Abstract
In recent years, the problems associated with bacterial resistance to antibiotics caused nanodrugs to be considered as a new way for infectious diseases treatment. The main purpose of this study was to develop a new agent against Pseudomonas aeruginosa, a very difficult bacterium to treat, based on azlocillin antibiotic and silver nanoparticles (AgNPs). Azlocillin was conjugated with AgNPs by chemical methods and its antimicrobial activity was studied against P. aeruginosa using well diffusion agar method. Then, minimum inhibitory concentration and minimum bactericidal concentration of the new conjugate was specified with macro‐dilution method. The animal study showed the considerable enhanced antibacterial effect of azlocillin in conjugation with AgNPs against P. aeruginosa in comparison with azlocillin alone, AgNPs alone and azlocillin in combination with AgNPs.
Inspec keywords: antibacterial activity, silver, nanoparticles, organic compounds, microorganisms, drugs, nanomedicine, biomedical materials, diseases, diffusion, nanofabrication
Other keywords: Ag, macrodilution method, minimum bactericidal concentration, minimum inhibitory concentration, well diffusion agar method, P. aeruginosa, antimicrobial activity, chemical methods, azlocillin antibiotic nanoparticles, infectious diseases treatment, nanodrugs, bacterial resistance, Pseudomonas aeruginosa, silver nanoparticles, antibacterial effect
1 Introduction
Pseudomonas aeruginosa, an opportunist pathogenic bacterium, has several virulence factors such as lipopolysaccharide, pilli and alginate that suppress the immune system [1]. In recent years, microbial drug resistance has been increased specially among the bacteria. Previously, a type of penicillin along with an aminoglycoside was used for treatment of clinical Pseudomonas infections effectively [2]. Azlocillin was one of the essential anti‐Pseudomonas penicillins that were used intravenously in respiratory and urinary systems as well as in septicemia caused by Pseudomonas infections. But today P. aeruginosa has found resistance to azlocillin [3]. The increasing of resistance in P. aeruginosa against antibiotics such as azlocillin has reduced the permeability of cell membrane, generated extracellular beta‐lactamase with plasmid and chromosomal origin, altered the amino glycoside chemical structure and activated the mechanism of multi‐drug disposals [4, 5].
Many scientists are interested in using inorganic nanoparticles such as silver nanoparticles (AgNPs) as antibacterial agents. AgNPs have a strong antimicrobial effect on gram‐positive and gram‐negative bacteria according to the literature [6]. AgNPs can be exploited in various fields, particularly medical and pharmaceutical due to their low toxicity on human cells, high thermal stability and low volatility [7, 8, 9]. In 2005, Jose Ruben Morones et al. [10] have established the bactericidal effect of AgNPs on four types of gram‐negative bacteria: Escherichia coli, Vibrio cholerae, P. aeruginosa and Scrub typhus. The previous studies showed that binding of AgNPs to other antimicrobial agents could be useful for increasing their antimicrobial activity. In 2005, Ping et al. [11] linked AgNPs with amoxicillin and noticed that the complex found the enhanced antimicrobial effect against E. coli in comparison with amoxicillin alone. In 2007, Edward Turos et al. combined polyacrylate nanoparticles to penicillin and used it against methicillin‐resistant Staphylococcus aureus (MRSA). They concluded that the combination of antibiotics with nanoparticles enhances the antibacterial activity of antibiotics [12].
In 2009, Greenhalgh et al. prepared penicillin‐conjugated polyacrylate nanoparticles as a new opportunity for development of a new anti‐MRSA agent. They reported great antibacterial activity of the prepared complex against MRSA in analogy with non‐conjugated form of the antibiotic [13]. In 2015, Ajalli et al. [14] indicated the enhanced effect of doxycycline in conjugation with AgNPs against Brucella abortus 544. The aim of this study was to enhance antibacterial effect of azlocillin against P. aeruginosa by conjugating it with AgNPs.
2 Material and methods
2.1 Materials and media
AgNPs were purchased from Nano Nasb Pars Company in the size range of 50–60 nm and concentration of 4000 ppm. The chemical materials and microbial media were obtained from Merck, Germany. P. aeruginosa PAO1 was obtained from Pasteur institute, Tehran, Iran. Azlocillin (CAS: 37091‐65‐90) was purchased from Sigma Aldrich, USA. Female BALB/c mice with 6–8 weeks old and 25 ± 5 g weight were purchased from Pasteur institute, Tehran, Iran.
2.2 Conjugation of azlocillin with AgNPs
2.2.1 Binding of 8‐amino‐1‐octanollinker to azlocillin sodium salt
Azlocillin sodium salt (1) (0.49 g) and 8‐amino‐1‐octanol (2) (0.2 g) were stirred in dry dimethylformamide (10 ml) and the solution was chilled to 0°C. Then, methylmorpholine (3) (0.18 ml), hydroxybenzotriazole (4) (0.26 gr) and ethyl‐3‐3‐dimethylaminopropylcarbodiimide (5) (0.31 g) were added to the solution. The reaction mixture was stirred under nitrogen atmosphere for 24 h at room temperature. The solvent was removed under reduced pressure. Then, the resulting viscous liquid was solved in dichloromethane (12.5 ml) and washed with a solution of citric acid (12.5 ml, 2.5%), saturated NaHCO3 (12.5 ml) and NaCl (12.5 ml) [15]. The organic layer was dried by anhydrous Na2 SO4 and after solvent evaporation, the azlocillin‐hydroxyoctyl compound (6) was obtained (Fig. 1).
Fig. 1.
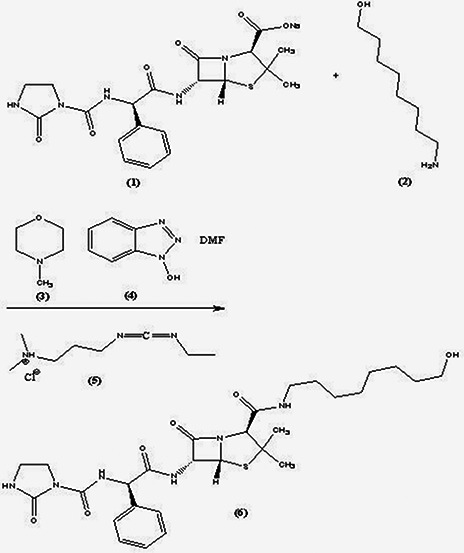
Binding of 8‐amino‐1‐octanol linker to azlocillin sodium salt
2.2.2 Conversion of azlocillin‐hydroxyoctyl compound to azlocillin‐octylmethanesulphonate compound
For conversion of the azlocillin‐hydroxyloctyl compound to thiol‐containing linker with binding ability to AgNPs, it must be converted to azlocillin‐octylmethanesulphonate (8). So, azlocillin‐hydroxyloctyl compound (6) (0.25 g), was dissolved in 5 ml dichloromethane and the solution was cooled to 0°C. Triethylamine (0.15 ml) and mesylchloride (7) (0.06 ml) were added to the stirred solution under nitrogen atmosphere for 2 h at 0°C [15]. Then, the solution was placed on ice and extracted by dichloromethane. The solvent was evaporated to obtain the product (8) (Fig. 2).
Fig. 2.
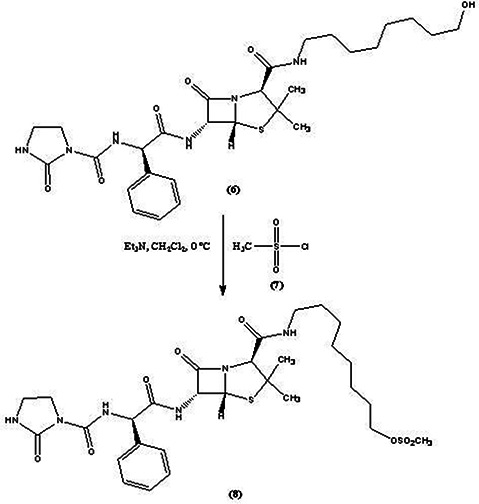
Conversion of azlocillin‐hydroxyoctyl compound to azlocillin‐octylmethanesulphonate compound
2.2.3 Conversion of azlocillin‐octylmethanesulphonate compound to azlocillin‐mercaptooctyl compound
Sodium hydrosulphide (0.02 g) was dissolved in tetrahydrofuran (2 ml) and sonicated. Then, the solution of azlocillin‐octylmethanesulphonate compound (8) (0.14 g) in tetrahydrofuran (1 ml) was added to the mixture. The reaction mixture was stirred for 24 h at room temperature. Then, 3 ml of water was added to the mixture and washed with ethyl acetate and NaCl [15]. The final solution was filtered and azlocillin‐mercaptooctyl compound (9) was obtained in a powder form (Fig. 3).
Fig. 3.
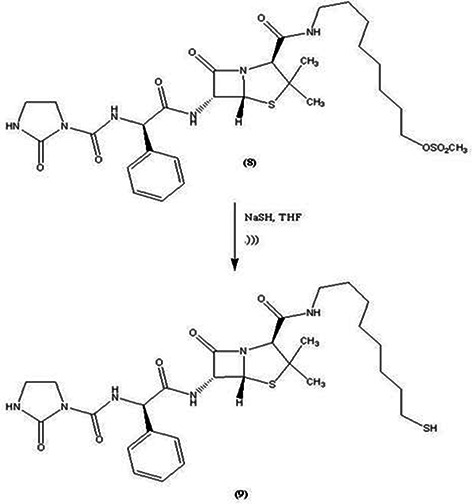
Conversion of azlocillin‐octylmethanesulphonate compound (8) to azlocillin‐mercaptooctyl compound (9)
2.2.4 Coating of AgNPs with glutathione (GSH)
About 350 µl of colloidal AgNPs, 350 µl of GSH (0.1 M) and 100 µl of Tris‐HCl at pH = 7–7.5 were mixed. The reaction mixture was placed on a shaker for 3 days at ambient temperature [15]. The product was precipitated, washed with methanol (3X) and collected by centrifugation at 6000g to obtain GSH‐AgNPs. Then, the GSH‐AgNPs was resuspended in 50% aqueous glycerol (Fig. 4).
Fig. 4.
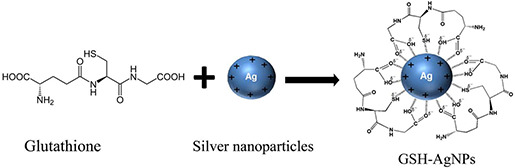
Coating of silvernanoparticles with GSH (GSH‐SNP)
2.2.5 Conjugation of GSH‐AgNPs with linker containing azlocillin
GSH‐AgNPs (700 µl) and azlocillin‐mercaptooctyl compound (9) (700 µl) were combined in methanol and glycerol (700 µl) was added to the mixture. After 10 days, the supernatant was removed and the product was washed with methanol (3X) to remove excess azlocillin‐mercaptooctyl compound and GSH to obtain azlocillin‐mercaptooctyl‐GSH‐AgNPs (azlocillin–AgNPs) conjugate (Fig. 5) [15]. In order to remove the non‐conjugated AgNPs and excess azlocillin antibiotic, the prepared solution was subsequently centrifuged at 4°C for 5 min at 12,000 rpm. The supernatant was discarded and the precipitate was dissolved in phosphate buffer saline. The process was repeated three times for complete removal of excess AgNPs and azlocillin antibiotic molecules [15].
Fig. 5.
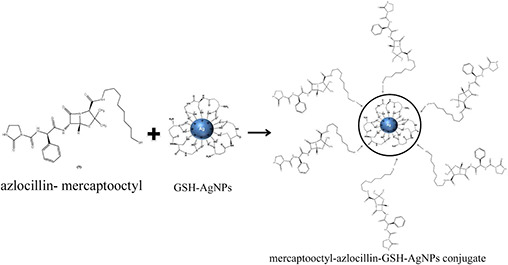
Conjugation of GSH‐AgNPs with linker containing azlocillin
2.3 Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy was performed using a Jasco FTIR 6300 spectrometer for GSH, AgNPs coated with GSH, mercaptooctyl‐azlocillin and GSH ‐AgNPs‐mercaptooctyl‐azlocillin conjugate to confirm the formation of prepared compounds.
2.4 Antibacterial activity
The antibacterial activity of azlocillin–AgNPs conjugate was monitored against P. aeruginosa using the well diffusion agar method. Mueller‐Hinton agar media with 5 mm diameter wells were prepared and 1.5 × 108 CFU/ml of P. aeruginosa suspension was cultivated in plates with sterile swab. Then, the different concentrations of the trials (2, 4, 8, 16, 32, 64 and 128 ppm) including azlocillin–AgNPs conjugate, azlocillin alone, AgNPs alone, azlocillin in combination with AgNPs and normal saline (as a control) were loaded in the wells. The plates were incubated at 37° C for 24 h, and finally the zone of growth inhibition was measured with a ruler. This process was repeated three times [12, 16].
2.5 Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determination
The dilutions of azlocillin–AgNPs conjugate were prepared in Mueller‐Hinton broth in tubes to prepare the different concentrations of 2, 4, 6, 8, 10, 12, 14 and 16 ppm. Then, 5 × 105 CFU/ml of P. aeruginosa suspension was added to each tube and incubated at 37°C for 24 h. Next, the tubes were examined for turbidity. The lowest concentration of azlocillin–AgNPs conjugate that inhibited growth of the bacteria was designated as MIC. For calculation of MBC of azlocillin–AgNPs conjugate, 0.1 ml of inoculums from each tube was sub‐cultured on Mueller‐Hinton agar plates [12, 16]. The number of colonies on agar after 24 h of incubation at the same conditions was counted and compared with the number of CFU/ml in the original inoculums. The lowest concentration of azlocillin–AgNPs conjugate that could purge 99.9% of primary bacteria was determined as MBC. This process was replicated three times. For comparison study, the above steps were repeated for determination of MIC and MBC of AgNPs alone, azlocillin alone and azlocillin–AgNPs combination.
2.6 Mouse model
Twenty five female BALB/c mice were divided into five groups of five. The groups were treated with azlocillin–AgNPs conjugate, azlocillin alone, AgNPs alone, azlocillin–AgNPs combination and normal saline (as a control). The mice were injected intra‐peritoneal with suspension of 5 × 105 CFU/ml of P. aeruginosa on the first day. Then, 0.5 ml of MBC concentration of the above trials was injected intra‐peritoneally to each group separately on the second day. After 7 days, the mice were killed with an anaesthetic agent. Then, the spleen of each mouse was removed aseptically and homogenised in 10 ml of sterile saline buffer. Next, the suspensions of spleens were cultured on plates containing Mueller‐Hinton agar media. The plates were incubated at 37°C for 24 h. Then, the bacteria colonies were counted [12, 16]. The experiments involving BALB/c mice were approved by the Animal Care Committee of Tarbiat Modares University, Tehran, Iran.
2.7 Data analysis and statistics
The results were presented as mean ± SD. Statistical comparisons were performed using of analysis of variance, whenever applicable, by SPSS 18 software. P ‐value in this test was <0.05.
3 Results
3.1 FTIR spectroscopy
Fig. 6 a shows the IR spectra for GSH and GSH‐AgNPs. The S–H stretching vibration (2524 cm−1 band), which appears in GSH spectrum was missed in GSH‐AgNPs spectrum, because of the new S–Ag0 bond in GSH‐AgNPs compound. In GSH, the band at 1709 cm−1 is attributed to the ‐COOH group of the glycine residue, which is absent in GSH‐AgNPs. This pattern indicates the interaction of –COOH with metal ions. Fig. 6 b shows the IR spectra of mercaptooctyl‐azlocillin and mercaptooctyl‐azlocillin‐GSH‐AgNPs compounds. The presence of some peaks related to the functional groups of azlocillin‐mercaptooctyl compound (1700, 1730 cm−1 related to C = O stretching vibration of amides and 3000–3300 cm−1 related to N–H stretching vibration) in mercaptooctyl‐ azlocilin‐GSH‐AgNPs spectra revealed the participation of mercaptooctyl‐azlocillin compound in final synthesised product.
Fig. 6.
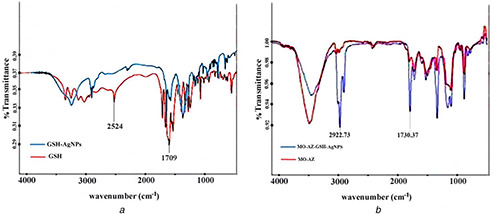
IR spectra for GSH and GSH‐AgNPs
(a) FTIR comparison of GSH and GSH‐AgNPs, (b) FTIR comparison of the mercaptooctyl‐azlocillin with mercaptooctyl‐azlocillin‐GSH‐AgNPs conjugates
3.2 Antibacterial activity
The antibacterial susceptibility test was performed on Mueller‐Hinton agar using well diffusion agar method. The results showed that the antimicrobial effect of azlocillin–AgNPs conjugate against P. aeruginosa was greater than AgNPs alone, azlocillin alone or their combination form (Table 1). The difference between the diameter of inhibition zone in the prepared nanodrug and other trials was shown in the image of agar plates too (Fig. 7). The findings indicated that by increasing the concentration of azlocillin–AgNPs conjugate (up to 64 ppm), the diameter of inhibition zone increases.
Table 1.
Antibacterial effect of azlocillin–AgNPs conjugate, AgNPs alone, azlocillin alone and azlocillin–AgNPs combination against P. aeruginosa in different concentration
| Concentration, ppm | Diameter of inhibition zone, mm | |||
|---|---|---|---|---|
| Azlocillin | AgNPs | Azlocillin and AgNPs combination | Azlocillin–AgNPs conjugate | |
| 2 | 10±1 | 5±1 | 14±2 | 17.33±3 |
| 4 | 15±2 | 8±1 | 18±3 | 20.47±3 |
| 8 | 20±2 | 10±1 | 24±3 | 28.66±3 |
| 16 | 25±2 | 15±2 | 32±4 | 37±4 |
| 32 | 30±5 | 20±2 | 38±4 | 52.33±5 |
| 64 | 35±5 | 25±2 | 44±5 | 63±5 |
| 128 | 42±2 | 30±2 | 51±5 | 68±14 |
Fig. 7.
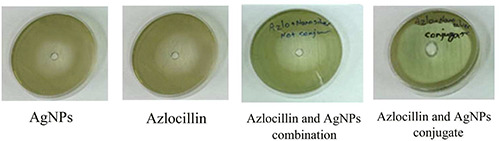
Agar plate images for comparing the antibacterial effect of AgNPs alone, azlocillin alone, azlocillin and AgNPs in combination form and azlocillin–AgNPs conjugate against P. aeruginosa (from left to right)
3.3 MIC and MBC
Table 2 shows the MIC and MBC of azlocillin–AgNPs conjugate, AgNPs alone, azlocillin alone and azlocillin–AgNPs combination against P. aeruginosa. The results showed the enhanced antibacterial effect of azlocillin in conjugation with AgNPs in comparison with other trials. It can be seen that the concentration of the prepared conjugate has been reduced considerably in comparison with AgNPs and azlocillin alone or their combination form for the same antibacterial effect.
Table 2.
MIC and MBC azlocillin–AgNPs conjugate, AgNPs alone and azlocillin alone
| Trials | MIC, ppm | MBC, ppm |
|---|---|---|
| azlocillin–AgNPs conjugate | 4 | 6 |
| azlocillin and AgNPs combination | 6 | 8 |
| azlocillin | 8 | 10 |
| AgNPs | 10 | 12 |
3.4 Mouse model
The results of colony counting in the spleen culture were listed in Table 3. This study demonstrated that azlocillin–AgNPs conjugate can strongly reduce the colonisation of P. aeruginosa. As it can be seen there is a significant difference between the main group and other trial groups in killing of bacteria that approves the enhanced antibacterial effect of azlocillin in conjugation with AgNPs.
Table 3.
Average of P. aeruginosa colonies in spleen suspension culture in different trials
| Trials | CFU/spleen |
|---|---|
| azlocillin–AgNPs conjugate | (29±5)×103 * |
| azlocillin and AgNPs combination | (52±17)×106 |
| AgNPs | (110±4)×108 |
| azlocillin | (89±8)×108 |
| normal saline (control) | (200±37)×1010 |
* Indicates a significant difference compared with other groups.
4 Discussion
According to the treatment problems and increasing the resistance of P. aeroginosa to different antibiotics there is the necessity of finding a new health care system with more usages and less side effects and our intension of this research was to define a new nanodrug to cure the diseases caused by P. aeroginosa. AgNPs have shown antimicrobial activity and are recently being used to overcome some infectious diseases [17]. In 2013, Alizadeh et al. [18] showed intramacrophage antimicrobial effect of AgNPs against Brucella melitensis 16M, also, they reported non‐toxicity of AgNPs on peritoneal macorophages. The previous studies showed that the binding of AgNPs to other antimicrobial agents could be useful for increasing of antimicrobial activity [19]. In 2013, Kora and Rastogi [19] indicated enhanced antibacterial activity of capped AgNPs in combination with antibiotics, on model gram‐negative and gram‐positive bacteria. AgNPs have high affinity for binding to sulphur groups. So, azlocillin carrying out sulphur groups was attached to AgNPs. We used GSH for conjugation of AgNPs to azlocillin. GSH is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side‐chain and the amine group of cysteine which is attached by normal peptide linkage to glycine [20]. In addition, for stable connection between the AgNPs and azlocillin, the mercaptooctyl linker was used to stabilise the binding. Then, to confirm the binding of linker to the antibiotic and verifying the conjugation of AgNPs with azlocillin, FTIR spectroscopy was performed.
The antibacterial susceptibility test performed on Mueller‐Hinton agar showed the antimicrobial effect of azlocillin–AgNPs conjugate against P. aeroginosa. This study showed the enhanced bactericidal effect of azlocillin in conjugation with AgNPs in comparison with azlocillin alone, AgNPs alone and even more than azlocillin–AgNPs combination (Table 1). The findings of agar well diffusion experiment also indicated that with increasing of the concentration of azlocillin–AgNPs conjugate up to 64 ppm, the bacterial growth decreases very fast (dose dependency). There was not seen any increasing in antibacterial activity of the new conjugate in the concentrations above 64 ppm.
A comparison study was performed to determine the MIC and MBC of azlocillin in conjugation with AgNPs, azlocillin alone, AgNPs alone and in their combination form. The MIC and MBC of azlocillin–AgNPs conjugate against P. aeruginosa were found to be 4 and 6, respectively. These figures are considerably less than the same figures determined for AgNPs and azlocillin alone or in their combination form (Table 2). The enhanced antibacterial effect of azlocillin in conjugation with AgNPs reduces the necessary dose for killing the bacteria and consequently the side effects of excess use of the antibiotic that is important factor in treating infectious diseases [21].
The animal study was the last experiment to approve the superior bactericidal effect of azlocillin in conjugation with AgNPs compared with azlocillin and AgNPs alone or in their combination form. This study demonstrated that there were considerable differences between the main group (treated with azlocillin–AgNPs conjugate) and other experimental groups in colonisation ability in the spleen tissue (Table 3). The findings showed that the numbers of bacteria (colonies) grown out in the main group was significantly less than the groups treated with AgNPs alone or azlocillin alone. It can be seen that although the combination of azlocillin with AgNPs increases the antibacterial effect of azlocilln significantly in comparison with azlocillin alone, but there is more enhanced antibacterial effect when azlocillin is conjugated with AgNPs. The same effect was seen in well diffusion agar and MIC and MBC determination tests between azloicilln in combination with AgNPs and in conjugation with AgNPs.
5 Conclusion
The results showed the enhanced antibacterial activity of azlocillin in conjugation with AgNPs in comparison with azlocillin alone, AgNPs alone or their combination form against P. aeruginosa in vitro and in vivo.
6 References
- 1. Cobb L.M. Mychaleckyj J.C. Wozniak D.J. et al.: ‘ Pseudomonas aeruginosa flagellin and alginate elicit very distinct gene expression patterns in airway epithelial cells: implications for cystic fibrosis disease’, J. Immunol., 2004, 173, pp. 5659 –5670 [DOI] [PubMed] [Google Scholar]
- 2. Brooks G.F. Butel J.S. Morse S.A.: ‘Jawets Melnick and Adelberg's medical microbiology’ (McGraw Hill., 2004, 23rd edn), pp. 262 –267, 380–420 [Google Scholar]
- 3. Cattoir V.: ‘Efflux‐ mediated antibiotics resistance in bacteria’, Pathol. Biol., 2004, 52, pp. 607 –616 [DOI] [PubMed] [Google Scholar]
- 4. Aeschlimann J.R.: ‘The role of multidrug efflux pumps in the antibiotic resistance of Pseudomonas aeruginosa and other gram‐negative bacteria: insights form the Society of Infectious Diseases Pharmacists’, Pharmacotherapy, 2003, 23, pp. 916 –924 [DOI] [PubMed] [Google Scholar]
- 5. Kreuter J.: ‘Nanoparticles‐a historical perspective’, Int. J. Pharm., 2007, 331, pp. 1 –10 [DOI] [PubMed] [Google Scholar]
- 6. Mahapatra S.S. Karak N.: ‘SNPs in hyperbranched polyamine: synthesis, characterization and antibacterial activity’, J. Mater. Chem. Phys., 2008, 112, pp. 1114 –1119 [Google Scholar]
- 7. Sondi I. Salopek‐Sondi B.: ‘SNPs as antimicrobial agent: a case study on E. coli as a model for gram‐negative bacteria’, J. Colloids Interface Sci., 2004, 275, pp. 177 –182 [DOI] [PubMed] [Google Scholar]
- 8. Elechiguerra J.L. Burt J.L. Morones J.R. et al.: ‘Interaction of SNPs with HIV‐I’, J. Nanobiotechnol., 2005, 3, pp. 3155 –3165 [Google Scholar]
- 9. Joerger R. Klaus T. Granqvist C.G.: ‘Biologically produced silver–carbon composite materials for optically functional thin‐ film coating’, Adv. Mater., 2000, 12, pp. 407 –409 [Google Scholar]
- 10. Morones J.R. Elechiguerra J.L. Camacho A. et al.: ‘The bactericidal effect of SNPs’, Nanotechnology, 2005, 16, pp. 2346 –2353 [DOI] [PubMed] [Google Scholar]
- 11. Ping L.I. Juan L.I. Changzhu W.U. et al.: ‘Synergistic antibacterial effects of β ‐lactam antibiotic combined with SNPs’, Nanotechnology, 2005, 16, pp. 1912 –1917 [Google Scholar]
- 12. Turos E. Reddy G.S. Greenhalgh K. et al.: ‘Penicillin‐bound polyacrylate nanoparticles: restoring the activity of [beta]‐lactam antibiotics against MRSA: a review’, Bioorg. Med. Chem. Lett., 2007, 17, pp. 3468 –3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenhalgh K. Turos E.: ‘In vivo studies of polyacrylate nanoparticle emulsions for topical and systemic applications’, Nanomedicine, 2009, 5, pp. 46 –54 [DOI] [PubMed] [Google Scholar]
- 14. Ajalli M. Salouti M. Alizadeh H. et al.: ‘Antibacterial activity of doxycycline conjugated with SNPs against Brucella Bacteriain vitro and in vivo’, Int. J. BPAS, 2015, 4, pp. 5539 –5550 [Google Scholar]
- 15. Kudgus R.A.: ‘Gold nanocrystal therapeutics: treatment of multidrug resistant pathogens and disrupting protein/protein interactions’. A dissertation submitted to the Graduate Faculty of North Carolina State University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy, 2010.
- 16. Seleem M.N. Munusamy P. Ranjan A. et al.: ‘Silica‐antibiotic hybrid nanoparticles for targeting intracellular pathogens’, Anti. Agents Chemother., 2009, 53, pp. 4270 –4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X.F. Liu Z.G.. Shen W. et al.: ‘Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches’, Int. J. Mol. Sci., 2016, 17, p. 1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alizadeh H. Salouti M. Shapouri R. et al.: ‘Intramacrophage antimicrobial effect of silver nanoparticles against Brucella melitensis 16M’, Jundishapur J. Microbiol., 2013, 7, pp. 1035 –1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kora A.J. Rastogi L.: ‘Enhancement of antibacterial activity of capped silver nanoparticles in combination with antibiotics, on model gram‐negative and gram‐positive bacteria’, Bioinorg. Chem. Appl., 2013, 10.1155/2013/871097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pompella A. Visvikis A. Paolicchi A. et al.: ‘The changing faces of glutathione, a cellular protagonist’, Biochem. Pharmacol., 2003, 66, pp. 1499 –1503 [DOI] [PubMed] [Google Scholar]
- 21. Sadeghi B. Jamali M. Kia S.H. et al.: ‘Synthesis and characterization of SNPs for antibacterial activity’, Int. J. Nano Dimens., 2010, 1, pp. 119 –124 [Google Scholar]


