Abstract
Age has a major effect on brain volume. However, the normative studies available are constrained by small sample sizes, restricted age coverage and significant methodological variability. These limitations introduce inconsistencies and may obscure or distort the lifespan trajectories of brain morphometry. In response, we capitalized on the resources of the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) Consortium to examine age‐related trajectories inferred from cross‐sectional measures of the ventricles, the basal ganglia (caudate, putamen, pallidum, and nucleus accumbens), the thalamus, hippocampus and amygdala using magnetic resonance imaging data obtained from 18,605 individuals aged 3–90 years. All subcortical structure volumes were at their maximum value early in life. The volume of the basal ganglia showed a monotonic negative association with age thereafter; there was no significant association between age and the volumes of the thalamus, amygdala and the hippocampus (with some degree of decline in thalamus) until the sixth decade of life after which they also showed a steep negative association with age. The lateral ventricles showed continuous enlargement throughout the lifespan. Age was positively associated with inter‐individual variability in the hippocampus and amygdala and the lateral ventricles. These results were robust to potential confounders and could be used to examine the functional significance of deviations from typical age‐related morphometric patterns.
Keywords: brain morphometry, ENIGMA, longitudinal trajectories, multisite
We analyzed subcortical volumes from 18,605 healthy individuals from multiple cross‐sectional cohorts to infer age‐related trajectories between the ages of 3 and 90 years.

1. INTRODUCTION
Over the last 20 years, studies using structural magnetic resonance imaging (MRI) have confirmed that brain morphometric measures change with age. In general, whole brain, global and regional gray matter volumes increase during development and decrease with aging (Brain Development Cooperative Group, 2012; Driscoll et al., 2009; Fotenos, Snyder, Girton, Morris, & Buckner, 2005; Good et al., 2001; Pfefferbaum et al., 2013; Pomponio et al., 2019; Raz et al., 2005; Raznahan et al., 2014; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003; Walhovd et al., 2011). However, most published studies are constrained by small sample sizes, restricted age coverage and methodological variability. These limitations introduce inconsistencies and may obscure or distort the lifespan trajectories of brain structures. To address these limitations, we formed the Lifespan Working group of the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) Consortium (Thompson et al., 2014, 2017) to perform large‐scale analyses of brain morphometric data extracted from MRI images using standardized protocols and unified quality control procedures, harmonized and validated across all participating sites.
Here we focus on ventricular, striatal (caudate, putamen, nucleus accumbens), pallidal, thalamic, hippocampal and amygdala volumes. Subcortical structures are crucial for normal cognitive and emotional adaptation (Grossberg, 2009). The striatum and pallidum (together referred to as basal ganglia) are best known for their role in action selection and movement coordination (Calabresi, Picconi, Tozzi, Ghiglieri, & Di Filippo, 2014) but they are also involved in other aspects of cognition particularly memory, inhibitory control, reward and salience processing (Chudasama & Robbins, 2006; Richard, Castro, Difeliceantonio, Robinson, & Berridge, 2013; Scimeca & Badre, 2012; Tremblay, Worbe, Thobois, Sgambato‐Faure, & Féger, 2015). The role of the hippocampus has been most clearly defined in connection to declarative memory (Eichenbaum, 2004; Shohamy & Turk‐Browne, 2013) while the amygdala has been historically linked to affect processing (Kober et al., 2008). The thalamus is centrally located in the brain and acts as a key hub for the integration of motor and sensory information with higher‐order functions (Sherman, 2005; Zhang, Snyder, Shimony, Fox, & Raichle, 2010). The role of subcortical structures extends beyond normal cognition because changes in the volume of these regions have been reliably identified in developmental (Ecker, Bookheimer, & Murphy, 2015; Krain & Castellanos, 2006), psychiatric (Hibar et al., 2016; Kempton et al., 2011; Schmaal et al., 2016; van Erp et al., 2016) and degenerative disorders (Risacher et al., 2009).
Using data from 18,605 individuals aged 3–90 years from the ENIGMA Lifespan working group we delineated the association between age and subcortical volumes from early to late life in order to (a) identify periods of volume change or stability, (b) provide normative, age‐adjusted centile curves of subcortical volumes and (c) quantify inter‐individual variability in subcortical volumes which is considered a major source of inter‐study differences (Dickie et al., 2013; Raz, Ghisletta, Rodrigue, Kennedy, & Lindenberger, 2010).
2. MATERIALS AND METHODS
2.1. Study samples
The study data derive from 88 samples comprising 18,605 healthy participants, aged 3–90 years, with near equal representation of men and women (48% and 52%) (Table 1, Figure 1). At the time of scanning, participating individuals were screened to exclude the presence of mental disorders, cognitive impairment or significant medical morbidity. Details of the screening process and eligibility criteria for each research group are shown in Table S1).
TABLE 1.
Characteristics of the included samples
| Sample | Age, mean, years | Age, SD, years | Age range | Sample size N | Number of males | Number of females | |
|---|---|---|---|---|---|---|---|
| ABIDE | 17 | 7.8 | 6 | 56 | 534 | 439 | 95 |
| ADHD NF | 13 | 1 | 12 | 15 | 13 | 7 | 6 |
| ADNI | 76 | 5.1 | 60 | 90 | 150 | 70 | 80 |
| ADNI2GO | 73 | 6.1 | 56 | 89 | 133 | 55 | 78 |
| AMC | 23 | 3.4 | 17 | 32 | 92 | 60 | 32 |
| Barcelona 1.5T | 15 | 1.8 | 11 | 17 | 30 | 14 | 16 |
| Barcelona 3T | 15 | 2.1 | 11 | 17 | 44 | 24 | 20 |
| Betula | 61 | 12.9 | 25 | 81 | 234 | 104 | 130 |
| BIG 1.5T | 28 | 13.3 | 13 | 77 | 1,288 | 628 | 660 |
| BIG 3T | 24 | 7.9 | 18 | 69 | 1,276 | 540 | 736 |
| BIL&GIN | 27 | 7.8 | 18 | 57 | 444 | 217 | 227 |
| Bonn | 39 | 6.5 | 29 | 50 | 174 | 174 | 0 |
| BRAINSCALE | 10 | 1.4 | 9 | 15 | 270 | 125 | 145 |
| BRCATLAS | 38 | 15.8 | 18 | 80 | 153 | 77 | 76 |
| CAMH | 41 | 17.6 | 18 | 86 | 128 | 65 | 63 |
| Cardiff | 25 | 7.4 | 18 | 58 | 316 | 87 | 229 |
| CEG | 16 | 1.7 | 13 | 19 | 32 | 32 | 0 |
| CIAM | 27 | 5 | 19 | 40 | 30 | 16 | 14 |
| CLING | 25 | 5.3 | 18 | 58 | 320 | 131 | 189 |
| CODE | 40 | 13.3 | 20 | 64 | 74 | 31 | 43 |
| COMPULS/TS Eurotrain | 11 | 1 | 9 | 13 | 53 | 36 | 17 |
| Dublin (1) | 37 | 13 | 17 | 65 | 52 | 23 | 29 |
| Dublin (2) | 30 | 8.3 | 19 | 52 | 92 | 51 | 41 |
| Edinburgh | 24 | 2.9 | 19 | 31 | 55 | 35 | 20 |
| ENIGMA‐HIV | 25 | 4.4 | 19 | 33 | 31 | 16 | 15 |
| ENIGMA‐OCD (AMC/Huyser) | 14 | 2.6 | 9 | 17 | 23 | 9 | 14 |
| ENIGMA‐OCD (IDIBELL) | 33 | 10.1 | 18 | 61 | 65 | 29 | 36 |
| ENIGMA‐OCD (Kyushu/Nakao) | 39 | 12.5 | 22 | 63 | 40 | 15 | 25 |
| ENIGMA‐OCD (London Cohort/Mataix‐Cols) | 37 | 11.2 | 21 | 63 | 32 | 11 | 21 |
| ENIGMA‐OCD (van den Heuvel 1.5T) | 31 | 7.6 | 21 | 53 | 48 | 18 | 30 |
| ENIGMA‐OCD (van den Heuvel 3T) | 39 | 11.2 | 22 | 64 | 35 | 16 | 19 |
| ENIGMA‐OCD‐3T‐CONTROLS | 31 | 10.6 | 19 | 56 | 27 | 10 | 17 |
| FBIRN | 37 | 11.2 | 19 | 60 | 173 | 123 | 50 |
| FIDMAG | 38 | 10.2 | 19 | 64 | 122 | 53 | 69 |
| GSP | 26 | 14.9 | 18 | 89 | 1962 | 860 | 1,102 |
| HMS | 40 | 12.2 | 19 | 64 | 55 | 21 | 34 |
| HUBIN | 42 | 8.9 | 19 | 56 | 99 | 66 | 33 |
| IDIVAL (1) | 65 | 10.2 | 49 | 87 | 31 | 10 | 21 |
| IDIVAL (3) | 30 | 7.7 | 19 | 50 | 114 | 69 | 45 |
| IDIVAL(2) | 28 | 7.6 | 15 | 52 | 79 | 49 | 30 |
| IMAGEN | 14 | 0.4 | 13 | 16 | 1744 | 864 | 880 |
| IMH | 32 | 10 | 20 | 59 | 79 | 50 | 29 |
| IMpACT‐NL | 37 | 12 | 19 | 63 | 134 | 52 | 82 |
| Indiana 1.5T | 60 | 11 | 37 | 79 | 41 | 7 | 34 |
| Indiana 3T | 27 | 18.8 | 6 | 73 | 197 | 95 | 102 |
| Johns Hopkins | 44 | 12.5 | 20 | 65 | 87 | 41 | 46 |
| KaSP | 27 | 5.7 | 20 | 43 | 32 | 15 | 17 |
| Leiden | 17 | 4.8 | 8 | 29 | 565 | 274 | 291 |
| MAS | 78 | 4.5 | 70 | 89 | 361 | 137 | 224 |
| MCIC | 33 | 12 | 18 | 60 | 93 | 63 | 30 |
| Melbourne | 20 | 3 | 15 | 26 | 102 | 54 | 48 |
| METHCT | 27 | 7.3 | 18 | 53 | 62 | 48 | 14 |
| MHRC | 22 | 2.9 | 16 | 28 | 52 | 52 | 0 |
| Moods | 33 | 9.8 | 18 | 51 | 310 | 146 | 164 |
| NCNG | 50 | 16.7 | 19 | 79 | 311 | 92 | 219 |
| NESDA | 40 | 9.8 | 21 | 56 | 65 | 22 | 43 |
| NeuroIMAGE | 17 | 3.7 | 8 | 29 | 376 | 172 | 204 |
| Neuroventure | 14 | 0.6 | 12 | 15 | 137 | 62 | 75 |
| NTR (1) | 15 | 1.4 | 11 | 18 | 34 | 11 | 23 |
| NTR (2) | 34 | 10.3 | 19 | 57 | 105 | 39 | 66 |
| NTR (3) | 30 | 5.9 | 20 | 42 | 29 | 11 | 18 |
| NU | 41 | 18.8 | 17 | 68 | 15 | 1 | 14 |
| NUIG | 37 | 11.5 | 18 | 58 | 89 | 50 | 39 |
| NYU | 31 | 8.7 | 19 | 52 | 51 | 31 | 20 |
| OATS (1) | 71 | 5.3 | 65 | 84 | 94 | 27 | 67 |
| OATS (2) | 68 | 4.4 | 65 | 81 | 33 | 13 | 20 |
| OATS (3) | 69 | 4.3 | 65 | 81 | 128 | 44 | 84 |
| OATS (4) | 70 | 4.6 | 65 | 89 | 95 | 23 | 72 |
| OLIN | 36 | 12.8 | 21 | 87 | 594 | 236 | 358 |
| Oxford | 16 | 1.4 | 14 | 19 | 38 | 18 | 20 |
| PING | 12 | 4.9 | 3 | 21 | 518 | 271 | 247 |
| QTIM | 23 | 3.4 | 16 | 30 | 342 | 112 | 230 |
| Sao Paolo 1 | 27 | 5.8 | 17 | 43 | 69 | 45 | 24 |
| Sao Paolo 3 | 30 | 8.1 | 18 | 50 | 83 | 44 | 39 |
| SCORE | 25 | 4.3 | 19 | 39 | 44 | 17 | 27 |
| SHIP 2 | 55 | 12.3 | 31 | 84 | 368 | 206 | 162 |
| SHIP TREND | 50 | 13.9 | 21 | 81 | 788 | 439 | 349 |
| StagedDep | 47 | 8 | 27 | 59 | 84 | 20 | 64 |
| Stanford | 37 | 10.7 | 19 | 61 | 54 | 20 | 34 |
| STROKEMRI | 42 | 21.3 | 18 | 77 | 47 | 17 | 30 |
| Sydney | 37 | 21.1 | 12 | 79 | 147 | 58 | 89 |
| TOP | 35 | 9.8 | 18 | 73 | 296 | 155 | 141 |
| Tuebingen | 40 | 12.1 | 24 | 61 | 53 | 24 | 29 |
| UMC Utrecht 1.5T | 32 | 12.1 | 17 | 66 | 289 | 171 | 118 |
| UMCU 3T | 45 | 15.2 | 19 | 81 | 109 | 52 | 57 |
| UNIBA | 27 | 8.7 | 18 | 63 | 130 | 66 | 64 |
| UPENN | 36 | 13.6 | 16 | 85 | 185 | 85 | 100 |
| Yale | 14 | 2.2 | 10 | 18 | 23 | 12 | 11 |
| Total | 31 | 18.4 | 3 | 90 | 18,605 | 8,980 | 9,625 |
Abbreviations: ABIDE = Autism Brain Imaging Data Exchange; ADNI = Alzheimer's Disease Neuroimaging Initiative; ADNI2GO = ADNI‐GO and ADNI‐2; ADHD‐NF = Attention Deficit Hyperactivity Disorder‐Neurofeedback Study; AMC = Amsterdam Medisch Centrum; Basel = University of Basel; Barcelona = University of Barcelona; Betula = Swedish longitudinal study on aging, memory, and dementia; BIG = Brain Imaging Genetics; BIL&GIN = a multimodal multidimensional database for investigating hemispheric specialization; Bonn = University of Bonn; BrainSCALE = Brain Structure and Cognition: an Adolescence Longitudinal twin study; CAMH = Centre for Addiction and Mental Health; Cardiff = Cardiff University; CEG = Cognitive‐experimental and Genetic study of ADHD and Control Sibling Pairs; CIAM = Cortical Inhibition and Attentional Modulation study; CLiNG = Clinical Neuroscience Göttingen; CODE = formerly Cognitive Behavioral Analysis System of Psychotherapy (CBASP) study; Dublin = Trinity College Dublin; Edinburgh = The University of Edinburgh; ENIGMA‐HIV = Enhancing NeuroImaging Genetics through Meta‐Analysis‐Human Immunodeficiency Virus Working Group; ENIGMA‐OCD = Enhancing NeuroImaging Genetics through Meta‐Analysis‐ Obsessive Compulsive Disorder Working Group; FBIRN = Function Biomedical Informatics Research Network; FIDMAG = Fundación para la Investigación y Docencia Maria Angustias Giménez; GSP = Brain Genomics Superstruct Project; HMS = Homburg Multidiagnosis Study; HUBIN = Human Brain Informatics; IDIVAL = Valdecilla Biomedical Research Institute; IMAGEN = the IMAGEN Consortium; IMH=Institute of Mental Health, Singapore; IMpACT = The International Multicentre persistent ADHD Genetics Collaboration; Indiana = Indiana University School of Medicine; Johns Hopkins = Johns Hopkins University; KaSP = The Karolinska Schizophrenia Project; Leiden = Leiden University; MAS = Memory and Ageing Study; MCIC = MIND Clinical Imaging Consortium formed by the Mental Illness and Neuroscience Discovery (MIND) Institute now the Mind Research Network; Melbourne = University of Melbourne; Meth‐CT = study of methamphetamine users, University of Cape Town; MHRC = Mental Health Research Center; Muenster = Muenster University; N = number; NESDA = The Netherlands Study of Depression and Anxiety; NeuroIMAGE = Dutch part of the International Multicenter ADHD Genetics (IMAGE) study; Neuroventure: the imaging part of the Co‐Venture Trial funded by the Canadian Institutes of Health Research (CIHR); NCNG = Norwegian Cognitive NeuroGenetics sample; NTR = Netherlands Twin Register; NU = Northwestern University; NUIG = National University of Ireland Galway; NYU = New York University; OATS = Older Australian Twins Study; Olin = Olin Neuropsychiatric Research Center; Oxford = Oxford University; QTIM = Queensland Twin Imaging; Sao Paulo = University of Sao Paulo; SCORE = University of Basel Study; SHIP‐2 and SHIP TREND = Study of Health in Pomerania; Staged‐Dep = Stages of Depression Study; Stanford = Stanford University; StrokeMRI = Stroke Magnetic Resonance Imaging; Sydney = University of Sydney; TOP = Tematisk Område Psykoser (Thematically Organized Psychosis Research); TS‐EUROTRAIN = European‐Wide Investigation and Training Network on the Etiology and Pathophysiology of Gilles de la Tourette Syndrome; Tuebingen = University of Tuebingen; UMCU = Universitair Medisch Centrum Utrecht; UNIBA = University of Bari Aldo Moro; UPENN = University of Pennsylvania; Yale = Yale University.
FIGURE 1.
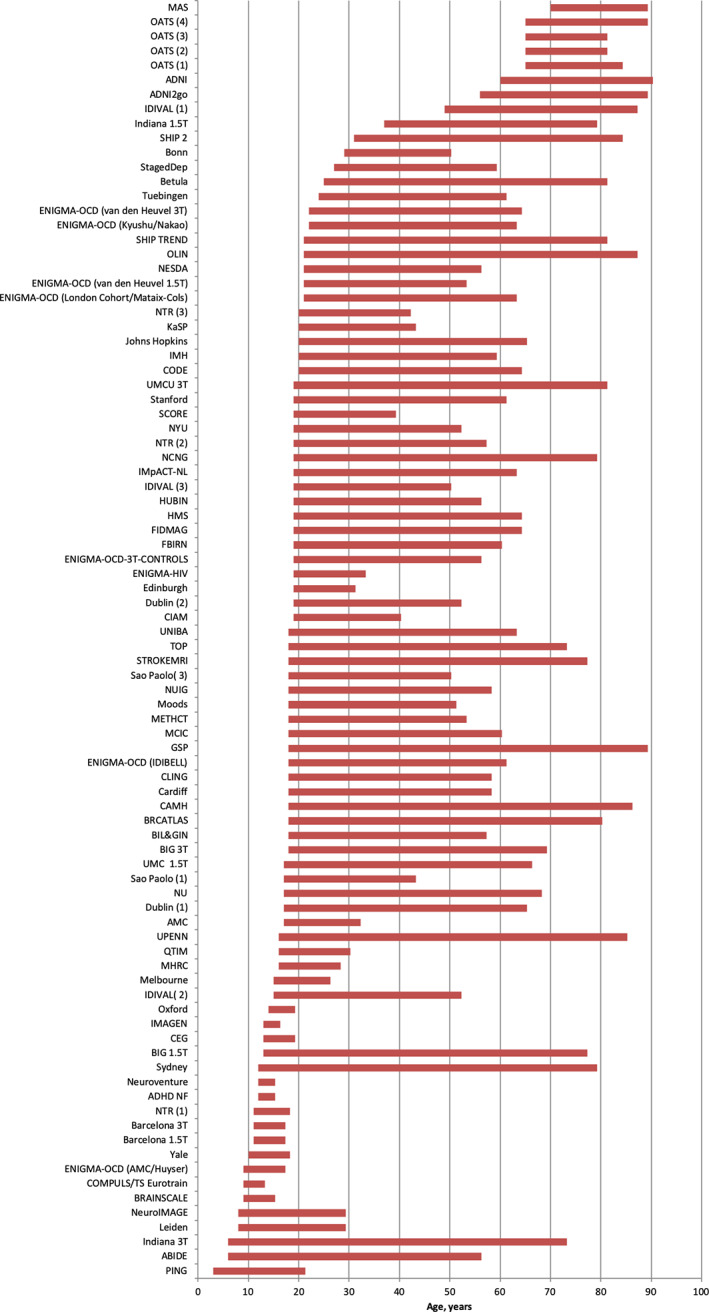
ENIGMA lifespan samples. Details of each sample are provided Table 1 and in the supplemental material. Abbreviations are provided in Table 1
2.2. Neuroimaging
Detailed information on scanner vendor, magnet strength and acquisition parameters for each sample are presented in Table S1. For each sample, the intracranial volume (ICV) and the volume of the basal ganglia (caudate, putamen, pallidum, nucleus accumbens), thalamus, hippocampus, amygdala and lateral ventricles were extracted using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) from high‐resolution T1‐weighted MRI brain scans (Fischl, 2012; Fischl et al., 2002). Prior to data pooling, images were visually inspected at each site to exclude participants whose scans were improperly segmented. After merging the samples, only individuals with complete data were included outliers were identified and excluded using Mahalanobis distances. All analyses described below were repeated for ICV‐unadjusted volumetric measures which yielded identical results and are only presented as a separate supplement.
Approximately 20% of the samples had a multi‐scanner design. During data harmonization the scanner was modeled as a site. In each site, the intracranial volume (Figure S1) was used to adjust the subcortical volumes via a formula based on the analysis of the covariance approach: “adjusted volume = raw volume – b × (ICV – mean ICV)”, where b is the slope of regression of a region of interest volume on ICV (Raz et al., 2005). The values of the subcortical volumes were then harmonized between sites using the ComBat method in R (Fortin et al., 2017, 2018; Radua et al., 2020). Originally developed to adjust for batch effect in genetic studies, ComBat uses an empirical Bayes to adjust for inter‐site variability in the data, while preserving variability related to the variables of interest.
2.3. Fractional polynomial regression analyses
The effect of age on each ICV‐ and site‐adjusted subcortical volume was modeled using high order fractional polynomial regression (Royston & Altman, 1994; Sauerbrei, Meier‐Hirmer, Benner, & Royston, 2006) in each hemisphere. Because the effect of site (scanner and Freesurfer version) was adjusted using ComBat, we only included sex as a covariate in the regression models. Fractional polynomial regression is currently considered the most advantageous modeling strategy for continuous variables (Moore, Hanley, Turgeon, & Lavoie, 2011) as it allows testing for a wider range of trajectory shapes than conventional lower‐order polynomials (e.g., linear or quadratic) and for multiple turning points (Royston & Altman, 1994; Royston, Ambler, & Sauerbrei, 1999). For each subcortical structure, the best model was obtained by comparing competing models of up to three power combinations. The powers used to identify the best fitting model were −2, −1, −0.5, 0.5, 1, 2, 3 and the natural logarithm (ln) function. The optimal model describing the association between age and each of the volumes was selected as the lowest degree model based on the partial F‐test (if linear) or the likelihood‐ratio test. To avoid over‐fitting at ages with more data points, we used the stricter .01 level of significance as the cut‐off for each respective likelihood‐ratio tests, rather than adding powers, until the .05 level was reached. For ease of interpretation we centered the volume of each structure so that the intercept of a fractional polynomial was represented as the effect at zero for sex. Fractional polynomial regression models were fitted using Stata/IC software v.13.1 (Stata Corp., College Station, TX). Standard errors were also adjusted for the effect of site in the FP regression.
We conducted two supplemental analyses: (a) we specified additional FP models separately for males and females and, (b) we calculated Pearson's correlation coefficient between subcortical volumes and age in the early (6–29 years), middle (30–59 years), and late‐life (60–90 years) age‐group. The results of these analyses have been included in the supplemental material.
2.4. Inter‐individual variability
Inter‐individual variability was assessed using two complimentary approaches. First, for each subcortical structure we compared the early (6–29 years), middle (30–59 years) and late‐life (60–90 years) age‐groups in terms of their mean inter‐individual variability; these groups were defined following conventional notions regarding periods of development, midlife and aging. The variance of each structure in each age‐group was calculated as
where e represents the residual variance of each individual (i) around the nonlinear best fitting regression line, and n the number of observations in each age‐group (t). The residuals (e i ) were normally distributed suggesting good fit of the model without having over‐ or under‐fitted the data. Upon calculating the square root of the squared residuals we used the natural logarithm to account for the positive skewness of the new distribution. Then the mean inter‐individual variability between early (6–29 years), middle (30–59 years) and late‐life (60–90 years) age‐groups was compared using between‐groups omnibus tests for the residual variance around the identified best‐fitting nonlinear fractional polynomial model of each structure. We conducted 16 tests (one for each structure) and accordingly the critical alpha value was set at 0.003 following Bonferroni correction for multiple comparisons.
The second approach entailed the quantification of the mean individual variability of each subcortical structure through a meta‐analysis of the SD of the adjusted volumes according to the method proposed by Senior, Gosby, Lu, Simpson, and Raubenheimer (2016).
2.5. Centile curves
Reference curves for each structure by sex and hemisphere were produced from ICV‐ and site‐adjusted volumes as normalized growth centiles using the parametric Lambda (λ), Mu (μ), Sigma (σ) (LMS) method (Cole & Green, 1992) implemented using the Generalized Additive Models for Location, Scale and Shape (GAMLSS) in R (http://cran.r-project.org/web/packages/gamlss/index.html) (Rigby & Stasinopoulos, 2005; Stasinopoulos & Rigby, 2007). LMS allows for the estimation of the distribution at each covariate value after a suitable transformation and is summarized using three smoothing parameters, the Box‐Cox power λ, the mean μ and the coefficient of variation σ. GAMLSS uses an iterative maximum (penalized) likelihood estimation method to estimate λ, μ and σ as well as distribution dependent smoothing parameters and provides optimal values for effective degrees of freedom (edf) for every parameter (Indrayan, 2014). This procedure minimizes the Generalized Akaike Information Criterion (GAIC) goodness of fit index; smaller GAIC values indicate better fit of the model to the data. GAMLSS is a flexible way to derive normalized centile curves as it allows each curve to have its own number of edf while overcoming biased estimates resulting from skewed data
3. RESULTS
3.1. Fractional polynomial regression analyses
The volume of the caudate, putamen, globus pallidus and nucleus accumbens peaked early during the first decade of life and showed a linear decline immediately thereafter (Figure 2, Figures S2–S4). The association between age and the volumes of the thalamus, hippocampus and amygdala formed a flattened, inverted U‐curve (Figure 3, Figures S5 and S6). Specifically, the volumes of these structures were largest during the first 2–3 decades of life, remained largely stable until the sixth decade and declined gradually thereafter (Table S2). The volume of the lateral ventricles increased steadily with age bilaterally (Figure S7). The smallest proportion of variance explained by age and its FP derivatives was noted in the right amygdala (7%) and the largest in the lateral ventricles bilaterally (38%) (Table S2).
FIGURE 2.
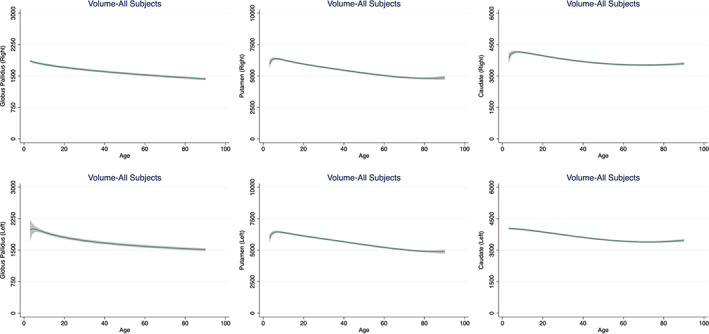
Fractional polynomial plots for the volume of the basal ganglia. Fractional Polynomial plots of adjusted volumes (mm3) against age (years) with a fitted regression line (solid line) and 95% confidence intervals (shaded area)
FIGURE 3.
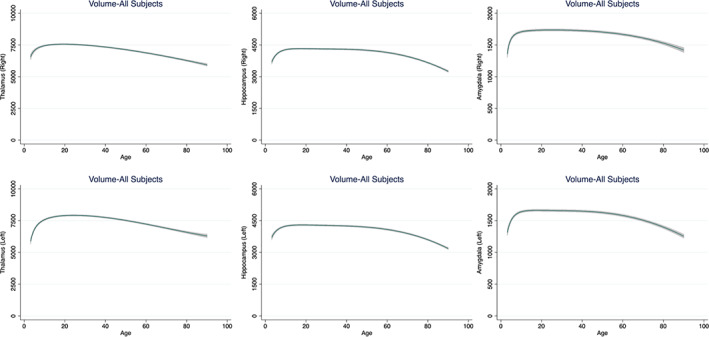
Fractional polynomial plots for the volume of the thalamus, hippocampus and amygdala. Fractional polynomial plots of adjusted volumes (mm3) against age (years) with a fitted regression line (solid line) and 95% confidence intervals (shaded area)
Striatal volumes correlated negatively with age throughout the lifespan with the largest coefficients observed in the middle‐life age‐group (r = −0.39 to −0.20) and the lowest (|r| < 0.05) in the late‐life age‐group, particularly in the caudate. The volumes of the thalamus, the hippocampus and the amygdala showed small positive correlations with age (r ≈ 0.16) in the early‐life age‐group. In the middle‐life age‐group, the correlation between age and subcortical volumes became negative (r = −0.30 to −0.27) for the thalamus but remained largely unchanged for the amygdala and the hippocampus. In the late‐life age‐group, the largest negative correlation coefficients between age and volume were observed for the hippocampus bilaterally (r = −0.44 to −0.39). The correlation between age and lateral ventricular volumes bilaterally increased throughout the lifespan from r = 0.19 to 0.20 in early‐life age‐group to r = 0.40 to 0.45 in the late‐life age‐group (Table S3). No effect of sex was noted for any pattern of correlation between subcortical volumes and age in any age‐group.
Inter‐individual variability: For each structure, the mean inter‐individual variability in volume in each age‐group is shown in Table S5. Inter‐individual variance was significantly higher for the hippocampus, thalamus amygdala and lateral ventricles bilaterally in the late‐life age‐group compared to both the early‐ and middle‐life group. These findings were recapitulated when data were analyzed using a meta‐analytic approach (Figure S8).
Normative Centile Curves: Centile normative values for each subcortical structure stratified by sex and hemisphere are shown in Figure 4 and Tables S6–S8.
FIGURE 4.
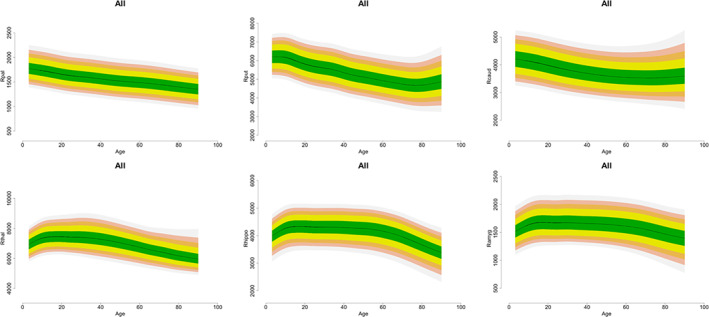
Centile values for subcortical volumes; Additional details in Tables S6‐S9
4. DISCUSSION
We analyzed subcortical volumes from 18,605 healthy individuals from multiple cross‐sectional cohorts to infer age‐related trajectories between the ages of 3 and 90 years. Our lifespan perspective and our large sample size complement and enrich previous age‐related findings in subcortical volumes.
We found three distinct patterns of association between age and subcortical volumes. The volume of the lateral ventricles increased monotonically with age. Striatal and pallidal volumes peaked in childhood and declined thereafter. The volumes of the thalamus, hippocampuus and amygdala peaked later and showed a prolonged period of stability lasting until the sixth decade of life, before they also started to decline. These findings are in line with those of Pomponio et al. (2019), who also used harmonized multi‐site MRI data from 10,323 individuals aged 3–96 years, and those reported by Douaud et al. (2014) who analyzed volumetric data from 484 healthy participants aged 8 to 85 years. Notably, both studies reported similarity in the age‐related changes of the thalamus, hippocampus and the amygdala. Our results also underscore the significantly steeper negative association between subcortical volumes and age from the sixth decade of life onwards. This effect seemed relatively more pronounced for the hippocampus, compared to the other subcortical regions, as observed in other studies (Jernigan et al., 2001; Pomponio et al., 2019; Raz et al., 2010).
The trajectories of subcortical volumes are shaped by genetic and nongenetic exposures, biological or otherwise (Eyler et al., 2011; Somel et al., 2010; Wardlaw et al., 2011). Our findings of higher inter‐individual variability with age in the volumes of the thalamus, hippocampus and amygdala suggest that these structures may be more susceptible to person‐specific exposures, or late‐acting genes, particularly from the sixth decade onwards.
The unique strengths of this study are the availability of age‐overlapping cross‐sectional data from healthy individuals, lifespan coverage and the use of standardized protocols for volumetric data extraction across all samples. Study participants in each site were screened to ensure mental and physical wellbeing at the time of scanning using procedures considered as standard in designating study participants as healthy controls. Although health is not a permanent attribute, it is extremely unlikely given the size of the sample that the results could have been systematically biased by incipient disease
A similar longitudinal design would be near infeasible in terms of recruitment and retention both of participants and investigators. Although multisite studies have to account for differences in scanner type and acquisition, lengthy longitudinal designs encounter similar issues due to inevitable changes in scanner type and strength and acquisition parameters over time. In this study, the use of age‐overlapping samples from multiple different countries has the theoretical advantage of diminishing systematic biases reflecting cohort and period effects (Glenn, 2003; Keyes, Utz, Robinson, & Li, 2010) that are likely to operate in single site studies.
In medicine, biological measures from each individual are typically categorized as normal or otherwise in reference to a population derived normative range. This approach is yet to be applied to neuroimaging data, despite the widespread use of structural MRI for clinical purposes and the obvious benefit of a reference range from the early identification of deviance (Dickie et al., 2013; Pomponio et al., 2019). Alzheimer's disease provides an informative example as the degree of baseline reduction in medial temporal regions, and particularly the hippocampus, is one of the most significant predictors of conversion from mild cognitive impairment to Alzheimer's disease (Risacher et al., 2009). The data presented here demonstrate the power of international collaborations within ENIGMA for analyzing large‐scale datasets that could eventually lead to normative range for brain volumes for well‐defined reference populations. The centile curves presented here are a first‐step in developing normative reference values for neuroimaging phenotypes and further work is required in establishing measurement error and functional significance (see Supplement). These curves are not meant to be used clinically or to provide valid percentile measures for a single individual.
In conclusion, we used existing cross‐sectional data to infer age‐related trajectories of regional subcortical volumes. The size and age‐coverage of the analysis sample has the potential to disambiguate uncertainties regarding developmental and aging changes in subcortical volumes while the normative centile values could be further developed and evaluated.
CONFLICT OF INTEREST
H.‐J. G.: Travel grants and speaker honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag; research funding from Fresenius Medical Care. O. A. A.: Consultant to HealthLytix, speaker honorarium from Lundbeck. A. M. D.: Founder and member of the Scientific Advisory Board CorTechs Labs, Inc where he holds equity; member of the Scientific Advisory of Human Longevity Inc; research grants with General Electric Healthcare.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
This study presents independent research funded by multiple agencies. The funding sources had no role in the study design, data collection, analysis, and interpretation of the data. The views expressed in the manuscript are those of the authors and do not necessarily represent those of any of the funding agencies. Dr. Dima received funding from the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, the Psychiatry Research Trust and 2014 NARSAD Young Investigator Award. Dr. Frangou received support from the National Institutes of Health (R01 MH104284, R01MH113619, R01 MH116147), the European Community's Seventh Framework Programme (FP7/2007–2013) (grant agreement n°602450). This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai, USA. Dr. Agartz was supported by the Swedish Research Council (grant numbers: 521‐2014‐3487 and 2017‐00949). Dr. Alnæs was supported by the South Eastern Norway Regional Health Authority (grant number: 2019107). Dr. O Andreasen was supported by the Research Council of Norway (grant number: 223273) and South‐Eastern Norway Health Authority (grant number: 2017‐112). Dr. Cervenka was supported by the Swedish Research Council (grant number 523‐2014‐3467). Dr. Crespo‐Facorro was supported by the IDIVAL Neuroimaging Unit for imaging acquisition; Instituto de Salud Carlos III (grant numbers: PI020499, PI050427, PI060507, PI14/00639 and PI14/00918) and the Fundación Instituto de Investigación Marqués de Valdecilla (grant numbers: NCT0235832, NCT02534363, and API07/011). Dr. Gur was supported by the National Institute of Mental Health (grant numbers: R01MH042191 and R01MH117014). Dr. James was supported by the Medical Research Council (grant no G0500092). Dr. Saykin received support from U.S. National Institutes of Health grants R01 AG19771, P30 AG10133 and R01 CA101318. Dr. Thompson, Dr. Jahanshad, Dr. Wright, Dr. Medland, Dr. O Andreasen, Dr. Rinker, Dr. Schmaal, Dr. Veltam, Dr. van Erp, and D.P.H. were supported in part by a Consortium grant (U54 EB020403 to P.M.T.) from the NIH Institutes contributing to the Big Data to Knowledge (BD2K) Initiative. FBIRN sample: Data collection and analysis was supported by the National Center for Research Resources at the National Institutes of Health (grant numbers: NIH 1 U24 RR021992 (Function Biomedical Informatics Research Network) and NIH 1 U24 RR025736‐01 (Biomedical Informatics Research Network Coordinating Center; http://www.birncommunity.org). FBIRN data was processed by the UCI High Performance Computing cluster supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000153. Brainscale: This work was supported by Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO 51.02.061 to H.H., NWO 51.02.062 to D.B., NWO‐ NIHC Programs of excellence 433‐09‐220 to H.H., NWO‐MagW 480‐04‐004 to D.B., and NWO/SPI 56‐464‐14192 to D.B.); FP7 Ideas: European Research Council (ERC‐230374 to D.B.); and Universiteit Utrecht (High Potential Grant to H.H.). UMCU‐1.5T: This study is partially funded through the Geestkracht Programme of the Dutch Health Research Council (Zon‐Mw, grant No 10‐000‐1001), and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and mental health care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Center and the mental health institutions: GGZ Ingeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Centre, GGZ Noord Holland Noord. Groningen: University Medical Center Groningen and the mental health institutions: Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht and Parnassia psycho‐medical center The Hague. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGzE, GGZ Breburg, GGZ Oost‐Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan, Virenze riagg, Zuyderland GGZ, MET ggz, Universitair Centrum Sint‐Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint‐Truiden, PZ Sancta Maria Sint‐Truiden, GGZ Overpelt, OPZ Rekem. Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal and Delta.). UMCU‐3T: This study was supported by NIMH grant number: R01 MH090553 (to RAO). The NIMH had no further role in study design, in the collection, analysis and interpretation of the data, in the writing of the report, and in the decision to submit the paper for publication. Netherlands Twin Register: Funding was obtained from the Netherlands Organization for Scientific Research (NWO) and The Netherlands Organization for Health Research and Development (ZonMW) grants 904‐61‐090, 985‐10‐002, 912‐10‐020, 904‐61‐193,480‐04‐004, 463‐06‐001, 451‐04‐034, 400‐05‐717, 400‐07‐080, 31160008, 016‐115‐035, 481‐08‐011, 056‐32‐010, 911‐09‐032, 024‐001‐003, 480‐15‐001/674, Center for Medical Systems Biology (CSMB, NWO Genomics), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI‐NL, 184.021.007 and 184.033.111); Spinozapremie (NWO‐ 56‐464‐14192), and the Neuroscience Amsterdam research institute (former NCA). The BIG database, established in Nijmegen in 2007, is now part of Cognomics, a joint initiative by researchers of the Donders Centre for Cognitive Neuroimaging, the Human Genetics and Cognitive Neuroscience departments of the Radboud University Medical Centre, and the Max Planck Institute for Psycholinguistics. The Cognomics Initiative is supported by the participating departments and centers and by external grants, including grants from the Biobanking and Biomolecular Resources Research Infrastructure (Netherlands) (BBMRI‐NL) and the Hersenstichting Nederland. The authors also acknowledge grants supporting their work from the Netherlands Organization for Scientific Research (NWO), that is, the NWO Brain & Cognition Excellence Program (grant 433‐09‐229), the Vici Innovation Program (grant 016‐130‐669 to BF) and #91619115. Additional support is received from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreements n° 602805 (Aggressotype), n° 603016 (MATRICS), n° 602450 (IMAGEMEND), and n° 278948 (TACTICS), and from the European Community's Horizon 2020 Programme (H2020/2014–2020) under grant agreements n° 643051 (MiND) and n° 667302 (CoCA). Betula sample: Data collection for the BETULA sample was supported by a grant from Knut and Alice Wallenberg Foundation (KAW); the Freesurfer segmentations were performed on resources provided by the Swedish National Infrastructure for Computing (SNIC) at HPC2N in Umeå, Sweden. Indiana sample: This sample was supported in part by grants to BCM from Siemens Medical Solutions, from the members of the Partnership for Pediatric Epilepsy Research, which includes the American Epilepsy Society, the Epilepsy Foundation, the Epilepsy Therapy Project, Fight Against Childhood Epilepsy and Seizures (F.A.C.E.S.), and Parents Against Childhood Epilepsy (P.A.C.E.), from the Indiana State Department of Health Spinal Cord and Brain Injury Fund Research Grant Program, and by a Project Development Team within the ICTSI NIH/NCRR Grant Number RR025761. MHRC study: It was supported in part by RFBR grant 20‐013‐00748. PING study: Data collection and sharing for the Pediatric Imaging, Neurocognition and Genetics (PING) Study (National Institutes of Health Grant RC2DA029475) were funded by the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health & Human Development. A full list of PING investigators is at http://pingstudy.ucsd.edu/investigators.html. QTIM sample: The authors are grateful to the twins for their generosity of time and willingness to participate in our study and thank the many research assistants, radiographers, and other staff at QIMR Berghofer Medical Research Institute and the Centre for Advanced Imaging, University of Queensland. QTIM was funded by the Australian National Health and Medical Research Council (Project Grants No. 496682 and 1009064) and US National Institute of Child Health and Human Development (RO1HD050735). Lachlan Strike was supported by a University of Queensland PhD scholarship. Study of Health in Pomerania (SHIP): this is part of the Community Medicine Research net (CMR) (http://www.medizin.uni-greifswald.de/icm) of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg‐ West Pomerania. MRI scans in SHIP and SHIP‐TREND have been supported by a joint grant from Siemens Healthineers, Erlangen, Germany and the Federal State of Mecklenburg‐West Pomerania. This study was further supported by the DZHK (German Centre for Cardiovascular Research), the German Centre of Neurodegenerative Diseases (DZNE) and the EU‐JPND Funding for BRIDGET (FKZ:01ED1615). TOP study: this was supported by the European Community's Seventh Framework Programme (FP7/2007–2013), grant agreement n°602450. The Southern and Eastern Norway Regional Health Authority supported Lars T. Westlye (grant no. 2014‐097) and STROKEMRI (grant no. 2013‐054). HUBIN sample: HUBIN was supported by the Swedish Research Council (K2007‐62X‐15077‐04‐1, K2008‐62P‐20597‐01‐3, K2010‐62X‐15078‐07‐2, K2012‐61X‐15078‐09‐3), the regional agreement on medical training and clinical research between Stockholm County Council, and the Karolinska Institutet, and the Knut and Alice Wallenberg Foundation. The BIG database: this was established in Nijmegen in 2007, is now part of Cognomics, a joint initiative by researchers of the Donders Centre for Cognitive Neuroimaging, the Human Genetics and Cognitive Neuroscience departments of the Radboud university medical centre, and the Max Planck Institute for Psycholinguistics. The Cognomics Initiative is supported by the participating departments and centres and by external grants, including grants from the Biobanking and Biomolecular Resources Research Infrastructure (Netherlands) (BBMRI‐NL) and the Hersenstichting Nederland. The authors also acknowledge grants supporting their work from the Netherlands Organization for Scientific Research (NWO), that is, the NWO Brain & Cognition Excellence Program (grant 433‐09‐229), the Vici Innovation Program (grant 016‐130‐669 to BF) and #91619115. Additional support is received from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreements n° 602805 (Aggressotype), n° 603016 (MATRICS), n° 602450 (IMAGEMEND), and n° 278948 (TACTICS), and from the European Community's Horizon 2020 Programme (H2020/2014–2020) under grant agreements n° 643051 (MiND) and n° 667302 (CoCA).
Dima D, Modabbernia A, Papachristou E, et al. Subcortical volumes across the lifespan: Data from 18,605 healthy individuals aged 3–90 years. Hum Brain Mapp. 2022;43:452–469. 10.1002/hbm.25320
Funding information National Institute of Mental Health, Grant/Award Numbers: MH104284, MH116147, R01MH113619, R01 MH090553, R01MH117014, R01MH042191; Karolinska Institutet; Stockholm County Council; Southern and Eastern Norway Regional Health Authority; German Centre for Cardiovascular Research; DZHK; Siemens Healthineers; University of Queensland; US National Institute of Child Health and Human Development, Grant/Award Number: RO1HD050735; Australian National Health and Medical Research Council; Eunice Kennedy Shriver National Institute of Child Health & Human Development; National Institute on Drug Abuse, Grant/Award Numbers: UL1 TR000153, 1 U24 RR025736‐01, 1 U24 RR021992; Brain Injury Fund Research Grant Program; Indiana State Department of Health Spinal Cord; Parents Against Childhood Epilepsy; Epilepsy Therapy Project, Fight Against Childhood Epilepsy and Seizures; Epilepsy Foundation; American Epilepsy Society; Knut and Alice Wallenberg Foundation; European Community's Horizon 2020 Programme; Vici Innovation Program; NWO Brain & Cognition Excellence Program; Netherlands Organization for Scientific Research; Hersenstichting Nederland; Netherlands Organization for Health Research and Development; Geestkracht Programme of the Dutch Health Research Council, Grant/Award Number: 10‐000‐1001; Universiteit Utrecht; FP7 Ideas: European Research Council; Nederlandse Organisatie voor Wetenschappelijk Onderzoek; National Center for Advancing Translational Sciences; National Institutes of Health; National Center for Research Resources; Consortium grant, Grant/Award Number: U54 EB020403; U.S. National Institutes of Health, Grant/Award Numbers: R01 CA101318, P30 AG10133, R01 AG19771; Medical Research Council, Grant/Award Number: G0500092; Fundación Instituto de Investigación Marqués de Valdecilla, Grant/Award Numbers: API07/011, NCT02534363, NCT0235832; Instituto de Salud Carlos III, Grant/Award Numbers: PI14/00918, PI14/00639, PI060507, PI050427, PI020499; the Research Council, Grant/Award Number: 223273; South Eastern Norway Regional Health Authority, Grant/Award Numbers: 2017‐112, 2019107; Swedish Research Council; European Community's Seventh Framework Programme, Grant/Award Number: 602450; King's College London; South London and Maudsley NHS Foundation Trust; Biomedical Research Centre; National Institute for Health Research
Contributor Information
Danai Dima, Email: danai.dima@city.ac.uk.
Sophia Frangou, Email: sophia.frangou@mssm.edu.
DATA AVAILABILITY STATEMENT
The ENIGMA Lifespan Working Group welcomes expression of interest from researchers in the field who wish to use the ENIGMA samples. Data sharing is possible subsequent to consent for the principal investigators of the contributing datasets. Requests should be directed to the corresponding authors.
REFERENCES
- Brain Development Cooperative Group . (2012). Total and regional brain volumes in a population‐based normative sample from 4 to 18 years: The NIH MRI Study of Normal Brain Development. Cerebral Cortex, 22, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi, P. , Picconi, B. , Tozzi, A. , Ghiglieri, V. , & Di Filippo, M. (2014). Direct and indirect pathways of basal ganglia: A critical reappraisal. Nature Neuroscience, 17, 1022–1030. [DOI] [PubMed] [Google Scholar]
- Chudasama, Y. , & Robbins, T. W. (2006). Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological Psychology, 73, 19–38. [DOI] [PubMed] [Google Scholar]
- Cole, T. J. , & Green, P. J. (1992). Smoothing reference centile curves: The LMS method and penalized likelihood. Statistics in Medicine, 11, 1305–1319. [DOI] [PubMed] [Google Scholar]
- Dickie, D. A. , Job, D. E. , Gonzalez, D. R. , Shenkin, S. D. , Ahearn, T. S. , Murray, A. D. , & Wardlaw, J. M. (2013). Variance in brain volume with advancing age: Implications for defining the limits of normality. PLoS One, 8, e84093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud, G. , Groves, A. R. , Tamnes, C. K. , Westlye, L. T. , Duff, E. P. , Engvig, A. , … Johansen‐Berg, H. (2014). A common brain network links development, aging, and vulnerability to disease. Proceedings of the National Academy of Sciences of the United States of America, 111, 17648–17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll, I. , Davatzikos, C. , An, Y. , Wu, X. , Shen, D. , Kraut, M. , & Resnick, S. M. (2009). Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology, 72, 1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker, C. , Bookheimer, S. Y. , & Murphy, D. G. (2015). Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet Neurology, 14, 1121–1134. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H. (2004). Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron, 44, 109–120. [DOI] [PubMed] [Google Scholar]
- Eyler, L. T. , Prom‐Wormley, E. , Fennema‐Notestine, C. , Panizzon, M. S. , Neale, M. C. , Jernigan, T. L. , … Kremen, W. S. (2011). Genetic patterns of correlation among subcortical volumes in humans: Results from a magnetic resonance imaging twin study. Human Brain Mapping, 32, 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fortin, J. P. , Cullen, N. , Sheline, Y. I. , Taylor, W. D. , Aselcioglu, I. , Cook, P. A. , … Shinohara, R. T. (2018). Harmonization of cortical thickness measurements across scanners and sites. NeuroImage, 167, 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, J. P. , Parker, D. , Tunc, B. , Watanabe, T. , Elliott, M. A. , Ruparel, K. , … Shinohara, R. T. (2017). Harmonization of multi‐site diffusion tensor imaging data. NeuroImage, 161, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos, A. F. , Snyder, A. Z. , Girton, L. E. , Morris, J. C. , & Buckner, R. L. (2005). Normative estimates of cross‐sectional and longitudinal brain volume decline in aging and AD. Neurology, 64, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Glenn, N. D. (2003). Distinguishing age, period, and cohort effects. In Mortimer J. T. & Shanahan M. J. (Eds.), Handbook of the life course (pp. 465–476). New York: Springer US. [Google Scholar]
- Good, C. D. , Johnsrude, I. S. , Ashburner, J. , Henson, R. N. , Friston, K. J. , & Frackowiak, R. S. (2001). A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Grossberg, S. (2009). Cortical and subcortical predictive dynamics and learning during perception, cognition, emotion and action. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , van Erp, T. G. , Rasmussen, J. , Leonardo, C. D. , Faskowitz, J. , … Andreassen, O. A. (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21, 1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrayan, A. (2014). Demystifying LMS and BCPE methods of centile estimation for growth and other health parameters. Indian Pediatrics, 51, 37–43. [DOI] [PubMed] [Google Scholar]
- Jernigan, T. L. , Archibald, S. L. , Fenema‐Notestine, C. , Gamst, A. C. , Stout, J. C. , Bonner, J. , & Hesselink, J. R. (2001). Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging, 22, 581–594. [DOI] [PubMed] [Google Scholar]
- Kempton, M. J. , Salvador, Z. , Munafò, M. R. , Geddes, J. R. , Simmons, A. , Frangou, S. , & Williams, S. C. (2011). Structural neuroimaging studies in major depressive disorder. Meta‐analysis and comparison with bipolar disorder. Archives of General Psychiatry, 68, 675–690. [DOI] [PubMed] [Google Scholar]
- Keyes, K. M. , Utz, R. L. , Robinson, W. , & Li, G. (2010). What is a cohort effect? Comparison of three statistical methods for modelling cohort effects in obesity prevalence in the United States, 1971–2006. Social Science & Medicine, 70, 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober, H. , Barrett, L. F. , Joseph, J. , Bliss‐Moreau, E. , Lindquist, K. , & Wager, T. D. (2008). Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. NeuroImage, 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain, A. L. , & Castellanos, F. X. (2006). Brain development and ADHD. Clinical Psychology Review, 26, 433–444. [DOI] [PubMed] [Google Scholar]
- Moore, L. , Hanley, J. A. , Turgeon, A. F. , & Lavoie, A. (2011). A comparison of generalized additive models to other common modeling strategies for continuous covariates: Implications for risk adjustment. Journal of Biometrics and Biostatistics, 2, 109. [Google Scholar]
- Pfefferbaum, A. , Rohlfing, T. , Rosenbloom, M. J. , Chu, W. , Colrain, I. M. , & Sullivan, E. V. (2013). Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas‐based parcellation of MRI. NeuroImage, 65, 176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomponio, R. , Erus, G. , Habes, M. , Doshi, J. , Srinivasan, D. , Mamourian, E. , … Davatzikos, C. (2019). Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. NeuroImage, 208, 116450. 10.1016/j.neuroimage.2019.116450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J. , Vieta, E. , Shinohara, R. , Kochunov, P. , Quidé, Y. , Green, M. J. , … Pineda‐Zapata, J. (2020). Increased power by harmonizing structural MRI site differences with the ComBat batch adjustment method in ENIGMA. NeuroImage, 218, 116956. 10.1016/j.neuroimage.2020.116956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz, N. , Ghisletta, P. , Rodrigue, K. M. , Kennedy, K. M. , & Lindenberger, U. (2010). Trajectories of brain aging in middle‐aged and older adults: Regional and individual differences. NeuroImage, 51, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz, N. , Lindenberger, U. , Rodrigue, K. M. , Kennedy, K. M. , Head, D. , Williamson, A. , … Acker, J. D. (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15, 1676–1689. [DOI] [PubMed] [Google Scholar]
- Raznahan, A. , Shaw, P. W. , Lerch, J. P. , Clasen, L. S. , Greenstein, D. , Berman, R. , … Giedd, J. N. (2014). Longitudinal four‐dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences of the United States of America, 111, 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick, S. M. , Pham, D. L. , Kraut, M. A. , Zonderman, A. B. , & Davatzikos, C. (2003). Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. The Journal of Neuroscience, 23, 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, J. M. , Castro, D. C. , Difeliceantonio, A. G. , Robinson, M. J. , & Berridge, K. C. (2013). Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neuroscience and Biobehavioral Reviews, 37, 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby, R. A. , & Stasinopoulos, D. M. (2005). Generalized additive models for location, scale and shape. Applied Statistics, 54, 507–554. [Google Scholar]
- Risacher, S. L. , Saykin, A. J. , West, J. D. , Shen, L. , Firpi, H. A. , McDonald, B. C. , & Alzheimer's Disease Neuroimaging Initiative . (2009). Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Current Alzheimer Research, 6, 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston, P. , & Altman, D. G. (1994). Regression using fractional polynomials of continuous covariates: Parsimonious parametric modelling. Applied Statistics, 43, 429–467. [Google Scholar]
- Royston, P. , Ambler, G. , & Sauerbrei, W. (1999). The use of fractional polynomials to model continuous risk variables in epidemiology. International Journal of Epidemiology, 28, 964–974. [DOI] [PubMed] [Google Scholar]
- Sauerbrei, W. , Meier‐Hirmer, C. , Benner, A. , & Royston, P. (2006). Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. Computational Statistics and Data Analysis, 50, 3464–3485. [Google Scholar]
- Schmaal, L. , Veltman, D. J. , van Erp, T. G. , Sämann, P. G. , Frodl, T. , Jahanshad, N. , … Hibar, D. P. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Molecular Psychiatry, 21, 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca, J. M. , & Badre, D. (2012). Striatal contributions to declarative memory retrieval. Neuron, 75, 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior, A. M. , Gosby, A. K. , Lu, J. , Simpson, S. J. , & Raubenheimer, D. (2016). Meta‐analysis of variance: An illustration comparing the effects of two dietary interventions on variability in weight. Evolution, Medicine, and Public Health, 2016, 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, S. M. (2005). Thalamic relays and cortical functioning. Progress in Brain Research, 149, 107–126. [DOI] [PubMed] [Google Scholar]
- Shohamy, D. , & Turk‐Browne, N. B. (2013). Mechanisms for widespread hippocampal involvement in cognition. Journal of Experimental Psychology. General, 142, 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somel, M. , Guo, S. , Fu, N. , Yan, Z. , Hu, H. Y. , Xu, Y. , … Khaitovich, P. (2010). MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain. Genome Research, 20, 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasinopoulos, D. M. , & Rigby, R. A. (2007). Generalized additive models for location scale and shape (GAMLSS) in R. Journal of Statistical Software, 23, 1–46. [Google Scholar]
- Thompson, P. M. , Andreassen, O. A. , Arias‐Vasquez, A. , Bearden, C. E. , Boedhoe, P. S. , Brouwer, R. M. , … ENIGMA Consortium . (2017). ENIGMA and the individual: Predicting factors that affect the brain in 35 countries worldwide. NeuroImage, 145, 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. M. , Stein, J. L. , Medland, S. E. , Hibar, D. P. , Vasquez, A. A. , Renteria, M. E. , … Alzheimer's Disease Neuroimaging Initiative, EPIGEN Consortium, IMAGEN Consortium, Saguenay Youth Study (SYS) Group . (2014). The ENIGMA consortium: Large‐scale collaborative analyses of neuroimaging and genetic data. Brain Imaging and Behavior, 8, 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, L. , Worbe, Y. , Thobois, S. , Sgambato‐Faure, V. , & Féger, J. (2015). Selective dysfunction of basal ganglia subterritories: From movement to behavioral disorders. Movement Disorders, 30, 1155–1170. [DOI] [PubMed] [Google Scholar]
- van Erp, T. G. , Hibar, D. P. , Rasmussen, J. M. , Glahn, D. C. , Pearlson, G. D. , Andreassen, O. A. , … Turner, J. A. (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21, 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd, K. B. , Westlye, L. T. , Amlien, I. , Espeseth, T. , Reinvang, I. , Raz, N. , … Fjell, A. M. (2011). Consistent neuroanatomical age‐related volume differences across multiple samples. Neurobiology of Aging, 32, 916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw, J. M. , Bastin, M. E. , Valdés Hernández, M. C. , Maniega, S. M. , Royle, N. A. , Morris, Z. , … Deary, I. J. (2011). Brain aging, cognition in youth and old age and vascular disease in the Lothian Birth Cohort 1936: Rationale, design and methodology of the imaging protocol. International Journal of Stroke, 6, 547–559. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Snyder, A. Z. , Shimony, J. S. , Fox, M. D. , & Raichle, M. E. (2010). Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cerebral Cortex, 20, 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Data Availability Statement
The ENIGMA Lifespan Working Group welcomes expression of interest from researchers in the field who wish to use the ENIGMA samples. Data sharing is possible subsequent to consent for the principal investigators of the contributing datasets. Requests should be directed to the corresponding authors.


