Abstract
The aim of this study was to evaluate the effects of iron (Fe)/SDS and gold (Au) nanoparticles on growth and biosurfactant production of Pseudomonas aeruginosa PBCC5. The concentrations of the nanoparticles used were 1, 500 and 1000 mg/l. In this research, the surface tension of biosurfactant, dry weight of biosurfactant and biomass, emulsification indexes (E24) were measured and transmission electron microscopy analysis was used to monitor the nanoparticles. The test results showed that the effect of nanoparticles on the bacterial growth and biosurfactant production varied corresponding to the type and concentration of nanoparticles. Fe/SDS nanoparticles showed no bacterial toxicity when the concentration of nanoparticles was 1 mg/ml and increased the growth and biosurfactant production, 23.21 and 20.73%, respectively. While at higher concentrations (500, 1000 mg/l), the nanoparticles suppressed bacterial growth as well as biosurfactant production. Similarly, Au nanoparticles had no bacterial toxicity and also increased bacterial growth and biosurfactant production. The surface tensions of all samples decreased from 72 of distiled water to 32–35 mN/m.
Inspec keywords: nanoparticles, iron, gold, nanofabrication, nanomedicine, surfactants, biomedical materials, surface tension, renewable materials, transmission electron microscopy, microorganisms
Other keywords: Au nanoparticles, P. aeruginosa bacterial growth, biosurfactant production, Pseudomonas aeruginosa PBCC5, surface tension, biomass, emulsification indexes, dry weight, transmission electron microscopy, Fe‐SDS nanoparticles, distiled water, Fe, Au
1 Introduction
Biosurfactants are amphipathic compounds with a hydrophobic chain and a hydrophilic head. They can reduce the surface tension and interfacial tension, by forming water‐in‐oil or oil‐in‐water emulsion. Their hydrophilic head is generally a peptide (cationic or anionic) and their one or two chains are polysaccharidic. Biosurfactants have several industrial and environmental applications based on their detergency, emulsification and solubilisation of hydrophobic compounds [1, 2, 3, 4]. Biosurfactants have several advantages over chemical surfactants, particularly in relation to their low toxicity, high selectivity, environmental compatibility, biodegradability and specific activity at extreme temperatures, pH and salinity [5]. Biosurfactants are extracellular secondary metabolites which are produced from different species of microorganisms in the stationary phase. They have different molecular sizes depending on microorganism species that produced them. The function of biosurfactants in the microbial cell is regulatory factors related to stress conditions, swarming, virulence and quorum sensing and biofilm formation [6, 7, 8]. In recent years, several studies used new microorganisms and cheap substrates to produce cost‐effective biosurfactants [1, 2, 3, 9, 10, 11]. Different carbon sources have been used to optimise biosurfactant production, e.g. vegetable oils such as canola, corn, sunflower, soybean or waste vegetable oil [2, 11, 12, 13] and also glycerol, maltose and glucose [2].
The first time, it was reported that crystalline glycolipid isolated from Pseudomonas aeruginosa showed antibiotic activity against Tuberculosis in mice. Several years ago, biosurfactants and their great capabilities were not well known [14]. Glycolipids are a major class of biosurfactants and the main glycolipid is rhamnolipid produced by P. aeruginosa [7, 15, 16, 17]. Rhamnolipids are used in microbially enhanced oil recovery (MEOR) industry [6, 8, 18, 19, 20, 21] and also the removal of pollutants from the environment [22]. Ghurye et al. for the first time in 1994 used molasses for biosurfactant production [10]. Numerous studies reported the production of rhamnolipid biosurfactants in mediums containing molasses and other sources by P. aeruginosa [13, 23, 24]. P. aeruginosa is a Gram‐negative, rod‐shaped and monoflagellated bacteria. It is very a ubiquitous microorganism, for example, it has been found in environments such as soil, water, humans, animals, plants and hospital. In the late 19th century, P. aeruginosa was introduced as a separate bacterial species [25].
Nowadays, a study on the interaction of nanoparticles with bacteria is of increasing interest. Different reports also have shown that nanoparticles can improve the growth of bacteria and biosurfactant production. Gold (Au) nanoparticles have very low toxicity to microorganisms and have been used in biosensors and other biomedical applications [11, 26]. Some bacteria can acquire energy from the oxidation of Fe2+ –Fe3+. This ion is an essential element for bacterial growth and metabolism [27, 28].
Several studies have shown that iron (Fe), Au, Fe‐coated Au nanoparticles have different effects on bacteria growth and biosurfactant production. For example, in 2013 researchers reported Fe nanoparticles coated with Au reduce oxidation compared with the uncoated Fe nanoparticles. Fe/Au nanoparticles not only did not show any negative effect on bacteria but also they showed a positive effect on bacterial growth and production of biosurfactant [11, 26, 27, 28].
In this paper, the effects of Fe/SDS and Au nanoparticles on the growth of P. aeruginosa PBCC5 bacteria and its biosurfactant production were investigated. and biosurfactant production, biomass and Emulsification activity (E24) were measured.
2 Materials and methods
2.1 Chemicals
All reagents were commercially available and used without further purification (methanol, ethanol, chloroform, phosphate buffered saline solution (PBS), nutrient broth (NB), HCl. Nanoparticles used in this paper bought from US Research Nanomaterials, Inc. (Au) and Biological Engineering pars (Fe/SDS). Nanoparticles were sterilised using ethanol and centrifuged at 8000 rpm for 10 min. Following this, nanoparticles were dried at the sterile condition and finally suspended in PBS buffer and dispersed with ultrasonic bath for 30 min.
2.2 Microorganism
P. aeruginosa is a Gram‐negative and rod‐shaped bacterium. It is the most common bacterium that can be found in water and soil environments. P. aeruginosa PBCC5 was used in this paper. Partial sequence of P. aeruginosa strain PBCC% 16S ribosomal RNA gene is presented in Table 1. It was obtained from the soil of an oil‐rich area by the Department of Biotechnology Research Institute of Petroleum Industry, Tehran, Iran. Characterisation of P. aeruginosa PBCC5 was carried out using the method of Gram staining. The growth of pure cultures was measured as an increase in optical density (OD) of the culture. Total cell number was measured by direct counting using a light microscope with a counting grid.
Table 1.
P. aeruginosa strain PBCC5 16 S ribosomal RNA gene, partial sequence
| GenBank: FJ463254.1 |
|---|
| LOCUS: FJ463254, 1410 bp, DNA linear |
| ACCESSION: FJ463254 |
| VERSION: FJ463254.1 |
| SOURCE: P. aeruginosa |
| /mol_type = ″genomic DNA’ |
| /strain = ″PBCC5’ |
| /isolation_source = ″petroleum oil contaminated soil’ |
| /product = ″16S ribosomal RNA’ |
| ORIGIN |
| 1 gatgaaggga gcttgctcct ggattcagcg gcggacgggn |
| nnnnnnnnnn nnnnaatctg |
| 61 cctggtngtg ggggataacg tnnnnnnncg ggcgctaata |
| ccgcatacgt cctgagggag |
| 121 aaagtggggg atcttcggac ctcacgctat cagatgagcc |
| taggtcggat tagctagttg |
| 181 gtggggtaaa ggcctaccaa ggcgacgatc cgtaactggt |
| ctgagaggat gatcagtcac |
| 241 actggaactg agacacggtc cagactccta cgggaggcag |
| cagtggggaa tattggacaa |
| 301 tgggcgaaag cctgatccag ccatgccgcg tgtgtgaaga |
| aggtcttcgg attgtaaagc |
| 361 actttaagtt gggaggaagg gcagtaagtt aataccttgc |
| tgttttgacg ttaccaacag |
| 421 aataagcacc ggctaacttc gtgccagcag ccgcggtaat |
| acgaagggtg caagcgttaa |
| 481 tcggaattac tgggcgtaaa gcgcgcgtag gtggttcagc |
| aagttggatg tgaaatcccc |
| 541 gggctcaacc tgggaactgc atccaaaact actgagctag |
| agtacggtag agggtggtgg |
| 601 aatttcctgt gtagcggtga aatgcgtaga tataggaagg |
| aacaccagtg gcgaaggcga |
| 661 ccacctggac tgatactgac actgaggtgc gaaagcgtgg |
| ggagcaaaca ggattagata |
| 721 cccctggtag tccacgccgt aaacgatgtc gactagccgt |
| tgggatcctt gagatcttag |
| 781 tggcgcagct aacgcgataa gtcgaccgcc tggggagtac |
| ggccgcaagg ttaaaactca |
| 841 aatgaattga cgggggcccg cacaagcggt ggagcatgtg |
| gtttaattcg aagcaacgcg |
| 901 aagaacctta cctggccttg acatgctgag aactttccag |
| agatggattg gtgccttcgg |
| 961 gaactcagac acaggtgctg catggctgtc gtcagctcgt |
| gtcgtgagat gttgggttaa |
| 1021 gtcccgtaac gagcgcaacc cttgtcctta gttaccagca |
| cctcgggtgg gcactctaag |
| 1081 gagactgccg gtgacaaacc ggaggaaggt ggggatgacg |
| tcaagtcatc atggccctta |
| 1141 cggccagggc tacacacgtg ctacaatggt cggtacaaag |
| ggttgccaag ccgcgaggtg |
| 1201 gagctaatcc cataaaaccg atcgtagtcc ggatcgcagt |
| ctgcaactcg actgcgtgaa |
| 1261 gtcggaatcg ctagtaatcg tgaatcagaa tgtcacggtg |
| aatacgttcc cgggnnnnnn |
| 1321 nnnnnccgcc cgtcacacca tgggagtggg ttgctccann |
| nnnnnctagt ctaaccgcaa |
| 1381 gggggacggt taccacggag tgattcatga |
| — |
2.3 Cultivation condition
NB was used for seed culture. The cultures were grown overnight (16 h) at 38°C and 180 rpm [12, 26]. Flasks (250 ml) containing 60 ml of molasses were inoculated with 1% (v/v) seed culture. Molasses was provided by Marvdasht sugar factory in Shiraz, Iran. It was dissolved in tap water to get a concentration of 15% (w/v) and pH was adjusted to 7×1 M HCl. Flasks were autoclaved at 121°C for 20 min. The cultures with bacteria were incubated at 180 rpm and 38°C for 96 h (4 days).
2.4 Growth curve and biomass
P. aeruginosa PBCC5 was grown separately in sterilised NB provided overnight (16 h). Then, inoculums were transferred into sterilised 15% (w/v) molasses and stirred at 180 rpm and 38°C in a shaker incubator for 96 h. The growth curve of bacteria was achieved by measurement of OD (OD = 600 nm) for every 2 h in 4 days [26]. This curve showed that P. aeruginosa PBCC5 lagging phase was passed after 6–8 h of growth and logarithmic phase began. Bacteria in logarithmic phase quickly start to multiply and produce metabolites. Stationary phase began after 20–22 h of bacteria growth and biosurfactants were produced in this phase (Fig. 1). The amount of bacterial growth was measured by dry weight of biomass. The culture samples were centrifuged at 10,000 rpm for 20 min at 4°C (The cells were washed with distiled water and centrifuged repeatedly to remove medium compounds.). Then, sedimentation of cells was dried at 80°C in a hot oven and weighed [7, 9, 20]. Biomass was reported of g/l (dry weight).
Fig. 1.
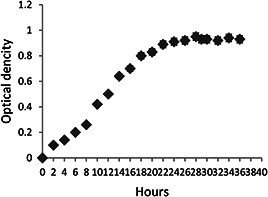
Growth curve of P. aeruginosa PBCC5 in molasses. OD of culture was measured every 2 h by spectrophotometer at a wavelength of 600 nm for 4 days. P. aeruginosa PBCC5 lagging phase was passed after 6–8 h of growth and logarithmic phase began. Stationary phase began after 20–22 h of bacteria growth
2.5 Interaction of nanoparticles and bacteria
The concentrations of nanoparticles used for interaction tests were Fe/SDS (1, 500 and 1000 mg/l) and Au (500 and 1000 mg/l). Both of nanoparticles were sonicated in PBS buffer for 30 min and then were added to the bacteria culture [11]. Samples were compared with a control sample (a flask containing cells plus media without nanoparticles).
2.6 Biosurfactant production
The amount of biosurfactant was measured by extraction of rhamnolipids from the culture medium. Culture samples were centrifuged at 10,000 rpm and 4°C for 20 min to remove the cells as well as nanoparticles. The supernatant was separated using 1 M HCl acidic precipitation (pH: 2) and it was kept in the refrigerator at 4°C overnight for better sedimentation of rhamnolipids. Then, it was centrifuged at 8000 rpm for 20 min and the resultant sediment was washed with methanol and chloroform solvents with a volume ratio of 1:2. Then, the washed sediments were dried in a rotary vacuum evaporator and weighed [13, 16].
2.7 Emulsification activity (E24)
About 2 ml of gas oil was added to 2 ml of supernatant with a volume ratio of 1:1. The mixture was vortexed with a high rate for 2 min [6, 28, 29]. The samples were kept for 24 h stabile and the emulsification indexes (E24) were estimated after 24 h using this formula
2.8 Surface tension
The culture mediums on the fourth day (96 h) were centrifuged at 10,000 rpm for 20 min in order to remove the cells and then surface tension of supernatant was measured by tensiometer KRUESS KLOT K9 and reported as mN/m. An efficient biosurfactant can reduce the surface tension of water from 72 to 35 mN/m [1, 30].
2.9 Microscopic study
About 1 ml of culture was centrifuged at 1000 rpm for 10 min and the settled cells were suspended in PBS buffer. The morphology of the bacteria and their interactions with both of nanoparticles were observed using transmission electron microscopy (TEM) after 12 h (log phase) of bacteria and nanoparticles inoculation [11].
3 Results and discussion
3.1 Emulsification index (E24)
E24 was used to stabilise the biosurfactant after 24 h (E24). Emulsification activity was measured daily (24, 48, 72 and 96 h). Emulsification was not observed in any of the samples on the first day (24 h). Samples exposed to Au nanoparticles had significant changes compared with control sample. It can be said that none of the concentrations of Au nanoparticles was ineffective on emulsion indexes. Emulsification indexes reduced with increasing the concentration of Fe/SDS nanoparticles from 500 to 1000 mg/l compared with control sample on the second day (48 h) while for sample exposed to 1 mg/l Fe/SDS, the emulsification index was higher than for control sample. It is likely that this concentration of Fe/SDS (1 mg/l) causes bacteria to reach stationary phase sooner and produce biosurfactant. Any changes in emulsification indexes were observed on fourth day (96 h). According to the results of E24, it can be said that the low concentration of Fe/SDS (1 mg/l) nanoparticles caused the bacteria to reach stationary phase sooner and enhanced production of biosurfactant, while higher concentrations of nanoparticles (500, 1000 mg/l) decreased biosurfactant production in the first 48 h (Fig. 2).
Fig. 2.
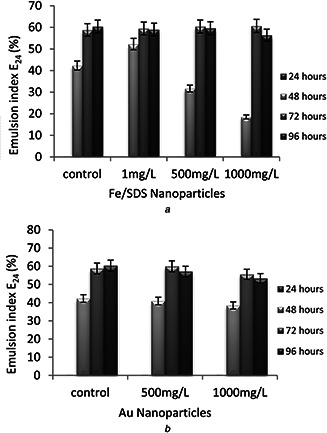
Higher Nanoparticle concentrations
(a) Effects of Fe/SDS nanoparticles on emulsification activity (E24), (b) Effect of Au nanoparticles on emulsification activity (E24)
In 2012, Bendaha et al. [29] reported biosurfactant produced by P. aeruginosa P.B.2 bacteria showed E24 = 56.32% and other strain P. fluorescens P.V. 10 showed E24 = 56.443%. For a sample of 1 mg/l Fe/SDS, biosurfactant produced by P. aeruginosa PBCC5 showed E24 = 52.27% at the second day of incubation. In this research, all of the samples had good emulsification activity in all days but the best emulsification index belonged to the sample exposed to 1 mg/l Fe/SDS nanoparticles for two days, which was because of the faster biosurfactant production in this sample. The emulsification index for this sample was E24 = 52.27% after 48 h.
3.2 Surface tension
Surface tensions of all samples were measured after 96 h of incubation. The results showed that all samples decreased the surface tension from 72 of distiled water to 32–35 mN/m. According to these results, it was demonstrated that both nanoparticles (Au and Fe/SDS) at different concentrations did not have a negative effect on surface tension. It was due to the amount of produced biosurfactant was reached to critical micelle concentration (Fig. 3).
Fig. 3.
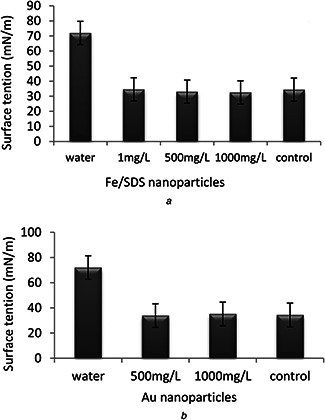
Surface tension
(a) Effect of Fe/SDS nanoparticle concentration on the surface tension of biosurfactants after 96 h of incubation, (b) Effect of Au nanoparticle concentration on the surface tension of biosurfactants after 96 h of incubation. Surface tension was measured after 96 h of co‐incubation. All samples decreased the surface tension from 72 of distiled water to 32–35 mN/m
These results were acceptable in terms of purity and it was stated by EL‐Sheshtawy et al. [30] that a good biosurfactant can reduce the surface tension of water from 72 to 35 mN/m. In addition, they reported biosurfactant produced by P. aeruginosa ATCC‐10145 decreased the surface tension from 72 distiled water to 32 mN/m. Even this strain had higher reduction than other strain Bacillus subtilis NCTC‐104.
3.3 Nanoparticles effect on biomass and biosurfactant production
The growth of bacterial cells was showed by biomass after 96 h (4 days) co‐incubation with nanoparticles. According to the result, the concentrations of nanoparticles had different effects on bacterial growth. At a concentration of 1 mg/l, Fe/SDS nanoparticles not only did not cause toxicity to the bacteria but also increased the growth amount to 23.21% compared with control sample. With increasing concentration of Fe/SDS nanoparticles, the growth of cells was reduced and thus the biosurfactant production decreased. This may be due to the cell wall penetration or breakage and oxidative stresses caused by the high concentration of Fe/SDS. Au nanoparticles increased cell growth at concentrations of 500 and 1000 mg/l by 16 and 5%, respectively. Probably these concentrations of nanoparticles could enhance the nutrition of medium to activate the bacteria (Fig. 4).
Fig. 4.
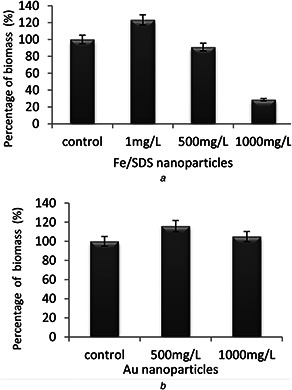
Nanoparticles effect on biomass production
(a) Effect of Fe/SDS nanoparticles on the bacterial growth after 96 h of exposure, (b) Effect of Au nanoparticles on the bacterial growth after 96 h of exposure
Biosurfactant production was measured after 96 h co‐incubation with both nanoparticles in the fermentation medium of bacteria. According to the results shown in Fig. 5, the most biosurfactant production belonged to the sample exposed to 1 mg/l Fe/SDS. In this sample, biosurfactant production was 20.73% higher compared with control sample. Both increments of cells growth and more secretion biosurfactant are responsible for this enhancement. Fe/SDS nanoparticles at concentrations of 500 and 1000 mg/l were toxic to the bacteria. These concentrations of nanoparticles had negative effects on the bacterial cell which may be due to the breakdown of the cell walls or reduction of bacterial growth. Biosurfactant production in the presence of 500 and 1000 mg/l Au nanoparticles increased 14.63 and 4.87% compared with control sample. This is may be due to the low toxicity of Au nanoparticles even in high concentrations. The important point is that, with increasing concentration of Au nanoparticles from 500 to 1000 mg/l, the increase in cell growth and biosurfactant production was lower, which indicated that at concentrations above 1000 mg/l, Au nanoparticles have a negative effect on cell growth (Fig. 5).
Fig. 5.
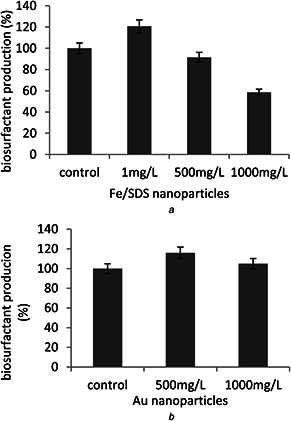
Nanoparticles effect on biosurfactant production
(a) Biosurfactant production by bacteria after 4 days of exposure to the various amounts of Fe/SDS nanoparticles, (b) Biosurfactant production by the bacteria after 4 days of exposure to the various amounts of Au nanoparticles
These obtained results were similar to the observation made by Liu et al. [11, 27] on Serratia bacteria. Liu reported Au/Fe nanoparticles showed less toxicity compared with Fe nanoparticles on Serratia. About 1 mg/l of Fe was not harmful to the Serratia while concentrations higher than 10 mg/l of Fe led to a sharp decrease in the bacteria density. This could be due to minimal interaction at lower concentrations of nanoparticles with bacteria.
In another work reported by Kiran et al. [28], the Fe nanoparticles up to a concentration of 10 mg/l did not show toxicity on Nocardiopsis bacteria. This could be due to the minimal interaction between the bacteria and nanoparticles at a lower concentration. For Fe/SDS nanoparticles concentrations higher than 1 mg/l, caused higher toxicity to the bacteria, this may be due to the cell wall penetration or breakage and oxidative stresses caused by the Fe/SDS nanoparticles.
As shown in Figs. 4 a and 5 b, Au nanoparticles did not influence the bacteria growth and biosurfactant production in the range of 500 and 1000 mg/l, which was similar to the observations by Liu et al. [11, 27] and Chatterjee et al. [26]. It can be seen that Fe/SDS nanoparticles were more toxic than Au nanoparticles. This is probably because Fe nanoparticles are more reactive than Au nanoparticles. Au nanoparticles have very low biological toxicity and are widely used in biological application [11, 26].
3.4 Microscopic observation
The result of scanning electron microscopy (SEM) showed that both of nanoparticles (Fe/SDS, Au) had a spherical shape. Au nanoparticles were about 50 nm and Fe/SDS nanoparticles were about 20 nm in diameter (Figs. 6 and 7).
Fig. 6.
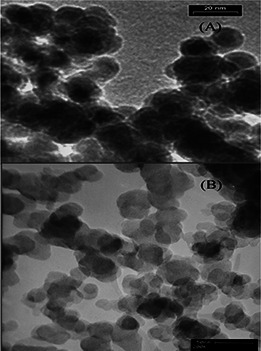
SEM of Fe/SDS and Au nanoparticles
(a) SEM image of spherical shape Fe/SDS nanoparticles shows the size of the nanoparticles to be 20 nm, (b) SEM image of spherical shape Au nanoparticles shows the size of the nanoparticles to be 50 nm
Fig. 7.
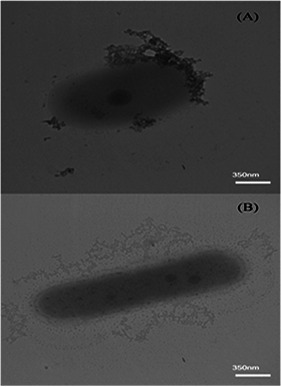
Microscopic observation
(a) TEM images of P. aeruginosa PBCC5 grown in the presence of Fe/SDS nanoparticles, (b) TEM images of P. aeruginosa in the presence of PBCC5 Au nanoparticles. Also, FTIR analysis of biosurfactant is shown in Fig. 8, as well
3.5 Structure of biosurfactant
Structure of our biosurfactant was also characterised by Fourier transform infrared (FTIR) spectrometry (Fig. 8).
Fig. 8.
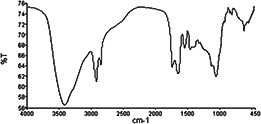
FTIR analysis of biosurfactant
Sample details:
| Water vapour | passed |
| Carbon dioxide | passed |
| baseline low | passed |
| baseline high | passed |
| baseline slope | passed |
| strong bands | passed |
| weak bands | caution |
| high noise | passed |
| fringes | warning |
| vignetting | passed |
| blocked beam | passed |
| negative bands | passed |
| zero transmission | passed |
| stray light | passed |
| window cutoff | passed. |
4 Conclusion
Today, the negative effects of chemical surfactants in industries, especially in the environmental industry have been proved. The use of biosurfactants as good alternatives to chemical surfactants is important in various industries. The association of biotechnology is expected to overcome the challenges of biosurfactant production in industrial scale. In this research, the effects of Fe/SDS and Au nanoparticles on P. aeruginosa PBCC5 growth and biosurfactant production were investigated. Both of nanoparticles had various effects on P. aeruginosa PBCC5 growth and rhamnolipids production based on type and concentration of nanoparticles. In other words, bacterial growth and biosurfactant production were dependent on nanoparticles concentration. Experimental evidence indicated that Fe/SDS nanoparticles at a concentration of 1 mg/l not only did not have any negative effect on the bacterial growth but also increased the growth of bacteria and biosurfactant production. The sample incubated with 1 mg/l of Fe/SDS nanoparticles had higher biosurfactant production and growth rate by 20.73 and 23.21%, respectively, compared with control. According to the obtained results, it is concluded that low concentrations of Fe/SDS nanoparticles are beneficial for biosurfactant production. The concentrations of 500 and 1000 mg/l of Fe/SDS nanoparticles had a negative effect on bacterial growth. Reduction of bacterial cells growth in high concentrations of Fe/SDS resulted in the reduction of biosurfactant production. Therefore, it can be said that high concentrations of Fe/SDS nanoparticles are harmful to bacterial cells and suppress biosurfactant production. Au nanoparticles not only had no toxicity to the bacteria growth and biosurfactant production but also they accelerated bacterial growth and biosurfactant production. These findings proved that nanoparticles could enhance the nourishment medium which prompts bacteria and also damage cell membrane at high concentration. Although it is possible that nanoparticles be effective on cells function or proteins level and genes; however, this is not discovered yet.
5 References
- 1. Fracchia L. Cavallo M. Giovanna Martinotti M. et al.: ‘Biosurfactants and bioemulsifiers biomedical and related applications – present status and future potentials’ (Del Piemonte Orientale University Press, Intechopen, Rijeka, Croatia, 2012), pp. 325 –370 [Google Scholar]
- 2. Gomathy C. Senthilkumar R.: ‘Production of rhamnolipid biosurfactant from a marine Pseudomonas aeruginosa ’, Int. J. Res. Environ. Sci. Technol., 2013, 3, (3), pp. 86 –91 [Google Scholar]
- 3. Pereira J. Gudina E. Costa R. et al.: ‘Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications’, Fuel Res., 2013, 111, pp. 259 –268 [Google Scholar]
- 4. Kaskatepe B. Yildiz S. Gumustas M. et al.: ‘Biosurfactant production by Pseudomonas aeruginosa in kefir and fish meal’, Braz. J. Microbiol., 2015, 46, (3), pp. 855 –859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marti M.E. Colonna W.J. Patra P. et al.: ‘Production and characterization of microbial biosurfactants for potential use in oil‐spill remediation’, Enzyme Microbial. Technol. Res., 2014, 55, pp. 31 –39 [DOI] [PubMed] [Google Scholar]
- 6. Rikalovic M.G.. Cvijovic G.G.. Vrvic M.M. et al.: ‘Production and characterization of rhamnolipids from Pseudomonas aeruginosa san‐ai’, J. Serb. Chem. Soc., 2012, 77, (1), pp. 27 –42 [Google Scholar]
- 7. Singh V.: ‘Biosurfactant – isolation, production, purification & significance’, Int. J. Sci. Res. Publ., 2012, 2, (7), pp. 1 –4 [Google Scholar]
- 8. Reis R.S. Pacheco G.J. Pereira A.G. et al.: ‘Biosurfactants: production and applications’ (University of Sydney, Australia Press Biodegradation – Life of Science, Intechopen, Rijeka, Croatia, 2013), chapter 2, pp. 31 –61 [Google Scholar]
- 9. Gudiña E.J. Teixeira J.A. Rodrigues L.R.: ‘Biosurfactants produced by marine microorganisms with therapeutic applications’, Mar. drugs, 2016, 14, (38), pp. 1 –15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makkar R. Cameotra S. Banat I.: ‘Advances in utilization of renewable substrates for biosurfactant production’, Springer Open J., 2011, 1, (5), pp. 1 –19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lia J. Vipulanandan C.: ‘Effects of Au/Fe and Fe nanoparticles on Serratia bacterial growth and production of biosurfactant’, Mater. Sci. Eng., 2013, 33, pp. 1 –28 [DOI] [PubMed] [Google Scholar]
- 12. Amani H. Muller M.M. Syldatk C. et al.: ‘Production of microbial rhamnolipid by Pseudomonas aeruginosa MM1011 for ex situ enhanced oil recovery’, Springer Sci., 2013, 170, pp. 1080 –1093 [DOI] [PubMed] [Google Scholar]
- 13. Bagheri Lofabad T. Partovi M. Bahmaei M.: ‘Rhamnolipid biosurfactant production by Pseudomonas aeruginosa MR01 using vegetable oil refinery wastes’, New Cell. Mol. Biotechnol. J. Res., 2013, 2, (9), pp. 91 –99 [Google Scholar]
- 14. Bagheri Lotfabad T. Shahceraghi F. Shooraj F.: ‘Assessment of antibacterial capability of rhamnolipids produced by two indigenous Pseudomonas aeruginosa strains’, Jundishapur J. Microbiol., 2013, 6, (1), pp. 29 –35 [Google Scholar]
- 15. Amini F. Samadi N. Harande M. et al.: ‘Optimization of the production of rhamnolipids by Pseudomonas aeruginosa strains’, Nutr. Sci. Food Technol., 2009, 4, (1), pp. 33 –38 [Google Scholar]
- 16. Naghizadeh S. Rashedi H. Yazdian F. et al.: ‘Recovery and purification of rhamnolipid from fermentation broth, by use of a nanotechnology process’, New Biotechnol., 2012, 29, pp. 23 –26 [Google Scholar]
- 17. Ebrahimi F. Naghizadeh S. Rashedi H. et al.: ‘Capability evaluation of rhamnolipid biosurfactant purified by magnetic iron oxide nanoparticles for emulsification of water/n‐decane mixture’, New Biotechnol., 2012, 29, pp. 23 –26 [Google Scholar]
- 18. Hashemi Z. Fooladi J. Ebrahimipour G. et al.: ‘Isolation and identification of crude oil degrading and biosurfactant producing bacteria from the oil contaminated soils of Gachsaran’, Appl. Food Biotechnol., 2016, 3, (2), pp. 83 –89 [Google Scholar]
- 19. Reddy G.S. Saisree M. Pallavi P.: ‘Isolation, purification and production of biosurfactant by microorganism for enhanced oil recovery’, J. Chem. Pharm. Res., 2016, 8, (1), pp. 254 –259 [Google Scholar]
- 20. Sahebnazar Z.: ‘Optimization and production of biosurfactant for use in MEOR and the effect of nanoparticles on this process’, PhD thesis, Shiraz University, Department of Chemical Engineering Oil and Gas, 2016. [Google Scholar]
- 21. Rocha C.A. Pedregosa A.M. Laborda F.: ‘Biosurfactant‐mediated biodegradation of straight and methyl‐branched alkanes by Pseudomonas aeruginosa ATCC 55925’, AMB Express Res., 2011, 1, (9), pp. 1 –10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosa C. Freire D. Ferraz H.: ‘Biosurfactant microfoam: application in the removal of pollutants from soil’, J. Environ. Chem. Eng., 2015, 3, pp. 89 –94 [Google Scholar]
- 23. Rashedi H. Assadi M.M. Bonakdarpour B. et al.: ‘Environmental importance of rhamnolipid production from molasses as a carbon source’, Int. J. Environ. Sci. Technol., 2005, 2, (1), pp. 59 –62 [Google Scholar]
- 24. Raza Z.A.. Khan M.S. Khalid Z.M.: ‘Physicochemical and surface‐active properties of biosurfactant produced using molasses by a Pseudomonas aeruginosa mutant’, J. Environ. Sci. Health Res., 2007, 42, (1), pp. 73 –80 [DOI] [PubMed] [Google Scholar]
- 25. Bhawsar N. Singh M.: ‘Isolation and characterization of Pseudomonas aeruginosa from waste soybean oil as biosurfactants which enhances biodegradation of industrial waste with special reference to Kosmi Dam, Betul District, (M.P.)’, Int. J. Adv. Res., 2014, 2, (6), pp. 778 –783 [Google Scholar]
- 26. Chatterjee S. Bandyopadhyay A. Sarkar K.: ‘Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application’, J. Nanobiotechnol., 2011, 9, (34), pp. 1 –7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J. Vipulanandan C. Cooper T.F. et al.: ‘Effects of Fe nanoparticles on bacterial growth and biosurfactant production’, J. Nanoparticle Res., 2013, 15, (1405), pp. 1 –13 [Google Scholar]
- 28. Kiran G. Nishanth L. Priyadharshini S. et al.: ‘Effect of Fe nanoparticle on growth and glycolipid biosurfactant production under solid state culture by marine Nocardiopsis sp’, MSA13A BMC Biotechnol. Res., 2014, 14, (48), pp. 1 –10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bendaha M. Mebrek S. Naimi M. et al.: ‘Isolation and comparison of rhamnolipids production in Pseudomonas aeruginosa P.B:2 and Pseudomonas fluorescens P.V:10’, Sci. Rep. Res., 2012, 1, (12), pp. 1 –7 [Google Scholar]
- 30. EI‐Sheshtawy H.S. Doheim M.: ‘Selection of Pseudomonas aeruginosa for biosurfactant production and studies of its antimicrobial activity’, Egypt. J. Pet. Res. DOI: 10.1016/j.ejpe.2014.02.001 [Google Scholar]


