age of onset (i.e., prior to age 21). Despite the large study sample size and homogeneous analysis protocols, no statistically significant group differences emerged for any of the other subcortical structures. It is possible, however, that such aggregate measures of volume are either insensitive to local volumetric effects or that they obscure heterogeneous local effects by averaging out more complex shape effects. In this respect, the analysis of shape parameters represents a complementary approach to volumetric analyses.
Indeed, MDD is most likely characterized by specific associations with functionally distinct subregions within the hippocampus, amygdala, striatum, and other subcortical structures (e.g., thalamus). It is important to note, however, that characterizing local patterns in subcortical surfaces has traditionally been challenging due to the lack of identifiable surface landmarks that are more common in cortical surfaces (e.g., deep sulcal patterns). Thus, the lack of detectable volumetric differences in the amygdala, caudate, putamen, and NAcc between MDD and CTL in our previous meta‐analytic study may be due to the fact that we did not use shape analyses to examine these important subdivisions (Schmaal et al., 2016). Furthermore, local variations in shape measures may provide critical insight into the anatomical relation between a subcortical structure and important clinical variables, such as illness onset and recurrence, as well as detect granular changes that may be particularly helpful in the context of monitoring intervention targets with more specificity.
To address these knowledge gaps, we conducted a multisite meta‐analytic investigation to test whether MDD patients, and whether specific subgroups of MDD based on important clinical characteristics, show differences from controls in subcortical shape. Specifically, we applied meta‐analytic models on effect sizes generated from 10 study cohorts from six different countries participating in the MDD Working Group of the international ENIGMA consortium. Each study site applied a well‐validated harmonized preprocessing pipeline and conducted statistical models on high‐resolution T1‐weighted MRIs, yielding site‐level summary statistics of volume and shape for seven bilateral subcortical regions from 1,781 patients diagnosed with MDD and 2,953 healthy controls (CTL).
Guided by findings from our prior meta‐analysis in which we reported that the most robust difference between individuals with MDD and CTL was smaller hippocampal volume (Schmaal et al., 2016), and from recent work indicating that the cornu ammonis subfields (CA) 1–4, dentate gyrus (DG), and the subiculum (SUB) are associated with exposure to aversive stressful experiences (Teicher, Anderson, & Polcari, 2012; Treadway et al., 2015) and MDD (Cao et al., 2017; Cole et al., 2010; Han, Won, Sim, & Tae, 2016; Huang et al., 2013; Roddy et al., 2018; Treadway et al., 2015), we hypothesized that patients with MDD would exhibit reductions in these hippocampal subregions. Given previously documented effects of age of illness onset and recurrence of illness on subcortical volumes (primarily the amygdala and hippocampus; Hamilton, Siemer, & Gotlib, 2008; Schmaal et al., 2016), we also sought to stratify groups according to these clinical characteristics: early (prior to age 21) versus later (after age 21) onset MDD and first‐episode versus recurrent‐episode. We also report results from additional exploratory analyses of medicated and nonmedicated patients (each compared separately to CTL) and of dimensional associations between subcortical shape and depression severity (clinician‐rated as well as self‐reported) among patients with MDD.
2. MATERIALS AND METHODS
2.1. Samples
Ten participating sites in the MDD Working Group of ENIGMA consortium (Schmaal et al., 2016, 2017; Thompson et al., 2014) applied harmonized preprocessing and statistical models on structural T1‐weighted MRIs, yielding site‐level summary statistics of subcortical volume and shape from a total of 4,734 participants (1,781 patients with MDD and 2,953 CTL). Detailed demographics, clinical characteristics, and exclusion criteria for study enrollment for each site are presented in Supporting Information Table S1. All participating sites obtained approval from their respective local institutional review boards and ethics committees. All study participants provided written consent at their local site.
2.2. Clinical variables of interest
We selected specific clinical variables of interest based on prior work demonstrating their effects on aggregate subcortical volumes in MDD (Hamilton et al., 2008; Schmaal et al., 2016). Specifically, we considered participants with earlier or adolescent onset (EO) to be those who developed their first episode at or before age 21, and participants with later or adult onset (LO) to be those who developed their first episode after age 21 (Schmaal et al., 2016). We defined recurrent‐episode MDD (RECUR) to be those who experienced more than one major depressive episode (Schmaal et al., 2016).
Because not all sites used the same depression severity scales, as a supplemental analysis we assessed the severity of depressive symptoms at the time of scan as measured by the clinician‐rated 17‐item Hamilton Depression Rating Scale (HDRS‐17; Hamilton, 1960) or the 21‐item self‐report Beck's Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and their linear associations with subcortical shape metrics for the subset of sites that had this information. Similarly, because the majority of sites did not include detailed information on lifetime medication usage, dosage, or adherence, as a supplemental analysis we also compared MDD groups on the basis of antidepressant medication usage at the time of scan.
2.3. Image processing and analysis
All participating sites collected anatomical T1‐weighted MRI brain scans locally at each site, where they were analyzed using the fully‐automated and validated segmentation software FreeSurfer version 5.3 (Fischl, 2002), with the exception of three sites which used version 5.0 or 5.1 (see Table S1). A subset of these subcortical measures has been previously published (Frodl et al., 2016; Renteria et al., 2017; Schmaal et al., 2016); however, none of these prior meta‐analyses from the ENIGMA MDD Working Group conducted shape analyses. Detailed information on the number of sites that overlap between the present investigation and the subcortical paper ENIGMA MDD (Schmaal et al., 2016) is presented in Table S2. Image acquisition parameters and software descriptions for each sample are presented in Supporting Information Table S1. The seven bilateral subcortical segmentations were: the nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus (as well as lateral ventricles and total intracranial volume, ICV). All segmentations were visually inspected for accuracy following standardized protocols (http://enigma.ini.usc.edu/protocols/imaging-protocols/).
We analyzed shape using the ENIGMA‐Shape protocol (http://enigma.usc.edu/ongoing/enigma-shape-analysis/), for which test–retest reliability has been previously validated (Hibar et al., 2015). Briefly, shapes were extracted using the FreeSurfer 5.3 parcellation, followed by a topological correction and mild smoothing based on the topology‐preserving level set algorithm (Gutman et al., 2015). As in prior work, after registering shapes to standardized templates, we then defined two vertex‐wise measures of space morphometry which facilitated comparisons of subcortical shape: radial distance, as derived from the medial model (Gutman et al., 2015; Gutman, Wang, Rajagopalan, Toga, & Thompson, 2012), which yields a measure of “shape thickness,” and the Jacobian determinant, as derived from tensor‐based morphometry (TBM; Gutman et al., 2015; Wang et al., 2011), which yields a metric of localized tissue reduction or enlargement of surface area (relative to the respective template shape). Because the Jacobian represents the ratio of the area in the individual shape relative to the area in the template at the corresponding vertex, and is not Gaussian in distribution, we used the logarithm of the Jacobian in all analyses examining shape surface area. A useful feature of the ENIGMA‐Shape pipeline is that results are based on bilateral shape measures (i.e., templates for corresponding left and right regions are vertex‐wise registered after reflecting one of them, and summed vertex‐wise). Importantly, our registration algorithm provides a unique and stable matching between datasets, allowing us to efficiently meta‐analyze the effects of MDD across datasets (as in Roshchupkin et al., 2016). See “Image processing and analysis” under the Supporting Information for more details on the pipeline for subcortical shape analysis and on quality control procedures.
Each of the 10 study sites applied the subcortical shape pipeline and ran a priori statistical models (for details, see “Statistical framework for meta‐analyses”, below) that were guided by discussions with ENIGMA‐MDD members and previous work (Schmaal et al., 2016, 2017) to generate summary statistics for inclusion in our meta‐analyses.
2.4. Site‐specific statistical models
To harmonize analyses across sites, a set of standardized scripts to compute mass univariate statistics was distributed to all participating sites via the ENIGMA‐Git page (https://github.com/ENIGMA-git/ENIGMA/tree/master/WorkingGroups). Each study site performed mass univariate (per‐vertex, per‐measure) analysis for all the linear models proposed in the present study (see Table 1). Specifically, for our primary statistical models of interest, subcortical shape measures of thickness (radial distance) and surface area (log of the Jacobian determinant) were the outcome variables, and a binary group indicator variable (e.g., 0 = CTL, 1 = MDD) was the predictor of interest, with age, sex (as a factor), and total ICV as covariates. Our planned comparisons included the following: MDD versus CTL; early‐onset MDD (EO) versus CTL; later‐onset MDD (LO) versus CTL; EO versus LO; recurrent episode MDD (RECUR) versus CTL; first episode MDD (FIRST) versus CTL; RECUR versus FIRST. We also tested whether sex and age significantly interacted with diagnostic group. Although demographic variables for MDD versus CTL were matched at the site‐level, such matching was not necessarily preserved for subgroup comparisons (e.g., EO vs. CTL). Thus, in all analyses, age, sex (as a binary factor), and total ICV were included as covariates.
TABLE 1.
Summary of results
| Statistical model | # of first group/# of second group/total sample size | # of sites | Global‐FDR correction results for thickness (Cohen's d/% affected/I 2) | Global‐FDR correction results for surface area (Cohen's d/% affected/I 2) |
|---|---|---|---|---|
| MDD versus CTL a | 1,781/2,953/4,734 | 10 | n.s. | n.s. |
| EO versus CTL | 476/2,879/3,355 | 9 |
Hipp: −0.172/4.51%/1.41 Amyg: −0.164/4.23%/0.04 |
Hipp: −0.180/22.52%/2.79 Amyg: −0.168/6.01%/1.36 |
| LO versus CTL | 1,028/2,879/3,907 | 9 | n.s. | n.s. |
| EO versus LO | 476/1,028/1,504 | 9 | n.s. | n.s. |
| RECUR versus CTL | 1,273/2,953/4,226 | 10 | n.s. | n.s. |
| FIRST versus CTL | 500/2,879/3,379 | 9 | n.s. | n.s. |
| RECUR versus FIRST | 1,174/500/1,674 | 9 |
Hipp: −0.173/1.61%/6.27 Amyg: −0.174/3.45%/7.21 Thal: 0.177/6.79%/0.90 |
Hipp: −0.174/1.94%/0.47 Amyg: −0.183/0.52%/0 Thal: 0.176/7.68%/5.78 |
| MED versus CTL | 976/2,879/3,855 | 9 |
Hipp: −0.139/2.99%/7.92 Caudate: −0.133/9.73%/8.24 |
Hipp: −0.136/9.32%/7.87 Caudate: −0.140/2.31%/2.72 NAcc: −0.143/22.10%/13.1 |
| NON versus CTL | 797/2,933/3,730 | 9 | n.s. | n.s. |
| HDRS‐17 b | 720 | 4 | n.s. | n.s. |
| BDI b | 760 | 6 | n.s. | n.s. |
Note: Statistical models in bold indicate primary analyses. All site‐specific analyses included age, sex (as a factor), and intracranial volume (ICV) as covariates and all meta‐analytic models pooled each sample's effect sizes (i.e., d or r) using an inverse variance‐weighted random effects model. For more information on each study site, please see Table S1. Thickness is measured by radial distance and surface area is measured using tensor‐based morphometry. See Figures 1 and 2 for more details on results from the primary analyses surviving global‐FDR correction, Figures S3–S7 for more details of results from the primary analyses surviving local‐FDR correction, and Figures S8–S10 for results on the supplemental analyses involving medication usage.
Abbreviations: BDI, Beck's Depression Inventory; CTL, healthy controls; EO, early‐onset MDD (≤21 years old); FIRST, first‐episode MDD; HDRS‐17, Hamilton Depression Rating Scale (17 items); LO, later‐onset MDD (>22 years old); MDD, major depressive disorder; MED, medicated at time of scan; n.s., no significant effects; NON, not medicated at time of scan; RECUR. recurrent‐episode MDD.
Interactions between age and sex (separately) were also tested.
Dimensional analyses conducted within MDD only.
Finally, we conducted exploratory analyses testing for the associations (i.e., linear correlations) with HDRS‐17 and BDI scores (separately) within the MDD group only, as well as comparing groups based on antidepressant usage at the time of scan (MED vs. CTL, and NON vs. CTL) to explain variation in subcortical shape measurements. These analyses were exploratory as only a subset of sites collected the relevant information that permitted us to conduct these analyses.
2.5. Meta‐analytic framework and correction for multiple comparisons
The resulting group‐level maps of effect sizes (i.e., Cohen's d for the group comparisons and Pearson's r for the dimensional analyses), regression parameters, and confidence intervals, as well as basic site information, were aggregated for mass univariate meta‐analysis. As performed in Schmaal et al. (2017, 2016), we conducted meta‐analyses which pooled each site's effect sizes, for each region, using an inverse variance‐weighted random‐effects model as implemented in the R package metafor (version 1.9‐1) and fit with REML (https://cran.r-project.org/). One advantage of random effects models is that they allow effect sizes to vary across studies due to study‐specific differences (e.g., mean age); random effects models therefore weight within‐study as well as between‐study variance in the pooled effect size estimates to mitigate bias or undue influence from the largest samples in the meta‐analysis (Borenstein, Hedges, Higgins, & Rothstein, 2010).
Maps of p‐values resulting from the meta‐analysis were corrected for multiple comparisons using a modified searchlight false discovery rate (FDR) procedure set to p < .05 (for details on procedures and code, see Langers, Jansen, & Backes, 2007). We applied this correction globally across all seven bilateral subcortical regions and measures (thickness, surface area) for each linear model. See “Meta‐analytic framework and correction for multiple comparisons” in the Supporting Information for more details. For comprehensiveness, we also conducted and report local FDR‐corrected results for each of the seven bilateral subcortical structures (i.e., corrected independently in each subcortical region and for each shape metric) in the Supporting Information. Finally, we also report I 2 values for each of our significant effects, which reflect the amount of heterogeneity relative to the total amount of variance in the observed effects.
3. RESULTS
3.1. Global FDR‐corrected effects
3.1.1. MDD versus CTL (and interaction effects with age and sex)
There were no significant differences between MDD and CTL, and no significant interactions between diagnostic group and age or sex.
3.1.2. Age of onset groups
There were no significant differences between LO and CTL, and no significant differences between EO and LO. Relative to CTL, EO had lower thickness in the hippocampus (Cohen's d = −0.17) and amygdala (Cohen's d = −0.16), and smaller surface area in the hippocampus (Cohen's d = −0.18) and amygdala (Cohen's d = −0.17). The strongest effects were primarily in the surface area of the SUB, CA1, and BLA. See Table 1 and Figure 1 for more details. Please see Figure S1 for a map of I 2 values for the surface area results for EO versus CTL.
FIGURE 1.
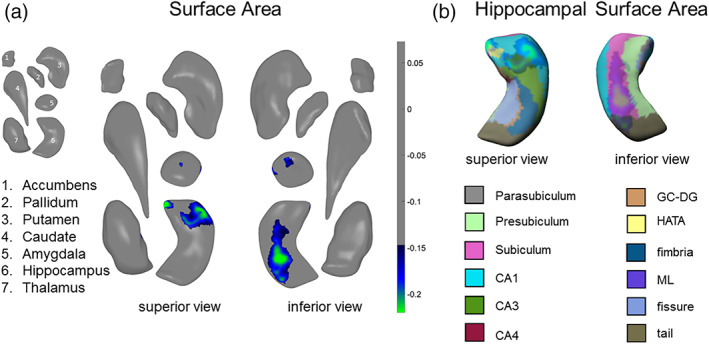
Global‐FDR corrected results for EO versus CTL. (a) Surface area effects in subregions of the amygdala and hippocampus from a superior view (left) and an inferior view (right). (b) Surface area effects overlaid on the FreeSurfer v. 6.0 hippocampal subfield atlas (mirrored). Colored bars correspond to range of effect sizes (Cohen's d). All results are based on bilateral shape measures (i.e., templates for corresponding left and right regions are vertex‐wise registered after reflecting one of them, and summed vertex‐wise). See Table 1 for more information
3.1.3. Recurrence status
There were no significant differences between RECUR and CTL, and no significant differences between FIRST and CTL. Relative to FIRST, RECUR had lower thickness in the hippocampus (Cohen's d = −0.17) and amygdala (Cohen's d = −0.17), and smaller surface area in the hippocampus (Cohen's d = −0.17) and amygdala (Cohen's d = −0.18). These effects were primarily in the surface area of the CA1 and BLA. Relative to FIRST, RECUR also had both greater thickness (Cohen's d = 0.18) and greater surface area (Cohen's d = 0.18) in the medial posterior thalamus. See Table 1 and Figure 2 for more details. Please see Figure S2 for a map of I 2 values for the surface area results for RECUR versus FIRST.
FIGURE 2.
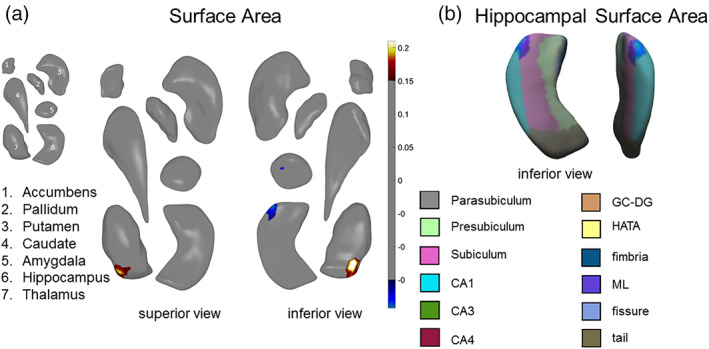
Global‐FDR corrected results for RECUR versus FIRST. (a) Surface area effects in subregions of the amygdala, hippocampus, and thalamus from a superior view (left) and an inferior view (right). (b) Surface area effects overlaid on the FreeSurfer v. 5.3 hippocampal subfield atlas. Color bars correspond to range of effect sizes (Cohen's d). All results are based on bilateral shape measures (i.e., templates for corresponding left and right regions are vertex‐wise registered after reflecting one of them, and summed vertex‐wise). See Table 1 for more information
3.2. Supplemental results assessing associations with depression severity
There were no significant associations with depressive symptom severity using HDRS‐17 or BDI scores in any subcortical structural outcome measures.
3.3. Supplemental results of group comparisons based on local‐FDR correction
Overall, the results from the local‐FDR corrected results were consistent with the global‐FDR corrected results, including smaller thickness and surface area of the SUB and BLA in EO relative to CTL and smaller BLA thickness in RECUR relative to FIRST. Not surprisingly, we observed more significant results with the local‐FDR correction. Notably, MDD relative to CTL exhibited smaller surface area in SUB, BLA, and NAcc shell. Please see Table S2 for a summary of local FDR‐corrected thresholds and Figures S3–S7 in the Supporting Information for more details.
3.4. Supplemental results of group comparisons based on antidepressant usage
Please see Table S1 for a summary of results comparing MED, NON, and CTL, and Figures S8–S10 in the Supporting Information for more details.
4. DISCUSSION
The present study is the largest investigation of subcortical shape in MDD to date with a total of 1,781 patients with MDD and 2,953 healthy controls. We identified reductions in the thickness and surface area of the subiculum (SUB) and the cornu ammonis (CA) one subfields of the hippocampus and the basolateral amygdala (BLA) in patients with an adolescent age of onset (i.e., prior to age 21 years) compared to healthy controls. Further, patients with recurrent depression (i.e., more than one episode of MDD) exhibited lower thickness and surface area primarily in CA1 and BLA compared to patients experiencing their first episode of depression.
Our findings build significantly from our initial study from the ENIGMA MDD Working Group (Schmaal et al., 2016). First, we report depression‐related effects in the shape of structures—namely, the amygdala and thalamus—that did not yield statistically significant volumetric differences as a function of depression status (Schmaal et al., 2016). Second, we provide information on which aspects of gray matter morphology (thickness, surface area) are impacted by depression. Third, we report that the subiculum of the hippocampus and the basolateral amygdala specifically are affected by early onset and recurrent depression. Thus, our results reveal depression‐related differences that are manifested in nuanced changes in subcortical morphometry. Importantly, these patterns may offer insight into important clinical influences (e.g., early onset, recurrent episodes) on the brain basis of MDD.
In line with findings from our previous findings (Schmaal et al., 2016), we found significant reductions in hippocampal volume that were primarily driven by patients who had an age of onset of depression prior to 21 years and/or patients experiencing recurrent episodes of MDD. Our present finding that early‐onset MDD is characterized by smaller surface area of the SUB and CA1 compared to healthy controls suggests that excessive or dysregulated stress responses play a key role in the development of MDD, consistent with broader theoretical literature (Hammen, 2005). Our results are theoretically consistent with preclinical and clinical models of MDD that posit that stress‐induced increases in glucocorticoid levels shrink dendrites and reduce the number of spines in the hippocampus, resulting in atrophy (McEwen et al., 2015; Tata & Anderson, 2010). Postmortem data indicate that the human SUB may contain a higher density of glucocorticoid binding sites than CA1–4 or even the DG (Kim, Pellman, & Kim, 2015; Sarrieau et al., 1986). The SUB also receives input from other subfields of the hippocampus (especially CA1), has reciprocal connections with the hypothalamic nuclei, and sends projections to several subcortical and cortical targets, making it a key structure that regulates the HPA axis (Lowry, 2002; O'Mara, 2005). In light of the extant literature focused on hippocampal subfields in the context of stress and MDD (Bearden et al., 2009; Cao et al., 2017; Cole et al., 2010; Han et al., 2016; Huang et al., 2013; Roddy et al., 2018; Teicher et al., 2012; Treadway et al., 2015), at least two potentially complementary explanations emerge: first, stress‐induced neurotoxicity due to HPA‐axis dysregulation prior to disease onset may have led to the observed structural deficits. Second, in light of our findings that recurrent MDD is associated with lower thickness and smaller surface area in CA1, the hippocampal alterations we report may also be a consequence of the stress associated with experiencing multiple episodes of depression. Unfortunately, an insufficient number of sites provided information on the number and timing of depressive episodes, making it challenging to dissociate the effects of early onset from recurrent depression. Future longitudinal data are ultimately needed to disentangle these possibilities.
Patients with recurrent MDD also showed reduced basolateral amygdala (BLA) and enlarged medial posterior thalamus relative to those in their first episode. Our finding of reduced BLA in patients with recurrent MDD relative to those in their first episode clarifies conflicting data in the extant literature on the effects of MDD on amygdala volume and is consistent with the role of the BLA in responding to threats and stressors in the environment (Terburg et al., 2018). Indeed, previous studies have documented that age of onset, number of episodes, and antidepressant medication affect amygdala volume in people with MDD (Hamilton et al., 2008; Kronenberg et al., 2009; Rubinow et al., 2016; Schmaal et al., 2016; van Eijndhoven et al., 2009). Interestingly, in our exploratory analyses examining patients who were medicated (at the time of scan) and also those who were not medicated versus CTL, we did not find evidence of enlarged amygdala volume, as was reported in a meta‐analysis from a decade ago (Hamilton et al., 2008). Given the partial overlap in sites and samples (see Table S2), it is not surprising our results are more aligned with our prior meta‐analysis of aggregate subcortical volumes, where we reported a trend that individuals with MDD exhibit reduced amygdala volume compared to healthy controls (Schmaal et al., 2016).
Our finding of greater medial posterior thalamic thickness and surface area in recurrent patients with MDD relative to first‐episode patients is an intriguing result that requires more research. While one study of postmortem samples reported more neurons in the mediodorsal and anteromedial nuclei of the thalamus in people diagnosed with MDD relative to healthy controls (Young, Holcomb, Yazdani, Hicks, & German, 2004), others have reported larger thalamic volumes in first‐episode treatment‐naïve patients with MDD (Qiu et al., 2014; Zhao et al., 2014). Interestingly, in a meta‐analysis by Bora et al., late‐life depression was associated with smaller thalamic volume (Bora, Harrison, Davey, Yucel, & Pantelis, 2012). Lithium usage is associated with larger thalamic volumes in patients with bipolar disorder (Lopez‐Jaramillo et al., 2017; Lyoo et al., 2010), but it is unclear from our data as well as in the current literature what the role of mood stabilizing medications are on brain structure in patients with MDD. As we report in the Supporting Information, patients receiving antidepressant treatment at the time of scan did not differ, on average, in thalamus thickness or surface area compared to healthy controls. It will be important for future research to carefully consider the role of subregions of the thalamus in MDD and determine how illness recurrence and/or medication usage affects morphometry of this structure.
Using a less stringent statistical threshold for significance (i.e., local‐FDR correction only), we also report that patients with MDD show lower caudate thickness and smaller surface area in the shell of the nucleus accumbens (NAcc‐s) compared to CTL (in addition to smaller surface area of SUB and BLA). As with the amygdala, while we did not detect overall statistically significant differences in nucleus accumbens volume between MDD and CTL (Schmaal et al., 2016), the use of shape analysis was more sensitive in identifying nuanced differences between groups. The NAcc‐s enjoys robust connections with the orbitofrontal cortex (OFC), with prior research suggesting that both structures underlie disturbed reward processing and decision making in MDD (Drevets, 2007; Kumar et al., 2019). Similarly, extensive work has demonstrated that the caudate plays a critical role in reward‐based reinforcement learning, with neurons in this structure relaying signals that code for expectation violation and reward prediction errors (Arulpragasam et al., 2018; Haber & Knutson, 2010; O'Doherty, 2004; Tricomi & Lempert, 2014). Smaller putamen and caudate volumes have been observed not only in adults with depression (Pizzagalli et al., 2009) but also in young adolescents with parental history of MDD (Pagliaccio et al., 2019), suggesting that these morphological characteristics may represent risk markers. While more research in this area is clearly needed, our results are consistent with neurobiological models of anhedonia and melancholic MDD, which are characterized by more persistent episodes of depression (and indeed, we find smaller NAcc‐s in recurrent patients relative to controls in our local‐FDR corrected results), that implicate dopaminergic dysfunction specifically in mesolimbic pathways (Heller et al., 2009; Misaki, Suzuki, Savitz, Drevets, & Bodurka, 2016; Whitton, Treadway, & Pizzagalli, 2015).
Overall, our effect sizes are modest; nevertheless, they are comparable to what we have reported in prior meta‐analytic investigations comparing MDD and CTL in subcortical and cortical regions (Schmaal et al., 2016, 2017). Given the heterogeneity of MDD as a disorder (e.g., atypical depression) and the likely clinical heterogeneity across the different study sites (e.g., illness duration, medication usage), it may be that several of the findings we report here represent nuanced variations in subcortical subregions as a function of specific clinical characteristics in MDD, and that combining across these distinct clinical subgroups resulted in smaller effect sizes. For instance, early‐onset depression may affect the hippocampus specifically due to early and/or chronic exposure to stress (as well as experiencing recurrent episodes, which are themselves stressful experiences) whereas later‐onset depression may affect the striatum due to experiences affecting reward‐based circuitry specifically (e.g., anhedonia, motivational loss, etc.). Future research is needed to comprehensively parse subtypes of MDD and collect more detailed information on important clinical characteristics in order for us to link specific clinical profiles with their neurobiological substrates.
4.1. Strengths, limitations, and future directions
As the first multisite meta‐analytic study of subcortical shape in MDD, major strengths of our investigation include the large number of observations sampled from several sites across the world combined with the use of standardized quality control procedures across all of these sites. Despite the standardized preprocessing protocols and statistical analyses, one limitation of our meta‐analytic investigation is that we combined pre‐existing data across worldwide samples; thus, data collection protocols (e.g., scan sequences, depression measurements) were not harmonized. Therefore, there may be important sources of heterogeneity in both imaging acquisition protocols and in clinical assessments that will need to be considered in future investigations utilizing mega‐analyses. Indeed, there are several advantages to mega‐analyses over meta‐analyses, including greater flexibility in model specifications (e.g., relaxation of model assumption such as within‐study normality, inclusion of covariates at the level of individuals as well as at the level of sites, etc.) and potentially greater statistical power to detect certain effects (e.g., higher order interactions) (Boedhoe et al., 2019; Burke, Ensor, & Riley, 2017; Debray, Moons, Abo‐Zaid, Koffijberg, & Da Riley, 2013). Nevertheless, there are several analytic advantages to meta‐analyses over mega‐analyses, including the ability to assess the robustness and generalizability of findings across cohorts (see Table 1, Figures S1 and S2), as well as handling site‐specific covariates to account for local population substructure (Burke et al., 2017; Schmaal et al., 2020).
Even though all sites performed quality control tests according to the ENIGMA‐Shape Quality Control guide, a limitation of our study is the reliance on an automated segmentation tool (i.e., FreeSurfer) that has been shown to systematically overestimate the size of the hippocampus, amygdala, and other structures in comparison to manual tracing (Makowski et al., 2018; Schmidt et al., 2018; Schoemaker et al., 2016). However, a recent study reported that despite this bias, agreement between FreeSurfer and tracing methods, as well as measures of spatial overlap, were high (r: 0.70–0.72; Schmidt et al., 2018). Another recent study also reported high test–retest reliability for FreeSurfer estimates of the twelve subfields of the hippocampus, with the exception of the hippocampal fissure (ICC: 0.66–0.96; Whelan et al., 2016). Although this issue is outside of the scope of the present investigation, it is critical for the field to identify which subcortical (and cortical) regions may be adequately segmented by automated procedures and which may show systematic biases (or may only exhibit such biases with certain clinical conditions). Indeed, this is an explicit goal and active area of future research for the ENIGMA Consortium (Thompson et al., 2014, 2019).
It is important to note that with respect to hippocampal differences between MDD and CTL, the results of the present study differed from our previous study on subcortical volume, in that we identified group differences in subfields of this structure with local‐FDR correction only whereas the most robust subcortical volumetric difference between groups was smaller hippocampal volume in MDD (Schmaal et al., 2016). One reason for this discrepancy is that the samples differed between the two studies, both in terms of total sample size (Schmaal et al. examined a total N = 8,927) and in the specific sites included. As detailed in Table S2, five of the sites (n = 1909) that were included in this study were not included in our previous study. Given the smaller sample size of the present study, statistical power is also likely an issue, particularly since we applied vertex‐wise correction for determining significance thresholds, resulting in more stringent p‐values. Despite these differences, the effect size for smaller hippocampal surface in MDD versus CTL in our study was d = −0.11, which is comparable to the effect size of our previous volumetric analyses (d = −0.14). Moreover, both studies report that early‐onset depression robustly affected hippocampal morphology. Thus, despite the differing samples and distinct methodological approaches, the shape and volumetric analyses do broadly share consistent results with respect to MDD‐related hippocampal effects.
While previous studies on overall volume in subcortical regions have identified important neurobiological correlates of MDD, investigating the shape of these structures may represent an important direction for future research focused on understanding structural abnormalities in psychiatric disease, as such findings are able to complement volumetric analyses that are unable to show surface abnormalities. Such an approach is particularly important for structures whereby distinct subfields or regions exhibit distinct functionality (e.g., BLA vs. CMA; Mosher et al., 2010) and promises to provide insight into circuit‐level connections that can be investigated further with other imaging modalities (e.g., diffusion‐weighted MRI, resting‐state MRI), as well as in preclinical animal models.
Finally, as we alluded to previously, investigating the effects of antidepressant medication was challenging in the present study, as the majority of sites did not collect detailed information on history, duration/adherence, type, and dosage of antidepressant treatment. Future research studies focused on collecting detailed information on lifetime medication usage in patients with MDD are needed to better understand how various antidepressants affect brain structure.
4.2. Conclusions
We identified reductions in stress‐sensitive subfields of the hippocampus, particularly in the subiculum and CA1, and in the basolateral amygdala in MDD patients with an earlier onset of depression and in MDD patients with recurrent episodes, compared to healthy controls and first‐episode patients, respectively. Examining nuances in subcortical shape may help disentangle the complex clinical influences on the brain basis of MDD (e.g., structural correlates with important clinical variables, such as illness onset and recurrence), as well as provide the ability to detect fine‐grained changes that show promise in the context of monitoring intervention targets with more specificity.
Supporting information
Data S1 Supporting Information.
Table S1 Uploaded as a separate document.
ACKNOWLEDGMENTS
This work was supported by NIH grants U54 EB020403 and R01 MH116147 to P. M. T.
The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research net (CMR) (http://www.medizin.uni-greifswald.de/icm) of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg‐West Pomerania. MRI scans in SHIP and SHIP‐TREND have been supported by a joint grant from Siemens Healthineers, Erlangen, Germany and the Federal State of Mecklenburg‐West Pomerania.
The FOR2107 cohort was supported by the German Research Foundation (DFG, grant FOR2107 DA1151/5‐1 and DA1151/5‐2 to U. D.; SFB‐TRR58, Projects C09 and Z02 to U. D.; grant FOR2107 KR 3822/7‐2 to A. K.; FOR2107 KI 588/14‐2 to T. K., FOR2107 NE 2254/1‐2 to I. N., and FOR2107 JA 1890/7‐2 to A. J.), the Interdisciplinary Center for Clinical Research (IZKF) of the Medical Faculty of Münster (grant Dan3/012/17 to U. D.).
DIP‐Groningen cohort was supported by the Gratama Foundation, the Netherlands (2012/35 to N. G.).
The CODE cohort was collected from studies funded by Lundbeck and the German Research Foundation (WA 1539/4‐1, SCHN 1205/3‐1, SCHR443/11‐1).
The Magdeburg‐Sexpect cohort was supported by the German Research Foundation (DFG‐SFB779/TPA06).
L. S. is supported by a NHMRC Career Development Fellowship (1140764). T. C. H. is supported in part by NIH grant K01 MH117442. N. J. is supported by NIH grants R01 MH117601, R01 AG059874, and U54 EB020403. P. M. T. is supported in part by NIH grants U54 EB020403, R01 MH116147, R56 AG058854, R01 MH111671 and P41 EB015922. C. R. K. C. is supported in part by T32 AG058507, T32 MH073526, and U54 EB02403. H. J. G. is supported in part by the German Research Foundation (DFG), the German Ministry of Education and Research (BMBF), the DAMP Foundation, Fresenius Medical Care, the EU Joint Programme Neurodegenerative Disorders (JPND), and the European Social Fund (ESF). I. H. G. is supported in part by NIH grant R37 MH101495. P. G. S. is supported in part by the German Research Foundation (DFG, SA 1358/2‐1) and the Max Planck Institute of Psychiatry, Munich.
H. J. G. has received travel grants and speakers honoraria from Fresenius Medical Care and Janssen Cilag. K. S. has consulted for Roche Pharmaceuticals and Servier Pharmaceuticals. P. M. T. and C. R. K. C. have received partial research support from Biogen, Inc. unrelated to the topic of this manuscript. All other authors declare no biomedical conflicts of interest.
The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Ho TC, Gutman B, Pozzi E, et al. Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Hum Brain Mapp. 2022;43:341–351. 10.1002/hbm.24988
Funding information National Health and Medical Research Council, Grant/Award Number: 1140764; National Institute of Aging, Grant/Award Numbers: R01AG059874, R56AG058854, T32AG058507; National Institute of Biomedical Imaging and Bioengineering, Grant/Award Numbers: P41EB015922, U54EB020403; National Institute of Mental Health, Grant/Award Numbers: K01MH117442, R01MH117601, R01MH111671, R01MH116147, T32MH073526, R37MH101495; Biogen, Inc.; Roche Pharmaceuticals and Servier Pharmaceuticals; Fresenius Medical Care and Janssen Cilag; Max Planck Institute of Psychiatry, Munich; European Social Fund (ESF); EU Joint Programme Neurodegenerative Disorders (JPND); Fresenius Medical Care; DAMP Foundation; German Ministry of Education and Research (BMBF); Lundbeck; Gratama Foundation, Grant/Award Number: 2012/35; Interdisciplinary Center for Clinical Research (IZKF) of the Medical Faculty of Münster, Grant/Award Number: Dan3/012/17; German Research Foundation, Grant/Award Numbers: SA 1358/2‐1, DFG‐SFB779/TPA06, SCHR443/11‐1, SCHN 1205/3‐1, WA 1539/4‐1, FOR2107 JA 1890/7‐2, FOR2107 NE 2254/1‐2, FOR2107 KI 588/14‐2, FOR2107 KR 3822/7‐2, SFB‐TRR58, DA1151/5‐2, FOR2107 DA1151/5‐1; Federal State of Mecklenburg‐West Pomerania; Siemens Healthineers; German Federal State of Mecklenburg‐West Pomerania
Contributor Information
Tiffany C. Ho, Email: tiffany.ho@ucsf.edu.
Boris Gutman, Email: bgutman1@iit.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the investigators of each of the sites who have contributed data to this meta‐analytic study. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Arulpragasam, A. R. , Cooper, J. A. , Nuutinen, M. R. , Treadway, M. T. (2018). Corticoinsular circuits encode subjective value expectation and violation for effortful goal‐directed behavior. Proceedings of the National Academy of Sciences of the United States of America, 115(22), E5233–5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden, C. E. , Thompson, P. M. , Avedissian, C. , Klunder, A. D. , Nicoletti, M. , Dierschke, N. , … Soares, J. C. (2009). Altered hippocampal morphology in unmedicated patients with major depressive illness. ASN Neuro, 1, e00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. , Ward, C. H. , Mendelson, M. , Mock, J. , & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Boedhoe, P. S. W. , Heymans, M. W. , Schmaal, L. , Abe, Y. , Alonso, P. , Ameis, S. H. , … Twisk, W. R. J. (2019). An empirical comparison of meta‐ and mega‐analysis with data from the ENIGMA obsessive‐compulsive disorder working group. Frontiers in Neuroinformatics, 12, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora, E. , Harrison, B. J. , Davey, C. G. , Yucel, M. , & Pantelis, C. (2012). Meta‐analysis of volumetric abnormalities in cortico‐striatal‐pallidal‐thalamic circuits in major depressive disorder. Psychological Medicine, 42, 671–681. [DOI] [PubMed] [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. , & Rothstein, H. R. (2010). A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods, 1, 97–111. [DOI] [PubMed] [Google Scholar]
- Burke, D. L. , Ensor, J. , & Riley, R. D. (2017). Meta‐analysis using individual participant data: One‐stage and two‐stage approaches, and why they may differ. Statistics in Medicine, 36, 855–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Passos, I. C. , Mwangi, B. , Amaral‐Silva, H. , Tannous, J. , Wu, M. J. , … Soares, J. C. (2017). Hippocampal subfield volumes in mood disorders. Molecular Psychiatry, 22(9), 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J. , Toga, A. W. , Hojatkashani, C. , Thompson, P. , Costafreda, S. G. , Cleare, A. J. , … Walsh, N. D. (2010). Subregional hippocampal deformations in major depressive disorder. Journal of Affective Disorders, 126(1–2), 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. J. , Pizzagalli, D. , Nitschke, J. B. , & Putnam, K. (2002). Depression: Perspectives from affective neuroscience. Annual Review of Psychology, 53, 545–574. [DOI] [PubMed] [Google Scholar]
- Debray, T. P. A. , Moons, K. G. M. , Abo‐Zaid, G. M. A. , Koffijberg, H. , & Da Riley, R. (2013). Individual participant data meta‐analysis for a binary outcome: One‐stage or two‐stage? PLoS One, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets, W. C. Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences. 2007;1121(1):499–527. [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2002). FreeSurfer. NeuroImage, 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl, T. , Janowitz, D. , Schmaal, L. , Tozzi, L. , Dobrowolny, H. , Stein, D. J. , … Grabe, H. J. (2016). Childhood adversity impacts on brain subcortical structures relevant to depression. Journal of Psychiatric Research, 86, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman, B. A. , Jahanshad, N. , Ching, C. R. , Wang, Y. , Kochunov, P. V. , Nichols, T. E. , & Thompson, P. M. (2015). Medial demons registration localizes the degree of genetic influence over subcortical shape variability: An N = 1480 meta‐analysis. In IEEE International Symposium on Biomedical Imaging 2015 (pp. 1402–1406). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman, B. A. , Wang, Y. , Rajagopalan, P. , Toga, A. W. , & Thompson, P. M. (2012). Shape matching with medial curves and 1‐D group‐wise registration. In 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI) (pp. 716–719). IEEE. [Google Scholar]
- Haber, S. N. , & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, J. P. , Siemer, M. , & Gotlib, I. H. (2008). Amygdala volume in major depressive disorder: A meta‐analysis of magnetic resonance imaging studies. Molecular Psychiatry, 13, 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen, C. (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293–319. [DOI] [PubMed] [Google Scholar]
- Han, K. M. , Won, E. , Sim, Y. , & Tae, W. S. (2016). Hippocampal subfield analysis in medication‐naive female patients with major depressive disorder. Journal of Affective Disorders, 194, 21–29. [DOI] [PubMed] [Google Scholar]
- Heller, A. S. , Johnstone, T. , Shackman, A. J. , Light, S. N. , Peterson, M. J. , Kolden, G. G. , … Davidson, R. J. (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto‐striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America, 106, 22445–22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Stein, J. L. , Renteria, M. E. , Arias‐Vasquez, A. , Desrivieres, S. , Jahanshad, N. , … Medland, S. E. (2015). Common genetic variants influence human subcortical brain structures. Nature, 520, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Coupland, N. J. , Lebel, R. M. , Carter, R. , Seres, P. , Wilman, A. H. , & Malykhin, N. V. (2013). Structural changes in hippocampal subfields in major depressive disorder: A high‐field magnetic resonance imaging study. Biological Psychiatry, 74(1), 62–68. [DOI] [PubMed] [Google Scholar]
- Kim, E. J. , Pellman, B. , & Kim, J. J. (2015). Stress effects on the hippocampus: A critical review. Learning & Memory, 22, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg, G. , Tebartz van Elst, L. , Regen, F. , Deuschle, M. , Heuser, I. , & Colla, M. (2009). Reduced amygdala volume in newly admitted psychiatric in‐patients with unipolar major depression. Journal of Psychiatric Research, 43, 1112–1117. [DOI] [PubMed] [Google Scholar]
- Kumar, P. , Pisoni, A. , Bondy, E. , Kremens, R. , Singleton, P. , Pizzagalli, D. A. , Auerbach, R. P. Delineating the social valuation network in adolescents. Social cognitive and affective neuroscience. 2019;14(11):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langers, D. R. , Jansen, J. F. , & Backes, W. H. (2007). Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. NeuroImage, 38, 43–56. [DOI] [PubMed] [Google Scholar]
- Lopez‐Jaramillo, C. , Vargas, C. , Diaz‐Zuluaga, A. M. , Palacio, J. D. , Castrillon, G. , Bearden, C. , Vieta, E. (2017). Increased hippocampal, thalamus and amygdala volume in long‐term lithium‐treated bipolar I disorder patients compared with unmedicated patients and healthy subjects. Bipolar Disorders, 19, 41–49. [DOI] [PubMed] [Google Scholar]
- Lowry, C. A. (2002). Functional subsets of serotonergic neurones: Implications for control of the hypothalamic‐pituitary‐adrenal axis. Journal of Neuroendocrinology, 14, 911–923. [DOI] [PubMed] [Google Scholar]
- Lyoo, I. K. , Dager, S. R. , Kim, J. E. , Yoon, S. J. , Friedman, S. D. , Dunner, D. L. , & Renshaw, P. F. (2010). Lithium‐induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: A longitudinal brain imaging study. Neuropsychopharmacology, 35, 1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski, C. , Béland, S. , Kostopoulos, P. , Bhagwat, N. , Devenyi, G. A. , Malla, A. K. , … Chakravarty, M. M. (2018). Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: Comparing automated approaches to manual delineation. NeuroImage, 170, 182–198. [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. , Bowles, N. P. , Gray, J. D. , Hill, M. N. , Hunter, R. G. , Karatsoreos, I. N. , Nasca, C. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, R. S. , Cha, D. S. , Soczynska, J. K. , Woldeyohannes, H. O. , Gallaugher, L. A. , Kudlow, P. , … Baskaran, A. (2013). Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions. Depression and Anxiety, 30, 515–527. [DOI] [PubMed] [Google Scholar]
- Misaki, M. , Suzuki, H. , Savitz, J. , Drevets, W. C. , & Bodurka, J. (2016). Individual variations in nucleus accumbens responses associated with major depressive disorder symptoms. Scientific Reports, 6, 21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher, C. P. , Zimmerman, P. E. , & Gothard, K. M. (2010). Response characteristics of basolateral and centromedial neurons in the primate amygdala. The Journal of Neuroscience, 30, 16197–16207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, J. P. Reward representations and reward‐related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology, 2004;14(6), 769–776. [DOI] [PubMed] [Google Scholar]
- O'Mara, S. (2005). The subiculum: What it does, what it might do, and what neuroanatomy has yet to tell us. Journal of Anatomy, 207, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio, D. , Alqueza, K. L. , Marsh, R. , Auerbach, R. P. Brain volume abnormalities in youth at high risk for depression: adolescent brain and cognitive development study. Journal of the American Academy of Child & Adolescent Psychiatry. 2019. 10.1016/j.jaac.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli, D. A. , Holmes, A. J. , Dillon, D. G. , Goetz, E. L. , Birk, J. L. , Bogdan, R. , … Fava, M. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry, 166, 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, L. , Lui, S. , Kuang, W. , Huang, X. , Li, J. , Zhang, J. , … Gong, Q. (2014). Regional increases of cortical thickness in untreated, first‐episode major depressive disorder. Translational Psychiatry, 4, e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria, M. E. , Schmaal, L. , Hibar, D. P. , Couvy‐Duchesne, B. , Strike, L. T. , Mills, N. T. , … Hickie, I. B. (2017). Subcortical brain structure and suicidal behaviour in major depressive disorder: A meta‐analysis from the ENIGMA‐MDD working group. Translational Psychiatry, 7, e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy, D. W. , Farrell, C. , Doolin, K. , Roman, E. , Tozzi, L. , Frodl, T. , … O'Hanlon, E. (2018). The hippocampus in depression: More than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biological Psychiatry, 85(6), 487–497. [DOI] [PubMed] [Google Scholar]
- Roshchupkin, G. V. , Gutman, B. A. , Vernooij, M. W. , Jahanshad, N. , Martin, N. G. , Hofman, A. , … Adams, H. H. H. (2016). Heritability of the shape of subcortical brain structures in the general population. Nature Communications, 7, 13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow, M. J. , Mahajan, G. , May, W. , Overholser, J. C. , Jurjus, G. J. , Dieter, L. , … Stockmeier, C. A. (2016). Basolateral amygdala volume and cell numbers in major depressive disorder: A postmortem stereological study. Brain Structure & Function, 221, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrieau, A. , Dussaillant, M. , Agid, F. , Philibert, D. , Agid, Y. , & Rostene, W. (1986). Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post‐mortem brain. Journal of Steroid Biochemistry, 25, 717–721. [DOI] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. P. , Samann, P. G. , Hall, G. B. , Baune, B. T. , Jahanshad, N. , … Veltman, D. J. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 22, 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Pozzi, E. , Ho, T. C. , van Velzen, L. S. , Veer, I. M. , Opel, N. , … Veltman, D. J. (2020). ENIGMA MDD: Seven years of global neuroimaging studies of major depression through worldwide data sharing. PsyArXiv. 10.31234/osf.io/6j2rw [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Veltman, D. J. , van Erp, T. G. , Samann, P. G. , Frodl, T. , Jahanshad, N. , … Veltman, D. J. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Molecular Psychiatry, 21(6), 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M. F. , Storrs, J. M. , Freeman, K. B. , Jack, C. R., Jr. , Turner, S. T. , Griswold, M. E. , & Mosley, T. H., Jr. (2018). A comparison of manual tracing and FreeSurfer for estimating hippocampal volume over the adult lifespan. Human Brain Mapping, 39(6), 2500–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker, D. , Buss, C. , Head, K. , Sandman, C. A. , Davis, E. P. , Chakravarty, M. M. , … Pruessner, J. C. (2016). Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage, 129, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata, D. A. , & Anderson, B. J. (2010). The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: Implications for hippocampal volume reductions in depression. Physiology & Behavior, 99, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , Anderson, C. M. , & Polcari, A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences of the United States of America, 109, E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg, D. , Scheggia, D. , Triana Del Rio, R. , Klumpers, F. , Ciobanu, A. C. , Morgan, B. , … van Honk, J. (2018). The basolateral amygdala is essential for rapid escape: A human and rodent study. Cell, 175(723–735), e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. , Jahanshad, N. , Ching, C. R. K. , Salminen, L. , Thomopoulos, S. I. , Bright, J. , … Zelman, V. (2019). ENIGMA and Global Neuroscience: A Decade of Large‐Scale Studies of the Brain in Health and Disease across more than 40 Countries. 10.31234/osf.io/qnsh7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. M. , Stein, J. L. , Medland, S. E. , Hibar, D. P. , Vasquez, A. A. , Renteria, M. E. , … Drevets, W. (2014). The ENIGMA Consortium: Large‐scale collaborative analyses of neuroimaging and genetic data. Brain Imaging and Behavior, 8, 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway, M. T. , Waskom, M. L. , Dillon, D. G. , Holmes, A. J. , Park, M. T. M. , Chakravarty, M. M. , … Pizzagalli, D. A. (2015). Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biological Psychiatry, 77, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi, E. , Lempert, K. M. Value and probability coding in a feedback‐based learning task utilizing food rewards. Journal of Neurophysiology, 2014;113(1), 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijndhoven, P. , van Wingen, G. , van Oijen, K. , Rijpkema, M. , Goraj, B. , Jan Verkes, R. , … Tendolkar, I. (2009). Amygdala volume marks the acute state in the early course of depression. Biological Psychiatry, 65, 812–818. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Song, Y. , Rajagopalan, P. , An, T. , Liu, K. , Chou, Y. Y. , … Alzheimer's Disease Neuroimaging Initiative . (2011). Surface‐based TBM boosts power to detect disease effects on the brain: An N = 804 ADNI study. NeuroImage, 56, 1993–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan, C. D. , Hibar, D. P. , van Velzen, L. S. , Zannas, A. S. , Carrillo‐Roa, T. , McMahon, K. , … Iglesias, J. E. (2016). Heritability and reliability of automatically segmented human hippocampal formation subregions. NeuroImage, 128, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton, A. E. , Treadway, M. T. , & Pizzagalli, D. A. (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody, M. L. , & Gibb, B. E. (2015). Integrating NIMH research domain criteria (RDoC) into depression research. Current Opinion in Psychology, 4, 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2017). Depression and other common mental disorders: Global health estimates (Vol. 2018). Geneva: World Health Organization. [Google Scholar]
- Young, K. A. , Holcomb, L. A. , Yazdani, U. , Hicks, P. B. , & German, D. C. (2004). Elevated neuron number in the limbic thalamus in major depression. The American Journal of Psychiatry, 161, 1270–1277. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. J. , Du, M. Y. , Huang, X. Q. , Lui, S. , Chen, Z. Q. , Liu, J. , … Gong, Q. Y. (2014). Brain grey matter abnormalities in medication‐free patients with major depressive disorder: A meta‐analysis. Psychological Medicine, 44, 2927–2937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information.
Table S1 Uploaded as a separate document.
Data Availability Statement
The data that support the findings of this study are available upon request from the investigators of each of the sites who have contributed data to this meta‐analytic study. The data are not publicly available due to privacy or ethical restrictions.


