Abstract
The biosynthesis of silver nanoparticles (AgNPs) is substantial for its applications in different fields. The Moringa oleifera leaves were used as reducing and stabilising agent for the biosynthesis of AgNPs. The synthesised AgNPs were characterised through UV–visible spectroscopy, zeta analyser, scanning electron microscopy (SEM) and energy dispersive Xray (EDX). In this study, effects of the synthesised AgNPs were also evaluated on nucellus tissues germination frequency and biochemical parameters of plant tissues. Nucellus tissues of Citrus reticulata were inoculated on MS medium supplemented with 10, 20, 30 and 40 µg/ml suspension of the synthesised AgNPs. Green synthesised AgNPs enhanced the in vitro germination because of low toxicity and nonfriendly issues. Significant results were obtained for germination parameters i.e. root and shoot length and seedling vigour index in response to 30 µg/ml suspension of green synthesised AgNPs. The 30 µ/ml suspension of AgNPs also enhanced antioxidant activity (41%) and SOD activity (0.36 nM/min/mg FW) while total phenolic content (4.7 µg/mg FW) and total flavonoid content (1.1 µg/mg FW) was significantly high when MS medium was fortified with 40 µg/ml suspension of the synthesised AgNPs. The content of total protein was significant (558 µg/BSA Eq/mg FW) in control plantlets as compared to the other treatments.
Inspec keywords: antibacterial activity, ultraviolet spectra, X‐ray chemical analysis, proteins, microorganisms, biochemistry, nanofabrication, silver, nanotechnology, visible spectra, surface plasmon resonance, nanoparticles, suspensions, nanomedicine, scanning electron microscopy, electrokinetic effects
Other keywords: green synthesised silver nanoparticles, superoxide dismutase activity, biochemical profile, UV–visible spectroscopy, Citrus reticulata, green synthesised suspension, EDX detector, zeta potential, scanning electron microscopy, SEM, energy dispersive X‐ray, EDX, total phenolic content, total flavonoid content, size 423.0 nm to 425.0 nm, size 8.0 nm to 28.0 nm
1 Introduction
The use of nanotechnology is extensively increasing in the last decade in the different fields like biotechnology, medical and energy sector, information technology, consumer goods, and agriculture. Nanotechnology deals with the production of minute particles having dimension <1 µm [1]. The nanoparticles (NPs) can be synthesised from a wide variety of materials and they can expand their actions, depending on the size, shape, and chemical composition of the particles [2]. NPs are synthesised by microwave radiation [3], UV irradiation [4], γ‐ray irradiation [5], electrochemical reduction [6], photochemical reduction [7], and chemical reduction [8]. Among them, chemical synthesis is the most commonly used method having some shortcomings such as high cost, toxicity, and non‐friendly issues [9]. Alternatives include the biological synthesis of NPs by different biological agents, i.e. microorganisms, yeast, fungi, plant extracts, and sometimes intact plants [10].
Citrus is one of the most important horticultural and economically important tree fruit crop in the world [11, 12, 13]. Citrus belongs to family rutaceae and includes Citrus reticulata, Citrus sinensis, and Citrus limon which comprise 18% of the total fruit production in the world [14]. According to an estimate, ∼95% of the total C. reticulata (Kinnow) all over the world is produced in Pakistan [15]. Kinnow is a rich source of phenolics, flavonoids, terpenes, and some other biologically active ingredients. Kinnow is nutritionally very important because of rich source of vitamin C in conjugation with sugar, organic acids, amino acids, and minerals [16].
Plant tissue culture is considered as an alternative tool to the conventional breeding practices. It expands the quality of economically important plants and establishes the high‐frequency regeneration protocol. In vitro regeneration is very beneficial for the production of various secondary metabolites and antioxidants under stress because conventional breeding practices are imperilled to land availability, weather, and varieties of pests which adversely affect the qualities of harvested plants [17].
Reactive oxygen species (ROS) are produced under stress condition to tackle the new environment; however, they also interact with biological molecules and inhibit growth and differentiation. Two types of secondary metabolites are produced under unfavourable condition in order to nullify the damaging effect of ROS, i.e. non‐enzymatic compounds and anti‐oxidative enzymes [18]. Non‐enzymatic compounds contribute in quenching toxic‐free radicals, apoptosis induction, enzyme activation, gene expression, and immune system stimulation [19]. Similarly, antioxidative enzymes play vital role in morphogenesis, plant development, and conversion of oxygen radical to hydrogen peroxide [20]. Generally, a strong relationship exists between antioxidant activity and non‐enzymatic components [21].
So far, few studies have been carried out on the applications of nanomaterials in plant tissue culture. An increase in root and shoot length and seedling vigour index (SVI) was observed in Artemisia absinthium seeds exposed to various metallic NPs [22]. Zaka et al. [23] reported the seed germination and biochemical profile of Eruca sativa exposed to various NPs. The NPs do not directly influence the plants but indirectly alters the mechanism and this change in growth behaviour was observed after a certain time period [24].
The understanding of how NPs are involved in controlling the growth and development in plants is still to be explored and requires more comprehensive research work. The prime objective of this research work is to find the effect of various concentrations of green synthesised silver nanoparticles (AgNPs) on root length, shoot length, SVI, total phenolic content (TPC), total flavonoid content (TFC), superoxide dismutase (SOD) activity, content of total protein (CTP), and antioxidant activity in C. reticulata. There are very few reports which are available on the applications of chemically synthesised NPs on the in vitro germination and biochemical profiling of some plant species. To the best of our knowledge, this is the first study on the application of green synthesised AgNPs on in vitro germination and to explore the probable effects on the secondary metabolites production and antioxidant activity of C. reticulata.
2 Materials and methods
2.1 Green synthesis of AgNPs
The green synthesis of AgNPs was done according to the protocol described by Hussain et al. [25]. Silver nitrate solution (SNS) was reduced by using Moringa oleifera leaves extract as main reducing and stabilising agent. The aqueous extract of M. oleifera was prepared by boiling 50 g leaves in 500 ml distilled water for 10 min. The extract was filtered thrice to remove all the remains of contaminants. A solution of silver nitrate (5 mM) was prepared by dissolving 0.85 g/l of distilled water. SNS was then continuously boiled for 5 min and reduced stepwise by the addition of plant extract until the colour of solution turned to dark brown. As soon as colour changed to dark brown, solution was centrifuged four times at 14,000 rpm for 10 min. Pellets were collected and supernatants were discarded. This process was repeated four times to remove the unreacted silver salt and plant extract. The synthesised AgNPs were used for characterisation as well as accessing germination parameters and biochemical profiling in C. reticulata.
2.2 Characterisation of the green synthesised AgNPs
2.2.1 UV–Visible spectroscopy
The reduction in silver nitrate (AgNO3) to AgNPs was observed by recording UV–Visible spectrum from National Centre of Physics (NCP), Islamabad. The synthesised AgNPs were added in sterilised water and then subjected to ultra‐sonication for 5–10 min. UV–Visible spectrum analysis was done by recording the spectrum from 300 to 700 nm.
2.2.2 Zeta analyser
The size range of the green synthesised AgNPs was determined through zeta analyser from Nuclear Institute of Biotechnology and Genetic Engineering (NIBGE), Faisalabad. The synthesised NPs were ultra‐sonicated for 15 min and then size range was determined through zeta potential.
2.2.3 Scanning electron microscopy
The structural analysis of the synthesised AgNPs was examined by scanning electron microscopy (SEM) by utilising SIGMA model operated at 5 kV, magnification ×10k from the Institute of Space and Technology (IST), Islamabad. Then film of the sample was prepared on a carbon‐coated copper grid by just dropping the suspension of AgNPs in water on the grid, extra solution was removed by utilising the blotting paper and then the film on the SEM grid was allowed to dry by putting it under mercury lamp for 5 min. The sample surface images were taken at different magnifications.
2.2.4 Energy dispersive X‐ray spectroscopy
The elemental analysis of the green synthesised AgNPs was also done from the Institute of Space and Technology (IST), Islamabad. Energy dispersive X‐ray (EDX) detector was utilised for the elemental analysis of the green synthesised AgNPs by dropping the synthesised NPs on carbon film.
2.3 Plant source and surface sterilisation
Immature fruits of C. reticulata were collected from the Bhalwal region of district Sargodha. Seeds were harvested from the C. reticulata fruits and nucellus tissues were separated and surface sterilised by following the protocol described by Hussain et al. [15].
2.4 Nucellus tissues germination protocol
AgNPs were suspended directly in distilled water by sonication. About 10, 20, 30, and 40 µg/ml suspension of AgNPs were separately augmented to the MS medium with the help of micropipette (Table 1). Sterilised nucellus tissues were inoculated on the MS medium under completely sterilised conditions. The germination frequency was recorded after a period of 14 days. The whole experiment was conducted for 42 days and data was recorded at 7 days interval.
Table 1.
Layout
| Treatments | Concentrations |
|---|---|
| T0 | control |
| T1 | AgNPs (10 µg/ml) |
| T2 | AgNPs (20 µg/ml) |
| T3 | AgNPs (30 µg/ml) |
| T4 | AgNPs (40 µg/ml) |
2.5 Nucellus tissues germination parameters
Different germination parameters were recorded, i.e. germination frequency, root length, shoot length, and SVI.
2.5.1 Nucellus tissue germination frequency
The germination frequency was recorded after every week. The germination frequency was calculated by utilising the following formula [26]
2.5.2 Root and shoot length
Root and shoot length was recorded in centimetre (cm) after every week and first data was collected after 14 days.
2.5.3 Seedling vigour index
SVI was calculated by following the protocol described by Abdul‐Baki and Anderson [27] and expressed as index number as described by Ushahra and Malik [28]
2.6 Biochemical parameters
2.6.1 Antioxidant activity
The antioxidant activity of the plant extracts was determined by using the method of Abbasi et al. [29] with slight alterations. Approximately 0.5 ml of the DPPH (2,2‐diphenyl‐1‐picrylhydrazyl) solution was mixed with 4 ml of methanol augmented with 10 mg of dried plant tissue. The resulting reaction mixture was vortexed for 15 s followed by incubation at room temperature for a period of 30 min. Finally, the absorbance of samples was recorded at 517 nm by using UV/Visible spectrophotometer. The antioxidant activity was calculated by using the following equation
where As is the absorbance of solution after the addition of extract and Ab the absorbance of control.
2.6.2 TPC determination
For the quantification of TPC, Folin–Ciocalteu reagent was used according to the protocol described by Velioglu et al. [30] with slight alterations. Approximately, 0.75 ml of the Folin–Ciocalteu reagent was mixed with 100 μl of the plant extract followed by incubation for 5 min at 22°C. Thereafter, Na2 CO3 solution (0.75 ml) was added to the mixture and kept for 90 min at 22°C. Finally, the absorbance of the sample was recorded at 725 nm by using UV–Visible spectrophotometer.
2.6.3 TFC determination
TFCs were determined by using the protocol described by Chang et al. [31] with minor modifications. Briefly, quercetin (10 mg) was dissolved in 80% C2 H5 OH and further dilutions were prepared. The resulting standard solution was mixed with 0.1 ml of 1 M potassium acetate, 0.1 ml of 10% aluminium chloride, 1.5 ml of 95% ethanol, and 2.8 ml of distilled water followed by incubation for 30 min at room temperature. Finally, the absorbance of mixture was recorded at 415 nm by using UV/Visible spectrophotometer.
2.6.4 Total protein content determination
For the quantification of total protein content, protocol described by Lowry et al. [32] was used with some modifications. Enzyme extract was prepared in the same way as for antioxidant assay. The absorbance was then recorded at 650 nm by using spectrophotometer.
2.6.5 SOD activity
SOD assay was performed according to the method of Ullah et al. [33] with minor modifications. The mixture was prepared using 1 mM EDTA, 0.75 mM NBT, 50 mM phosphate buffer (pH 7), 130 mM methionine, and 0.02 mM riboflavin. The mixture was exposed to fluorescent light for 7 min and then the absorbance was recorded at 560 nm. SOD activity was calculated applying the Lambert–Beer law
where A is the absorbance, the extinction coefficient, L the length of each wall, and C the concentration of enzymes.
2.7 Statistical analysis
The experimental design consisted of three replicates and each experiment was repeated twice. The results were interpreted as mean standard deviation. Student's t ‐test was selected for the statistical analysis of experimental data and each experimental value was compared with the corresponding control value.
3 Results and discussion
3.1 Synthesis and characterisation of AgNPs
The addition of M. oleifera leaves extract to SNS resulted in the change in colour to dark brown. To study the initial synthesis of AgNPs, the product produced by the reaction of plant extract and SNS is principally analysed through UV–Visible spectrum on HALO DB‐20 spectrophotometer. The surface plasmon resonance (SPR) at 423–425 nm indicated the formation of AgNPs. Fig. 1 shows the UV–Visible spectrum of the synthesised AgNPs. Different characterisation peaks usually in the range of 410–480 nm are obvious for AgNPs synthesis [34, 35]. However, various wavelengths may attribute different sizes and shapes of AgNPs [36]. The synthesised AgNPs were also characterised through zeta potential for determining the size range. The zeta analyser elucidated that the size of synthesised AgNPs ranged from 8 to 28 nm (Fig. 2). Structural analysis of the green synthesised AgNPs was done through scanning electron microscope (SEM). The SEM image represents the rectangular segments fused together (Fig. 3). Similar shape of the synthesised AgNPs was also reported by Anjum and Abbasi [37] in an attempt to find the structural analysis by utilising WPE and CE. Elemental analysis of the synthesised AgNPs was done by the EDX detector (Fig. 4). The presence of metallic silver ions in the M. oleifera leaves extract mediated AgNPs was confirmed by using EDX. EDX spectrum showed absorption peak of silver in the range of 3–4 keV while silver nanocrystal showed absorption peaks in the range of 2.5–4 keV. The present findings are in line with some other researchers who reported similar results in an attempt to find absorption peaks of metallic silver ions by using the EDX detector [38, 39]. Impurities in the sample are attributed with the organic compounds which are involved in the reduction in AgNO3 to AgNPs
Fig. 1.
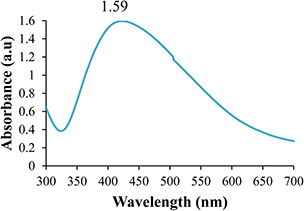
UV–Visible spectrum of green synthesised AgNPs
Fig. 2.
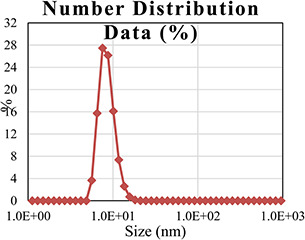
Size distribution of green synthesised AgNPs through zeta analyser
Fig. 3.
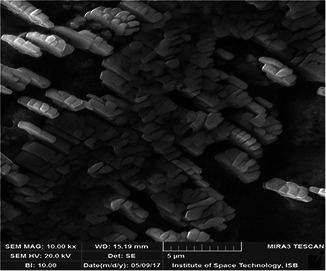
SEM micrograph of green synthesised AgNPs
Fig. 4.
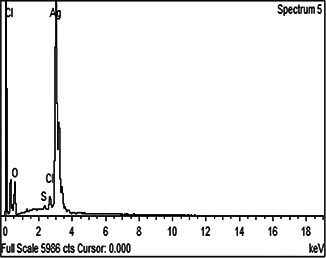
EDX spectrum of green synthesised AgNPs
3.2 Effect of green synthesised AgNPs on the nucellus tissue germination
Seed germination is an efficient phytotoxicity test because it is rapid, easy, low cost, and most suitable for unstable samples [40, 41]. Percentage germination of nucellus tissues was recorded at different day intervals i.e. 14, 21, 28, 35, and 42 days and first data was recorded after 2 weeks of incubation on the MS medium. The effect of different treatments of green synthesised AgNPs on the in vitro germination of nucellus tissues is shown in Table 2. The nucellus tissues augmented on the MS medium without the suspension of green synthesised AgNPs were taken as control. The germination frequency was progressively increased in all the applied treatments with the passage of time. The maximum germination percentage was recorded when MS medium was fortified with 30 µg/ml suspension of the green synthesised AgNPs at different day intervals, i.e. 14, 21, 28, 35, and 42 days as compared to the other concentrations of the synthesised AgNPs. It was found that 40 µg/ml suspension of the green synthesised AgNPs show inhibitory effects on nucellus tissues germination at different day intervals. Toxicity of NPs depends on the size, shape, chemical composition, and release of toxic ions from the synthesised NPs [2]. AgNPs can act as Trojan horse because it passes certain obstacles and release silver ions in aqueous state with the passage of time [42].
Table 2.
Germination frequency of nucellus tissues of C. reticulata in response to different treatments of green synthesised AgNPs
| Sr. no. | Tr. | Germination frequency | ||||
|---|---|---|---|---|---|---|
| After 14 days | After 21 days | After 28 days | After 35 days | After 42 days | ||
| 01 | T0 | 66.3 ± 3.31 | 71.4 ± 3.57 | 78.2 ± 3.91 | 83.7 ± 4.18 | 88.6 ± 4.43 |
| 02 | T1 | 58.8 ± 2.94 | 74.1 ± 3.70 | 83.5 ± 4.17 | 92.1 ± 4.59 | 93.4 ± 4.67 |
| 03 | T2 | 72.6 ± 3.63 | 78.3 ± 3.91 | 88.4 ± 4.42 | 92.1 ± 4.60 | 94.5 ± 4.72 |
| 04 | T3 | 76.9 ± 3.84 | 81.7 ± 4.08 | 90.2 ± 4.51 | 93.6 ± 4.68 | 98.4 ± 4.92 |
| 05 | T4 | 52.6 ± 2.63 | 62.1 ± 3.10 | 71.7 ± 3.58 | 75.5 ± 3.77 | 80.8 ± 4.04 |
3.3 Effect of green synthesised AgNPs on root length, shoot length, and SVI
After exposing the nucellus tissues of C. reticulata to different treatments of green synthesised AgNPs, significant root and shoot length and SVI were observed at different day intervals such as 14, 21, 28, 35, and 42 days (Table 3). The effect of different treatments of the green synthesised AgNPs on root and shoot length and SVI varied greatly depending on the concentration of the synthesised AgNPs and number of days passed after inoculation. It was observed that 30 µg/ml suspensions of the AgNPs have positive effect on root and shoot length and SVI as compared to the other treatments of the synthesised AgNPs. Root inhibition varied greatly among the NPs, type of plants, and partially correlated to NPs concentration [40]. The maximum root length (4.1 cm), shoot length (6.1 cm), and SVI was observed when MS medium was augmented with 30 µg/ml suspension of the M. oleifera leaves extract mediated AgNPs after 42 days of the experiment. The present outcomes are in agreement with Savithramma et al. [43] who reported that AgNPs pierced and persuaded pores in seeds resulted in the rapid influx of nutrients. The influx of nutrients induced rapid germination of seed. The 40 µg/ml suspension of the synthesised AgNPs have reduced the root and shoot length and SVI and showed more stress as compared to the other treatments of the synthesised AgNPs. The findings of Lee et al. [44] also affirmed our results.
Table 3.
Effect of different treatments of green synthesised AgNPs on root length, shoot length, and SVI of C. reticulata
| Germination frequency | Tr. | Root length, cm | Shoot length, cm | SVI |
|---|---|---|---|---|
| after 14 days | T0 | 1.1 ± 0.05 | 2.3 ± 0.11 | 225.4 ± 11.2 |
| T1 | 1.0 ± 0.05 | 2.1 ± 0.10 | 182.3 ± 9.1 | |
| T2 | 1.4 ± 0.07 | 2.5 ± 0.12 | 210.5 ± 10.5 | |
| T3 | 1.9 ± 0.09 | 2.7 ± 0.13 | 353.7 ± 17.6 | |
| T4 | 1.7 ± 0.08 | 2.2 ± 0.11 | 205.1 ± 10.2 | |
| after 21 days | T0 | 1.5 ± 0.07 | 2.8 ± 0.14 | 307.0 ± 15.3 |
| T1 | 1.6 ± 0.08 | 2.7 ± 0.13 | 318.6 ± 15.9 | |
| T2 | 2.1 ± 0.10 | 3.0 ± 0.15 | 399.3 ± 19.9 | |
| T3 | 2.4 ± 0.12 | 3.5 ± 0.17 | 482.0 ± 24.1 | |
| T4 | 1.9 ± 0.09 | 3.2 ± 0.16 | 316.7 ± 15.8 | |
| after 28 days | T0 | 1.8 ± 0.09 | 3.1 ± 0.15 | 383.2 ± 19.1 |
| T1 | 1.9 ± 0.09 | 3.3 ± 0.16 | 434.2 ± 21.7 | |
| T2 | 2.4 ± 0.12 | 3.8 ± 0.19 | 548.1 ± 27.4 | |
| T3 | 2.7 ± 0.13 | 4.0 ± 0.20 | 604.3 ± 30.2 | |
| T4 | 2.2 ± 0.11 | 3.7 ± 0.18 | 423.0 ± 21.1 | |
| after 35 days | T0 | 2.4 ± 0.12 | 3.5 ± 0.17 | 493.8 ± 24.6 |
| T1 | 2.6 ± 0.13 | 4.0 ± 0.20 | 606.5 ± 30.3 | |
| T2 | 2.7 ± 0.13 | 4.3 ± 0.21 | 644.7 ± 32.2 | |
| T3 | 3.3 ± 0.16 | 4.9 ± 0.24 | 767.5 ± 38.3 | |
| T4 | 3.0 ± 0.15 | 4.4 ± 0.22 | 558.7 ± 27.9 | |
| after 42 days | T0 | 2.8 ± 0.14 | 4.2 ± 0.21 | 620.2 ± 31.0 |
| T1 | 3.4 ± 0.17 | 4.6 ± 0.23 | 747.2 ± 37.3 | |
| T2 | 3.5 ± 0.17 | 5.4 ± 0.27 | 841.1 ± 42.0 | |
| T3 | 4.1 ± 0.20 | 6.1 ± 0.30 | 1003.7 ± 50.1 | |
| T4 | 3.7 ± 0.18 | 5.3 ± 0.26 | 727.2 ± 36.3 |
3.4 Antioxidant activity
Among the different methods used for antioxidants determination, DPPH radical scavenging assay (RSA) is the simplest, rapid, efficient, and inexpensive method for the determination of antioxidant activity in plant cell cultures [45]. In the present study, the effect of green synthesised AgNPs on the DPPH RSA was investigated after 42 days of the experiment (Fig. 5). Different treatments of the synthesised AgNPs significantly affected the antioxidant activity in C. reticulata. It was observed that DPPH RSA was significant (41%) when MS medium was augmented with 30 µg/ml suspension of the green synthesised AgNPs as compared to the other treatments. The 10 µg/ml suspension of AgNPs have the reduced antioxidant activity as compared to the other treatments. Similar results were reported by other researchers in an attempt to find the effect of AgNPs on the antioxidant activity in Silybum marianum, A. absinthium, and C. reticulata [22, 46, 47].
Fig. 5.
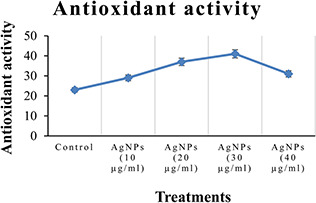
Percentage DPPH RSA of C. reticulata against different treatments of green synthesised AgNPs
3.5 TPC and TFC
In this study, TPC and TFC were also investigated in response to different treatments of the green synthesised AgNPs (Fig. 6). It was observed that TPC (4.7 µg/mg FW) and TFC (1.1 µg/mg FW) in relation to dry mass was significantly high in plantlets treated with 40 µg/ml suspension of the green synthesised AgNPs. TFC show dependency and linear correlation with TPC. However, 10 µg/ml suspension of the green synthesised AgNPs‐treated plantlets showed comparatively less production of non‐enzymatic components. The elicitors not only affect the organogenic potential but also the synthesis and production of non‐enzymatic components and antioxidative enzymes [48]. Furthermore, different researchers have also reported the TPC and TFC in the in vitro cultures of different medicinal plants [25, 49]. Applications of flavonoids‐based AgNPs resulted in the enhanced production of non‐enzymatic components of defence system [50].
Fig. 6.
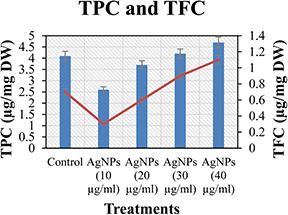
TPC and TFC of C. reticulata against different treatments of green synthesised AgNPs
3.6 CTP and SOD activity
As shown in Fig. 7, the effect of different treatments of the green synthesised AgNPs on CTP and SOD activity was also evaluated in plantlets after 42 days of experiment. CTP is comparatively lesser in plantlets treated with 30 µg/ml suspension of the synthesised AgNPs as compared to the control and other treatments of the green synthesised AgNPs. Plantlets which were not treated with suspension of AgNPs showed significant CTP (558 µg/BSA Eq/mg FW) as compared to the other treatments. Moreover, the present findings are in agreement with Tariq et al. [51] who reported almost similar CTP on in vitro cultures of A. absinthium. Unlike the CTP, plantlets treated with the suspension of different suspensions of green synthesised AgNPs showed significant SOD activity as compared to the control. The incorporation of 30 µg/ml suspension of the synthesised AgNPs to the medium enhanced the SOD activity (0.36 nM/min/mg FW) as compared to the other treatments. The differences in pattern are mainly due to size, shape, and composition of NPs. The enzymatic role of SOD is widely reported in different medicinal plants [25, 52, 53].
Fig. 7.
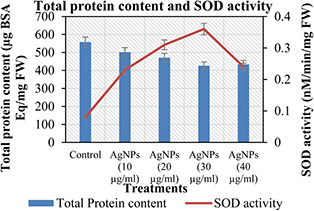
Total protein content and SOD activity of C. reticulata against different treatments of green synthesised AgNPs
4 Conclusion
The biosynthesis of AgNPs by utilising M. oleifera leaves extract has potent in vitro germination, growth, and biochemical effect on C. reticulata. It was found that effects of different treatments of green synthesised AgNPs on germination, growth, and biochemical profile of C. reticulata mericlones were significantly positive. Once the NPs get entry into seeds, then they have long‐lasting effects on seed germination and growth. Furthermore, it can also considerably alter the biochemical profile. So far few studies have been conducted regarding nanomaterials as elicitors of secondary metabolites and antioxidant activity in the mericlones. These preliminary findings pave the way for more comprehensive study about the ecotoxicity of NPs and understanding the mechanism at molecular level that would provide basis to recognise their chemistry for future challenges.
5 Acknowledgment
This work was financially supported by the PMAS Arid Agriculture University Rawalpindi, Pakistan, and their support is gratefully acknowledged.
6 References
- 1. Sun T. Zhang Y.S. Pang B. et al.: ‘Engineered nanoparticles for drug delivery in cancer therapy’, Angew Chem. Int., 2014, 53, pp. 12320 –12364 [DOI] [PubMed] [Google Scholar]
- 2. Brunner T.J. Wick P. Manser P.: ‘In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility’, Environ. Sci. Technol., 2006, 40, pp. 4374 –4381 [DOI] [PubMed] [Google Scholar]
- 3. Tsuji M. Nishizawa Y. Matsumoto K. et al.: ‘Rapid synthesis of silver nanostructures by using microwave‐polyol method with the assistance of Pt seeds and polyvinylpyrrolidone’, Colloids Surfaces A Physicochem. Eng. Asp., 2007, 293, pp. 185 –194 [Google Scholar]
- 4. Cheng D. Zhou X. Xia H. et al.: ‘Novel method for the preparation of polymeric hollow nanospheres containing silver cores with different sizes’, Chem. Mater., 2005, 17, pp. 3578 –3581 [Google Scholar]
- 5. El‐Batal A.I. Haroun B.M. Farrag A.A. et al.: ‘Synthesis of silver nanoparticles and incorporation with certain antibiotic using gamma irradiation’, Br. J. Pharm. Res., 2014, 4, p. 1341 [Google Scholar]
- 6. Liu Y.C. Lin L.H.: ‘New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectro chemical methods’, Electrochem. Commun., 2004, 6, pp. 1163 –1168 [Google Scholar]
- 7. Henglein A.: ‘Colloidal silver nanoparticles: photochemical preparation and interaction with O2, CCl4, and some metal ions’, Chem. Mater., 1998, 2, pp. 444 –450 [Google Scholar]
- 8. Yu D.G.: ‘Formation of colloidal silver nanoparticles stabilized by Na + ‐poly (gamma‐glutamic acid) silver nitrate complex via chemical reduction process’, Colloids Surf. B. Biointerfaces, 2007, 59, pp. 171 –178 [DOI] [PubMed] [Google Scholar]
- 9. Tao A. Sinsermsuksakul P. Yang P.: ‘Polyhedral silver nanocrystals with distinct scattering signatures’, Angew Chem. Int., 2006, 45, pp. 4597 –4601 [DOI] [PubMed] [Google Scholar]
- 10. Park Y. Noh H.J. Han L.: ‘ Artemisia capillaris extracts as a green factory for the synthesis of silver nanoparticles with antibacterial activities’, J. Nanosci. Nanotechnol., 2012, 12, pp. 7087 –7095 [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y. Sun Y. Xi W. et al.: ‘Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits’, Food Chem., 2014, 145, pp. 674680 [DOI] [PubMed] [Google Scholar]
- 12. Tao N. Jia L. Zhou H.: ‘Anti‐fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum ’, Food Chem., 2014, 153, pp. 265 –271 [DOI] [PubMed] [Google Scholar]
- 13. María A.F. Micheloud N.G. Roeschlin R.A. et al.: ‘Surface barriers of mandarin ‘Okitsu’ leaves make a major contribution to canker disease resistance’, Am Phytopathol. Society J., 2014, 104, pp. 970 –976 [DOI] [PubMed] [Google Scholar]
- 14. Rashid U. Ibrahim M. Yasin S. et al.: ‘Biodiesel from Citrus reticulata (mandarin orange) seed oil, a potential non‐food feedstock’, Ind. Crops Prod., 2013, 45, pp. 355 –359 [Google Scholar]
- 15. Hussain M. Raja N.I. Iqbal M. et al.: ‘Plantlets regeneration via somatic embryogenesis from the nucellus tissues of Kinnow Mandarin (Citrus reticulata L.)’, Am. J. Plant Sci., 2016, 7, pp. 798 –805 [Google Scholar]
- 16. Niaz A.C. Maken M.N. Malik S.A.: ‘Native home, historical background and importance of citrus fruits in Pakistan’. Proc. 1st Int. Conf. on Citriculture. University of Agriculture, Faisalabad, 2004, pp. 48 –56 [Google Scholar]
- 17. Sa G. Mi M. He‐Chun Y.: ‘Effects of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L.’, Plant Sci., 2001, 160, (4), pp. 691 –698 [DOI] [PubMed] [Google Scholar]
- 18. Valko M. Leibfritz D. Moncol J. et al.: ‘Free radicals and antioxidants in normal physiological functions and human disease’, Int. J. Biochem. Cell Biol., 2006, 7, pp. 45 –78 [DOI] [PubMed] [Google Scholar]
- 19. Joo S.S. Kim Y. Lee D.I.: ‘Antimicrobial and antioxidant properties of secondary metabolites from white rose flower’, Plant Pathology J., 2010, 26, pp. 57 –62 [Google Scholar]
- 20. Manohari J.S.S.D. Indra M. Muthuchelian K.: ‘Enzymatic activities of mother plant and tissue cultured plants of Cassia siamea (Fabaceae)’, J. Biosci. Res., 2011, 2, pp. 183 –188 [Google Scholar]
- 21. Amid A. Johan N.N. Jamal P. et al.: ‘Observation of antioxidant activity of leaves, callus and suspension culture of Justicia gendarusa ’, African J. Biotech., 2011, 10, pp. 18653 –18656 [Google Scholar]
- 22. Hussain M. Raja N.I. Mashwani Z.R. et al.: ‘In vitro seed germination and biochemical profiling of Artemisia absinthium exposed to various metallic nanoparticles’, 3 Biotech., 2017, 7, p. 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaka M. Abbasi B.H. Rahman L. et al.: ‘Synthesis and characterisation of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa ’, IET Nanobiotechnol., 2016, 10, (3), pp. 1 –7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Navarro E. Piccapietra F. Wagner B.: ‘Toxicity of silver nanoparticles to Chlamydomonas reinhardtii ’, Environ. Sci. Technol., 2008, 42, pp. 8959 –8964 [DOI] [PubMed] [Google Scholar]
- 25. Hussain M. Raja N.I. Mashwani Z.R. et al.: ‘In vitro germination and biochemical profiling of Citrus reticulata in response to green synthesized zinc and copper nanoparticles’, IET Nanobiotechnol., 2017, 11, (7), pp. 790 –796 [Google Scholar]
- 26. Iqbal M. Asif S. Ilyas N. et al.: ‘Effect of plant derived smoke on germination and post germination expression of wheat (Triticum aestivum L.)’, Am. J. Plant Sci., 2016, 7, pp. 806 –813 [Google Scholar]
- 27. Abdul‐Baki A.A. Anderson J.D.: ‘Vigor determination in soybean and seed multiple criteria’, Crop. Sci., 1973, 13, pp. 630 –633 [Google Scholar]
- 28. Ushahra J. Malik C.P.: ‘Putrescine and ascorbic acid mediated enhancement in growth and antioxidant status of eruca sativa varieties’, Tech. J. Biotechnol., 2013, 2, pp. 53 –64 [Google Scholar]
- 29. Abbasi B.H. Khan M.A. Mahmood T. et al.: ‘Shoot regeneration and free radical scavenging activity in Silybum marianum L.’, Plant Cell Tiss. Org. Cult., 2010, 101, pp. 371 –376 [Google Scholar]
- 30. Velioglu Y.S. Mazza G. Gao L. et al.: ‘Antioxidant activity and total phenolics in selected fruits, vegetables and grains products’, J. Agri. Food Chem., 1998, 46, pp. 4113 –4117 [Google Scholar]
- 31. Chang C. Yang M.H. Wen H.M. et al.: ‘Estimation of total flavonoid content in propolis by two complimentary colorimetric method’, J. Food Drug anal., 2002, 10, pp. 178 –182 [Google Scholar]
- 32. Lowry O.H. Rosebrough N.J. Farr A.I. et al.: ‘Protein measurement with the folin phenol reagent’, J. Bio. Biochem., 1951, 193, (1), pp. 265 –275 [PubMed] [Google Scholar]
- 33. Ullah N. Haq I.U. Safdar N.: ‘Physiological and biochemical mechanisms of allelopathy mediated by the allelochemical extracts of Phytolacca latbenia (Moq.) H. Walter’, Toxicol. Ind. Health., 2015, 31, pp. 931 –937 [DOI] [PubMed] [Google Scholar]
- 34. Nazeruddin G. Prasad N. Waghmare S.R.: ‘Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti‐microbial activity’, J. Alloys Comp., 2014, 583, pp. 272 –277 [Google Scholar]
- 35. Gogoi N. Babu P.J. Mahanta C. et al.: ‘Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities’, Mater. Sci. Eng. C., 2015, 46, pp. 463 –469 [DOI] [PubMed] [Google Scholar]
- 36. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, pp. 2638 –2650 [Google Scholar]
- 37. Anjum S. Abbasi B.H.: ‘Thidiazuron‐enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L.’, Int. J. Nanomed., 2016, 11, pp. 715 –728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar R. Roopan S.M. Prabhakarn A. et al.: ‘Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles’, Spectrochim Acta. A. Mol. Biomol. Spectrosc., 2012, 90, pp. 173 –176 [DOI] [PubMed] [Google Scholar]
- 39. Mie R. Samsudin M.W. Din L.B. et al.: ‘Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum ’, Int. J. Nanomed., 2014, 9, pp. 121 –127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munzuroglu O. Geckil H.: ‘Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus ’, Arch. Environ. Contam. Toxicol., 2002, 43, pp. 203 –213 [DOI] [PubMed] [Google Scholar]
- 41. Wang X. Sun C. Gao S.: ‘Validation of germination rate and root elongation as an indicator to assess phytotoxicity with Cucumis sativus ’, Chemosp, 2001, 44, pp. 1711 –1721 [DOI] [PubMed] [Google Scholar]
- 42. Park E.J. Yi J. Kim Y.: ‘Silver nanoparticles induce cytotoxicity by a Trojan‐horse type mechanism’, Toxicol. In Vitro, 2010, 24, pp. 872 –878 [DOI] [PubMed] [Google Scholar]
- 43. Savithramma N. Ankanna S. Bhumi G.: ‘Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata – an endemic and endangered medicinal tree taxon’, Nano Vis., 2012, 2, pp. 61 –66 [Google Scholar]
- 44. Lee W. An Y. Yoon H.: ‘Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant uptake for water insoluble nanoparticles’, Environ. Toxicol. Chem., 2008, 27, pp. 1915 –1921 [DOI] [PubMed] [Google Scholar]
- 45. Abouzid S.F. El‐Bassuon A.A. Nasib A. et al.: ‘Withaferin a production by root cultures of Withania coagulans ’, Int. J. Appl. Res. Nat. Prod., 2010, 3, pp. 23 –27 [Google Scholar]
- 46. Khan M.S. Zaka M. Abbasi B.H. et al.: ‘Seed germination and biochemical profile of Silybum marianum exposed to monometallic and bimetallic alloy nanoparticles’, IET Nanobiotechnol., 2016, 10, (6), pp. 1 –8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hussain M. Raja N.I. Mashwani Z.R. et al.: ‘Green synthesis and characterisation of silver nanoparticles and their effects on antimicrobial efficacy and biochemical profiling in Citrus reticulata ’, IET Nanobiotechnol., 2018, 12, pp. 16, doi: 10.1049/iet‐nbt.2017.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hemm M.R. Rider S.D. Ogas J. et al.: ‘Light induces phenylpropanoid metabolism in Arabidopsis roots’, Plant J., 2004, 38, pp. 765 –778 [DOI] [PubMed] [Google Scholar]
- 49. Costa P. Gonçalves S. Valentao P. et al.: ‘ Thymus lotocephalus wild plants and in vitro cultures produce different profiles of phenolic compounds with antioxidant activity’, Food Chem., 2012, 135, pp. 1253 –1260 [DOI] [PubMed] [Google Scholar]
- 50. Hussain M. Raja N.I. Iqbal M. et al.: ‘Applications of plant flavonoids in the green synthesis of colloidal silver nanoparticles and impacts on human health’, Iran J. Sci. Technol. Trans. Sci., 2017. Available at 10.1007/s40995-017-0431-6 [DOI] [Google Scholar]
- 51. Tariq U. Ali M. Abbasi B.H.: ‘Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L.’, J. Photochem. Photobiol., B: Biol., 2014, 130, pp. 264 –271 [DOI] [PubMed] [Google Scholar]
- 52. Priyadarshini S. Deepesh B. Zaidi M.G.H.: ‘Silver nanoparticle‐mediated enhancement in growth and antioxidant status of Brassica juncea ’, Appl. Biochem. Biotech., 2012, 167, pp. 2225 –2233 [DOI] [PubMed] [Google Scholar]
- 53. Fazal H. Abbasi B.H. Ahmad N. et al.: ‘Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L.’, Appl. Biochem. Biotechnol., 2016, 180, pp. 1076 –1092 [DOI] [PubMed] [Google Scholar]


