Abstract
Published studies indicate that virtually any kind of botanical material can be exploited to make biocompatible, safe, and cost‐effective silver nanoparticles. This hypothesis is supported by the fact that plants possess active bio‐ingredients that function as powerful reducing and coating agents for Ag+. In this respect, a phytomediation method provides favourable monodisperse, crystalline, and spherical particles that can be easily purified by ultra‐centrifugation. However, the characteristics of the particles depend on the reaction conditions. Optimal reaction conditions observed in different experiments were 70–95 °C and pH 5.5–8.0. Green silver nanoparticles (AgNPs) have remarkable physical, chemical, optical, and biological properties. Research findings revealed the versatility of silver particles, ranging from exploitation in topical antimicrobial ointments to in vivo prosthetic/organ implants. Advances in research on biogenic silver nanoparticles have led to the development of sophisticated optical and electronic materials with improved efficiency in a compact configuration. So far, eco‐toxicity of these nanoparticles is a big challenge, and no reliable method to improve the toxicity has been reported. Therefore, there is a need for reliable models to evaluate the effect of these nanoparticles on living organisms.
1. INTRODUCTION
Nanotechnology is the study of extremely small entities, and it is interdisciplinary in nature including material science, biological, biotechnology, and engineering [1]. The value of metal NPs was USD 10.92 Billion, in 2016, and it is projected to increase up to USD 25.26 Billion, in 2022 [2]. Among the array of NPs, metallic AgNPs have significance in multiple areas such as in bio‐labelling, biosensors, and filters [3,4]. Their antimicrobial potency makes these particles efficient bio‐remediators, water purifiers, and antibiotics [5, 6, 7]. In this respect, several physical and chemical methods are available for the preparation of nano‐silver. However, these methods are prone to various snags such as elevated cost and energy dissipation, in addition to the use of certain noxious reagents as passivators (a type of corrosion inhibitors), particularly mercapto‐acetate, thiourea, and thiophenol [1,8,9].
Physical methods are more attractive because they lead to high purity AgNPs as compared with products of other methods. However, the primary hindrance in the application of these methods at an industrial scale is their high‐energy requirements and lower yield. Consequently, the green synthesis of metallic NPs is gaining attention among scientists because these nanoparticles are cheaper, easy to synthesis, and are eco‐friendly [10]. Moreover, the green synthesis of NPs can be easily scaled up to produce large quantities. In addition, no toxic stabilizers and reductants are involved during the green synthesis of AgNPs [11]. Furthermore, the principals of green chemistry are used for the fabrication and designing of the nanoscale products to avoid their harmful effects due to the extensive use of these products [12]. For the green synthesis of these nanoparticles, polysaccharides, bacteria, fungi, and plant extracts are used as stabilisers and reducing agents [13]. In this context, plant extracts are a very simple method for making nanoparticles because it does not involve complex process of preserving and culturing biotic cells. Moreover, silver nanoparticles obtained using plant extracts are harmless for the therapeutic practices in humans [14,15]. In addition, stabilisation, fabrication, and reduction of AgNPs using plant extracts can be achieved by using the biochemical constituents present in these extracts. Therefore, plants are considered as one of the best resources for large‐scale synthesis of metal oxides and metal nanoparticles with distinct size and morphology [16].
Recent findings revealed developed and exciting biological procedures to generate nano‐silver via microbes or botanic materials as potential bio‐reducers and bio‐cappers [13,17]. Along this line, AgNPs are used for precise bimolecular detection, antimicrobials, and diagnostics [18]. These particles possess several unique properties, which make them gaining the attention as antifungal, larviciadal, antibacterial, and antioxidant agents [19,20]. They are also being used in the field of solar cells, agriculture, pollution control, waste management, medicine, and forensic science [21]. In comparison with microbial biosynthesis, phyto‐synthesis has furnished a novel dimension to material chemistry by manufacturing biocompatible silver nano‐structures. This method is green, economical, and reproducible, due to the diversified availability of the materials, ease of manipulation, and minimal impediments [22, 23, 24, 25, 26, 27, 28]. In this regard, phyto‐synthesised AgNPs containing alkaloids and flavonoids can be used for bactericidal activity of pathogens found in humans [15]. Microbes such as bacteria and fungi have dynamic capacity to reduce silver ions (Ag+) to particles (Ago), however, more investigations are required to reap crystalline, monodispersed, and uniform conformation. In addition, isolation and purification of intra‐ and extra‐cellular synthesised microbial nano‐silver from the biological system is an immense challenge [29,30]. Based on the above discussion, herein, it is designed to assess the phyto‐synthesis of nano‐silver, their multiple approaches of purification, characterisation, and optimisation, and finally their applications and toxicity in medical, industrial, and environmental sectors.
2. PHYTO‐FABRICATION OF NANO‐SILVER
According to the literature, phyto‐compounds that act as reducing agents for conversion of Ag + to Ago are polysaccharides, proteins, polyphenols, alkaloids, and flavonoids, among others. These biomolecules occur in significant amounts in plant parts such as leaves, roots, stem, bark, flowers, and fruits. Biocompatible AgNPs were earlier mass produced using leaves broth of Acalypha indica (Indian nettle) [31], Camellia sinensis (tea) [32], Capsicum annuum (chili) [33], Cymbopogon flexuosus (lemon grass) [34], Datura metel (devils trumpet) [34], Medicago sativa (alfalfa) [34], Pelargonium graveolens (geranium) [34], Diospyros kaki (persimmon) [35], Ginkgo biloba (maiden‐hair tree) [36], Magnolia kobus (Kobushi) [35], Pinus densiflora (pine) [15], Platanus orientalis (oriental plant) [35], Euphorbia hirta (asthma‐plant) [36], Eucalyptus citriodora (lemon eucalyptus) [37], Ficus benghalensis (Figure 1) [37], Garcinia mangostana (mangosteen) [38], Ocimum sanctum (basil) [38], Acacia nilotica [39], Cyperus conglomeratus [40], Ziziphus mauritiana Lam. [41], Sesbania grandiflora [42], Psidium guajava [43], aqueous root extracts of Arachis hypogaea (ground nut) [44], Catharanthus roseus (Madagascar periwinkle) [45], Mammea suriga (surangi) [46], Cannabis sativa (marijuana) [47], Vetiveria zizanioides (vertiver) [47], and Zingiber officinale (andraka) [48]. Broth from the bark of Cacumen platycladi (Thujae), Cinnamon zeylanicum (cinnamon), Cochlospermum gossypium (gum kondagogu), and Pinus eldarica (Elder) also acts as potential ionic silver reducers [49].
FIGURE 1.
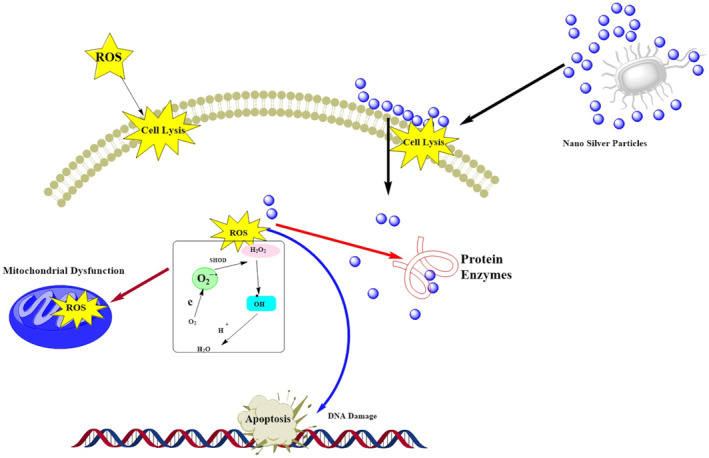
Schematic of antimicrobial activity associated with Ag nano‐silver
In a similar fashion, aqueous flower extracts from Couroupita guianensis [50], Nyctanthes arbortristis [51], and Tagetes erecta (Marigold) [52], Cananga odorata [53] have been verified to phyto‐synthesise biocompatible nano‐silver particles possessing good antimicrobial properties. Furthermore, aqueous fruit extracts from Andean blackberry [54], Citrus limon [55], Citrus reticulate [55], Citrus sinensis [55], Tanacetum vulgare (tansy fruit) [56] and Vitis vinifera (grape vine) [57], Artemisia nilagirica [58], Phyllanthus reticulatus, and Conyza bonariensis [59], Ficus retusa [60], Momordica charantia [61], Ocimum gratissimum [62], Ficus benghalensis [63], Elephantopus scaber, and Azadirachta indica [64], and Gardenia jasminoides Ellis [65] were reported to contain compounds that can reduce silver ions. On the other hand, Eucalyptus leaf extract [66], Coccinia grandis fruit extract [67], Reetha and Shikakai leaf extract [68], Alchornea laxiflora leaf extract [69] were also documented to have reducing phyto‐compounds that aid in the fabrication of silver nanoparticles which can be exploited for various purposes. Table 1 summarizes the studies on phytofabrication potentials of various medicinal herbs.
TABLE 1.
Summary of the literature on phytofabrication studies, the applied plants along with the targeted applications
| Plants | Plant Part | Size (nm) | Shape | Application | Reference |
|---|---|---|---|---|---|
| Annona muricata | Fruit | 60.12 | Spherical | Antimicrobial | [70] |
| Portulaca oleracea L | Stems, leaves and roots | 175,136,146 | Multiple | N.D | [71] |
| Allium giganteum | Shoots | 12 | Spherical | Antibacterial and anticancer | [72] |
| Allium saralicum | Leaves | ‐ | ‐ | Antioxidant, antibacterial and antifungal | [73] |
| Tropaeolum majus L. | Leaves | 35‐55 | Round | Antioxidant, antibacterial, antifungal and anticancer | [74] |
| Piper betle | Leaves | 3‐37 | Spherical | Medical and pharmaceutical | [75] |
| Allium sativum | Root | 7.3 | Spherical | Antibacterial | [76] |
| N. jatamansi | Rhizomes | 10‐15 | Round | Anti‐inflammatory and ant biofilm | [77] |
| Annona reticulata | Leaves | 6‐8 | ‐ | Antibacterial | [78] |
| Justicia spicigera | 80‐‐100 | Spherical | Antimicrobial | [79] | |
| Salvia hispanica L | Seeds | 1‐30 | Spherical | Antibacterial | [80] |
| Pinus densiflora | Leaves | 15‐50 | Cubic | Cosmetics, foods, and medical | [35] |
| Pithecellobium dulce | Leaves | 62 | Spherical rods | Larvicidal | [81] |
| Falcaria vulgaris | Leaves | A rane | ‐ | Antibacterial | [82] |
| Tarragon | Leaves | 25 | Spherical | Antibacterial | [83] |
| Selaginella | Leaves | 5‐10 | Round | Anti, icrobial | [84] |
| Rumex dantatus | Root | 25‐70 | ‐ | Antibacterial | [85] |
| Coptis chinesis | Leaves | 6‐45 | Round | Chemotherapy | [86] |
| Aloe vera | Gel | 66.6 | Spherical | Antioxidant | [87] |
| Anthoceros | Whole plant | 20‐50 | Cuboidal and triangula | ‐ | [88] |
| Salvia officinalis | Leaf | 41 | Spherical | Antiplasmodial | [89] |
| Morus nigra | Leaves | 23 | Cubical | Antibacterial | [90] |
| Helianthus annus | Leaves | 19 | Multiple | Antibacterial | [91] |
| Memecylon umbellatum Burm F | Leaves | 7‐23 | Round | Antibacterial/antitumour | [92] |
| Calotropis gigantean | Leaves | 83.7, 5.9,11.8 | Spherical | Antibacterial | [93] |
The synthesis of NPs using a single active substance is getting attention of scientists because it has been observed that the purification of such particles is easy. However, further research is required for the applications of such particles in medical fields. Some recent studies have shown that flavonoids present in plant extracts are the main compounds, which contribute the bio reduction of metal ions to nanoparticles. The phytochemicals such as alkaloids, proteins, phenols, alcohols, terpenoids, and flavonoids are generally present in plant materials, and it has been observed that these materials are actively involved in stabilisation and reduction of metal ions [94,95].
2.1. Mechanism of Photosynthesis of AgNPs
Numerous studies suggested the synthesis of NPs [96, 97, 98, 99], however, the exact mechanism about synthesis is still not known. Several studies were carried out at the National Chemical Laboratoy (NCL), Pune, India, to find the mechanism for the synthesis of silver and gold nanoparticles from different plants including chickpea [100], neem [101], tamarind [102], and lemon grass [103]. They reported that terpiniods present in plant material are the main compounds that contribute to reduction of silver ions. Moreover, they also reported that proteins are the main capping agents in gold nanoparticles [104]. When the Azadirachta indica leaf extract was used for synthesising metallic and bimetallic nanoparticles, it was observed that reducing sugars are the main components involved in reduction of metal ions as well as stabilisation of these nanoparticles [101]. Similarly, reducing susgars were also involved in the formation of AuNPs from lemon grass [103].
2.2. Factors affecting the synthesis of nanoparticle
There are many factors that influence the synthesis of NPs including temperature, pressure, light intensity, and incubation time. Moreover, some other factors including concentration of leaf extract, concentration of AgNO3, reaction time, reaction temperature, pH of the reaction, and other light reactions affect the size, dimension, and shape of these NPs. In addition, the conditions of light such as tube light, blue light, bulb light, and red light also influence the synthesis of these NPs.
2.3. Temperature
Temperature is the main factor, which affects the synthesis of AgNPs. Generally, synthesis of nanoparticles takes place at room temperature, which is a time‐consuming process. However, the temperature can be increased to lower the time. In general, the synthesis is performed at a temperature ranging from 30 to 100°C. The synthesis at higher temperature results in reduction of Ag+ ions as well as homogeneous nucleation of silver nuclei, resulting in smaller size silver NPs [105]. The rate of NPs synthesis decreases and the stability increases with the increase of reaction temperature. Moreover, the size of the silver NPs decreases with the increase of reaction temperature.
2.4. Volume of extract
The volume of leaf extract affects the time required for the formation of NPs as the leaf extract is the main part of reaction of silver ion. Usually 1, 5, 10, 15, 20, and 100 ml of extract volume can be used to analyse the effect. The volume of leaf extract affects the particle size of the silver NPs. The reduction of Ag+ ions is significantly affected by the concentration of leaf extract that is the contents of anthocyanin, phenol, tannins, polyphenols, and polysaccharides [106].
2.5. pH
pH can affect the electrical loads of biomolecules which consequently can affect their stability, capping ability as well as their growth. Research findings indicated that the acidic pH is not suitable for the synthesis of NPs because under acidic conditions the transition from light green to dark brown takes longer time. The size of the NPs formed under acidic conditions is usually larger as compared with‐ the ones synthesised under basic conditions. Moreover, the alkaline conditions also favour reduction as well as the stabilising capacity of antioxidants [106].
2.6. Silver nitrate (AgNO3) concentration
The AgNO3 concentration is measured in 0.1–1 mM or higher concentrations such as 10, 50, and 100 mM. However, 1 mM concentration has been found best as it was observed previously that the particles synthesised using 0.1 mM AgNO3 are very minute and cannot be seen with a naked eye. Therefore, reactants are required in very small concentrations. The higher concentrations of reactants results in an unsuccessful reduction of Ag+.
2.7. Stirring time of reaction
It is the time required for the synthesis of silver NPs, starting from the reactants added to beaker. The stirring time allow the proper reaction between reducing complex components of leaf extract with silver salt. If the concentration of phytochemicals or secondary metabolite is higher in a plant, it will reduce the silver salt in less time and vice versa. The NPs formation takes place quickly if the plant contains less secondary metabolites [106].
3. PURIFICATION OF NANO‐SILVER
There are numerous purification techniques, which are currently used for the purification of NPs. These techniques include centrifugation, precipitation by antisolvent, flocculation using photolyzable surfactant, or temperature‐induced separation. However, most of these techniques are time consuming [107]. Moreover, they require higher amounts of solvent as well as higher energy, and they are inefficient in removing small molecule and inorganic impurities [108,109].
Published research indicated that the recommended procedure for isolation and purification of nano‐silver from a complex reaction mixture is ultra‐centrifugation at high speeds (200,000 rpm) for 15 min, or sucrose density gradient centrifugation [109,110]. In addition, a new technique termed as differential thawing was recently introduced for the purification of nano‐silver, which involves the freeze‐drying of prepared nano‐silver solution in a deep freezer overnight, and then thawing the icy nano‐silver at 28 °C. However, centrifugation is a more preferable approach than differential thawing because the latter yields larger and polydispersed particles due to agglomeration [111].
Multifarious purification protocols that provide tremendous outcomes depending on the feasibility of the wet laboratory have been investigated. Out of many, sucrose density gradient centrifugation, flow centrifugation, and repeated ultra‐centrifugation at 10,000 rpm for 10 min are preferred, due to their ease in handling, less laborious, speedy, and budget‐friendly results. According to earlier studies, green nanoparticles from leaves of Eugenia jambolana, Piliostigma thonningii, and Azadirachta indica were purified following sucrose density centrifugation method which provided purified AgNPs in estimated range of 10–100 nm [112,113]. In an another study, crude phyto‐AgNPs from Tagetes erecta flowers were purified following ultracentrifugation for 10 min at ≤ 50F0B0C. The repeated centrifugation for 10 min yielded excellent pelleted particles, which were dried and stored at 4F0B0C. The purified pellets were characterised to be monodispersed, polycrystalline, and almost spherical in morphology owing to a size of about 1–90 nm, thus qualifying them as ultra‐fine particles [52]. Purification of nanoparticles is a crucial step for proper characterisation and function of green AgNPs. The excess ligand and impurities hinder the precise function of AgNPs. To cope all this, a novel strategy of continuous flow purification was introduced in February 2020, which claimed to eliminate 56.73% of the hindering ligands while maintaining the desired structure of biosynthesised AgNPs. In addition, the purified AgNPs can be effortlessly dried via freeze‐drying (–20F0B0C or below) or spread drying (≤50°C) method [114].
4. CHARACTERISATION OF NANO‐SILVER
Nano‐silver is characterised by numerous spectroscopic techniques including UV–Vis spectroscopy, scanning electron microscopy (SEM), energy dispersed X‐ray spectroscopy (EDX), X‐ray diffraction spectroscopy (XRD), transmission electron microscopy (TEM), atomic force microscopy (AFM), zeta potential, dynamic light scattering (DLS), DLS, energy‐dispersive spectra (EDS), nuclear magnetic resonance spectroscopy (NMR), UV–Vis spectroscopy, surface‐enhanced Raman spectroscopy, high‐resolution transmission electron microscopy (HR‐TEM), AFM, X‐ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), and surface‐enhanced Raman spectroscopy [115,116]. These techniques are useful in investigating the precise and controlling synthesis of AgNPs. Parameters such as surface area, morphology, topology, porosity, crystallinity, dispersion, and the size of a sole particle are analysed via these techniques. In addition, nanocomposites, nanotubes, and nanowires orientation and intercalation can be determined by means of the aforementioned techniques [117]. For instance, the diameter and conformation of nano‐silver are investigated by SEM, TEM, and AFM, which normally varies from 10 to 500 nm in size having complex morphology of spherical to complex rods, triangles, trapezoids, and prisms. On the other hand, the crystalline structures are probed by XRD, whereas size distribution is assessed by DLS.
Furthermore, EDX spectroscopy can be used to investigate the elemental status of biogenic nano‐silver, whereas UV–Vis spectrophotometry confirms the explicit sample formation by scrutinizing absorbance values based on λ max showing plasmon resonance in the range of 350–500 nm [118]. In this respect, Saratale et al. used Acacia nilotica leaf (ANL) extract to produce silver nanoparticles. In this case, the involvement of phytoconstituents was analysed using energy‐dispersive spectra (EDS) and Fourier transform infrared spectroscopy (FTIR) which confirms the involvement of ANL in stabilisation, reduction, and capping of the silver nanoparticles matrix. Characterisation of silver nanoparticles was carried out with the aid of X‐ray diffraction (XRD) and high‐resolution transmission electron microscopy (HR‐TEM); results showed that the average particle size of the silver nanoparticles was ∼20 nm with spherical shape and crystalline structure [39].
5. OPTIMISATION OF NANO‐SILVER PARTICLES
Optimising crucial parameters such as substrate concentrations, pH, and temperature are the principle keys to obtain accurate, monodispersed, crystalline, and nano‐sized particles. A study by Ahmad and colleagues showed that the substrate concentration is directly proportional to the rate of reaction [119]. An increase in substrate concentration from 1 to 5 mM AgNO3, caused a rapid nano‐silver synthesis, however, the stability was more controlled at 1 mM substrate concentration; where stable, monodispersed, and small particles of AgNPs were produced at an average size of 10 nm. In comparison, higher substrate concentrations led to the formation of unstable, polydispersed, large aggregated nano‐silver with a diameter of ≥100 nm [63]. Similarly, the pH and temperature of reaction mixture are also considered as prime factors for precise phytosynthesis of nano‐silver. It was shown that green AgNPs obtained at temperatures ≥100°C and pH 4, were unstable large particles of size ≥55 nm. The favourable conditions that assist in brisk biosynthesis were temperature 70–95°C and slightly acidic to neutral pH that is 5.5–8, which produces small nanoparticles with increased surface area [63,120].
5.1. Biocompatibility of AgNPs
Nano‐silver has garnered prodigious attention across the globe because of their dynamic applications in multifarious spheres. Their unique physicochemical characteristics have been applied in several biomedical devices ranging from a simple wound dressing to complicated pacemakers. Their multiple modes of action along with cost effective, eco‐friendly and least toxic nature had made their utilisation unlimited because of their promising outcomes [121]. Along with many pros of green AgNPs, there are certain controversies arising in regard to their biocompatibility with normal animal cells peculiarly to humans. Various present day studies suggest that sustained exposure to AgNPs can cause their accumulation in vital organs and complicate the therapeutic scenario [122, 123, 124]. It is evidenced that inhaled AgNPs may cause lung injuries by damaging the alveoli. Similarly, deposition of AgNPs is also reported in nervous, cardiac, renal, and hepatic tissues [125, 126, 127]. Cytotoxic evaluation evinced that AgNPs have potential to bio‐transform the natural environment of living cells by generation of oxidative stress that interact with biological macromolecules [128,129]. In another molecular analysis, it was manifested that AgNPs have the capability to modify mitochondrial membrane permeability and accumulation of reactive oxidative species which in turn cause profuse DNA damage [130,131]. Many studies proved that AgNP exposure can induce a decrease in cell viability through different cellular mechanisms. One of these mechanisms is represented by the induction of apoptosis‐related genes and the activation of apoptosis mechanism. Also, it was proven that nano‐silver can cause the formation and intracellular accumulation of ROS, modification of mitochondrial membrane permeability, and DNA damage [132]. The in vitro toxicity of AgNPs has been investigated in several research studies, but there is still a lack of consistent and reliable data. Furthermore, genotoxic, cytotoxic, and mutagenic investigations validated that toxicity of biogenic AgNPs is related to surface chemistry, morphology, size, therapeutic concentration, dispersion rate, and physicochemical characteristics [133]. In vivo toxicity of AgNPs is categorised according to the site of impairment that is caused. The various modes of exposure to AgNPs, their biodistribution, and their mechanisms underlying the effects are documented in literature [133]. As AgNPs are preferably utilised in most of the modern day technology, the continuous exposure may pose an alarming condition to human health. There is a consistent requirement of more cytotoxic and genotoxic research to be conducted to understand the underlying side effects of the phyto‐fabricated silver nanoparticle [134, 135, 136].
5.2. Stability of AgNPs
Stability of AgNPs can be improved by applying different analytical techniques. These techniques include fluorescence correlation spectroscopy (FCS), DLS, flow field fractionation (FFFF), AFM, and nanoparticle tracking analysis (NTA). The area stabilisation and surface charge of AgNPs can be understood using Zeta potential and BET surface area measurements. Besides surface charge, steric stabilisation of AgNPs could also occur [137]. The stability of AgNPs in aqueous solutions is affected by the coating of chemical materials. Most of the stability analyses of AgNPs have been done using citrate‐coated AgNPs [138, 139, 140, 141]. A previous study on the stability of citrate‐coated AgNPs in synthetic seawater, natural freshwater, and stimulated estuarine waters has shown a stability of few days of these materials [138].
5.3. Stability of Phytofabricated AgNPs
Phyto‐synthesised AgNPs were investigated for their long‐term stability at variable fluctuation conditions. Aqueous extracts from Parachlorella kessleri were utilised to synthesize AgNPs. Different spectrophotometric analyses demonstrated that the rate of synthesis is directly proportional to freshness of sample and amount of AgNPs solution. From scanning and transmission electron microscopy, it was concluded that a pH range of 8–10 provided fine, stable monodispersed polyhedron particles in the diameter of 5–60 nm. In contrast, acidic pH of reaction afforded minimally stable particles of irregular shape and size, thus losing their integrity at 10th day of the experiment. In addition, temperature and light fluctuations also play a vital role in the stability of greenly synthesised nano‐silver. Reactions temperature below 20F0B0C and excessive interaction of light tend to disintegrate the stability of potential nanoparticles. Furthermore, storage temperature of ≤5°C provides best long‐term stability. The AgNPs remained desirably spherical, ultra‐fine (5–20 nm), and stable without agglomeration even after more than 6 months [142,143].
5.4. Reproducibility of Phytofabricated AgNPs
Biofabrication of silver nanoparticles by biological reducing agents including plant, bacterial, and fungal extracts is a substitute to chemical and physical methods, although preparation of bacterial and fungal extracts requires high maintenance of cultures and are time consuming as compared with plants. Many plant species, algae and their endophytes such as Averrhoa carambola, Solidago altissima, Chlorella vulgaris, Calophyllum apetalum, and Padina tetrastromatica have been successfully used for the synthesis of AgNPs [144]. Phytofabrication of AgNPs using aqueous extracts from whole parts or plant parts showed promising medicinal potency in remediating many ailments such as malaria rheumatism, microbial infections, leprosy, and even cancer. Plant extracts provide bioactive ingredients, which act as stable bio‐factories for recyclable and reproducible synthesis of AgNPs in an economic and eco‐friendly manner with aced medicinal properties [145].
5.5. Dispersity of Phytofabricated AgNPs
Biogenic AgNPs are considered to be amphipillic particles because of their affinity towards both polar and non‐polar solvents. According to various biological investigations, dissolution of AgNPs in number of solvents ranging from water to n‐hexane has broadened up multiple experimental vistas to work on and to make the phyto‐fabricated AgNPs commercially available for multitude of products [145].
5.6. Uniform size of Phytofabricated AgNPs
According to a present day investigation, it is validated that fine control over the nanoparticle's size is achieved by varying the concentration of tannic acid (a phytoactive compound), which results in uniform nanoparticles in the range of 18–30 nm in diameter with a standard deviation of less than 15%. Phyto‐reduction method is a preferred route because of profuse amounts of eco‐friendly and cost effective bio‐reducing and bio‐capping agents, which provide the desired morphology. It is also known that utilisation of various stabilizing agents such as sodium borohydride, polyvinylpyrrolidone, and tri‐sodium citrate provides uniform spherical morphologies in the diameter of about 15–90 nm. It was also observed that spherical AgNPs exhibited a better antibacterial activity because of their proficient mode of penetration plus multiple target affinity [146, 147, 148, 149, 150].
6. BIOLOGICAL COMPETENCE OF NANO‐SILVER
In the past few years, explorations on green AgNPs have expanded to diverse fields such as medicine, agriculture, environment, and many other industries [2]. Documented applications of phyto‐based AgNPs in the above‐mentioned domains are discussed in the following sections.
7. MEDICINAL APPLICATIONS
7.1. Antimicrobial
Centuries ago, silver was acknowledged as a notable antimicrobial agent whether in the form of colloids, salts, or pure solutions. This attribute was inherited in AgNPs with superior characteristics. Among all of the metals which are considered as anti‐bacterial agents, AgNPs have been proven to be the most effective for a wide range of microorganisms [151, 152, 153]. These particles have the ability to control the microbial growth in human infection. The antimicrobial effectiveness of silver nanoparticles mainly depends on their size and shape. The smaller size AgNPs are more effective as an antimicrobial agent as compared with the larger one. According to previous reviews, AgNPs is a broad‐spectrum antibiotic that inhibit pathogenic microorganisms. The amount of green AgNPs is directly proportional to the antimicrobial effect. If the size of AgNPs is smaller, the antimicrobial prospects will be superior due to higher affinity and extended surface area towards microbial membranes [154]. Conformation also plays a crucial part in contributing to antimicrobial property. Criterion was tested by Sadeghi and coworkers who postulated that nanoplates are excellent bactericidal agents against Escherichia coli and Staphylococcus aureus [155]. Previous experiments hypothesised that a combination therapy of AgNPs with antibiotics such as amoxicillin can be utilised to manage resistant microbial infections [156]. Similarly, findings by Kim and coworkers revealed that the combination therapy restrains the mycelia growth of several resistant fungi such as Candida and Trichophyton species [157].
AgNPs have the potential to kill both gram‐positive and ‐negative bacterial strains [158, 159, 160]. However, nanoparticles are considered more effective for killing gram‐negative bacteria as compared with the gram‐positive bacterial strains. This could be attributed to the fact that the gram‐positive bacteria have a thick cell wall consisting of different peptidoglycan layers 920–80 nm) in addition to one cytoplasmic membrane. On the other hand, gram‐negative bacteria contain an external layer of lipopolysaccharide (LPS) followed by peptidoglycan, which is a very thin layer, and the last layer is of plasma membrane [161,162]. Multi‐drug bacteria, which are now well recognised, have become a major threat to the human health [163]. The coating of AgNPs with hydrophobic and cationic functional groups is helpful in suppressing the growth of gram positive, gram negative, and multi‐drug‐resistant bacteria [164]. AgNPs are also effective in overcoming these multi‐drug bacteria. The AgNPs release Ag+ ions during their antibacterial activity, which interact with bacterial protein particularly the thiol group. Due to this interaction, the DNA replication gets disturbed which results in lysis of bacteria [165, 166]. The antimicrobial activity of the AgNPs depends upon the size, dose, charge, and shape [167,168].
Antimicrobial mechanisms of biogenic AgNPs is not conventionally elucidated, but it is inferred that nano‐silver possesses affinity towards almost all microorganisms. These particles have the capacity to induce cell membrane permeability via (a) free radical emergence or structural alterations, (b) inhibition of DNA replication via the inhibition of vital enzymes or phosphate containing bases, and (c) modulation of signal transduction to alter phosphotyrosine profile of microbial peptides. All these changes incite microbial cell apoptosis, thus conferring bactericidal and fungicidal effects (Figure 1) [169].
Silver nanoparticles are capable of binding with the cell wall of bacteria and then penetrate inside the cell. After penetration, they cause changes in cell wall structure, which includes permeability as well as death of the cell [144]. These particles enter by endocytosis after accumulating on cell surface [170]. Particles also have the ability to form free radicals, which cause death of the cell. Some previous studies have shown the formation of free radicals by silver nanoparticles through spin resonance spectroscopy during their contact with bacteria. These radicals then damage the cell wall and make them porous [171,172]. This causes the death of these cells. Soft bases have the capacity to react with soft acids. Cells are formed from phosphorous and sulphur, which are soft bases and the silver is soft acid. Sulphur and phosphorous are the major components of DNA and silver nanoparticles act on DNA and cause its destruction which ultimately cause the cell death [173].
Reactive oxygen species are also formed when silver particles encounter bacterial cell wall, which may be generated by the inhibition of respiratory enzymes by silver ions. They attack the cell and cause its death [174,175].
7.2. Antiviral
AgNPs have antiviral attributes and they are well known for their anticancer activity [176]. In this respect, infections of herpes simplex virus type 1 (HSV‐1) can be inhibited by AgNPs because these nanoparticles block the attachment and control the entrance of the virus into the cells. Moreover, they control the virus by preventing its spread from cell to cell. In addition, phyto‐generated AgNPs act as a robust antiviral agent against a wide array of strains such as human immunodeficiency virus type 1 (HIV‐1), HSV‐1, Hepatitis B virus (HBV), respiratory syncytial virus (RSV), and monkey‐pox virus [177,178]. Taylor and coworkers demonstrated that the antiviral efficacy of AgNPs is more intense than that of silver salt solutions. Silver salt solution releases only Ag+ ions, whereas the eco‐friendly nanoparticles solution imparts dynamic Ago (atomic) and Ag+ (ionic) clusters [179].
In a similar fashion, Lara and colleagues showed that AgNPs confine the replication of HIV‐1 at stage 1 by binding to viral glycoproteins, which halt the viral attachment, penetration, and fusion to CD‐4 T‐lymphocytes, hence inhibiting infection [180]. Shown in Figure 2a is the antiviral mechanism of AgNPs impact on HIV‐1 glycoprotein, whereas Figure 2b presents a table of amino acid residue related to HIV glycoprotein.
FIGURE 2a.
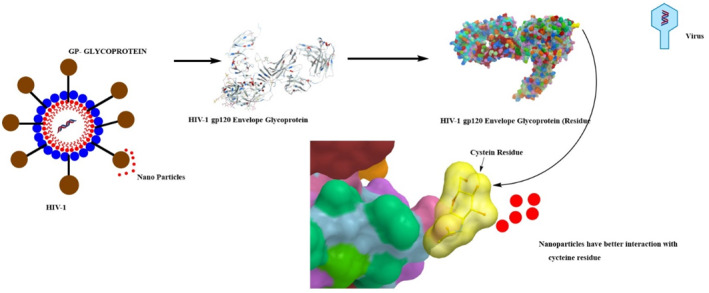
Antiviral mechanism of Ag naoparticles impact on HIV‐1 glycoprotein. Ag has the strongest interaction with cys residue
FIGURE 2b.
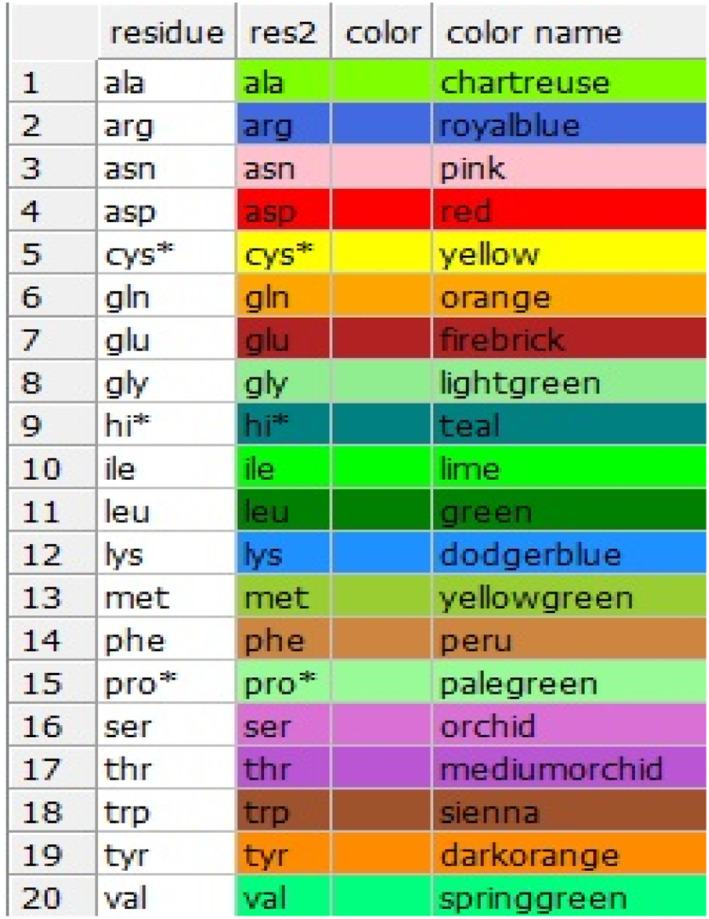
Colourful table of amino acid residue related to HIV glycoprotein, yellow colour is linked to cycteine residue
A recent study has also shown the potential of these NPs as a candidate for 2019‐nCoV treatment [181]. Zacher [182] proposed that the silver nanoparticles can be used to treat COVID‐19 at an early stage. The nanoparticles‐based vaccine is more effective in boosting immunity as compared with other conventional antigen‐based vaccines [181].
7.3. Anti‐inflammatory
Biosynthesised AgNPs were approved as an eminent anti‐inflammatory agent. Experiments involving animal models such as allergic rhinitis mouse revealed that the administered dose of botanic AgNPs inhibits the expression of ovalbumin‐specific immunoglobulin E, interleukin‐4 and ‐10, goblet cell hyperplasia, and inflammatory cell infiltration [183]. Contact dermatitis experimentations on swine models showed that administered doses of silver nanoparticles altered the expression of pro‐inflammatory cytokines tumour necrosis factor‐α (TNF‐α) and transforming growth factor‐β (TGF‐β) (Figure 3) [184]. Furthermore, clinical studies on humans showed anti‐inflammatory activity in response to chronic leg ulcers. The ameliorative effect of AgNPs occurred via the inflammatory cell apoptosis, reduction in the mast cells and lymphocytes infiltration, and by limiting the release of metalloproteinases and cytokines [185,186]. The anti‐inflammatory properties of the AgNPs are directly related to the size of these particles and larger diameter particles possess better anti‐inflammatory properties compared with the smaller one [187]. Moreover, the NPs prepared by the combination of extracts from different plants showed better anti‐inflammatory properties compared with the ones which were prepared by only one plant [188].
FIGURE 3.
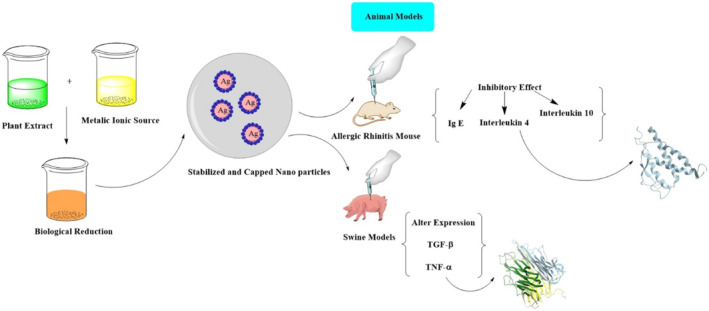
Production of Ag nanoparticle and its effect in two animal models; allergic rhinitis Mouse and Swine
7.4. Wound Dressers
Anti‐coat was the initial nano‐silver product to be recommended by the pharmaceutical industry to medicate chronic dermal wounds and infections such as pemphigus, ulcers, gangrenes, and other toxic epidermal necrolysis [189]. It was formulated by the Canadian scientist Dr. R. Burrell, and later clinically tested by Chinese scientists. The appraisal demonstrated that topical application of biogenic AgNPs dressings promotes quick re‐epithelialisation along with antimicrobial properties [190,191]. Further studies produced a remedy, which was formulated by the combination of AgNPs and chitosan polymer. This antimicrobial bandaid with low tissue absorbance was used to heal deep wounds [192]. AgNPs have shown a cytocompatibility with tested cell lines. These particles have higher moisture contents and good transparency level, which they are considered as a good option for treating chronic wounds that have higher microbial bioburden [193]. Figure 4 portrays the mechanism of wound healing using patch containing Ag nanoparticles.
FIGURE 4.
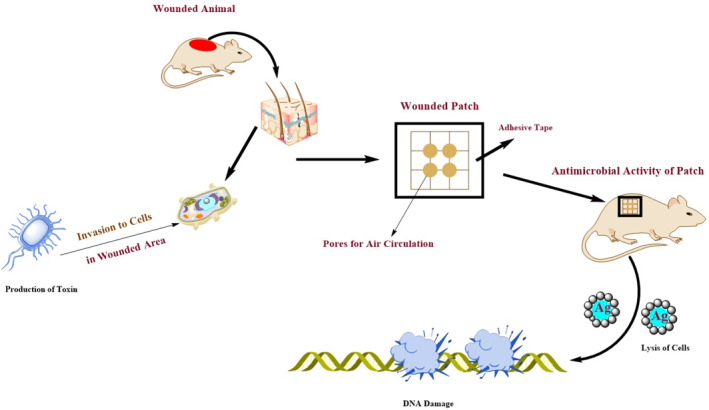
Schematic medication of wounded area by patch including Ag nanoparticles [223]
7.5. Medical implants
A preliminary implantation device that contained elemental silver (Ag+) was a prosthetic silicon heart valve, which possessed excellent antimicrobial and anti‐inflammatory potencies [194]. However, the metallic silver coating caused hypersensitivity, thus inhibiting normal function of fibroblasts leading to paravalvular leakage in convalescents [195]. To resolve this drawback, Andara and coworkers, engineered heart valves and stents coated with green AgNPs and diamond‐like carbon. These coated cardiovascular implants proved to be safe and have the desired antimicrobial and anti‐thrombogenic properties [196]. AgNPs precisely provide the sustained exposure to infarcted heart through direct intramyocardial injection [197]. Furthermore, their hemodynamic and mechanical mechanisms were investigated by creating multiple coats of nano‐silver implants [198].
Aside from cardiovascular implants, AgNP‐coated catheters were also extensively investigated to prevent serious catheter‐associated infections. Along this line, there are two commercially available AgNP‐coated catheters that is silver‐line (Spiegelberg GmbH and Co. KG, Hamburg, Germany) and ON‐Q Silver Soaker™ (I‐Flow Corporation, CA, USA). These catheters were accepted worldwide as they were validated to reduce post‐surgical infections while having least or no side effects. In vitro studies on animal models demonstrated that plastic catheters filmed with AgNPs possess bacteriostatic effects up to 72 h [198]. Similarly, clinical studies on 19 nano‐silver‐catheterised convalescents validated clear cerebrospinal fluid cultures along with negative catheter‐associated ventriculitis [199].
7.6. Dental implants
Biogenic phyto‐AgNPs can be used in dental medicine as part of dental fillers or complex orthodontic implants [200,201]. Incorporation of AgNPs at different concentrations with composite resins proved useful, and the mechanical and antimicrobial properties of the product improved significantly. The endodontic filling material containing AgNPs provide better antibacterial properties against Enterococcus faecalis, Streptococcus milleri, and S. aureus. It inhibits the proliferation of caries‐associated bacteria such as Streptococcus mutans, Streptococcus milleri, Enterococcus faecalis, and in some cases Staphylococcus aureus (Figure 5) [202]. A recent study has shown that coating of the silver nanoparticles with silica form Ag@SiO2 further improves their antimicrobial activity and no cytotoxicity on human dental cells was observed [203].
FIGURE 5.
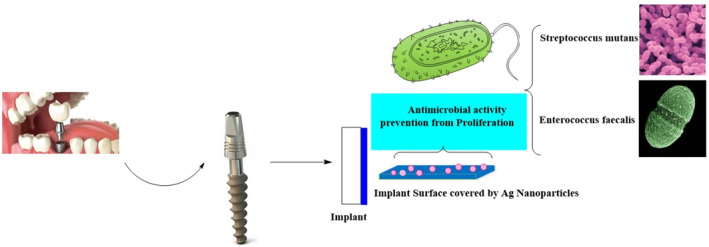
Schematic presentation of an implant surface integrated by Ag nanoparticles and its antimicrobial activity
7.7. Other medical advancements
Biosynthesised AgNPs, as part of biosensors, have recently been used in the diagnosis of cancers and Alzheimer's disease [204]. They are also used as bio‐tags for quantitative analysis of a process, and as carriers of therapeutic agents [205]. Their antimicrobial properties are also used in eye care products such as contact lens solution, and are expected to be used in the next generation of implantable medical devices [206]. N. khasiana extract has recently been used for production of silver nanoparticles. Performance analysis of these particles has shown that they can be used for the treatment of Alzheimer's disease [207].
7.7.1. Fabrics
AgNPs have been widely used in diverse consumer products including clothes, laboratory gowns, and socks as well as medical products including dressing bandages, surgical gowns, and facemasks due to their antimicrobial potency. The NPs coating on cloth surface is achieved using numerous techniques including pad dry cure method, sol–gel method, and sonochemical method [208,209]. Several studies have claimed that the NP‐coated fabrics have the potential to protect from ultraviolet radiations as well as their antimicrobial ability [210,211]. Previous studies had shown that coated NPs are particularly suitable in protecting against S.aureus and E.coli pathogens [212,213].
8. AGRICULTURAL APPLICATIONS
AgNPs show promising outcomes in agricultural practices by promoting crop conservation, animal vitality, and fisheries [214]. They function as active biosensors to detect pests/pathogens, monitor packaged products, and standardise the release of reactive substances [215,216]. Biogenic AgNPs capsules are used as positive genetic carriers to transport agricultural ingredients to produce novel stress‐ and disease‐resistant crops plus sensing favourable field environment for excessive crop production (Figure 6) [217,218]. These particles are also being used as antimicrobial as well as ethylene inhibitors in agriculture industry to increase their post‐harvest quality [219]. Recently, A. aspera and S. dulcis have been used to prepare fungicides [220]. These fungicides can be used as an alternative to synthetic fungicides for agricultural applications.
FIGURE 6.
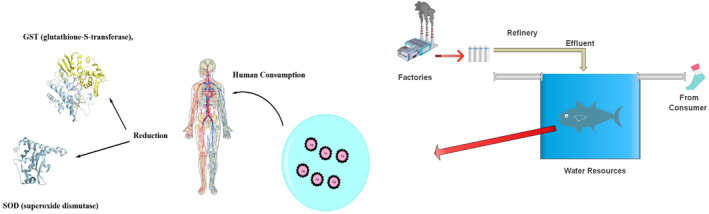
Schematic presentation of accumulation of Ag nanoparticles in marine sources and some of their impacts on humans [224]
9. ENVIRONMENTAL APPLICATIONS
Published research pertaining to silver nanotechnology revealed that approximately one‐third of environmental silver is dispersed through excessive utilisation of nanoparticles. This silver gets into the atmosphere and wastewater via washing off consumer goods that are loaded with nano‐silver such as cosmetics, toiletries, fabrics, and various household appliances [221]. In addition, silver nanoparticles can be used as catalyst in power generation equipment of automobiles, which reacts with hazardous gases such as nitrous oxide and carbon monoxide to reduce their environmental impacts. Silver nanoparticles can also be used in wastewater treatment plants to remove different types of hazardous pollutants and pathogens from water. They are effective in the removal of microorganisms, minerals and pesticides from wastewater to purify it for drinking purposes [161]. A report on sewage sludge showed that silver has entered water systems and was even found to be aggregated into sediments and soil; however, environmental risks are not well‐characterised. It is presumed that the accumulation of these silver particles in the bodies of aquatic such as mollusks can disturb the food chain and ecosystem. This bioaccumulation can inhibit the growth of beneficial bacteria too. Therefore, new protocols should be developed for qualitative and quantitative analysis of environmentally released silver to protect the biosphere [222]. Aerva lanata synthesised AgNPs have been proven effective in degradation of dyes, and have shown potential for environmental applications.
Figure 7 depicts the mechanism of bio‐sensing activity when Ag nanoparticles are deposited on a surface.
FIGURE 7.

Schematic representation of silver nano‐biosensor: stage 1) stabilisation of silver nanoparticles on the surface, stage 2) Bio‐functionalisation of the surface and an increase in refractive index, stage 3) reaction with a ligand analyte and shifting the blue curve to higher wavelength (showing bio‐sensing activity) [225]
10. OTHER RECENT ADVANCEMENTS
Green AgNPs can be used to manufacture conductive inks due to their exclusive thermal and electrical conductivities. They are combined with vanadium oxide, which increases shelf‐life and efficiency of batteries. They are also used in optical spectroscopies such as metal‐enhanced fluorescence (MEF) and surface‐enhanced Raman scattering (SERS) due to their increased affinity to capture light energy [225].
11. NANO‐SILVER TOXICITY
Due to the increasing prevalence of AgNPs in consumer goods, there is an urgent need to understand their biological mechanisms and hitherto unreported health risks. For centuries, colloidal silver solutions were utilised in folk medicine to treat various chronic infections but the deposition of Ag+ in tissues led to a cosmetic malady called ‘Argyria’ or ‘argyrosis’. Under these conditions, the skin turns blue or bluish‐grey. On the other hand, experimentation on AgNPs led to the finding that nanoparticles are superior over silver salt solutions and colloidal silver because they demonstrated effective antimicrobial properties at less dosage, which can assist in the treatment of infections without any reported side effects.
Research findings showed that AgNPs can act as anti‐inflammatory, genotoxic, mutagenic, cytotoxic, and cytostatic agents. Although these properties might be interpreted as useful for immune potentiation or tumour control for normal cells and DNA, they can still be deleterious. AgNPs can penetrate the cell wall and create oxidative stress, and the resulting reactive oxygen species (ROS) can cause dysregulated enzyme cascades and undesirable immune activation.
12. CONCLUSIONS
In summary, findings from this literature review suggest that phyto‐fabrication of AgNPs is an ideal strategy to generate large numbers of eco‐friendly nanoparticles in a cost‐effective way. Based on the AgNPs plasmonic resonance, purified dry pellets of AgNPs have λ max values in the range of 350–500 nm, with a variably simple to complex morphologies in the range of 10–500 nm diameters, depending on experimental conditions. These green‐synthesised AgNPs exhibit distinctive biological traits which can be used in assorted pharmaceutical and engineering industries to make commercial novel and effective consumer items. However, more detailed studies related to the toxicity of AgNPs are required to establish their safety and efficacy.
ACKNOWLEDGEMENTS
This work was supported by Higher Education Commission (HEC) of Pakistan under project No: 7343/KPK/NRPU/R&D/HEC/2017.
REFERENCES
- 1. Yaqoob, A.A. , et al.: Silver nanoparticles: various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 1–10 (2020) [Google Scholar]
- 2. Curtis, A. , Wilkinson, C. : 'Nanotechniques and approaches in biotechnology. Trends. Biotechnol. 19, 97–101 (2001) [DOI] [PubMed] [Google Scholar]
- 3. Lee, S.H. , Jun, B.H. : Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci. 20(4), 865 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Markets, M. : Metal nanoparticles market by metal (platinum, gold, silver, iron, titanium, copper, nickel), end‐use industry (pharmaceutical & healthcare, electrical & electronics, catalyst, personal care & cosmetics), and region‐global forecast to 2022. Mark. Res. Rep. 14, 4489142 (2018) [Google Scholar]
- 5. Ahmeda, A. , Zangeneh, A. , Zangeneh, M.M. : Characterization and anti‐acute T cell leukemia properties of silver nanoparticles synthesized by a green approach for bioremediation applications: Introducing a new chemotherapeutic drug for clinical trial studies. Appl. Organomet. Chem. 34(3), e5374 (2020) [Google Scholar]
- 6. Shepard, Z.J. , Lux, E.M. , Oyanedel‐Craver, V.A. : Performance of silver nanoparticle‐impregnated ovoid ceramic water filters. Environ. Sci. Nano. 7, 1772–1780 (2020). 10.1039/D0EN00115E [DOI] [Google Scholar]
- 7. Keshari, A. , Srivastava, R. , Yadav, S. : Synergistic activity of green silver nanoparticles with antibiotics. Nanomed. Res. J. 5(1), 44–54 (2020) [Google Scholar]
- 8. Garg, D. , Sarkar, A. , Chand, P. : Synthesis of silver nanoparticles utilizing various biological systems: mechanisms and applications—a review. Prog. Biomater. 9(3), 81–95 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sripriya, N. , et al.: Encapsulated enhanced silver nanoparticles biosynthesis by modified new route for nano‐biocatalytic activity. Biocatal. Agric. Biotechnol. 18, 101045 (2019) [Google Scholar]
- 10. Saratale, R.G. , et al.: Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2)', Artificial. Cells. Nanomed. Biotechnol. 46, 211–222 (2018) [DOI] [PubMed] [Google Scholar]
- 11. Gour, A. , Jain, N.K. : Advances in green synthesis of nanoparticles'. Artificial. Cells. Nanomed. Biotechnol. 47, 844–851 (2019) [DOI] [PubMed] [Google Scholar]
- 12. Abikoye, E.T. , et al.: Biosynthesis of silver nanoparticles in improved strain of Auricularia polytricha—an edible mushroom from Nigeria and its antimicrobial activities. Conven. J. Physiol. Life Sci. 7(1), 47–55 (2019) [Google Scholar]
- 13. Saleh, G.M. : Green synthesis concept of nanoparticles from environmental bacteria and their effects on pathogenic bacteria. Iraqi. J. Sci. 4, 1289–1297 (2020) [Google Scholar]
- 14. Dahlous, K.A. , et al.: Eco‐friendly method for silver nanoparticles immobilized decorated silica: synthesis & characterization and preliminary antibacterial activity. J. Taiwan. Ins. Chem. Eng. 95, 324–331 (2019) [Google Scholar]
- 15. Sidra, N. , et al.: Environmentally benign and economical phytofabrication of silver nanoparticles using Juglans regia leaf extract for antibacterial study. J. Electronic. Mater. 48, 3562–3569 (2019) [Google Scholar]
- 16. Iravani, S. , et al.: Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharmaceut. Sci. 9, 385–406 (2014) [PMC free article] [PubMed] [Google Scholar]
- 17. Milner, M.J. , Kochian, L. : Investigating heavy‐metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Botany 102, 3–13 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bilal, M. , et al.: Development of silver nanoparticles loaded chitosan‐alginate constructs with biomedical potentialities. Int. J. Biol. Macromol. 105, 393–400 (2017) [DOI] [PubMed] [Google Scholar]
- 19. Abdel‐Aziz, M.S. , et al.: Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi. Chem. Soc. 18, 356–363 (2014) [Google Scholar]
- 20. Bharathi, D. , Vasantharaj, S. , Bhuvaneshwari, V. : Green synthesis of silver nanoparticles using Cordia dichotoma fruit extract and its enhanced antibacterial, anti‐biofilm and photo catalytic activity. Mater. Res. Exp. 5, 055404 (2018) [Google Scholar]
- 21. Basu, S. , Maji, P. , Ganguly, J. : Rapid green synthesis of silver nanoparticles by aqueous extract of seeds of Nyctanthes arbor‐tristis. Appl. Nanosci. 6, 1–5 (2016) [Google Scholar]
- 22. Sagadevan, S. , et al.: Exploring the therapeutic potentials of phyto‐mediated silver nanoparticles formed via Calotropis procera (Ait.) R. Br. root extract. J. Exp. Nanosci. 15(1), 217–232 (2020) [Google Scholar]
- 23. Abbasi, B.A. , et al.: Environmentally friendly green approach for the fabrication of silver oxide nanoparticles: characterization and diverse biomedical applications. Microsc. Res. Tech. (2020). 10.1002/jemt.23522 [DOI] [PubMed] [Google Scholar]
- 24. Maity, T.R. , et al.: Evaluation of Piper betle mediated silver nanoparticle in post‐harvest physiology in relation to vase life of cut spike of Gladiolus. Bull. Nat. Res. Cent. 43(1), 1–11 (2019) [Google Scholar]
- 25. Alshehri, M.A. , et al.: Phytochemical analysis of Rhazya stricta extract and its use in fabrication of silver nanoparticles effective against mosquito vectors and microbial pathogens. Sci. Total Environ. 700, 134443 (2020) [DOI] [PubMed] [Google Scholar]
- 26. Yaqoob, A.A. , Umar, K. , Ibrahim, M.N.M. : Silver nanoparticles: various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 10, 1369–1378 (2020) [Google Scholar]
- 27. Sastry, S : Green synthesis and characterization of silver nano particles. J. Water. Environ. Nanotech. 5(1), 81–91 (2020) [Google Scholar]
- 28. Korbekandi, H. , Iravani, S. , Abbasi, S. : Production of nanoparticles using organisms. Crit. Rev. Biotech. 29, 279–306 (2009) [DOI] [PubMed] [Google Scholar]
- 29. Kathiresan, K. , et al.: Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment’. Colloid. Surface. B. 71, 133–137 (2009) [DOI] [PubMed] [Google Scholar]
- 30. Shaligram, S. , et al.: Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process. Biochem. 44, 939–943 (2009) [Google Scholar]
- 31. Krishnaraj, C. , et al.: Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids. Surface B. 76, 50–56 (2010) [DOI] [PubMed] [Google Scholar]
- 32. Vilchis‐Nestor, A. , et al.: A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Mater. Lett. 62, 3103–3105 (2008) [Google Scholar]
- 33. Li, S. , et al.: Green synthesis of silver nanoparticles using Capsicum annuum L. extract'. Green Chem. 9, 852–858 (2007) [Google Scholar]
- 34. Kesharwani, J. , et al.: Phyto‐fabrication of silver nanoparticles by leaf extract of Datura metel, hypothetical mechanism involved in synthesis. J. Bionanosci. 3, 1–6 (2009) [Google Scholar]
- 35. Song, Y. , Kim, B. : Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess. Biosyst. Eng. 32, 79–84 (2009) [DOI] [PubMed] [Google Scholar]
- 36. Elumalai, K. , et al.: Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J. Pharmaceut. Sci. Res. 2, 549–554 (2010) [Google Scholar]
- 37. Ravindra, S. , et al.: Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via green approach. Colloids. Surf. A. Physicochem. Eng. Asp. 367, 31–40 (2010) [Google Scholar]
- 38. Veerasamy, R. , et al.: Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities’. J. Saudi. Chem. Soc. 15, 113–120 (2011). [Google Scholar]
- 39. Saratale, R.G. , et al.: Phyto‐fabrication of silver nanoparticles by Acacia nilotica leaves: investigating their antineoplastic, free radical scavenging potential and application in H2O2 sensing. J. Taiwan. Ins. Chem. Eng. 99, 239–249 (2019) [Google Scholar]
- 40. Al‐Nuairi, A.G. , et al.: Biosynthesis, characterization, and evaluation of the cytotoxic effects of biologically synthesized silver nanoparticles from Cyperus conglomeratus root extracts on breast cancer cell line MCF‐7. Biol. Trace Elem. Res. 1–10 (2019) [DOI] [PubMed] [Google Scholar]
- 41. Al‐Bahrani, R.M. , et al.: Phyto‐fabrication, characteristics and anti‐candidal effects of silver nanoparticles from leaves of Ziziphus mauritiana Lam. Acta. Pharma. Sci. 56(3), 85–92 (2018) [Google Scholar]
- 42. Mallikarjuna, K. : Phyto‐synthesis and antibacterial studies of bio‐based silver nanoparticles using Sesbania grandiflora (Avisa) leaf tea extract. Mater. Res. Exp. 5, 015054 (2018) [Google Scholar]
- 43. Wang, L. , et al.: Characterization, antioxidant and antimicrobial activities of green synthesized silver nanoparticles from Psidium guajava L. leaf aqueous extracts. Mater. Sci. Eng. 86, 1–8 (2018) [DOI] [PubMed] [Google Scholar]
- 44. Sankaranarayanan, A. , et al.: Green synthesis of silver nanoparticles using Arachis hypogaea (ground nut) root extract for antibacterial and clinical applications. J. Clust. Sci. 2, 1–12 (2016) [Google Scholar]
- 45. Rajagopal, T. , et al.: Synthesis of silver nanoparticles using Catharanthus roseus root extract and its larvicidal effects. J. Environ. Biol. 36, 1283–1289 (2015) [PubMed] [Google Scholar]
- 46. Poojary, M. , Passamonti, P. , Adhikari, A.V. : Green synthesis of silver and gold nanoparticles using root bark extract of Mammea suriga: characterization, process optimization, and their antibacterial activity. BioNanoSci. 6, 110–120 (2016) [Google Scholar]
- 47. Swain, S. , et al.: Green synthesis of gold nanoparticles using root and leaf extracts of Vetiveria zizanioides and Cannabis sativa and its antifungal activities. BioNanoSci. 6, 205–213 (2016). [Google Scholar]
- 48. Velmurugan, P. , et al.: Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioproc. Biosys. Eng. 37, 1935–1943 (2014) [DOI] [PubMed] [Google Scholar]
- 49. Iravani, S. , Zolfaghari, B. : Green synthesis of silver nanoparticles using Pinus eldarica bark extract. BioMed. Res. Int. 5 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kumar, T. , et al.: Evaluation of silver nanoparticles synthetic potential of Couroupita guianensis Aubl., flower buds extract and their synergistic antibacterial activity. 3 Biotech. 6(1), 92 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gogoi, N. , et al.: Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater. Sci. Eng. 46, 463–469 (2015) [DOI] [PubMed] [Google Scholar]
- 52. Padalia, H. , et al.: Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 8, 732–741 (2015) [Google Scholar]
- 53. Hulkoti, N.I. , Taranath, T.C. : ‘Biosynthesis of silver nanoparticles using Cananga odorata (lam.) Hook. f. & thomson. Stem broth–an eco‐friendly Approach. Int. J. Pharm. Biol. Sci. 9, 113–124 (2018) [Google Scholar]
- 54. Kumar, B. , et al.: Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi. J. Biol. Sci. 24, 45–50 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sujitha, M. , Kannan, S. : Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 102, 15–23 (2013) [DOI] [PubMed] [Google Scholar]
- 56. Dubey, S. , Lahtinen, M. : Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Proc. Biochem. 45, 1065–1071 (2010) [Google Scholar]
- 57. Gnanajobitha, G. , et al.: Fruit‐mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J. Nanostruc. Chem. 3, 67 (2013) [Google Scholar]
- 58. Nalini, M. , et al.: Effect of phyto‐synthesized silver nanoparticles on developmental stages of malaria vector, Anopheles stephensi and dengue vector, Aedes aegypti. Egypt. J. Basic Appl. Sci. 4, 212–218 (2017) [Google Scholar]
- 59. Potbhare, A.K. , et al.: Phytosynthesis of nearly monodisperse CuO nanospheres using Phyllanthus reticulatus/Conyza bonariensis and its antioxidant/antibacterial assays. Mater. Sci. Eng. 99, 783–793 (2019) [DOI] [PubMed] [Google Scholar]
- 60. Zayed, M.F. , et al.: Ficus retusa‐stabilized gold and silver nanoparticles: controlled synthesis, spectroscopic characterization, and sensing properties. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 214, 496–512 (2019) [DOI] [PubMed] [Google Scholar]
- 61. Jha, M. , Shimpi, N.G. , Sonawane, S.S. : Green synthesis of zero valent colloidal nanosilver targeting A549 lung cancer cell: in vitro cytotoxicity. J. Genetic. Eng. Biotechnol. 16, 115–124 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Das, B. , et al.: Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 10, 862–876 (2015) [Google Scholar]
- 63. Nayak, R. , et al.: Green synthesis of silver nanoparticle by Penicillium purpurogenum NPMF: the process and optimization. J. Nanopart. Res. 1, 9 (2011) [Google Scholar]
- 64. Hajra, A. , Mondal, N.M. : Phytofabrication of silver nanoparticles using Elephantopus scaber and Azadirachta indica leaf extract and its effect on larval and pupal mortality of Culex quinquefasciatus. Asian. Pac. J. Trop. Dis. 6, 979–986 (2016) [Google Scholar]
- 65. Saravanakumar, K. , et al.: Green synthesis and characterization of biologically active nanosilver from seed extract of Gardenia jasminoides Ellis. J. Photochem. Photobiol. Biol. 185, 126–135 (2018) [DOI] [PubMed] [Google Scholar]
- 66. Liu, Y. , Jin, X. , Chen, Z. : The formation of iron nanoparticles by Eucalyptus leaf extract and used to remove Cr (VI). Sci. Total Environ. 627, 470–479 (2018) [DOI] [PubMed] [Google Scholar]
- 67. Devi, T.B. , Ahmaruzzaman, M. : Facile preparation of copper nanaoparticles using Coccinia grandis fruit extract and its application towards the reduction of toxic nitro compound. Mater. Today. Proc. 5, 2098–104 (2018) [Google Scholar]
- 68. Sur, U.K. , et al.: Green synthesis of silver nanoparticles using the plant extract of Shikakai and Reetha. Mater. Today. Proc. 5, 2321–2329 (2018) [Google Scholar]
- 69. Olajire, A.A. , Kareem, A. , Olaleke, A. : Green synthesis of bimetallic Pt@ Cu nanostructures for catalytic oxidative desulfurization of model oil. J. Nanostruc. Chem. 7, 159–70 (2017) [Google Scholar]
- 70. Gavamukulya, Y. , et al.: Green synthesis and characterization of highly stable silver nanoparticles from ethanolic extracts of fruits of Annona muricata. J. Inorg. Organomet. Polym. Mater. 30(4), 1231–1242 (2020) [Google Scholar]
- 71. Asghari, G. , Varshosaz, J. , Shahbazi, N. : Synthesis of silver nanoparticle using Portulaca oleracea L. extracts. Nanomed. J. 1(2), 94–99 (2014) [Google Scholar]
- 72. Taghavizadeh Yazdi, M.E. , Hamidi, A. , Amiri, M.S. : Eco‐friendly and plant‐based synthesis of silver nanoparticles using Allium giganteum and investigation of its bactericidal, cytotoxicity, and photocatalytic effects. Mater. Technol. 34(8), 490–497 [Google Scholar]
- 73. Zangeneh, M.M. , Bovandi, S. , Gharehyakheh, S. : Green synthesis and chemical characterization of silver nanoparticles obtained using Allium saralicum aqueous extract and survey of in vitro antioxidant, cytotoxic, antibacterial and antifungal properties. Appl. Organomet. Chem. 33(7), e4961 (2019) [Google Scholar]
- 74. Valsalam, S. , Agastian, P. , Arasu, M.V. : Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in‐vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B 191, 65–74 (2019) [DOI] [PubMed] [Google Scholar]
- 75. Mallikarjuna K., et al.: Phytofabrication and characterization of silver nanoparticles from Piper betle broth. Res. J. Nanosci. Nanotechnol. 2(1), 17–23 (2012) [Google Scholar]
- 76. Rastogi, L. , Arunachalam, J. : Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater. Chem. Phys. 129(1‐2), 558–563 (2011) [Google Scholar]
- 77. Muthuraman, M.S. , Nithya, S. , Christena, L.R. : Green synthesis of silver nanoparticles using Nardostachys jatamansi and evaluation of its anti‐biofilm effect against classical colonizers. Microb. Pathog. 126, 1–5 (2019) [DOI] [PubMed] [Google Scholar]
- 78. Manikandan, R. , Anjali, R. , Beulaja, M. : Synthesis, characterization, anti‐proliferative and wound healing activities of silver nanoparticles synthesized from Caulerpa scalpelliformis. Process. Biochem. 79, 135–141 (2019) [Google Scholar]
- 79. Bernardo‐Mazariegos, E. , Valdez‐Salas, B. , González‐Mendoza, D. : Silver nanoparticles from Justicia spicigera and their antimicrobial potentialities in the biocontrol of foodborne bacteria and phytopathogenic fungi. Revista. Argentina. De. Microbiol. 51(2), 103–109 (2019) [DOI] [PubMed] [Google Scholar]
- 80. Hernández‐Morales, L. , et al.: Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl. Surface Sci. 489, 952–961 (2019) [Google Scholar]
- 81. Raman, N. , Sudharsan, S. , Veerakumar, V. : Pithecellobium dulce mediated extra‐cellular green synthesis of larvicidal silver nanoparticles. Spectrochim. Acta A. 96, 1031–1037 (2012) [DOI] [PubMed] [Google Scholar]
- 82. Kohsari, I. , Mohammad‐Zadeh, M. , Minaeian, S. : In vitro antibacterial property assessment of silver nanoparticles synthesized by Falcaria vulgaris aqueous extract against MDR bacteria. J. Sol. Gel. Sci. Technol. 90(2), 380–389 (2019) [Google Scholar]
- 83. Omidi, S. , Sedaghat, S. , Tahvildari, K. : Biosynthesis of silver nanocomposite with Tarragon leaf extract and assessment of antibacterial activity. J. Nanostruc. Chem. 8(2), 171–178 (2018) [Google Scholar]
- 84. Dakshayani, S.S. , Marulasiddeshwara, M.B. , Kumar, S. : Antimicrobial, anticoagulant and antiplatelet activities of green synthesized silver nanoparticles using Selaginella (Sanjeevini) plant extract. Int. J. Biol. Macromol. 131, 787–797 (2019) [DOI] [PubMed] [Google Scholar]
- 85. Rashid, S. , Azeem, M. , Khan, S. A. : Characterization and synergistic antibacterial potential of green synthesized silver nanoparticles using aqueous root extracts of important medicinal plants of Pakistan. Colloids Surface B. 179, 317–325 (2019) [DOI] [PubMed] [Google Scholar]
- 86. Pei, J. , Fu, B. , Jiang, L. : Biosynthesis, characterization, and anticancer effect of plant‐mediated silver nanoparticles using Coptis chinensis. Int. J. Nanomed. 14, 1969 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sohal, J.K. , et al.: Determination of antioxidant potential of biochemically synthesized silver nanoparticles using Aloe vera gel extract. Plant. Sci. Today. 6(2), 208–217 (2019) [Google Scholar]
- 88. Kulkarni, A.P. , Srivastava, A.A. , Harpale, P.M. : 'Plant mediated synthesis of silver nanoparticles‐tapping the unexploited sources. J. Nat. Prod. Plant Resour. 1(4), 100–107 (201)
- 89. Okaiyeto, K. , Hoppe, H. , Okoh, A.I. : Plant‐based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: characterization and its antiplasmodial activity. J. Clust. Sci. 1–9 (2020) [Google Scholar]
- 90. Awwad, A.M. , Salem, N.M. : Green synthesis of silver nanoparticles by mulberry leaves extract. Nanosci. Nanotechnol. 2(4), 125–128 (2012) [Google Scholar]
- 91. Leela, A. , Vivekanandan, M. : Tapping the unexploited plant resources for the synthesis of silver nanoparticles. Afr. J. Biotechnol. 7(17), 3162–3165 (2008) [Google Scholar]
- 92. AlSalhi, M.S. , et al.: 'Synthesis of silver nanoparticles using plant derived 4‐N‐methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi. J. Biol. Sci. 26(5), 970–978 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sivakumar, J. , et al.: Biosynthesis of silver nanoparticles using Calotropis gigantean leaf. Afr. J. Basic Appl. Sci. 3(6), 265–270 (2011) [Google Scholar]
- 94. Jayaprakash, N. , et al.: Green synthesis of Ag nanoparticles using tamarind fruit extract for the antibacterial studies. J. Photochem. Photobiol. A. 169, 178–185 (2017) [DOI] [PubMed] [Google Scholar]
- 95. Khodadadi, B. , Bordbar, M. , Nasrollahzadeh, M. : Green synthesis of Pd nanoparticles at Apricot kernel shell substrate using Salvia hydrangea extract: catalytic activity for reduction of organic dyes. J. Colloid. Interface. Sci. 490, 1–10 (2017) [DOI] [PubMed] [Google Scholar]
- 96. Rai, M. , Yadav, A. , Gade, A. : CRC 675—current trends in phytosynthesis of metal nanoparticles. Crit. Rev. Biotechnol. 28(4), 277–284 (2008) [DOI] [PubMed] [Google Scholar]
- 97. Park, Y. , et al.: Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 5(3), 69–78 (2011) [DOI] [PubMed] [Google Scholar]
- 98. Durán, N. , Seabra, A. B. : Metallic oxide nanoparticles: state of the art in biogenic syntheses and their mechanisms. Appl. Microbiol. Biotechnol. 95(2), 275–288 (2012) [DOI] [PubMed] [Google Scholar]
- 99. Durán, N. , et al.: Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 90(5), 1609–1624 (2011) [DOI] [PubMed] [Google Scholar]
- 100. Rautaray, D. , et al.: Biological synthesis of stable vaterite crystals by the reaction of calcium ions with germinating chickpea seeds. Cryst. Growth Des. 5(2), 399–402 (2005) [Google Scholar]
- 101. Shankar, S.S. , et al.: Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 275(2), 496–502 (2004) [DOI] [PubMed] [Google Scholar]
- 102. Ankamwar, B. , Chaudhary, M. , Sastry, M. : Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth. React. Inorg. Met.‐Org. Nano.‐Met. Chem. 35, 19–26 (2005) [Google Scholar]
- 103. Shankar, S.S. , et al.: Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared‐absorbing optical coatings. Chem. Mater. 17(3), 566–572 (2005) [Google Scholar]
- 104. Shankar, S.S. , Ahmad, A. , Sastry, M. : Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Prog. 19(6), 1627–1631 (2003) [DOI] [PubMed] [Google Scholar]
- 105. Lade, B.D. : Biochemical and molecular approaches for characterization of wound stress induced antimicrobial secondary metabolites in Passiflora foetida linn., Ph.D. thesis,. Biotechnology, Sant Gadge Baba Amravati University, Amravati: (2017) [Google Scholar]
- 106. Lade, B.D. , Shanware, A.S. : Phytonanofabrication: methodology and factors affecting biosynthesis of nanoparticles in nanosystems. IntechOpen; (2020) [Google Scholar]
- 107. Hollamby, M.J. , et al.: Separation and purification of nanoparticles in a single step. Langmuir. 26(10), 6989–6994 (2010) [DOI] [PubMed] [Google Scholar]
- 108. Dahl, J.A. , Maddux, B.L. , Hutchison, J.E. : Toward greener nanosynthesis. Chem. Rev. 107(6), 2228–2269 (2007) [DOI] [PubMed] [Google Scholar]
- 109. Gurunathan, S. , et al.: Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surface B. 74, 328–335 (2009) [DOI] [PubMed] [Google Scholar]
- 110. Raut, R. , et al.: Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr. Nanosci. 5, 117–122 (2009). 10.2174/157341309787314674 [DOI] [Google Scholar]
- 111. Kowshik, M. , et al.: Extracellular synthesis of silver nanoparticles by a silver‐tolerant yeast strain MKY3. Nanotechnology 14, 1 (2002) [Google Scholar]
- 112. Ahmad, S. , et al.: Green nanotechnology: a review on green synthesis of silver nanoparticles ‐ an ecofriendly approach. Int. J. Nanomed. 14, 5087–5107 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shittu, O.K. , Ihebunna, O. : Purification of simulated waste water using green synthesized silver nanoparticles of Piliostigma thonningii aqueous leave extract. Nanosci. Nanotechnol. 8(4), 045003 (2017) [Google Scholar]
- 114. Khositanon, C. , Adpakpang, K. , Bureekaew, S. : Continuous‐flow purification of silver nanoparticles and its integration with flow synthesis. J. Flow. Chem. 10(2020), 353–362 (2020) [Google Scholar]
- 115. Yoosaf, K. , et al.: Phyto‐synthesis and structural characterization of catalytically active gold nanoparticles biosynthesized using Delonix regia leaf extract. J. Phys. Chem. 1287, 111 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang, Y. , et al.: Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid. Interface Sci. 325, 371 (2008). [DOI] [PubMed] [Google Scholar]
- 117. Choi, Y. , Ho, N. , Tung, C. : Sensing phosphatase activity by using gold nanoparticles. Angew. Chem. Int. 707, 46 (2007) [DOI] [PubMed] [Google Scholar]
- 118. Kholoud, M. , et al.: 'Synthesis and applications of silver nanoparticles. Arab. J. Chem. 3, 135–140 (2010) [Google Scholar]
- 119. Ahmad, A. , et al.: Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surface B. 28, 313–318 (2003) [DOI] [PubMed] [Google Scholar]
- 120. Iravani, S. : Green synthesis of metal nanoparticles using plants. Green Chem. 13, 2638–2650 (2011) [Google Scholar]
- 121. Khan, I. , Saeed, K. , Khan, I. : Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 12, 908–931 (2017) [Google Scholar]
- 122. McShan, D. , Ray, P.C. , Yu, H : Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 22, 116–127 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dos Santos, C.A. , et al.: Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 103, 1931–1944 (2014) [DOI] [PubMed] [Google Scholar]
- 124. Sudha, A. , Jeyakanthan, J. , Srinivasan, P : Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Effic. Technol. 3, 506–515 (2017) [Google Scholar]
- 125. Ribeiro, C. , et al.: Evaluation of cell toxicity and DNA and protein binding of green synthesized silver nanoparticles. Biomed. Pharmacother. 101, 137–144 (2018) [DOI] [PubMed] [Google Scholar]
- 126. Lin, C.X. , et al.: The acute toxic effects of silver nanoparticles on myocardial transmembrane potential, INA and IK1 channels and heart rhythm in mice. Nanotoxicology. 11, 827–837 (2017) [DOI] [PubMed] [Google Scholar]
- 127. Burdus, A. , Gherasim, O. , Mihai, A. : Biomedical applications of silver nanoparticles: an up‐to‐date overview. Nanomaterials. 8(681), 1–25 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Senthil, B. , et al.: Non‐cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B Biol. 177, 1–7 (2017) [DOI] [PubMed] [Google Scholar]
- 129. Kora, A.J. , Sashidhar, R.B. : Biogenic silver nanoparticles synthesized with rhamnogalacturonan gum: antibacterial activity, cytotoxicity and its mode of action. Arab. J. Chem. 11, 313–323 (2018) [Google Scholar]
- 130. Durán, N. , et al.: Silver nanoparticle protein corona and toxicity: a mini‐review. J. Nanobiotechnol. 13, 55 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gliga, A.R. , et al.: Size‐dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 11, 1–11 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Galbiati, V. , et al.: Invitro assessment of silver nanoparticles immunotoxicity. Food Chem. Toxicol. 112, 363–375 (2018) [DOI] [PubMed] [Google Scholar]
- 133. Vance, M.E. , et al.: Beilstein J. Nanotechnol. 6, 1769–1780 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. De‐Matteis, V. : Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and invitro/invivo toxicity evaluation. Toxics. 5, 1–29 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ferdous, Z. , Abderrahim Nemmar, A. : Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 21(7), 2375 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Brown, P.K. , et al.: Silver nanoscale antisense drug delivery system for photoactivated gene silencing. ACS Nano. 7, 2948–2959 (2013) [DOI] [PubMed] [Google Scholar]
- 137. Levard, C. , Hotze, E.M. , Lowry, G.V. : Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol. 46(13), 6900–6914 (2012) [DOI] [PubMed] [Google Scholar]
- 138. Chinnapongse, S.L. , MacCuspie, R.I. , Hackley, V.A. : Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci. Total Environ. 409(12), 2443–2450 (2011) [DOI] [PubMed] [Google Scholar]
- 139. Zook, J.M. , et al.: Measuring silver nanoparticle dissolution in complex biological and environmental matrices using UV–visible absorbance. Anal. Bioanal. Chem. 401(6), 1993 (2011) [DOI] [PubMed] [Google Scholar]
- 140. Zook, J.M. , MacCuspie, R.I. , Locascio, L.E. : Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology. 5(4), 517–530 (2011) [DOI] [PubMed] [Google Scholar]
- 141. MacCuspie, R.I. , Allen, A.J. , Hackley, V.A. : Dispersion stabilization of silver nanoparticles in synthetic lung fluid studied under in situ conditions. Nanotoxicology. 5(2), 140–156 (2011) [DOI] [PubMed] [Google Scholar]
- 142. Oksana, V. , Anna, M. : Limitations and possibilities of green synthesis and long‐term stability of colloidal Ag nanoparticles. AIP Conf. Proc. 1918(1), 1–8 (2017) [Google Scholar]
- 143. Velgosov, O. , Anna, S. , Mražíková Briančin, J. : Effect of P. kessleri extracts treatment on AgNPs synthesis', Inorganic. Nano‐Metal Chem. 50(9), 842–852 (2020) [Google Scholar]
- 144. Mittal, J. , Batra, A. , Singh, A. : Phytofabrication of nanoparticles through plant as nanofactories. Nanosci. Nanotechnol. 5(4), 1–11 (2014) [Google Scholar]
- 145. Mohanpuria, P. , Rana, N.K. , Yadav, S.K : 'Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 10, 507–517 (2008) [Google Scholar]
- 146. Chelah, N.A. , Johan, M. : Facile shape control synthesis and optical properties of silver nanoparticles stabilized by Daxad 19 surfactant. Appl. Surface Sci. 257(17), 7494–7500 (2011) [Google Scholar]
- 147. Lee, K.S. , El‐Sayed, M.A. : Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 110(2006), 19220–19225 (2006) [DOI] [PubMed] [Google Scholar]
- 148. Dadosh, T. : 'Synthesis of uniform silver nanoparticles with a controllable size. Mater. Lett. 63(26), 2236–2238 (2009) [Google Scholar]
- 149. Mousavi‐Khattat, M. , Keyhanfar, M. , Razmjou, A. : A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles, Artificial Cells. Nanomed. Biotech. 46(3), 1022–S1031 (2018) [DOI] [PubMed] [Google Scholar]
- 150. Raza, M.A. , et al.: Size‐ and shape‐dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials. 6(4), 74 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Surmeneva, M.A. , et al.: Incorporation of silver nanoparticles into magnetron‐sputtered calcium phosphate layers on titanium as an antibacterial coating. Colloids Surface B. 156, 104–113 (2017) [DOI] [PubMed] [Google Scholar]
- 152. Abdalla, S.S. , Katas, H. , Azmi, F. : Antibacterial and anti‐biofilm biosynthesised silver and gold nanoparticles for medical applications: mechanism of action, toxicity and current status. Curr. Drug Deliv. 17(2), 88–100 (2020) [DOI] [PubMed] [Google Scholar]
- 153. Deng, Y. , Si, Y. , Sun, G. : Fibrous materials for antimicrobial applications, In Handbook of Fibrous Materials (pp. 927–951) (2020) [Google Scholar]
- 154. Kim, J.S. , Kuk, E. , Yu, K.N. : Antimicrobial effects of silver nanoparticles. Nanomedicine. 3, 95–101 (2007) [DOI] [PubMed] [Google Scholar]
- 155. Sadeghi, B. , Garmaroudi, F.S. , Hashemi, M. : Comparison of the antibacterial activity on the nanosilver shapes: nanoparticles, nanorods and nanoplates. Adv. Powder Technol. 23, 22–26 (2012) [Google Scholar]
- 156. Li, P. , et al.: Synergistic antibacterial effects of β‐lactam antibiotic combined with silver nanoparticles. Nanotechnology. 16, 1912–1917 (2005) [Google Scholar]
- 157. Kim, K.J. , et al.: Antifungal effect of silver nanoparticles on dermatophytes. J. Microbiol. Biotechnol. 18, 1482–1484 (2008) [PubMed] [Google Scholar]
- 158. Pallavicini, P. , Dacarro, G. , Taglietti, A. : Self‐assembled monolayers of silver nanoparticles: from intrinsic to switchable inorganic antibacterial surfaces. Eur. J. Inorg. Chem. 4846–4855 (2018) [Google Scholar]
- 159. Kędziora, A. , et al.: Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 19, 444 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yusuf, M. : Silver nanoparticles: synthesis and applications. In Handbook of Ecomaterials. (pp. 23–43) (2020) [Google Scholar]
- 161. Slavin, Y.N. , et al.: Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15, 65 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Fröhlich, E. , Fröhlich, E. : Cytotoxicity of nanoparticles contained in food on intestinal cells and the gut microbiota. Int. J. Mol. Sci. 17, 509 (2016). 10.3390/ijms17040509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sharma, R.K. , et al.: Immunomodulatory activities of curcumin‐stabilized silver nanoparticles: efficacy as an antiretroviral therapeutic. Immunol. Invest. 46, 833–846 (2017) [DOI] [PubMed] [Google Scholar]
- 164. Li, X. , et al.: Functional gold nanoparticles as potent antimicrobial agents against multi‐drug‐resistant bacteria. ACS Nano. 8(10), 10682–10686 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Saratale, G.D. , et al.: Anti‐diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J. Clust. Sci. 28, 1709–1727 (2017) [Google Scholar]
- 166. Lakshminarayanan, R. , et al.: Recent advances in the development of antimicrobial nanoparticles for combating resistant pathogens. Adv. Healthc. Mater. 7, 1701400 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dakal, T.C. , et al.: Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 7, 1831 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hong, X. , et al.: Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave‐assisted method. Environ. Sci. Pollut. Res. Int. 23, 4489–4497 (2016) [DOI] [PubMed] [Google Scholar]
- 169. Ahmad, B. , et al.: Green synthesis, characterisation and biological evaluation of AgNPs using Agave americana, Mentha spicata and Mangifera indica aqueous leaves extract. IET. Nanobiotechnol. 10, 281–287 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sondi, I. , Salopek‐Sondi, B. : Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram‐negative bacteria. J. Colloid Interface Sci. 275(1), 177–182 (2004) [DOI] [PubMed] [Google Scholar]
- 171. Danilczuk, M. , et al.: Conduction electron spin resonance of small silver particles. Spectrochim. Acta. 63(1), 189–191 (2006) [DOI] [PubMed] [Google Scholar]
- 172. Kim, J.S. , et al.: Antimicrobial effects of silver nanoparticles. Nanomedicine. 3(1), 95–101 (2007). [DOI] [PubMed] [Google Scholar]
- 173. Hatchett, D.W. , White, H.S. : Electrochemistry of sulfur adlayers on the low‐index faces of silver. J. Phys. Chem. 100(23), 9854–9859 (1996) [Google Scholar]
- 174. Feng, Q.L. , et al.: A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 52(4), 662–668 (2000) [DOI] [PubMed] [Google Scholar]
- 175. Matsumura, Y. , et al.: Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 69(7), 4278–4281 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Garg, D. , et al.: Synthesis of silver nanoparticles utilizing various biological systems: mechanisms and applications—a review. Prog. Biomater. 1–15 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dutta, R. , Roy, S. , Datta, C. : Silver nanoparticle as antiviral agent and its uses. Nano Trends. 22(1), 28–34 (2020) [Google Scholar]
- 178. Ge, L. , et al.: Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int. J. Nanomed. 9, 2399–2407 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Taylor, P.L. , Ussher, L. , Burrell, E. : Impact of heat on nanocrystalline silver dressings. Biomaterial. 26, 7221–7229 (2005) [DOI] [PubMed] [Google Scholar]
- 180. Lara, H. , et al.: Mode of antiviral action of silver nanoparticles against HIV‐1. J. Nanobiotechnol. 8, 1 (2010). 10.1186/1477-3155-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Siadati, S. A. , Afzali, M. , Sayyadi, M. : Could silver nano‐particles control the 2019‐nCoV virus? an urgent glance to the past. Chem. Rev. Lett. 3(1), 9–11 (2020) [Google Scholar]
- 182. Zachar, O. : Formulations for COVID‐19 treatment via silver nanoparticles Inhalation Delivery (2020) [Google Scholar]
- 183. Shin, S.H. , Ye, M.K. : The effect of nano‐silver on allergic rhinitis model in mice. Clin. Exp. Otorhinolaryngol. 5, 222–227 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Nadworny, P.L. , et al.: Anti‐inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine. 4, 241–251 (2008) [DOI] [PubMed] [Google Scholar]
- 185. Sibbald, R.G. , et al.: Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Ad. Skin. Wound. Care. 20, 549–558 (2008) [DOI] [PubMed] [Google Scholar]
- 186. Castillo, P.M. , Herrera, J.L. , Fernandez‐Montesinos, R. : Tiopronin monolayer‐protected silver nanoparticles modulate IL‐6 secretion mediated by Toll‐like receptor ligands. Nanomedicine. 3(5), 627–635 (2008). 10.2217/17435889.3.5.627 [DOI] [PubMed] [Google Scholar]
- 187. Krajewska, J. B. , Długosz, O. , Sałaga, M. : Silver nanoparticles based on blackcurrant extract show potent anti‐inflammatory effect in vitro and in DSS‐induced colitis in mice. Int. J. Pharmaceut. 119549 (2020) [DOI] [PubMed] [Google Scholar]
- 188. Helmy, A. , et al.: The synergistic effect of biosynthesized silver nanoparticles from a combined extract of parsley, corn silk, and gum Arabic: in vivo antioxidant, anti‐inflammatory and antimicrobial activities. Mater. Res. Express. 7(2), 025002 (2020) [Google Scholar]
- 189. Chaloupka, K. , Malam, Y. , Seifalian, A.M. : Nanosilver as a new generation of nano‐product in biomedical applications. Trends. Biotechnol. 28, 580–588 (2010) [DOI] [PubMed] [Google Scholar]
- 190. Chen, J. , et al.: Effect of silver nanoparticle dressing on second degree burn wound. Zhonghua Wai Ke Za Zhi. 44, 50–52 (2008) [PubMed] [Google Scholar]
- 191. Huang, Y. , Li, X. , Liao, Z. : A randomized comparative trial between Anti‐coat and SD‐Ag in the treatment of residual burn wounds, including safety analysis. Burns. 33(2), 161–166 (2007). 10.1016/j.burns.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 192. Lu, S. , Gao, W. , Gu, H.Y. : Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns. 34, 623–628 (2008) [DOI] [PubMed] [Google Scholar]
- 193. Gupta, A. , et al.: Synthesis of silver nanoparticles using curcumin‐Cyclodextrins loaded into bacterial Cellulose‐based Hydrogels for wound dressing applications. Biomacromolecules. 21(5), 1802–1811 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Grunkemeier, L. , Jin, Y. , Starr, A. : Prosthetic heart valves: objective performance criteria versus randomized clinical trial. Ann. Thorac. Surg. 82, 776–780 (2006) [DOI] [PubMed] [Google Scholar]
- 195. Jamieson, R. , Fradet, G.J. , Abel, J. : Seven‐year results with the St. Jude medical silzone mechanical prosthesis. J. Thorac. Cardiovasc. Surg. 137, 1109–1115 (2009) [DOI] [PubMed] [Google Scholar]
- 196. Andara, M. , Agarwal, A. , Scholvin, D. : Hemo‐compatibility of diamond like carbon–metal composite thin films. Diamond. Mater. 15, 1941–1948 (2006) [Google Scholar]
- 197. Fan, C. , et al.: Nanoparticle‐mediated drug delivery for treatment of ischemic heart disease. Front. Bioeng. Biotechnol. 8, 687 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ghanbari, H. , et al.: Polymeric heart valves: new materials, emerging hopes. Trend. Biotechnol. 27, 359–367 (2009) [DOI] [PubMed] [Google Scholar]
- 199. Lackner, P. , Beer, R. , Broessner, G. : Efficacy of silver nanoparticles‐impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit. Care. 8, 360–365 (2008) [DOI] [PubMed] [Google Scholar]
- 200. Yamamoto, K. , et al.: Antibacterial activity of silver ions implanted in SiO2 filler on oral Streptococci. Dental. Mater. 12, 227–229 (1996) [DOI] [PubMed] [Google Scholar]
- 201. Ahn, J. , et al.: Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 25, 206–213 (2009) [DOI] [PubMed] [Google Scholar]
- 202. Magalhães, R. , Santos, B. , Lopes, G. : Nanosilver application in dental cements. ISRN Nanotech. 1–6 (2012) [Google Scholar]
- 203. Rodrigues, M.C. , et al.: Biogenic synthesis and antimicrobial activity of silica‐coated silver nanoparticles for esthetic dental applications. J. Dent. 103327 (2020) [DOI] [PubMed] [Google Scholar]
- 204. Liu, J. , Zhao, Y. , Guo, Q. : TAT‐modified nanosilver for combating multidrug‐resistant cancer. Biomaterial. 33, 6155–6161 (2012) [DOI] [PubMed] [Google Scholar]
- 205. Skirtach, G. , et al.: Remote activation of capsules containing Ag nanoparticles and IR dye by laser light. Langmuir. 20, 6988–6992 (2004) [DOI] [PubMed] [Google Scholar]
- 206. Etheridge, M.L. , et al.: The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 9(1), 1–14 (2013). 10.1016/j.nano.2012.05.013.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhang, X. , Li, Y. , Hu, Y. : Green synthesis of silver nanoparticles and their preventive effect in deficits in recognition and spatial memory in sporadic Alzheimer's rat model. Colloid Surface A. 605(20), 125288 (2020) [Google Scholar]
- 208. Morais, D.S. , Guedes, R.M. , Lopes, M.A. : Antimicrobial approaches for textiles: from research to market. Material. 9(6), 498 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zahran, M.K. , Ahmed, H.B. , El‐Rafie, M.H. : Surface modification of cotton fabrics for antibacterial application by coating with AgNPs–alginate composite. Carbohydr. Polym. 108, 145–152 (2013) [DOI] [PubMed] [Google Scholar]
- 210. Sivakumar, A. , et al.: UV protection and self‐cleaning finish for cotton fabric using metal oxide nanoparticles (2013) [Google Scholar]
- 211. Wang, M. , et al.: The green adsorption of chitosan tripolyphosphate nanoparticles on cotton fiber surfaces. Carbohydr. Polym. 101(30), 812–818 (2014) [DOI] [PubMed] [Google Scholar]
- 212. Nateghi, M.R. , Shateri‐Khalilabad, M. : Silver nanowire‐functionalized cotton fabric. Carbohydr. Polym. 117, 160–168 (2015) [DOI] [PubMed] [Google Scholar]
- 213. Zheng, Y. , et al.: Coating fabrics with gold nanorods for colouring, UV‐protection, and antibacterial functions. Nanoscale. 5(2), 788–795 (2013) [DOI] [PubMed] [Google Scholar]
- 214. Thornton, P. : Livestock production: recent trends, future prospects. Philos. Trans. R. Soc. B. 365, 2853–2867 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Boom, R. : Nanotechnology in food production. Nanotechnology in the Agri‐food sector: Implications for the future. Wiley‐VCH, Weinheim: (2011). 9–58 [Google Scholar]
- 216. Senturk, A. , Yalcýn, B. , Otles, S. : Nanotechnology as a food perspective. J. Nanomat. Mol. Nanotechnol. 2(6) (2013) [Google Scholar]
- 217. Forsberg, M. , Lauwere, C. : Integration needs in assessments of nanotechnology in food and agriculture. Etikk. i. Praksis. 1, 38–54 (2013) [Google Scholar]
- 218. Zhao, M. , Liu, L. , Her, R. : Nano‐sized delivery for agricultural chemicals. In: Tiddy, G , Tan, R (eds.) NanoFormulation, pp. 256–265.Royal Society of Chemistry, Cambridge: (2012) [Google Scholar]
- 219. Naing, A.H. , Kim, C.K. : Application of nano‐silver particles to control the postharvest biology of cut flowers: a review. Sci. Horticul. 270, 109463 (2020) [Google Scholar]
- 220. Le, N.T.T. , et al.: Silver nanoparticles ecofriendly synthesized by Achyranthes aspera and Scoparia dulcis leaf broth as an effective fungicide. Appl. Sci. 10(7), 2505 (2020) [Google Scholar]
- 221. Keat, C.L. : Biological properties of “naked” metal nanoparticles. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2, 47 (2008) [DOI] [PubMed] [Google Scholar]
- 222. Maureen, R.G. , Vallyathan, V. : Nanoparticles: health effects—pros and cons. Environ. Health. Pers. 114, 1818–1825 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Haseeb, M.T. , et al.: Linseed hydrogel‐mediated green synthesis of silver nanoparticles for antimicrobial and wound‐dressing applications. Int. J. Nanomed. 12, 2845–2855 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Saleem Khan, M. , et al.: Toxicity of silver nanoparticles in fish: a critical review. J. Biodiv. Environ. Sci. 212–227 (2015) [Google Scholar]
- 225. Sotiriou, G.A. , Pratsinis, E. : Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 1, 3–10 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]


