Abstract
Drought adversely affects the yield, quality and nutritional value of the crop plants. Additionally, it causes unrest in society and economic loss to the farmers and governments. The present study involved the applications of nanoparticles to induce drought tolerance and improve yield in the wheat (Triticum aestivum L.) plants. Green chemical methods were used for the synthesis of silver nanoparticles (AgNPs) and copper nanoparticles (CuNPs). CuNPs were used in 0, 3, 5 and 7 mg/L and 0, 10, 20 and 30 mg/L of AgNPs were tested at −4, −6 and −8 bars osmotic potential in laboratory experiments and 40%, 60% and 80% field capacity (FC) in the glasshouse experiments were maintained. The solution culture experiments revealed significantly higher chlorophyll stability index (CSI), leaf succulence (LS) and leaf K (LK) content in plants treated with 03 mg/L of CuNPs and 10 mg/L of AgNPs, indicated the positive role of CuNPs and AgNPs in drought tolerance of wheat. A similar trend was observed for stomatal conductance (SC) and morphological parameters with the applications of Cu and Ag nanoparticles at different levels of field capacity. The results of this study provide the experimental evidence to use CuNPs and AgNPs to induce drought resistance and improve yield in the wheat plants by a satisfactory increase in nutrients uptake and water retention.
1. INTRODUCTION
Food security is an important issue in the modern world. Loss of the food during the process of cultivation, wrong seed selection, natural disasters and the poor environmental conditions of an area play a major role to affect the crop physiology that ameliorates grain yield and the quality of the product [1]. Drought is abiotic stress and affecting the wheat crop worldwide. It is one of the leading causes of food insecurity, an increase in inflation, the poor mental stability of the farmers and leads to a great loss to the economy of a country [2]. The literature has many reports which show that the drought can lead to an increase in the accumulation of the heavy metals in crops that eventually affect the crop quality and the quantity [3]. It was observed that most of the changes in the climate of an area are mainly associated with global warming and drought. It was predicted that in the first quarter of the 21st century about 1.8 billion world population will be under absolute water shortage and scarcity, while 65% of the population will be under partial‐water shortage, which is a very big and alarming number [4].
Wheat is a very important staple food, inhabitants of many regions including South Asia, America and Europe, use wheat and the product of the wheat to fulfil their nutritional requirements [5]. The importance of wheat as a staple food necessitates utilising modern technologies to boost wheat production and nutritional values [6]. An important constraint for low productivity is the plant stress which can be managed through nanoparticle applications [7]. The most important abiotic stress for wheat in semi‐arid regions is drought. In Pakistan, 15 million hectares area under wheat cultivation, is being adversely affected by the drought [8].
Nanotechnology is a modern discipline of science that has applications in almost every field including agriculture [9]. The use of nano‐fertilisers, nano‐pesticides, nano‐biosensors, nano‐metrological instruments are helping farmers to not only enhance the crop yield and quality by improving the soil conditions but it also helps by predicting the weather forecast of an area and using sensors for automation of agriculture sector [10, 11, 12]. The green synthesis of metal nanoparticles has advantages over using hazardous chemicals because of the ease of reaction conditions, energy‐intensive, cost‐effective and results in the synthesis of biocompatible nanoparticles [13]. The nanoparticles promote the uptake of nitrate reductase enzyme in the embryo which enhances the abilities of seeds' to absorb a great amount of water and other nutrients. The water absorbance plays a promising role to break the seed coat and the nutrients uptake help plants to overcome drought [11].
Cu is a very important naturally occurring plant micronutrient that acts as an enzyme and co‐enzyme in the various metabolic processes of plants. Cu plays a crucial role to help lignification of cell walls via the polyphenol metabolism, and thus influences the water balance in plants. It also contributes towards the process of photosynthesis, respiration, carbohydrate synthesis and protein metabolism [14, 15, 16]. Cu also contributes greatly to chlorophyll biosynthesis [17].
Ag is another important metal and is bactericidal and antimicrobial. It plays a promising role to control the pests’ population because of its cytotoxic nature but the limited amount of Ag can be beneficial and stimulate the process of growth and development [18]. Silver contributes greatly to bioremediate soil. AgNPs play a promising role to improve seed germination, plant growth, photosynthetic efficiency and curb microbial growths on plant body [19].
The physical, optical and chemical properties of the elements change remarkably by their conversion into nanoforms [20]. Nanoparticles greatly influence the physiological and morphological states of the crop plants and it also helps to improve the soil conditions. Different concentrations of NPs have a different impact on plants' quality and growth parameters [5, 6].
Some studies were performed previously to study the effects of CuNPs and AgNPs to increase drought resistance in wheat plants [16, 21, 22, 23]. However, they did not perform in‐depth analysis to provide detailed information regarding the effects of nanoparticles on physiological and morphological traits of wheat plants. The present study was designed to apply different concentrations of CuNPs and AgNPs in soil and hydroponic solution to induce drought resistance in the wheat plants under osmotic stress and evaluation of stimulatory effects of nanoparticles to promote yield under different field capacities. The schematic layout of the work is given in Figure 1.
FIGURE 1.

Schematic layout of the work
2. MATERIALS AND METHODS
2.1. Green synthesis of silver and copper nanoparticles and spectrophotometric analysis
AgNPs were prepared by the reduction of silver nitrate (AgNO3) by tri‐sodium citrate dihydrate (Na3C6H5O7.2H2O). A total of 157.4 mg of AgNO3 was reduced by using 294 mg of sodium citrate through continuous stirring (600–700 rpm) at 70°C on a magnetic stirrer [24, 25, 26].
CuNPs were synthesised by following the plant‐mediated green synthesis method. Onion (Allium cepa. L.) aqueous extract was used as an agent to reduce and stabilise CuSO4.5H2O into a nano‐copper. An onion extract was prepared in a 1 to 10 ratio of the plant material and distilled water. Five hundred milligram of CuSO4 was dissolved per litre of distilled water. This solution was reduced by the stepwise addition of 250 ml of onion extract along with the continuous stirring (300–400 rpm) by magnetic stirrer at 100 °C on a water bath [14, 15, 16].
Initially, the synthesis of AgNPs and CuNPs was confirmed by observing a change in the colour of the reaction mixture followed by measuring the absorbance of light wavelength between 200 and 900 nm by using a UV‐Visible spectrophotometer (Shimadzu 1601, Japan) [27].
2.2. Scanning electron microscopic imaging and particle size analysis
Structural evaluation of nanoparticles was performed on a scanning electron microscope (SEM) JEOL 7500F HRSEM. Copper grids were used to mount the sample by using the drop coating method [13].
The size distribution of nanoparticles and zeta potential (ionic charge) was evaluated by using a dynamic light scattering technique (Malvern Instruments particle sizer, Zetasizer Nano S, Malvern Instruments, UK). The sample was prepared in Milli‐Q® water [28].
2.3. Preparation of plant specimens and experimental setup
Experiments were conducted to determine the effects of AgNPs and CuNPs on wheat seedlings grown under various osmotic stress conditions and on yield parameters. The seeds of wheat cultivar Punjab‐2011 were obtained from the National Agriculture Research Centre (NARC). The seeds were sterilised with ethanol for 5 min and chemicals from the seed surfaces were removed through washing with the distilled water. The seedlings were then shifted to the chiller for cold treatment for 3–4 days at chilling temperature to enhance seed germination.
2.4. Measurement of physiological parameters
After processing, the seeds were transferred to the plastic pots filled with a known volume of the MS medium (Murashige and Skoog) containing 10, 20 and 30 mg/L of AgNPs and 3, 5 and 7 mg/L of CuNPs and osmotic stress of −6, −8 and −10 bars were induced by mixing polyethylene glycol (PEG‐6000) in liquid medium at 20 °C and was measured by using an osmometer. The NPs were applied hydroponically in MS medium of the plant pots. The treatments were arranged according to two factorial fashion using a completely randomised design (CRD) in three replicates [5, 8, 29, 30].
2.4.1. Plant physiological parameters
The data on chlorophyll contents (CC), chlorophyll stability index (CSI), leaf succulence (LS) and leaf K (LK) contents of wheat plants treated hydroponically with CuNPs and AgNPs were recorded.
2.4.2. Chlorophyll contents measurement
The CC were measured by SPAD‐502, Konica Minolta Sensing, Inc. Japan. The CC of the wheat plant were measured after 80 days of plant growth. Plant leaves were extracted with the solution of (80% v/v) acetone and the temperature was maintained at 4 °C for 24 h under dark which was followed by observing samples on a spectrophotometer at 470, 647 and 664.5 nm [29].
2.4.3. Chlorophyll stability index measurement
The wheat leaf samples were collected after 80 days of cultivation. One gram of fresh leaf samples were heated at 65 °C in 25 ml of distilled water on the water bath. The chlorophyll was extracted by using 80% of acetone in 40 ml quantity and blended for 5 min and filtered by using a Whatman filter paper. The filter paper was washed thrice to remove any residues of the chlorophyll from the surface. The extracted samples were read at 660 nm wavelength of light. The CSI was calculated by using the following formula [31];
2.4.4. Leaf succulence measurement
The leaf area, fresh and dry weight of a known mass of leaves were recorded. The leaves were subjected to oven drying at 70 °C for a period until no further reduction in weight was recorded. The LS was recorded by using the following equation [32];
2.4.5. Leaf potassium contents and stomatal conductance measurement
The leaf potassium contents were determined with a flame photometer using a standard procedure [14] and stomatal conductance (SC) was measured by Leaf Porometer (AP4) at a flag leaf stage.
2.5. Measurement of morphological parameters
The greenhouse experiments were conducted to document the effects of various concentrations of AgNPs and CuNPs on wheat growth and yield attributes grown under different field capacity (FC) levels. The seedlings were shifted to earthen pots (10 seedlings per pot) in a greenhouse after cold treatment containing 0.58 g of urea (46% N), 0.52 g of DAP (18% N, 46% P2O5) and 0.46 g of KCl (52% K2O). The pots were filled with a known mass of soil (12 kg) and FC was determined with the gravimetric method. The FC levels included 40%, 60% and 80% and 0, 10, 20 and 30 mg/L concentrations of AgNPs and 0, 3, 5 and 7 mg/L concentrations of CuNPs were applied. The seedlings were thinned to five per pot at 40 days after shifting. The treatments were arranged according to CRD factorial design. The yield attributes such as spike length, number of spikelets and grains per spike, hundred grains weight and grains yield per pot were recorded [19, 33, 34].
2.6. Statistical analysis
All experiments were performed in triplicates. The mean and SD of the results was calculated. The multifactorial analysis of variance (ANOVA) was performed by using Statistix 8.1® software. The data was further subjected to perform the least significance difference (LSD) test at 5% probability level for parameters for which the F value was found significant.
3. RESULTS AND DISCUSSION
3.1. Synthesis of Ag and Cu nanoparticles and spectrophotometric analysis
Change in the colour of the reaction mixture was considered as an initial sign for the synthesis of CuNPs and AgNPs which is a response of the interaction of nanoparticles with the electromagnetic light waves [35]. The results of the spectrophotometric analysis expressed a surface plasmon resonance (SPR) band at 485 and 550 nm characteristic to manifest the synthesis of AgNPs and CuNPs, respectively (Figure 2(a, b). The SPR band is a result of the oscillating electromagnetic light waves with the vibrating free electrons of AgNPs and CuNPs [13]. A characteristic greenish‐yellow and brown colour were observed from CuNPs and AgNPs, respectively (inset Figure 3).
FIGURE 2.
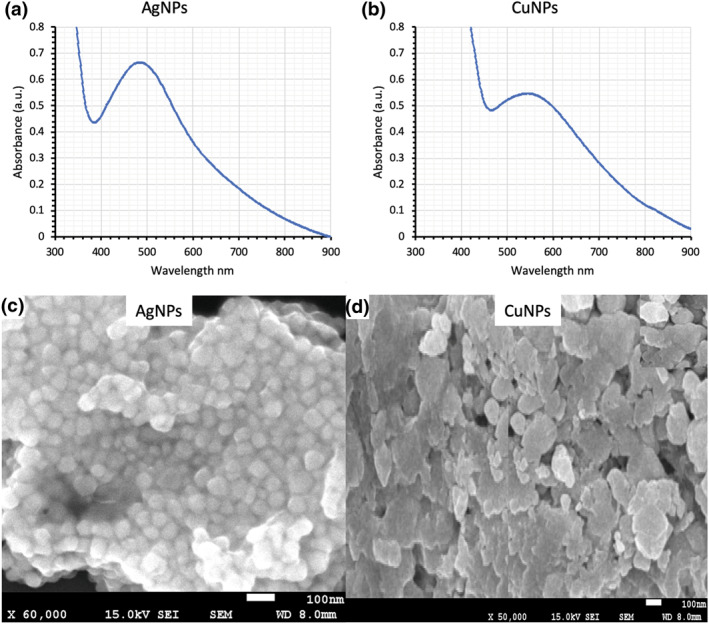
Spectrophotometric analysis of (a) AgNPs and (b) CuNPs. Scanning electron microscopic images of (c) AgNPs and (d) CuNPs
FIGURE 3.
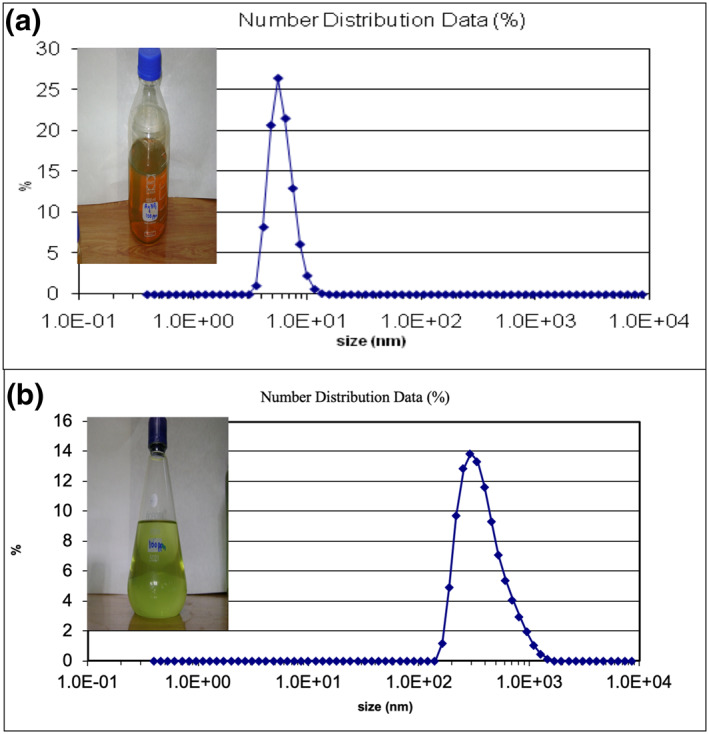
Particle size analysis of (a) AgNPs and (b) CuNPs by using the DLS technique. Insets are showing the colloidal solution of Ag and Cu NPs
A previously published paper expressed the characteristic SPR band between 400 and 500 nm which represents the synthesis of AgNPs [27] while the SPR band at 650 nm was observed to confirm the synthesis of CuNPs which is in favour of our study [36].
3.2. Structural evaluation and particle size analysis of nanoparticles
The SEM images of AgNPs expressed that they are anisotropic, nearly spherical while some cubical nanoparticles were also found (Figure 2(c)). The CuNPs were observed anisotropic and polydisperse (Figure 2(d)). The particle size analysis of nanoparticles in Brownian motion was performed by using a dynamic light scattering technique and it was observed that the AgNPs have a size of ≤10 nm (Figure 3(a)) while CuNPs existed between 100 and 1000 nm (Figure 3(b)).
The particle size analysis was performed at the room temperature after synthesis and with a gap of 3 months after synthesis by using the DLS machine to check the stability of nanoparticles. There was no change in the size of nanoparticles before and after a gap of three months which represents the stability of nanoparticles. The stability of nanoparticles was further confirmed by measuring the zeta potential (ζ). The ionic charge on the surface of AgNPs was measured at −12.79 mV, which shows that the AgNPs have a negative charge on their surface which provides them the repulsive forces and help them to stay stable for a longer period. The zeta potential of CuNPs was measured at −54.29 mV. The negative value of the CuNPs zeta potential also represents that the nanoparticles are stable. Our results are in favour of previously published literature [35, 37].
These results show that the nanoparticles are small and stable enough to be used for biological applications on crop plants. The small size of nanoparticles helps them to absorb easily on the cell's surface and pass the plasma cell membrane barrier to perform their biochemical functions.
3.3. Effects of CuNPs and AgNPs on various physiological parameters of wheat under different osmotic stress levels
Water deficiency is a major problem in the modern world. The use of classical agronomic practices is obsolete and are not much futile to control drought [8, 38, 39]. The researchers are focusing on nanotechnology due to their diverse applications to treat drought which is increasing day by day due to upheave in population and global warming [40]. The data presented in Tables 1 and 2, show the effects of CuNPs and AgNPs on chlorophyll stability (CSI), LS and LK contents (LK) under different osmotic stress levels (−6, −8 and −10). It was observed that different concentrations of AgNPs (0, 10, 20 and 30 mg/L) and CuNPs (0, 3, 5 and 7 mg/L) under different osmotic stress levels have a very different effect on physiological attributes of the wheat plants which mainly affect the quality and quantity of the seeds. The concentration of the CuNPs and AgNPs were designed according to the response of the wheat plants to the initial treatment received.
TABLE 1.
Effect of CuNPs on various physiological traits under different levels of osmotic stress
| Conc. (mg/L) | Chlorophyll stability index (%) | Means | Leaf succulence (mg/cm2) | Means | Leaf K (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stress (bars) | Stress (bars) | Stress (bars) | ||||||||||
| −6 | −8 | −10 | −6 | −8 | −10 | −6 | −8 | −10 | Means | |||
| 0 | 48.33bc | 40.00efg | 35.33gh | 41.22b | 10.60b | 9.37bc | 5.97ef | 8.64b | 1.15b | 1.10bc | 0.87de | 1.04b |
| 3 | 58.30a | 52.00b | 46.58bcd | 52.32a | 12.87a | 10.33b | 5.93ef | 9.71a | 1.34a | 1.11b | 0.96cd | 1.14a |
| 5 | 45.33cde | 42.55def | 39.73fg | 42.54b | 9.07bc | 8.00cd | 4.90f | 7.32c | 1.08bc | 1.05bc | 0.65f | 0.93c |
| 7 | 36.67gh | 32.33h | 31.67h | 33.56c | 8.60c | 6.53de | 4.70f | 6.61c | 0.87de | 0.80e | 0.50g | 0.72d |
| Means | 47.18a | 41.72b | 38.33c | 10.28a | 8.56b | 5.38c | 1.11a | 1.02b | 0.75c | |||
| LSD (conc.) = 3.23 | LSD (conc.) = 0.92 | LSD (conc.) = 0.08 | ||||||||||
| LSD (stress) = 2.80 | LSD (stress) = 0.80 | LSD (stress) = 0.07 | ||||||||||
| LSD (conc. × stress) = 5.60 | LSD (conc. × stress) = 1.59 | LSD (conc. × stress) = 0.14 | ||||||||||
Means values denoted by various lower case alphabets differed significantly at 5% probability levels using the LSD test (p < 0.05).
TABLE 2.
Effect of AgNPs on various physiological traits under different levels of osmotic stress
| Conc. (mg/L) | Chlorophyll stability index (%) | Means | Leaf succulence (mg/cm2) | Means | Leaf K (mg/kg) | Means | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stress (bars) | Stress (bars) | Stress (bars) | ||||||||||
| −6 | −8 | −10 | −6 | −8 | −10 | −6 | −8 | −10 | ||||
| 0 | 57.00b | 52.67bc | 47.67cd | 52.44b | 11.67de | 9.53fgh | 7.63hi | 9.61c | 1.54b | 1.35cd | 1.19ef | 1.36b |
| 10 | 66.69a | 62.80a | 56.54b | 62.01a | 17.63a | 15.30b | 8.50gh | 13.81a | 1.84a | 1.54b | 1.42bc | 1.60a |
| 20 | 48.67cd | 47.86cd | 44.94d | 47.16c | 14.53bc | 12.70cd | 7.57hi | 11.62b | 1.53b | 1.23de | 1.13ef | 1.29b |
| 30 | 36.67e | 34.31ef | 29.00f | 33.33d | 11.40def | 9.90efg | 6.30i | 9.20c | 1.42bc | 1.15ef | 1.09f | 1.22c |
| Means | 52.26a | 49.41b | 44.54c | 13.81a | 11.88b | 7.50c | 1.58a | 1.32b | 1.21c | |||
| LSD (conc.) = 3.23 | LSD (conc.) = 1.06 | LSD (conc.) = 0.07 | ||||||||||
| LSD (stress) = 2.80 | LSD (stress) = 1.23 | LSD (stress) = 0.07 | ||||||||||
| LSD (conc. × stress) = 5.60 | LSD (conc. × stress) = 2.13 | LSD (conc. × stress) = 0.13 | ||||||||||
Means values denoted by various lower case alphabets differed significantly at 5% probability levels using the LSD test (p < 0.05).
The wheat plants were introduced to drought stress by increasing the osmotic potential by the application of polyethylene glycol to the hydroponic culture medium. It was observed that the control experimental plants grown in drought stress had drastic effects on physiological parameters and they declined gradually with increasing the osmotic potential. The CSI, LS and leaf potassium contents showed a marked decrease from 48.33% to 35.33%, 10.60 to 5.97 mg/cm2 and 1.15 to 0.87 mg/kg, respectively when no CuNPs treatment was given (p < 0.05). The growth of wheat plants increased remarkably which have shown in the form of an increase in physiological parameters when 03 mg/L colloidal solution of CuNPs were used (Figures 4 and 5). The CSI, LS and LK contents increased at 03 mg/L and better growth was observed at −6 bar osmotic potential which decreased gradually by increasing osmotic stress. At the same time, the CuNPs treated plants exhibit better stress tolerance represented by physiological traits. The CSI was at maximum by using 03 mg/L of CuNPs and decreased at higher concentrations. It was increased by 20.6% at −6 bars, 30.0% at −8 bars and 31.8% at −10 bars regarding their respective controls (p < 0.05). The highest chlorophyll stability index (58.30%) was recorded at −6 bars when 03 mg/L of CuNPs were used and the lowest values were produced from 7 mg/L of CuNPs along with three osmotic stress levels. The application of CuNPs improved the LS under osmotic stress conditions. Like CSI, 03 mg/L was again the optimum CuNPs concentration for improving LS. Although improvement in LS was gradually decreased at higher osmotic stress. The leaf potassium contents were improved by applying 03 mg/L of CuNPs. It was maximum (1.34 mg/kg) at −6 bars and decreased at higher osmotic stress levels (p < 0.05). It indicates that 03 mg/L concentration of CuNPs improved the potassium uptake and mobility in plants. These parameters modulate photosynthesis directly or indirectly and help plants to tolerate drought stress. The K plays a very important role to maintain turgor pressure. It also contributes to the ionic exchange and opening and closing of the stomatal guard cells. The K also acts as a co‐enzyme. The leaf potassium contents determine the cell's turgor pressure, stomatal activity and water relation. The decreased leaf potassium contents are due to reduced potassium mobility in plants [4]. The plants treated with 03 mg/L of CuNPs improved the CSI and SC under drought conditions over control. Some previous studies manifested Cu as a very important micronutrient that contributes to its potential in the hydrolysis of water during the process of photosynthesis. The copper is taken up by wheat plants in the form of Cu2+. The copper is a very important component of plastocyanin in photosynthetic electron transport chains of photosystems. The photosynthetic cells usually have higher copper contents. The potential of Cu as an electron carrier in redox reactions of the electron transport chain also helps plants to tolerate drought stress conditions [17]. It is also evident that Cu contributes to plant growth and help in the process of wheat reproduction [41].
FIGURE 4.
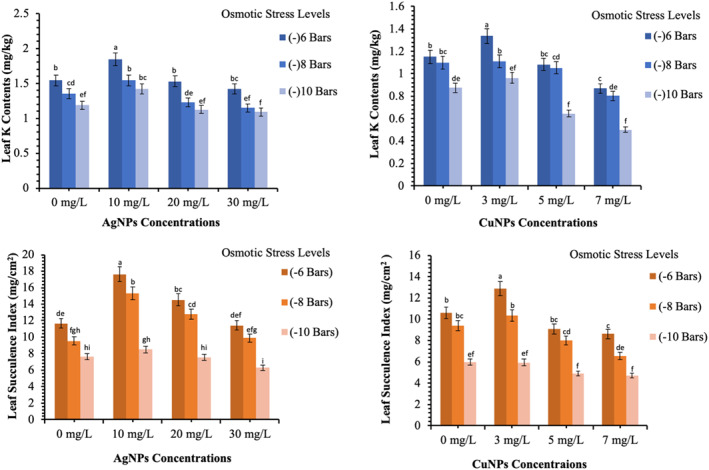
Effects of the AgNPs and CuNPs on the physiological characteristics of wheat under osmotic stress. Different alphabets represent that results are significantly different (p < 0.05)
FIGURE 5.
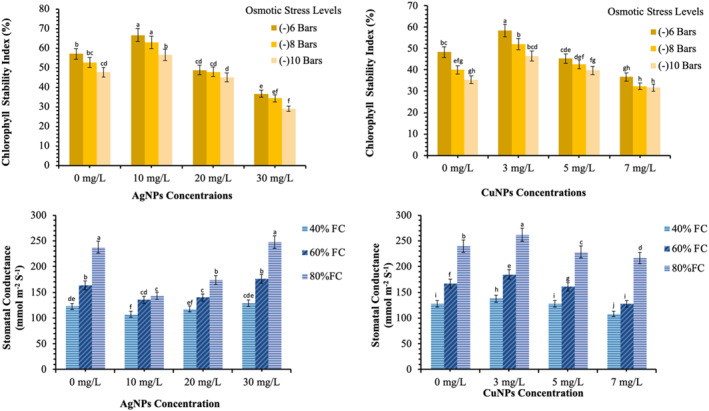
Effects of the AgNPs and CuNPs on the physiological characteristics of wheat under different osmotic stress levels. Different alphabets represent that results are significantly different (p < 0.05)
The application of 10 mg/L of AgNPs improved the CSI, LS and leaf potassium contents over control from 52.44% to 62.01%, 9.61 to 13.8 mg/cm2 and 1.36 to 1.60 mg/kg, respectively (Table 2) (p < 0.05). The application of 10 mg/L of AgNPs at −6, −8 and −10 bars produced 19.48%, 14.07% and 19.32% of LK contents over their respective control (Figures 4 and 5) (p < 0.05).
The higher concentration of AgNPs beyond 10 mg/L, decreased the CSI. The lowest (29.00%) and highest (66.69%) CSI were recorded for 30 mg/L of AgNPs at −10 bars and 10 mg/L of AgNPs at −6 bars osmotic potential, respectively (p < 0.05). A similar trend was followed for LS. The maximum LS (17.63 mg/cm2) was reported by using 10 mg/L of AgNPs at −6 bars and minimum LS (6.30 mg/cm2) was observed by using 10 mg/L of AgNPs at −10 bars of osmotic potential. The leaf potassium contents were similarly affected by AgNPs applications at various stress levels. The treatment of wheat plants with silver nanoparticles can improve the electron exchange efficiency in cells which reduces the formation of reactive oxygen species (ROS) through arresting the electron leakage. The sharp increase in chlorophyll b and substantial improvement in chlorophyll a content in silver nanoparticles treated seedlings was also mentioned in previous reports [11, 39].
The plants respond to various environmental stresses through modification in an internal process. The wheat sown under different osmotic stress levels led to decreased physiological and morphological attributes [16, 30, 31]. The reduced values for leaf chlorophyll and LS under drought conditions have already been confirmed. The LS is positively influenced by leaf water potential which decreases at limited moisture supply. The control experimental plants without nanoparticles reported significantly low physiological parameters (p < 0.05). The drought reduced the growth of wheat. However, the nanoparticles application at certain concentrations was very helpful to induce drought tolerance. It may be because small size nanoparticles that easily enter the epidermal cells for osmotic adjustments [1, 5, 11, 29].
3.4. Effects of CuNPs and AgNPs on stomatal conductance of wheat at different field capacities
SC is an important process to determine the photosynthetic rate and most likely to be affected first in drought. The SC also plays a very important role to determine the efficiency of the stomatal apparatus. It also helps to explain the photosynthetic abilities and respiratory quotient of a particular plant. The physiological stress including drought greatly alters the SC which affects the growth and the quality of the crop plant. The SC was decreased at low field capacity (Figure 5). The CuNPs applications mitigated the adverse effects of drought by improving the SC by 8.0%, 10.5% and 9.3% at 40%, 60% and 80% FC levels, respectively (p < 0.05); 3 mg/L of CuNPs found very effective to increase the SC at all field capacities. The highest values for stomatal conductance (262.12 mmol m−2 s−1) was observed by using 03 mg/L of CuNPs at 80% FC. However, the further increase in the concentration beyond 03 mg/L, significantly reduced the stomatal conductance (p < 0.05).
The data presented in Figure 5 indicate the significant differences between AgNPs‐treated plants and control for the stomatal conductance (p < 0.05). In contrast to CuNPs, the AgNPs were inhibitory at lower concentrations and stimulatory at higher concentrations [4, 5, 6, 11]. Therefore, plants treated with 30 mg/L of AgNPs produced the maximum figures regarding the SC.
3.5. Effects of CuNPs and AgNPs on morphological parameters of wheat under different field capacities
The morphological attributes of the plants change with physiological mechanisms. The morphological traits of the wheat plants were measured in terms of spike length, spikelets per spike, the number of the grains per spike, weight of the grains and number of the grains per pot (Figures 6 and 7). It was observed that increasing the FC by the addition of fertilisers along with the application of the CuNPs and AgNPs greatly improved the morphological parameters. The highest values for spike length (12.67 cm), spikelets per spike (12.33 spike−1), gains per spike (17.33 spike−1), 100‐grains weight (10.50 g) and grains yield per pot (14.59 g pot−1) was achieved by the application of 03 mg/L of CuNPs at 80% FC. Non‐significant differences at 05 mg/L of CuNPs and control were observed at 40% and 60% of FC. The plants treated with 03 mg/L of CuNPs significantly improved the yield by 22.3%, 43.4% and 18.0% with respect to control at 40%, 60%, and 80% FC levels, respectively (p < 0.05).
FIGURE 6.
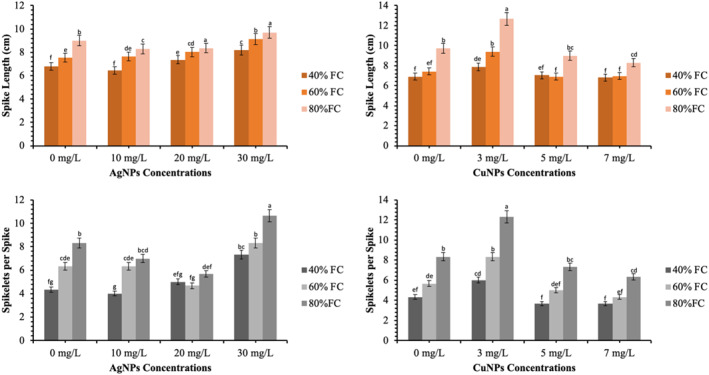
Effects of the AgNPs and CuNPs on the morphological characteristics of wheat under different field capacities (FC) levels. Different alphabets represent that results are significantly different (p < 0.05)
FIGURE 7.
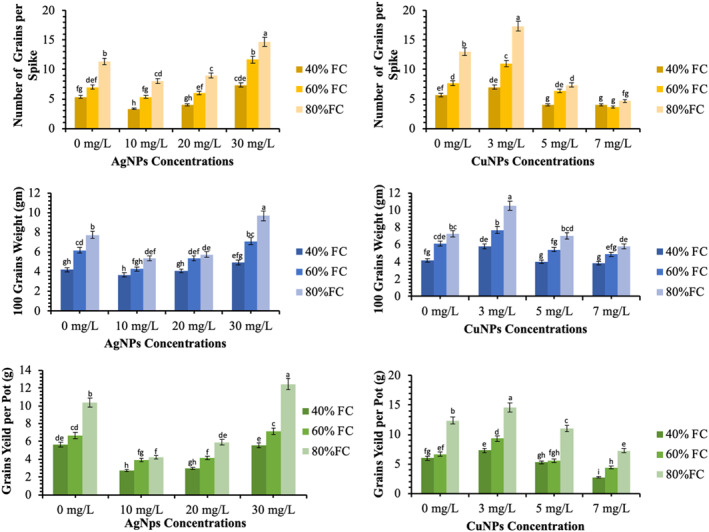
Effects of the AgNPs and CuNPs on the morphological characteristics of wheat under different field capacities (FC) levels. Different alphabets represent that results are significantly different (p < 0.05)
The data presented in Figures 6 and 7 indicate significant differences between AgNPs‐treated plants and control for grain yield (p < 0.05). In contrast to CuNPs, the AgNPs were inhibitory at low concentrations and stimulatory at the higher concentrations. Therefore, plants treated with 30 mg/L of AgNPs produced the maximum figures regarding morphological parameters. However, the 30 mg/L of AgNPs produced significant differences over control for the morphological growth components (p < 0.05). Figure 8 represents the photographic comparison of different morphological traits of the wheat plants after soil application of CuNPs and AgNPs.
FIGURE 8.

Photographic images showing a difference in the growth of wheat plants with and without treatment (a) treatment of plants with 30 mg/L of AgNPs at 80% FC (b) treatment of plants with 3 mg/L of CuNPs at 80% FC
It can be concluded that the increase in the CC, CSI, LS and LK contents due to the applications of CuNPs and AgNPs, have a strong impact on the morphological parameters of the wheat plants. Our results are in favour of some previously published scientific reports [4, 14, 39, 40, 42, 43].
4. CONCLUSION
Herein, we reported the hydroponic applications of CuNPs and AgNPs in different concentrations to induce drought tolerance in wheat plants grown under different osmotic stress levels and soil applications of nanomaterials to improve the yield at varying field capacities. An increase in the physiological parameters such as CSI, LS and LK contents was observed by the application of nanoparticles which indirectly maintained plant morphological characteristics favouring the use of CuNPs and AgNPs to induce drought resistance in wheat. The CuNPs at a low concentration of 03 mg/L and the highest field capacity (80%) improved morphological attributes of plants significantly different from the control (p < 0.05). Unlikely, AgNPs showed inhibitory effects at the lowest concentration (10 mg/L) and stimulatory or growth‐promoting effects at the higher concentration (30 mg/L) to improve the yield. Even a very slight increase in the growth of plants under drought by using NPs can be very promising to increase the yield per hector, decrease the food scarcity, destress farmers, can increase export and add a marginal digit to the country's GDP.
Farooq Ahmed and Bilal Javed contributed equally to this study, so consider both as first authors.
REFERENCES
- 1. Afzal, S. , et al.: A comparative screening of abiotic stress tolerance in early flowering rice mutants. J. Biotechnol. 302(July), 112–122 (2019) [DOI] [PubMed] [Google Scholar]
- 2. Ghasemlou, F. , Amiri, H. : Alleviation of drought stress effects on Verbascum nudicuale by methyl jasmonate and titanium dioxide nanoparticles. Alleviation of the effects of on drought stress Verbascum nudicuale by methyl jasmonate and titanium dioxide nanoparticles. Iran. J. Plant Physiol. 9(8), 2911–2920 (2019) [Google Scholar]
- 3. Rastogi, A. , et al.: Application of silicon nanoparticles in agriculture. 3 Biotech. 9(3), 1–11 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sallam, A. , et al.: Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 20(13), 1–36 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathur, S. , Raikalal, P. , Jajoo, A. : Physiological Responses of Wheat to Environmental Stresses (2019) [Google Scholar]
- 6. Thorny Chanu, T. , Upadhyaya, H. : Zinc Oxide Nanoparticle‐Induced Responses on Plants. Elsevier Inc. (2019) [Google Scholar]
- 7. Karamian, R. , Ghasemlou, F. , Amiri, H. : Physiological evaluation of drought stress tolerance and recovery in Verbascum sinuatum plants treated with methyl jasmonate. salicylic acid and titanium dioxide nanoparticles. Plant Biosyst. 154(3), 277–287 (2019) [Google Scholar]
- 8. Adrees, M. , et al.: Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere. 238 (2020) [DOI] [PubMed] [Google Scholar]
- 9. Shukla, P. , et al.: Nanotechnology in sustainable agriculture: studies from seed priming to post‐harvest management. Nanotechnol. Environ. Eng. 4(1), 1–15 (2019) [Google Scholar]
- 10. Dimetry, N.Z. , Hussein, H.M. : Role of nanotechnology in agriculture with special reference to pest control. Int. J. PharmTech Res. 9(10), 121–144 (2016) [Google Scholar]
- 11. Khan, Z. , Upadhyaya, H. : Impact of Nanoparticles on Abiotic Stress Responses in Plants. Elsevier Inc. (2019) [Google Scholar]
- 12. Manna, I. , Bandyopadhyay, M. : A review on the biotechnological aspects of utilizing engineered nanoparticles as delivery systems in plants, vol. 17, pp. 100167. Elsevier B.V. (2019) [Google Scholar]
- 13. Javed, B. , et al.: Understanding the potential of bio‐fabricated non‐oxidative silver nanoparticles to eradicate Leishmania and plant bacterial pathogens. Appl. Nanosci. 10(6), 2057–2067 (2020) [Google Scholar]
- 14. Cumplido‐Nájera, C.F. , et al.: The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Hortic. (Amsterdam). 245, 82–89 (2019) [Google Scholar]
- 15. Tamez, C. , et al.: Uptake, transport, and effects of nano‐copper exposure in zucchini (Cucurbita pepo). Sci. Total Environ. 665, 100–106 (2019) [DOI] [PubMed] [Google Scholar]
- 16. Taran, N. , et al.: Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 12(60), 1–6 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Droppa, M. , Horváth, G. : The role of copper in photosynthesis. Plant Sci. 2008, 37–41 (2013) [Google Scholar]
- 18. Javed, B. , Nadhman, A. , Mashwani, Z.U.R. : Phytosynthesis of Ag nanoparticles from Mentha longifolia : their structural evaluation and therapeutic potential against HCT116 colon cancer, Leishmanial and bacterial cells. Appl. Nanosci. (2020) [Google Scholar]
- 19. Jhanzab, H.M. , et al.: Proteomic analysis of the effect of inorganic and organic chemicals on silver nanoparticles in wheat. Int. J. Mol. Sci. 20(4), 1–19 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Javed, B. , et al.: One‐pot phytosynthesis of nano‐silver from Mentha longifolia L.: their characterization and evaluation of photodynamic potential. Mater. Res. Express Pap. 7(5), 1–9 (2020) [Google Scholar]
- 21. Almutairi, Z.M. , Alharbi, A. : Effect of silver nanoparticles on seed germination of crop plants. J. Adv. Agric. 10(1), 283–288 (2015) [Google Scholar]
- 22. Hafeez, A. , et al.: Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 1(1), 6–11 (2015) [Google Scholar]
- 23. Yasmeen, F. , et al.: Effect of silver, copper and iron nanoparticles on wheat germination. Int. J. Biosci. 6(4), 112–117 (2015) [Google Scholar]
- 24. Singh, R. , et al.: Bacteriagenic Silver Nanoparticles: Synthesis, Mechanism, and Applications, vol. 99, pp. 4579–4593. Springer Verlag; (2015) [DOI] [PubMed] [Google Scholar]
- 25. Behravan, M. , et al.: Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 124, 148–154 (2019) [DOI] [PubMed] [Google Scholar]
- 26. Parthiban, E. , et al.: Green synthesis of silver‐nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti‐microbial activity on human pathogens. Biotechnol. Reports. 21, e00297 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Javed, B. , Nadhman, A. , Mashwani, Z.U.R. : Optimization, characterization and antimicrobial activity of silver nanoparticles against plant bacterial pathogens phyto‐synthesized by Mentha longifolia. Mater. Res. Express. 7, 1–12 (2020) [Google Scholar]
- 28. Javed, B. , Mashwani, Z. : Synergistic effects of physicochemical parameters on bio‐fabrication of mint silver nanoparticles: structural evaluation and action against HCT116 colon cancer cells. Int. J. Nanomedicine. 15, 3621–3637 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan, Z.S. , et al.: The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 26(19), 19859–19870 (2019) [DOI] [PubMed] [Google Scholar]
- 30. Behboudi, F. , et al.: Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 42(13), 1439–1451 (2019) [Google Scholar]
- 31. Vinaya Rai, R.S. , Parthiban, K.T. : Studies on the drought tolerance of Eucalyptus at seedling stage. J. Trop. For. Sci. 8(2), 155–160 (1995) [Google Scholar]
- 32. Qi, C.H. , et al.: Increase in aquaporin activity is involved in leaf succulence of the euhalophyte Suaeda salsa under salinity. Plant Sci. 176(2), 200–205 (2009) [Google Scholar]
- 33. Ali, S. , et al.: Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 140(May), 1–8 (2019) [DOI] [PubMed] [Google Scholar]
- 34. Azeez, L. , et al.: Zero‐valent silver nanoparticles attenuate Cd and Pb toxicities on Moringa oleifera via immobilization and induction of phytochemicals. Plant. Physiol. Biochem. 139(March), 283–292 (2019) [DOI] [PubMed] [Google Scholar]
- 35. Nindawat, S. , Agrawal, V. : Fabrication of silver nanoparticles using Arnebia hispidissima (Lehm.) A. DC. root extract and unravelling their potential biomedical applications. Artif. Cells. Nanomedicine Biotechnol. 47(1), 166–180 (2019) [DOI] [PubMed] [Google Scholar]
- 36. Shende, S. , et al.: Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 31(6), 865–873 (2015) [DOI] [PubMed] [Google Scholar]
- 37. Kaur, P. , Thakur, R. , Chaudhury, A. : Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green. Chem. Lett. Rev. 9(1), 33–38 (2016) [Google Scholar]
- 38. Hojjat, S.S. : The Effect of silver nanoparticle on lentil Seed Germination under drought stress. Int. J. Farming Allied Sci. 208–212 (2016) [Google Scholar]
- 39. Dimkpa, C.O. , et al.: Zinc oxide nanoparticles alleviate drought‐induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 688, 926–934 (2019) [DOI] [PubMed] [Google Scholar]
- 40. Kranjc, E. , Drobne, D. : Nanomaterials in plants: a review of hazard and applications in the agri‐food sector. Nanomaterials. 9(8), 1–33 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown, J.C. , Clark, R.B . Copper as essential to wheat reproduction. Plant Soil. 523(5187), 509–523 (1977) [Google Scholar]
- 42. Dimkpa, C.O. , et al.: Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain Dev. 37(1), 1–13 (2017) [Google Scholar]
- 43. Malea, P. , et al.: Zinc uptake, photosynthetic efficiency and oxidative stress in the seagrass Cymodocea nodosa exposed to ZnO nanoparticles. Materials (Basel). 12(13), 1–15 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]


