Abstract
Many fundamental cellular processes such as division, polarization, endocytosis, and motility require the assembly, maintenance and disassembly of actin filament (F-actin) networks at specific locations and times within the cell. The particular function of each network is governed by F-actin organization, size and density, as well as actin filament dynamics. The distinct characteristics of different F-actin networks are determined through the coordinated action of specific sets of actin binding proteins (ABPs). Furthermore, cells typically assemble and use multiple F-actin networks simultaneously within the same cytoplasm, so networks must self-organize from a common pool of shared actin monomers (G-actin) and overlapping sets of ABPs. Recent advances in multi-color imaging and analysis of ABPs and their associated F-actin networks in cells, as well as the development of sophisticated in vitro reconstitutions of networks with ensembles of ABPs, has allowed the field to start uncovering the underlying principles by which cells self-organize diverse F-actin networks to execute basic cellular functions.
Self-organization and homeostasis in cell biology
Cells are made up of complex mixtures of biomolecules, including nucleic acids, proteins, lipids, and carbohydrates that assemble into large and dynamic macromolecular assemblies, organelles, and membrane-bound compartments. Despite the vast number of component parts, cells maintain a remarkable degree of organization and stability across a wide range of sizes and shapes. A cell’s interior organization facilitates essential cellular processes, but our current understanding of the molecular mechanisms that determine the size and shape of cells and organelles is inadequate to completely explain observed cellular phenomena. How are these large, macromolecular structures built and maintained?
To address central questions about the organization of living cells, biologists have begun to apply the principles of self-organization, first developed in chemistry and physics (Karsenti, 2008; Misteli, 2001; Wedlich-Söldner and Betz, 2018). In cell biology, self-organization refers to the capacity of a macromolecular structure, such as an organelle, to determine its own size and shape based on the physical interactions of its component parts (Misteli, 2001). The process of self-organization involves the association of molecules into adaptable steady-state structures (Karsenti, 2008; Misteli, 2001). A self-organizing system generally meets three main criteria: 1) the resulting macromolecular structure is dynamic, with the ability to vary in size, density, and shape, 2) the self-organized structure exchanges energy and matter with its environment, and 3) an overall stable configuration is generated from dynamic component parts (Karsenti, 2008; Misteli, 2001).
In cell biology, self-organization is thought to regulate the assembly of several important cellular structures, including the mitotic spindle, the nucleolus, and the actin and microtubule cytoskeletons, thereby mediating many essential cellular processes (Dundr et al., 2000; Glick, 2002; Heald et al., 1996; Karsenti and Vernos, 2001). For example, bipolar spindles self-organize in the absence of external cues when chromatin, microtubules, and microtubule-associated proteins physically interact (Heald et al., 1996; Karsenti and Vernos, 2001). Beyond the mitotic spindle, it is important that we broaden our understanding of cellular self-organization to better elucidate how cells achieve stability on the whole-cell level.
This review will focus on the assembly, maintenance and architecture of the actin cytoskeleton, which exhibits many hallmarks of a self-organizing system. First, the actin cytoskeleton is remarkably dynamic, as actin filaments are constantly growing, disassembling, and undergoing turnover to adopt various architectures. These dynamic actin filaments assemble into a diverse array of stable, functionally distinct, higher-order networks that continuously exchange actin subunits and actin-binding proteins (ABPs) with the cytoplasm. A fundamental question in cell biology is how a single actin subunit building block can assemble into so many structurally and functionally diverse actin cytoskeleton networks, and a significant key to understanding this phenomenon lies in its ability to self-organize.
What is the physiological relevance of actin cytoskeletal self-organization? The actin cytoskeleton plays a central role in determining cell shape and polarity, providing structural support, and facilitating essential cellular processes such as cell division and motility. To support these functions, actin filament (F-actin) networks must assemble and disassemble at the correct time and place, and with the proper organization and dynamics within a crowded cytoplasm. It has been largely accepted that signaling cascades control activation of actin assembly factors, thereby regulating actin assembly spatially and temporally. However, we have recently proposed a revised model, whereby following their activation at specific locations in the cell, F-actin networks exist in homeostasis, where their size and density are also regulated in part by a limited cytoplasmic pool of globular actin monomers that becomes depleted as F-actin networks grow (Burke et al., 2014; Suarez et al., 2015; Suarez and Kovar, 2016). Thus, while actin homeostasis has historically referred to the cellular ratio of G-actin to F-actin, we have expanded this model to include the idea that actin filaments are distributed between functionally diverse competing F-actin networks (Chesarone and Goode, 2009; Gao and Bretscher, 2008; Hotulainen and Lappalainen, 2006; Kovar et al., 2011; Nakano and Mabuchi, 2006; Suarez and Kovar, 2016).
In addition to actin filaments, higher-order F-actin networks are composed of many ABPs that work together to define the organization, structure and turnover of the network (Supplemental Figure 1). While we know a lot about how individual ABPs associate with and influence F-actin, how ensembles of ABPs build distinct F-actin networks simultaneously is less well-understood. Here, we describe how self-organization and homeostasis may contribute to higher-order F-actin network behavior in a variety of cell types and discuss how competitive and cooperative interactions between ABPs contribute to F-actin network self-organization.
F-actin assembly, disassembly, and network organization
Although G-actin spontaneously assembles into F-actin, actin assembly and disassembly are tightly regulated in space and time in cells by a wide range of ABPs with complementary biochemical properties (Supplemental Figure 1). Cytoplasmic actin concentrations range from ~50–200 μM, approximately half of which appears to be unassembled. Most unpolymerized actin is bound to actin monomer-binding proteins that prevent their unregulated spontaneous assembly, namely profilin and β-thymosins (Supplemental Figure 1) (Kaiser et al., 1999). These monomer-binding proteins are just one class of actin-binding proteins (ABPs) that collectively regulate actin assembly and disassembly in time and space. Many additional ABPs work together to nucleate and elongate F-actin from the pool of unpolymerized profilin-actin and organize higher order F-actin networks through a variety of activities including stabilizing, severing, crosslinking, and capping actin filaments (for a more detailed description of ABPs, follow the Supplemental Materials link in the online version of this article or at http://www.annualreviews.org/).
Functionally Distinct F-actin networks
The diversity of actin assembly factors and ABPs (described further in Supplemental Materials) gives rise to a wide array of F-actin networks, each organized with architectures and properties to perform specific functions in the cell (Figure 1). These cellular roles include providing structural support, generating polarity, promoting endocytosis, cell motility, and cell division. Each of these functions requires networks with distinct qualities dictated by how the filaments are assembled, the rate of filament turnover, how F-actin is organized, and if or how they are bundled. F-actin networks differ in structure and molecular components depending on particular cellular requirements. In the following sections, we will highlight some of the model systems and networks in which actin cytoskeleton self-organization has been investigated.
Figure 1. Diverse F-Actin Networks in Animal and Fission Yeast Cells:
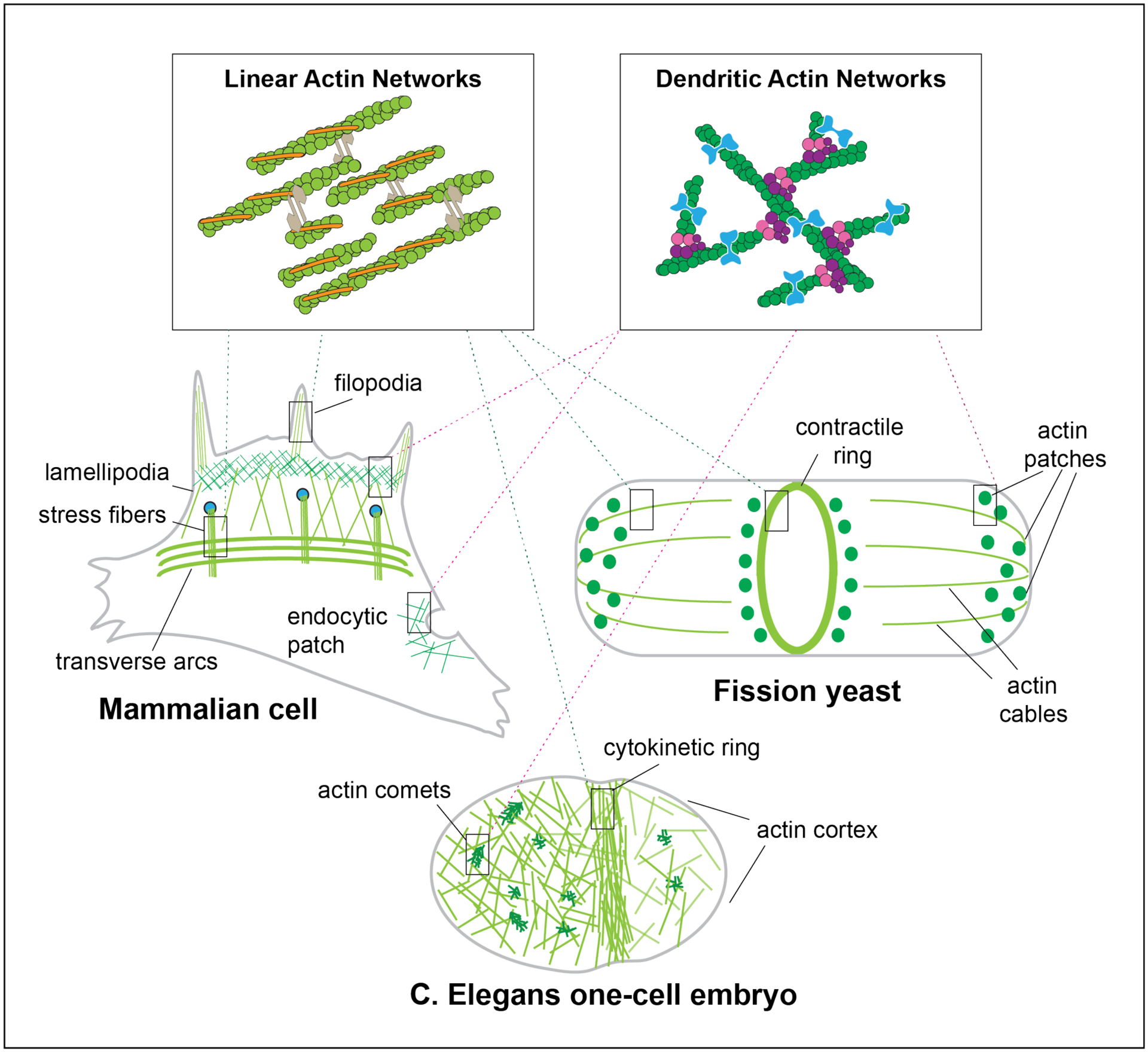
Distinct cell types assemble dendritic and linear F-actin networks for distinct fundamental processes. Animal cells have many F-actin networks, with linear networks found in filopodia and stress fibers and branched networks in lamellipodia and endocytic patches. Yeast have three prominent networks, with linear actin filaments making up polarizing actin cables and the cytokinetic contractile ring, and arborized networks composing the endocytic actin patches. Similarly, the early C. Elegans one-cell embryo has linear filaments that make up the cytokinetic ring, as well as both linear and branched F-actin networks at the cell cortex. Furthermore, actin comets are likely made of branched actin networks.
Fission yeast F-actin networks
Because of the complexity of the actin cytoskeleton in multi-cellular systems, in which cells assemble numerous networks from a large variety of ABPs with multiple isoforms, budding and fission yeasts have been widely used to investigate complexities of actin cytoskeletal network behavior and function (Mishra et al., 2014). Compared to multicellular organisms, fission yeast has a simplified actin cytoskeleton with ~40 ABPs and a single actin isoform. Furthermore, vegetatively growing fission yeast cells build only three primary F-actin networks, each assembled by a distinct assembly factor (Figure 1): endocytic actin patches (Arp2/3 complex), the cytokinetic contractile ring (formin Cdc12) and polarizing actin cables (formin For3) (Kovar et al., 2011).
Actin patches assemble at the tips of interphase cells and the midzone of dividing cells to mediate endocytosis (Balasubramanian et al., 2004; Gachet and Hyams, 2005). Assembly of actin patches occurs in an ordered fashion, with proteins promoting membrane invagination arriving first, followed by adapter proteins and regulators of actin assembly, namely Arp2/3 complex and its NPFs (Sirotkin et al., 2010). The mechanism by which Arp2/3 complex-mediated actin assembly results in vesicle internalization is not completely understood, but models predict that actin patches contain ~150 short actin filaments (100–200 nm) that are quickly capped by capping protein. These short, branched filaments are crosslinked by fimbrin Fim1 (Akamatsu et al., 2020; Lacy et al., 2019).
Fission yeast cytokinesis has long served as a leading model for identifying and characterizing important players in contractile ring formation. The prevailing mechanism of contractile ring assembly in fission yeast, called Search, Capture, Pull, and Release, involves the assembly of pre-ring cytokinesis nodes containing type-II myosin Myo2, formin Cdc12, and other scaffolding and signaling proteins (Laporte et al., 2011; Vavylonis et al., 2008; Wu et al., 2003). Myo2 exerts pulling forces on actin filaments elongated by Cdc12 on nearby nodes, driving node coalescence (Coffman et al., 2009; Wu et al., 2003). These node-node contacts are released by cofilin-mediated severing, and the cycle repeats to generate a mature contractile ring of antiparallel actin filaments bundled by the dynamic crosslinking protein α-actinin Ain1 (Nakano and Mabuchi, 2006; Wu et al., 2001).
Actin cables serve as polarized tracks for type V myosin-based transport of cargo to the growing tips of fission yeast cells. Actin cables are composed of short bundles of parallel F-actin assembled by the formin For3, which associates with the cortex at the cells tips to nucleate and elongate F-actin (Feierbach and Chang, 2001; Kamasaki et al., 2005; Nakano et al., 2002; Wang and Vavylonis, 2008). Less is generally known about the assembly and ABP composition of actin cables compared to actin patches and the contractile ring, as cables are more difficult to image in live cells. Tropomyosin Cdc8 and coronin Crn1 localize to actin cables, but the ABP responsible for bundling filaments in cables is not known.
Animal cell F-actin networks
Although the underlying mechanistic principles of actin filament organization and dynamics are conserved, the metazoan actin cytoskeleton is highly specialized and actin filaments are organized into distinct subcellular domains that differ from the networks found in yeast cells (Figure 1). Contrasting with the three well-defined F-actin networks in vegatitive yeast cells, a typical animal cell has 18–20 F-actin networks with multiple actin isoforms, which are organized by hundreds of different ABPs (Blanchoin et al., 2014; Chhabra and Higgs, 2007). While considerable work remains to determine the specific identity and behavior of F-actin structures in different cells, we will highlight some well-characterized networks that have been used to investigate competition and self-organization of the actin cytoskeleton in animal cells.
One prominent feature of animal cells is the cortical F-actin network found adjacent to the plasma membrane composed of both branched and linear actin filaments (Bovellan et al., 2014). In specific subregions of the membrane, this cortical F-actin network is organized into structures needed for diverse cellular functions (Figure 1) including cell motility (lamellipodia, lamella, filopodia, blebs), endocytosis (phagosomes, endocytic patches), absorption (microvilli), exocytosis, and invasion (podosomes, invadosomes). Additionally, F-actin structures link the plasma membrane to intercellular structures such as stress fibers, as well as circumferential F-actin networks linked to adherens junctions. Each of these described networks have multiple specific assembly factors (Campellone and Welch, 2010), and a plethora of ABPs with complementary properties that generate distinct actin filament organizations that are tailored for each network’s cellular function. Despite the complexity in animal cells, in subdomains and at particular times in the cell cycle, distinct F-actin networks have been identified and the properties dictating the kinetics and dynamics of these networks are starting to be appreciated from a systems-wide perspective.
Due to their role in motility, several F-actin networks at the leading edge of motile cells have been well-studied and characterized, namely the lamellipodia and filopodia (Figure 1). The lamellipodia is a flattened, protrusive extension of the plasma membrane made up of dendritic F-actin networks (Svitkina and Borisy, 1999). The assembly of this arborized network is controlled by the concerted action of multiple nucleators, but it is clear that Arp2/3 complex is required for lamellipodia formation in many cell types (Nicholson-Dykstra and Higgs, 2008; Suraneni et al., 2012; Wu et al., 2012) as loss of Arp2/3 has effects on motility and causes large scale reorganization of F-actin networks in the lamellipodia (Anderson et al., 2017). Additionally, recent work has demonstrated a requirement for formins FMNL2 and FMNL3 in the lamellipodia (Kage et al., 2017). Emerging from the lamellipodia are protrusive, finger-like structures called filopodia that are made up of bundled, linear actin filaments that serve as guidance structures that sense and respond to the surrounding environment (Bentley and Toroian-Raymond, 1986). The linear F-actin networks of the filopodia are polymerized (nucleated and elongated, or elongated from Arp2/3 complex) by assembly factors such as formins and Ena/VASP (Chesarone and Goode, 2009), and bundled by F-actin crosslinking proteins such as fascin (Jansen et al., 2011; Vignjevic et al., 2006). As is obvious from this brief description of the cellular structures required for motility at the leading edge, even specific subcellular structures in animal cells have a mix of actin assembly factors, ABPs, and architectures within each structure. Despite this complexity, the lamellipodia has proved a useful candidate for examining self-organization of the F-actin cortex of animal cells, which will be further elucidated below. While these studies have provided initial insight, due to the sheer number of networks in animal cells, investigating F-actin network self-organization at the cellular level remains a significant challenge.
In order to bridge the gap between the relative simplicity of yeast and mammalian cells, metazoan model systems where F-actin network self-organization can be interrogated on a systems-based level needs to be pursued. One promising candidate is the C. elegans one-cell embryo (Figure 1), which has a moderate number of four to five F-actin networks (Davies et al., 2014; Velarde et al., 2007), three actin isoforms (of five isoforms total), and moderate number of ABPs, which typically have one to five isoforms (Willis et al., 2006).
Self-Organization
There are a number of mechanisms that dictate when, where, and how distinct F-actin networks are established in the cell. The question as to how functionally and structurally diverse assemblies of actin filaments arise within a common cytoplasm has long been a prominent question in cell biology. Early studies into the hair cells of the chick auditory organ noted distinct F-actin networks that arose over developmental time and likely were due to multiple mechanisms, including regulation of actin nucleation as well as the ABPs associated with each network (Tilney and Tilney, 1984). In the intervening years, there has been significant focus on network specificity arising from regulated signaling that activates F-actin assembly at particular times and locations within the cell. Recent work has detailed additional mechanisms that affect network identity, including competition for actin monomers as well as cooperation and competition between ABPs that generate unique compositions of proteins localized to specific networks. Additionally, there is evidence that other factors, such as mechanical strain (Wioland et al., 2019), actin filament helicity (Jegou and Romet-Lemonne, 2019), and F-actin network architecture (Boujemaa-Paterski et al., 2017; Gressin et al., 2015; Reymann et al., 2010) can affect the structure and function of networks as well as the proteins associated with each network. Historically, F-actin networks were studied in isolation, but now the focus is turning to the interplay between networks, highlighting how specific subgroups of ABPs co-localize to a particular structure and collectively influence F-actin organization. In the following sections, we will specifically highlight how one mechanism of self-organization, competition and cooperation, affects the distribution of actin monomers and ABPs to give rise to the functionally diverse filament structures found in the cell.
Competition for actin monomers
The size of individual F-actin networks in the cell is determined by the rate of actin filament nucleation, rates of filament elongation, and rates of network disassembly. A long-standing model has been that F-actin network size and density are regulated exclusively through the activation of actin assembly factors by GTPase signaling cascades, F-actin barbed end capping, and F-actin disassembly factors (Campellone and Welch, 2010; Chesarone and Goode, 2009; Michelot and Drubin, 2011; Pollard, 2016). A key premise of this model is that functionally diverse F-actin networks within a common cytoplasm do not influence the size and density of each other, as the pool of actin monomers is large and unlimited. While the activation of actin assembly factors by GTPases has an important role in determining the time and place of cellular actin nucleation, many studies also provide examples of cross-talk and competition between different F-actin networks. Specifically, disruption of one network by affecting specific nucleation factors frequently results in an increase in the size and/or density of other F-actin networks in a cell (Dimchev et al., 2017; Gao and Bretscher, 2008; Kumari et al., 2020; Nicholson-Dykstra and Higgs, 2008; Rotty et al., 2015; Sagot et al., 2002; Wu et al., 2012).
Through systematic evaluation of this competition, we found that the depletion of Arp2/3 complex-generated actin patch networks in fission yeast results in a dramatic increase in F-actin assembled at the contractile ring or actin cables mediated by the formins Cdc12 and For3 (Burke et al., 2014). Importantly, ectopic F-actin assembly due to inhibition of Arp2/3 complex fails to occur if patch disassembly is inhibited, preventing release of actin patch monomers and re-incorporation into the remaining F-actin networks. Conversely, genetic depletion of formins increases the density of Arp2/3 complex-mediated actin patches. Furthermore, increasing actin expression favors Arp2/3 complex actin patches, whereas lowering actin expression favors assembly of formin-mediated contractile rings. These results suggest an important regulatory mechanism whereby the actin cytoskeleton is in homeostasis, characterized by an intrinsic competition for a common pool of G-actin that helps set the number and size of diverse F-actin networks in fission yeast.
This evidence supports the model that competition for a limited concentration of actin between rival F-actin networks contributes to the regulation of their size and density (Figure 2) (Chesarone and Goode, 2009; Gao and Bretscher, 2008; Hotulainen and Lappalainen, 2006; Kovar et al., 2011). By systematically inhibiting rival actin assembly factors, resulting in over-consumption of actin by remaining F-actin networks, several groups have now demonstrated that self-organized F-actin networks are in homeostasis, whereby they indirectly influence each other’s size/density by competing for limiting actin subunits in lamellipodia (Lomakin et al., 2015; Rotty et al., 2015) and fission yeast actin networks (Burke et al., 2014; Suarez et al., 2015). Although competition for limiting actin cannot explain F-actin network size scaling on its own, this homeostatic mechanism appears likely to contribute to network sizing from fission yeast to animal cells (Antkowiak et al., 2019; Billault-Chaumartin and Martin, 2019; Chan et al., 2019; Davidson et al., 2018; Dimchev et al., 2017; Faust et al., 2019; Hotulainen and Lappalainen, 2006; Kumari et al., 2020; Lomakin et al., 2015; Sarmiento et al., 2008; Wu et al., 2012).
Figure 2. Cooperative and Competitive Interactions in F-Actin Networks in Animal and Fission Yeast:
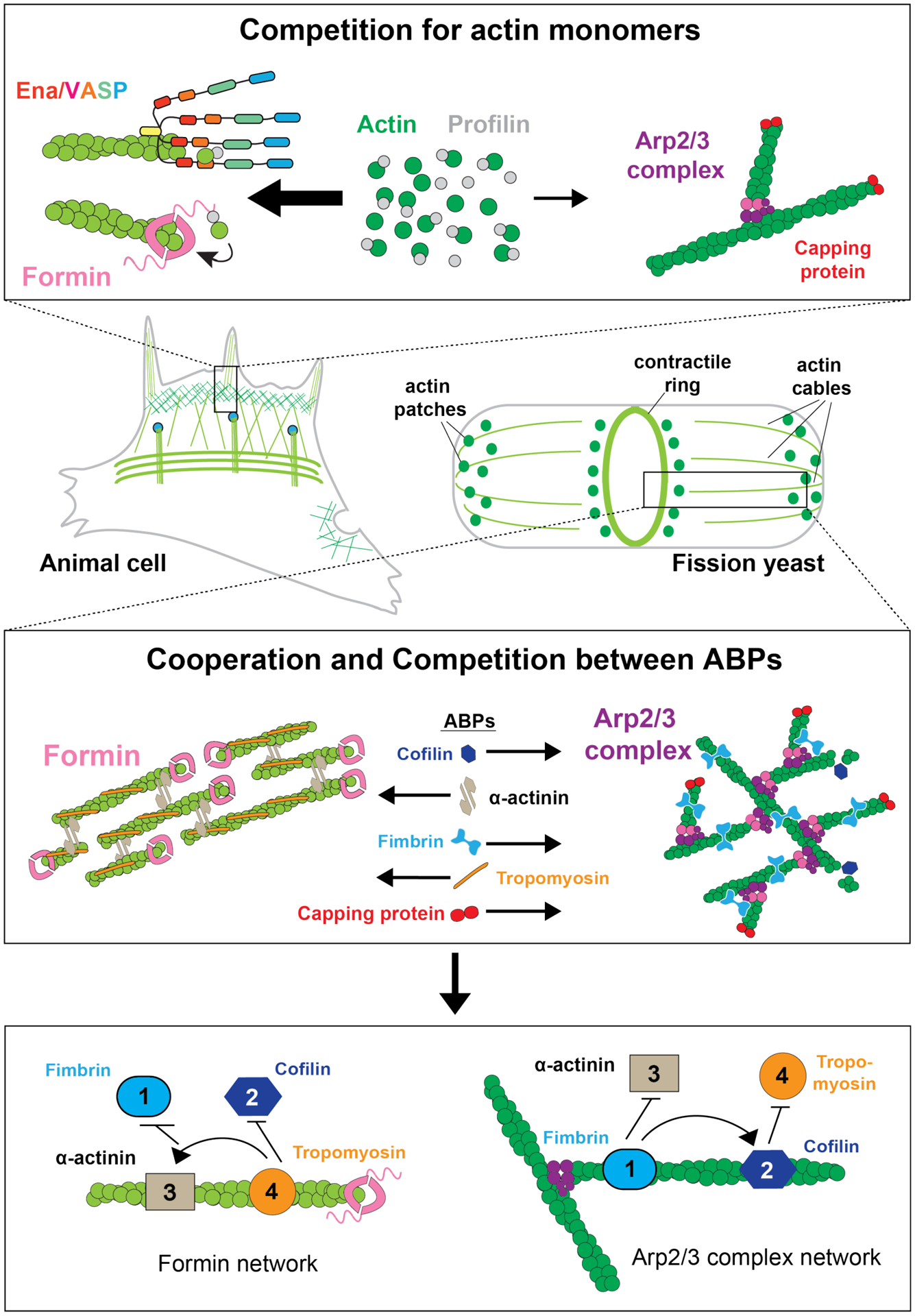
To self-organize multiple functionally diverse F-actin networks simultaneously from a common pool of components, both animal and fission yeast cells must (A) properly allocate actin monomers between competing networks, and (B) sort unique subsets of actin-binding proteins, which establish the structure and dynamics of each network. (C) ABPs compete and cooperate with each other, leading to a potential ABP hierarchy, where upstream proteins either recruit or inhibit the access of downstream proteins to specific F-actin networks.
Since the initial proposal of this model, there have been a number of studies that have identified mechanisms that contribute to the proper distribution of monomers to competitive F-actin networks. Several studies have focused on competition for polymerization-competent actin monomers and found that the small G-actin binding protein, profilin, plays a key role in mediating this competition (Figure 2). In fission yeast, profilin is required for formin to compete effectively with Arp2/3 complex for limited G-actin and to assemble F-actin for contractile ring formation in dividing cells (Suarez et al., 2015). Similarly, in the lamellipodia of fibroblasts, profilin favors Ena/VASP to assemble linear actin arrays over dendritic networks assembled by Arp2/3 complex activation (Rotty et al., 2015). A recent paper also focused on the lamellipodia of enterocytes found that profilin promotes the assembly of linear actin filaments (likely by another linear filament assembly factor, Cobl) in microvilli in competition with the branched network generated by the Arp2/3 complex in lamellipodia (Faust et al., 2019).
A key aspect of these studies is that profilin reduces Arp2/3 complex-mediated branch formation by approximately 5-fold and high profilin::actin ratios reduces the amount of actin monomers incorporated into Arp2/3 complex-generated branched networks, thereby favoring (and/or allowing) linear filament assembly via a number of different nucleators such as formin and/or Ena/VASP (Figure 2). An important test of this model is that inhibition of Arp2/3 complex (pharmacologically or genetically) suppresses genetic depletion of profilin in fission yeast, allowing formin-mediated contractile ring assembly at severely reduced profilin levels (Balasubramanian et al., 1996, 1996; Suarez et al., 2015). In this scenario, we have proposed that profilin competes with WASP (fission yeast Wsp1) for binding actin monomers (Suarez et al., 2015). Recent work has questioned this mechanism because the proline-rich region of WASP upstream of the VCA region could bind profilin::actin and promote actin use by the VCA of WASP in filament nucleation and elongation along with the Arp2/3 complex (Bieling et al., 2018). At least in yeast, this mechanism seems unlikely, as Arp2/3 complex-mediated branched actin assembly activated by full-length recombinant WASP, is inhibited by profilin::actin to the same extent as VCA alone (Suarez et al., 2015). This was also observed with full-length Scar and Arp2/3 complex from Acanthamoeba (Machesky et al., 1999). While branched assembly by Arp2/3 complex occurs with profilin::actin, the rate is reduced as compared to actin alone. In organisms with multiple profilin isoforms (Neidt et al., 2009), specific isoforms have differing effects on formin-mediated assembly rates of actin filaments. Likewise, different profilin isoforms in mammalian systems may be tuned to promote assembly depending on the NPF, linear assembly factor, and Arp2/3 complex isoforms present, and this potential specialization warrants future investigation.
There remain some outstanding questions and alternative interpretations postulated for the role of profilin in mediating homeostasis between competing F-actin networks in cells. One proposed mechanism is that high concentrations of profilin may bind the barbed end of the actin filaments and compete with other barbed-end binding proteins, affecting branch assembly and filament elongation (Carlier and Shekhar, 2017; Pernier et al., 2016). Recent quantification of profilin::actin concentrations in animal cells suggest the amount of free profilin in the cell is not high enough (>10 μM) for profilin to competitively bind the barbed end of actin filaments (Funk et al., 2019). Another alternative considers the cellular profilin pool to be finite and lower than the total actin concentration. In experimental conditions where transient network disassembly is induced, the released actin monomers may overwhelm profilin’s capacity to bind actin monomers (Funk et al., 2019). The observed ectopic actin nucleation could be caused by excess free actin monomers that trigger assembly through spontaneous nucleation and formin-specific nucleation pathways (Higashida et al., 2013). This proposed effect does not consider results where an increase in profilin concentration via overexpression or injection can promote linear networks (formin, Ena/VASP, or Cobl) over Arp2/3 complex-mediated networks. Nevertheless, development of techniques that allow for quantification of monomer and filamentous actin states in vivo during assembly, disassembly, and reorganization are needed to understand their effect on local F-actin network dynamics (Isogai and Danuser, 2018; Skruber et al., 2018). These tools will be vital to future studies investigating in more detail how cells respond to changes in actin expression, how robustly they can maintain homeostasis or changes in actin levels, and whether they can actively control actin expression to regulate network competition.
Recent work modeling the limiting-pool mechanism found evidence that both supports (Antkowiak et al., 2019) and questions (Mohapatra et al., 2017) this mechanism, depending on the assumptions upon which the models are built. Modeling competing networks of similar sizes with differing assembly rates (Mohapatra et al., 2017), the authors found the network with the faster assembly rate will consume all the building blocks. But, F-actin networks of similar size could be generated if the network lifetime is of the same order as the time required to construct the network, which is likely the case in the cell (Suarez et al., 2017). In combined in vitro/in silico work that lends support to the limiting-pool mechanism (Antkowiak et al., 2019), Antkowiak et al. found that when evaluating the effect of profilin, capping protein, and ADF/cofilin on network assembly rates that profilin had the most profound effect, in agreement with Suarez et al. (Suarez et al., 2015) and Rotty et al. (Rotty et al., 2015). Pairing modeling with in vitro and in vivo work has been a powerful method to uncover principles governing actin assembly in motility and cytokinesis and will continue to inform our understanding of inter-network competition.
The role of capping protein and disassembly factors in monomer availability
An important aspect of F-actin network competition and regulation is how the dimensions of networks are established and maintained. While F-actin network size is partially dictated by the activity of actin assembly factors and competition for actin monomers, the restriction of barbed-end elongation and disassembly of networks also play important roles. Filament nucleation and elongation rates are directly proportional to the concentration of polymerization competent monomers, as such, the rate that F-actin is recycled into G-actin likely affects subsequent network assembly (Pollard, 2016). The activities of capping protein and disassembly factors are crucial for regeneration of polymerization competent monomers, therefore these factors can directly affect the competition for actin monomers detailed above (Carlier and Shekhar, 2017; Suarez et al., 2017).
A role for capping protein in F-actin network homeostasis
One mechanism to modulate F-actin network size is to restrict the elongation of filament barbed ends by barbed-end cappers. Capping protein is the major player in this process and binds tightly to F-actin barbed ends (Kd ~ 0.1nM) (Schafer et al., 1996). In Arp2/3 complex-generated networks, most barbed ends are rapidly capped by capping protein, generating a dense dendritic network of short filaments needed for generating protrusive force (Akin and Mullins, 2008). In linear networks generated by barbed-end associated proteins such as formins and Ena/VASP, capping protein is in competition with these factors for the barbed end (Bear et al., 2002). Capping protein can be regulated by a number of factors that inhibit its interaction with the barbed end either via steric (myostrophin (V1) and phosphatidylinositol-4,5- bisphosphate) or allosteric mechanisms (CARMIL/LRRC16A) (Edwards et al., 2014). A positive regulator of capping protein is twinfilin, which binds competitively with CARMIL, protecting capping protein from barbed end displacement by CARMIL, and can also relieve the inhibition by V1 (Johnston et al., 2018). The various mechanisms that modify capping protein’s association with the barbed ends can affect monomeric G-actin availability, affecting competition between networks based on the specific proteins associated with each distinct network.
There is evidence for a role for capping protein in mediating competition between linear and branched F-actin networks, but this competition appears to be context dependent. Loss of capping protein might cause unrestricted growth from filament barbed ends in Arp2/3 complex-generated networks and rapidly deplete the monomer pool, affecting the availability of G-actin for linear filament assembly. Supporting this model, deletion of capping protein in budding and fission yeast increases actin incorporation into Arp2/3 complex-generated patches by at least 35% and decreases the number of formin-mediated actin cables (Amatruda et al., 1990; Berro and Pollard, 2014; Kovar et al., 2005; Sizonenko et al., 1996). The evidence for capping protein mediating competition between networks is less clear in mammalian cells, possibly due to more complex F-actin networks. Depletion of capping protein in melanoma cells and neurons decreases F-actin in Arp2/3 complex assembled lamellipodia and increases filopodia number five-fold (Mejillano et al., n.d.; Sinnar et al., 2014). As described above, lamellipodia and filopodia of mammalian cells are in close association, which may cause these observed results. It is possible that loss of capping protein facilitates filament elongation, further assisted by Ena/VASP and formins, which would then be rapidly bundled by fascin, generating filopodial-like structures. These results demonstrate a role for capping protein in inter-network competition, but also highlight that this role depends on network organization.
Disassembly Factors Affect Actin Monomer Availability
The assembly of new F-actin networks requires the disassembly of previous networks in order to preserve the cellular pool of unpolymerized G-actin (Carlier and Shekhar, 2017; Suarez et al., 2017). Therefore, it would be expected that the rate of filament disassembly would affect the availability of actin monomers, and as such, affect the rate of filament assembly in the cell. Members of the ADF/Cofilin family have been identified as major contributors to actin filament turnover in cells (Bamburg, 1999; Blanchoin et al., 2014; Blanchoin and Pollard, 1999; Carlier et al., 1997; Lappalainen and Drubin, 1997); acting by severing and depolymerizing actin filaments, thereby generating ADP-actin monomers. ADF/Cofilin localizes both to branched F-actin networks in cells (Theriot, 1997) as well as linear networks such as the filopodia in mammalian cells (Breitsprecher et al., 2011) and the cytokinetic ring in yeast (Chen and Pollard, 2011). While ADF/Cofilin is required at both branched and linear networks in the cell, the rate of disassembly is highly dependent on the ABPs localized to each network.
In vitro, cofilin alone has a low rate of severing and depolymerization, which does not match rates observed in vivo (Miyoshi and Watanabe, 2013; Suarez et al., 2011; Wioland et al., 2017). Work from many labs over the last 20 years has highlighted cooperation between actin disassembly factors that promotes actin filament severing and depolymerization. ADF/Cofilin often acts in synergy with actin-interacting protein 1 (AIP1) (Okada et al., 1999; Rodal et al., 1999), coronin (Brieher et al., 2006), twinfilin, and Srv2/adenylyl cyclase-associated protein (Hilton et al., 2018; Johnston et al., 2015). One suite of proteins that enhances ADF/cofilin severing activity includes coronin (Gandhi et al., 2009), which promotes cofilin binding to actin filaments, and AIP1 (Balcer et al., 2003; Kueh et al., 2008), which cooperates with cofilin and coronin to sever filaments (Jansen et al., 2015). Recently, the mechanism underlying cooperation between cofilin and Srv2/adenylyl cyclase-associated protein (CAP) to promote depolymerization from the actin filament pointed end was shown by structural (Kotila et al., 2019) and biochemical (Shekhar et al., 2019) methods. These mechanisms for F-actin disassembly have primarily been characterized on linear actin filaments in vitro, but there is evidence that these factors may have differential preference on dendritic networks. For example, networks consisting of linear filaments require ADF/cofilin and AIP1 for disassembly, whereas Arp2/3 networks don’t appear to require AIP1 (Gressin et al., 2015). Furthermore, the ADF/cofilin family member GMF (Gandhi et al., 2010) and coronin (Sirotkin et al., 2010) are found specifically in arborized networks to promote debranching, which may represent an early step in network disassembly. The cooperation of these factors and the unique makeup of the actin disassembly factors in each network could modify the local availability of G-actin and allow for rapid remodeling of individual networks.
In addition to cooperative interactions that promote severing and depolymerization, there is evidence for competitive interactions between ADF/cofilin and ABPs that affect filament depolymerization. Myosin can bind to actin filaments and compete with cofilin for binding (Wiggan et al., 2012) and in some cases displace cofilin (Elam et al., 2013), generating boundaries of cofilin-bound versus bare actin filaments that promote severing. In contrast to an increase in severing with myosin, non-muscle myosin-bundled stress fibers made up of linear actin filaments are protected from disassembly (Lomakin et al., 2015). Inhibition of myosin via blebbistatin resulted in rapid disassembly of stress fibers, liberation of actin monomers, and assembly of actin into dendritic networks at the leading edge. Other competitive and cooperative interactions with proteins such as tropomyosin and fimbrin that affect binding of cofilin to actin will be further discussed below.
The net rate of F-actin disassembly is also affected by the density and architecture of the network as well as the local concentration of F-actin and ADF/cofilin. ADF/cofilin can be locally depleted from solution by binding to F-actin, resulting in denser networks having greater depletion of ADF/cofilin, but sparser networks will exhibit faster filament turnover and disassembly (Manhart et al., 2019). Network architecture affects disassembly rate, with ADF/cofilin binding more sparsely to branched networks, and promoting increased disassembly as compared to linear networks (Gressin et al., 2015). Bundled linear actin networks appear to be resistant to severing by cofilin (Michelot et al., 2007), but subsequent work evaluating F-actin bundled by fascin found that cofilin-mediated disassembly was enhanced (Breitsprecher et al., 2011), indicating a cooperation between an actin filament bundling factor and cofilin. Furthermore, mechanical stress in highly cross-linked actin networks can enhance the rate of ADF/cofilin severing of actin filaments (Wioland et al., 2019).
These interacting networks of proteins and conditions that mediate the activity of ADF/cofilin highlight the numerous and complicated array of mechanisms available in the cell that allow for regulation of F-actin network disassembly. Specific proteins needed to promote filament disassembly could affect the rate of turnover in different regions, thereby affecting the local actin monomer concentration. The question remains as to if these conditions cause local enrichment of monomers or if diffusion is fast enough to render the effect of network-specific turnover moot in the cell. Supporting rapid monomer diffusion upon network disassembly, Higashida et al. found that when F-actin networks are disrupted by microneedle manipulation, cytoplasmic G-actin levels increase (Higashida et al., 2013). Furthermore, it remains unclear if there is any degree of crosstalk between networks to regulate F-actin disassembly to maintain actin monomer levels (Carlier and Shekhar, 2017). As we gain a better understanding of the cooperative and competitive milieu in which ADF/cofilin operates, it will be important to incorporate the effect of these interactions on homeostatic maintenance of F-actin network size and organization.
Competition and cooperation between actin binding proteins
Activation of specific actin assembly factors and subsequent recruitment of specific sets of ABPs leads to the formation of F-actin networks with the correct size, density and architectures to carry out distinct cellular processes. However, multiple different F-actin networks are assembled simultaneously within a common cytoplasm. Thus, the mechanisms that govern how specific sets of ABPs localize to the correct F-actin network are unclear. To explain how ABPs sort to the appropriate network at the right time and place, several non-mutually exclusive mechanisms have been proposed that can be grouped into two categories: extrinsic and intrinsic factors.
Extrinsic vs. Intrinsic regulation
Extrinsic factors that contribute to ABP sorting include external signals that act on an ABP. Just as upstream signals activate actin assembly factors, models of extrinsic regulation postulate that upstream signals regulate ABP localization to distinct cellular regions and F-actin networks. Examples of upstream signals include post-translational modifications (PTMs) or ion-binding that influence ABP activity in different cell cycle stages or subcellular compartments. For example, villin proteins mediate a calcium-dependent remodeling of F-actin by severing and bundling F-actin in A. thaliana pollen tubes responding to self-incompatibility. Budding yeast fimbrin Sac6 is phosphorylated in a cell cycle-dependent manner, enhancing its ability to associate with actin filaments (Miao et al., 2016), but how this phosphorylation promotes Sac6’s localization to endocytic actin patches is unclear.
Conversely, the intrinsic model of ABP sorting relies on qualities inherent to ABPs themselves that influence how, when, or where they associate with actin filaments. As all ABPs associated with actin networks bind F-actin, important intrinsic features that could affect ABP sorting include the number, type, orientation, or spacing of actin-binding modules. For example, the actin-binding domains alone of α-actinin and filamin are sufficient to localize to the proper cellular actin networks in Dictyostelium (Washington and Knecht, 2008). These intrinsic features could alter 1) an ABP’s preference for a specific F-actin architecture (branched vs. straight filaments), 2) an ABP’s affinity for a specific type of actin (ATP vs ADP-associated, length), or 3) the cooperative binding or kinetics of an ABP’s association with F-actin. Additionally, intrinsic factors could modify actin filaments by changing the twist and/or flexibility of individual filaments or altering characteristics of higher-order F-actin networks, such as the spacing, orientation, or density of filaments within the network, thereby making these filaments more amenable for ABP-binding.
Competition and cooperation between actin binding proteins
Recent evidence suggests that competition between ABPs for binding F-actin is a major factor that helps drive their sorting to different F-actin networks (Figure 2). For example, competition between capping protein and formin (or Ena/VASP (Bear et al., 2002)), barbed end-binding proteins, has been well-characterized in vitro (Bombardier et al., 2015; Harris et al., 2004; Kovar et al., 2003; Moseley et al., 2004; Shekhar et al., 2015; Zigmond et al., 2003). It was previously thought that once either capping protein or formin associate with a barbed end, it prevents the other from influencing polymerization. However, more recent evidence suggests that formin and capping proteins may bind the barbed end simultaneously, creating a “decision complex” that allows fine-tuning of filament length (Bombardier et al., 2015; Shekhar et al., 2015). In fission yeast cells, capping protein associates with Arp2/3 complex-mediated filaments, preserving the identity of branched networks and confining formin to other actin networks (Billault-Chaumartin and Martin, 2019). Further study will elucidate how cells balance formin and capping protein activities to promote F-actin assembly at the proper time and place.
Intrinsic factors also mediate the sorting of ABPs to distinct stretches of bundled filaments. Two crosslinking proteins, fascin and α-actinin, sort themselves into mutually exclusive domains on the same bundle in vitro (Winkelman et al., 2016). Fascin generates F-actin bundles with a narrow 8 nm spacing, while α-actinin creates more widely spaced bundles with filaments ~40 nm apart. Importantly, espin and fimbrin, also compact crosslinking proteins, sort to fascin-coated domain of the bundle, which suggests that filament spacing may be a key structural mechanism of ABP sorting in bundled networks (Winkelman et al., 2016). The ability to reconstitute this ABP sorting from purified proteins highlights the importance of intrinsic regulation, as no extrinsic signaling is required to generate mutually exclusive domains of fascin and α-actinin.
Another example of competition between specific ABPs involves fission yeast fimbrin Fim1, a crosslinking protein that localizes primarily to endocytic actin patches, and the contractile ring-associated tropomyosin Cdc8. Fim1 inhibits Cdc8’s association with actin filaments in vitro and actin patches in fission yeast cells (Christensen et al., 2017; Skau and Kovar, 2010). Cdc8, as well as other tropomyosins, also protects F-actin from cofilin Adf1-mediated severing (Gateva et al., 2017; Nishida et al., 1984; Skau and Kovar, 2010), so another important role for Fim1 is to promote F-actin turnover by preventing Cdc8’s association with actin patches. Furthermore, Cdc8 differentially regulates three fission yeast myosins: Myo1 (a class I myosin that serves as an Arp2/3 complex NPF in actin patches), Myo51, and Myo52 (class V myosins that localize to the contractile ring and actin cables, respectively) (Clayton et al., 2010). Interestingly, different mammalian tropomyosin isoforms sort to different actin filament populations and differentially regulate how ABPs access F-actin, with some isoforms activating myosin activity, and others protecting filaments from severing by ADF/cofilin (Gateva et al., 2017). While fission yeast Cdc8 promotes motor activity of Myo51 and Myo52 at formin-mediated F-actin networks and blocks Myo1-binding at actin patches, Fim1 restores Myo1 association with Arp2/3 complex-mediated filaments in actin patches by antagonizing Cdc8 (Clayton et al., 2010). Further study in fission yeast revealed a complicated web of interactions that leads to sorting of fission yeast Fim1, Cdc8, Adf1, and α-actinin Ain1 to the proper F-actin networks (Figure 2). Multi-color TIRF microscopy with fluorescently-labeled ABPs showed that while Cdc8 readily associates with single actin filaments, Fim1 can actively displace Cdc8 from actin filaments as it bundles them (Christensen et al., 2017).
Supporting previous findings (Skau and Kovar, 2010), it was shown that Cdc8, like some mammalian tropomyosin isoforms (Gateva et al., 2017), also competes with Adf1 by inhibiting its initial association with F-actin, but Adf1 displaces Cdc8 from older stretches of F-actin at later time points (Christensen et al., 2017). Interestingly, Fim1 also displays a mutually exclusive binding pattern with Adf1, despite both being actin patch-associated proteins in fission yeast. The competitive interaction between Fim1 and Adf1 actually leads to a more densely bundled actin network, as Adf1-mediated severing of actin bundles rapidly generates new, elongating barbed ends. The synergistic interaction between Adf1 and Fim1 allows them to compete with Cdc8, as Cdc8 is completely prevented from associating with F-actin in the presence of both Adf1 and Fim1 (Christensen et al., 2017) (Figure 2). These results suggest that competitive, pairwise interactions between ABPs could mediate their own sorting to distinct actin networks or influence how other ABPs associate with F-actin. What are the molecular mechanisms that govern these competitive interactions?
One intrinsic property that contributes to ABP sorting is cooperative association with actin filaments, which occurs when the binding of one molecule increases the likelihood that another will bind. For example, cooperative binding by crosslinking proteins like fascin and α-actinin contributes to the formation of F-actin bundles with a specific spacing that is optimal for recruiting other crosslinkers that maintain that spacing (Winkelman et al., 2016). Tropomyosin Cdc8 exhibits a high degree of end-to-end cooperativity that promotes its polymerization along actin filaments as well as indirect cooperativity via the actin filament that enhances its association with F-actin (Christensen et al., 2017). Cdc8’s cooperative binding makes it a great “gatekeeping” protein that rapidly coats F-actin to influence how other ABPs can access actin filaments. However, Cdc8’s cooperativity also promotes its rapid removal from F-actin, which likely explains its inability to compete with Fim1. Once Fim1 crosslinks actin filaments, Cdc8 is unable to displace it, leading to Cdc8’s exclusion from Fim1-associated actin networks like actin patches. However, if Fim1 outcompetes Cdc8 for binding F-actin, why is Cdc8 abundant at the fission yeast contractile ring, while Fim1 is practically absent?
Subsequent work revealed that Cdc8 collaborates with other contractile ring proteins to better compete against Fim1. α-Actinin Ain1, the ABP primarily responsible for bundling F-actin in the fission yeast contractile ring (Wu et al., 2001), also competes with Fim1 for binding F-actin both in vitro and in fission yeast cells. Fim1 outcompetes Ain1 and prevents it from associating with actin filaments, likely because Fim1 is a more stable bundler, while Ain1 generates dynamic bundles due to its shorter residence time on F-actin (Christensen et al., 2019; Li et al., 2016). Interestingly, in contrast to Fim1, Cdc8 actually enhances Ain1’s ability to bundle F-actin by increasing the number of Ain1 binding events along actin bundles (Christensen et al., 2019). As a result, the combination of Cdc8 and Ain1 is better able to compete against Fim1, as they synergize to increase the amount of Cdc8 present on bundles and decrease Fim1’s association with actin filaments (Figure 2). This synergy could explain why Fim1 associates poorly with the contractile ring in fission yeast cells and hints at a large, complex network of interactions between ABPs that ensures their sorting to specific F-actin networks.
While Cdc8 and Ain1 combine to more effectively compete with Fim1, they only reduce Fim1 association with F-actin bundles by 40% in vitro (Christensen et al., 2019). Furthermore, there is a higher cellular concentration of Fim1 than Ain1 or Cdc8 (Wu and Pollard, 2005). Thus, a reasonable assumption is that Ain1 and Cdc8 only prevent a portion of Fim1 molecules from associating with the contractile ring in vivo. Thus, there are likely a variety of different mechanisms that contribute to ABP sorting. One possibility is that Fim1 activity is regulated by post-translational modifications (PTMs). It is known that budding yeast fimbrin Sac6 is phosphorylated by Cdk1 at different stages of the cell cycle, increasing its ability to bind F-actin (Miao et al., 2016). Many fission yeast ABPs, including Fim1, are thought to be similarly post-translationally modified, which could affect the degree of activity of cytoplasmic Fim1 (Kettenbach et al., 2015). Furthermore, phosphorylation of Cdc8 weakens its association with F-actin, promoting filament disassembly by allowing cofilin-mediated severing (Palani et al., 2019). Thus, phosphorylation serves as an important mode of regulating multiple fission yeast ABPs and is likely important for regulating ABP activity and localization in higher eukaryotes as well (Arber et al., 1998; Elam et al., 2017; Yang et al., 1998). Another possibility is that mechanical stresses, such as those applied to actin filaments by type-II myosins during contractile ring assembly and constriction, can affect how ABPs association with actin filaments. Some ABPs, including mammalian α-actinin 4, act as mechanosensors that are recruited more strongly to actin filaments under tension (Schiffhauer et al., 2016). Furthermore, because actin filaments in fission yeast actin patches are short and branched, while those in the contractile ring are longer and straight, it is also possible that different actin filament architectures in competing F-actin networks contribute to ABP sorting by promoting recruitment of specific ABPs.
These competitive and cooperative interactions suggest a potential ABP hierarchy, where upstream gatekeeper proteins associate with specific F-actin networks and recruit some downstream ABPs while inhibiting the association of others, ultimately resulting in the association of the correct subset of ABPs with each network. However, these competitive interactions could also occur as a web of interactions, where various small interactions between ABPs combine with other biases toward particular F-actin networks, perhaps due to an affinity toward actin filaments in networks of different architectures and/or generated by a specific assembly factor. Future work will determine how the interplay between these non-mutually exclusive mechanisms promotes ABP association with the proper F-actin network at the correct time and place in different cell types.
Assembly Factors
A relatively under-investigated aspect of F-actin network self-organization is the effect of the specific assembly factor on recruitment of downstream ABPs. It has been proposed that a particular assembly factor could impart structural information such as a specific twist or conformational change upon the assembled actin filament that promotes subsequent ABP association (Kovar et al., 2011; Michelot and Drubin, 2011). Support for this mechanism was first appreciated in early experiments using the Arp2/3 complex activator, ActA from the intracellular pathogen Listeria monocytogenes. Beads coated with ActA incubated in cellular extract could generate F-actin networks with the appropriate suite of ABPs needed for motility (Cameron et al., 1999). Furthermore, incubating beads with the budding yeast Arp2/3 complex activator Las17 in yeast cellular extracts gnerates Arp2/3 complex generated networks with ABPs specific to actin patches (Michelot et al., 2010). Similarly, incubating beads with the formin Bni1 in mitotic yeast cell extracts generates cable-like actin networks containing cable-specific ABPs (Johnson et al., 2014; Miao et al., 2013; Yonetani and Chang, 2010). These results suggest that activation of the appropriate assembly factor is all that is required for recruitment of appropriate ABPs to specific networks. In fission yeast, the ABP tropomyosin (Cdc8) localizes to the filaments in the contractile ring nucleated by the formin Cdc12, or to actin cables assembled by the formin For3 depending on the acetylation status of Cdc8 (Coulton et al., 2010). If the localization of the assembly factor is switched, the localization of the acetylated or unacetylated Cdc8 likewise follows the appropriate assembly factor, suggesting that localization of post-translationally modified Cdc8 is dependent on the formin assembling the actin filaments (Johnson et al., 2014).
What might be the mechanism by which a difference in actin filament structure could be conferred by different assembly factors? Many formins, including mDia and budding yeast Bni1 rotate along the long-pitch helix of F-actin (Mizuno et al., 2011). If the rotational freedom of either the growing filament (Mizuno et al., 2011; Yu et al., 2018, 2017) or the movement of the formin is limited (Mizuno et al., 2011; Suzuki et al., 2020), processivity of the formin is affected. In the cell, where formins can be anchored to the lipid membrane, or filaments can be bundled by ABPs, restriction of this rotation may cause under-twisting of the actin filament (Jegou and Romet-Lemonne, 2019), which could affect the binding of ABPs such as cofilin (Mizuno et al., 2018). Conversely, Arp2/3 complex-mediated F-actin networks might present an architecture that is slightly preferred by upstream ABPs, providing a mechanism for the initial bias of fission yeast fimbrin (Fim1) for actin patches described above (Figure 2). The field has recently begun to appreciate that differences in actin filament helicity can have effects on the organization of actin networks (Jegou and Romet-Lemonne, 2019; Watanabe et al., 2018), and we expect this to be an exciting area of future investigation.
Conclusions
The actin cytoskeleton in the cell consists of numerous F-actin networks with distinct architectures and dynamics tailored to specific functions as diverse as cell motility, cytokinesis, endocytosis, and polarization. F-actin networks were historically evaluated in isolation, providing detailed information about the protein interactions needed to build and remodel each network. As a more complete picture of the components that make up these networks is established, the field has started to examine how cross-talk and competition between networks affect network size, molecular composition, dynamics and function in the cell. In this review, we have highlighted recent work focused on how different types of self-organization at the level of actin monomers, actin filaments, and ABPs converge to generate the many distinct cellular F-actin networks. With increasingly complex in vitro reconstitution of F-actin networks with large ensembles of ABPs, paired with in vivo manipulation and quantitative visualization of ABP dynamics in a range of model systems and modeling, we expect further insights into the complicated web of mechanisms that give rise to diverse networks needed for basic cellular functions.
Supplementary Material
Acknowledgments
We thank members of the Kovar lab for helpful comments and discussions, including Caitlin Anderson and Cristian Suarez. Our work on actin cytoskeleton self-organization is supported by National Institute for Health Grant R01 GM079265 (to D.R.K.) and the Department of Defense Army Research Office’s MURI grant W911NF1410403 (to D.R.K.).
Abbreviations:
- ABP
actin-binding protein
- F-actin
filamentous actin
- G-actin
globular actin
References
- Akamatsu M, Vasan R, Serwas D, Ferrin MA, Rangamani P, Drubin DG. 2020. Principles of self-organization and load adaptation by the actin cytoskeleton during clathrin-mediated endocytosis. eLife 9:e49840. doi: 10.7554/eLife.49840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin O, Mullins RD. 2008. Capping Protein Increases the Rate of Actin-Based Motility by Promoting Filament Nucleation by the Arp2/3 Complex. Cell 133:841–851. doi: 10.1016/j.cell.2008.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda JF, Cannon JF, Tatchell K, Hug C, Cooper JA. 1990. Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature 344:352–354. doi: 10.1038/344352a0 [DOI] [PubMed] [Google Scholar]
- Anderson KL, Page C, Swift MF, Suraneni P, Janssen MEW, Pollard TD, Li R, Volkmann N, Hanein D. 2017. Nano-scale actin-network characterization of fibroblast cells lacking functional Arp2/3 complex. Journal of Structural Biology 197:312–321. doi: 10.1016/j.jsb.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkowiak A, Guillotin A, Sanders MB, Colombo J, Vincentelli R, Michelot A. 2019. Sizes of actin networks sharing a common environment are determined by the relative rates of assembly. PLOS Biology 17:e3000317. doi: 10.1371/journal.pbio.3000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393:805–809. doi: 10.1038/31729 [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Bi E, Glotzer M. 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr Biol 14:R806–818. doi: 10.1016/j.cub.2004.09.022 [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Feoktistova A, McCollum D, Gould KL. 1996. Fission yeast Sop2p: a novel and evolutionarily conserved protein that interacts with Arp3p and modulates profilin function. EMBO J 15:6426–6437. [PMC free article] [PubMed] [Google Scholar]
- Balcer HI, Goodman AL, Rodal AA, Smith E, Kugler J, Heuser JE, Goode BL. 2003. Coordinated Regulation of Actin Filament Turnover by a High-Molecular-Weight Srv2/CAP Complex, Cofilin, Profilin, and Aip1. Current Biology 13:2159–2169. doi: 10.1016/j.cub.2003.11.051 [DOI] [PubMed] [Google Scholar]
- Bamburg JR. 1999. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15:185–230. doi: 10.1146/annurev.cellbio.15.1.185 [DOI] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109:509–521. doi: 10.1016/s0092-8674(02)00731-6 [DOI] [PubMed] [Google Scholar]
- Bentley D, Toroian-Raymond A. 1986. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature 323:712–715. doi: 10.1038/323712a0 [DOI] [PubMed] [Google Scholar]
- Berro J, Pollard TD. 2014. Local and global analysis of endocytic patch dynamics in fission yeast using a new “temporal superresolution” realignment method. Mol Biol Cell 25:3501–3514. doi: 10.1091/mbc.E13-01-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling P, Hansen SD, Akin O, Li T-D, Hayden CC, Fletcher DA, Mullins RD. 2018. WH2 and proline-rich domains of WASP-family proteins collaborate to accelerate actin filament elongation. EMBO J 37:102–121. doi: 10.15252/embj.201797039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billault-Chaumartin I, Martin SG. 2019. Capping Protein Insulates Arp2/3-Assembled Actin Patches from Formins. Current Biology 29:3165–3176.e6. doi: 10.1016/j.cub.2019.07.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. 2014. Actin Dynamics, Architecture, and Mechanics in Cell Motility. Physiological Reviews 94:235–263. doi: 10.1152/physrev.00018.2013 [DOI] [PubMed] [Google Scholar]
- Blanchoin L, Pollard TD. 1999. Mechanism of interaction of Acanthamoeba actophorin (ADF/Cofilin) with actin filaments. J Biol Chem 274:15538–15546. doi: 10.1074/jbc.274.22.15538 [DOI] [PubMed] [Google Scholar]
- Bombardier JP, Eskin JA, Jaiswal R, Corrêa IR, Xu M-Q, Goode BL, Gelles J. 2015. Single-molecule visualization of a formin-capping protein ‘decision complex” at the actin filament barbed end.’ Nat Commun 6. doi: 10.1038/ncomms9707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boujemaa-Paterski R, Suarez C, Klar T, Zhu J, Guérin C, Mogilner A, Théry M, Blanchoin L. 2017. Network heterogeneity regulates steering in actin-based motility. Nat Commun 8:655. doi: 10.1038/s41467-017-00455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovellan M, Romeo Y, Biro M, Boden A, Chugh P, Yonis A, Vaghela M, Fritzsche M, Moulding D, Thorogate R, Jégou A, Thrasher AJ, Romet-Lemonne G, Roux PP, Paluch EK, Charras G. 2014. Cellular Control of Cortical Actin Nucleation. Current Biology 24:1628–1635. doi: 10.1016/j.cub.2014.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Koestler SA, Chizhov I, Nemethova M, Mueller J, Goode BL, Small JV, Rottner K, Faix J. 2011. Cofilin cooperates with fascin to disassemble filopodial actin filaments. Journal of Cell Science 124:3305–3318. doi: 10.1242/jcs.086934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Kueh HY, Ballif BA, Mitchison TJ. 2006. Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J Cell Biol 175:315–324. doi: 10.1083/jcb.200603149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TA, Christensen JR, Barone E, Suarez C, Sirotkin V, Kovar DR. 2014. Homeostatic Actin Cytoskeleton Networks Are Regulated by Assembly Factor Competition for Monomers. Current Biology 24:579–585. doi: 10.1016/j.cub.2014.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LA, Footer MJ, van Oudenaarden A, Theriot JA. 1999. Motility of ActA protein-coated microspheres driven by actin polymerization. Proceedings of the National Academy of Sciences 96:4908–4913. doi: 10.1073/pnas.96.9.4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Welch MD. 2010. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11:237–251. doi: 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. 1997. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136:1307–1322. doi: 10.1083/jcb.136.6.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M-F, Shekhar S. 2017. Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat Rev Mol Cell Biol 18:389–401. doi: 10.1038/nrm.2016.172 [DOI] [PubMed] [Google Scholar]
- Chan F-Y, Silva AM, Saramago J, Pereira-Sousa J, Brighton HE, Pereira M, Oegema K, Gassmann R, Carvalho AX. 2019. The ARP2/3 complex prevents excessive formin activity during cytokinesis. MBoC 30:96–107. doi: 10.1091/mbc.E18-07-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Pollard TD. 2011. Actin filament severing by cofilin is more important for assembly than constriction of the cytokinetic contractile ring. J Cell Biol 195:485–498. doi: 10.1083/jcb.201103067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone MA, Goode BL. 2009. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol 21:28–37. doi: 10.1016/j.ceb.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra ES, Higgs HN. 2007. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol 9:1110–1121. doi: 10.1038/ncb1007-1110 [DOI] [PubMed] [Google Scholar]
- Christensen JR, Hocky GM, Homa KE, Morganthaler AN, Hitchcock-DeGregori SE, Voth GA, Kovar DR. 2017. Competition between Tropomyosin, Fimbrin, and ADF/Cofilin drives their sorting to distinct actin filament networks. Elife 6. doi: 10.7554/eLife.23152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JR, Homa KE, Morganthaler AN, Brown RR, Suarez C, Harker AJ, O’Connell ME, Kovar DR. 2019. Cooperation between tropomyosin and α-actinin inhibits fimbrin association with actin filament networks in fission yeast. eLife 8:e47279. doi: 10.7554/eLife.47279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JE, Sammons MR, Stark BC, Hodges AR, Lord M. 2010. Differential regulation of unconventional fission yeast myosins via the actin track. Curr Biol 20:1423–1431. doi: 10.1016/j.cub.2010.07.026 [DOI] [PubMed] [Google Scholar]
- Coffman VC, Nile AH, Lee I-J, Liu H, Wu J-Q. 2009. Roles of formin nodes and myosin motor activity in Mid1p-dependent contractile-ring assembly during fission yeast cytokinesis. Mol Biol Cell 20:5195–5210. doi: 10.1091/mbc.e09-05-0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton AT, East DA, Galinska-Rakoczy A, Lehman W, Mulvihill DP. 2010. The recruitment of acetylated and unacetylated tropomyosin to distinct actin polymers permits the discrete regulation of specific myosins in fission yeast. Journal of Cell Science 123:3235–3243. doi: 10.1242/jcs.069971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Amato C, Thomason PA, Insall RH. 2018. WASP family proteins and formins compete in pseudopod- and bleb-based migration. J Cell Biol 217:701–714. doi: 10.1083/jcb.201705160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T, Jordan SN, Chand V, Sees JA, Laband K, Carvalho AX, Shirasu-Hiza M, Kovar DR, Dumont J, Canman JC. 2014. High-Resolution Temporal Analysis Reveals a Functional Timeline for the Molecular Regulation of Cytokinesis. Developmental Cell 30:209–223. doi: 10.1016/j.devcel.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimchev G, Steffen A, Kage F, Dimchev V, Pernier J, Carlier M-F, Rottner K. 2017. Efficiency of lamellipodia protrusion is determined by the extent of cytosolic actin assembly. MBoC 28:1311–1325. doi: 10.1091/mbc.e16-05-0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr M, Misteli T, Olson MO. 2000. The dynamics of postmitotic reassembly of the nucleolus. J Cell Biol 150:433–446. doi: 10.1083/jcb.150.3.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA. 2014. Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol 15:677–689. doi: 10.1038/nrm3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam WA, Cao W, Kang H, Huehn A, Hocky GM, Prochniewicz E, Schramm AC, Negrón K, Garcia J, Bonello TT, Gunning PW, Thomas DD, Voth GA, Sindelar CV, De La Cruz EM. 2017. Phosphomimetic S3D cofilin binds but only weakly severs actin filaments. J Biol Chem 292:19565–19579. doi: 10.1074/jbc.M117.808378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam WA, Kang H, De la Cruz EM. 2013. Biophysics of actin filament severing by cofilin. FEBS Lett 587:1215–1219. doi: 10.1016/j.febslet.2013.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust JJ, Millis BA, Tyska MJ. 2019. Profilin-Mediated Actin Allocation Regulates the Growth of Epithelial Microvilli. Current Biology 29:3457–3465.e3. doi: 10.1016/j.cub.2019.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B, Chang F. 2001. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr Biol 11:1656–1665. [DOI] [PubMed] [Google Scholar]
- Funk J, Merino F, Venkova L, Heydenreich L, Kierfeld J, Vargas P, Raunser S, Piel M, Bieling P. 2019. Profilin and formin constitute a pacemaker system for robust actin filament growth. eLife 8:e50963. doi: 10.7554/eLife.50963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet Y, Hyams JS. 2005. Endocytosis in fission yeast is spatially associated with the actin cytoskeleton during polarised cell growth and cytokinesis. J Cell Sci 118:4231–4242. doi: 10.1242/jcs.02530 [DOI] [PubMed] [Google Scholar]
- Gandhi M, Achard V, Blanchoin L, Goode BL. 2009. Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Mol Cell 34:364–374. doi: 10.1016/j.molcel.2009.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Smith BA, Bovellan M, Paavilainen V, Daugherty-Clarke K, Gelles J, Lappalainen P, Goode BL. 2010. GMF is a cofilin homolog that binds Arp2/3 complex to stimulate filament debranching and inhibit actin nucleation. Curr Biol 20:861–867. doi: 10.1016/j.cub.2010.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Bretscher A. 2008. Analysis of Unregulated Formin Activity Reveals How Yeast Can Balance F-Actin Assembly between Different Microfilament-based Organizations. Mol Biol Cell 19:1474–1484. doi: 10.1091/mbc.E07-05-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateva G, Kremneva E, Reindl T, Kotila T, Kogan K, Gressin L, Gunning PW, Manstein DJ, Michelot A, Lappalainen P. 2017. Tropomyosin Isoforms Specify Functionally Distinct Actin Filament Populations In Vitro. Curr Biol 27:705–713. doi: 10.1016/j.cub.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS. 2002. Can the Golgi form de novo ? Nat Rev Mol Cell Biol 3:615–619. doi: 10.1038/nrm877 [DOI] [PubMed] [Google Scholar]
- Gressin L, Guillotin A, Guérin C, Blanchoin L, Michelot A. 2015. Architecture Dependence of Actin Filament Network Disassembly. Current Biology 25:1437–1447. doi: 10.1016/j.cub.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Harris ES, Li F, Higgs HN. 2004. The Mouse Formin, FRLα, Slows Actin Filament Barbed End Elongation, Competes with Capping Protein, Accelerates Polymerization from Monomers, and Severs Filaments. J Biol Chem 279:20076–20087. doi: 10.1074/jbc.M312718200 [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. 1996. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382:420–425. doi: 10.1038/382420a0 [DOI] [PubMed] [Google Scholar]
- Higashida C, Kiuchi T, Akiba Y, Mizuno H, Maruoka M, Narumiya S, Mizuno K, Watanabe N. 2013. F- and G-actin homeostasis regulates mechanosensitive actin nucleation by formins. Nat Cell Biol 15:395–405. doi: 10.1038/ncb2693 [DOI] [PubMed] [Google Scholar]
- Hilton DM, Aguilar RM, Johnston AB, Goode BL. 2018. Species-Specific Functions of Twinfilin in Actin Filament Depolymerization. J Mol Biol 430:3323–3336. doi: 10.1016/j.jmb.2018.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Lappalainen P. 2006. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173:383–394. doi: 10.1083/jcb.200511093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai T, Danuser G. 2018. Discovery of functional interactions among actin regulators by analysis of image fluctuations in an unperturbed motile cell system. Philos Trans R Soc Lond, B, Biol Sci 373. doi: 10.1098/rstb.2017.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Collins A, Chin SM, Ydenberg CA, Gelles J, Goode BL. 2015. Single-molecule imaging of a three-component ordered actin disassembly mechanism. Nat Commun 6:7202. doi: 10.1038/ncomms8202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Collins A, Yang C, Rebowski G, Svitkina T, Dominguez R. 2011. Mechanism of actin filament bundling by fascin. J Biol Chem 286:30087–30096. doi: 10.1074/jbc.M111.251439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegou A, Romet-Lemonne G. 2019. The many implications of actin filament helicity. Seminars in Cell & Developmental Biology S1084952118303069. doi: 10.1016/j.semcdb.2019.10.018 [DOI] [PubMed] [Google Scholar]
- Johnson M, East DA, Mulvihill DP. 2014. Formins determine the functional properties of actin filaments in yeast. Curr Biol 24:1525–1530. doi: 10.1016/j.cub.2014.05.034 [DOI] [PubMed] [Google Scholar]
- Johnston AB, Collins A, Goode BL. 2015. High-speed depolymerization at actin filament ends jointly catalysed by Twinfilin and Srv2/CAP. Nat Cell Biol 17:1504–1511. doi: 10.1038/ncb3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AB, Hilton DM, McConnell P, Johnson B, Harris MT, Simone A, Amarasinghe GK, Cooper JA, Goode BL. 2018. A novel mode of capping protein-regulation by twinfilin. Elife 7. doi: 10.7554/eLife.41313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage F, Winterhoff M, Dimchev V, Mueller J, Thalheim T, Freise A, Brühmann S, Kollasser J, Block J, Dimchev G, Geyer M, Schnittler H-J, Brakebusch C, Stradal TEB, Carlier M-F, Sixt M, Käs J, Faix J, Rottner K. 2017. FMNL formins boost lamellipodial force generation. Nat Commun 8:14832. doi: 10.1038/ncomms14832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser DA, Vinson VK, Murphy DB, Pollard TD. 1999. Profilin is predominantly associated with monomeric actin in Acanthamoeba. J Cell Sci 112 (Pt 21):3779–3790. [DOI] [PubMed] [Google Scholar]
- Kamasaki T, Arai R, Osumi M, Mabuchi I. 2005. Directionality of F-actin cables changes during the fission yeast cell cycle. Nat Cell Biol 7:916–917. doi: 10.1038/ncb1295 [DOI] [PubMed] [Google Scholar]
- Karsenti E 2008. Self-organization in cell biology: a brief history. Nat Rev Mol Cell Biol 9:255–262. doi: 10.1038/nrm2357 [DOI] [PubMed] [Google Scholar]
- Karsenti E, Vernos I. 2001. The Mitotic Spindle: A Self-Made Machine. Science 294:543–547. doi: 10.1126/science.1063488 [DOI] [PubMed] [Google Scholar]
- Kettenbach AN, Deng L, Wu Y, Baldissard S, Adamo ME, Gerber SA, Moseley JB. 2015. Quantitative phosphoproteomics reveals pathways for coordination of cell growth and division by the conserved fission yeast kinase pom1. Mol Cell Proteomics 14:1275–1287. doi: 10.1074/mcp.M114.045245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotila T, Wioland H, Enkavi G, Kogan K, Vattulainen I, Jégou A, Romet-Lemonne G, Lappalainen P. 2019. Mechanism of synergistic actin filament pointed end depolymerization by cyclase-associated protein and cofilin. Nat Commun 10:5320. doi: 10.1038/s41467-019-13213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Kuhn JR, Tichy AL, Pollard TD. 2003. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J Cell Biol 161:875–887. doi: 10.1083/jcb.200211078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Sirotkin V, Lord M. 2011. Three’s company: the fission yeast actin cytoskeleton. Trends Cell Biol 21:177–187. doi: 10.1016/j.tcb.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Wu J-Q, Pollard TD. 2005. Profilin-mediated Competition between Capping Protein and Formin Cdc12p during Cytokinesis in Fission Yeast. Mol Biol Cell 16:2313–2324. doi: 10.1091/mbc.E04-09-0781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh HY, Brieher WM, Mitchison TJ. 2008. Dynamic stabilization of actin filaments. PNAS 105:16531–16536. doi: 10.1073/pnas.0807394105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Jiu Y, Carman PJ, Tojkander S, Kogan K, Varjosalo M, Gunning PW, Dominguez R, Lappalainen P. 2020. Tropomodulins Control the Balance between Protrusive and Contractile Structures by Stabilizing Actin-Tropomyosin Filaments. Current Biology 30:767–778.e5. doi: 10.1016/j.cub.2019.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy MM, Baddeley D, Berro J. 2019. Single-molecule turnover dynamics of actin and membrane coat proteins in clathrin-mediated endocytosis. eLife 8:e52355. doi: 10.7554/eLife.52355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte D, Coffman VC, Lee I-J, Wu J-Q. 2011. Assembly and architecture of precursor nodes during fission yeast cytokinesis. J Cell Biol 192:1005–1021. doi: 10.1083/jcb.201008171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen P, Drubin DG. 1997. Cofilin promotes rapid actin filament turnover in vivo. Nature 388:78–82. doi: 10.1038/40418 [DOI] [PubMed] [Google Scholar]
- Li Y, Christensen JR, Homa KE, Hocky GM, Fok A, Sees JA, Voth GA, Kovar DR. 2016. The F-actin bundler α-actinin Ain1 is tailored for ring assembly and constriction during cytokinesis in fission yeast. Mol Biol Cell 27:1821–1833. doi: 10.1091/mbc.E16-01-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin AJ, Lee K-C, Han SJ, Bui DA, Davidson M, Mogilner A, Danuser G. 2015. Competition of two distinct actin networks for actin defines a bistable switch for cell polarization. Nat Cell Biol 17:1435–1445. doi: 10.1038/ncb3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD. 1999. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA 96:3739–3744. doi: 10.1073/pnas.96.7.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart A, Icheva TA, Guerin C, Klar T, Boujemaa-Paterski R, Thery M, Blanchoin L, Mogilner A. 2019. Quantitative regulation of the dynamic steady state of actin networks. eLife 8:e42413. doi: 10.7554/eLife.42413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. n.d. Lamellipodial Versus Filopodial Mode of the Actin Nanomachinery: Pivotal Role of the Filament Barbed End 11. [DOI] [PubMed] [Google Scholar]
- Miao Y, Han X, Zheng L, Xie Y, Mu Y, Yates JR, Drubin DG. 2016. Fimbrin phosphorylation by metaphase Cdk1 regulates actin cable dynamics in budding yeast. Nat Commun 7:11265. doi: 10.1038/ncomms11265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Wong CCL, Mennella V, Michelot A, Agard DA, Holt LJ, Yates JR, Drubin DG. 2013. Cell-cycle regulation of formin-mediated actin cable assembly. Proc Natl Acad Sci USA 110:E4446–4455. doi: 10.1073/pnas.1314000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot A, Berro J, Guérin C, Boujemaa-Paterski R, Staiger CJ, Martiel J-L, Blanchoin L. 2007. Actin-Filament Stochastic Dynamics Mediated by ADF/Cofilin. Current Biology 17:825–833. doi: 10.1016/j.cub.2007.04.037 [DOI] [PubMed] [Google Scholar]
- Michelot A, Costanzo M, Sarkeshik A, Boone C, Yates JR, Drubin DG. 2010. Reconstitution and protein composition analysis of endocytic actin patches. Curr Biol 20:1890–1899. doi: 10.1016/j.cub.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot A, Drubin DG. 2011. Building Distinct Actin Filament Networks in a Common Cytoplasm. Curr Biol 21:R560–R569. doi: 10.1016/j.cub.2011.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Huang J, Balasubramanian MK. 2014. The yeast actin cytoskeleton. FEMS Microbiol Rev 38:213–227. doi: 10.1111/1574-6976.12064 [DOI] [PubMed] [Google Scholar]
- Misteli T 2001. The concept of self-organization in cellular architecture. J Cell Biol 155:181–186. doi: 10.1083/jcb.200108110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Watanabe N. 2013. Can filament treadmilling alone account for the F-actin turnover in lamellipodia?: Treadmilling and Alternative Mechanisms Regulating F-actin Turnover. Cytoskeleton 70:179–190. doi: 10.1002/cm.21098 [DOI] [PubMed] [Google Scholar]
- Mizuno H, Higashida C, Yuan Y, Ishizaki T, Narumiya S, Watanabe N. 2011. Rotational movement of the formin mDia1 along the double helical strand of an actin filament. Science 331:80–83. doi: 10.1126/science.1197692 [DOI] [PubMed] [Google Scholar]
- Mizuno H, Tanaka K, Yamashiro S, Narita A, Watanabe N. 2018. Helical rotation of the diaphanous-related formin mDia1 generates actin filaments resistant to cofilin. Proc Natl Acad Sci USA 115:E5000–E5007. doi: 10.1073/pnas.1803415115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra L, Lagny TJ, Harbage D, Jelenkovic PR, Kondev J. 2017. The Limiting-Pool Mechanism Fails to Control the Size of Multiple Organelles. Cell Systems 4:559–567.e14. doi: 10.1016/j.cels.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. 2004. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell 15:896–907. doi: 10.1091/mbc.E03-08-0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Imai J, Arai R, Toh-E A, Matsui Y, Mabuchi I. 2002. The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J Cell Sci 115:4629–4639. [DOI] [PubMed] [Google Scholar]
- Nakano K, Mabuchi I. 2006. Actin-depolymerizing protein Adf1 is required for formation and maintenance of the contractile ring during cytokinesis in fission yeast. Mol Biol Cell 17:1933–1945. doi: 10.1091/mbc.e05-09-0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidt EM, Scott BJ, Kovar DR. 2009. Formin differentially utilizes profilin isoforms to rapidly assemble actin filaments. J Biol Chem 284:673–684. doi: 10.1074/jbc.M804201200 [DOI] [PubMed] [Google Scholar]
- Nicholson-Dykstra SM, Higgs HN. 2008. Arp2 depletion inhibits sheet-like protrusions but not linear protrusions of fibroblasts and lymphocytes. Cell Motil Cytoskeleton 65:904–922. doi: 10.1002/cm.20312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida E, Maekawa S, Sakai H. 1984. Cofilin, a protein in porcine brain that binds to actin filaments and inhibits their interactions with myosin and tropomyosin. Biochemistry 23:5307–5313. doi: 10.1021/bi00317a032 [DOI] [PubMed] [Google Scholar]
- Okada K, Obinata T, Abe H. 1999. XAIP1: a Xenopus homologue of yeast actin interacting protein 1 (AIP1), which induces disassembly of actin filaments cooperatively with ADF/cofilin family proteins. J Cell Sci 112 (Pt 10):1553–1565. [DOI] [PubMed] [Google Scholar]
- Palani S, Köster DV, Hatano T, Kamnev A, Kanamaru T, Brooker HR, Hernandez-Fernaud JR, Jones AME, Millar JBA, Mulvihill DP, Balasubramanian MK. 2019. Phosphoregulation of tropomyosin is crucial for actin cable turnover and division site placement. J Cell Biol 218:3548–3559. doi: 10.1083/jcb.201809089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernier J, Shekhar S, Jegou A, Guichard B, Carlier M-F. 2016. Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility. Dev Cell 36:201–214. doi: 10.1016/j.devcel.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. 2016. Actin and Actin-Binding Proteins. Cold Spring Harb Perspect Biol 8:a018226. doi: 10.1101/cshperspect.a018226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymann A-C, Martiel J-L, Cambier T, Blanchoin L, Boujemaa-Paterski R, Théry M. 2010. Nucleation geometry governs ordered actin networks structures. Nat Mater 9:827–832. doi: 10.1038/nmat2855 [DOI] [PubMed] [Google Scholar]
- Rodal AA, Tetreault JW, Lappalainen P, Drubin DG, Amberg DC. 1999. Aip1p interacts with cofilin to disassemble actin filaments. J Cell Biol 145:1251–1264. doi: 10.1083/jcb.145.6.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotty JD, Wu C, Haynes EM, Suarez C, Winkelman JD, Johnson HE, Haugh JM, Kovar DR, Bear JE. 2015. Profilin-1 Serves as a Gatekeeper for Actin Assembly by Arp2/3-Dependent and -Independent Pathways. Developmental Cell 32:54–67. doi: 10.1016/j.devcel.2014.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. 2002. An actin nucleation mechanism mediated by Bni1 and profilin. Nat Cell Biol 4:626–631. doi: 10.1038/ncb834 [DOI] [PubMed] [Google Scholar]
- Sarmiento C, Wang W, Dovas A, Yamaguchi H, Sidani M, El-Sibai M, DesMarais V, Holman HA, Kitchen S, Backer JM, Alberts A, Condeelis J. 2008. WASP family members and formin proteins coordinate regulation of cell protrusions in carcinoma cells. The Journal of Cell Biology 180:1245–1260. doi: 10.1083/jcb.200708123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Jennings PB, Cooper JA. 1996. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol 135:169–179. doi: 10.1083/jcb.135.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffhauer ES, Luo T, Mohan K, Srivastava V, Qian X, Griffis ER, Iglesias PA, Robinson DN. 2016. Mechanoaccumulative Elements of the Mammalian Actin Cytoskeleton. Curr Biol 26:1473–1479. doi: 10.1016/j.cub.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S, Chung J, Kondev J, Gelles J, Goode BL. 2019. Synergy between Cyclase-associated protein and Cofilin accelerates actin filament depolymerization by two orders of magnitude. Nat Commun 10:5319. doi: 10.1038/s41467-019-13268-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S, Kerleau M, Kühn S, Pernier J, Romet-Lemonne G, Jégou A, Carlier M-F. 2015. Formin and capping protein together embrace the actin filament in a ménage à trois. Nat Commun 6. doi: 10.1038/ncomms9730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnar SA, Antoku S, Saffin J-M, Cooper JA, Halpain S. 2014. Capping protein is essential for cell migration in vivo and for filopodial morphology and dynamics. Mol Biol Cell 25:2152–2160. doi: 10.1091/mbc.E13-12-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin V, Berro J, Macmillan K, Zhao L, Pollard TD. 2010. Quantitative Analysis of the Mechanism of Endocytic Actin Patch Assembly and Disassembly in Fission Yeast. MBoC 21:2894–2904. doi: 10.1091/mbc.e10-02-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizonenko GI, Karpova TS, Gattermeir DJ, Cooper JA. 1996. Mutational analysis of capping protein function in Saccharomyces cerevisiae. Mol Biol Cell 7:1–15. doi: 10.1091/mbc.7.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skau CT, Kovar DR. 2010. Fimbrin and tropomyosin competition regulates endocytosis and cytokinesis kinetics in fission yeast. Curr Biol 20:1415–1422. doi: 10.1016/j.cub.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skruber K, Read T-A, Vitriol EA. 2018. Reconsidering an active role for G-actin in cytoskeletal regulation. J Cell Sci 131. doi: 10.1242/jcs.203760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, Carroll RT, Burke TA, Christensen JR, Bestul AJ, Sees JA, James ML, Sirotkin V, Kovar DR. 2015. Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev Cell 32:43–53. doi: 10.1016/j.devcel.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, Kovar DR. 2016. Internetwork competition for monomers governs actin cytoskeleton organization. Nat Rev Mol Cell Biol 17:799–810. doi: 10.1038/nrm.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, McCall PM, Gardel ML, Kovar DR. 2017. When Is “Enough” Enough? Cell Systems 4:480–482. doi: 10.1016/j.cels.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Suarez C, Roland J, Boujemaa-Paterski R, Kang H, McCullough BR, Reymann A-C, Guérin C, Martiel J-L, De la Cruz EM, Blanchoin L. 2011. Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr Biol 21:862–868. doi: 10.1016/j.cub.2011.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraneni P, Rubinstein B, Unruh JR, Durnin M, Hanein D, Li R. 2012. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J Cell Biol 197:239–251. doi: 10.1083/jcb.201112113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki EL, Chikireddy J, Dmitrieff S, Guichard B, Romet-Lemonne G, Jégou A. 2020. Geometrical Constraints Greatly Hinder Formin mDia1 Activity. Nano Lett 20:22–32. doi: 10.1021/acs.nanolett.9b02241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG. 1999. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol 145:1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot JA. 1997. Accelerating on a treadmill: ADF/cofilin promotes rapid actin filament turnover in the dynamic cytoskeleton. J Cell Biol 136:1165–1168. doi: 10.1083/jcb.136.6.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS. 1984. Observations on how actin filaments become organized in cells. The Journal of Cell Biology 99:76s–82s. doi: 10.1083/jcb.99.1.76s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D, Wu J-Q, Hao S, O’Shaughnessy B, Pollard TD. 2008. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 319:97–100. doi: 10.1126/science.1151086 [DOI] [PubMed] [Google Scholar]
- Velarde N, Gunsalus KC, Piano F. 2007. Diverse roles of actin in C. elegans early embryogenesis. BMC Dev Biol 7:142. doi: 10.1186/1471-213X-7-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. 2006. Role of fascin in filopodial protrusion. J Cell Biol 174:863–875. doi: 10.1083/jcb.200603013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Vavylonis D. 2008. Model of For3p-mediated actin cable assembly in fission yeast. PLoS ONE 3:e4078. doi: 10.1371/journal.pone.0004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington RW, Knecht DA. 2008. Actin binding domains direct actin-binding proteins to different cytoskeletal locations. BMC Cell Biology 9. doi: 10.1186/1471-2121-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Tohyama K, Yamashiro S. 2018. Mechanostress resistance involving formin homology proteins: G- and F-actin homeostasis-driven filament nucleation and helical polymerization-mediated actin polymer stabilization. Biochemical and Biophysical Research Communications 506:323–329. doi: 10.1016/j.bbrc.2018.09.189 [DOI] [PubMed] [Google Scholar]
- Wedlich-Söldner R, Betz T. 2018. Self-organization: the fundament of cell biology. Philos Trans R Soc Lond, B, Biol Sci 373. doi: 10.1098/rstb.2017.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggan O, Shaw AE, DeLuca JG, Bamburg JR. 2012. ADF/Cofilin Regulates Actomyosin Assembly through Competitive Inhibition of Myosin II Binding to F-Actin. Developmental Cell 22:530–543. doi: 10.1016/j.devcel.2011.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis JH, Munro E, Lyczak R, Bowerman B. 2006. Conditional dominant mutations in the Caenorhabditis elegans gene act-2 identify cytoplasmic and muscle roles for a redundant actin isoform. Mol Biol Cell 17:1051–1064. doi: 10.1091/mbc.e05-09-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JD, Suarez C, Hocky GM, Harker AJ, Morganthaler AN, Christensen JR, Voth GA, Bartles JR, Kovar DR. 2016. Fascin- and α-Actinin-Bundled Networks Contain Intrinsic Structural Features that Drive Protein Sorting. Current Biology 26:2697–2706. doi: 10.1016/j.cub.2016.07.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wioland H, Guichard B, Senju Y, Myram S, Lappalainen P, Jégou A, Romet-Lemonne G. 2017. ADF/Cofilin Accelerates Actin Dynamics by Severing Filaments and Promoting Their Depolymerization at Both Ends. Current Biology 27:1956–1967.e7. doi: 10.1016/j.cub.2017.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wioland H, Jegou A, Romet-Lemonne G. 2019. Torsional stress generated by ADF/cofilin on cross-linked actin filaments boosts their severing. Proc Natl Acad Sci USA 116:2595–2602. doi: 10.1073/pnas.1812053116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Asokan SB, Berginski ME, Haynes EM, Sharpless NE, Griffith JD, Gomez SM, Bear JE. 2012. Arp2/3 Is Critical for Lamellipodia and Response to Extracellular Matrix Cues but Is Dispensable for Chemotaxis. Cell 148:973–987. doi: 10.1016/j.cell.2011.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Bähler J, Pringle JR. 2001. Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol Biol Cell 12:1061–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-Q, Kuhn JR, Kovar DR, Pollard TD. 2003. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell 5:723–734. [DOI] [PubMed] [Google Scholar]
- Wu J-Q, Pollard TD. 2005. Counting cytokinesis proteins globally and locally in fission yeast. Science 310:310–314. doi: 10.1126/science.1113230 [DOI] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. 1998. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393:809–812. doi: 10.1038/31735 [DOI] [PubMed] [Google Scholar]
- Yonetani A, Chang F. 2010. Regulation of cytokinesis by the formin cdc12p. Curr Biol 20:561–566. doi: 10.1016/j.cub.2010.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Le S, Efremov AK, Zeng X, Bershadsky A, Yan J. 2018. Effects of Mechanical Stimuli on Profilin- and Formin-Mediated Actin Polymerization. Nano Lett 18:5239–5247. doi: 10.1021/acs.nanolett.8b02211 [DOI] [PubMed] [Google Scholar]
- Yu M, Yuan X, Lu C, Le S, Kawamura R, Efremov AK, Zhao Z, Kozlov MM, Sheetz M, Bershadsky A, Yan J. 2017. mDia1 senses both force and torque during F-actin filament polymerization. Nat Commun 8:1650. doi: 10.1038/s41467-017-01745-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH, Evangelista M, Boone C, Yang C, Dar AC, Sicheri F, Forkey J, Pring M. 2003. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr Biol 13:1820–1823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


