Abstract
Schizophrenia is associated with widespread alterations in subcortical brain structure. While analytic methods have enabled more detailed morphometric characterization, findings are often equivocal. In this meta‐analysis, we employed the harmonized ENIGMA shape analysis protocols to collaboratively investigate subcortical brain structure shape differences between individuals with schizophrenia and healthy control participants. The study analyzed data from 2,833 individuals with schizophrenia and 3,929 healthy control participants contributed by 21 worldwide research groups participating in the ENIGMA Schizophrenia Working Group. Harmonized shape analysis protocols were applied to each site's data independently for bilateral hippocampus, amygdala, caudate, accumbens, putamen, pallidum, and thalamus obtained from T1‐weighted structural MRI scans. Mass univariate meta‐analyses revealed more‐concave‐than‐convex shape differences in the hippocampus, amygdala, accumbens, and thalamus in individuals with schizophrenia compared with control participants, more‐convex‐than‐concave shape differences in the putamen and pallidum, and both concave and convex shape differences in the caudate. Patterns of exaggerated asymmetry were observed across the hippocampus, amygdala, and thalamus in individuals with schizophrenia compared to control participants, while diminished asymmetry encompassed ventral striatum and ventral and dorsal thalamus. Our analyses also revealed that higher chlorpromazine dose equivalents and increased positive symptom levels were associated with patterns of contiguous convex shape differences across multiple subcortical structures. Findings from our shape meta‐analysis suggest that common neurobiological mechanisms may contribute to gray matter reduction across multiple subcortical regions, thus enhancing our understanding of the nature of network disorganization in schizophrenia.
Keywords: schizophrenia, structure, subcortical shape
Our manuscript reports the first, large prospective subcortical shape meta‐analysis to date, comparing individuals with schizophrenia and healthy controls. Twenty‐one institutions worldwide contributed 24 cross‐sectional study samples to this study via the Enhancing Neuro Imaging Genetics Through Meta‐Analysis (ENIGMA) Consortium, Schizophrenia Working Group, totaling 2,833 individuals with schizophrenia and 3,929 healthy controls. The study utilized a prospective meta‐analysis approach with state‐of‐the‐art harmonized shape analysis protocols. We report robust patterns of subcortical shape and asymmetry differences, and associations with medication and symptoms that spanned multiple neighboring subcortical structures, which have not been previously reported in individual studies with consistency.
1. INTRODUCTION
Deep‐brain nuclei are implicated in a range of emotional, cognitive, motor, and sensory processes (Haijma et al., 2013; Levitt, Bobrow, Lucia, & Srinivasan, 2010; van Erp et al., 2016), with perturbations resulting in a host abnormal behavioral features (Bonelli & Cummings, 2007). Schizophrenia is associated with abnormalities in volume (Haijma et al., 2013; Levitt et al., 2010; van Erp et al., 2016) and shape measures (Csernansky et al., 1998; Gutman et al., 2015; Harms et al., 2007; Lee et al., 2004; Mamah et al., 2007; McClure et al., 2013; Narr et al., 2001; Roalf et al., 2015) of these subcortical structures, as well as their asymmetry (Okada et al., 2016; L. Wang, Joshi, Miller, & Csernansky, 2001). However, substantial heterogeneity in reported findings (see, e.g., Levitt et al., 2010 for a review) has inspired efforts to harmonize studies via collaborative meta‐analyses. Meta‐analyses aim to distill true effects from heterogeneous findings, a crucial task for advancing existing theories and for hypothesis generation (Berman & Parker, 2002). Prospective meta‐analyses allow for collaborative and harmonized analyses across diverse data sets (Jahanshad & Thompson, 2017; Thompson et al., 2014; van Erp et al., 2016). Using this approach, a recent meta‐analysis investigated subcortical volumes in over 4,500 individuals from 15 data sets, and found that schizophrenia patients had smaller hippocampus, amygdala, thalamus and nucleus accumbens, and larger pallidum as compared to healthy control participants, whereas caudate and putamen did not show group differences (van Erp et al., 2016).
Given the anatomical complexity of subcortical structures, studies of shape—defined as the inward and outward variations of surface‐based structural boundaries, as well as their curvatures, expansions, and contractions—can detect and characterize more nuanced disease‐related structural morphometry patterns relative to whole‐structure volume (Levitt et al., 2010). In particular, subcortical structural shape is consistently altered in subtle, yet significant, ways in individuals with schizophrenia (Csernansky, Wang, Joshi, Ratnanather, & Miller, 2004; Mamah, Alpert, Barch, Csernansky, & Wang, 2016; Yang et al., 2012). However, inconsistent findings for specific nuclei have been observed, likely due to the small sample sizes of individual studies as well as investigators' preferred choices of atlases, computational methods, statistical models, and specialized quantitative phenotypes.
For example, a review of shape analysis research in psychosis‐spectrum disorders reveals patient sample sizes and types ranging from 20 (Li et al., 2015) to 126 (Qiu et al., 2010) in “strict” schizophrenia samples, 28 (Qiu, Gan, Wang, & Sim, 2013) to 35 (Coscia et al., 2009) in first‐episode samples, 60 in an antipsychotic‐naïve schizophrenia sample, and another study of 86 early‐onset subjects (Chakravarty et al., 2015). While the choices for computational approaches in shape analysis are not as varied relative to volumetric procedures, there still exists some variability including: the radial distance method (Coscia et al., 2009; Thompson et al., 2003); FSL FIRST (Danivas, Kalmady, Venkatasubramanian, & Gangadhar, 2013; Li et al., 2015; Smith et al., 2004); large deformation diffeomorphic metric mapping (LDDMM; (Csernansky et al., 2004; Khan, Wang, & Beg, 2008; Qiu et al., 2010; Qiu et al., 2013; Qiu & Miller, 2008; Vaillant, Qiu, Glaunes, & Miller, 2007; Wang et al., 2008; Womer et al., 2014); and the MAGeT automatic segmentation method (Chakravarty et al., 2013) with a modified surface‐based approach (Chakravarty et al., 2015; Lerch et al., 2008). The heterogeneity in these approaches has yielded both consistent (e.g., thalamus: Coscia et al., 2009; Csernansky, Schindler, et al., 2004; Danivas et al., 2013; Harms et al., 2007; basal ganglia: Chakravarty et al., 2015; Mamah et al., 2016; Wang et al., 2008; hippocampus: Qiu et al., 2013; Wang et al., 2001) and inconsistent findings (e.g., Li et al., 2015), suggesting a need for synthesizing the literature to better capture the global effects of psychosis on these important brain regions.
Given the variability described above, a new approach to meta‐analysis of structural shape is needed to overcome the limitations of a traditional, retrospective meta‐analysis to characterize subcortical structural shape alterations in schizophrenia. The ENIGMA consortium (Thompson et al., 2020) recently developed a prospective shape meta‐analysis approach by harmonizing atlases, computational methods, and statistical models prior to hypothesis testing (Gutman et al., 2015; Gutman, Wang, Rajagopalan, Toga, & Thompson, 2012; Roshchupkin et al., 2016). Here we report the first, large prospective subcortical shape meta‐analysis comparing individuals with schizophrenia and healthy control participants using standardized normalization to a common surface‐based subcortical atlas, harmonized quality assurance procedures, and a common set of linear statistical models. In this approach, shape analysis was first independently performed for each data set, followed by a meta‐analysis on the aggregated group‐level results. Two measures of shape were computed; the first was a measure of distance from the medial axis of each structure which we called thickness, while the second was a measure of surface contraction or expansion as calculated by the log Jacobian determinant.
Based on previous literature findings, we hypothesized a predominance of concordant reductions in thickness and contractions across the surfaces of the hippocampus, amygdala, nucleus accumbens, and thalamus. Furthermore, we hypothesized a predominance of concordant increases in thickness and expansions across the surfaces of the putamen and pallidum, in individuals with schizophrenia as compared with control participants. Since atypical antipsychotic drugs have been associated with both increases (Haijma et al., 2013; Konradi & Heckers, 2001; Roiz‐Santianez, Suarez‐Pinilla, & Crespo‐Facorro, 2015) and reductions (Dorph‐Petersen et al., 2005; Ho, Andreasen, Ziebell, Pierson, & Magnotta, 2011; Li et al., 2018; Roiz‐Santianez et al., 2014; Roiz‐Santianez et al., 2015) in subcortical volumes, as well as shape alterations (Mamah et al., 2012), we also attempted to resolve these disparate findings by testing for effects of antipsychotic drug exposure (using chlorpromazine dose equivalents) on shape.
2. MATERIALS AND METHODS
Twenty‐four worldwide cross‐sectional study samples from 21 institutions (Figure S1)—2,833 individuals with schizophrenia and 3,929 healthy control participants—contributed to the collaborative shape meta‐analysis via the ENIGMA Schizophrenia Working Group. Each study sample was collected with participants' written informed consent approved by local Institutional Review Boards. The characteristics of these cohorts are provided in Table 1 including weighted age, age of illness onset, duration of illness, and symptom severity. The weighted total scores for positive and negative syndrome scale (PANSS), scale for the assessment of negative symptoms (SANS), and scale for the assessment of positive symptoms (SAPS) across data sets were 16.6 (range of means across data sets: 13.7–22.9), 21.3 (range: 5.5–38.9) and 17.1 (range: 9.0–23.2), respectively. For data sets that recorded current antipsychotic type and dose, we determined the percentage of patients on second‐generation (atypical; 77%), first‐generation (typical; 15%), both (3%) or none (5%), and chlorpromazine dose equivalents (from 11 data sets) based on Woods (2003) (http://www.scottwilliamwoods.com/files/Equivtext.doc).
TABLE 1.
Sample characteristics
| Mean (range of means) | Schizophrenia | Healthy control |
|---|---|---|
| N | 2,833 | 3,929 |
| % male | 66.9% (54.4–100%) | 54.9% (38.6–100%) |
| Age, years | 35.1 (16.3–43.9) | 33.2 (16.2–43.6) |
| Age at onset, years | 23.9 (20.7–28.9) | ‐ |
| Duration of illness, years | 11.3 (0.7–31.5) | ‐ |
| PANSS total | 16.6 (13.7–22.9) | ‐ |
| SANS total | 21.3 (5.5–38.9) | ‐ |
| SAPS total | 17.1 (9.0–23.2) | ‐ |
| Chlorpromazine dose equivalent | 376 (166.1–634.6) | ‐ |
Note: Age, age at onset and duration of illness, total PANSS (Positive and Negative Syndrome Scale), SANS (Scale for the Assessment of Negative Symptoms), SAPS (Scale for the Assessment of Positive Symptoms), and chlorpromazine dose equivalents were all weighted by sample size. Range is reported on range of means across the data sets. The 100% extreme in % male was due to the all‐male sample from the Mental Health Research Center, Moscow.
To assess subcortical shape measures, we followed the validated ENIGMA‐Shape pipeline (http://enigma.usc.edu/ongoing/enigma-shape-analysis/) (Ching et al., 2020; Chye et al., 2020; T. C. Ho et al., 2020; Roshchupkin et al., 2016). A set of standardized scripts to compute mass univariate statistics was distributed to all sites performing the analysis locally via the ENIGMA‐Git webpage (https://github.com/ENIGMA-git/ENIGMA/tree/master/WorkingGroups). To harmonize analysis across sites, the distributed script accessed a static online list of models agreed upon by the ENIGMA‐Schizophrenia Working Group. Below, we summarize the pipeline.
FreeSurfer parcellation. Left and right thalamus, caudate, putamen, pallidum, accumbens, hippocampus and amygdala parcellations, their volumes, and intracranial volumes (ICVs), were obtained with FreeSurfer (http://surfer.nmr.mgh.harvard.edu) from high‐resolution T1‐weighted structural brain scans. For details on study type (single site or multisite), scanner vendor/strength/sequence, acquisition parameters, FreeSurfer versions, and parcellation quality control, we refer the reader to van Erp et al. (2016) table S3b.
Surface triangulation. The boundary of each individual FreeSurfer‐parcellated subcortical region was represented by a triangulated surface model. The surface was spherically inflated and registered to a region‐specific average model by matching local geometric features (Wang et al., 2011). In this way, each subject was represented by a set of shapes with vertex‐to‐vertex correspondence to a standard template surface. Visual quality control of the surfaces was performed by individual raters according to the ENIGMA‐Shape Quality Control guide (http://enigma.ini.usc.edu/ongoing/enigma-shape-analysis/). Beyond the guide, sites performed local quality control, assisted by experienced raters at the University of Southern California Imaging Genetics Center for particularly difficult cases. More information on shape quality control and failure rates is available in Supplementary Materials.
Surface registration. A single template—the default ENIGMA shape atlas—was used by all ENIGMA‐Schizophrenia sites participating in the shape project. The ENIGMA‐Shape atlas was constructed by averaging surface models of 200 unrelated individuals (100 M/100 F, mean age: 22.9 [2.8] years) from the Queensland Twin Imaging Study (QTIM, Renteria et al., 2014). QTIM data were not used in this study beyond atlas construction.
Shape and asymmetry computation. Following registration, a medial curve was computed for the individual shape models (Gutman et al., 2012), and their registrations were further refined (Gutman et al., 2012). Two measures of shape were calculated at each vertex (Wang et al., 2011). The first, which we called “thickness,” was the distance to the medial curve. For a cylindrical structure, the medial curve is its center axis, and our measure of thickness measures the length of the radius of each circular cross‐section. The second quantity was the Jacobian determinant, which we called “contraction or expansion,” was defined as the ratio of the triangular area relative to the triangular area in the template at corresponding vertices. As the Jacobian tends to be non‐Gaussian, we used its logarithm transform in all analyses. Vertex‐wise maps were then compared to test for group differences (see below). We reasoned that when significant thinning and contraction (or thickening and expansion) occurred concordantly in the same vertices, subvolume changes were likely occurring nearer the structural surface. In contrast, when measures of thickness and contraction/expansion were present in a discordant fashion, we reasoned that subvolume changes could be occurring farther from the surface and closer to the medial curve. We computed the degree of concordance for each of the structures. Finally, the ENIGMA‐Shape atlas contains corresponding vertices across the left and right hemispheres allowing calculation of asymmetry indices for the absolute difference between left and right thickness and the log Jacobian determinant at each vertex.
Statistical analysis. Vertex‐wise mass univariate analysis per shape/asymmetry measure was independently performed first for each data set. Resulting group‐level maps of effect sizes (Cohen's d; Cohen, 1992), regression parameters, and confidence intervals were then aggregated for mass univariate meta‐analysis. We followed the same meta‐analytic strategy as in van Erp et al. (2016), using inverse variance‐based mixed effects sample weighting with as implemented in the R metafor package. Further details are available in Supplementary Materials. The following effects were tested using linear models: effect of diagnosis on thickness (Model 1), log Jacobian determinant (Model 2), asymmetry index of thickness (Model 3), asymmetry index of log Jacobian determinant (Model 4), and effect of chlorpromazine dose equivalents in individuals with schizophrenia (Model 5). In all models, sex and age were accounted for by including their linear, quadratic, and interaction terms: sex, age, age x sex, age2, and age2 x sex. Intracranial Volume (ICV) was also included in all models as a covariate. Maps of p‐values resulting from the meta‐analysis were corrected for multiple comparisons (number of structures, vertices, and measures) using a modified searchlight false discovery rate (FDR) procedure (Kriegeskorte, Goebel, & Bandettini, 2006; Langers, Jansen, & Backes, 2007). The correction was applied globally across all structures and measures for each linear model. Distance in the searchlight procedure was defined as the Euclidean distance between atlas vertices, with distance between vertices of different structures set to infinity. We note that this is a more conservative correction than one that assumes spatial correlation between boundaries of different structures, though less conservative than the original Benjamini and Hochberg procedure (Hochberg & Benjamini, 1990).
3. RESULTS
3.1. Effect of diagnosis on bilateral shape features
Patterns of predominant concave shape differences were observed across multiple, neighboring subcortical structures. An examination of the aggregate surfaces of the hippocampus, amygdala, accumbens, and the thalamus indicate a predominance of regions showing thinning (Cohen's d range = −.03 to −.17; Figure 1a) and surface contractions (Cohen's d range = −.01 to −.04; Figure 1b) in individuals with schizophrenia as compared with control subjects. In contrast, an examination of the aggregate surfaces of the caudate, putamen and the pallidum, indicate a predominance of regions showing thickening (Cohen's d range = .03 to .17; Figure 1a) and surface expansions (Cohen's d range = .01 to .04; Figure 1b). The magnitude of size for all above comparisons are characterized as small effects (Cohen, 1992).
FIGURE 1.
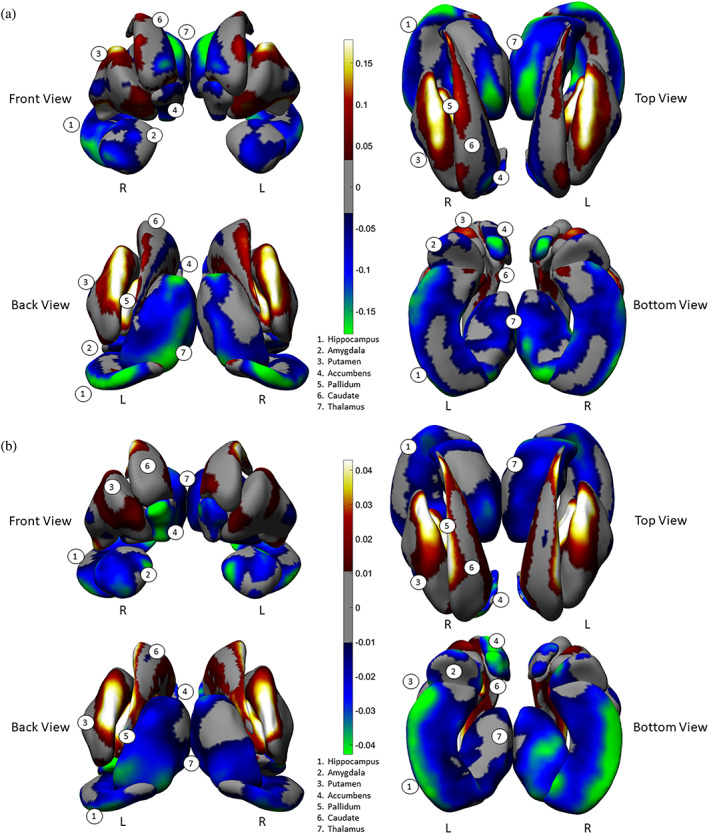
Vertex‐wise effects of diagnosis (i.e., schizophrenia vs. control) on each hemisphere for (a) thickness, (b) surface dilation/contraction (log Jacobian determinant). The effects are tested in models that included diagnosis sex, age, age x sex, age2, age2 x sex, and ICV. Vertex‐wise effect sizes (Cohen's d, see text) are visualized on subcortical surfaces. The subcortical structures—1. hippocampus, 2. amygdala, 3. putamen, 4. accumbens, 5. pallidum, 6. caudate, and 7. thalamus—are shown as a group situated in template space, from front, back, top, and bottom viewpoints of the brain. L = left hemisphere. R = right hemisphere. Color scale indicates the intensity of effect sizes. Cooler colors (i.e., negative effect sizes) indicate thinning, surface contraction for schizophrenia as compared to controls, and warmer colors (i.e., positive effect sizes) indicate thickening, surface dilation. Gray color indicates nonsignificant surface vertices after multiple comparison correction
Group differences in the two shape measures did not always overlap with each other. For example, surface contraction without thinning was observed in the superior portions of the hippocampal body (i.e., close to the CA2‐4 + DG subfields, Figure S2). In addition, the caudate showed thinning along with surface expansion in its medial regions (Figure S7A,B). Nonetheless, the proportion of concordance of these two measures accounted for the majority of shape differences. A more detailed description of the spatial patterns and the degree of concordance of shape measures for each individual structure can be found in the Supplementary Materials.
We assessed an overall effect size for thinning/thickening and surface contraction/expansion for each subcortical structure collapsed across the hemispheres. We did this by first obtaining surface indices for which the above linear models were significant on measures of interhemispheric means, then separately computing an average for vertices carrying positive effect sizes and an average for negative effect sizes (see Cohen's d, Figure 2a,b). With regard to thinning vs. thickening (Figure 2a), only the amygdala and accumbens showed thinning (i.e., negative effect sizes only) in schizophrenia participants. For the hippocampus and thalamus, surface regions with thinning were larger in extent (based on effect size) compared with regions showing thickening. The caudate, putamen, and pallidum showed more thickening than thinning. With regard to surface contraction vs. expansion (Figure 2b), only the hippocampus and accumbens showed surface contraction in schizophrenia participants. Surface regions with larger contraction relative to expansion (based on effect size) included the amygdala and thalamus. The caudate and putamen showed more expansion than contraction, and the pallidum showed only expansion compared to control participants. See Supplementary Materials for more detailed descriptions. Again, the magnitude of all effects above were characterized as small in size.
FIGURE 2.
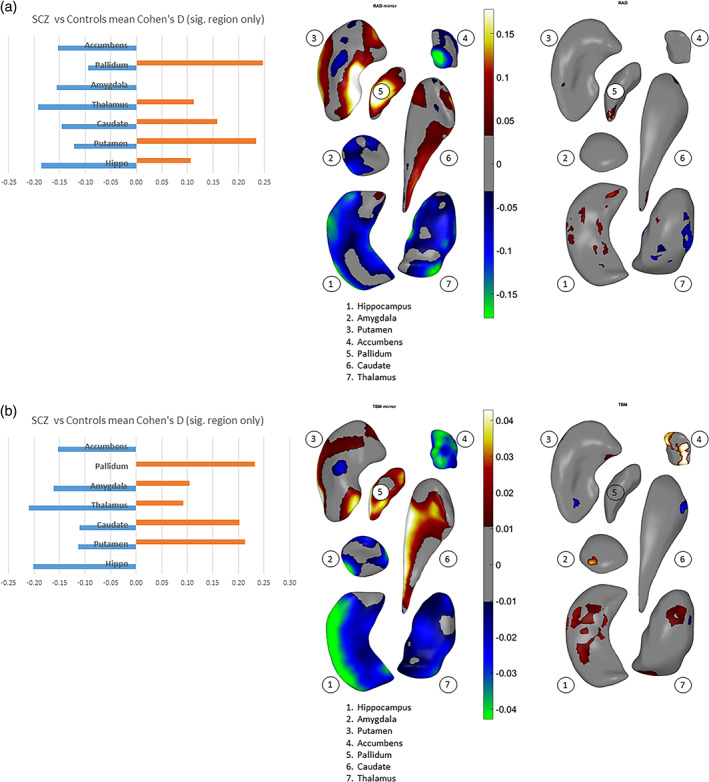
Overall and vertex‐wise effects of diagnosis (i.e., schizophrenia vs. control) across hemispheres for (a) thickness, (b) surface dilation/contraction (log Jacobian determinant). The effects are tested in models that included diagnosis sex, age, age x sex, age2, age2 x sex, and ICV. In the left column, mean positive effect sizes and mean negative effect sizes across each subcortical structure surface (see text) are shown as bar plots. The middle column shows the vertex‐wise effects of diagnosis on interhemispheric means (see text). The right column shows the vertex‐wise effects of diagnosis on interhemispheric absolute differences (reproduced from Figure 3a,b right columns). The subcortical structures—1. hippocampus, 2. amygdala, 3. putamen, 4. accumbens, 5. pallidum, 6. caudate, and 7. thalamus—are positioned generally from a bottom viewpoint, with some slightly rotated about their own principal axis to be oblique, for better exposure: caudate—pi/7 or about 25°, accumbens—pi/10 or 18°, pallidum—pi/3 or 60°. Color scale indicates the intensity of effect sizes. Cooler colors (i.e., negative effect sizes) indicate reduced asymmetry for schizophrenia as compared to controls, and warmer colors (i.e., positive effect sizes) indicate exaggerated asymmetry. Gray color indicates nonsignificant surface vertices after multiple comparison correction
3.2. Effect of diagnosis on shape asymmetry
Significant group differences in asymmetry patterns were also observed for both shape measures. Our interpretation of shape asymmetry was based on how the asymmetry index was calculated, which was the absolute difference in effect size for the structures between left and right hemispheres. The asymmetry index for thinning/thickening (Figure 3a) was greater for individuals with schizophrenia as compared to control subjects for the hippocampus, amygdala, nucleus accumbens, and putamen (see red color on the surfaces, Cohen's d = .05 to .1). For the thalamus, the index was predominantly smaller, as seen in blue color (Cohen's d = −0.05 to −0.15). For the pallidum and the caudate nucleus, group differences in the asymmetry index was negligible. The asymmetry index of surface contraction/expansion (Figure 3b) was greater for individuals with schizophrenia as compared with control subjects for the hippocampus, amygdala, putamen, and nucleus accumbens (Cohen's d = .01 to .04). For the thalamus, the index was predominantly greater for individuals with schizophrenia as compared with control subjects, with smaller proportions of the surface showing smaller indices (Cohen's d = −.01 to −.03). For the pallidum and caudate nucleus, group differences in the asymmetry index was negligible.
FIGURE 3.
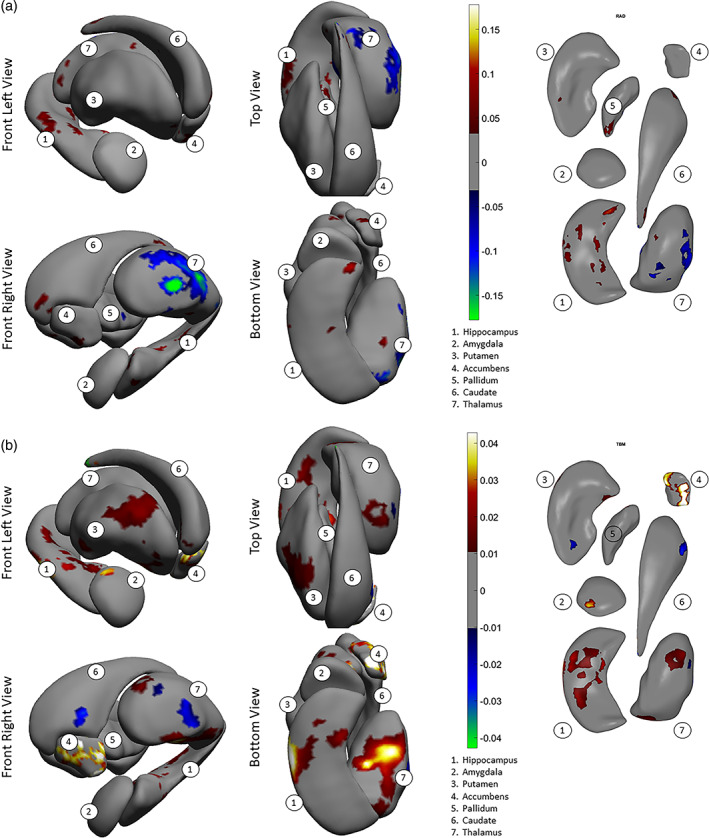
Effects of diagnosis (i.e., schizophrenia vs. control) for (a) asymmetry index of thickness, (b) asymmetry index of surface dilation/contraction (log Jacobian determinant). Vertex‐wise asymmetry indices for thickness and surface dilation/contraction were calculated as the absolute values of left‐versus‐right differences. The effects are tested in models that included diagnosis sex, age, age x sex, age2, age2 x sex, and ICV. Effect sizes (Cohen's d, see text) are visualized on subcortical surfaces. In the left two columns, the subcortical structures—1. hippocampus, 2. amygdala, 3. putamen, 4. accumbens, 5. pallidum, 6. caudate, and 7. thalamus—are shown as a group situated in template space, from front left, front right, top and bottom viewpoints of the brain. L = left hemisphere. R = right hemisphere. In the right column, the subcortical structures are positioned generally from a bottom viewpoint, with some slightly rotated about their own principal axis to be oblique, for better exposure: caudate—pi/7 or about 25°, accumbens—pi/10 or 18°, pallidum—pi/3 or 60°. Color scale indicates intensity of effect sizes. Cooler colors (i.e., negative effect sizes) indicate reduced asymmetry for schizophrenia as compared with controls, and warmer colors (i.e., positive effect sizes) indicate exaggerated asymmetry. Gray color indicates nonsignificant surface vertices after multiple comparison correction
Consistent with the shape difference maps, patterns of shape asymmetry differences also demonstrated continuity across multiple, neighboring structures. For example, one pattern extended across the hippocampus, amygdala, to the thalamus, showing predominantly exaggerated asymmetry for individuals with schizophrenia as compared with control subjects (Figure 3b, bottom and front views), and another encompassed the ventral striatum and ventral and dorsal thalamus, revealing predominantly diminished normal asymmetry for individuals with schizophrenia (Figure 3b, front view). Additionally, the determination that these asymmetry differences indicated a leftward versus rightward, or an exaggerated versus diminished normal asymmetry, was made in the context of group differences across both hemispheres; please see Supplementary Materials for these observations.
3.3. Clinical correlates
In a prior study of structural volumes, we did not detect any significant correlations between chlorpromazine dose equivalents or psychopathology measures and volume differences in a large subset of the patients from 15 of 21 samples analyzed here (van Erp et al., 2016). However, in the present study, using our shape meta‐analysis, we found small but statistically significant relationships between higher chlorpromazine dose equivalents and shape‐derived thinning (Cohen's d = −.00008 to −.00014, Figure 4a) and surface contraction (Cohen's d = −.00005 to −.00004, Figure 4b) in the hippocampus and amygdala, and also in the caudate, accumbens, and thalamus. In addition, we found small but statistically significant relationships between elevated positive symptom (SAPS total) scores and thinning (Cohen's d = −.00002.5 to −.00005, Figure 5a) and contraction (Cohen's d = −.00005 to −.00014, Figure 5b) in the hippocampus, amygdala, caudate, and thalamus.
FIGURE 4.
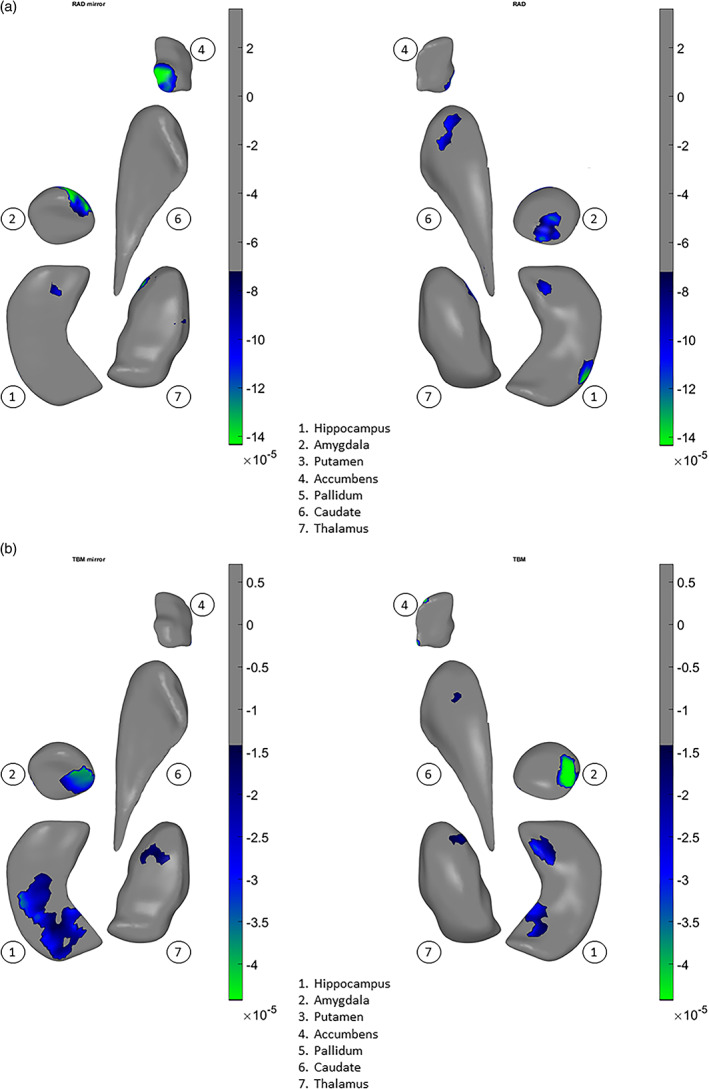
Overall and vertex‐wise effects of chlorpromazine dose equivalents across both hemispheres for (a) thickness, (b) surface dilation/contraction (log Jacobian determinant). The effects are tested in models that included diagnosis sex, age, age x sex, age2, age2 x sex, and ICV. Small but statistically significant relationships are found between higher chlorpromazine dose equivalents and locally reduced thickness (a) and surface contraction (b) in the hippocampus, amygdala, caudate, accumbens, and thalamus. In the left column, the subcortical structures—1. hippocampus, 2. amygdala, 4. accumbens, 6. caudate, and 7. thalamus—are positioned generally from a bottom viewpoint, with some slightly rotated about their own principal axis to be oblique, for better exposure: caudate—pi/7 or about 25°, accumbens—pi/10 or 18°. In the right column, the subcortical structures are positioned generally from a top viewpoint with the same rotations. Color scale indicates intensity of effect sizes. Cooler colors indicate negative associations, that is, higher chlorpromazine dose equivalents are associated with reduced surface measures. Gray color indicates nonsignificant surface vertices after multiple comparison correction. Putamen and pallidum are not shown as no effects were found for these subcortical structures
FIGURE 5.
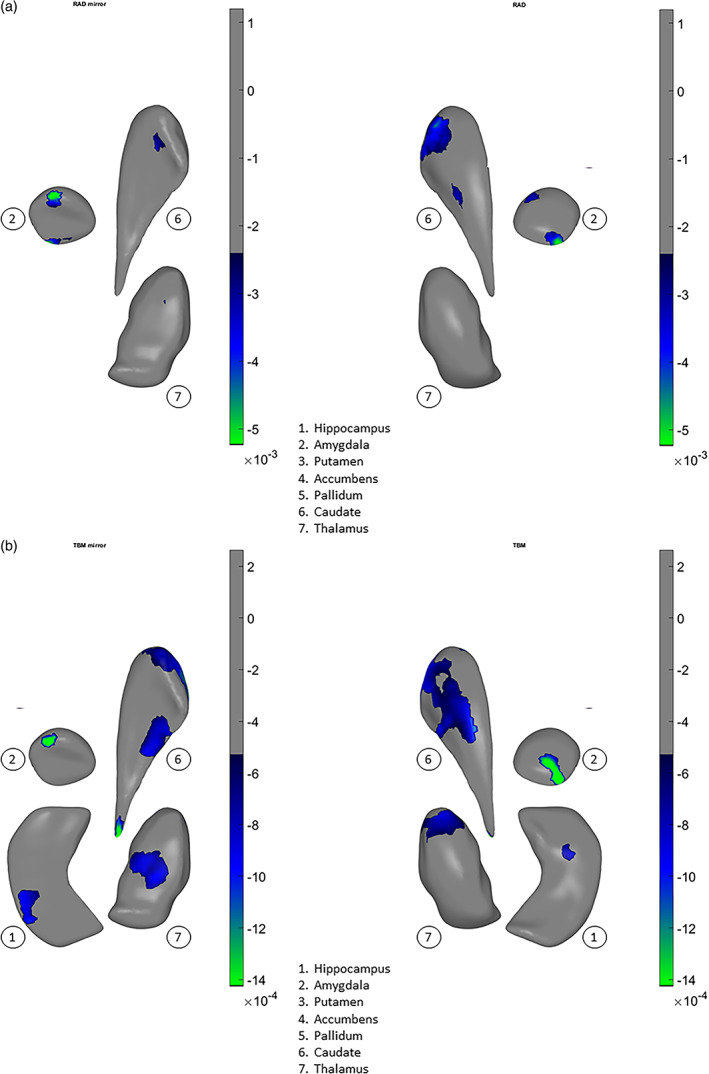
Overall and vertex‐wise effects of SAPS total scores across both hemispheres for (a) thickness, (b) surface dilation/contraction (log Jacobian determinant). The effects are tested in models that included diagnosis sex, age, age x sex, age2, age2 x sex, and ICV. Small but statistically significant relationships are found between higher SAPS total scores and locally reduced thickness in the amygdala, caudate, and thalamus (a), and surface contraction (b) in the hippocampus, amygdala, caudate, and thalamus. In the left column, the subcortical structures—1. hippocampus (panel b, surface contraction only), 2. amygdala, 6. caudate, and 7. thalamus—are positioned generally from a bottom viewpoint, with some slightly rotated about their own principal axis to be oblique, for better exposure: caudate—pi/7 or about 25°, accumbens—pi/10 or 18°. In the right column, the subcortical structures are positioned generally from a top viewpoint with the same rotations. Color scale indicates the intensity of effect sizes. Cooler colors indicate negative associations, that is, higher chlorpromazine dose equivalents are associated with reduced surface measures. Gray color indicates nonsignificant surface vertices after multiple comparison correction. Accumbens, putamen, and pallidum are not shown as no effects were found for these subcortical structures
4. DISCUSSION
In this meta‐analysis of subcortical shape variations in schizophrenia, we found a predominance of concave, disease‐related, shape differences in the bilateral amygdala, hippocampus, accumbens, and thalamus. As there was a concordance of thinning and surface contraction, these surface changes may reflect losses of subvolumes relatively near the structural surfaces. In turn, we found a predominance of convex, disease‐related, shape differences in the bilateral putamen and the pallidum. Notably, the caudate showed a mix of both concave and convex shape differences. In the majority of these instances, differences in thinning/thickening and contraction/expansion were concordant (i.e., overlapping), which suggested to us that these changes more likely reflected variable subvolume changes relatively close to the structural surfaces.
The ranking of effect sizes (i.e., Cohen's d) corroborated the shape observations, and were consistent with prior volume‐based meta‐analyses (Haijma et al., 2013; van Erp et al., 2016). In a meta‐analysis of overlapping subjects, the volumes of the hippocampus, amygdala, accumbens, and the thalamus were reported to be smaller in individuals with schizophrenia compared to healthy control participants, while the volumes of the putamen and the pallidum were larger, and the volume of the caudate did not differ (van Erp et al., 2016). In a meta‐analysis of over 18,000 subjects, Haijma et al. also reported volume reductions in the hippocampus, amygdala, accumbens, and the thalamus for individuals with schizophrenia compared with healthy control participants, with no volumetric differences in the caudate and putamen, and enlargement in the pallidum (Haijma et al., 2013). Our meta‐analysis also revealed patterns of shape differences that were contiguous across neighboring structures (Figures S9–S11). For example, concave shape differences extended across the amygdala, hippocampus, accumbens, and thalamus, with larger differences crossing between the amygdala and hippocampus, between the hippocampus and thalamus, and between the caudate and accumbens (Figures S9 and S11). These shape differences confirmed findings previously reported by multiple individual shape studies; that is, in the anteriolateral (CA1) and subicular portions of the hippocampus (Csernansky et al., 2002; Lee et al., 2004; McClure et al., 2013), and anterior and pulvinar portions of the thalamus (Csernansky, Schindler, et al., 2004); see supplemental materials for a more in‐depth summary of literature findings.
Previous work has observed that when compared with healthy control participants, individuals with schizophrenia show smaller neuron sizes in the CA1 and subiculum subfields of the hippocampus (Arnold et al., 1995), reduced total number of neurons in the lateral nucleus of amygdala (Kreczmanski et al., 2007), and in the anteroventral, anteromedial, and mediodorsal nuclei and pulvinar of the thalamus (Dorph‐Petersen & Lewis, 2017; Young, Manaye, Liang, Hicks, & German, 2000). Microscopic reductions in neuron size or total number of neurons may manifest in macroscopical reductions in gray matter volume measured in MRI. Smaller neuronal size may reflect structural and/or metabolic compromise in the soma, or reduction in the synapses and dendrites that are metabolically supported (Arnold et al., 1995). Furthermore, post mortem studies of individuals with schizophrenia show reduced pre‐ and postsynaptic markers in hippocampal pyramidal neurons (Glantz & Lewis, 1997; Harrison, 1999b; Harrison & Eastwood, 1998), as well as shorter length, number, and complexity of dendritic processes (Garey et al., 1998; Rosoklija et al., 2000).
Regarding the caudate and putamen, postmortem studies have reported both larger (Beckmann & Lauer, 1997) as well as smaller disease‐related changes in total neuron number (Kreczmanski et al., 2007). Consistent with this mix of findings, we observed both convex and concave shape differences in these structures (Figure S11). In addition, the regions where convex or concave differences were found tended to be contiguous with the loss and expansion patterns observed in neighboring structures. For example, convex shape differences extended across the caudate and putamen, and adjoining regions, whereas the dorsal concave shape differences in the caudate extended across to the neighboring thalamus. Our observed shape differences are consistent with these patterns of neuronal deficits reported in postmortem studies of individuals with schizophrenia, suggesting that common or similar disease mechanisms may contribute to gray matter volume reduction across multiple subcortical regions.
With regard to shape asymmetry, we also observed patterns that extended across multiple, neighboring structures. One predominantly exaggerated asymmetry pattern was observed across the hippocampus, amygdala, and thalamus in individuals with schizophrenia compared to control participants, while a pattern of diminished asymmetry in individuals with schizophrenia encompassed the ventral striatum and ventral and dorsal thalamus. Exaggerated asymmetry patterns in schizophrenia across multiple structures have not been reported by previous, volume‐based meta‐analyses, perhaps due to their subtlety. In the aforementioned meta‐analysis of over 18,000 subjects, Haijma et al. reported no statistically significant differences in global left‐versus‐right subcortical volume effect sizes between individuals with schizophrenia and healthy control participants (Haijma et al., 2013), though left‐sided decreases in hippocampus and amygdala volumes were greater than the right‐sided decreases. In a meta‐analysis comparing 884 individuals with schizophrenia and 1,680 healthy control participants, Okada et al. also reported increased leftward asymmetry only for the pallidum volume in schizophrenia, but not for other subcortical structures (Okada et al., 2016). Our observed exaggerated asymmetry across the multiple subcortical structures, that is, the hippocampus, amygdala, and thalamus, may be partially attributable to a genetic mechanism that controls the development of cerebral asymmetry in schizophrenia (Brun et al., 2008; Crow et al., 1989; Satizabal et al., 2019; Thompson et al., 2001; van der Meulen et al., 2020). Diminished asymmetry such as the effects we observed in the ventral striatum and ventral and dorsal thalamus might be a result of epigenetic dysregulation (Abdolmaleky et al., 2019). Environmental insults during neurodevelopment could also lead to disturbed brain asymmetry observed later in life (Algan & Rakic, 1997; Schindler et al., 2002). In addition, abnormal developments in neuroanatomical asymmetry may underlie the disturbed asymmetry patterns in brain network connectivity in individuals with schizophrenia (Gomez‐Gastiasoro et al., 2019; Guo, Han, Li, & Reddick, 2019; McKenna, Babb, Miles, Goff, & Lazar, 2020; Wang et al., 2019). Ongoing studies of populations of subjects at genetic risk for developing schizophrenia, such as relatives of patients with schizophrenia (de Zwarte et al., 2019; Harms et al., 2007; Mamah et al., 2008; Zhu et al., 2018), may help elucidate the specific roles of genetic influence and environmental factors that contribute to the development of schizophrenia.
The brain's deeper structures are integral components of distributed functional network circuitry. In particular, the thalamus and striatum have direct, excitatory connections with the cortex, while the pallidum receives inhibitory signals from the striatum and limbic structures, and in turn, passes inhibitory signals to the thalamus. Information from different cortical areas, including the prefrontal, frontal, motor, and sensory areas, is received by the striatum via excitatory projections, and then passed on to the thalamus via inhibitory projections from the pallidum. The thalamus projects to the cortex, completing a cortico‐basal ganglia‐thalamo‐cortical loop (Kandel, Schwartz, & Jessell, 1991). The hippocampus and amygdala also have reciprocal excitatory connections with the medial prefrontal cortex (Barbas & Blatt, 1995; Carmichael & Price, 1995), and project to the anterior nucleus of the thalamus as part of Papez' circuit (Jankowski et al., 2013). Structural neuroimaging studies have demonstrated that schizophrenia is associated with widespread gray matter reduction in the brain, predominantly in medial temporal, frontal, and parietal cortical regions, as well in the midline limbic structures and the deep thalamic and striatal nuclei (Bora et al., 2011; Fornito, Yucel, Patti, Wood, & Pantelis, 2009; Honea, Crow, Passingham, & Mackay, 2005; Keshavan, Prasad, & Pearlson, 2007; Lawrie & Abukmeil, 1998; Levitt et al., 2010; McCarley et al., 1999; Palaniyappan, Balain, & Liddle, 2012; Pantelis et al., 2009; Pearlson & Calhoun, 2007; Pearlson & Marsh, 1999; Schmitt, Hasan, Gruber, & Falkai, 2011; Shenton, Dickey, Frumin, & McCarley, 2001; Shenton, Whitford, & Kubicki, 2010). These multifocal brain abnormalities are hypothesized to reflect a “fragmentation of brain pathologies” that ultimately leads to breakdowns in network organization (Shenton et al., 2001; van den Berg, Gong, Breakspear, & van Leeuwen, 2012). Advances in structural and functional neuroimaging studies continue to provide evidence that schizophrenia is a disorder with subtle, multifocal abnormalities involving distributed changes in brain network architecture (Wang & Csernansky, 2014). Our shape meta‐analysis findings help us understand this network disorganization in schizophrenia by isolating the specific locations of structural subvolume abnormalities on the surface of deep‐brain structures to identify which afferent and efferent connections are affected (Bullmore, Frangou, & Murray, 1997; Friston & Frith, 1995; Meyer‐Lindenberg et al., 2001; Volkow et al., 1988; Weinberger, Berman, Suddath, & Torrey, 1992).
Focal subcortical shape deformities in individuals with schizophrenia, such as the anterior‐lateral, posterior‐lateral aspects of the hippocampus, are known to correlate with focal thinning of cortical regions involved in both dorsal and ventral visual processes (Qiu et al., 2010), as well as lower white matter integrity as measured by fractional anisotropy (Qiu et al., 2010). These findings are consistent with cellular studies showing that the number of neurons expressing NADPH‐d enzyme is lower in the hippocampus (Akbarian et al., 1993) as well as in the dorsolateral prefrontal cortex (Akbarian et al., 1993), of subjects with schizophrenia, suggesting the presence of a common pathological process that has interrupted normal neurodevelopment (Akbarian, Vinuela, et al., 1993). Cobia et al. (2017) showed that inward deformity of thalamic shape in the pulvinar region correlated with reduced thickness of the frontal, temporal and parietal cortices, and longitudinal changes in these thalamic and cortical subregions were similarly correlated. The thalamic surface zones reported by Cobia et al. (2017) strongly overlapped with thalamic regions that were the thinnest in our meta‐analysis. The pulvinar of the thalamus has broad distributions of connections across the association cortex (Sherman & Guillery, 2011), and its abnormalities were found to be related to cortical abnormalities in individuals with schizophrenia (Sherman & Guillery, 2011). Therefore, our shape findings provide further structural evidence that the development of multiple brain structures are affected in schizophrenia, which support changes in functional brain networks.
Our shape meta‐analysis found that higher chlorpromazine dose equivalents were related to concave shape differences primarily in the hippocampus and amygdala, but also in the caudate, accumbens, and thalamus. The literature on the association between subcortical structure and second‐generation antipsychotic treatment reflects conflicting findings. In van Erp et al., where an overlapping sample was analyzed for subcortical volumes (van Erp et al., 2016), no relationship was found between chlorpromazine dose equivalents and differences in subcortical volumes. However, the volumetric meta‐analysis by Haijma et al. (2013) found that a higher dose of atypical antipsychotics was associated with larger caudate volumes. While some studies have found that antipsychotics were related to increases in gray matter volume or attenuated volume reduction in subcortical structures (see Konradi & Heckers, 2001; Roiz‐Santianez et al., 2015 for reviews), others have reported that volume reductions in the hippocampus (Li et al., 2018) and caudate (Roiz‐Santianez et al., 2014) were associated with antipsychotic drug treatment (Dorph‐Petersen et al., 2005; Ho et al., 2011; Roiz‐Santianez et al., 2015). In a shape analysis by Mamah et al. (2012), schizophrenia patients who were taking olanzapine showed inward shape changes in the hippocampus as compared with healthy controls, but changes that were relatively smaller than inward shape observed in patients taking first‐generation antipsychotics. In a meta‐analysis of 778 schizophrenia patients, a longer duration of illness was correlated with increased globus pallidus volumes (Hashimoto et al., 2018). In rodent studies, antipsychotic drugs have been shown to affect synaptic ultrastructure, but not neuronal number or size. Atypical antipsychotics tended to upregulate dendritic spine formation and synaptogenesis, whereas typical antipsychotic drugs tended to downregulate them or have no significant effect (Critchlow, Maycox, Skepper, & Krylova, 2006; Delotterie et al., 2010). Following atypical antipsychotic treatment, increased synapses have been observed in the striatum (see Harrison, 1999a for a review) and the hippocampus (Park et al., 2013), whereas typical antipsychotics were not associated with similar changes (Critchlow et al., 2006). Further examination of the mechanisms of antipsychotic drugs' effects on brain structures may help explain the findings of relationships between drug classes and brain changes (i.e., concave shape differences or volume reductions associated with higher doses of first‐ or second‐ generation drugs) (Li et al., 2018).
Our shape meta‐analysis showed that higher positive symptom severity (SAPS total) scores were associated with concave shape differences in the hippocampus, amygdala, caudate, and thalamus. In the abovementioned volumetric meta‐analysis in an overlapping sample (van Erp et al., 2016), no relationships between subcortical volumes and severity of positive or negative symptoms were found. Moreover, in the literature, the association between subcortical structure and psychopathological symptoms has been largely absent (Gong, Lui, & Sweeney, 2016; Haukvik, Hartberg, & Agartz, 2013; Levitt et al., 2010; Li et al., 2018). While the inconsistent relationship in the literature suggests that subcortical structures may not reflect the intensity of psychopathology (Gong et al., 2016), our findings are consistent with the few studies that have shown a relationship between increased positive symptom severity and reduced accumbens volume (Mamah et al., 2007), longitudinal thalamic shape deformation (Wang et al., 2008), and reductions in cortical gray matter measures (Chen et al., 2014).
Future studies in structural shape should consider the following limitations with respect to our particular analytic tools: In the hippocampus, we found concave changes more prominently in the lateral (proximal to the CA1 hippocampal subfield) and medial regions (proximal to the subiculum). These patterns were more posterior than the more anterior shape deformities reported by groups using diffeomorphic surface mapping methods (Csernansky et al., 2002; Qiu et al., 2010). One explanation may be that the FreeSurfer software, used by every site in our meta‐analysis, generates subcortical gray matter boundaries that are sometimes noisy (Wang et al., 2009). While this noise may not affect whole‐structure volume measurements (see volume meta‐analysis paper by van Erp et al. (2016), it may influence shape measurements such as ours, based on vertex‐wise information. The nature of the meta‐analysis in this study did not permit the implementation of methodologies that could have generated smooth subcortical surfaces. In addition, harmonization of heterogeneous clinical variables (such as medication, psychopathological symptoms) is challenging, given the differences in outcome measures/variables used.
There is a growing literature of subtypes of schizophrenia. Using MRI, EEG, ocular motor, and cognition measures, the B‐SNIP consortium reported three biotypes that differed on social‐relational negative symptoms (Clementz et al., 2020). We and others have reported subtypes as defined by the degree of neuropsychological impairment who differed also in their cortical neuroanatomy (Cobia, Csernansky, & Wang, 2011). McCutcheon, Abi‐Dargham, and Howes (2019), however, reported no evidence of subtypes based on treatment responses in a meta‐analysis. The ENIGMA consortium provides an ideal setting for future, prospective meta‐analytical studies to define and validate subtypes of schizophrenia based on genetics, cognition and other biomarker measures (Smeland et al., 2017; T. Wang et al., 2017). Using polygenic risk scores (PGRS), a recent review of associations between functional MRI activity and polygenic risk for schizophrenia reports that although genetic modulation on brain function for schizophrenia occurs predominantly in frontal areas, it also impacts the task‐dependent recruitment across multiple brain regions (Purcell et al., 2009). The ENIGMA consortium is therefore also ideal for meta‐analytic studies that can provide anatomical and connectomic mechanisms for the widespread cognitive deficits observed in schizophrenia (Smeland et al., 2017). Further, while antipsychotic drugs and behavioral treatments have not been effective in relieving negative symptoms and cognitive impairment in schizophrenia (Green, Kern, & Heaton, 2004; Nuechterlein et al., 2011), cognitive behavior therapy could lead to increased activation in the thalamus (Kumari et al., 2010) (see Howells, Baldwin, and Kingdon (2016) for a review). Treatment modalities such as aerobic exercise have been shown to improve positive and negative symptoms and quality of life (Dauwan, Begemann, Heringa, & Sommer, 2016; Firth, Cotter, Elliott, French, & Yung, 2015; Vancampfort, Rosenbaum, Ward, & Stubbs, 2015). Aerobic exercise has been demonstrated to improve a range of cognitive functions such as working memory and verbal learning (Vakhrusheva, Marino, Stroup, & Kimhy, 2016) and brain structure (Kandola, Hendrikse, Lucassen, & Yucel, 2016; Malchow et al., 2016; Pajonk et al., 2010) among individuals with schizophrenia. The mechanism by which aerobic exercise renders these beneficial effects are not established but is reportedly related to improvement in neurogenesis and neuroplasticity processes, particularly in the hippocampus (Falkai et al., 2013; Vakhrusheva et al., 2016). Therefore, future studies may provide further mechanistic basis and targets for potential interventions.
Finally, as data were merged from multiple sites, we chose a statistical design based on meta‐analysis to generate consensus maps from the various data sets analyzed. Other approaches are possible, and in Radua et al. (2020) we compared meta‐analysis with mega‐analysis, in which individual level data from all sites are centralized, and site‐level effects are be modeled as random effects. In a meta‐analysis, which we used here, it is not assumed that the data from each site have the same mean and variance, as models are fitted locally and effect sizes are combined, with a site‐dependent weight. Although we did not do this here, additional adjustments can be made to the data from each site to further harmonize it, including ComBat and its variants (Fortin et al., 2018; Pomponio et al., 2020), hierarchical Bayes models (Kia et al., 2020), and generative adversarial networks (Liu et al., 2021). It is still an open question how to adjust site‐level data to increase the distributional overlap across sites, especially when inclusion criteria differ somewhat and latent confounds at each site can lead to under‐ or overadjustment of data (Kia et al., 2020). As such we used a meta‐analysis, which gives comparable results to other methods in recent comparisons (Radua et al., 2020).
In conclusion, our meta‐analysis of subcortical shapes revealed strong patterns of shape differences and shape asymmetry differences that provide finer‐scale information than prior studies of structural volumes, and spanned multiple neighboring subcortical structures. Findings from our shape meta‐analysis suggest that common mechanisms may contribute to gray matter reduction across multiple subcortical regions, which may enhance our understanding of the nature of network disorganization in schizophrenia.
5. COLLABORATORS
The authors also would like to acknowledge the following members of the Karolinska Schizophrenia Project (KaSP) Consortium: Lars Farde, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; Lena Flyckt, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; Helena Fatouros‐Bergman, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; Simon Cervenka, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; Karin Collste, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; Pauliina Victorsson, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; Göran Engberg, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; Sophie Erhardt, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; Lilly Schwieler, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; Anna Malmqvist, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; Mikael Hedberg, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; Funda Orhan, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; Carl M Sellgren, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; Fredrik Piehl, Neuroimmunology Unit, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Ingrid Agartz, NORMENT, Division of Mental Health and Addiction, Oslo University Hospital & Institute of Clinical Medicine, University of Oslo.
CONFLICT OF INTERESTS
One of the authors (TGMvE) has had a research contract with Otsuka Pharmaceutical. One of the authors (AP) has served as a consultant for Boehringer Ingelheim. One of the authors (DJS) has received research grants and/or honoraria from Lundbeck and Sun. One of the authors (DHM) has served as a consultant for Boehringer Ingelheim, Aptinyx, and Greenwich Biosciences. One of the authors (SC) has received grant support from AstraZeneca as co‐investigator, and has served as a speaker for Otsuka Pharmaceuticals. Authors PMT, CRKC, and NJ received a research grant from Biogen, Inc. (Boston) for research unrelated to this manuscript. The remaining authors declare no potential conflict of interest.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under Award Number U54EB020403 and R01MH116147. For additional grant support and acknowledgments, see Supplementary Materials.
Gutman, B. A. , van Erp, T. G. M. , Alpert, K. , Ching, C. R. K. , Isaev, D. , Ragothaman, A. , Jahanshad, N. , Saremi, A. , Zavaliangos‐Petropulu, A. , Glahn, D. C. , Shen, L. , Cong, S. , Alnæs, D. , Andreassen, O. A. , Doan, N. T. , Westlye, L. T. , Kochunov, P. , Satterthwaite, T. D. , Wolf, D. H. , … Wang, L. (2021). A meta‐analysis of deep brain structural shape and asymmetry abnormalities in 2,833 individuals with schizophrenia compared with 3,929 healthy volunteers via the ENIGMA Consortium . Human Brain Mapping, 43(1), 352–372. 10.1002/hbm.25625
Funding information Center for Integrated Healthcare, U.S. Department of Veterans Affairs, Grant/Award Number: I01 CX000497; Commonwealth Health Research Board, Grant/Award Number: HRA_POR/2011/100; Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Numbers: 478466/2009, 480370/2009; Department of Energy, Labor and Economic Growth, Grant/Award Number: DE‐FG02‐99ER62764; Forskningsrådet om Hälsa, Arbetsliv och Välfärd, Grant/Award Numbers: K2009‐62X‐15077‐06‐3, K2012‐61X‐15077‐09‐3, 523‐2014‐3467, 2009‐7053, 2013‐2838; Fundação Amazônia Paraense de Amparo à Pesquisa, Grant/Award Numbers: 2009/14891‐9, 2010/18672‐7, 2012/23796‐2, 2013/039; Instituto de Salud Carlos III, Grant/Award Numbers: FIS 00/3095, 01/3129, PI020499, PI060507, PI10/001; National Health and Medical Research Council, Grant/Award Numbers: 1009064, 496682; National Institutes of Health, Grant/Award Numbers: 1RC1MH089257, MH 60722, MH019112, MH064045, MH085096, MH098130, MO1 RR025758, P41RR14075, P50 MH071616, R01 DA053028, R01 EB020062, R01 HD050735, R01 MH056584, R01 MH084803, R01 MH116147, R01 MH117601, R01EB005846, R01EB015611, R01MH074797, R21 MH097196, R21MH097196, R37MH43375, S10 OD023696, T32 AG058507, T32 MH073526, TR000153, U01 MH097435, U24 RR021382A, U24 RR021992, U24 RR025736, U24 RR21992, U24RR021992, U54 EB020403, U54EB020403, UL1 TR000153; National Science Foundation, Grant/Award Numbers: 1636893, 1734853; Norges Forskningsråd, Grant/Award Numbers: 213837, 217776, 223273; Science Foundation Ireland, Grant/Award Numbers: 08/IN.1/B1916, 12/IP/1359; Wellcome Trust, Grant/Award Number: 072894/2/03/Z
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abdolmaleky, H. M. , Gower, A. C. , Wong, C. K. , Cox, J. W. , Zhang, X. , Thiagalingam, A. , … Thiagalingam, S. (2019). Aberrant transcriptomes and DNA methylomes define pathways that drive pathogenesis and loss of brain laterality/asymmetry in schizophrenia and bipolar disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 180(2), 138–149. 10.1002/ajmg.b.32691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian, S. , Bunney, W. E., Jr. , Potkin, S. G. , Wigal, S. B. , Hagman, J. O. , Sandman, C. A. , & Jones, E. G. (1993). Altered distribution of nicotinamide‐adenine dinucleotide phosphate‐diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Archives of General Psychiatry, 50(3), 169–177. [DOI] [PubMed] [Google Scholar]
- Akbarian, S. , Vinuela, A. , Kim, J. J. , Potkin, S. G. , Bunney, W. E., Jr. , & Jones, E. G. (1993). Distorted distribution of nicotinamide‐adenine dinucleotide phosphate‐diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Archives of General Psychiatry, 50(3), 178–187. [DOI] [PubMed] [Google Scholar]
- Algan, O. , & Rakic, P. (1997). Radiation‐induced, lamina‐specific deletion of neurons in the primate visual cortex. The Journal of Comparative Neurology, 381(3), 335–352. [DOI] [PubMed] [Google Scholar]
- Arnold, S. E. , Franz, B. R. , Gur, R. C. , Gur, R. E. , Shapiro, R. M. , Moberg, P. J. , & Trojanowski, J. Q. (1995). Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical‐hippocampal interactions. The American Journal of Psychiatry, 152(5), 738–748. 10.1176/ajp.152.5.738 [DOI] [PubMed] [Google Scholar]
- Barbas, H. , & Blatt, G. J. (1995). Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus, 5(6), 511–533. 10.1002/hipo.450050604 [DOI] [PubMed] [Google Scholar]
- Beckmann, H. , & Lauer, M. (1997). The human striatum in schizophrenia. II. Increased number of striatal neurons in schizophrenics. Psychiatry Research, 68(2–3), 99–109. [DOI] [PubMed] [Google Scholar]
- Berman, N. G. , & Parker, R. A. (2002). Meta‐analysis: Neither quick nor easy. BMC Medical Research Methodology, 2, 10. 10.1186/1471-2288-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli, R. M. , & Cummings, J. L. (2007). Frontal‐subcortical circuitry and behavior. Dialogues in Clinical Neuroscience, 9(2), 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora, E. , Fornito, A. , Radua, J. , Walterfang, M. , Seal, M. , Wood, S. J. , … Pantelis, C. (2011). Neuroanatomical abnormalities in schizophrenia: A multimodal voxelwise meta‐analysis and meta‐regression analysis. Schizophrenia Research, 127(1–3), 46–57. [DOI] [PubMed] [Google Scholar]
- Brun, C. , Lepore, N. , Pennec, X. , Chou, Y. Y. , Lee, A. D. , Barysheva, M. , … Thompson, P. M. (2008). A tensor‐based morphometry study of genetic influences on brain structure using a new fluid registration method. Medical Image Computing and Computer‐Assisted Intervention, 11(Pt 2), 914–921. 10.1007/978-3-540-85990-1_110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore, E. T. , Frangou, S. , & Murray, R. M. (1997). The dysplastic net hypothesis: An integration of developmental and dysconnectivity theories of schizophrenia. Schizophrenia Research, 28(2–3), 143–156. [DOI] [PubMed] [Google Scholar]
- Carmichael, S. T. , & Price, J. L. (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology, 363(4), 615–641. 10.1002/cne.903630408 [DOI] [PubMed] [Google Scholar]
- Chakravarty, M. M. , Rapoport, J. L. , Giedd, J. N. , Raznahan, A. , Shaw, P. , Collins, D. L. , … Gogtay, N. (2015). Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: A longitudinal study. Human Brain Mapping, 36(4), 1458–1469. 10.1002/hbm.22715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty, M. M. , Steadman, P. , van Eede, M. C. , Calcott, R. D. , Gu, V. , Shaw, P. , … Lerch, J. P. (2013). Performing label‐fusion‐based segmentation using multiple automatically generated templates. Human Brain Mapping, 34(10), 2635–2654. 10.1002/hbm.22092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Deng, W. , Gong, Q. , Huang, C. , Jiang, L. , Li, M. , … Li, T. (2014). Extensive brain structural network abnormality in first‐episode treatment‐naive patients with schizophrenia: Morphometrical and covariation study. Psychological Medicine, 44(12), 2489–2501. 10.1017/S003329171300319X [DOI] [PubMed] [Google Scholar]
- Ching, C. R. K. , Gutman, B. A. , Sun, D. , Villalon Reina, J. , Ragothaman, A. , Isaev, D. , … Bearden, C. E. (2020). Mapping subcortical brain alterations in 22q11.2 deletion syndrome: Effects of deletion size and convergence with idiopathic neuropsychiatric illness. The American Journal of Psychiatry, 177(7), 589–600. 10.1176/appi.ajp.2019.19060583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chye, Y. , Mackey, S. , Gutman, B. A. , Ching, C. R. K. , Batalla, A. , Blaine, S. , … Garavan, H. (2020). Subcortical surface morphometry in substance dependence: An ENIGMA addiction working group study. Addiction Biology, 25(6), e12830. 10.1111/adb.12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz, B. A. , Trotti, R. L. , Pearlson, G. D. , Keshavan, M. S. , Gershon, E. S. , Keedy, S. K. , … Tamminga, C. A. (2020). Testing psychosis phenotypes from bipolar‐schizophrenia network for intermediate phenotypes for clinical application: Biotype characteristics and targets. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(8), 808–818. 10.1016/j.bpsc.2020.03.011 [DOI] [PubMed] [Google Scholar]
- Cobia, D. J. , Csernansky, J. G. , & Wang, L. (2011). Cortical thickness in neuropsychologically near‐normal schizophrenia. Schizophrenia Research, 133(1–3), 68–76. 10.1016/j.schres.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobia, D. J. , Smith, M. J. , Salinas, I. , Ng, C. , Gado, M. , Csernansky, J. G. , & Wang, L. (2017). Progressive deterioration of thalamic nuclei relates to cortical network decline in schizophrenia. Schizophrenia Research, 180, 21–27. 10.1016/j.schres.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. 10.1037//0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Coscia, D. M. , Narr, K. L. , Robinson, D. G. , Hamilton, L. S. , Sevy, S. , Burdick, K. E. , … Szeszko, P. R. (2009). Volumetric and shape analysis of the thalamus in first‐episode schizophrenia. Human Brain Mapping, 30(4), 1236–1245. 10.1002/hbm.20595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow, H. M. , Maycox, P. R. , Skepper, J. N. , & Krylova, O. (2006). Clozapine and haloperidol differentially regulate dendritic spine formation and synaptogenesis in rat hippocampal neurons. Molecular and Cellular Neurosciences, 32(4), 356–365. 10.1016/j.mcn.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Crow, T. J. , Ball, J. , Bloom, S. R. , Brown, R. , Bruton, C. J. , Colter, N. , … Roberts, G. W. (1989). Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Archives of General Psychiatry, 46(12), 1145–1150. 10.1001/archpsyc.1989.01810120087013 [DOI] [PubMed] [Google Scholar]
- Csernansky, J. G. , Joshi, S. , Wang, L. , Haller, J. W. , Gado, M. , Miller, J. P. , … Miller, M. I. (1998). Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proceedings of the National Academy of Sciences of the United States of America, 95(19), 11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky, J. G. , Schindler, M. K. , Splinter, N. R. , Wang, L. , Gado, M. , Selemon, L. D. , … Miller, M. I. (2004). Abnormalities of thalamic volume and shape in schizophrenia. The American Journal of Psychiatry, 161(5), 896–902. 10.1176/appi.ajp.161.5.896 [DOI] [PubMed] [Google Scholar]
- Csernansky, J. G. , Wang, L. , Jones, D. , Rastogi‐Cruz, D. , Posener, J. A. , Heydebrand, G. , … Miller, M. I. (2002). Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. The American Journal of Psychiatry, 159(12), 2000–2006. [DOI] [PubMed] [Google Scholar]
- Csernansky, J. G. , Wang, L. , Joshi, S. C. , Ratnanather, J. T. , & Miller, M. I. (2004). Computational anatomy and neuropsychiatric disease: Probabilistic assessment of variation and statistical inference of group difference, hemispheric asymmetry, and time‐dependent change. NeuroImage, 23(Suppl 1), S56–S68. [DOI] [PubMed] [Google Scholar]
- Danivas, V. , Kalmady, S. V. , Venkatasubramanian, G. , & Gangadhar, B. N. (2013). Thalamic shape abnormalities in antipsychotic naive schizophrenia. Indian Journal of Psychological Medicine, 35(1), 34–38. 10.4103/0253-7176.112198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwan, M. , Begemann, M. J. , Heringa, S. M. , & Sommer, I. E. (2016). Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: A systematic review and meta‐analysis. Schizophrenia Bulletin, 42(3), 588–599. 10.1093/schbul/sbv164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwarte, S. M. C. , Brouwer, R. M. , Agartz, I. , Alda, M. , Aleman, A. , Alpert, K. I. , … van Haren, N. E. M. (2019). The association between familial risk and brain abnormalities is disease specific: An ENIGMA‐relatives study of schizophrenia and bipolar disorder. Biological Psychiatry, 86(7), 545–556. 10.1016/j.biopsych.2019.03.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delotterie, D. , Ruiz, G. , Brocard, J. , Schweitzer, A. , Roucard, C. , Roche, Y. , … Andrieux, A. (2010). Chronic administration of atypical antipsychotics improves behavioral and synaptic defects of STOP null mice. Psychopharmacology, 208(1), 131–141. 10.1007/s00213-009-1712-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph‐Petersen, K. A. , & Lewis, D. A. (2017). Postmortem structural studies of the thalamus in schizophrenia. Schizophrenia Research, 180, 28–35. 10.1016/j.schres.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph‐Petersen, K. A. , Pierri, J. N. , Perel, J. M. , Sun, Z. , Sampson, A. R. , & Lewis, D. A. (2005). The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology, 30(9), 1649–1661. 10.1038/sj.npp.1300710 [DOI] [PubMed] [Google Scholar]
- Falkai, P. , Malchow, B. , Wobrock, T. , Gruber, O. , Schmitt, A. , Honer, W. G. , … Cannon, T. D. (2013). The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: A randomized controlled MRI study. European Archives of Psychiatry and Clinical Neuroscience, 263(6), 469–473. 10.1007/s00406-012-0383-y [DOI] [PubMed] [Google Scholar]
- Firth, J. , Cotter, J. , Elliott, R. , French, P. , & Yung, A. R. (2015). A systematic review and meta‐analysis of exercise interventions in schizophrenia patients. Psychological Medicine, 45(7), 1343–1361. 10.1017/S0033291714003110 [DOI] [PubMed] [Google Scholar]
- Fornito, A. , Yucel, M. , Patti, J. , Wood, S. J. , & Pantelis, C. (2009). Mapping grey matter reductions in schizophrenia: An anatomical likelihood estimation analysis of voxel‐based morphometry studies. Schizophrenia Research, 108(1–3), 104–113. [DOI] [PubMed] [Google Scholar]
- Fortin, J. P. , Cullen, N. , Sheline, Y. I. , Taylor, W. D. , Aselcioglu, I. , Cook, P. A. , … Shinohara, R. T. (2018). Harmonization of cortical thickness measurements across scanners and sites. NeuroImage, 167, 104–120. 10.1016/j.neuroimage.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , & Frith, C. D. (1995). Schizophrenia: A disconnection syndrome? Clinical Neuroscience, 3(2), 89–97. [PubMed] [Google Scholar]
- Garey, L. J. , Ong, W. Y. , Patel, T. S. , Kanani, M. , Davis, A. , Mortimer, A. M. , … Hirsch, S. R. (1998). Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry, 65(4), 446–453. 10.1136/jnnp.65.4.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz, L. A. , & Lewis, D. A. (1997). Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Archives of General Psychiatry, 54(10), 943–952. 10.1001/archpsyc.1997.01830220065010 [DOI] [PubMed] [Google Scholar]
- Gomez‐Gastiasoro, A. , Zubiaurre‐Elorza, L. , Pena, J. , Ibarretxe‐Bilbao, N. , Rilo, O. , Schretlen, D. J. , & Ojeda, N. (2019). Altered frontal white matter asymmetry and its implications for cognition in schizophrenia: A tractography study. NeuroImage: Clinical, 22, 101781. 10.1016/j.nicl.2019.101781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Q. , Lui, S. , & Sweeney, J. A. (2016). A selective review of cerebral abnormalities in patients with first‐episode schizophrenia before and after treatment. The American Journal of Psychiatry, 173(3), 232–243. 10.1176/appi.ajp.2015.15050641 [DOI] [PubMed] [Google Scholar]
- Green, M. F. , Kern, R. S. , & Heaton, R. K. (2004). Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophrenia Research, 72(1), 41–51. 10.1016/j.schres.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Guo, J. , Han, Y. , Li, Y. , & Reddick, W. E. (2019). Reduced brain microstructural asymmetry in patients with childhood leukemia treated with chemotherapy compared with healthy controls. PLoS One, 14(5), e0216554. 10.1371/journal.pone.0216554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman, B. A. , Jahanshad, N. , Ching, C. R. , Wang, Y. , Kochunov, P. V. , Nichols, T. E. , & Thompson, P. M. (2015). Medial demons registration localizes the degree of genetic influence over subcortical shape variability: An N= 1480 meta‐analysis. The IEEE International Symposium on Biomedical Imaging, 2015, 1402–1406. 10.1109/ISBI.2015.7164138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman, B. A. , Wang, Y. , Rajagopalan, P. , Toga, A. W. , & Thompson, P. M. (2012). Shape matching with medial curves and 1‐D group‐wise registration. Paper presented at the 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI), 2‐5 May 2012.
- Haijma, S. V. , Van Haren, N. , Cahn, W. , Koolschijn, P. C. , Hulshoff Pol, H. E. , & Kahn, R. S. (2013). Brain volumes in schizophrenia: A meta‐analysis in over 18 000 subjects. Schizophrenia Bulletin, 39(5), 1129–1138. 10.1093/schbul/sbs118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms, M. P. , Wang, L. , Mamah, D. , Barch, D. M. , Thompson, P. A. , & Csernansky, J. G. (2007). Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. The Journal of Neuroscience, 27(50), 13835–13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, P. J. (1999a). The neuropathological effects of antipsychotic drugs. Schizophrenia Research, 40(2), 87–99. 10.1016/s0920-9964(99)00065-1 [DOI] [PubMed] [Google Scholar]
- Harrison, P. J. (1999b). The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain, 122(Pt 4), 593–624. 10.1093/brain/122.4.593 [DOI] [PubMed] [Google Scholar]
- Harrison, P. J. , & Eastwood, S. L. (1998). Preferential involvement of excitatory neurons in medial temporal lobe in schizophrenia. Lancet, 352(9141), 1669–1673. 10.1016/S0140-6736(98)03341-8 [DOI] [PubMed] [Google Scholar]
- Hashimoto, N. , Ito, Y. M. , Okada, N. , Yamamori, H. , Yasuda, Y. , Fujimoto, M. , … Cocoro . (2018). The effect of duration of illness and antipsychotics on subcortical volumes in schizophrenia: Analysis of 778 subjects. NeuroImage: Clinical, 17, 563–569. 10.1016/j.nicl.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukvik, U. K. , Hartberg, C. B. , & Agartz, I. (2013). Schizophrenia – What does structural MRI show? Tidsskrift for Den Norske Legeforening, 133, 850–853. [DOI] [PubMed] [Google Scholar]
- Ho, B. C. , Andreasen, N. C. , Ziebell, S. , Pierson, R. , & Magnotta, V. (2011). Long‐term antipsychotic treatment and brain volumes: A longitudinal study of first‐episode schizophrenia. Archives of General Psychiatry, 68(2), 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, T. C. , Gutman, B. , Pozzi, E. , Grabe, H. J. , Hosten, N. , Wittfeld, K. , … Schmaal, L. (2020). Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Human Brain Mapping. 10.1002/hbm.24988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg, Y. , & Benjamini, Y. (1990). More powerful procedures for multiple significance testing. Statistics in Medicine, 9(7), 811–818. [DOI] [PubMed] [Google Scholar]
- Honea, R. , Crow, T. J. , Passingham, D. , & Mackay, C. E. (2005). Regional deficits in brain volume in schizophrenia: A meta‐analysis of voxel‐based morphometry studies. The American Journal of Psychiatry, 162(12), 2233–2245. [DOI] [PubMed] [Google Scholar]
- Howells, F. M. , Baldwin, D. S. , & Kingdon, D. G. (2016). Can cognitive behaviour therapy beneficially influence arousal mechanisms in psychosis? Human Psychopharmacology, 31(1), 64–69. 10.1002/hup.2499 [DOI] [PubMed] [Google Scholar]
- Jahanshad, N. , & Thompson, P. M. (2017). Multimodal neuroimaging of male and female brain structure in health and disease across the life span. Journal of Neuroscience Research, 95(1–2), 371–379. 10.1002/jnr.23919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski, M. M. , Ronnqvist, K. C. , Tsanov, M. , Vann, S. D. , Wright, N. F. , Erichsen, J. T. , … O'Mara, S. M. (2013). The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Frontiers in Systems Neuroscience, 7, 45. 10.3389/fnsys.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel, E. , Schwartz, J. , & Jessell, T. (Eds.). (1991). Principles of neural science. Norwald: Connecticut: Appleton & Lange. [Google Scholar]
- Kandola, A. , Hendrikse, J. , Lucassen, P. J. , & Yucel, M. (2016). Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: Practical implications for mental health treatment. Frontiers in Human Neuroscience, 10, 373. 10.3389/fnhum.2016.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan, M. S. , Prasad, K. M. , & Pearlson, G. (2007). Are brain structural abnormalities useful as endophenotypes in schizophrenia? International Review of Psychiatry, 19(4), 397–406. [DOI] [PubMed] [Google Scholar]
- Khan, A. R. , Wang, L. , & Beg, M. F. (2008). FreeSurfer‐initiated fully‐automated subcortical brain segmentation in MRI using large deformation diffeomorphic metric mapping. NeuroImage, 41(3), 735–746. 10.1016/j.neuroimage.2008.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia, S. M. , Huijsdens, H. , Dinga, R. , Wolfers, T. , Mennes, M. , Andreassen, O. A. , . . . Marquand, A. F. (2020). Hierarchical bayesian regression for multi‐site normative modeling of neuroimaging data. arXiv:2005.12055
- Konradi, C. , & Heckers, S. (2001). Antipsychotic drugs and neuroplasticity: Insights into the treatment and neurobiology of schizophrenia. Biological Psychiatry, 50(10), 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczmanski, P. , Heinsen, H. , Mantua, V. , Woltersdorf, F. , Masson, T. , Ulfig, N. , … Schmitz, C. (2007). Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain, 130(Pt 3), 678–692. 10.1093/brain/awl386 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte, N. , Goebel, R. , & Bandettini, P. (2006). Information‐based functional brain mapping. Proceedings of the National Academy of Sciences of the United States of America, 103(10), 3863–3868. 10.1073/pnas.0600244103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari, V. , Antonova, E. , Fannon, D. , Peters, E. R. , Ffytche, D. H. , Premkumar, P. , … Kuipers, E. (2010). Beyond dopamine: Functional MRI predictors of responsiveness to cognitive behaviour therapy for psychosis. Frontiers in Behavioral Neuroscience, 4, 4. 10.3389/neuro.08.004.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langers, D. R. , Jansen, J. F. , & Backes, W. H. (2007). Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. NeuroImage, 38(1), 43–56. 10.1016/j.neuroimage.2007.07.031 [DOI] [PubMed] [Google Scholar]
- Lawrie, S. M. , & Abukmeil, S. S. (1998). Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. The British Journal of Psychiatry, 172, 110–120. [DOI] [PubMed] [Google Scholar]
- Lee, J. M. , Kim, S. H. , Jang, D. P. , Ha, T. H. , Kim, J. J. , Kim, I. Y. , … Kim, S. I. (2004). Deformable model with surface registration for hippocampal shape deformity analysis in schizophrenia. NeuroImage, 22(2), 831–840. 10.1016/j.neuroimage.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Lerch, J. P. , Carroll, J. B. , Spring, S. , Bertram, L. N. , Schwab, C. , Hayden, M. R. , & Henkelman, R. M. (2008). Automated deformation analysis in the YAC128 Huntington disease mouse model. NeuroImage, 39(1), 32–39. 10.1016/j.neuroimage.2007.08.033 [DOI] [PubMed] [Google Scholar]
- Levitt, J. J. , Bobrow, L. , Lucia, D. , & Srinivasan, P. (2010). A selective review of volumetric and morphometric imaging in schizophrenia. Current Topics in Behavioral Neurosciences, 4, 243–281. [DOI] [PubMed] [Google Scholar]
- Li, W. , Li, K. , Guan, P. , Chen, Y. , Xiao, Y. , Lui, S. , … Gong, Q. (2018). Volume alteration of hippocampal subfields in first‐episode antipsychotic‐naive schizophrenia patients before and after acute antipsychotic treatment. NeuroImage: Clinical, 20, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Black, M. , Xia, S. , Zhan, C. , Bertisch, H. C. , Branch, C. A. , & DeLisi, L. E. (2015). Subcortical structure alterations impact language processing in individuals with schizophrenia and those at high genetic risk. Schizophrenia Research, 169(1–3), 76–82. 10.1016/j.schres.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Maiti, P. , Thomopoulos, S. , Zhu, A. , Chai, Y. , Kim, H. , & Jahanshad, N. (2021). Style transfer using generative adversarial networks for multi‐site MRI harmonization. bioRxiv, 2021.2003.2017.435892. 10.1101/2021.03.17.435892 [DOI] [PMC free article] [PubMed]
- Malchow, B. , Keeser, D. , Keller, K. , Hasan, A. , Rauchmann, B. S. , Kimura, H. , … Falkai, P. (2016). Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophrenia Research, 173(3), 182–191. 10.1016/j.schres.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Mamah, D. , Alpert, K. I. , Barch, D. M. , Csernansky, J. G. , & Wang, L. (2016). Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. NeuroImage: Clinical, 11, 276–286. 10.1016/j.nicl.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah, D. , Harms, M. P. , Barch, D. , Styner, M. , Lieberman, J. A. , & Wang, L. (2012). Hippocampal shape and volume changes with antipsychotics in early stage psychotic illness. Frontiers in Psychiatry, 3, 96. 10.3389/fpsyt.2012.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah, D. , Harms, M. P. , Wang, L. , Barch, D. , Thompson, P. , Kim, J. , … Csernansky, J. G. (2008). Basal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biological Psychiatry, 64(2), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah, D. , Wang, L. , Barch, D. , de Erausquin, G. A. , Gado, M. , & Csernansky, J. G. (2007). Structural analysis of the basal ganglia in schizophrenia. Schizophrenia Research, 89(1–3), 59–71. 10.1016/j.schres.2006.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley, R. W. , Wible, C. G. , Frumin, M. , Hirayasu, Y. , Levitt, J. J. , Fischer, I. A. , & Shenton, M. E. (1999). MRI anatomy of schizophrenia. Biological Psychiatry, 45(9), 1099–1119 doi:S0006‐3223(99)00018‐9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure, R. K. , Styner, M. , Maltbie, E. , Lieberman, J. A. , Gouttard, S. , Gerig, G. , … Zhu, H. (2013). Localized differences in caudate and hippocampal shape are associated with schizophrenia but not antipsychotic type. Psychiatry Research, 211(1), 1–10. 10.1016/j.pscychresns.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon, R. A. , Abi‐Dargham, A. , & Howes, O. D. (2019). Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends in Neurosciences, 42(3), 205–220. 10.1016/j.tins.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna, F. , Babb, J. , Miles, L. , Goff, D. , & Lazar, M. (2020). Reduced microstructural lateralization in males with chronic schizophrenia: A diffusional kurtosis imaging study. Cerebral Cortex, 30(4), 2281–2294. 10.1093/cercor/bhz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Lindenberg, A. , Poline, J. B. , Kohn, P. D. , Holt, J. L. , Egan, M. F. , Weinberger, D. R. , & Berman, K. F. (2001). Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. The American Journal of Psychiatry, 158(11), 1809–1817. [DOI] [PubMed] [Google Scholar]
- Narr, K. L. , Thompson, P. M. , Sharma, T. , Moussai, J. , Blanton, R. , Anvar, B. , … Toga, A. W. (2001). Three‐dimensional mapping of temporo‐limbic regions and the lateral ventricles in schizophrenia: Gender effects. Biological Psychiatry, 50(2), 84–97. [DOI] [PubMed] [Google Scholar]
- Nuechterlein, K. H. , Subotnik, K. L. , Green, M. F. , Ventura, J. , Asarnow, R. F. , Gitlin, M. J. , … Mintz, J. (2011). Neurocognitive predictors of work outcome in recent‐onset schizophrenia. Schizophrenia Bulletin, 37(Suppl 2), S33–S40. 10.1093/schbul/sbr084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, N. , Fukunaga, M. , Yamashita, F. , Koshiyama, D. , Yamamori, H. , Ohi, K. , … Hashimoto, R. (2016). Abnormal asymmetries in subcortical brain volume in schizophrenia. Molecular Psychiatry, 21(10), 1460–1466. 10.1038/mp.2015.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk, F. G. , Wobrock, T. , Gruber, O. , Scherk, H. , Berner, D. , Kaizl, I. , … Falkai, P. (2010). Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry, 67(2), 133–143. 10.1001/archgenpsychiatry.2009.193 [DOI] [PubMed] [Google Scholar]
- Palaniyappan, L. , Balain, V. , & Liddle, P. F. (2012). The neuroanatomy of psychotic diathesis: A meta‐analytic review. Journal of Psychiatric Research, 46(10), 1249–1256. [DOI] [PubMed] [Google Scholar]
- Pantelis, C. , Yucel, M. , Bora, E. , Fornito, A. , Testa, R. , Brewer, W. J. , … Wood, S. J. (2009). Neurobiological markers of illness onset in psychosis and schizophrenia: The search for a moving target. Neuropsychology Review, 19(3), 385–398. 10.1007/s11065-009-9114-1 [DOI] [PubMed] [Google Scholar]
- Park, S. W. , Lee, C. H. , Cho, H. Y. , Seo, M. K. , Lee, J. G. , Lee, B. J. , … Kim, Y. H. (2013). Effects of antipsychotic drugs on the expression of synaptic proteins and dendritic outgrowth in hippocampal neuronal cultures. Synapse, 67(5), 224–234. 10.1002/syn.21634 [DOI] [PubMed] [Google Scholar]
- Pearlson, G. D. , & Calhoun, V. (2007). Structural and functional magnetic resonance imaging in psychiatric disorders. Canadian Journal of Psychiatry, 52(3), 158–166. [DOI] [PubMed] [Google Scholar]
- Pearlson, G. D. , & Marsh, L. (1999). Structural brain imaging in schizophrenia: A selective review. Biological Psychiatry, 46(5), 627–649. [DOI] [PubMed] [Google Scholar]
- Pomponio, R. , Erus, G. , Habes, M. , Doshi, J. , Srinivasan, D. , Mamourian, E. , … Davatzikos, C. (2020). Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. NeuroImage, 208, 116450. 10.1016/j.neuroimage.2019.116450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, S. M. , Wray, N. R. , Stone, J. L. , Visscher, P. M. , O'Donovan, M. C. , Sullivan, P. F. , … International Schizophrenia, C . (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature, 460(7256), 748–752. 10.1038/nature08185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, A. , Gan, S. C. , Wang, Y. , & Sim, K. (2013). Amygdala‐hippocampal shape and cortical thickness abnormalities in first‐episode schizophrenia and mania. Psychological Medicine, 43(7), 1353–1363. 10.1017/S0033291712002218 [DOI] [PubMed] [Google Scholar]
- Qiu, A. , & Miller, M. I. (2008). Multi‐structure network shape analysis via normal surface momentum maps. NeuroImage, 42(4), 1430–1438. 10.1016/j.neuroimage.2008.04.257 [DOI] [PubMed] [Google Scholar]
- Qiu, A. , Tuan, T. A. , Woon, P. S. , Abdul‐Rahman, M. F. , Graham, S. , & Sim, K. (2010). Hippocampal‐cortical structural connectivity disruptions in schizophrenia: An integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. NeuroImage, 52(4), 1181–1189. 10.1016/j.neuroimage.2010.05.046 [DOI] [PubMed] [Google Scholar]
- Radua, J. , Vieta, E. , Shinohara, R. , Kochunov, P. , Quide, Y. , Green, M. J. , … Collaborators, E. C . (2020). Increased power by harmonizing structural MRI site differences with the ComBat batch adjustment method in ENIGMA. NeuroImage, 218, 116956. 10.1016/j.neuroimage.2020.116956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria, M. E. , Hansell, N. K. , Strike, L. T. , McMahon, K. L. , de Zubicaray, G. I. , Hickie, I. B. , … Wright, M. J. (2014). Genetic architecture of subcortical brain regions: Common and region‐specific genetic contributions. Genes, Brain, and Behavior, 13(8), 821–830. 10.1111/gbb.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf, D. R. , Vandekar, S. N. , Almasy, L. , Ruparel, K. , Satterthwaite, T. D. , Elliott, M. A. , … Gur, R. E. (2015). Heritability of subcortical and limbic brain volume and shape in multiplex‐multigenerational families with schizophrenia. Biological Psychiatry, 77(2), 137–146. 10.1016/j.biopsych.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz‐Santianez, R. , Ayesa‐Arriola, R. , Tordesillas‐Gutierrez, D. , Ortiz‐Garcia de la Foz, V. , Perez‐Iglesias, R. , Pazos, A. , … Crespo‐Facorro, B. (2014). Three‐year longitudinal population‐based volumetric MRI study in first‐episode schizophrenia spectrum patients. Psychological Medicine, 44(8), 1591–1604. 10.1017/S0033291713002365 [DOI] [PubMed] [Google Scholar]
- Roiz‐Santianez, R. , Suarez‐Pinilla, P. , & Crespo‐Facorro, B. (2015). Brain structural effects of antipsychotic treatment in schizophrenia: A systematic review. Current Neuropharmacology, 13(4), 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshchupkin, G. V. , Gutman, B. A. , Vernooij, M. W. , Jahanshad, N. , Martin, N. G. , Hofman, A. , … Adams, H. H. H. (2016). Heritability of the shape of subcortical brain structures in the general population. Nature Communications, 7, 13738. 10.1038/ncomms13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoklija, G. , Toomayan, G. , Ellis, S. P. , Keilp, J. , Mann, J. J. , Latov, N. , … Dwork, A. J. (2000). Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: Preliminary findings. Archives of General Psychiatry, 57(4), 349–356. 10.1001/archpsyc.57.4.349 [DOI] [PubMed] [Google Scholar]
- Satizabal, C. L. , Adams, H. H. H. , Hibar, D. P. , White, C. C. , Knol, M. J. , Stein, J. L. , … Ikram, M. A. (2019). Genetic architecture of subcortical brain structures in 38,851 individuals. Nature Genetics, 51(11), 1624–1636. 10.1038/s41588-019-0511-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, M. K. , Wang, L. , Selemon, L. D. , Goldman‐Rakic, P. S. , Rakic, P. , & Csernansky, J. G. (2002). Abnormalities of thalamic volume and shape detected in fetally irradiated rhesus monkeys with high dimensional brain mapping. Biological Psychiatry, 51(10), 827–837. 10.1016/s0006-3223(01)01341-5 [DOI] [PubMed] [Google Scholar]
- Schmitt, A. , Hasan, A. , Gruber, O. , & Falkai, P. (2011). Schizophrenia as a disorder of disconnectivity. European Archives of Psychiatry and Clinical Neuroscience, 261(Suppl 2), S150–S154. 10.1007/s00406-011-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton, M. E. , Dickey, C. C. , Frumin, M. , & McCarley, R. W. (2001). A review of MRI findings in schizophrenia. Schizophrenia Research, 49(1–2), 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton, M. E. , Whitford, T. J. , & Kubicki, M. (2010). Structural neuroimaging in schizophrenia: From methods to insights to treatments. Dialogues in Clinical Neuroscience, 12(3), 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, S. M. , & Guillery, R. W. (2011). Distinct functions for direct and transthalamic corticocortical connections. Journal of Neurophysiology, 106(3), 1068–1077. [DOI] [PubMed] [Google Scholar]
- Smeland, O. B. , Frei, O. , Kauppi, K. , Hill, W. D. , Li, W. , Wang, Y. , … Neuro, C. C. W. G. (2017). Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal‐numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry, 74(10), 1065–1075. 10.1001/jamapsychiatry.2017.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Thompson, P. M. , Cannon, T. D. , Narr, K. L. , van Erp, T. , Poutanen, V. P. , Huttunen, M. , … Toga, A. W. (2001). Genetic influences on brain structure. Nature Neuroscience, 4(12), 1253–1258. 10.1038/nn758 [DOI] [PubMed] [Google Scholar]
- Thompson, P. M. , Hayashi, K. M. , de Zubicaray, G. , Janke, A. L. , Rose, S. E. , Semple, J. , … Toga, A. W. (2003). Dynamics of gray matter loss in Alzheimer's disease. The Journal of Neuroscience, 23(3), 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. M. , Jahanshad, N. , Ching, C. R. K. , Salminen, L. E. , Thomopoulos, S. I. , Bright, J. , … Consortium, E. (2020). ENIGMA and global neuroscience: A decade of large‐scale studies of the brain in health and disease across more than 40 countries. Translational Psychiatry, 10(1), 100. 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. M. , Stein, J. L. , Medland, S. E. , Hibar, D. P. , Vasquez, A. A. , Renteria, M. E. , … Alzheimer's Disease Neuroimaging Initiative, E. C. I. C. S. Y. S. G . (2014). The ENIGMA Consortium: Large‐scale collaborative analyses of neuroimaging and genetic data. Brain Imaging and Behavior, 8(2), 153–182. 10.1007/s11682-013-9269-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant, M. , Qiu, A. , Glaunes, J. , & Miller, M. I. (2007). Diffeomorphic metric surface mapping in subregion of the superior temporal gyrus. NeuroImage, 34(3), 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrusheva, J. , Marino, B. , Stroup, T. S. , & Kimhy, D. (2016). Aerobic exercise in people with schizophrenia: Neural and neurocognitive benefits. Current Behavioral Neuroscience Reports, 3(2), 165–175. 10.1007/s40473-016-0077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg, D. , Gong, P. , Breakspear, M. , & van Leeuwen, C. (2012). Fragmentation: Loss of global coherence or breakdown of modularity in functional brain architecture? Frontiers in Systems Neuroscience, 6, 20. 10.3389/fnsys.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen, M. , Wierenga, L. M. , Achterberg, M. , Drenth, N. , Van, I. M. H. , & Crone, E. A. (2020). Genetic and environmental influences on structure of the social brain in childhood. Developmental Cognitive Neuroscience, 44, 100782. 10.1016/j.dcn.2020.100782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp, T. G. , Hibar, D. P. , Rasmussen, J. M. , Glahn, D. C. , Pearlson, G. D. , Andreassen, O. A. , … Turner, J. A. (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21(4), 585. 10.1038/mp.2015.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort, D. , Rosenbaum, S. , Ward, P. B. , & Stubbs, B. (2015). Exercise improves cardiorespiratory fitness in people with schizophrenia: A systematic review and meta‐analysis. Schizophrenia Research, 169(1–3), 453–457. 10.1016/j.schres.2015.09.029 [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Wolf, A. P. , Brodie, J. D. , Cancro, R. , Overall, J. E. , Rhoades, H. , & Van Gelder, P. (1988). Brain interactions in chronic schizophrenics under resting and activation conditions. Schizophrenia Research, 1(1), 47–53. [DOI] [PubMed] [Google Scholar]
- Wang, L. , & Csernansky, J. G. (2014). Recent advances in neuroimaging biomarkers of schizophrenia. In Janicak P. G., Marder S. R., Tandon R., & Goldman M. (Eds.), Schizophrenia (pp. 71–103). New York: Springer. [Google Scholar]
- Wang, L. , Joshi, S. C. , Miller, M. I. , & Csernansky, J. G. (2001). Statistical analysis of hippocampal asymmetry in schizophrenia. NeuroImage, 14(3), 531–545. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Khan, A. , Csernansky, J. G. , Fischl, B. , Miller, M. I. , Morris, J. C. , & Beg, M. F. (2009). Fully‐automated, multi‐stage hippocampus mapping in very mild Alzheimer disease. Hippocampus, 19(6), 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Mamah, D. , Harms, M. P. , Karnik, M. , Price, J. L. , Gado, M. H. , … Csernansky, J. G. (2008). Progressive deformation of deep brain nuclei and hippocampal‐amygdala formation in schizophrenia. Biological Psychiatry, 64(12), 1060–1068. 10.1016/j.biopsych.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Zhang, J. , Liu, Z. , Crow, T. J. , Zhang, K. , Palaniyappan, L. , … Li, T. (2019). "brain connectivity deviates by sex and hemisphere in the first episode of schizophrenia"‐A route to the genetic basis of language and psychosis? Schizophrenia Bulletin, 45(2), 484–494. 10.1093/schbul/sby061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. , Zhang, X. , Li, A. , Zhu, M. , Liu, S. , Qin, W. , … Liu, B. (2017). Polygenic risk for five psychiatric disorders and cross‐disorder and disorder‐specific neural connectivity in two independent populations. NeuroImage: Clinical, 14, 441–449. 10.1016/j.nicl.2017.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Song, Y. , Rajagopalan, P. , An, T. , Liu, K. , Chou, Y. Y. , … Alzheimer's Disease Neuroimaging, I . (2011). Surface‐based TBM boosts power to detect disease effects on the brain: An N=804 ADNI study. NeuroImage, 56(4), 1993–2010. 10.1016/j.neuroimage.2011.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger, D. R. , Berman, K. F. , Suddath, R. , & Torrey, E. F. (1992). Evidence of dysfunction of a prefrontal‐limbic network in schizophrenia: A magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. The American Journal of Psychiatry, 149(7), 890–897. [DOI] [PubMed] [Google Scholar]
- Womer, F. Y. , Wang, L. , Alpert, K. I. , Smith, M. J. , Csernansky, J. G. , Barch, D. M. , & Mamah, D. (2014). Basal ganglia and thalamic morphology in schizophrenia and bipolar disorder. Psychiatry Research, 223(2), 75–83. 10.1016/j.pscychresns.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. W. (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of Clinical Psychiatry, 64(6), 663–667. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Nuechterlein, K. H. , Phillips, O. R. , Gutman, B. , Kurth, F. , Dinov, I. , … Narr, K. L. (2012). Disease and genetic contributions toward local tissue volume disturbances in schizophrenia: A tensor‐based morphometry study. Human Brain Mapping, 33(9), 2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. A. , Manaye, K. F. , Liang, C. , Hicks, P. B. , & German, D. C. (2000). Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biological Psychiatry, 47(11), 944–953. [DOI] [PubMed] [Google Scholar]
- Zhu, F. , Liu, F. , Guo, W. , Chen, J. , Su, Q. , Zhang, Z. , … Zhao, J. (2018). Disrupted asymmetry of inter‐ and intra‐hemispheric functional connectivity in patients with drug‐naive, first‐episode schizophrenia and their unaffected siblings. eBioMedicine, 36, 429–435. 10.1016/j.ebiom.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


