Abstract
The number of patients with cancer in Africa has been predicted to increase from 844 279 in 2012 to more than 1·5 million in 2030. However, many countries in Africa still lack access to radiotherapy as a part of comprehensive cancer care. The objective of this analysis is to present an updated overview of radiotherapy resources in Africa and to analyse the gaps and needs of the continent for 2030 in the context of the UN Sustainable Development Goals. Data from 54 African countries on teletherapy megavoltage units and brachytherapy afterloaders were extracted from the Directory for Radiotherapy Centres, an electronic, centralised, and continuously updated database of radiotherapy centres. Cancer incidence and future predictions were taken from the GLOBOCAN 2018 database of the International Agency for Research on Cancer. Radiotherapy need was estimated using a 64% radiotherapy utilisation rate, while assuming a machine throughput of 500 patients per year. As of March, 2020, 28 (52%) of 54 countries had access to external beam radiotherapy, 21 (39%) had brachytherapy capacity, and no country had a capacity that matched the estimated treatment need. Median income was an important predictor of the availability of megavoltage machines: US$1883 (IQR 914–3269) in countries without any machines versus $4485 (3079–12480) in countries with at least one megavoltage machine (p=0·0003). If radiotherapy expansion continues at the rate observed over the past 7 years, it is unlikely that the continent will meet its radiotherapy needs. This access gap might impact the ability to achieve the Sustainable Development Goals, particularly the target to reduce preventable, premature mortality by a third, and meet the target of the cervical cancer elimination strategy of 90% with access to treatment. Urgent, novel initiatives in financing and human capacity building are needed to change the trajectory and provide comprehensive cancer care to patients in Africa in the next decade.
Introduction
Substantial progress has been made in global oncology in recent years with numerous initiatives launched at country, regional, and international levels to tackle the growing burden of cancer.1–5 In particular, the UN Agenda 2030 for Sustainable Development with its Sustainable Development Goals has placed non-communicable diseases on the global agenda with several targets addressing cancer.6
In the framework of the 2030 Agenda, the third Sustainable Development Goal relates to improving health and wellbeing and includes the targets of reducing premature mortality from non-communicable diseases, improving access to universal health care and essential health services, and improving the training and retention of the health workforce in developing countries, all of which relate to cancer care and radiotherapy.6 In particular, target 3.4 reads: “By 2030, reduce by one third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being.” The success of countries in meeting these targets will partly depend on adequate access to radiotherapy machines and personnel, particularly for the treatment of cervical cancer, for which radiotherapy is often an essential, curative modality, and for treating cancer-related pain and the symptoms of advanced disease, for which radiotherapy can offer effective palliation.7
Radiotherapy is increasingly recognised as an essential component of comprehensive, global cancer care. The Global Taskforce in Radiotherapy for Cancer Control published robust findings from a situational analysis of global radiotherapy and the economic case for expanded access in 2015.1 WHO has not only expanded its list of essential medicines related to cancer care, but also published a comprehensive list of priority devices, including recommendations for radiotherapy machines and technical specifications, elaborated in collaboration with the International Atomic Energy Agency (IAEA).8–10
However, despite this progress, a chasm remains between radiotherapy need and current capacity in Africa. In 2012, only 20 of 52 African countries for which data were available had radiotherapy facilities.11 Although several nations have initiated or increased radiotherapy services since 2012, there have also been temporary or seemingly permanent lapses in existing capacity.12,13 In this context, the predicted increase in new cases of cancer has not flagged. The International Agency for Research on Cancer has reported an increase in new cancer cases in Africa from 844 279 in 2012 to more than 1·1 million in 2020 and predicts more than 1·5 million in 2030.14 Thus, despite an increase in capacity, the gap in radiotherapy resources has widened and will continue to widen given the projected increase in cancer burden.15
The aim of this Policy Review is to present an updated picture of the radiotherapy resources in Africa and analyse the gaps and needs of the continent for 2030—in particular, how radiotherapy gaps will impact achievement of the third Sustainable Development Goal and target 3.4,6 and achievement of the cervical cancer elimination strategy.16
Methods
Data sources and collection
We created a database of 54 African countries using open-source data, including data on population size, gross national income, gross domestic product (GDP), cancer incidence and mortality, and radiotherapy equipment. Region definitions and country names were taken from the Statistics Division of the UN Department of Economic and Social Affairs. Population and GDP per capita estimates were taken from the World Bank. We obtained current and projected data about cancer incidence at the country level from the GLOBOCAN 2018 database.
The primary data source for radiotherapy equipment was the Directory for Radiotherapy Centres (DIRAC), an electronic, centralised, and continuously updated database of radiotherapy centres created and maintained by the Division of Human Health of the IAEA. Data for Africa were extracted in March, 2020, to analyse the trends and match with GLOBOCAN projections for 2020. Historical DIRAC data from 2012 were also available. Data in the primary extraction included the operational status and number of megavoltage units—cobalt-60 units and linear accelerators— and low-dose-rate and high-dose-rate brachytherapy installations. In all countries, we verified current machine number and functional status, and checked completeness and accuracy of the data, by contacting personnel based in the radiotherapy centres or country experts, or by reviewing travel and meeting reports from IAEA staff.
Estimates of radiotherapy coverage and need
External beam radiotherapy
Estimations for patients needing radiotherapy entails taking the estimated total number of cancer cases per country and the radiotherapy utilisation rate. On the basis of the 2013 Collaboration for Cancer Outcomes Research and Evaluation radiotherapy utilisation rate model, the global rate was 50% and ranged from 47% in upper-middle-income countries to 53% in lower-middle-income countries.17 To preserve comparability with our previous analysis,11,17,18 we used an optimal radiotherapy utilisation rate of 64% as the average for African countries, which accounts for retreatment. We also report results for a radiotherapy utilisation rate of 50%, when appropriate, for easy reference to other sources.
To calculate the demand of megavoltage units, we assumed a machine throughput of 500 patients per year and a standard 8 h working day to preserve comparability with previous estimates.19–22 If the estimated number of patients requiring radiotherapy in a country is less than 500, the megavoltage need was estimated at 1 unit.
Thus, applying the radiotherapy utilisation rate to the total GLOBOCAN population-based estimated number of cancer cases gives the number of patients requiring radiotherapy. The number of megavoltage units needed results from dividing the number of patients requiring radiotherapy by the machine throughput.
The number of megavoltage units per million people is a commonly used estimator of teletherapy radiotherapy capacity that can be correlated with gross national income per capita.15,23,24 However, the demand for megavoltage units in countries is difficult to calculate on a population basis because the estimated number of cancer cases per population varies considerably between countries, from 57·2 cases per 100 000 people in The Gambia to 213 per 100 000 people in South Africa.14 In this situation, the number of megavoltage units per 1000 cancer cases might be a more robust indicator of radiotherapy demand than megavoltage units per 100 000 people, and so was also calculated and reported.
Coverage was defined as the proportion of the cancer cases needing radiotherapy that can be treated with the installed capacity, assuming an optimal radiotherapy utilisation rate of 50% and 64% and a machine throughput of 500 patients per year.25
To calculate the projected needs and gaps by 2030, we repeated the method, substituting the number of cancer cases with the projections given in GLOBOCAN 2018.
Brachytherapy
The brachytherapy analysis considered only the needs for cervical cancer. We estimated brachytherapy treatment capacity for cervical cancer in our previous analysis using a formula developed by Tatsuzaki and Levin.26 The total number of cervical cancer patients that can be treated per year is the sum of low-dose-rate capacity ([number of low-dose-rate installations multiplied by 50] + [number of low-dose-rate afterloaders multiplied by 80]) plus the high-dose-rate capacity (number of high-dose-rate afterloaders multiplied by 500). Quantitative estimates of brachytherapy capacity that are inclusive of all clinical indications have not been developed.
The brachytherapy utilisation rate for cervical cancer in Africa is not well established; the optimal rate depends on the casemix (International Federation of Gynecology and Obstetrics stage IA disease: 14%; stage IB–IIA: 47%; stage IIB–IVA: 100%; stage IVB: 0%).27 The actual brachytherapy utilisation rate depends on the casemix and also on the available resources (eg, access to surgery, chemotherapy, and radiotherapy). Robust modelling has been done for some high-income countries,28 but information for Africa is lacking.
Statistical analysis
Descriptive statistics and data visualisation were used to present aggregated and country-level data. To give an idea of geographical access to radiotherapy on the continent, we mapped the radiotherapy centres with the number of megavoltage units and used geographic information system software to visualise the population distribution relative to the radiotherapy centres.
Regional comparisons were done using one-way, non-parametric ANOVA and Kruskal-Wallis H-test for analysis of variance. We used a simple linear regression to relate installed capacity per million people (megavoltage units or brachytherapy afterloaders) and coverage (percentage of need covered) to GDP per capita among countries with any external beam radiotherapy or brachytherapy availability.
Statistical analyses were done with STATA 14, Tableau, and QGIS. The final dataset was captured on March 15, 2020, and the analysis was finalised on March 30, 2020.
Results
Current status of external beam radiotherapy and brachytherapy resources in Africa
Of the 54 African countries included in the analysis, complete data on machine availability, demographics, income, and cancer cases were available for 50 countries (93%). Income data were unavailable for Eritrea, Somalia, and South Sudan. Population data were unavailable for Eritrea. GLOBOCAN 2018 does not contain data on cancer case estimates for Seychelles.
As of March, 2020, there were 430 megavoltage units in Africa. Compared with 2012, there has been a 45% increment in the installed capacity. External beam radiotherapy was available in 28 (52%) of 54 countries, compared with 23 (43%) in 2012. Around half of the installed units were concentrated in two countries: Egypt (119 units) and South Africa (97 units). The number of megavoltage units per million people varied from 0·02 in Ethiopia, Nigeria, and Uganda to 2·37 in Mauritius. Megavoltage units per 1000 cancer cases for countries with radiotherapy services ranged between 0·01 in Ethiopia and 1·38 in Tunisia. Only three countries (Gabon, Mauritius, and Tunisia) had at least one megavoltage unit per 1000 cancer cases (figure 1). Average coverage in 2020 was 29·9%, with only one country (Tunisia) above 100%. Compared with 2012, coverage increased by only 2·7 percentage points in 2020, due to the higher increase in the cancer burden (32%) relative to the increase in the population (19%; table 1).
Figure 1: External beam radiotherapy availability in Africa in 2020.
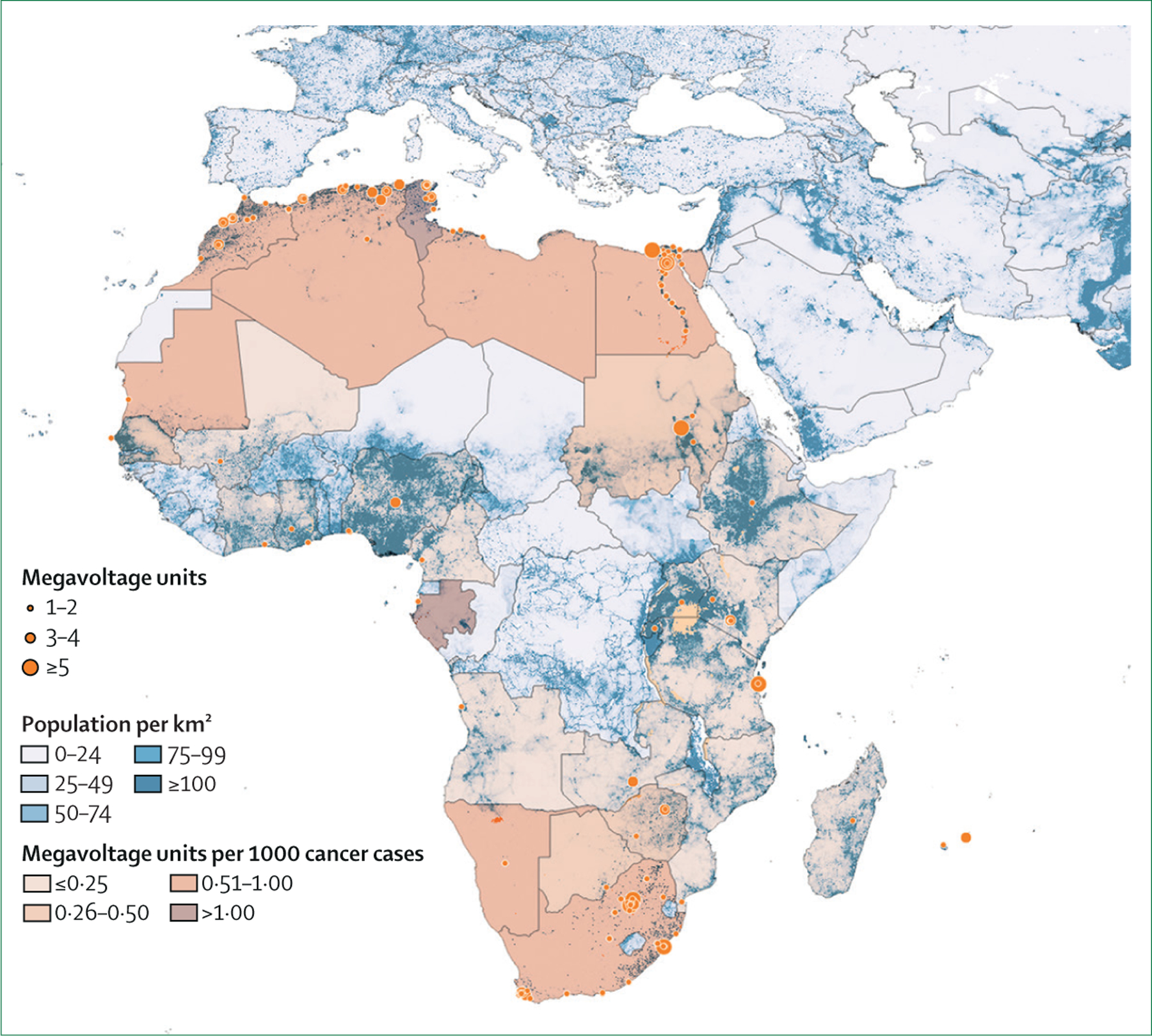
Blue layer represents population density. Orange dots represent radiotherapy centre locations, and size indicates megavoltage units per centre. Orange layer represents capacity in megavoltage units per 1000 cancer cases.
Table 1:
Overview of change in radiotherapy availability in Africa
| 2012* | 2020† | Change | |
|---|---|---|---|
| Population (million) | 1121 | 1340 | +19·5% |
| Megavoltage units | 294 | 430 | +46·3% |
| Cobalt-60 units | 89 | 64 | −28·1% |
| Linear accelerators | 205 | 366 | +78·5% |
| Ratio of cobalt-60 units to linear accelerators | 1:2·3 | 1:5·7 | ·· |
| Total cancer cases | 844 279* | 1122 495† | +33·0% |
| Cervical cancer cases | 71 017* | 127 233† | +79·2% |
| Megavoltage units per million people | 0·26 | 0·32 | +23·1% |
| Megavoltage units per 1000 cancer cases | 0·33 | 0·38 | +15·2% |
| Coverage‡ | 27·2% | 29·9% | +2·7§ |
| Brachytherapy capacity (patients with cervical cancer per year) | 24 300 | 36 100 | +48·6% |
| Coverage of cervical cancer needs | 45·0% | 37·8% | −7·2§ |
GLOBOCAN 2012 data for 2012.
GLOBOCAN 2018 projection for 2020.
Coverage assuming a radiotherapy utilisation rate of 64%.
Data are percentage point increases or decreases.
In 2020, linear accelerators accounted for 85% of 430 megavoltage units. Since 2012, the number of teletherapy cobalt-60 units decreased by 28%, while the number of linear accelerators increased by 78%. Currently, there are around 5·7 linear accelerators for each teletherapy cobalt-60 unit (table 1). 50% of countries have both linear accelerators and cobalt-60 units. 11 countries (20%) have only linear accelerators and three countries (11%) have only cobalt-60 units.
Regional differences and trends over time (2012–20) are summarised in table 2. All regions show a net increase in the availability of megavoltage units. Despite this increase in capacity, the increase in coverage was modest (except in northern Africa) and even decreased in southern Africa (table 2). Differences in distribution between regions were statistically significant (p=0·0061).
Table 2:
Regional summary of radiotherapy access in 2020
| Megavoltage units | Megavoltage units per million | Megavoltage units per 1000 cancer cases | Coverage* | |||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2020 | 2012 | 2020 | 2012 | 2020 | 2012 | 2020 | |
| Eastern Africa | 22 | 40 | 0·061 | 0·090 | 0·081 | 0·113 | 6·34% | 8·85% |
| Central Africa | 6 | 8 | 0·043 | 0·044 | 0·072 | 0·078 | 5·66% | 6·10% |
| Northern Africa | 159 | 258 | 0·794 | 1·050 | 0·764 | 0·914 | 59·70% | 71·29% |
| Southern Africa | 94 | 100 | 1·564 | 1·481 | 1·134 | 0·833 | 88·61% | 65·11% |
| Western Africa | 13 | 24 | 0·039 | 0·059 | 0·065 | 0·098 | 5·08% | 7·70% |
Coverage assuming a radiotherapy utilisation rate of 64%.
The availability of external beam radiotherapy services was analysed by income level (figure 2). There was a significant difference in median income between countries with and without external beam radiotherapy capacity. Median GDP per capita among countries without any external beam radiotherapy capacity was US$1883 (IQR 914–3269), compared with $4485 (3079–12480) for countries with at least one megavoltage unit (p=0·0003).
Figure 2: GDP and megavoltage units per 1000 cancer cases.

Income variables were log-transformed to analyse linear fit. AGO=Angola. BWA=Botswana. CIV=Côte d’Ivoire. CMR=Cameroon. DZA=Algeria. EGY=Egypt. ETH=Ethiopia. GAB=Gabon. GDP=gross domestic product. GHA=Ghana. KEN=Kenya. LBY=Libya. MAR=Morocco. MDG=Madagascar. MLI=Mali. MOZ=Mozambique. MRT=Mauritania. MUS=Mauritius. NAM=Namibia. NGA=Nigeria. PPP=purchasing power parity. RWA=Rwanda. SDN=Sudan. SEN=Senegal. TUN=Tunisia. TZA=Tanzania. UGA=Uganda. ZAF=South Africa. ZMB=Zambia. ZWE=Zimbabwe.
The number of megavoltage units per 1000 cancer cases was correlated with GDP per capita (r²=0·631, p<0·0001; figure 2). We noted that each $1000 increment in GDP per capita is correlated with a 50% increase in megavoltage units per 1000 cancer cases. In absolute numbers, this percentage represents an increase from 0·27 to 0·32 megavoltage units per 1000 cancer cases for countries with a GDP per capita around the median ($4485); 0·20 to 0·25 megavoltage units per 1000 cancer cases for countries around the lower quartile ($3079); and 0·65 to 0·70 megavoltage units per 1000 cancer cases for countries around the upper quartile ($12 480). The linear regression model accounts for 63% of the total variance, suggesting that other factors not captured might explain variability in the availability of megavoltage units at the country level.
Regarding the provision of brachytherapy services, only 21 (39%) of 54 countries in Africa had available resources. These resources included 102 individual brachytherapy installations, both high-dose-rate and low-dose-rate. The largest number of brachytherapy installations were in South Africa (24), Egypt (23), and Algeria (12), together accounting for almost 60% of total capacity. High-dose-rate brachytherapy accounts for 68% (70 afterloaders) of the total brachytherapy capacity in Africa. Assuming all available brachytherapy resources were dedicated to cervical cancer, all patients had access to them, and the brachytherapy utilisation rate was at least 75% (given the frequent late-stage presentation of this cancer), the current brachytherapy capacity could treat 36 100 patients with cervical cancer per year. In this best-case scenario, brachytherapy capacity covers 37% of the total need on the continent.
Current and projected gaps: radiotherapy need in Africa in 2030
The current gap for megavoltage units is immense, with an estimated deficit of 1018 megavoltage units from the number needed to serve more than 1·1 million patients with cancer in 2020. By region, the required number of additional megavoltage units to cover the current demand is 412 units in eastern Africa, 308 in western Africa, 123 in northern Africa, 122 in central Africa, and 53 in southern Africa. The need in western Africa is concentrated in Nigeria (147 additional megavoltage units needed), which accounts for 47% of the need in western Africa and 14% of all need in Africa (table 3).
Table 3:
Country megavoltage machine status, and current and projected needs per cancer incidence
| Cancer cases (2018) | Projected cancer cases (2020) | Supply of megavoltage units (2020) | Megavoltage units per 1000 cancer cases (2020) | Demand for megavoltage units (2020) | Projected cancer cases (2030) | Demand for megavoltage units (2030) | |
|---|---|---|---|---|---|---|---|
| Northern Africa | |||||||
| Algeria | 53 076 | 56 670 | 53 | 0·94 | 72 | 76 784 | 98 |
| Egypt | 128 892 | 136 434 | 119 | 0·87 | 174 | 183 039 | 234 |
| Libya | 6308 | 6778 | 6 | 0·74 | 8 | 9744 | 12 |
| Morocco | 52 783 | 56 115 | 49 | 0·87 | 71 | 74 323 | 95 |
| Sudan | 25 746 | 27 411 | 8 | 0·29 | 35 | 37 470 | 48 |
| Tunisia | 15 894 | 16 698 | 23 | 1·38 | 21 | 21 506 | 27 |
| Western Africa | |||||||
| Benin | 8036 | 8585 | 0 | 0 | 11 | 12 055 | 15 |
| Burkina Faso | 11 643 | 12 451 | 0 | 0 | 16 | 17 806 | 23 |
| Cape Verde | 641 | 666 | 0 | 0 | 1 | 903 | 1 |
| Côte d’Ivoire | 14 484 | 15 298 | 2 | 0·13 | 19 | 20 581 | 26 |
| The Gambia | 705 | 753 | 0 | 0 | 1 | 20 581 | 1 |
| Ghana | 22 823 | 24 178 | 5 | 0·21 | 31 | 33 232 | 42 |
| Guinea | 7274 | 7707 | 0 | 0 | 10 | 10 641 | 13 |
| Guinea-Bissau | 1037 | 1101 | 0 | 0 | 1 | 1520 | 2 |
| Liberia | 2808 | 2991 | 0 | 0 | 4 | 4181 | 5 |
| Mali | 13 114 | 13 926 | 1 | 0·07 | 18 | 19 431 | 25 |
| Mauritania | 2733 | 2935 | 2 | 0·68 | 4 | 4166 | 5 |
| Niger | 8674 | 9324 | 0 | 0 | 12 | 13 468 | 17 |
| Nigeria | 115 950 | 122 728 | 10 | 0·08 | 157 | 165 466 | 211 |
| Senegal | 10 549 | 11 260 | 4 | 0·36 | 14 | 16 002 | 20 |
| Sierra Leone | 4125 | 4354 | 0 | 0 | 5 | 5855 | 7 |
| Togo | 4745 | 5068 | 0 | 0 | 6 | 7076 | 9 |
| Central Africa | |||||||
| Angola | 15 949 | 17 263 | 5 | 0·29 | 22 | 25 562 | 33 |
| Cameroon | 15 769 | 16 776 | 1 | 0·06 | 21 | 23 390 | 30 |
| Central African Republic | 2618 | 2711 | 0 | 0 | 3 | 3522 | 4 |
| Chad | 7491 | 7990 | 0 | 0 | 10 | 11 220 | 14 |
| Congo (Brazzaville) | 2415 | 2584 | 0 | 0 | 3 | 3706 | 5 |
| DR Congo | 48 980 | 52 400 | 0 | 0 | 67 | 74 110 | 95 |
| Equatorial Guinea | 787 | 830 | 0 | 0 | 1 | 1116 | 2 |
| Gabon | 1677 | 1768 | 2 | 1·13 | 2 | 2427 | 3 |
| São Tomé and Príncipe | 118 | 128 | 0 | 0 | 1 | 181 | 1 |
| Eastern Africa | |||||||
| Burundi | 8682 | 9312 | 0 | 0 | 12 | 13 247 | 17 |
| Comoros | 510 | 541 | 0 | 0 | 1 | 748 | 1 |
| Djibouti | 674 | 717 | 0 | 0 | 1 | 970 | 1 |
| Eritrea | 3144 | 3327 | 0 | 0 | 4 | 4557 | 6 |
| Ethiopia | 67 573 | 72 249 | 2 | 0·01 | 92 | 10 1351 | 129 |
| Kenya | 47 887 | 51 905 | 13 | 0·25 | 66 | 77 894 | 99 |
| Madagascar | 18 074 | 19 393 | 2 | 0·10 | 25 | 27 682 | 35 |
| Malawi | 19 767 | 21 209 | 0 | 0 | 27 | 30 390 | 39 |
| Mauritius | 2861 | 3007 | 3 | 1·00 | 4 | 3714 | 5 |
| Mozambique | 25 631 | 27 295 | 1 | 0·04 | 35 | 37 568 | 48 |
| Rwanda | 10 704 | 11 521 | 2 | 0·17 | 15 | 16 625 | 21 |
| Seychelles | ·· | ·· | 0 | 0 | ·· | ·· | ·· |
| Somalia | 9942 | 10 582 | 0 | 0 | 14 | 14 589 | 21 |
| South Sudan | 9398 | 9989 | 0 | 0 | 13 | 13 530 | 19 |
| Tanzania | 42 060 | 45 061 | 7 | 0·16 | 58 | 64 558 | 83 |
| Uganda | 32 617 | 35 140 | 1 | 0·03 | 45 | 51 944 | 66 |
| Zambia | 12 052 | 12 932 | 3 | 0·23 | 17 | 18 789 | 24 |
| Zimbabwe | 17 465 | 18 536 | 7 | 0·38 | 24 | 25 725 | 33 |
| Southern Africa | |||||||
| Botswana | 1953 | 2080 | 1 | 0·48 | 3 | 2841 | 4 |
| Eswatini | 1074 | 1136 | 0 | 0 | 1 | 1522 | 2 |
| Lesotho | 1888 | 1937 | 0 | 0 | 2 | 2326 | 3 |
| Namibia | 2200 | 2337 | 2 | 0·86 | 3 | 3210 | 4 |
| South Africa | 107 467 | 112 499 | 97 | 0·86 | 144 | 142 477 | 182 |
According to GLOBOCAN 2018, by 2030, the estimated number of new cancer cases per year in Africa will be 1·5 million, an increase of about 36% from the estimations for 2020. The radiotherapy utilisation rate will depend on the casemix, tumour stage at presentation, and rate of re-irradiation. Therefore, it is difficult to give an overall estimation for the radiotherapy utilisation rate in 2030, but a range of 50%–60% seems realistic.29 As such, between 750 000 and 900 000 new patients will need radiotherapy in 2030. Assuming a machine throughput of 450–500 courses per year, the number of megavoltage units needed in 2030 will be between 1500 and 2000, representing an increase above current capacity of between 250% and 365%.
Regarding cervical cancer, by 2030, around 174 000 new cases will be diagnosed according to GLOBOCAN 2018, representing a 37% increase from 2020. Assuming that the majority of patients with cervical cancer present at advanced stages, and a brachytherapy utilisation rate of 75%, the number of patients needing brachytherapy will be around 130 000. If there is a full transition to high-dose-rate brachytherapy by 2030, around 197 afterloaders will be needed, which is approximately 3 times the current capacity.
Discussion
Access to radiotherapy around the world remains scarce, even in high-income countries.30,31 The current situation in Africa shows progress has been made, in absolute terms, for radiotherapy capacity when compared with the previous analysis from 2012 (table 1).11 However, the absolute increase in the number of megavoltage units between 2012 and 2020 has not kept up with the need for radiotherapy, driven by demographic growth and a higher burden of cancer on the continent, in particular cervical cancer, resulting from factors such as better diagnostics and improved control of infectious diseases. The increase in megavoltage units (45%) translates to only an extra 2·7% of patients with cancer being included in the coverage. Although this increase is an improvement, it is unlikely that by 2030 the needs of the continent will be met with this trajectory (table 2). For cervical cancer and the many other cancers that include radiotherapy as part of the curative treatment framework, this outcome will result in a striking excess in mortality. For the numerous patients that would benefit from palliative radiation for cord compression, painful bone metastases, or symptomatic brain metastases, this outcome means a striking excess in treatable suffering.
In addition, the geographical accessibility of radiotherapy is clearly not uniform (figure 1). Densely populated regions in western, central, and eastern Africa are served by a few small radiotherapy centres. A more detailed analysis of inequities in access to the provision of radiotherapy services is underway by the current authors that also explores country by country geographical variation.
Our data suggested that several countries had a transient decrease in capacity to deliver radiotherapy services between 2012 and 2020, which raises sustainability concerns. Uganda, Senegal, Madagascar, and Nigeria experienced interruptions that lasted longer than a year. Uganda has since regained equivalent capacity with one cobalt-60 machine and Senegal has established two radiotherapy centres. Nigeria lost more than half of its machine capacity, whereas Cameroon and Libya lost two thirds. 26 countries remained without radiotherapy. However, since this analysis was done, four countries have initiated (Rwanda, Côte d’Ivoire, and Mozambique) or re-established (Madagascar) capacity for external beam radiotherapy.
The curative treatment of cervical cancer relies on the provision of brachytherapy services. Since 2012, there has been aggregated progress, with an increase of 6% in the number of brachytherapy installations. However, only one additional country has gained brachytherapy capacity, and access remains very scarce. Moreover, most of the brachytherapy capacity is still concentrated in northern and southern Africa, and 33 countries remain without any brachytherapy capacity in Africa. The absence of robust modelling of the radiotherapy utilisation rate limits the applicability of our results but, given the importance of cervical cancer on the continent, the scarcity of brachytherapy capacity will negatively impact cancer outcomes.
The strong, direct correlation between national income and the availability of megavoltage units per 1000 cancer cases suggests that wealth predicates availability and is a precondition for access. Although this finding has been shown before,15,23,24,31,32 it does suggest that low-income countries with no access to radiotherapy need strategies to include it in basic packages through universal health-care coverage. Such strategies would avoid catastrophic out-of-pocket expenditure and ensure sustainability and expansion of the services. The large, initial capital expenditure is the procurement and commissioning of megavoltage external beam radiotherapy machines and brachytherapy afterloaders.1,33 However, the costs for equipment maintenance and training and retaining staff are equally important. Partnerships between national governments and non-governmental agencies (eg, universities, international non-governmental organisations, private sector businesses) have been essential for the implementation and maintenance of radiotherapy machines and clinical services in Africa, both financially and in terms of technical expertise.33–39 The development of models for partnerships to be adapted by countries without existing infrastructure should be considered.
Related to the discussion of cost is the specific consideration of cobalt-60 teletherapy units, whose use has declined proportionally in favour of linear accelerators. Cobalt-60 units cost less to purchase and maintain than linear accelerators, but they require adjustments to provide highly conformal radiation and are associated with higher skin and soft tissue toxicity for deep-seated tumours (eg, cervical cancer) than linear accelerators are. Additionally, the safe transportation and storage of cobalt-60 material is politically complex. Few comparative studies of cobalt-60 units and linear accelerators exist, but the two units might be similar in terms of quality for superficial treatments (eg, treatment of cancers within the breast) or simple palliative treatments. Further consideration of the possible role of cobalt-60 units is needed.
Other factors not analysed in the current study might explain the variation in the availability of radiotherapy equipment, such as health expenditure, the taxation system, the distribution of the population in rural and urban areas, or the situation of human resources in the country. Future analyses should investigate additional factors that are more consequential in starting, scaling up, and sustaining a national radiotherapy programme.
Our results can be used to inform the development agenda for 2030. Some progress towards target 3.4 of the third Sustainable Development Goal has been made, in particular in mortality due to cancer. However, new policies have to be implemented to accelerate this decline in mortality, in order to achieve the target by 2030.40 The success of countries in meeting these targets will in part depend on adequate and sustainable access to radiotherapy machines and personnel. Regarding the importance of cervical cancer in Africa, the World Health Assembly 2020 adopted the global strategy to accelerate the elimination of cervical cancer as a public health problem and its associated goals and targets for the period 2020–30.41 This global strategy includes a goal of 90% access to treatment for women with a diagnosis of infiltrative cervical cancer.16 In this context, our results give further reason for national health systems to reach the proposed metrics. This aim will not be possible without a more robust yearly increase in publicly accessible external beam radiotherapy and brachytherapy centres than is described in this analysis.
Our analysis has several limitations. First, there is some uncertainty about the completeness and accuracy of the DIRAC data due to the voluntary and self-reported nature of the database. Transient service interruption and downtime can be recorded in DIRAC, but this functionality is rarely used. In addition, Africa presents a very dynamic situation, with many installations happening every year and changes or substitutions of equipment. Estimating needs is subject to further uncertainties, as the optimal radiotherapy utilisation rate depends both on the casemix of cancer types and the prevalence of each stage at presentation. There is still no robust data modelling of casemix in Africa and, therefore, our estimations are subject to uncertainties. In particular, the influence of both geographical accessibility and casemixes that differ substantially from expected use patterns might be significant and cannot be accounted for here. The GLOBOCAN 2018 current and projected cancer cases might also be imprecise given the ongoing development of cancer registries in Africa.42 Additionally, our estimates assume an 8 h working day to preserve comparability with previous estimates for Africa. Estimates of overall need would certainly be lower if 12 h or 16 h working days were used. However, with machine breakdowns and uncertain case throughput, it is not clear that 12 h or 16 h working days with consistent delivery of radiotherapy would be feasible. Finally, access to quality data for human resources and machine staffing is limited and, as such, these data cannot be used to calculate real gaps in staffing. However, a substantial number of current staff will not necessarily be practising in 2030 and, even if full staff capacity were present for current machines, this current number would be a small fraction of future need.43 We suggest that additional, country-based and region-based assessments of need and capacity be done to guide policy and practice.
In conclusion, access to radiotherapy in Africa continues to be a considerable challenge. This radiotherapy access chasm will impact the ability to achieve the Sustainable Development Goals regarding non-communicable diseases and the campaign to eliminate cervical cancer. The IAEA, through its various programmes and initiatives, has worked to improve access to radiotherapy globally for many decades. The agency provides longitudinal, comprehensive support for equipment selection, staff training, ongoing quality assurance, and development of international policy recommendations.11,38,39,44–46 Ongoing efforts include support for the first radiotherapy facilities in several countries in Africa, the development of AFRONET (an international network to share clinical cases and best practices for treatment), quality assurance support for machine commissioning and maintenance, and policy initiatives to eliminate cervical cancer and improve multidisciplinary breast cancer care. Barriers to the expansion of these efforts have included a scarcity of global funding sources for radiotherapy. Urgent, novel efforts, particularly regarding financing of services—for example, a multilateral global fund for cancer care that includes radiotherapy—are needed to change the trajectory and provide comprehensive cancer care to millions.
Search strategy and selection criteria.
To provide context for this policy update, we searched MEDLINE for indexed abstracts related to cancer control in Africa. The strategy was intended to be broad so as to capture all relevant material. We used the MeSH search terms “developing countries”, “Africa”, “radiotherapy”, and “neoplasms”. Articles unrelated to Africa or population health were excluded. Our search covered articles published in English or French from database inception to Sept 30, 2018.
Acknowledgments
There was no funding source for this study. All authors had full access to the full data in this study and accept the responsibility for the decision to submit for publication.
Footnotes
Declaration of interests
We declare no competing interests.
For the region definitions and country names of the UN Statistics Division see https://unstats.un.org/unsd/methodology/m49/
For the World Bank estimates see https://datatopics.worldbank.org/world-development-indicators/
For the GLOBOCAN database see https://gco.iarc.fr/today/home
For the DIRAC database see https://dirac.iaea.org/
For the Tableau software see http://qgis.org/
For the QGIS project see https://qgis.org/en/site/
References
- 1.Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015; 16: 1153–86. [DOI] [PubMed] [Google Scholar]
- 2.Jaffray DA, Atun R, Barton M, et al. Radiation therapy and the global health agenda. Clin Oncol (R Coll Radiol) 2015; 27: 67–69. [DOI] [PubMed] [Google Scholar]
- 3.Rodin D, Abdel-Wahab M, Lievens Y. Global radiotherapy challenge: turning data into action. Lancet Glob Health 2018; 6: S15–16. [Google Scholar]
- 4.Elmore SNC, Grover S, Bourque J-M, et al. Global palliative radiotherapy: a framework to improve access in resource-constrained settings. Ann Palliat Med 2019; 8: 274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Sukhun S, de Lima Lopes G Jr, Gospodarowicz M, Ginsburg O, Yu PP. Global health initiatives of the international oncology community. Am Soc Clin Oncol Educ Book 2017; 37: 395–402. [DOI] [PubMed] [Google Scholar]
- 6.UN. The Sustainable Development Goals report 2020. 2020. https://unstats.un.org/sdgs/report/2020/The-Sustainable-Development-Goals-Report-2020.pdf (accessed June 1, 2021).
- 7.Hanna TP, Shafiq J, Delaney GP, Barton MB. The population benefit of radiotherapy for cervical cancer: local control and survival estimates for optimally utilized radiotherapy and chemoradiation. Radiother Oncol 2015; 114: 389–94. [DOI] [PubMed] [Google Scholar]
- 8.Shulman LN, Wagner CM, Barr R, et al. Proposing essential medicines to treat cancer: methodologies, processes, and outcomes. J Clin Oncol 2016; 34: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. WHO medical device technical series: WHO list of priority medical devices for cancer management. 2017. https://apps.who.int/iris/handle/10665/255262 (accessed Aug 16, 2021).
- 10.WHO. WHO model lists of essential medicines. June 23, 2019. https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06 (accessed June 1, 2021).
- 11.Abdel-Wahab M, Bourque J-M, Pynda Y, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol 2013; 14: e168–75. [DOI] [PubMed] [Google Scholar]
- 12.Elmore SN, Sethi RV, Kavuma A, Kanyike DM. Broken machines or broken systems: the road to meaningful global radiotherapy access. J Glob Oncol 2016; 3: 438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irabor OC, Nwankwo KC, Adewuyi SA. The stagnation and decay of radiation oncology resources: lessons from Nigeria. Int J Radiat Oncol Biol Phys 2016; 95: 1327–33. [DOI] [PubMed] [Google Scholar]
- 14.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 15.Zubizarreta EH, Fidarova E, Healy B, Rosenblatt E. Need for radiotherapy in low and middle income countries—the silent crisis continues. Clin Oncol (R Coll Radiol) 2015; 27: 107–14. [DOI] [PubMed] [Google Scholar]
- 16.Simelela PN. WHO global strategy to eliminate cervical cancer as a public health problem: an opportunity to make it a disease of the past. Int J Gynaecol Obstet 2021; 152: 1–3. [DOI] [PubMed] [Google Scholar]
- 17.Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol 2014; 112: 140–44. [DOI] [PubMed] [Google Scholar]
- 18.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005; 104: 1129–37. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblatt E Planning national radiotherapy services. Front Oncol 2014; 4: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunscombe P, Grau C, Defourny N, et al. Guidelines for equipment and staffing of radiotherapy facilities in the European countries: final results of the ESTRO-HERO survey. Radiother Oncol 2014; 112: 165–77. [DOI] [PubMed] [Google Scholar]
- 21.Bentzen SM, Heeren G, Cottier B, et al. Towards evidence-based guidelines for radiotherapy infrastructure and staffing needs in Europe: the ESTRO QUARTS project. Radiother Oncol 2005; 75: 355–65. [DOI] [PubMed] [Google Scholar]
- 22.International Atomic Energy Agency. Setting up a radiotherapy programme: clinical, medical physics, radiation protection and safety aspects. February, 2008. https://www-pub.iaea.org/MTCD/Publications/PDF/pub1296_web.pdf (accessed June 1, 2021).
- 23.Rosenblatt E, Izewska J, Anacak Y, et al. Radiotherapy capacity in European countries: an analysis of the Directory of Radiotherapy Centres (DIRAC) database. Lancet Oncol 2013; 14: e79–86. [DOI] [PubMed] [Google Scholar]
- 24.Levin V, Tatsuzaki H. Radiotherapy services in countries in transition: gross national income per capita as a significant factor. Radiother Oncol 2002; 63: 147–50. [DOI] [PubMed] [Google Scholar]
- 25.International Atomic Energy Agency. Planning national radiotherapy services: a practical tool. Vienna, Austria: International Atomic Energy Agency, 2010. [Google Scholar]
- 26.Tatsuzaki H, Levin CV. Quantitative status of resources for radiation therapy in Asia and Pacific region. Radiother Oncol 2001; 60: 81–89. [DOI] [PubMed] [Google Scholar]
- 27.Thompson S, Delaney G, Gabriel GS, Jacob S, Das P, Barton M. Estimation of the optimal brachytherapy utilization rate in the treatment of carcinoma of the uterine cervix: review of clinical practice guidelines and primary evidence. Cancer 2006; 107: 2932–41. [DOI] [PubMed] [Google Scholar]
- 28.Thompson SR, Delaney GP, Gabriel GS, Jacob S, Das P, Barton MB. Estimation of the optimal brachytherapy utilization rate in the treatment of gynecological cancers and comparison with patterns of care. Int J Radiat Oncol Biol Phys 2013; 85: 400–05. [DOI] [PubMed] [Google Scholar]
- 29.Yap ML, Hanna TP, Shafiq J, et al. The benefits of providing external beam radiotherapy in low- and middle-income countries. Clin Oncol (R Coll Radiol) 2017; 29: 72–83. [DOI] [PubMed] [Google Scholar]
- 30.Borras JM, Lievens Y, Dunscombe P, et al. The optimal utilization proportion of external beam radiotherapy in European countries: an ESTRO-HERO analysis. Radiother Oncol 2015; 116: 38–44. [DOI] [PubMed] [Google Scholar]
- 31.Zubizarreta E, Van Dyk J, Lievens Y. Analysis of global radiotherapy needs and costs by geographic region and income level. Clin Oncol (R Coll Radiol) 2017; 29: 84–92. [DOI] [PubMed] [Google Scholar]
- 32.Mousa AG, Bishr MK, Mula-Hussain L, Zaghloul MS. Is economic status the main determinant of radiation therapy availability? The Arab world as an example of developing countries. Radiother Oncol 2019; 140: 182–89. [DOI] [PubMed] [Google Scholar]
- 33.Balogun O, Rodin D, Ngwa W, Grover S, Longo J. Challenges and prospects for providing radiation oncology services in Africa. Semin Radiat Oncol 2017; 27: 184–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaghloul MS, Bishr MK. Radiation oncology in Egypt: a model for Africa. Int J Radiat Oncol Biol Phys 2018; 100: 539–44. [DOI] [PubMed] [Google Scholar]
- 35.Leng J, Ntekim AI, Ibraheem A, Anakwenze CP, Golden DW, Olopade OI. Infrastructural challenges lead to delay of curative radiotherapy in Nigeria. JCO Glob Oncol 2020; 6: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubagumya F, Costas-Chavarri A, Manirakiza A, et al. State of cancer control in Rwanda: past, present, and future opportunities. JCO Glob Oncol 2020; 6: 1171–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Efstathiou JA, Heunis M, Karumekayi T, et al. Establishing and delivering quality radiation therapy in resource-constrained settings: the story of Botswana. J Clin Oncol 2016; 34: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Wahab M, Fidarova E, Polo A. Global access to radiotherapy in low- and middle-income countries. Clin Oncol (R Coll Radiol) 2017; 29: 99–104. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Wahab M, Grover S, Zubizarreta EH, Polo Rubio JA. Addressing the burden of cervical cancer through IAEA global brachytherapy initiatives. Brachytherapy 2020; 19: 850–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett JE, Stevens GA, Mathers CD, et al. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018; 392: 1072–88. [DOI] [PubMed] [Google Scholar]
- 41.World Health Assembly. Global strategy to accelerate the elimination of cervical cancer as a public health problem and its associated goals and targets for the period 2020–2030. August 3, 2020. https://apps.who.int/gb/ebwha/pdf_files/WHA73/A73_R2-en.pdf (accessed June 1, 2021).
- 42.Omonisi AE, Liu B, Parkin DM. Population-based cancer registration in sub-Saharan Africa: its role in research and cancer control. JCO Glob Oncol 2020; 6: 1721–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elmore SN, Prajogi GB, Polo A, Zubizarreta E. The global radiation oncology workforce in 2030: estimating physician training needs and proposing solutions to scale up capacity in low- and middle-income countries. Appl Radiat Oncol 2019; 8: 10–16. [Google Scholar]
- 44.Enwerem-Bromson N, Abdel-Wahab M. Expanding global access to radiotherapy: the IAEA perspective. Lancet Oncol 2015; 16: 1151–52. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Wahab M, Zubizarreta E, Polo A, Meghzifene A. Improving quality and access to radiation therapy—an IAEA perspective. Semin Radiat Oncol 2017; 27: 109–17. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Wahab M, Rosenblatt E, Prajogi B, Zubizarretta E, Mikhail M. Opportunities in telemedicine, lessons learned after COVID-19 and the way into the future. Int J Radiat Oncol Biol Phys 2020; 108: 438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


