Abstract
Novel coronavirus (COVID-19) outbreak, has raised a calamitous situation all over the world and has become one of the most acute and severe ailments in the past hundred years. The prevalence rate of COVID-19 is rapidly rising every day throughout the globe. Although no vaccines for this pandemic have been discovered yet, deep learning techniques proved themselves to be a powerful tool in the arsenal used by clinicians for the automatic diagnosis of COVID-19. This paper aims to overview the recently developed systems based on deep learning techniques using different medical imaging modalities like Computer Tomography (CT) and X-ray. This review specifically discusses the systems developed for COVID-19 diagnosis using deep learning techniques and provides insights on well-known data sets used to train these networks. It also highlights the data partitioning techniques and various performance measures developed by researchers in this field. A taxonomy is drawn to categorize the recent works for proper insight. Finally, we conclude by addressing the challenges associated with the use of deep learning methods for COVID-19 detection and probable future trends in this research area. The aim of this paper is to facilitate experts (medical or otherwise) and technicians in understanding the ways deep learning techniques are used in this regard and how they can be potentially further utilized to combat the outbreak of COVID-19.
Keywords: Coronavirus, COVID-19, deep learning, deep transfer learning, diagnosis, x-ray, computer tomography
I. Introduction
Novel coronavirus (COVID-19), resulting from a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a pandemic worldwide in recent times [1], [2]. The number of infected cases as well as the death rate is increasingrapidly. As of the writing of this manuscript, it is reportedthat more than 108,000,000 people have been infected withCOVID-19, the death cases are around 2,400,000, and thenumber of recovered patients is around 80,000,000 globally[3]. The universal transmission of COVID-19 has put a large portion of the world’s population into quarantine and ravaged numerous industrial sectors which in turn caused a worldwide financial crisis.
The most typical signs of the novel coronavirus include fever, dry cough, myalgia, dyspnea, and headache [4], [5] but in some scenarios, no symptoms are visible (asymptomatic) that make the disease an even bigger threat to public health. The reverse transcript polymerase chain reaction (RT-PCR) is considered as the gold standard for COVID-19 diagnosis [6]. However, the lack of resources and strict test environment requirements restrict fast and effective screening of suspicious cases. Furthermore, RT-PCR inspection also experiences false negative rates in some cases [7]. Unfortunately, the only solution to effectively combat this transmissible disease, is through clinical vaccines as well as precise drug/therapy practices, which are not yet available.
COVID-19 has proven to be amongst the most dangerous ailments that have posed a severe threat to human civilization. With the evolution of modern technology in the past few decades, ingenious solutions have been created to assist disease diagnosis, prevention as well as control which leverage smart healthcare tools and facilities [8]–[11]. Specifically, for COVID-19 diagnosis, different imaging modalities like CT and X-ray are considered among the most effective techniques [12]–[14], When available, CT screening is preferred in comparison with X-rays because of its versatility and three-dimensional pulmonary view [15], [16] though X-rays are must more affordable and widely available. These traditional medical imaging modalities play a vital role in the control of the pandemic.
Artificial Intelligence (AI), an evolving software technology in the area of medical image analysis has also directly helped combating the novel coronavirus [17]–[19] by efficiently providing high quality diagnosis results and dramatically reducing or eliminating man power. Very recently, deep learning and machine learning, two major areas of AI have become very popular in medical applications. Deep learning based support systems are developed for COVID-19 diagnosis using both CT and X-ray samples [20]–[23]. Some of the systems are developed based on pre-trained model with transfer learning [24], [25] and a few of them are introduced using customized networks [26]–[28]. Machine learning [29], [30], and data science [31] are also the diverse areas that are actively used for corona diagnosis, prognosis, prediction, and outbreak forecasting. Computer vision [32] has also contributed for the reduction of the severity of this pandemic. Moreover, Internet of things (IoT) [33], [34], big data [35], [36], and smartphone technology [37], [38] are extensively utilized to enable innovative solutions to fight against the spread of COVID-19.
The main aim of the paper is to review the recent developments of deep learning based COVID-19 diagnosis systems based upon data collected from medical imaging samples. A taxonomy is presented that classifies the reviewed systems based on pre-trained model with deep transfer learning and customized deep learning techniques. We review the most vital schemes developed for the diagnosis of COVID-19 highlighting some aspects such as the data used for experiments, the data splitting technique, the proposed architecture for detection, and the evaluation metrics. An open discussion also presents the challenges of deep learning based systems and projects future works.
Key contributions of this review are summarized as follows:
-
(i)
To systematically review the state-of-the-art developments of deep learning based COVID-19 diagnosis systems from CT and X-Ray medical imaging samples.
-
(ii)
To present the reviewed works and the relevant information in a clear, concise, and accessible manner by considering some key elements like the data used for experiments, the data partitioning method, the proposed architecture for diagnosis, and the performance evaluation metrics.
-
(iii)
To introduce a taxonomy of the reviewed literature for the proper insight of the developments.
-
(iv)
To highlight and discuss the challenging aspects of the current developments of deep learning based COVID-19 diagnosis systems.
-
(v)
To present future research directions for further development of efficient and reliable COVID-19 detection systems.
The rest of the paper reads as follows. Section II categorizes the reviewed systems for proper understanding. Section III explains the recently developed systems for COVID-19 diagnosis from both CT and X-ray samples using pre-trained model with deep transfer learning. Section IV demonstrates the custom network based COVID-19 diagnosis systems from both CT and X-ray. The discussions with challenges as well as possible future trends are depicted in section V. The limitations of the study are drawn in section VI and some concluding remarks are provided in section VII.
II. Taxonomy of Deep Learning Based COVID-19 Diagnosis Systems
Deep learning techniques are able to explain complex problems by learning from simple depictions. The main features that have made the deep learning methods popular are the capability of learning the exact representations and the property of learning the data in a deep manner where multiple layers are utilized sequentially [39], [40]. Deep learning methods are widely used in medical systems such as biomedicine [41], smart healthcare [42], drug discovery [43], medical image analysis [44], etc.
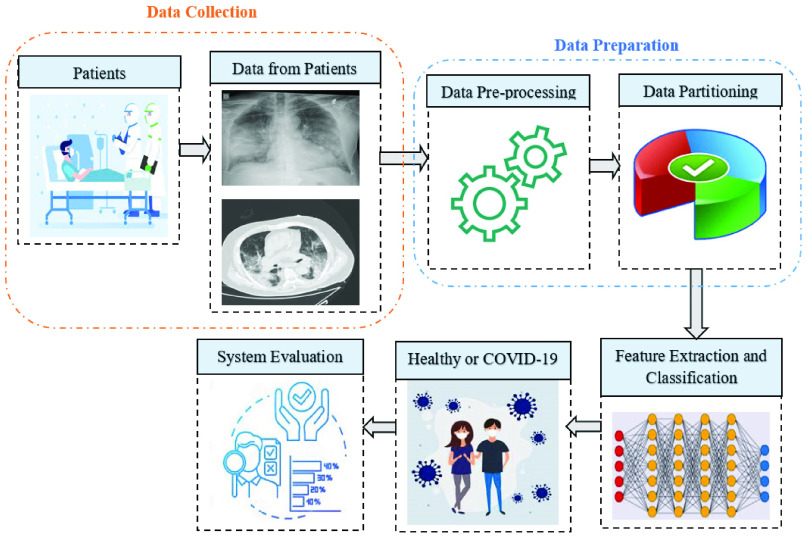
More recently, it is extensively used in the automated diagnosis of COVID-19 in patients. In general, deep learning based systems are comprised of several steps such as data collection, data preparation, feature extraction and classification, and performance evaluation. The general pipeline of a COVID-19 diagnosis system based on deep learning is illustrated in Fig. 1. At the data collection stage, the patients from the hospital environment are considered as a participant. The data may have different forms but for COVID-19 diagnosis, imaging techniques like CT and X-ray samples are taken. The following necessary step is the data preparation that converts the data into an appropriate format. In this step, data pre-processing includes some operations like noise removal, resizing, augmentation, and so on. The data partitioning step splits the data into training, validation, and testing set for the experiment. Generally, cross-validation technique is utilized for data partitioning. The training data is used to develop a particular model that is evaluated by validation data, and the performance of the developed model is appraised by test data. The major step of deep learning based COVID-19 diagnosis is the feature extraction and classification. In this stage, the deep learning technique automatically extracts the feature performing several operations repeatedly, and finally, the classification is done based on class labels (healthy or COVID-19). Lastly, the developed system is assessed by some evaluation metrics like accuracy, sensitivity, specificity, precision, F1-score, and so on.
FIGURE 1.
A general pipeline of deep learning based COVID-19 diagnosis system.
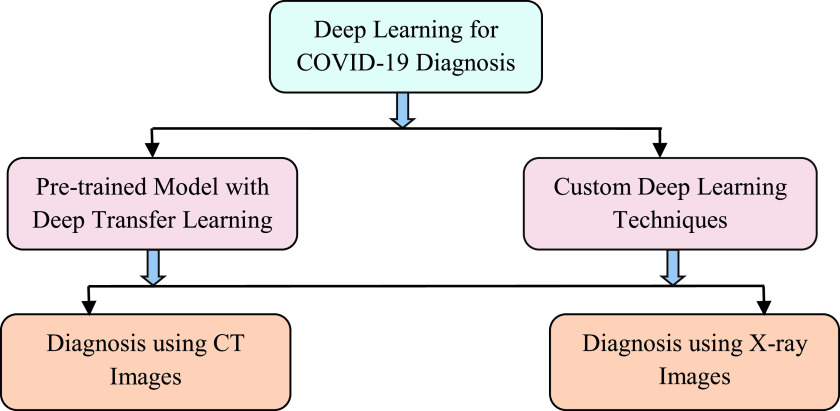
In this paper, a taxonomy of classifying COVID-19 diagnosis system is presented to facilitate the navigation of the landscape. Two different perspectives are applied which are related to the used deep learning techniques and the used imaging modalities (see Fig. 2). The deep learning methods are classified into two groups: pre-trained model with deep transfer learning and custom deep learning techniques. Additionally, each diagnostic approach based on deep learning architectures is divided into two categories: CT images and X-ray images. In this paper, we have reviewed a total of 45 COVID-19 diagnosis systems. Among them, 25 systems (55.55% of the total reviewed systems) used pre-trained models for diagnosis purposes and 20 (44.45% of the total reviewed systems) used custom deep learning techniques for COVID-19 diagnosis. From a different perspective, 25 reviewed systems used X-ray images (55.55% of the total reviewed systems) as data source, and the remaining 20 systems utilized CT scans (44.45% of the total reviewed systems).
FIGURE 2.
Taxonomy of the recent developed COVID-19 diagnosis systems using deep learning.
III. Pre-Trained Model With Deep Transfer Learning
A pre-trained model is one that has already been trained in fields similar to the context of the application. In transfer learning, weight and bias are transferred from a large trained model to a similar new model for testing or retraining. There are several advantages of using pre-trained model with deep transfer learning. In general, training a model from scratch for large datasets requires high computing power and is time-consuming [45], [46]. The pre-trained model with transfer learning enables the facility to speed up the convergence with network generalization [47], [48]. Numerous pre-trained models that are utilized in transfer learning are designed for the large convolutional neural network (CNN). There are several pre-trained models which are used for COVID-19 diagnosis such as AlexNet [49], GoogleNet [50], SqueezeNet [51], different versions of Visual Geometry Group (VGG) [52], diverse kinds of ResNet [53], Xception [54], different forms of inception [55], diverse types of MobileNet [56], DenseNet [57], U-Net [58], etc. Transfer learning has been efficiently used to detect 0 from CT and X-ray images. 3D CT images are processed differently than color X-Ray images. 3D CT images consist of a fixed number (16, 32, 64, 128, etc.) of slices based on the device and the settings. The individual slices are can be greyscale or color image in nature. In most cases, the slices are at first extracted and then treated as separate images [59], [60]. The slices with the most lung regions are selected while the others are discarded. In [61], the middle 50% slices from 3D CT scans are selected. The individual slices or features extracted from these slices are directly used for optimizing the pre-trained models. In other cases, 3D segmentation models such as U-Net models are used to segment and extract features of multiple Region of Interest (ROI) from 3D CT images [62]. These numeric features are then used to optimize the pre-trained models. The systems developed for COVID-19 diagnosis are described next.
A. Diagnosis Using Computer Tomography (CT) Images
1). Diagnosis Based on Multiple Source Data
Wu et al.
[59] introduced a deep learning based coronavirus screening framework using the concept of multi-view fusion. The system used a variant of CNN called ResNet50. The dataset is collected from two hospitals in China. A total of 495 images are taken into account for the experiment in which 368 are associated with confirmed COVID-19 cases, and 127 are of other pneumonia. In this scheme, the dataset is divided into a proportion of 80%, 10%, and 10% for training, testing, and validation respectively. Each of the images, considered in the system is resized into
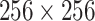 before the network development. In the test case, the developed system obtained accuracy of 76%, sensitivity of 81.1%, specificity of 61.5%, and Area under Curve (AUC) of 81.9%. The results are analyzed both for single-view and multi-view fusion model but the multi-view fusion model demonstrates superior performance. In another research work, Li et al.
[60] demonstrated an automatic system (COVNet) for the diagnosis of coronavirus from CT images using a deep learning technique which is a variant of CNN named ResNet50. The used dataset consists of 4536 chest CT samples, including 1296 samples for COVID-19, 1735 for community-acquired pneumonia (CAP), and 1325 for non-pneumonia. The dataset is partitioned into training and testing set in a proportion of 90% and 10% respectively. The experimental result showed that the system obtained sensitivity of 90%, specificity of 96%, and AUC of 96% for COVID-19 cases.
before the network development. In the test case, the developed system obtained accuracy of 76%, sensitivity of 81.1%, specificity of 61.5%, and Area under Curve (AUC) of 81.9%. The results are analyzed both for single-view and multi-view fusion model but the multi-view fusion model demonstrates superior performance. In another research work, Li et al.
[60] demonstrated an automatic system (COVNet) for the diagnosis of coronavirus from CT images using a deep learning technique which is a variant of CNN named ResNet50. The used dataset consists of 4536 chest CT samples, including 1296 samples for COVID-19, 1735 for community-acquired pneumonia (CAP), and 1325 for non-pneumonia. The dataset is partitioned into training and testing set in a proportion of 90% and 10% respectively. The experimental result showed that the system obtained sensitivity of 90%, specificity of 96%, and AUC of 96% for COVID-19 cases.
Afterward, for the proper diagnosis of COVID-19, Yousefzadeh et al. [61] introduced a deep learning framework called ai-corona which is worked based on CT images. The system is comprised of several variants of CNN named DenseNet, ResNet, Xception, and EfficientNetB0. The used dataset contained 2124 CT slices in overall where 1418 images are of non-COVID-19, and 706 slices are of COVID-19 infected cases. The dataset maintained a ratio of 80% and 20% for training and validation set respectively. The proposed system found accuracy of 96.4%, sensitivity of 92.4%, specificity of 98.3%, F1-score of 95.3%, and AUC of 98.9% from the experiment. Jin et al. [62] developed an artificial intelligence based coronavirus diagnosis system using a variant of CNN named ResNet152. The pre-trained model used 152 convolutional, subsampling, and fully-connected layers. The dataset is collected from three renowned Chinese hospitals and two publicly available databases. A total number of 1881 cases are considered where 496 cases are for COVID-19 infected patients, and 1385 are negative cases. The dataset is split randomly for experiments. The system achieved an accuracy of 94.98%, sensitivity of 94.06%, specificity of 95.47%, precision of 91.53%, F1-score of 92.78, and AUC of 97.91% from the experiment.
In another research, Xu et al. [63] developed a system for classifying healthy individuals from COVID-19 pneumonia and Influenza-A viral pneumonia utilizing CNN variants. The used pre-trained model in this system is Resnet18. The data is collected from three different hospitals in China. This study considers 618 CT images in which 219 images are obtained from patients infected with COVID-19, 224 from Influenza-A viral pneumonia, and 175 from normal individuals. To train the model, a total of 85.4% (528) images are used, and the remaining samples are used to test the developed model. The framework achieved 86.7% accuracy, 81.5% sensitivity, 80.8% precision, and 81.1% F1-score from the experiment. Furthermore, Jin et al. [64] introduced a medical system for COVID-19 screening using deep learning techniques. Their system used various pre-trained models of CNN like DPN-92, Inception-v3, ResNet-50, and Attention ResNet-50 with 3D U-Net++. The dataset is retrieved from different five hospitals in China. In this system, a total of 139 samples are used where 850 sample from COVID-19, and 541 samples from other cases which are considered as negative. The total data is randomly split into training and testing sets for performance evaluation. As the evaluation metrics, the system obtained sensitivity, specificity, and AUC of 97.4%, 92.2%, and 99.1% respectively using 3D Unet++-ResNet-50 which is considered as the best model. Moreover, Javaheri et al. [65] developed a deep learning approach called CovidCTNet for detecting coronavirus infection via CT images. The system used BCDU-Net architecture which is developed based on U-Net. The scheme distinguished COVID-19 from CAP as well as other lung disorders. For the experiment, the system used 89,145 CT images in total where 32,230 CT slices are confirmed with COVID-19, 25,699 CT slices are confirmed with CAP, and 31,216 CT slices are with healthy lungs or other disorder. The dataset is partitioned using hold-out method i.e. 90% is used for training and 10% is utilized for testing. It is obvious from the experimental results that the developed system obtained accuracy, sensitivity, specificity, AUC of 91.66%, 87.5%, 94%, and 95% respectively.
2). Diagnosis Based on Single Source Data
Ardakani et al. [66] proposed a system for the detection of COVID-19 using ten variants of CNN techniques in CT images. The used popular variants for diagnosis are AlexNet, VGG-16, VGG-19, SqueezeNet, GoogleNet, MobileNet-V2, ResNet-18, ResNet-50, ResNet-101, and Xception. In the proposed system, a total of 1020 CT samples are considered from the cases of COVID-19 and non-COVID-19. The dataset is split into training and validation set in a proportion of 80% and 20% respectively. Among the 10 networks, ResNet-101 and Xception performed comparatively better than the others. It is evident from the experimental results that the ResNet-101 model obtained accuracy of 99.51%, sensitivity of 100%, AUC of 99.4%, and specificity of 99.02%. In another network, Xception found the accuracy, sensitivity, AUC, and specificity of 99.02%, 98.04%, 87.3%, and 100% respectively. In another study, Chen et al. [71] introduced a deep learning based scheme in which a powerful pre-trained model named UNet++ was applied to high-resolution CT images for COVID-19 detection. Initially, UNet++ extracted valid regions in CT images. In this study, 46,096 images are collected from a hospital including 51 COVID-19 infected patients and 55 infected with other diseases. Among the dataset, 35,355 images are selected while eliminating low images using filtering and partitioned into training and testing set respectively. The sensitivity of 94.34%, specificity of 99.16%, accuracy of 98.85%, precision of 88.37%, and negative predictive value (NPV) of 99.61% are achieved. Further, Cifci [72] presented a scheme for the early diagnosis of coronavirus using various pre-trained models with deep transfer learning.The pre-trained models are AlexNet and Inception-V4 which are popular for medical image analysis. The study is carried out through CT images. To develop the system, 5800 CT images are retrieved from a public repository. As a training step, 4640 (80%) CT samples are used, while 1160 (20%) samples are used for testing. AlexNet performed comparatively better than Inception-V4 which is found through experimental results. AlexNet got an overall accuracy of 94.74% with sensitivity, and specificity of 87.37%, and 87.45% respectively.
Table 1 summarizes the aforementioned deep learning based COVID-19 diagnosis systems from CT samples using pre-trained model with deep transfer learning and describes some of the significant factors, such as data sources, number of images and classes, data partitioning technique, the used techniques for diagnosis, and the performance measures of the developed systems.
TABLE 1. Summary of Deep Learning Based COVID-19 Diagnosis in CT Images Using Pre-Trained Model With Deep Transfer Learning.
| Authors | Data Sources | Number of images | Number of classes | Partitioning | Techniques | Performances (%) |
|---|---|---|---|---|---|---|
| Wu et al. [59] | Two hospitals (China Medical University, Beijing Youan Hospital) | 495 (COVID-19=368, other pneumoniae 27) | 2 (COVID-19, other pneumonia) | Training=80%, Validation=10 %, Testing=10% | ResNet50 | Accuracy=76, Sensitivity=81.1, Specificity=61.5, AUC=81.9 |
| Li et al. [60] | Multiple hospitals environment | 4536 (COVID-19=1296, CAP=1735, non-pneumonia=1325) | 3 (COVID-19, CAP, non-pneumonia) | Training=90%, Testing=10% | ResNet50 | Sensitivity= 90, Specificity=96, AUC=96 |
| Yousefeadeh et al. [61] | Real-time data from hospital environment | 2124 (COVID-19=706, non-COVID-19=1418) | 2 (COVID-19, non-COVID-19) | Training=80%, Validation=20 % | DenseNet, ResNet, Xception, EfficientNetBO | Accuracy=96.4, Sensitivity= 92.4, Specificity=98.3, Fl-Score= 95.3, AUC=98.9, Kappa=91.7 |
| Jin et al. [62] | Three different hospitals (Wuhan Union Hospital, Western Campus of Wuhan Union Hospital, Jianghan Mobile Cabin Hospital), LIDC-IDRI [67], ILD-HUG [68] | 1881 (COVID-19 positive=496, COVID-19 negative=1385) | 2 (COVID-19 positive, COVID-19 negative) | Random partition | ResNetl52 | Accuracy=94.98, Sensitivity=94.06, Specificity=95.47, Precision= 91.53, Fl-Score= 92.78, AUC=97.91, NPV=96.86, Youden Index=89.53 |
| Xu et al. [63] | Zhejiang University, Hospital of Wenzhou, Hospital of Wenling | 618 (COVID-19=219, Influenza-A-viral-pneumonia=224, irrelevant-to-infection=175) | 3 (COVID-19, Influenza-A-viral-pneumonia, irrelevant-to-infection) | Training + Validaticm=85. 4%, Testing=14.6% | ResNetl8 | Accuracy=86.7, Sensitivity=81.5, Precision=80.8, Fl-Score=81.1 |
| Jin et al. [64] | Five different hospitals of China | 1391 (COVID-19 positive=850, COVID-19 negative=541) | 2 (COVID-19 positive, COVID-19 negative) | Random partition | DPN-92, Inception-v3, ResNet-50, Attention ResNet-50 with 3D U-Net++ | Sensitivity= 97.04, Specificity=92.2, AUC=99.1 |
| Javaheri et al. [65] | Five medical centers in Iran, SPIE-AAPM-NCI [69], LUNGx [70] | 89,145 (COVID-19=32,230, CAP=25,699, other diseases=31,216) | 3 (COVID-19, CAP, other diseases) | Training=90%, Validation=10 % | BCDU-Net (U-Net) | Accuracy=91.66, Sensitivity=87.5, Specificity=94, AUC=95 |
| Ardakani et al. [66] | Real-time data from hospital environment | 1020 (COVID-19=510, non-COVID-19=510) | 2 (COVID-19, non-COVID-19) | Training=80%, Validation=20 % | AlexNet, VGG-16, VGG-19, SqueezeNet, GoogleNet, MobileNet-V2, ResNet-18, ResNet-50, ResNet-101, Xception | Accuracy=99.51, Sensitivity=100, Specificity=99.02, Precision=99.27, AUC=99.4, NPV=100 |
| Chen et al. [71] | Renmin Hospital of Wuhan University | 35,355 | 2 (COVID-19, other diseases) | Random partition | UNet++ | Accuracy=98.85, Sensitivity=94.34, Specificity=99.16, Precision=88.37, AUC=99.4, NPV=99.61 |
| Cifci [72] | Different website's data from Kaggle | 5800 | 2 (COVID-19, other pneumonia) | Training=80%, Testing=20% | AlexNet, Inception-V4 | Accuracy=94.74, Sensitivity=87.37, Specificity=87.45 |
B. Diagnosis Using X-Ray Images
1). Diagnosis Based on Multiple Source Data
Apostolopoulos and Bessiana [73] developed a system for the automatic diagnosis of COVID-19 cases utilizing the concept of transfer learning with five variants of CNNs. The pre-trained models which are used in the system are VGG19, MobileNetv2, Inception, Xception, and Inception-ResNetv2. The system considered 1427 images including 224 for COVID-19, 700 for common pneumonia, and 504 for healthy cases in the first scenario. In the second scenario, 224 COVID-19 images, 714 bacterial and viral pneumonia images, and 504 healthy individual images are considered. The dataset was divided using the 10-fold cross-validation method. It was revealed that the highest accuracy of 96.78%, sensitivity of 98.66%, and specificity of 96.46% are obtained for the second dataset using MobileNetv2. In another research work, Loey et al. [74] introduced a novel system for the diagnosis of coronavirus using Generative Adversarial Network (GAN) and pre-trained models of CNN with deep transfer learning. The pre-trained models which are used in the proposed system are Alexnet, Googlenet, and Resnet18. As the number of X-ray images for COVID-19 is small, GAN is used to generate more samples for accurate detection of this virus. A total number of 307 images are considered including four classes like COVID-19, normal, pneumonia_bac, and pneumonia_vir. The system experimented on three different scenarios of the dataset depending on the consideration of class level. Considering four classes, Googlenet obtained the highest accuracy of 80.6%. Alexnet and Googlenet achieved accuracy of 85.2% and 100% respectively considering three and two classes.
Horry et al. [75] described a COVID-19 detection framework using the concept of pre-trained model in X-ray images. The proposed system used four popular pre-trained models like VGG, Inception, Xception, and Resnet with transfer learning. The used dataset consisted of 100 COVID-19 cases, 100 pneumonia, and 200 healthy cases for experiments. In this system, a ratio of 80:20 is preserved for training and testing set as a data partition. The experimental findings reveal that the system obtained precision, sensitivity, and F1-score of 83%, 80%, and 80% respectively using VGG19 which is measured as the highest performance in the study considering three-class data. Further, Ozcan [76] proposed a deep learning scheme with a combination of the grid search strategy and three pre-trained models of CNN (GoogleNet, ResNet18, and ResNet50). The grid search technique is used to select the best hyperparameter and the pre-trained models are utilized for feature extraction and classification. The system used three public datasets where the images are of 242 bacteria cases, 131 COVID-19 cases, 200 normal cases, and 148 viral cases. All the data are partitioned into training, testing, and validation set in a proportion of 50:30:20. The ResNet50 with grid search performed better and obtained accuracy of 97.69%, sensitivity of 97.26%, specificity of 97.90%, precision of 95.95%, and F1-score of 96.60%.
Sethy and Behra [77] introduced a system for the diagnosis of COVID-19 cases using pre-trained models of CNN and Support Vector Machine (SVM). The algorithm used eleven CNN pre-trained models for automatic extraction of features, and SVM for classification. In this system, two separate datasets were used where the first dataset included 25 positive COVID-19 and 25 negative X-ray images of COVID-19. A total of 133 images containing Middle East Respiratory Syndrome (MERS), SARS, and Acute Respiratory Distress Syndrome (ARDS) are used as positive samples and 133 normal X-ray images as negative samples in the second dataset. From the experimental results, it is found that Resnet50 with SVM obtained accuracy, False Positive Rate (FPR), Matthews Correlation Coefficient (MCC), and Kappa of 95.38%, 95.52%, 91.41%, and 90.76% respectively which the best is in the developed system for the first scenario of the dataset. Minaee et al. [78] proposed a framework named Deep-COVID using the concept of deep transfer learning for COVID-19 prediction in X-ray images. Four popular pre-trained models like ResNet18, ResNet50, SqueezeNet, and DenseNet-121 were considered in this study for COVID-19 diagnosis. In total, 5071 images are collected from different open-access resources. Among them, 2000 images with 31 COVID-19 cases were used for training, and 3000 images with 40 COVID-19 infected cases were used for testing in the experiments. The resulting dataset was named COVID-Xray-5k. The best performance obtained by the system is sensitivity of 100%, and specificity of 95.6% using SqueezeNet.
In another study, Punn and Agarwal [79] developed an automated COVID-19 diagnosis system using several pre-trained models like ResNet, Inception-v3, Inception ResNet-v2, DenseNet169, and NASNetLarge with a small number of X-ray images. The system used random oversampling and a weighted class loss function for fine-tuning known as transfer learning. In this system, a total of 1076 chest X-ray images are considered for experiments. The dataset is partitioned into 80%, 10%, and 10% ratios for training, testing, and validation set respectively. From the experimental results, it was shown that NASNetLarge performed comparatively better and achieved accuracy, precision, sensitivity, AUC, specificity, and F1-score of 98%, 88%, 91%, 99%, 98%, and 89% respectively. Afterward, Narin et al. [80] introduced a method for automatically classifying COVID-19 infected patients from X-ray images using the variants of CNN. The pre-trained models used are ResNet50, InceptionV3, and Inception-ResNetV2 which obtained higher predictive accuracy on a subset X-ray dataset. The system used a total of 100 X-ray images where 50 images were from COVID-19 patients while the remaining 50 from healthy individuals. The 5-fold cross-validation was used to partition the dataset for the experiment. The system achieved an accuracy of 98%, 97%, and 87% from ResNet50, InceptionV3, and Inception-ResNetV2 respectively in test cases. In terms of other evaluation metrics, the best performance was obtained using RecNet50 with a recall of 96%, specificity of 100%, precision of 100%, and F1-score of 98%.
Bukharia et al. [81] presented a COVID-19 diagnosis system using a variant of CNN named Resnet50. The system considered 278 X-ray images of three classes where 89 samples of COVID-19 infected, 93 samples of healthy participants, and 96 samples of pneumonia patients. The collected dataset was split into two sets like training and testing in a proportion of 80% (223 images), and 20% (55 images). The diagnosis process obtained accuracy, precision, recall, and F1-score of 98.18 %, 98.14%, 98.24%, and 98.19 % respectively from the experiment. Moreover, Abbas et al. [82] categorized COVID-19 infected patients, from healthy individuals using Decompose, Transfer, and Compose (DeTraC) deep ResNet18. The proposed DeTraC can fix any anomalies in the image dataset through the use of a class decomposition method to investigate class boundaries. In this system, a total of 196 images were utilized where 80 samples of normal patients, 105 samples of COVID-19, and 11 samples of SARS. The system generated 1764 samples from given samples using decomposition. The dataset was split into two groups, 70% for system training and 30% for evaluation. The proposed system achieved accuracy of 95.12%, sensitivity of 97.91%, specificity of 91.87%, and precision of 93.36% using DeTraC-ResNet18 framework.
In another research project, Moutounet-Cartan [83] developed a deep learning based system to diagnose the novel coronavirus as well as other pneumonia diseases from X-ray images. The system used the following variants of CNN architecture named VGG16, VGG19, InceptionResNetV2, InceptionV3, and Xception for diagnosis. In this study, in total 327 X-ray images were taken where 152 cases were from healthy people, 125 from COVID-19 cases, and the remaining 50 cases from other pneumonia diseases. The dataset is partitioned using the principle of 5-fold cross-validation. The system found VGG16 as the best performing model and obtained overall accuracy of 84.1%, sensitivity 87.7%, and AUC of 97.4% where the sensitivity and AUC were considered only for COVID-19 cases. Furthermore, Maguolo and Nanni [84] evaluated the performance of COVID-19 detection system from X-ray samples utilizing a popular pertained model named AlexNet. The system used four different publicly available datasets to evaluate the performance. A total of 339,271 images were taken where 144 images for COVID-19 patients, 108,948 samples of pneumonia and bacteria except COVID-19, 224,316 chest radiographs of bacteria and pneumonia, and 5,863 paediatric images viral and bacterial pneumonia. The dataset was partitioned into 10-fold cross-validation for training and testing. Using the concept of deep transfer learning, the system obtained the highest AUC of 99.97% in the study.
Ozturk et al. [85] presented a customized network (DarkCovidNet) for the automatic diagnosis of COVID-19 in raw chest X-ray samples utilizing deep neural networks. The proposed system used DarkNet as a classifier with 17 convolutional layers. In this system, two sources of the dataset were used which includes 127 images from the first source, and 500 normal and 500 pneumonia cases from frontal X-ray samples from the second source. The dataset was partitioned in 5-fold cross-validation technique. The obtained sensitivity, specificity, precision, F1-score, and accuracy of 95.13%, 95.3%, 98.03%, 96.51%, and 98.08% respectively for binary-class which are the highest in this study. Moreover, Luz et al. [86] presented an efficient deep learning scheme named EfficientNet for the detection of coronavirus pattern from X-ray radiographs. The main advantage of EfficientNet is that it used fewer parameters, approximately 30 times fewer parameters than the other pre-trained models. The system considered 30,663 images for the experiment where 183 cases were considered as COVID-19, 16,132 images as normal cases, and 14,348 images as other pneumonia cases. The system obtained overall accuracy of 93.9%, sensitivity of 96.8%, and precision of 100%.
2). Diagnosis Based on Single Source Data
Very recently, Hemdan et al. [87] proposed a system named COVIDX-Net to diagnose coronavirus using the variants of CNN in X-ray images. A total of seven pre-trained models are considered in this study. The dataset consisted of 50 images where 25 images are from healthy people and the remaining 25 samples from COVID-19 cases. For the experiment, the dataset was split into a proportion of 80% and 20% for the training and testing set respectively. The experimental results revealed that VGG19 and DenseNet outperformed the other pre-trained models with an accuracy of 90% and F1-score of 91%. InceptionV3 obtained the worst results.
Table 2 summarizes the aforementioned deep learning based COVID-19 diagnosis systems from X-ray samples using pre-trained model with deep transfer learning and describes some of the significant factors, such as data sources, number of images and classes, data partitioning technique, the used techniques for diagnosis, and the performance measures of the reported systems.
TABLE 2. Summary of Deep Learning Based COVID-19 Diagnosis in X-Ray Images Using Pre-Trained Model With Deep Transfer Learning.
| Authors | Data Sources | Number of images | Number of classes | Partitioning | Techniques | Performances (%) |
|---|---|---|---|---|---|---|
| Apostolopoulos and Bessiana [73] | COVID-19 X-ray image database [88], Kaggle dataset [89], Kermany et al. [90] | 1442 (COVID-19=224, pneumonia=714, normal=504) | 3 (COVID-19, pneumonia, normal) | 10- fold cross-validation | VGG19, MobileNetv2, Inception, Xception, Inception- ResNetv2 | Accuracy=96.78, Sensitivity=98.66, Specificity=96.46 |
| Loey et al. [74] | COVID-19 X-ray image database [88], Kermany et al. [90], Dataset [91] | 307 (COVID=69, normal=79, pneumonia bac= 79, pneumonia vii=7 9) | 4 (COVID, normal, pneumoniaba, pneumonia_vir) | Training=80%, Testing=10%, Validation= 10% | GAN, Alexnet, Googlenet, Resnetl8 | Accuracy=100, Sensitivity= 100, Precision= 100, Fl-Score= 100 |
| Horry etal. [75] | COVID-19 X-ray image database [88], NIH Chest X-Ray [92] | 400 (COVID-19=100, pneumonia=100, normal=200) | 3 (COVID-19, pneumonia, normal) | Training=80%, Testing=20% | VGG16, VGG19, ResNet50, InceptionV3, Xception | Sensitivity=80, Precision=83, Fl-Score=80 |
| Ozcan [76] | COVID-19 X-ray image database [88], Kaggle chest x-ray repository [93] | 721 (COVID- 19=131, bacteria=242, normal=200, virus=148) | 4 (COVID-19, normal, bacteria, virus) | Training=50%, Tcsting=30% Validation= 20% | GoogleNet, ResNetl8, ResNet50 | Accuracy=97.69, Sensitivity= 97.26, Specificity = 97.90, Precision= 95.95, Fl-Score= 96.60 |
| Sethy and Behra. [77] | COVID-19 X-ray image database [88], NIH Chest X-Ray [92], Kaggle chest x-ray repository [93] | 316 | 2(COVID-19+, COVID-19) | Training=60%, Testing=20% Validation= 20% | AlexNet, VGG16, VGG19, GoogleNet, ResNetl8, ResNet50, ResNetlOl, InceptionV3, InceptionResNet V2, DenseNet201, XceptionNet, SVM | Accuracy=95.38, Sensitivity= 97.47, Specificity= 93.47, Precision= 95.95, Fl-Score= 95.52, MCC=91.41, FPR=95.52, Kappa=90.76 |
| Minaee et al. [78] | COVID-19 X-ray image database [88], ChexPert [94] | 5071 (COVID-19=71, non-COVID=5000) | 2 (COVID-19, non-COVID) | Training=40%, Testing=60% | ResNetl8, ResNet50, SqueezeNet, DenseNet-121 | Sensitivity= 100, Specificity= 95.6, AUC=99.6 |
| Punnand Agarwal [79] | COVID-19 X-ray image database [88], RSNA Pneumonia Detection Challenge dataset [95] | 1076 (COVID-19=108, pneumonia=515, normal=453) | 3 (COVID-19, pneumonia, normal) | Training=80%, Testings 10%, Validation= 10% | ResNet, Inception-v3, Inception, ResNet-v2, DenseNetl69, NASNetL | Accuracy=98, Sensitivity= 91, Specificity= 91, Precision= 98, Fl-Score= 89, AUC=99 |
| Narin et al. [80] | COVID-19 X-ray image database [88], Kaggle chest x-ray repository [93] | 100 (COVID- 19=50, normal=50) | 2 (COVID-19, normal) | 5- fold cross-validation | ResNet50, InceptionV3, Inception-ResNetV2 | Accuracy=98, Sensitivity= 96, Specificity = 100, Precision= 100, Fl-Score= 98, AUC=100 |
| Bukharia et al. [81] | COVID-19 X-ray image database [88], NIH Chest X-Ray [92] | 278 (COVID-19=89, normal=93, pneumonia=96,) | 3 (COVID-19, normal, pneumonia,) | Training=80%, Testing=20% | ResNet50 | Accuracy=98.18, Sensitivity= 98.24, Precision= 98.14, Fl-Score= 98.19 |
| Abbas et al. [82] | COVID-19 X-ray image database [88], Japanese Society of Radiological Technology (JSRT) [96], [97] | 196 (COVID-19=105, normal=80, SARS=11) | 3 (COVID-19, normal, SARS) | Training=70%, Testing=30% | DeTraC-ResNetl8 | Accuracy=95.12, Sensitivity= 97.91, Specificity = 91.87, Precision= 93.36 |
| Moutounet-Cartan [83] | COVID-19 X-ray image database [88], Kermany et al. [90] | 327 (COVID-19=125, normal=152, pneumonia=50) | 3 (COVID-19, normal, pneumonia) | 5-fold cross-validation | VGG16, VGG19, InceptionResNet V2, InceptionV3, Xception | Accuracy= 84.1, Sensitivity=87.7, AUC=97.4 |
| Maguolo and Nanni [84] | COVID-19 X-ray image database [88], Kaggle chest x-ray repository [93], ChexPert [94], ChestX-ray8 [98] | 339,271 (COVH)-19=144, pneumonia=339, 127) | 2 (COVID-19, pneumonia) | 10-fold cross-validation | AlexNet | AUC=99.97 |
| Ozturk et al. [85] | COVID-19 X-ray image database [88], ChestX-ray8 [98] | 1127 (COVID=127, no-finding=500, pneumonia=500) | 3 (COVID, no- finding, pneumonia) | 5- fold cross-validation | DarkNet | Accuracy=98.08, Sensitivity=95.13, Specificity=95.3, Precision=98.03, Fl-Score=96.51 |
| Luz et al. [86] | COVID-19 X-ray image database [88], RSNA Pneumonia Detection Challenge dataset [95], COVIDx Dataset [99] | 30,663 (COVID-19=183, non-COVID pneumonia=14,3 48, healthy=16,132) | 3 (COVID-19, non-COVID pneumonia, healthy) | Training=90%, Testing=10% | EfHcientNet | Accuracy=93.9, Sensitivity=96.8, Precision=100 |
| Hemdan et al. [87] | COVID-19 X-ray image database [88] | 50 (COVID-19 =25, normal = 25) | 2 (COVID-19, normal) | Training=80%, Testing=20% | VGG19, DenseNetl21, InceptionV3, ResNetV2, InceptionResNet, Xception, MobileNetV2 | Accuracy=90, Sensitivity= 100, Specificity= 100, Precision= 100, Fl-Score= 91, AUC=90 |
IV. Custom Deep Learning Techniques
Custom deep learning techniques facilitate the development of user-friendly architecture with more consistent and accurate performance due to the attention to the specific application of interest. The custom networks are evolved with the use of a particular deep learning method [100] or the hybridization of deep learning algorithms [101], [102] or the hybridization of deep learning with other fields of AI such as machine learning, data mining, nature-inspired algorithms, etc. [103], [104]. No previous weights and bias are used in the customized network like pre-trained model hence it requires comparatively high computation power and time. The systems developed for COVID-19 diagnosis are outlined as follows.
A. Diagnosis Using Computer Tomography (CT) Images
1). Diagnosis Based on Multiple Source Data
Elghamrawy and Hassanien [105] proposed a scheme for the diagnosis and prediction of coronavirus infected patients using a combination of CNN and Whale Optimization Algorithm (WOA) from CT samples. The developed system had two key features, one focused on CNNs for the segmentation, and another one (WOA) proposed to predict the ability of the patient responding to treatment on the basis of different variables. The used dataset is collected from publicly available databases consisted of 617 CT scans. Among them, 134 images are excluded as it contains non-lung region. A total of 432 images confirmed of COVID-19 and 151 cases of other viral pneumonia were considered. To achieve better performance, the dataset was divided into a proportion of 65%, and 35% for training and testing respectively. The proposed system obtained overall accuracy, sensitivity, and precision of 96.40%, 97.25%, and 97.3% respectively for diagnosis. Further, He et al. [106] proposed a deep learning method named CRNet for the detection of COVID-19 using CT images. The system introduced a new transfer learning modality combined with contrastive self-supervised learning known as self-trans that learns effective and unbiased feature representations to minimize overfitting problems. In this system, a total of 746 CT images were analyzed where 349 were associated with COVID-19 cases, and 397 with non-COVID-19 cases. The dataset was formed by merging three publicly available datasets which was divided into three sets named training, testing, and validation set in a proportion of 60%, 25%, and 15% respectively. The proposed system obtained accuracy of 86%, F1-score of 85%, and AUC of 94% from the experimental results. In comparison with other prominent pre-trained models, the proposed system used comparatively fewer tuning parameters.
Afterwards, Wang et al. [107] introduced a scheme for COVID-19 diagnosis using a modified CNN technique named modified-Inception. The basic difference between Inception and modified-Inception is that modified-Inception reduces the dimension of attributes before final classification. In the proposed system, ROI is created on the given samples, the ROI images are converted into one-dimensional feature vectors using pre-trained inception architecture, and finally, classification is done in the fully connected layer. In the experiment, the scheme used 1040 CT images, in which 740 were tagged as COVID-19 positive and 325 as COVID-19 negative. The dataset was partitioned into training, testing, and validation set randomly. The experimental outcomes revealed that the scheme achieved accuracy, sensitivity, specificity, precision, and F1-score of 79.3%, 83%, 67%, 55%, and 63% respectively on the testing samples. Moreover, Liu et al. [108] developed an automatic COVID-19 diagnosis system using deep learning method via CT images. The system used modified DenseNet-264 (COVIDNet) for diagnosis where the model consisted of 4 dense blocks. Each block contained several number of units having two sequentially linked stacks with an instance normalization layer, a convolution layer, and ReLU activation layer in each unit where the feature maps are received from all previous units through dense connections. In this system, 920 COVID-19 and 1,073 non-COVID-19 cases were considered for the experiment. To obtain better performance, the dataset is partitioned into three sets namely training, testing, and validation in a proportion of 60%, 20%, and 20% respectively. The developed system obtained accuracy of 94.3%, AUC of 98.6%, sensitivity of 93.1%, specificity of 95.1%, precision of 93.9%, NPV of 94.5%, and F1-score of 93.5%.
In another study, Ying et al. [109] introduced a deep learning technique based on the Details Relation Extraction neural network (DRE-Net) named DeepPneumonia for the diagnosis of COVID-19 cases utilizing CT images. The proposed system is developed with the combination of ResNet50 and Feature Pyramid Network (enables to extract the best features from each sample). The dataset was collected from two popular hospitals in China. In this system, a total of 1990 image slices were taken where 777 images for COVID-19, 505 slices for bacterial pneumonia, and 708 samples from normal people. The dataset was split in a proportion of 60%, 30%, and 10% for training, testing, and validation set respectively. The proposed system obtained accuracy of 94%, sensitivity of 93%, precision of 96%, F1-score of 94%, and AUC of 99%. To detect COVID-19, Zheng et al. [110] proposed a 3D deep convolution neural network (DeCoVNet) from CT scans. In this system, UNet architecture generated 3D lung masks from the given samples, and the segmented regions are fed into the proposed architecture to predict infected regions. The proposed network is comprised of three segments like a vanilla 3D convolution, a batch norm layer, and a subsampling layer. The data for the study was collected from the hospital environment. A total of 630 CT samples were used for the experiment where 80% (499 images) were in the training set, and the rest 20% (131images) were used in testing. From the experimental outcome, accuracy, sensitivity, specificity, precision, NPV, AUC of 90.1%, 90.7%, 91.1%, 84%, 98.2%, and 95.9% are achieved.
Hasan et al. [111] proposed a hybrid system using the concept of Q-deformed entropy and deep learning features (QDE–DF) to differentiate COVID-19 infected people from pneumonia cases, and healthy people utilizing CT images. For deep features extraction, CNN and Q-deformed entropy were used, and LSTM was used to classify the cases from deep features. The key contribution of this system is to develop a novel architecture of CNN with a lesser number of parameters to reduce the over-fitting and the use of Q-deformed entropy to determine small alterations of the intensity of images. A total of 321 chest CT samples were used for this study, consisting of 118 CT samples of COVID-19 cases, 96 CT samples of pneumonia cases, and 107 CT samples of healthy individuals. Approximately, 16 attributes were extracted from each image using a feature extraction technique. To assess the developed system, the dataset was partitioned in a proportion of 70%, and 30% for training and testing set respectively. The system obtained accuracy of 99.68% which is considered as the highest in this study. Further, Amyar et al. [112] developed a scheme using deep learning method to diagnose COVID-19 patients from CT samples. The system consists of an encoder for reconstruction and two decoders for segmentation, and for classification purposes, a multi-layer perceptron is used. A common encoder is used for the three tasks where the CT samples are fed as inputs, two decoders are utilized for reconstruction and segmentation respectively, and the images are categorized based on the presence of COVID cases in multi-layer perceptron. The dataset used included 1044 cases where 449 cases were of confirmed COVID-19, 100 cases from healthy individuals, 98 samples were from confirmed lung cancer patients, and 397 from various other kinds of pathology. Collectively, 449 were associated with COVID-19 and 595 were not. The dataset was partitioned into training, validation, and testing set in a ratio of 80%, 10%, and 10% respectively. The proposed system received accuracy of 86%, sensitivity of 94%, specificity of 79%, AUC of 93%.
2). Diagnosis Based on Single Source Data
Singh et al. [120] classified COVID-19 infected (positive) cases from other (negative) cases using deep learning technique CNN. In this system, CNN’s initial parameters were tuned with the application of multi-objective differential evolution (MODE) and finally, the classification was done through CNN, Artificial Neural Network (ANN), and Artificial Neural Network Fuzzy Inference System (ANNFIS) where CNN obtained promising results. A total of 150 CT samples were taken where 75 samples for COVID-19 positive and 75 images for COVID-19 negative. Different variations in training and testing dataset ratio of 20:80 %, 30:70%, 40:60%, 50:50%, 60:40%, 70:30%, 80:20%, and 90:10%, respectively are taken to conduct the experiment. The best performed ratio for the proposed system is 90%, and 10% for training and testing set individually in the maximum cases. The system obtained accuracy of 93.25%, sensitivity of 90.70%, specificity of 90.72%, F1-score of 89.96%, and Kappa of 90.60% from the experiment. In another work, Farid et al. [122] introduced a new approach for classifying COVID-19 infections using the attributes from CT images. The image parameters were taken using four image filters in addition to following the developed hybrid composite extraction method. The extracted features were categorized using the Stack Hybrid Classification (SHC) technique where SHC integrated several models like ensemble learning to increase the performance of the developed system. The system considered two classes of data named COVID-19, and SARS, each of the class comprised of 51 images. The dataset was partitioned using 10-fold cross-validation technique to obtain a better outcome. The developed system obtained accuracy, precision, f1-score, and AUC of 94.11%, 99.4%, 94%, and 99.4% respectively.
Table 3 summarizes the aforementioned deep learning based COVID-19 diagnosis systems from CT samples using custom deep learning techniques and demonstrates some of the important factors, such as data sources, number of images and classes, data partitioning technique, diagnosis techniques, and the evaluation metrics of the developed systems.
TABLE 3. Summary of Deep Learning Based COVID-19 Diagnosis in CT Images Using Customized Network.
| Authors | Data Sources | Number of images | No. of classes | Partitioning | Techniques | Performances (%) |
|---|---|---|---|---|---|---|
| Elghamrawy and Hassanien [105] | Italian Society of Medical and Interventional Radiology: COVID-19 Database [113], COVID-CT [114] | 583 (COVID-19=432, viral pneumonias = 151) | 2 (COVID-19, viral pneumonia) | Training = 65%, Testing = 35% | WOA-CNN | Accuracy =96.40, Sensitivity =97.25, Precision =97.3 |
| He et al. [106] | Italian Society of Medical and Interventional Radiology: COVID-19 Database [113], Covid-19 [115], Eurorad [116], Coronacases [117] | 746 (COVID-19=349, non-COVID-19−=397) | 2 (COVID-19, non-COVID-19) | Training = 60%, Validation = 15% Testing = 25% | CRNet | Accuracy = 86, F1-Score = 85, AUC = 94 |
| Wang et al. [107] | Three different hospitals (Xi’an Jiaotong University, Nanchang University, Xi’anMedical College) | 1065 (COVID-19+=740, COVID-19−=325) | 2 (COVID-19+=, COVID-19−=) | Random partition | Modified-Inception | Accuracy =79.3, Sensitivity = 83, Specificity = 67, Precision = 55, NPV = 90, F1-Score = 63, AUC = 81, Kappa = 48, Yoden index = 50 |
| Liu et al. [108] | Ten designated COVID-19 hospitals in China | 1993 (COVID-19=920, non-COVID-19=1073) | 2 (COVID-19, non-COVID-19) | Training = 60%, Validation = 20%, Testing = 20% | Modified DenseNet-264 | Accuracy =94.3, Sensitivity =93.1, Specificity =95.1, Precision =93.9, F1-Score =93.5, AUC =98.6, NPV =94.5 |
| Ying et al. [109] | Two hospitals (Hospital of Wuhan University, Third Affiliated Hospital) | 1990 (COVID-19=777, bacterial pneumonia = 505, normal = 708) | 3 (COVID-19, bacterial pneumonia, normal) | Training = 60%, Validation = 10% Testing = 30% | DRE-Net | Accuracy =94.3, Sensitivity = 93, Precision = 96, F1-Score = 94, AUC = 99 |
| Zheng et al. [110] | Three different hospitals (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology) | 630 | 2 (COVID-positive, COVID-negative) | Training = 80%, Testing = 20% | DeCoVNet | Accuracy =90.1, Sensitivity =90.7, Specificity =91.1, Precision = 84, NPV =98.2, AUC =95.9 |
| Hasan et al. [111] | COVID-19 [115], SPIE-AAPM-NCI Lung Nodule Classification Challenge Dataset [118] | 321 (COVID-19=118, pneumonia = 96, healthy = 107) | 3 (COVID-19, pneumonia, healthy) | Training = 70%, Testing = 30% | QDE–DF | Accuracy =99.68 |
| Amyar et al. [112] | COVID-CT [114], COVID-19 CT segmentation dataset [119], a hospital named Henri Becquerel Center | 1044 (COVID-19=449, non-COVID-19=595) | 2 (COVID-19, non-COVID-19) | Training = 80%, Validation = 10% Testing = 10% | Encoder-Decoder with multi-layer perceptron | Accuracy = 86, Sensitivity = 94, Specificity = 79, AUC = 93, |
| Singh et al. [120] | COVID-19 patient chest CT images [121] | 150 (COVID-19+=75, COVID-19−=75) | 2 (COVID-19+=, COVID-19−=) | Various proportions of training and testing dataset | MODE-CNN | Accuracy =93.25, Sensitivity =90.70, Specificity =90.72, F1-Score =89.96, Kappa =90.60 |
| Farid et al. [122] | Kaggle benchmark dataset [123] | 102 (COVID-19=51, SARS = 51) | 2 (COVID-19, SARS) | 10-fold cross-validation | CNN | Accuracy =94.11, Precision =99.4, F1-Score = 94, AUC =99.4 |
B. Diagnosis Using X-Ray Images
1). Diagnosis Based on Multiple Source Data
Wong et al. [99] developed a coronavirus detection mechanism from chest X-ray data called COVID-Net. The proposed architecture used residual architecture that follows projection-expansion-projection-extension design pattern for classification. The system generated the dataset of COVIDx by combining and modifying two open-access datasets. In this study, the dataset consisted of a total of 13,800 chest x-ray samples from 13,645 patients. The system considered three classes by combining bacterial and viral classes into a negative case.
Among the total data, 90% was used for training and the rest 10% was utilized for validation. The proposed network obtained 92.4% accuracy in 10 iterations for test cases, and the sensitivity and precision of 80% and 88.9% were achieved in the case of COVID-19 class. In another study, Ucar and Korkmaz [124] developed a COVIDiagnosis-Net based on the Bayes-SqueezeNet for the diagnosis of coronavirus utilizing X-ray samples. To develop a lightweight and more efficient CNN model, the SqueezeNet convolution architecture utilizes the squeeze and enlarges the layers of the fire modules. The system used 1591 pneumonia cases with non-COVID-19, 45 COVID-19 cases, and 1203 uninfected normal patients in total as the dataset. The dataset is formed with the combination of three publicly available datasets. From the total data, 80% for training, 10% for validation, and 10% for testing are used in the proposed system. The experimental results obtained accuracy, correctness, completeness, specificity, f1-score, and MCC of 98.26%, 98.26%, 98.26%, 99.13%, 98.25%, and 97.39% individually in overall. Further, Khan et al. [125] proposed a deep CNN architecture named CoroNet for the diagnosis of COVID-19 infected patients from chest X-ray radiographs. Instead of traditional convolutions, the proposed network utilized depth-wise separable convolution layers with residual connections. In this system, a total of 1300 images were considered where 290 samples of COVID-19, 660 of bacterial pneumonia, 931 of viral pneumonia, and 1203 of normal patients. The dataset was split at a proportion of 80% and 20% for training and validation set respectively. The proposed system obtained accuracy, precision, sensitivity, and F1-score of 89.5%, 97%, 100%, and 98% respectively for COVID-19 class.
Recently, Rahimzadeh and Attar [126] proposed a modified CNN network for the diagnosis of novel coronavirus cases using X-ray samples. The system concatenated the two well-known architectures of CNN named Xception and ResNet50V2 that make the system robust using multiple feature extraction capability. The use of inception and residual layers enhanced the quality of generated semantic features that are highly suited for classification. Among the 15085 images, 180 were confirmed COVID-19, 6054 were pneumonia, and 8851 were normal cases. The scheme used 5-fold cross-validation for data partitioning. The network obtained accuracy of 99.50%, sensitivity of 80.53%, specificity of 99.56%, and precision of 35.27% for COVID-19 detection. Furthermore, Mukherjee et al. [127] proposed a system for the detection of novel coronavirus using shallow CNN in chest X-ray radiographs. The proposed architecture includes a single layer of convolution, followed by a layer of max-pooling, a 256-dimensional dense layer, and a layer of 2 dimensional output. The developed network is comparatively light-weight due to a small number of parameters. In this system, 130 positive COVID-19 cases, and 130 non-COVID cases were considered where the non-COVID cases include MERS, SARS, pneumonia, and normal chest X-rays. To obtain better performance, the dataset was split using 5-fold cross-validation. The performance of the system was evaluated by tuning the batch size of the CNN architecture. From the experimental results, it is found that the system obtained the highest accuracy, sensitivity, specificity, precision, F1-score, and AUC of 96.92%, 94.20%, 100%, 100%, 97.01%, and 99.22% respectively for batch size 50.
In another study, Li et al. [128] introduced a robust technique for automatic COVID-19 screening using discriminative cost-sensitive learning (DCSL), a combination of fine-grained classification and cost-sensitive learning. DCSL introduces a conditional center loss and score-level cost-sensitive learning to learn discriminative representation and expand the misclassifying cost of COVID-19 samples into other cases respectively. The used dataset consisted of 2,239 chest X-ray samples where 239 samples of COVID-19 cases, 1,000 samples from bacterial or viral pneumonia cases, and 1,000 samples of normal people. To obtain better performance, the dataset was partitioned using 5-fold cross-validation method. The proposed system achieved accuracy of 97.01%, precision of 97%, sensitivity of 97.09%, and F1-score of 96.98%. Khobahi et al. [129] developed a semi-supervised deep learning system based on AutoEncoders named CoroNet to detect COVID-19 infected patients. The highly-tailored network extracted the necessary attributes and classified them efficiently. The semi-supervised property of the developed architecture solved the problem associated with a small number of COVID-19 data. The proposed system merged three open-access datasets for experiments. In this scheme, 18,529 images of different categories were used. Among the images, 99 samples were of COVID-19 classes, 9579 were of non-COVID pneumonia, and 8851 samples were related to healthy cases. The dataset was split in a proportion of 90% and 10% for training and testing set respectively. Overall, the accuracy, precision, recall, and F1-score of 93.50%, 93.63%, 93.50%, and 93.51% were achieved from the experiment.
2). Diagnosis Based on Single Source Data
Alqudah et al. [130] suggested a hybrid method for the diagnosis of patients affected with coronavirus from X-ray data. The proposed system combined deep learning (CNN) and machine learning (SVM, RF) architecture. Deep learning was utilized both for feature extraction and classification purposes where machine learning was only used for classification task. In this system, 71 X-ray images of the chest were used in total where 48 are used for positive and 23 for negative. The system used 70% for training and 30% for testing and experiments were performed on several combinations like CNN-Softmax, CNN-SVM, and CNN-RF. The system obtained accuracy, sensitivity, specificity, and precision of 95.2%, 93.3%, 100%, and 100% respectively for CNN-Softmax classifier. Afterward, Farooq and Hafeez [131] presented a deep learning scheme with a pre-trained ResNet-50 network to detect the COVID-19 infected patients named COVID-ResNet. The proposed architecture includes discriminating the learning rate, looping the search of the learning rate, and fine-tuning to select the best parameters. The proposed system implemented a residual neural network with 50 layers in total. In this study, the dataset comprised of 13,800 chest X-ray samples from 13,645 patients in total. The system achieved accuracy of 96.23% overall with 41 iterations using the COVIDx dataset. The other evaluation metrics like sensitivity, precision, and F1-score of 100%, 100%, and 100% are obtained considering the COVID-19 case only. Further, Afshar et al. [132] developed a capsule network based system named COVID-CAPS for the diagnosis of COVID-19 patients using 3D X-ray samples. The developed network used 4 convolutional layers, and 3 capsule layers where the last capsule layer is used for classification and the remaining layers are utilized for features extraction. The proposed framework is well suited to work with a small dataset. The dataset used in the network was the COVIDx dataset, which is popular for COVID-19 research. The developed scheme used 13,800 chest x-ray images from 13,645 patients for experiments. Although there are four classes in the dataset, the system classifies the images into three classes considering bacterial and viral into one negative class. COVID-CAPS obtained accuracy, sensitivity, specificity, and AUC of 95.7%, 90%, 95.8%, and 0.97 respectively.
Table 4 summarizes the aforementioned deep learning based COVID-19 diagnosis systems from X-ray samples using custom deep learning techniques and demonstrates some of the important factors, such as data sources, number of images and classes, data partitioning technique, diagnosis techniques, and the evaluation metrics of the developed systems.
TABLE 4. Summary of Deep Learning Based COVID-19 Diagnosis in X-Ray Images Using Customized Network.
| Authors | Data Sources | Number of images | Number of classes | Partitioning | Techniques | Performances (%) |
|---|---|---|---|---|---|---|
| Wang et al. [99] | COVID-19 X-ray image database [88], RSNA Pneumonia Detection Challenge dataset [95] | 13, 800 | 3 (COVID-19, non-COVID-19, normal) | Training=90%, Testing=10% | COVID-Net (CNN) | Accuracy= 92.4, Sensitivity=80, Precision=88.9 |
| Ucar and Korkmaz [124] | COVID-19 X-ray image database [88], COVIDx Dataset [99], Kaggle chest X-ray pneumonia dataset [133] | 2839 (COVID-19=45, normal=1203, pneumoniae] 591) | 3 (COVID-19, normal, pneumonia) | Training=80%, Testing=10%, Validation=10% | Bayes-SqueezeNet | Accuracy=98.26, Specificity=99.13, Fl-Score=98.25, MCC=97.39, Correctness=98.26, Completeness=98.2 6 |
| Khan et al. [125] | COVID-19 X-ray image database [88], Kaggle chest x-ray repository [93] | 1251 (COVID-19=284, normal=310, pneumonia bacterial=330, pneumonia viral= 327) | 4 (COVID-19, normal, pneumonia bacterial, pneumonia viral) | Training=80%, Validation=20% | CoroNet (CNN) | Accuracy=89.5, Sensitivity=100, Precision=97, Fl-Score= 98 |
| Rahimzade h and Attar [126] | COVID-19 X-ray image database [88], RSNA Pneumonia Detection Challenge dataset [95] | 15085 (covro-19=180, pneumonia= 6054, normal= 8851) | 3 (COVID-19, pneumonia, normal) | 5- fold cross-validation | Concatenated CNN | Accuracy=99.50, Sensitivity=80.53, Specificity=99.56, Precision=35.27 |
| Mukheijee et al. [127] | covid-chestxray-dataset [88], Kaggle chest x-ray repository [93] | 260 (COVID-19=130, non-COVID=130) | 2 (COVID-19, non-COVID) | 5- fold cross-validation | Shallow CNN | Accuracy= 96.92, Sensitivity= 94.20, Specificity=100, Precision=100, Fl-Score=97.01, AUC=99.22 |
| Li et al. [128] | COVID-19 X-ray image database [88], Kaggle dataset [89], Kermany et al. [90] | 2239 (COVID-19=239, pneumonia=1000, normal=1000) | 3 (COVID-19, pneumonia, normal) | 5-fold cross-validation | DCSL | Accuracy=97.01, Sensitivity=97.09, Precision=97, Fl-Score=96.98 |
| Khobahi et al. [129] | COVID-19 X-ray image database [88], RSNA Pneumonia Detection Challenge dataset [95], COVIDx Dataset [99] | 18,529 (COVID-19=99, non-COVID-pneumonia=9579, healthy=8851) | 3 (COVID-19, non-COVID pneumonia, healthy) | Training=90%, Testing=10% | CoroNet (AutoEncoders) | Accuracy=93.50, Sensitivity=93.50, Precision=93.63, Fl-Score=93.51 |
| Alqudah et al. [130] | COVID-19 X-ray image database [88] | 71 (COVID-19=48, non-COVID-19=23) | 2 (COVID-19, non-COVID-19) | Training=70%, Testing=30% | CNN, SVM, RF | Accuracy=95.2, Sensitivity=93.3, Specificity=100, Precision= 100 |
| Farooq and Hafeez [131] | COVIDx Dataset [99] | 13, 800 | 4 (COVID-19, normal, bacterial, viral) | Training=90%, Testing=10% | COVID-ResNet (CNN) | Accuracy= 96.23, Sensitivity=100, Precision=100, Fl-Score=100 |
| Afshar et al. [132] | COVIDx Dataset [99] | 13, 800 | 3 (COVID-19, normal, non-COVID-19) | Training=90%, Testing=10% | COVID-CAPS (Capsule Network) | Accuracy 95.7, Sensitivity=90, Precision=95.8, AUC=97 |
V. Open Discussions, Challenges and Future Trends
This section demonstrates the discussions of reviewed systems, challenges, and the possible future trends of deep learning based systems for COVID-19 diagnosis.
A. Open Discussions
In this paper, 45 systems were reviewed where 25 systems used pre-trained model as a deep learning architecture and the remaining 20 utilized custom deep learning framework. The results of the individual system are presented for explanation. Two popular imaging techniques CT and X-ray are used for data samples.
Among the reviewed system, 25 systems are developed based on X-ray data and the rest 20 used CT samples. The majority of the systems used multiple source data and a few of them used single source data. We summarized the developed systems considering some features like the data sources, the number of images and classes, the data partitioning techniques, the used deep learning technique for diagnosis, and finally the evaluation metrics for performance measure. The data sources are the benchmark dataset or real-time data from hospital environment. Some of the systems used a huge number of images but the number of samples for COVID-19 cases is comparatively small. Both the binary and multi-class are considered throughout the review.
With respect to data partitioning, some of the systems used cross-validation techniques, and others used hold-out method. Both the pre-trained model and custom deep learning architecture are taken into consideration. Almost all the systems used CNN or variants of CNN for diagnosis. Some common evaluation metrics like accuracy, sensitivity, specificity, precision, F1-score, AUC, etc. are utilized throughout the whole review.
The summary for the CT scan based COVID-19 diagnosis utilizing pre-trained model as well as customized deep learning technique is illustrated in Table 1 and Table 3. It is evident from the results that most of the developed systems used real-time data from hospital environment of China and a few of the systems [62], [65], [105], [106], [111], [112], [120], and [122] used benchmark data. A few of the developed systems [66], [71], [72], [120], and [122] used data from a single source and the majority of the developed schemes used multiple source data. The datasets which are used multiple times in the reviewed systems are COVID-CT [114], and COVID-19 [115]. The reviewed systems which used maximum and minimum number of images for the experiment are [65], and [122] where the COVID-19 cases are 32,230 (augmented data), and 51 respectively. In case of the number of classes to be classified, most of the developed systems considered binary class (COVID-19, and non-COVID-19) while some of them [60], [63], [65], [109], and [111] considered multiple classes (COVID-19, pneumonia, and normal). The 10-fold [122] was taken into consideration in some cases, whereas some developed systems [62], [64], [71], and [107] used random partitioning, and the majority of them considered hold-out method for data splitting. As far as performance is concerned, the system developed in [66] obtained 100% sensitivity. Among the reviewed systems, most of the frameworks [60]–[62], [64], [66], [105], [108], [109], and [111] achieved comparatively higher accuracy, sensitivity, specificity, precision, F1-score and AUC having these measure (where applicable) greater than 90%. The highest accuracy of 99.51% and 99.68% were found at [66], [122] using pre-trained model and customized network respectively.
Table 2 and Table 4 depict the X-ray based diagnosis of COVID-19 using pre-trained model with deep transfer learning and customized deep learning architecture. Our analysis revealed that almost all the developed systems used a common dataset COVID-19 X-ray image database [88]. Some systems used Kermany et al. [90] dataset, and COVIDx Dataset [99] frequently for diagnosis. All the proposed schemes utilized benchmark data for the experiment, no system applied real-time data. The systems introduced in [87], [130], [131], and [132] used single source data whereas the rest of the reviewed systems considered data from multiple sources. The framework demonstrated in [84] considered the highest number of images where the lowest case was used in [87]. The number of COVID-19 cases for [84], and [87] is 144, and 25 respectively. Although some systems considered the maximum number of images but the total number of images for COVID-19 is comparatively small. In terms of number of class consideration, some of the systems [74], [76], [125], and [130] considered 4 classes, a few of them [73], [75], [79], [81]–[83], etc. used 3 classes, and the remaining reviewed systems utilized binary class for experiment. The cross-validation technique such as 10-fold [73], [84], and 5-fold [80], [83], [85], [126]–[128] are used in some cases, and other systems considered hold-out method for data partitioning.
It is quite challenging to single out one particular system among the reviewed ones in this paper as the dataset, experimental environment, and test cases are quite different. In the case of performance measures of the developed systems, 100% accuracy was achieved by the proposed systems in [74], 100% sensitivity in [74], [78], [87], [125], and [131], 100% specificity in [80], [87], [127], and [130], 100% precision in [74], [80], [87], [86], [127], [130], and [131], 100% F1-score [74], and [131], 100% AUC obtained in [80]. Most of the developed systems performed better in the case of precision measurement, and the second best is obtained in the case of sensitivity. It is found from the performance analysis that the technique introduced in [74] performed the best among the developed systems for COVID-19 diagnosis but the dataset used in this system is relatively small in size and collected from benchmark databases; not from the hospital environments. Most of the developed systems did not mention the computing time as they used different datasets for the experiment; a few of the systems calculated the computing time. The lowest testing time 3 seconds and 0.299704 seconds are found for CT and X-ray images developed in [60] and [130] respectively. Though the reviewed systems achieved comparatively better results for X-ray case both for pre-trained and custom network, the developed systems are not real-time tested with target people.
When comparing the pre-trained model to the custom network, some of the reviewed systems performed better in the latter. The developed systems’ respective performance varied (depending on the dataset) and is not comparable since different data sizes were used for almost every experiment. In terms of imaging modalities comparison, X-ray based systems performed comparatively better than CT based systems. However, most of the X-ray based frameworks used benchmark data while the real-time data from hospital environment was considered in the case of CT based systems. It is envisaged that the systems introduced using CT samples are conceivably applicable for real-time testing but the X-ray based schemes need real-time testing with target people before their application.
The frequently (more than once) used datasets in the reviewed systems are summarized in Table 5. The datasets are summarized in terms of some properties like the participants, the number of samples and classes, image size, and the accessing state whether it is public or private. From Table 5, it is shown that the several times used dataset is COVID-19 X-ray image database [88]; almost all the X-ray based systems used this dataset. Most of the datasets did not mention the participants clearly, the image size for all datasets is highly variable except NIH Chest X-Ray [92], and all the datasets are publicly available. The highest number of samples (224,316) are mentioned in ChexPert [94], although there are no COVID-19 cases. Some of the datasets ([92], [94], [98]) have 14 classes and all the classes are used as COVID-negative cases in the reviewed systems. Lastly, it is quite difficult to figure out the exact information of the used datasets as the information of the datasets is updated day by day with the increasing number of COVID-19 patients.
TABLE 5. Summary of the COVID-19 Diagnosis Datasets Used in the Reviewed Systems.
| Imaging Modalities | Dataset | Participants | Number of Samples | Number of classes | Image size | Accessing state |
|---|---|---|---|---|---|---|
| X-Ray | COVID-19 X-ray image database [88] | Websites and publications | 589 | COVID-19=434, Others=155 | Highly variable | Public |
| Kaggle dataset [89] | Articles and knowledge bases | 79 | 1 (COVID-19) | Various sizes | Public | |
| Kermany et al. [90] | 5,319 patients | 109,312 | 4 (CNV=37,456, DME=11,599, Drusen=8,867, Normal=51,390) | Different sizes | Public | |
| NIH Chest X-Ray [92] | N/M | 5,606 | 14 condition labels including Pneumonia and normal | 1024x1024 | Public | |
| Kaggle chest x-ray repository [93] | N/M | 5856 | 2 (Normal=1583, Pneumonia=4273) | Different sizes | Public | |
| ChexPert [94] | 65,240 patients | 224,316 | 14 sub-categories (No-finding, Edema, Pneumonia, etc.) | Various sizes | Public | |
| RSNA Pneumonia Detection Challenge dataset [95] | N/M | 30227 | 2 (Normal=8851, Infected= 21376) | Different sizes | Public | |
| ChestX-ray8 [98] | N/M | 108,948 | 14 different lung diseases and normal images | Various sizes | Public | |
| COVIDx Dataset [99] | 13,870 individuals | 13,975 | 3 (COVID-19= 358, Normal=8066, Pneumonia= 5,538) | Highly variable | Public | |
| CT | COVID-19 Database [113] | N/M | 68 | 1 (COVID-19) | Different sizes | Public |
| COVID-CT [114] | 271 patients | 812 | 2 (COVID-19=349, non-COVID-19=463) | Different sizes | Public | |
| COVID-19 [115] | N/M | 59 | 1 (COVID-19) | Various sizes | Public |
*N/M: Not Properly Mentioned, CNV: choroid neovascularization, DME: diabetic macular edema.
B. Challenges and Future Trends
There are many unique challenges for applying deep learning techniques and algorithms for the detection of novel coronavirus (COVID-19). Although deep learning based COVID-19 detection from chest CT and X-ray images show promising results, its widespread adaptation still faces various societal and technical pushbacks.
While deep learning techniques are highly automatable, it needs a large set of data to develop a robust system for diagnosis purposes. As COVID-19 is very new to research, the lack of standard data is a major challenge for diagnoses. On the other hand, the available imaging data for COVID-19 patients are incomplete, noisy, ambiguous, and inaccurately labeled in certain cases. To train a deep learning architecture with such massive and diverse data sets is very complex and a variety of problems must be resolved (e.g., data redundancy, sparsity, missing values). Almost all the reviewed systems used different data sets for the experiment. The developed systems collected data from internet sources, prepared it their way, and finally evaluated their systems using evaluation metrics. For this reason, it is quite difficult to conclude definitively which system yields the best result for COVID-19 detection.
Data shortage is a huge issue for deep learning based COVID-19 detection systems. Due to the relative newness of the COVID-19 pandemic, clinical data is still very rare and strongly regulated. Thus datasets related to these are also very few. The datasets also contain a limited number of COVID-19 cases (a couple of hundred samples in general). The small sized datasets are used in [59], [74], [75], [77], [80], [81], [83], [87], [111], [120], [122], [127], and [130]. A small sized dataset results in a low approximation in the training phase and leads to an optimistic as well as high variance estimation of the performance of deep learning based COVID-19 diagnosis systems in the testing phase. A limited number of data causes underfit or overfit problem depending on the nature of deep learning architecture that degrades the performance of the developed systems. Class imbalance is another big issue for deep learning based COVID-19 diagnosis systems. Data related to COVID-19 exist far less than other common lung diseases in chest X-ray and CT images. The data imbalance problem are found from the reviewed systems proposed in [61], [62], [64], [78], [79], [84]–[86], [124], [126], [128], and [129]. The imbalance in data very often raises bias during the training phase of deep learning techniques. With the fewer number of positive samples, it has become increasingly difficult to balance out the target sample. While both of the problems are found in the developed systems, the small sized dataset problem is more severe than class imbalance problem.
While accuracy is a viable metric to determine the performance of deep learning models, it cannot be used as the sole metric. Other metrics such as F1-score, sensitivity, specificity, ROC, AUC, confidence interval, etc. should also be used alongside the accuracy metric to determine the performance of deep learning models. The lack of confidence interval in particular is an issue for deep learning based COVID-19 diagnosis systems. Deep learning architecture provides the output as prediction confidence whereas the output indicator of a particular neuron is considered as a single probability. For COVID-19 diagnosis, the lack of confidence interval across a predicted value is usually not desirable.
From a technical perspective, PCR based methods such as RT-PCR and its derivatives are widely accepted and used around the world as the most reliable, safe, and fast COVID-19 detection method. The use of deep learning in medical applications is a very fast-growing research field and the development of such systems is still limited. Thus, deep learning based methods in their current forms cannot replace the PCR based methods. However, deep learning based systems can be used as assistive technology for healthcare professionals or initial screening methods for healthcare institutions. Another issue is the data loss associated with resizing image data. The medical images are of very high resolution. However, it is very hard to use these high resolution images for training deep learning models due to high computational costs. From a societal perspective, the majority of people around the world are not familiar with automated detection systems. Thus, deploying such automated systems in general requires adequate educational promotional actions beforehand.
To overcome these challenges, researchers may consider the design of optimized deep learning algorithms that can easily cope up with a small number of data [26]. A shallow long short-term memory (LSTM) [134] can be utilized to solve the limitations of a small dataset. In the absence of large-scale training datasets, leveraging the current deep learning architecture to extract features and conduct more learning in an end-to-end manner [135], [136] is an encouraging path. Freezing is a technique that provides the facilities to shrink the number of parameters in deep learning architecture in which the reduced parameters are hired from another network trained for similar purposes. While the number of parameters would reduce, it might be possible to achieve good performance from a small number of COVID-19 cases [137], [138]. Ensemble learning [139], [140], and multi-task learning [112], [141] are better suited for COVID-19 diagnosis in the context of a small number of data. In the case of ensemble learning, multiple architectures are developed instead of a single network and finally, the results of each network are combined. In multi-task architecture, diverse tasks are combined to take the facility of data annotations from one another.
Additionally, synthetic data generation might be a possible solution to overcome the challenges of deep learning based COVID-19 detection systems. The class imbalance and data shortage issue for COVID-19 diagnosis systems results in the necessity to use various balancing techniques such as GANs, augmentations, etc. for properly training the deep learning models. The most used techniques for data generation are data augmentation and GANs which are frequently utilized to solve the class imbalance problem as well as small sized dataset issue. The data augmentation technique [142], [143] generates new lesions from the given COVID-19 samples using flipping, rotation, cropping, random noise addition, etc. from the given images. But the overfitting problem may arise in the case of augmented data. GANs are the most sophisticated techniques for realistic synthetic data generation. From a small number of COVID-19 samples, GANs [74], [144] generate a large number of images that are used to train a deep learning system for the novel virus diagnosis. Further, weakly supervised deep learning methods [145], [146] would be a probable solution for limited training data. Furthermore, as the manually labeling of COVID-19 imaging data is costly, and lengthy process, the use of self-supervised deep learning techniques [147], [148] are highly recommended. In the future, efforts can be directed to create more datasets containing Sputum Smear Microscopy Images and Histopathology images of COVID-19 and other lung diseases. Also, cloud computing solutions can be used to effectively overcome the limited computational capacity problem.
VI. Limitations of the Study
The goal of this paper is to review and present some well-known deep learning based COVID-19 diagnosis systems based on CT and X-ray images. While many of the aspects discussed in the literature are highlighted here, there are still a few limitations that need to be addressed in future work. Firstly, only the COVID-19 diagnosis systems based on deep learning techniques are described, yet there are no specific descriptions of background knowledge on deep learning techniques highlighting mathematical representations. This work assumes a certain level of domain specific knowledge. Secondly, some specific aspects of the reviewed neural networks such as the number of layers, layer specification, learning rate, number of epochs, batch size, dropout layer, optimizer, and loss function, especially for custom architectures are not mentioned here, and instead the reader is invited to consult related references. Thirdly, although this review discusses COVID-19 diagnosis from a computer vision perspective, this article does not provide any qualitative results of diagnosis in CT or X-ray images. Fourthly, most of the reviewed systems present accuracy greater than 90% approximately whether it is pre-trained model or custom architecture both for CT and X-rays having a small or large number of data, but reliability of the reviewed systems in real-world is not properly assessed. Lastly, this work does not provide computer code or implemented examples that simulate some of the most significant results in reviewed COVID-19 diagnosis systems.
VII. Conclusion
COVID-19 remains an ongoing pandemic, creating new records daily for cumulative global infection numbers and death tolls. A consistent and accurate deep learning based automatic diagnosis of COVID-19 has already significantly assisted to diagnose this disease. This paper presents the recent works for COVID-19 diagnosis purposes using deep learning techniques from two types of imaging techniques like CT and X-ray samples. The review describes the systems which are developed based on pre-trained model with deep transfer learning and customized deep learning architecture for COVID-19 diagnosis. Two-leveled taxonomy was presented which explores the perspectives of deep learning techniques and imaging modalities. This paper outlines all the sources of used datasets which can be easily understood and accessed by the research community. The major challenge of the COVID-19 diagnosis systems based on deep learning is the lack of gold standards. Furthermore, possible solutions to overcome the current challenges are recommended so that it motivates and encourage researchers who would like to contribute in this area. It is prudent to note that deep learning techniques with imaging modalities offer only partial details about the infected patients. It is not really implied in the present study that the role of physicians or clinicians in clinical diagnosis can be replaced by deep learning techniques. In the near future, it is hoped that deep learning experts cooperate pro-actively with radiologists and medical experts to provide appropriate support systems for identifying COVID-19 infections, especially in the early stages of the disease, or gauging level of severity of the infection.
Biographies

Md. Milon Islam received the B.Sc. and M.Sc. degrees in computer science and engineering (CSE) from the Khulna University of Engineering & Technology, Khulna, Bangladesh, in 2016 and 2019, respectively. He is currently pursuing the Ph.D. degree with the Centre for Pattern Analysis and Machine Intelligence, Department of Electrical and Computer Engineering, University of Waterloo, Canada. In 2017, he joined the Department of Computer Science and Engineering, Khulna University of Engineering & Technology, as a Lecturer. He is also working as an Assistant Professor (on leave) of CSE with the Khulna University of Engineering & Technology. He has published several number of research papers in peer reviewed journals, book chapters and conferences. In addition, he is a reviewer of several reputed journals and conferences. His research interests include machine learning and its application, deep learning, intelligent systems design, health informatics, the Internet of Things (IoT), and to solve real life problems with the concept of computer science.

Fakhri Karray (Fellow, IEEE) is currently the University Research Chair Professor of electrical and computer engineering and the Co-Director of the Institute of Artificial Intelligence, University of Waterloo. He holds the Loblaw’s Research Chair of Artificial Intelligence. His research interests include intelligent systems and operational artificial intelligence as applied to autonomous machines/devices and man machine interaction systems through speech, gesture, and natural language. He has authored extensively in these areas and has disseminated his work in journals, conference proceedings, and textbooks. He is the coauthor of two dozen U.S. patents, has chaired/co-chaired several international conferences in his area of expertise and has served as keynote/plenary speaker on numerous occasions. He has served as the University of Waterloo’s Academic Advisor for Amazon’s Alexa Fund Fellowship Program. He is also a Fellow of the Canadian Academy of Engineering and the Engineering Institute of Canada. He has also served as the Associate Editor/Guest Editor for a variety of leading journals in the field, including the IEEE Transactions on Cybernetics, the IEEE Transactions on Neural Networks and Learning Systems, the IEEE Transactions on Mechatronics, and the IEEE Computational Intelligence Magazine. His work has been featured on Discovery Channel, CBC, Globe and Mail, The Record, Reuters, Daily Mail, Washington Post, Wired Magazine, and DigitalTrends portals.

Reda Alhajj (Senior Member, IEEE) is currently a Professor with the Department of Computer Science, University of Calgary, Canada. He is also affiliated with Department of Computer Engineering, Istanbul Medipol University, Turkey, and the Department of Health Informatics, University of Southern Denmark, Odense. He has served on the program committee of several international conferences. His research interests include data science and network science from management to integration and analysis. His current research interests include data management, analysis, and mining; social media and network analysis; systems and computational biology, bioinformatics, and health informatics; homeland security, terror and criminal networks, and so on; and sequence analysis with emphasis on domains, such as financial, weather, traffic, energy, and so on. He also leads a large research group of Ph.D. and M.Sc. candidates. He received best graduate supervision award and community service award at the University of Calgary. He is also a Founding Editor-in-Chief of the Springer premier journal Social Networks Analysis and Mining, a Founding Editor-in-Chief of Springer Series Lecture Notes on Social Networks, a Founding Editor-in-Chief of Springer journal Network Modeling Analysis in Health Informatics and Bioinformatics, a Founding Co-Editor-in-Chief of Springer Encyclopedia on Social Networks Analysis and Mining, a founding steering chair of the flagship conference “IEEE/ACM International Conference on Advances in Social Network Analysis and Mining”, and three accompanying symposiums FAB, FOSINT-SI, and HI-BI-BI. He is also a member of the editorial board of the Journal of Information Assurance and Security, Journal of Data Mining and Bioinformatics, Journal of Data Mining, Modeling and Management; he has been a guest editor of a number of special issues and edited a number of conference proceedings.

Jia Zeng received the Ph.D. degree in computer science from the University of Calgary, Canada. Her research interests include information retrieval, using machine learning and multi-agent system to identify motifs in human genomes, to conduct breast cancer classification, as well as other biomedical related topics. After receiving additional training at the Baylor College of Medicine under an interdisciplinary computational cancer biologist fellowship from the Cancer Prevention Institute of Texas, she joined the MD Anderson Cancer Center and started leading the efforts to build an overarching informatics infrastructure for personalized cancer medicine at the world’s leading cancer hospital.
Funding Statement
This work was supported in part by the Natural Sciences and Engineering Research Council (NSERC) Discovery Grant FK-2015-2020.
References
- [1].Wu F., Zhao S., Yu B., Chen Y. M., Wang W., and Song Z. G., “A new coronavirus associated with human respiratory disease in China,” Nature, vol. 579, no. 7798, pp. 265–269, Mar. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cucinotta D. and Vanelli M., “WHO declares COVID-19 a pandemic,” Acta Biomed., vol. 91, no. 1, pp. 157–160, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Worldometers. Accessed: Feb. 11, 2021. [Online]. Available: https://www.worldometers.info/coronavirus/
- [4].Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., and Zhang L., “Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China,” Lancet, vol. 395, pp. 497–506, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vetter P., Vu D. L., L’Huillier A. G., Schibler M., Kaiser L., and Jacquerioz F., “Clinical features of COVID-19,” BMJ, vol. 4, Apr. 2020, Art. no. m1470. [DOI] [PubMed] [Google Scholar]
- [6].Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., and Tao Q., “Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases,” Radiology, vol. 296, Feb. 2020, Art. no. 200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xie X., Zhong Z., Zhao W., Zheng C., Wang F., and Liu J., “Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: Relationship to negative RT-PCR testing,” Radiology, vol. 296, no. 2, pp. E41–E45, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar A., Gupta P. K., and Srivastava A., “A review of modern technologies for tackling COVID-19 pandemic,” Diabetes Metabolic Syndrome, Clin. Res. Rev., vol. 14, no. 4, pp. 569–573, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ting D. S. W., Carin L., Dzau V., and Wong T. Y., “Digital technology and COVID-19,” Nature Med., vol. 26, no. 4, pp. 459–461, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Islam M. M., Rahaman A., and Islam M. R., “Development of smart healthcare monitoring system in IoT environment,” Social Netw. Comput. Sci., vol. 1, no. 3, May 2020, Art. no. 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rahaman A., Islam M., Islam M., Sadi M., and Nooruddin S., “Developing IoT based smart health monitoring systems: A review,” Revue d’Intell. Artif., vol. 33, no. 6, pp. 435–440, Dec. 2019. [Google Scholar]
- [12].Kanne J. P., “Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: Key points for the radiologist,” Radiology, vol. 295, no. 1, pp. 16–17, Apr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rubin G. D., Ryerson C. J., Haramati L. B., and Sverzellati N., “The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the Fleischner Society,” Radiology, vol. 296, no. 1, pp. 172–180, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dong D.et al. , “The role of imaging in the detection and management of COVID-19: A review,” IEEE Rev. Biomed. Eng., vol. 14, pp. 16–29, Jan. 2021. [DOI] [PubMed] [Google Scholar]
- [15].Kim H., Hong H., and Yoon S. H., “Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: A meta-analysis,” Radiology, vol. 296, no. 3, pp. E145–E155, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ye Z., Zhang Y., Wang Y., Huang Z., and Song B., “Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review,” Eur. Radiol., vol. 30, no. 8, pp. 4381–4389, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shi F.et al. , “Review of artificial intelligence techniques in imaging data acquisition, segmentation, and diagnosis for COVID-19,” IEEE Rev. Biomed. Eng., vol. 14, pp. 4–15, Jan. 2021. [DOI] [PubMed] [Google Scholar]
- [18].McCall B., “COVID-19 and artificial intelligence: Protecting health-care workers and curbing the spread,” Lancet Digit. Health, vol. 2, no. 4, pp. e166–e167, Apr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vaishya R., Javaid M., Khan I. H., and Haleem A., “Artificial intelligence (AI) applications for COVID-19 pandemic,” Diabetes Metabolic Syndrome, Clin. Res. Rev., vol. 14, no. 4, pp. 337–339, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mei X., Lee H. C., Diao K., Huang M., Lin B., Liu C., and Xie Z., “Artificial intelligence–enabled rapid diagnosis of patients with COVID-19,” Nat. Med., vol. 26, no. 8, pp. 1224–1228, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wynants L., Van Calster B., Collins G. S., and Riley R. D., “Prediction models for diagnosis and prognosis of COVID-19: Systematic review and critical appraisal,” BMJ, vol. 4, Apr. 2020, Art. no. m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wynants L., Van Calster B., Bonten M. M. J., and Collins G. S., “Systematic review and critical appraisal of prediction models for diagnosis and prognosis of COVID-19 infection,” Brit. Med. J., vol. 369, Art. no. m1328, Apr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang L., Han R., Ai T., Yu P., Kang H., Tao Q., and Xia L., “Serial quantitative chest CT assessment of COVID-19: A deep learning approach,” Radiol., Cardiothoracic Imag., vol. 2, no. 2, Apr. 2020, Art. no. e200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Panwar H., Gupta P. K., Siddiqui M. K., Morales-Menendez R., and Singh V., “Application of deep learning for fast detection of COVID-19 in X-rays using nCOVnet,” Chaos, Solitons Fractals, vol. 138, Sep. 2020, Art. no. 109944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].El Asnaoui K. and Chawki Y., “Using X-ray images and deep learning for automated detection of coronavirus disease,” J. Biomol. Struct. Dyn., vol. 7, pp. 1–12, May 2020, doi: 10.1080/07391102.2020.1767212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Oh Y., Park S., and Ye J. C., “Deep learning COVID-19 features on CXR using limited training data sets,” IEEE Trans. Med. Imag., vol. 39, no. 8, pp. 2688–2700, Aug. 2020. [DOI] [PubMed] [Google Scholar]
- [27].Fan D.-P., Zhou T., Ji G.-P., Zhou Y., Chen G., Fu H., Shen J., and Shao L., “Inf-net: Automatic COVID-19 lung infection segmentation from CT images,” IEEE Trans. Med. Imag., vol. 39, no. 8, pp. 2626–2637, Aug. 2020. [DOI] [PubMed] [Google Scholar]
- [28].Pereira R. M., Bertolini D., Teixeira L. O., Silla C. N., and Costa Y. M. G., “COVID-19 identification in chest X-ray images on flat and hierarchical classification scenarios,” Comput. Methods Programs Biomed., vol. 194, Oct. 2020, Art. no. 105532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Albahri A. S., Hamid R. A., Alwan J. K., Al-qays Z. T., Zaidan A. A., Zaidan B. B., Albahri A. O. S., Alamoodi A. H., Khlaf J. M., Almahdi E. M., Thabet E., Hadi S. M., Mohammed K. I., Alsalem M. A., Al-Obaidi J. R., and Madhloom H. T., “Role of biological data mining and machine learning techniques in detecting and diagnosing the novel coronavirus (COVID-19): A systematic review,” J. Med. Syst., vol. 44, no. 7, Jul. 2020, Art. no. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Muhammad L. J., Islam M. M., Usman S. S., and Ayon S. I., “Predictive data mining models for novel coronavirus (COVID-19) infected patients’ recovery,” Social Netw. Comput. Sci., vol. 1, no. 4, 2020, Art. no. 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Latif S., Usman M., Manzoor S., Iqbal W., and Qadir J., “Leveraging data science to combat COVID-19: A comprehensive review,” IEEE Trans. Artif. Intell., vol. 1, no. 1, pp. 85–103, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ulhaq A., Born J., Khan A., Gomes D. P. S., Chakraborty S., and Paul M., “COVID-19 control by computer vision approaches: A survey,” IEEE Access, vol. 8, pp. 179437–179456, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singh R. P., Javaid M., Haleem A., and Suman R., “Internet of Things (IoT) applications to fight against COVID-19 pandemic,” Diabetes Metabolic Syndrome, Clin. Res. Rev., vol. 14, no. 4, pp. 521–524, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Swayamsiddha S. and Mohanty C., “Application of cognitive Internet of medical things for COVID-19 pandemic,” Diabetes Metabolic Syndrome, Clin. Res. Rev., vol. 14, no. 5, pp. 911–915, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shah P. and Patel C. R., “Prevention is better than cure: An application of big data and geospatial technology in mitigating pandemic,” Trans. Indian Nat. Acad. Eng., vol. 5, no. 2, pp. 187–192, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haleem A., Javaid M., Khan I. H., and Vaishya R., “Significant applications of big data in COVID-19 pandemic,” Indian J. Orthopaedics, vol. 54, no. 4, pp. 526–528, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Iyengar K., Upadhyaya G. K., Vaishya R., and Jain V., “COVID-19 and applications of smartphone technology in the current pandemic,” Diabetes Metabolic Syndrome, Clin. Res. Rev., vol. 14, no. 5, pp. 733–737, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Banskota S., Healy M., and Goldberg E., “15 smartphone apps for older adults to use while in isolation during the COVID-19 pandemic,” WestJEM, vol. 21, no. 3, pp. 514–525, Apr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goodfellow I., Bengio Y., and Courville A., Deep Learning—An MIT Press Book. Cambridge, MA, USA: MIT Press, 2016. [Google Scholar]
- [40].LeCun Y., Bengio Y., and Hinton G., “Deep learning,” Nature, vol. 521, pp. 436–444, May 2015. [DOI] [PubMed] [Google Scholar]
- [41].Wainberg M., Merico D., Delong A., and Frey B. J., “Deep learning in biomedicine,” Nat. Biotechnol., vol. 36, no. 9, pp. 829–838, Oct. 2018. [DOI] [PubMed] [Google Scholar]
- [42].Esteva A., Robicquet A., Ramsundar B., Kuleshov V., DePristo M., Chou K., Cui C., Corrado G., Thrun S., and Dean J., “A guide to deep learning in healthcare,” Nature Med., vol. 25, no. 1, pp. 24–29, Jan. 2019. [DOI] [PubMed] [Google Scholar]
- [43].Chen H., Engkvist O., Wang Y., Olivecrona M., and Blaschke T., “The rise of deep learning in drug discovery,” Drug Discovery Today, vol. 23, no. 6, pp. 1241–1250, Jun. 2018. [DOI] [PubMed] [Google Scholar]
- [44].Shen D., Wu G., and Suk H., “Deep learning in medical image analysis,” Annu. Rev. Biomed. Eng., vol. 19, pp. 221–248, Jun. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shin H.-C., Roth H. R., Gao M., Lu L., Xu Z., Nogues I., Yao J., Mollura D., and Summers R. M., “Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning,” IEEE Trans. Med. Imag., vol. 35, no. 5, pp. 1285–1298, May 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Litjens G., Kooi T., Bejnordi B. E., Setio A. A. A., Ciompi F., Ghafoorian M., van der Laak J. A. W. M., van Ginneken B., and Sánchez C. I., “A survey on deep learning in medical image analysis,” Med. Image Anal., vol. 42, pp. 60–88, Dec. 2017. [DOI] [PubMed] [Google Scholar]
- [47].Elhassouny A. and Smarandache F., “Trends in deep convolutional neural networks architectures: A review,” in Proc. Int. Conf. Comput. Sci. Renew. Energies (ICCSRE), Jul. 2019, pp. 1–8, doi: 10.1109/ICCSRE.2019.8807741. [DOI] [Google Scholar]
- [48].Alom M. Z., Taha T. M., Yakopcic C., Westberg S., Sidike P., Nasrin M. S., Hasan M., Van Essen B. C., Awwal A. A. S., and Asari V. K., “A State-of-the-Art survey on deep learning theory and architectures,” Electronics, vol. 8, no. 3, p. 292, Mar. 2019. [Google Scholar]
- [49].Das S.. (2017). CNN Architectures: LeNet, AlexNet, VGG, GoogLeNet, ResNet and More. [Online]. Available: https://medium.com/analytics-vidhya/cnns-architectures-lenet-alexnet-vgg-googlenet-resnet-and-more-666091488df5
- [50].Zeng G., He Y., Yu Z., Yang X., Yang R., and Zhang L., “Preparation of novel high copper ions removal membranes by embedding organosilane-functionalized multi-walled carbon nanotube,” J. Chem. Technol. Biotechnol., vol. 91, no. 8, pp. 2322–2330, Aug. 2016. [Google Scholar]
- [51].Iandola F. N., Han S., Moskewicz M. W., Ashraf K., Dally W. J., and Keutzer K., “SqueezeNet: AlexNet-level accuracy with 50x fewer parameters and < 0.5MB model size,” 2016, arXiv:1602.07360. [Online]. Available: http://arxiv.org/abs/1602.07360
- [52].Simonyan K. and Zisserman A., “Very deep convolutional networks for large-scale image recognition,” 2014, arXiv:1409.1556. [Online]. Available: http://arxiv.org/abs/1409.1556
- [53].He K., Zhang X., Ren S., and Sun J., “Deep residual learning for image recognition,” 2015, arXiv:1512.03385. [Online]. Available: http://arxiv.org/abs/1512.03385
- [54].Chollet F., “Xception: Deep learning with depthwise separable convolutions,” in Proc. IEEE Conf. Comput. Vis. Pattern Recognit. (CVPR), Jul. 2017, pp. 1800–1807. [Google Scholar]
- [55].Szegedy C., Ioffe S., Vanhoucke V., and Alemi A. A., “Inception-v4, inception-resnet and the impact of residual connections on learning,” in Proc. AAAI, 2017, pp. 4278–4284. [Google Scholar]
- [56].Qin Z., Zhang Z., Chen X., Wang C., and Peng Y., “Fd-mobilenet: Improved mobilenet with a fast downsampling strategy,” in Proc. 25th IEEE Int. Conf. Image Process. (ICIP), Oct. 2018, pp. 1363–1367. [Google Scholar]
- [57].Huang G., Liu Z., Van Der Maaten L., and Weinberger K. Q., “Densely connected convolutional networks,” in Proc. IEEE Conf. Comput. Vis. Pattern Recognit. (CVPR), Jul. 2017, pp. 2261–2269. [Google Scholar]
- [58].Ronneberger O., Fischer P., and Brox T., “U-Net: Convolutional networks for biomedical image segmentation,” in Proc. Int. Conf. Med. Image Comput. Comput.-Assist. Intervent., 2015, pp. 234–241. [Google Scholar]
- [59].Wu X., Hui H., Niu M., Li L., Wang L., He B., and Yang X., “Deep learning-based multi-view fusion model for screening 2019 novel coronavirus pneumonia: A multicentre study,” Eur. J. Radiol., vol. 128, Art. no.109041, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B., Bai J., and Lu Y., “Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT,” Radiology, vol. 19, Mar. 2020, Art. no. 200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yousefzadeh M., Esfahanian P., and Movahed S., “Ai-corona: Radiologist-assistant deep learning framework for COVID-19 diagnosis in chest ct scans,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.05.04.20082081v1 [DOI] [PMC free article] [PubMed]
- [62].Jin C.et al. , “Development and evaluation of an artificial intelligence system for COVID-19 diagnosis,” Nat. Commun., vol. 11, no. 1, p. 5088, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xu X., Jiang X., Ma C., Du P., Li X., Lv S., Yu L., Chen Y., Su J., Lang G., Li Y., Zhao H., Xu K., Ruan L., and Wu W., “Deep learning system to screen coronavirus disease 2019 pneumonia,” 2020, arXiv:2002.09334. [Online]. Available: http://arxiv.org/abs/2002.09334 [DOI] [PMC free article] [PubMed]
- [64].Jin S., Wang B., Xu H., Luo C., Wei L., Zhao W., and Hou X., “AI-assisted CT imaging analysis for COVID-19 screening: Building and deploying a medical AI system in four weeks,” MedRxiv, 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.03.19.20039354v1 [DOI] [PMC free article] [PubMed]
- [65].Javaheri T.et al. , “CovidCTNet: An open-source deep learning approach to identify COVID-19 using CT image,” 2020, arXiv:2005.03059. [Online]. Available: http://arxiv.org/abs/2005.03059
- [66].Ardakani A. A., Kanafi A. R., Acharya U. R., Khadem N., and Mohammadi A., “Application of deep learning technique to manage COVID-19 in routine clinical practice using CT images: Results of 10 convolutional neural networks,” Comput. Biol. Med., vol. 121, Jun. 2020, Art. no. 103795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].S. G. Armato, III, et al. , “The lung image database consortium (LIDC) and image database resource initiative (IDRI): A completed reference database of lung nodules on CT scans,” Med. Phys., vol. 38, no. 2, pp. 915–931, Jan. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Depeursinge A., Vargas A., Platon A., Geissbuhler A., Poletti P.-A., and Müller H., “Building a reference multimedia database for interstitial lung diseases,” Comput. Med. Imag. Graph., vol. 36, no. 3, pp. 227–238, Apr. 2012. [DOI] [PubMed] [Google Scholar]
- [69]. Armato R., Hadjiiski S. G., Tourassi L., Drukker G. D., Giger K., Li M. L., Farahani F. and Farahani L. P. G., Kirby K., and Clarke J. S., “SPIE-AAPM-NCI lung nodule classification challenge,” Cancer Imag. Arch., vol. 10, p. K9, Dec. 2015. [Google Scholar]
- [70].Armato S. G., Drukker K., Li F., Hadjiiski L., Tourassi G. D., Engelmann R. M., Giger M. L., Redmond G., Farahani K., Kirby J. S., and Clarke L. P., “LUNGx challenge for computerized lung nodule classification,” J. Med. Imag., vol. 3, no. 4, Dec. 2016, Art. no. 044506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chen J.et al. , “Deep learning-based model for detecting 2019 novel coronavirus pneumonia on high-resolution computed tomography,” Sci. Rep., vol. 10, no. 1, p. 19196, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cifci M. A., “Deep learning model for diagnosis of corona virus disease from CT images,” Int. J. Sci. Eng. Res., vol. 11, no. 4, pp. 273–278, 2020. [Google Scholar]
- [73].Apostolopoulos I. D. and Mpesiana T. A., “COVID-19: Automatic detection from X-ray images utilizing transfer learning with convolutional neural networks,” Phys. Eng. Sci. Med., vol. 43, no. 2, pp. 635–640, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Loey M., Smarandache F., and Khalifa N. E. M., “Within the lack of chest COVID-19 X-ray dataset: A novel detection model based on GAN and deep transfer learning,” Symmetry, vol. 12, no. 4, p. 651, Apr. 2020. [Google Scholar]
- [75].Horry M. J., Paul M., Ulhaq A., Pradhan B., and Saha M., “X-ray image based COVID-19 detection using pre-trained deep learning models,” Engrxiv, to be published. [Online]. Available: https://engrxiv.org/wx89s/
- [76].Ozcan T.. (2020). A Deep Learning Framework for Coronavirus Disease (COVID-19) Detection in X-Ray Images. [Online]. Available: https://www.researchsquare.com/article/rs-26500/v1 [Google Scholar]
- [77].Sethy S. K. B. and Kumar P.. (2020). Detection of Coronavirus Disease (COVID-19) Based on Deep Features. [Online]. Available: https://www.preprints.org/manuscript/202003.0300/v1 [Google Scholar]
- [78].Minaee S., Kafieh R., Sonka M., Yazdani S., and Jamalipour Soufi G., “Deep-COVID: Predicting COVID-19 from chest X-ray images using deep transfer learning,” Med. Image Anal., vol. 65, Oct. 2020, Art. no. 101794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Punn N. S. and Agarwal S., “Automated diagnosis of COVID-19 with limited posteroanterior chest X-ray images using fine-tuned deep neural networks,” Int. J. Speech Technol., vol. 15, pp. 1–14, Oct. 2020, doi: 10.1007/s10489-020-01900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Narin A., Kaya C., and Pamuk Z., “Automatic detection of coronavirus disease (COVID-19) using X-ray images and deep convolutional neural networks,” 2020, arXiv:2003.10849. [Online]. Available: http://arxiv.org/abs/2003.10849 [DOI] [PMC free article] [PubMed]
- [81].Bukhari S., Bukhari S., Syed A., and Shah S., “The diagnostic evaluation of Convolutional Neural Network (CNN) for the assessment of chest X-ray of patients infected with COVID-19,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.03.26.20044610v1
- [82].Abbas A., Abdelsamea M. M., and Gaber M. M., “Classification of COVID-19 in chest X-ray images using DeTraC deep convolutional neural network,” Appl. Intell., vol. 8, pp. 1–11, Sep. 2020. [Online]. Available: https://doi.org/10.1007/s10489-020-01829-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Moutounet-Cartan P. G. B., “Deep convolutional neural networks to diagnose COVID-19 and other pneumonia diseases from posteroanterior chest X-rays,” 2020, arXiv:2005.00845. [Online]. Available: https://arxiv.org/abs/2005.00845
- [84].Maguolo G. and Nanni L., “A critic evaluation of methods for COVID-19 automatic detection from X-ray images,” 2020, arXiv:2004.12823. [Online]. Available: http://arxiv.org/abs/2004.12823 [DOI] [PMC free article] [PubMed]
- [85].Ozturk T., Talo M., Yildirim E. A., Baloglu U. B., Yildirim O., and Rajendra Acharya U., “Automated detection of COVID-19 cases using deep neural networks with X-ray images,” Comput. Biol. Med., vol. 121, Jun. 2020, Art. no. 103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Luz E., Lopes Silva P., Silva R., Silva L., Moreira G., and Menotti D., “Towards an effective and efficient deep learning model for COVID-19 patterns detection in X-ray images,” 2020, arXiv:2004.05717. [Online]. Available: http://arxiv.org/abs/2004.05717
- [87].El-Din Hemdan E., Shouman M. A., and Esmail Karar M., “COVIDX-net: A framework of deep learning classifiers to diagnose COVID-19 in X-ray images,” 2020, arXiv:2003.11055. [Online]. Available: http://arxiv.org/abs/2003.11055
- [88].Cohen J. P., Morrison P., and Dao L., “COVID-19 image data collection,” 2020, arXiv:2003.11597. [Online]. Available: https://arxiv.org/abs/2003.11597
- [89].Kaggle Dataset. Accessed: Mar. 15, 2020. [Online]. Available: https://www.kaggle.com/andrewmvd/convid19-x-rays
- [90].Kermany D. S., Goldbaum M., Cai W., and Valentim C., “Identifying medical diagnoses and treatable diseases by image-based deep learning,” Cell, vol. 172, no. 5, pp. 1122–1131, 2018. [DOI] [PubMed] [Google Scholar]
- [91].Dataset. Accessed: Mar. 31, 2020. [Online]. Available: https://drive.google.com/uc?id=1coM7x3378f-Ou2l6Pg2wldaOI7Dntu1a
- [92].NIH Chest X-Ray. Accessed: Mar. 16, 2020. [Online]. Available: https://openi.nlm.nih.gov/
- [93].Kaggle Chest X-ray Repository. Accessed: Mar. 20, 2020. [Online]. Available: https://www.kaggle.com/paultimothymooney/chest-xray-pneumonia
- [94].Irvin J., Rajpurkar P., Ko M., Yu Y., Chute C., Ball R., Seekins J., Halabi S. S., Jones R., Larson D. B., C, Langlotz P., Patel B. N., and Lungren M. P., “CheXpert: A large chest radiograph dataset with uncertainty labels and expert comparison,” in Proc. AAAI Conf. Artif. Intell., vol. 33, Jul. 2019, pp. 590–597. [Google Scholar]
- [95].Radiological Society of North America. RSNA Pneumo-Nia Detection Challenge. Accessed: Mar. 10, 2020. [Online]. Available: https://www.kaggle.com/c/rsna-pneumonia-detection-challenge/data [Google Scholar]
- [96].Candemir S., Jaeger S., Palaniappan K., Musco J. P., Singh R. K., Xue Z., Karargyris A., Antani S., Thoma G., and McDonald C. J., “Lung segmentation in chest radiographs using anatomical atlases with nonrigid registration,” IEEE Trans. Med. Imag., vol. 33, no. 2, pp. 577–590, Feb. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jaeger S., Karargyris A., Candemir S., Folio L., Siegelman J., Callaghan F., Xue Z., Palaniappan K., Singh R. K., Antani S., Thoma G., Wang Y.-X., Lu P.-X., and McDonald C. J., “Automatic tuberculosis screening using chest radiographs,” IEEE Trans. Med. Imag., vol. 33, no. 2, pp. 233–245, Feb. 2014. [DOI] [PubMed] [Google Scholar]
- [98].Wang X., Peng Y., Lu L., Lu Z., Bagheri M., and Summers R. M., “ChestX-ray8: Hospital-scale chest X-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases,” in Proc. IEEE Conf. Comput. Vis. Pattern Recognit. (CVPR), Jul. 2017, pp. 3462–3471. [Google Scholar]
- [99].Wang L., Lin Z. Q., and Wong A., “COVID-net: A tailored deep convolutional neural network design for detection of COVID-19 cases from chest X-ray images,” Sci. Rep., vol. 10, no. 1, Dec. 2020, Art. no.19549,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Anwar S. M., Majid M., Qayyum A., Awais M., Alnowami M., and Khan M. K., “Medical image analysis using convolutional neural networks: A review,” J. Med. Syst., vol. 42, no. 11, Nov. 2018, Art. no. 226. [DOI] [PubMed] [Google Scholar]
- [101].Islam M. Z., Islam M. M., and Asraf A., “A combined deep CNN-LSTM network for the detection of novel coronavirus (COVID-19) using X-ray images,” Informat. Med. Unlocked, vol. 20, 2020, Art. no. 100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Liang G., Hong H., Xie W., and Zheng L., “Combining convolutional neural network with recursive neural network for blood cell image classification,” IEEE Access, vol. 6, pp. 36188–36197, 2018. [Google Scholar]
- [103].Jiang F., Jiang Y., Zhi H., Dong Y., Li H., and Ma S., “Artificial intelligence in healthcare: Past, present and future,” Stroke Vasc. Neurol., vol. 2, no. 4, pp. 230–243, Dec. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hosny A., Parmar C., Quackenbush J., Schwartz L. H., and Aerts H. J. W. L., “Artificial intelligence in radiology,” Nature Rev. Cancer, vol. 18, no. 8, pp. 500–510, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Elghamrawy A. E. and Hassanien S., “Diagnosis and prediction model for COVID-19 patient’s response to treatment based on convolutional neural networks and whale optimization algorithm using CT images,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.04.16.20063990v1
- [106].He X., Yang X., Zhang S., Zhao J., Zhang Y., Xing E., and Xie P., “Sample-efficient deep learning for COVID-19 diagnosis based on CT scans,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.04.13.20063941v1
- [107].Wang S., Kang B., Ma J., Zeng X., Xiao M., Guo J., and Cai M., “A deep learning algorithm using CT images to screen for Corona Virus Disease (COVID-19),” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.02.14.20023028v5 [DOI] [PMC free article] [PubMed]
- [108].Liu B., Liu P., Dai L., Yang Y., Xie P., Tan Y., Du J., and Shan W., “Assisting scalable diagnosis automatically via CT images in the combat against COVID-19,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.05.11.20093732v1 [DOI] [PMC free article] [PubMed]
- [109].Song Y., Zheng S., Li L., Zhang X., Zhang X., and Huang Z., “Deep learning enables accurate diagnosis of novel coronavirus (COVID-19) with CT images,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.02.23.20026930v1 [DOI] [PMC free article] [PubMed]
- [110].Zheng C., Deng X., Fu Q., Zhou Q., Feng J., Ma H., and Liu W., “Deep learning-based detection for COVID-19 from chest CT using weak label,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.03.12.20027185v2
- [111].Hasan A. M., AL-Jawad M. M., Jalab H. A., Shaiba H., Ibrahim R. W., and AL-Shamasneh A. R., “Classification of COVID-19 coronavirus, pneumonia and healthy lungs in CT scans using Q-Deformed entropy and deep learning features,” Entropy, vol. 22, no. 5, p. 517, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Amyar A., Modzelewski R., Li H., and Ruan S., “Multi-task deep learning based CT imaging analysis for COVID-19 pneumonia: Classification and segmentation,” Comput. Biol. Med., vol. 126, Nov. 2020, Art. no. 104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Italian Society of Medical and Interventional Radiology: COVID-19 Database. Accessed: Mar. 28, 2020. [Online]. Available: https://www.sirm.org
- [114].Yang X., He X., Zhao J., Zhang Y., Zhang S., and Xie P., “COVID-CT-dataset: A CT scan dataset about COVID-19,” 2020, arXiv:2003.13865. [Online]. Available: http://arxiv.org/abs/2003.13865
- [115].COVID-19. Accessed: Apr. 9, 2020. [Online]. Available: https://radiopaedia.org
- [116].Eurorad. Accessed: Apr. 9, 2020. [Online]. Available: https://www.eurorad.org/
- [117].Coronacases. Accessed: Apr. 9, 2020. [Online]. Available: https://coronacases.org/
- [118].Archive C. I.. (2019). SPIE-AAPM-NCI Lung Nodule Classification Challenge Dataset. Accessed: Apr. 1, 2020. [Online]. Available: https://www.cancerimagingarchive.net/ [Google Scholar]
- [119].COVID-19 CT Segmentation Dataset. Accessed: Apr. 15, 2020. [Online]. Available: http://medicalsegmentation.com/covid19/
- [120].Singh D., Kumar V., Vaishali, and Kaur M., “Classification of COVID-19 patients from chest CT images using multi-objective differential evolution-based convolutional neural networks,” Eur. J. Clin. Microbiol. Infect. Dis., vol. 39, no. 7, pp. 1379–1389, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Li X., Zeng X., Liu B., and Yu Y., “COVID-19 infection presenting with CT halo sign,” Radiol., Cardiothoracic Imag., vol. 2, no. 1, Jan. 2020, Art. no. e200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Farid A. A., Selim G. I., and Khater H. A. A.. (2020). A Novel Approach of CT Images Feature Analysis and Prediction to Screen for Corona Virus Disease (COVID-19). [Online]. Available: https://www.preprints.org/manuscript/202003.0284/v1 [Google Scholar]
- [123].Kaggle Benchmark Dataset. Accessed: Mar. 1, 2020. [Online]. Available: https://www.kaggle.com/andrewmvd/covid19-ct-scans
- [124].Ucar F. and Korkmaz D., “COVIDiagnosis-net: Deep Bayes-squeezenet based diagnosis of the coronavirus disease 2019 (COVID-19) from X-ray images,” Med. Hypotheses, vol. 140, Jul. 2020, Art. no. 109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Khan A. I., Shah J. L., and Bhat M. M., “CoroNet: A deep neural network for detection and diagnosis of COVID-19 from chest X-ray images,” Comput. Methods Programs Biomed., vol. 196, Nov. 2020, Art. no. 105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rahimzadeh M. and Attar A., “A modified deep convolutional neural network for detecting COVID-19 and pneumonia from chest X-ray images based on the concatenation of xception and ResNet50 V2,” Informat. Med. Unlocked, vol. 19, 2020, Art. no. 100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Himadri Mukherjee K. R., Ghosh Subhankar, Dhar Ankita, Obaidullah Sk. Md., Santosh KC. (2020). Shallow Convolutional Neural Network for COVID-19 Outbreak Screening using Chest X-rays. [Online]. Available: https://www.techrxiv.org/articles/preprint/Shallow_Convolutional_Neural_Network_for_COVID- 19_Outbreak_Screening_using_Chest_X-rays/12156522/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Li T., Han Z., Wei B., Zheng Y., Hong Y., and Cong J., “Robust screening of COVID-19 from chest X-ray via discriminative cost-sensitive learning,” 2020, arXiv:2004.12592. [Online]. Available: http://arxiv.org/abs/2004.12592
- [129].Shahin Khobahi M. S. and Agarwal C., “CoroNet: A deep network architecture for semi-supervised task-based identification of COVID-19 from chest X-ray images,” MedRxiv, Jan. 2020. [Online]. Available: https://www.medrxiv.org/content/10.1101/2020.04.14.20065722v1
- [130].Alqudah A. M., Qazan S., Alquran H., Qasmieh I. A., and Alqudah A.. (2020). COVID-2019 Detection Using X-Ray Images and Artificial Intelligence Hybrid Systems. [Online]. Available: https://www.researchgate.net/publication/340232556_Covid-2019_Detection_Using_X-Ray_Images_And_Artificial_Intelligence_Hybrid_Systems [Google Scholar]
- [131].Farooq M. and Hafeez A., “COVID-ResNet: A deep learning framework for screening of COVID19 from radiographs,” 2020, arXiv:2003.14395. [Online]. Available: http://arxiv.org/abs/2003.14395
- [132].Afshar P., Heidarian S., Naderkhani F., Oikonomou A., Plataniotis K. N., and Mohammadi A., “COVID-CAPS: A capsule network-based framework for identification of COVID-19 cases from X-ray images,” Pattern Recognit. Lett., vol. 138, pp. 638–643, Oct. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kermany K. G. M. and Zhang D., “Labeled optical coherence tomography (OCT) and chest X-ray images for classification,” Mendeley Data, vol. 2, p. 15, Jun. 2018. [Online]. Available: https://data.mendeley.com/datasets/rscbjbr9sj/3 [Google Scholar]
- [134].Swapnarekha H., Behera H. S., Nayak J., and Naik B., “Role of intelligent computing in COVID-19 prognosis: A state-of-the-art review,” Chaos, Solitons Fractals, vol. 138, Art. no.109947, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Song J., Wang H., Liu Y., Wu W., Dai G., Wu Z., Zhu P., Zhang W., Yeom K. W., and Deng K., “End-to-end automatic differentiation of the coronavirus disease 2019 (COVID-19) from viral pneumonia based on chest CT,” Eur. J. Nucl. Med. Mol. Imag., vol. 47, no. 11, pp. 2516–2524, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Signoroni A., Savardi M., Benini S., Adami N., Leonardi R., Gibellini P., Vaccher F., Ravanelli M., Borghesi A., Maroldi R., and Farina D., “End-to-end learning for semiquantitative rating of COVID-19 severity on chest X-rays,” 2020, arXiv:2006.04603. [Online]. Available: http://arxiv.org/abs/2006.04603
- [137].Tsiknakis N. and Trivizakis E., “Interpretable artificial intelligence framework for COVID-19 screening on chest X-rays,” Express Ther. Med., vol. 20, no. 2, pp. 727–735, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ezzat D., ell Hassanien A., and Aboul Ella H., “GSA-densenet121-COVID-19: A hybrid deep learning architecture for the diagnosis of COVID-19 disease based on gravitational search optimization algorithm,” 2020, arXiv:2004.05084. [Online]. Available: http://arxiv.org/abs/2004.05084 [DOI] [PMC free article] [PubMed]
- [139].Rajaraman S., Siegelman J., Alderson P. O., Folio L. S., Folio L. R., and Antani S. K., “Iteratively pruned deep learning ensembles for COVID-19 detection in chest X-rays,” IEEE Access, vol. 8, pp. 115041–115050, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Singh M. and Bansal S.. (2020). Transfer Learning Based Ensemble Support Vector Machine Model for Automated COVID-19 Detection Using Lung Computerized Tomography Scan Data. [Online]. Available: https://assets.researchsquare.com/files/rs-32493/v1_stamped [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Alom M. Z., Rahman M. M. S., Nasrin M. S., Taha T. M., and Asari V. K., “COVID_MTNet: COVID-19 detection with multi-task deep learning approaches,” 2020, arXiv:2004.03747. [Online]. Available: http://arxiv.org/abs/2004.03747
- [142].Rajaraman S. and Antani S., “Weakly labeled data augmentation for deep learning: A study on COVID-19 detection in chest X-rays,” Diag., vol. 10, no. 6, May 2020, Art. no. 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bai H. X., “AI augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other etiology on chest CT,” Radiology, vol. 7, Apr. 2020, Art. no. 201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Waheed A., Goyal M., Gupta D., Khanna A., Al-Turjman F., and Pinheiro P. R., “CovidGAN: Data augmentation using auxiliary classifier GAN for improved COVID-19 detection,” IEEE Access, vol. 8, pp. 91916–91923, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wang X., Deng X., Fu Q., Zhou Q., and Feng J., “A weakly-supervised framework for COVID-19 classification and lesion localization from chest CT,” IEEE Trans. Med. Imag., vol. 39, no. 8, pp. 2615–2625, May 2020. [DOI] [PubMed] [Google Scholar]
- [146].Hu S., Gao Y., Niu Z., Jiang Y., Li L., Xiao X., Wang M., Fang E. F., Menpes-Smith W., Xia J., Ye H., and Yang G., “Weakly supervised deep learning for COVID-19 infection detection and classification from CT images,” IEEE Access, vol. 8, pp. 118869–118883, 2020. [Google Scholar]
- [147].Abbas A., Abdelsamea M. M., and Gaber M., “4S-DT: Self supervised super sample decomposition for transfer learning with application to COVID-19 detection,” 2020, arXiv:2007.11450. [Online]. Available: http://arxiv.org/abs/2007.11450 [DOI] [PMC free article] [PubMed]
- [148].Stochiáoiu R. D., Petrica M., Rebedea T., Popescu I., and Leordeanu M., “A self-supervised neural-analytic method to predict the evolution of COVID-19 in Romania,” 2020, arXiv:2006.12926. [Online]. Available: http://arxiv.org/abs/2006.12926