Abstract
Nanomaterials play a vital role in textile industries due to their unique properties and applications. There is an increase in the use of nanoscale phyto products in textiles to control the bacterial infection in fabrics. Here, natural herbal nanoparticles of different sizes were prepared from shade‐dried Aloe vera plant leaves using ball milling technique without any additives. The amorphous herbal A. vera nanoparticles possess an average particle size of 40 ± 2 nm and UV‐absorption maximum at 269 nm. A. vera nanopowders–chitosan nanocomposites were prepared and coated on cotton fabrics using pad‐dry cure method. The evaluation of antibacterial activity against Escherichia coli (22.05 ± 0.06 mm) and Staphylococcus aureus (27.17 ± 0.02 mm), UV‐protection properties (UV‐protection factor = 57.2 ± 0.1), and superhydrophobic nature (155 ± 3°) of the prepared herbal nanoparticles and their composites were analysed by disc diffusion, UV–visible spectral analysis, and contact angle analysis. Understanding the functional properties of herbal nanoparticles, coated particles on fabrics highlights their potential applications in protective clothing with better antimicrobial properties, hydrophobicity, and UV‐protection properties. This study of using A. vera herbal nanoparticles in textiles significantly enhances the fabric performance to develop protective textile fabrics in defence and biomedical fields.
Inspec keywords: nanoparticles, particle size, nanofabrication, nanomedicine, antibacterial activity, biomedical materials, hydrophobicity, ultraviolet spectra, visible spectra, radiation protection, textile fibres, cotton fabrics, ball milling, X‐ray diffraction, light scattering, scanning electron microscopy, X‐ray fluorescence analysis, fluorescence, amorphous state, nanocomposites, filled polymers, protective coatings, curing, microorganisms, biodiffusion, contact angle, surface morphology, protective clothing
Other keywords: UV‐blocking, antimicrobial properties, disc diffusion, UV‐visible spectral analysis, contact angle analysis, morphological characteristics, protective clothing, protective textile fabrics, biomedical fields, superhydrophobic nature, UV‐protection factor, UV‐protection properties, Staphylococcus aureus, Escherichia coli, pad‐dry cure method, cotton fabrics, A. vera nanopowders‐chitosan nanocomposites, UV‐absorption maximum, average particle size, amorphous herbal A. vera nanoparticles, X‐ray fluorescence spectrometry, scanning electron microscopy, dynamic light scattering, UV‐visible spectrophotometry, X‐ray diffraction, ball milling, shade‐dried Aloe vera plant leaves, natural herbal nanoparticle size, bacterial infection, nanoscale phyto products, textile industries, nanomaterials, textile applications
1 Introduction
Technological aspects of nanoscience and technology currently needs novel and eco‐friendly synthesis of nanoparticles with unique and enhanced properties based on their distribution and morphology for wide range of applications in medicine, electronics, energy conservation, environmental science, agriculture etc. [1]. We can also find roots in the world of textile fabrication to enhance the physico‐mechanical and functional properties of the fabrics in terms of fabric strength, stiffness, crease recovery, fire‐resistant, UV protection, and antimicrobial activities [2, 3]. The nanoparticles coated on cotton fabrics are also durable because of the penetration of nanoparticles into the fabric surface due to their small size and high surface to volume ratio [4]. In recent years, there has been a large focus on the use of herbal nanoparticles to improve the functional and antimicrobial properties of cotton fabrics in medical textiles [5]. Synthesis of nanoparticles using chemical components and its byproducts cause troubles due to their instability and toxicity. These problems are overcome by the use of biological compounds, especially herbal plants, and also, it creates pathway to green nanotechnology [6]. The combination of nanotechnology and herbal medicine provides knowledge for the technology of making novel herbal nanoparticles as they show efficient bioavailability and no or less toxicity and side effects to organisms, with low cost.
One of the well‐known medical plants that belong to the Liliaceae family is Aloe vera. The terpenoids, flavonoids, tannins and saponins, anthraquinone derivatives, barbaloin, aloe‐emodin‐9‐anthrone, lsobarbaloin, and anthrone‐C‐glycosidesin A. vera possess broad‐spectrum antibacterial activity against pathogenic bacteria [4, 7, 8, 9, 10]. The A. vera gel contains antimicrobial compounds such as glucomannan and acemannan against Streptococcus sp., Serratia marcescens, Citrobacter sp., and Bacillus subtilis [5, 11, 12]. A. vera gel containing mannose‐6‐phosphate and glycoprotein showed wound‐healing activity when studied in rats and guinea pigs upon skin exposure to UV and gamma radiation, subjecting the skin to conditions such as cuts, burns, and eczema [13, 14, 15, 16, 17]. Although the specific role of A. vera has not been identified, administration of A. vera gel may generate free radical scavengers and antioxidant proteins, metallothionein on skin, which scavenges hydroxyl radicals and prevents suppression of superoxide dismutase and glutathione peroxidase. It reduces the production and liberation of skin keratinocyte derivative immunosuppressive cytokines such as interleukin‐10 (IL‐10), and hence it prevents UV‐induced suppression of delayed‐type allergic reaction [18, 19, 20, 21, 22, 23].
Biopolymers have been used in the textile and biomedical filed easy accessibility of low toxicity. The chitosan polymer is an effective biodegradable natural polymer for its antibacterial, haemostatic, and wound‐healing properties and applications in agricultural, textile, pharmaceutical, cosmetic, and food industries [24, 25, 26].
By keeping all the medicinal value of the products from A. vera, we aimed to develop A. vera ‐based herbal nanocomposites to improve the functional properties of the cotton fabrics. Hence, in this study, shade‐dried A. vera plant powder as a precursor is used to synthesise A. vera nanopowders. Then, via high‐energy milling approach, the prepared herbal nanoparticles are mixed with chitosan polymer and the obtained herbal nanocomposites are then meticulously characterised using a wide spectrum of characterisation techniques such as X‐ray diffraction (XRD), UV–visible spectrophotometry (UV–vis), dynamic light scattering (DLS), and scanning electron microscopy/energy‐dispersive X‐ray analysis (SEM–EDX). The nanocomposites coated on cotton fabrics are studied in detail to understand their UV protection, antibacterial and hydrophobic nature, and washing durability. Therefore, the antibacterial assessment of the herbal nanocomposite explains the potentiality of nanotechnology in a symbiosis between textile and pharmaceutical fields.
2 Materials and methods
2.1 Sample collection
Fresh leaves of A. vera plant were collected from in and around Tiruchengode, Tamil Nadu, India. The collected leaves were washed twice with tap water followed by washing with double‐distilled water for multiple times to remove dust from the surface of the leaves. Leaves were thoroughly washed and then the gel was carefully separated from the inner part of the leaf using scalpel. Then, the collected gel was air‐dried under shade at room temperature for 2 weeks.
2.2 Synthesis of herbal nanoparticles
Ten grams of shade‐dried A. vera gel were initially ground via top‐down approach using a mixer grinder to make coarse powder. The coarse powder was milled for 1 h using 20 mm sized balls (Zirconia) using Ball mill (PM100; Retsch, Germany) at 300 rpm to get fine powder. The obtained fine powder was again milled (10 mm balls: 300 rpm, 20:1 ball ratio) for 15 h. The powder was collected for further characterisation studies, followed by coating on fabrics as per the protocol reported in our earlier investigations [27, 28].
2.3 Characterisation studies
The crystalline phase finding of A. vera nanoparticles (AVNps) was carried out by XRD method. The X‐ray powder diffraction measurement was carried out using an X ‐ray powder diffractometer (Philips XRD; X’ PertPro; PANalytical, The Netherlands) with a long fine focus of Cu anode operated at 40 kV and 30 mA in Bragg–Brentano geometry. The powder XRD was obtained in the 2θ range from 10° to 80° in a step‐scan mode of 0.02°. The synthesis of AVNps was monitored periodically in a UV–vis spectrophotometer (Agilent Cary 8454, Singapore) operated from the UV to near‐infrared (180–800 nm) spectral region at a step size of 5 Å. The UV–vis spectra of the samples were monitored as a function of reaction time and particle dosage at a resolution of 1 nm.
The average particle size distribution of the prepared herbal nanoparticles was determined with a submicrometre particle size analyser (Nanophox, Sympatec, Germany) according to the DLS technique. The prepared AVNps were analysed using SEM‐EDX (JSM 6360; JEOL, Japan) to identify the morphology, microstructure, and elemental composition of the prepared samples. Hydrophobicity of the coated and un‐coated fabric surface was measured by water contact angle measurement (VCA Optima, ACT Product Inc., Japan studies). The hydrophobicity of coated and un‐coated fabrics was recorded by placing a drop of water on the fabrics both before and after the tenth wash.
2.4 Preparation of herbal nanoparticles–chitosan composite
The obtained herbal nanoparticles (1 g) were dissolved in 100 ml double‐distilled water and chitosan (1%) suspension and then it was sonicated for 30 min before usage [29]. The bleached woven cotton fabric was pre‐treated as substrates for coating. The cleaned fabrics were dried in an oven at 50°C for 5 min. The addition of chitosan is necessary to make the herbal nanoparticles stable in aqueous solution for a long period. A bleached (30 × 30 cm) cotton fabric was immersed four times in a homogeneous solution containing chitosan sol and A. vera –chitosan (nanocomposite). Then, the obtained solution was passed through a padding mangle at 35 rpm for 5 min with the help of a DC motor to collect the uniformly coated fabric. Thereafter, it was dried at 60°C for 10 min [30, 31, 32]. The chitosan/A. vera nanocomposite indicates the carboxylic groups of chitosan through electrostatic interaction to form the polyelectrolyte composites [33, 34].
2.5 UV protection on coated fabrics
The UV‐blocking properties of the fabric samples were tested by UV transmission spectra (Lambda 35; PerkinElmer, USA) with the wavelength ranging from 280 to 400 nm. The percentage of blocking of UV was calculated in accordance with ASTM D6603 standard [26]. UV‐protection factor (UPF) was obtained using the following relation:
| (1) |
where S (λ) is the solar spectral irradiance (W m−2 nm−1), E (λ) the relative erythemal spectral effectiveness, T (λ) the spectral transmittance, and Δ (λ) the wavelength interval.
2.6 Antimicrobial studies
2.6.1 Collection of microorganisms and culture maintenance
Two bacterial cultures, namely Gram‐positive Staphylococcus aureus (ATCC 6538P) and Gram‐negative Escherichia coli (ATCC 9677), were obtained from the National Collection of Industrial Microorganisms (NCIM) (National Chemical Laboratory, Pune, India). The bacterial cultures were periodically subcultured at 37°C for 24 h. The bacterial cultures were maintained on nutrient agar slant (HiMedia, India) for further experiments at 4°C.
2.6.2 Bacterial inoculum preparation
Bacterial inoculums were prepared by inoculating a loopful of test organisms into nutrient broth and incubating at 37°C for 5–8 h till a moderate turbidity was developed. A loopful of culture was uniformly swabbed on the agar plate.
2.6.3 Disc diffusion method
The qualitative antibacterial assessment was done using disc diffusion method. Coated and un‐coated fabrics were cut into different pieces (each piece with a size of 10 mm) and gently pressed transversely, across the inoculum of streaks to ensure intimate contact of fabrics with agar surface. The plates were then incubated at 37°C for 18–24 h and then they were examined for the inhibition of growth around the fabric edge.
2.7 Washing durability for herbal nanocomposite‐coated fabrics
The wash durability of un‐coated, chitosan‐coated, and A. vera composite fabrics was carried out as per the standard washing test No. 1 of IS: 687‐1979 using a neutral soap (5 gpl) at 40 ± 2°C for 20 min. After drying, the test fabrics and the control were assessed for antimicrobial activity using percentage reduction method.
2.8 Percentage reduction test
The coated and un‐coated fabrics were taken and cut into pieces according to standard size (10 mm radius) with round shape, as recommended by American Association of Textile Chemists and Colorists (AATCC). The samples were soaked with sterile AATCC bacteriostasis broth, and a loopful of test organisms (10−4 CFU/ml) was inoculated. The test samples were incubated at 37°C for 18 h; final number of cells in control and the test samples were calculated using a viable cell count method. After incubation, the number of colonies in each dilution plate was counted. The percentage in bacterial reduction was calculated using the following formula:
| (2) |
where A is the initial number of cells and B the final number of cells.
3 Results and discussion
The XRD pattern of the prepared herbal nanoparticles shown in Fig. 1 confirms the absence of any crystalline diffraction peaks at 2θ values in the range of 20°–30°. Thus, the XRD pattern confirms the amorphous nature of the nanoparticles prepared from A. vera gel [26]. The UV–vis spectra for A. vera herbal nanoparticles are given in Fig. 2. AVNps exhibit absorbance in the UV region at 269 nm which indicates that AVNps can be used for UV protection applications [28]. Generally, the absorption peaks of the herbal particles at UV region are due to the presence of active phytochemical constituents of the plants as reported from our previous report [28].
Fig. 1.
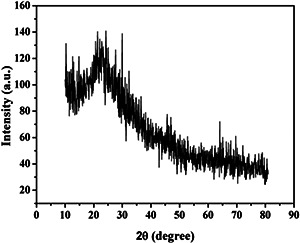
XRD spectrum of AVNps
Fig. 2.
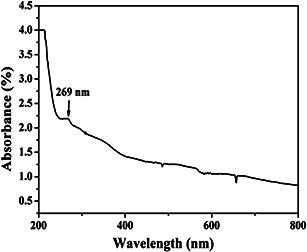
UV–vis spectrum of AVNps dispersed in water
The average particle size of the A. vera sample particles is found to be 40 ± 2 nm (Fig. 3). Since size is an important parameter towards biological response, antimicrobial activity is screened for the prepared herbal nanoparticles from A. vera gel. Antibacterial activity of the prepared AVNps is assessed by measuring the zone of inhibition observed at different concentrations, namely 25, 50, and 100 mg ml−1 of AVNps (Table 1).
Fig. 3.
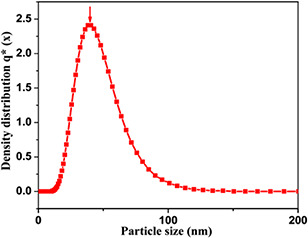
Particle size distribution curve of AVNps
Table 1.
Antimicrobial activity of the A. vera nanopowders from disc diffusion assay
| Herbal nanoparticles | Test organisms | Concentrations of A. vera gel nanopowders (zone of inhibition (mm)) | Standard antibiotic | ||
|---|---|---|---|---|---|
| 25 mg ml−1 | 50 mg ml−1 | 100 mg ml−1 | Amoxicillin (100 mg ml−1) | ||
| AVNps | E. coli | 16.31 ± 0.21 | 20.11 ± 0.07 | 22.05 ± 0.06 | 23.09 ± 0.41 |
| S. aureus | 23.61 ± 0.41 | 25.18 ± 0.03 | 27.17 ± 0.02 | 28.32 ± 0.17 | |
The well loaded with AVNps shows the maximum zone of inhibition (22.05 ± 0.06 and 27.17 ± 0.02 mm against E. coli and S. aureus, respectively, at 100 mg ml−1). These results indicate nearly closed to the standard antibiotic (amoxicillin) tested against E. coli and S. aureus (23.09 ± 0.41 and 28.32 ± 0.17 mm) which proves the excellent bacterial property of AVNps. The antimicrobial nanoparticles are further coated on cotton fabrics for enhancing the functional and biological properties of textiles. SEM image of the A. vera herbal nanoparticles is shown in Fig. 4 a. The topographical characterisation of the nanoparticles observed in SEM image shows the slightly agglomerated as well as discrete nanoparticles this also given information on the average particle size around 40 nm. The elemental composition of A. vera herbal nanopowders is analysed using the EDX spectrophotometer (Fig. 4 b). Besides C and Mg, peaks corresponding to elements such as K and Ca are also observed, as the plant products majorly contain K and Ca ions.
Fig. 4.
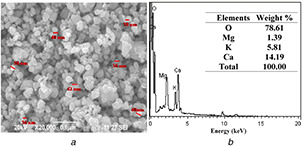
SEM and EDX images of AVNps
3.1 Characterisation of un‐coated and coated fabrics
This observation confirms the adhesion of nanocomposites on the fabric and uniform distributions on the surface as well as within the matrix. The weight of the coated fabrics, namely UC, CF‐Chi, and CF‐AVNps‐Chi, is 120.5 ± 0.5, 127.1 ± 0.5, and 135.9 ± 0.7 g sq ml−1, respectively. The weight ratio of CF‐AVNps‐Chi (composites) fabric is increased compared to that of UC and CF‐Chi, which is due to the addition of A. vera /chitosan nanocomposites. However, the thickness of the UC, CF‐Chi, and CF‐AVNps‐Chi was increased from 0.29 ± 0.1 (UC) to 0.33 ± 0.5 and 0.34 ± 0.5 mm, respectively, due to adhesion of nanoparticles on the surface of the fabric confirmed by our previous coated fabric characterisations [26]. The surface morphological features and EDS analysis of the un‐coated cotton fabrics (UC‐CF) are shown in Fig. 5. The SEM image and EDX of the chitosan‐coated (CF‐Chi) (Fig. 6 a) and AVNps‐Chi nanocomposite (CF‐AVNps‐Chi)‐coated (Fig. 6 b) unwashed cotton fabrics show cluster‐type polymer structures on the fabric surface. The chitosan polymer is strongly penetrated to the surface of the fabrics even after fifth and tenth washes (Figs. 7 a and 8 a).
Fig. 5.
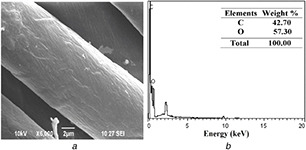
SEM and EDX images of un‐coated fabrics before wash
Fig. 6.
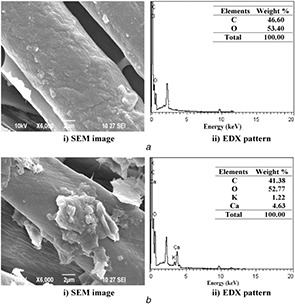
SEM and EDX images of coated fabrics before wash
Fig. 7.
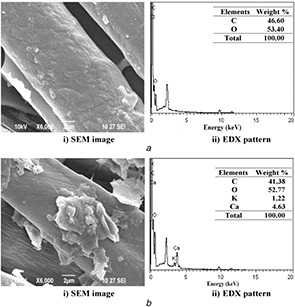
SEM and EDX images of
(a) CF‐Chi, (b) CF‐AVNps‐Chi‐coated fabrics after fifth wash
Fig. 8.
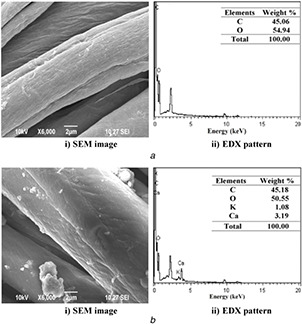
SEM and EDX images of
(a) CF‐Chi, (b) CF‐AVNps‐Chi‐coated fabrics after tenth wash
The wash durability (fastness) of CF‐Chi fabric surface is further confirmed through EDS measurements. Figs. 7 b and 8 b of different samples confirm the presence of uniform distribution of AVNps‐Chi nanocomposite and washes durability of fifth and tenth washes and their elemental composition analysis on coated fabrics.
The nano‐sized particles are embedded easily within the cellulose moieties of cotton fabrics which is strongly to adhered on the fabric. The chitosan/A. vera nanocomposite possesses carboxylic groups of chitosan which binds with fabric through electrostatic interaction and the Van der Waals attraction force [33, 35]. The above observations indicate that the CF‐Chi and CF‐AVNps‐Chi fabrics have strong adhesion on the fabrics, even after subsequent washes. The attachment of more nanocomposites on the textile substrate is due to the strong bond formation of polymeric nanocomposites with fabrics. On comparing with our previous study, it shows that the fabric coated with herbal nanoparticles shows has highly clustered structure even after the fifth and the tenth wash. A. vera nanocomposites are highly effective due to its low surface energy and high surface to volume ratio.
Hence, the aforementioned facts might have resulted in a deeper penetration of the herbal nanoparticles and high surface interaction even after fifth and tenth wash. The above observation confirms the enhanced adhesion of particles on the fabric the higher effectively off using this type of herbal nanoparticles coating while comparing the coated fabrics from the previous studies [26, 36]. Nevertheless, the percentage of nanoparticles coated on the fabric decreases significantly with an increase in the number of washings. However, concentration of the nanoparticles existing on the fabric surface is high enough to retain their functional properties even after the tenth wash. In many biomedical dressing applications, antimicrobial fabrics are used as primary wound‐dressing material as a disposable fabric [37]. However, our investigation proves that it is also possible to use the AVNps‐coated fabrics with improved wash durability and antimicrobial activity for long‐term applications.
UV protection of the coated and un‐coated fabrics is shown in Table 2. The CF‐AVNps‐Chi fabrics show high rate of UV‐B protection as compared to that of un‐coated and CF‐Chi fabrics. It is due to the presence of active phytochemical constituents in AVNps that effectively block the UV‐B radiations. This confirms the good UV‐protective properties of AVNps which is also confirmed from our previous observations of using Acalyph indica herbal nanocomposites‐coated fabrics [26, 38]. The high surface A. vera herbal nanoparticles may also has a high optical polarisation while prepared from high‐energy ball milling method.
Table 2.
UPF value for the herbal nanoparticles coated and un‐coated fabric samples
| Sample | UPF value | |
|---|---|---|
| before wash | ||
| UC‐CF | — | 13.9 ± 0.2 |
| CF‐Chi | — | 42.8 ± 0.3 |
| CF‐ AVNps‐Chi | — | 57.2 ± 0.1 |
| after fifth wash | ||
| CF | — | 11.2 ± 0.4 |
| CF‐Chi | — | 40.1 ± 0.2 |
| CF‐ AVNps‐Chi | — | 54.8 ± 0.1 |
| after tenth wash | ||
| CF | — | 10.8 ± 0.3 |
| CF‐Chi | — | 39.5 ± 0.1 |
| CF‐AVNps‐Chi | — | 52.1 ± 0.2 |
Moreover, the blocking rates of UV radiation for CF‐AVNps‐Chi fabric after the fifth and tenth washes are slightly reduced (up to 7 and 10%) than that of the unwashed cotton fabrics. The decrease in blocking rate of UV radiation is due to the slight removal of coating from the surface of fabrics upon consequent washes. Comparing the results with that of our previous study, the A. vera nanocomposite nanoparticles are highly UV‐protective on the coated fabric surface. UV‐blocking rate is high, evenafter the fifth and tenth washing when compared with previous reports investigated on Ac. indica nanoparticles‐coated fabrics [26, 38].
On the basis of the ASTM D6603 standard data, the calculated UPF for the fabrics used in UV blocking is much higher than 50. As expected, the values for CF and CF‐Chi fabrics are lower than 50 as compared to CF‐AVNps‐Chi fabrics. The UPF value for the CF, CF‐Chi, and CF‐AVNps‐Chi fabric samples are given in Table 2. The CF‐AVNps‐Chi fabric shows high UPF value of ∼50, indicating higher blocking capacity against UV radiation owing to the presence of AVNps on the surface of cotton fabrics. Hence, the CF‐AVNps‐Chi fabrics can effectively shield the UV radiation even after few washing cycles.
3.2 Antimicrobial assessment of AVNps composite and chitosan polymer‐coated fabrics
The antibacterial activities of CF‐Chi and CF‐AVNps‐Chi fabrics are shown in Fig. 9. As expected, a wider zone of inhibition is observed for CF‐AVNps‐Chi fabrics which confer more inhibitory action (33.13 ± 0.03 and 25.48 ± 0.32 mm) than CF‐Chi fabrics against E. coli. However, in the case of S. aureus, the difference in the magnitude of inhibition zone is slightly high (34 ± 0.51 and 26.53 ± 0.31 mm). In the case of UC‐CF fabrics, there is no evidence of bacterial inhibition (Fig. 9). In control, aqueous extract of A. vera nanopowders cause a zone of inhibition of 26.2 ± 0.02 and 27.17 ± 0.02 mm, respectively, against E. coli and S. aureus (Table 1).
Fig. 9.
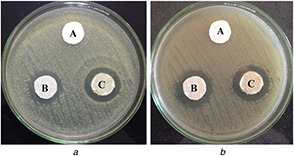
Antimicrobial activity of un‐coated (A), CF‐Chi (B), and CF‐AVNps‐Chi (C) coated fabrics against (a) E. coli, (b) S. aureus
A close observation of the obtained results indicates that CF‐AVNps‐Chi nanocomposite‐coated fabric is more efficient against E. coli and S. aureus. The obtained higher antimicrobial action of AVNps samples is due to the presence of phytochemical compounds which include several terpenoids, flavonoids, and tanninsand also saponins in the A. vera leaves, that possess wide range of antibacterial activity against pathogenic bacteria [7, 8]. Acemannan, a polysaccharide component, has indirect antimicrobial activity through its ability to stimulate phagocytic leucocytes [13, 14]. It is used for the treatment of wounds due to its good healing properties of existent vitamin D [15]. The above bacterial susceptibility investigations using AVNps and nanocomposites demonstrate the exotic medicinal properties and therapeutic uses.
Results of our previous studies show that the nanocomposite‐coated cotton fabric tends to show higher antibacterial property. It might also be because the herbal nanoparticles synthesised using this process tends to have a lower size distribution enabling the easy penetration of these nanoparticles inside the bacterial cells resulting in the inhibition of DNA synthesis as well as in higher cell death. Along with this, the structure of the nanoparticles also plays a major role in the antibacterial property because morphological difference in the nanocomposite sample also adds up to the higher antibacterial activity [26, 28].
3.3 Bacterial percentage reduction
The quantitative assessment of the antibacterial activity of the un‐coated and CF‐AVNps‐Chi nanocomposite‐coated cotton fabrics carried out by percentage reduction test is summarised in Table 3. The results show that a CF‐AVNps‐Chi fabric has higher bacterial reduction percentage (95–99%) than CF‐Chi (78–81%) fabrics, whereas UC‐CF has no bactericidal activity. After the fifth and tenth washes, growth reduction is decreased due to the removal of AVNps‐Chi nanocomposites from the cotton fabric surface.
Table 3.
Bacterial reduction percentage of A. vera chitosan‐coated fabrics
| No. of washes | Bacterial reduction percentage, % | |||
|---|---|---|---|---|
| CF‐Chi | CF‐ AVNps‐Chi | |||
| E. coli | S. aureus | E. coli | S. aureus | |
| 0 wash | 78 ± 0.12 | 81 ± 0.11 | 95 ± 0.45 | 99 ± 0.32 |
| fifth wash | 63 ± 0.31 | 67 ± 0.16 | 79 ± 0.15 | 85 ± 0.22 |
| tenthwash | 42 ± 0.43 | 37 ± 0.13 | 43 ± 0.17 | 45 ± 0.23 |
The nano‐sized particles embedded easily within the cellulose moieties of cotton fabrics which are strongly adhered on the fabric. The strong interaction of antimicrobial nanoscale A. vera particles provides better antimicrobial fabric with increased wash durability [39]. A similar observation is made in our previous investigation on cotton fabrics coated with Ac. indica herbal nanoparticles wherein consequent washes lead to the removal of nanoparticles from fabrics that result in a decrease in antimicrobial activity [26, 38]. Herbal particles loaded inside the polymeric matrix of chitosan coated on the fabric are found to be effective fabrics for long time durability of cotton fabrics [33].
3.4 Water‐repellent property
Surface contact angle is measured using sessile drop method to explain the comparison of the water‐repellent property of the un‐coated (UC‐CF) and coated fabrics (CF‐Chi and CF‐AVNps‐Chi) in Table 4. Generally, the un‐coated fabric shows high degree of hydrophilic nature due to the presence of the hydroxyl groups on its surface. As a result, as soon as the water droplet is placed on the cotton fabric, it gets absorbed easily by the fabric surface (Fig. 10).
Table 4.
UPF value for the herbal nanoparticles coated and un‐coated fabric samples
| Samples | Contact angle (°) |
|---|---|
| before wash | |
| UC‐CF | 0 |
| CF‐Chi | 125 ± 1 |
| CF‐ AVNps‐Chi | 155 ± 3 |
| fifth wash | |
| UC‐CF | 0 |
| CF‐Chi | 117 ± 2 |
| CF‐ AVNps‐Chi | 136 ± 1 |
| tenth wash | |
| UC‐CF | 0 |
| CF‐Chi | 102 ± 2 |
| CF‐ AVNps‐Chi | 127 ± 4 |
Fig. 10.
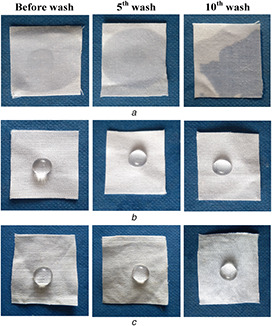
Photographic images of water droplet in textile surfaces of un‐coated and coated for before and after washes
In contrast, AVNp‐coated fabrics show super hydrophobicity due to lower surface energy of the coated fabric causing the reduction in the fabric pore sizes.
The water droplet on the fabric surface is easily absorbed on the un‐coated material. The AVNps‐coated fabrics shows an excellent water‐repellent property (super hydrophobic) (155 ± 3°). It is seen that the un‐coated fabric and the fabric coated with CF‐Chi have different contact angles such as 0° and 101 ± 2°, respectively. This result is comparatively high when compared with the fabric coated with other Ac. indica herbal nanoparticles in our previous studies. The superhydrophobic nature of the AVNps‐coated fabrics are due to the surface modification of fabrics with AVNps of having lower surface energy, uniform distribution, and binding [38].
Even after subsequent (5 and 10) washes, the fabric surface still has the superhydrophobic property (Table 3). From the aforementioned investigation, it is evident that the fabrics coated with the AVNps shows a superhydrophobic nature due to its excellent binding and the surface morphological changes on the fabrics as shown in SEM analysis (Fig. 6). Hence, we can conclude that all the coated fabrics retain the water‐resistive property even after subsequent washes.
4 Conclusion
Herbal nanoparticles are prepared from the shade‐dried gel of A. vera by ball milling (top‐down process) method and are used for coating on the cotton fabrics. The presence of nanoparticles on the fabric is confirmed by electron microscopy and spectroscopic studies. The UV–vis spectra of the AVNps show absorption maxima at 269 nm that confer better UV protection when it is coated on the fabrics. Significant amount of AVNps deposited on the fabric is found to remain evenafter ten washing.
The herbal nanoparticles‐coated cotton fabrics also exhibit better antimicrobial activity against S. aureus and E. coli. From the above results, it is known that the medicinal property of the natural phytochemical constituents of A. vera is enhanced at nanoscale and can be transferred to textile fabrics via coating of AVNps on the surface. Such coating also improves the functional properties of CF‐AVNps‐Chi nanocomposite‐coated fabrics in terms of antibacterial activities, washing durability, superhydrophobicity, and blocking of UV radiations. Therefore, the coating of A. vera ‐based herbal nanomaterials on the fabrics facilitates the application of natural products in biomedical and textile industries with improved physico‐chemical and functional properties.
5 Acknowledgment
The authors acknowledge the financial support provided by Board of Research and Nuclear Science (BRNS), Mumbai (Sanction no: 2013/34/30/BRNS/1127dt.19.9.2013).One of the authors (R.S) is thankful to the University Grants Commission (UGC), New Delhi for the award of Post‐Doctoral Fellowship for Women (F.15‐1/2015‐17/PDFWM‐2015‐17‐TAM‐36274 dt.12/10/2015) to carry out this research work.
6 References
- 1. Kumar C.S.S.R.: ‘Nanotechnology tools in pharmaceutical R&D’, Mater. Today., 2010, 12, pp. 24 –30 [Google Scholar]
- 2. Chen M. Wang S. Tan M. et al.: ‘Applications of nanoparticles in herbal medicine: zedoary turmeric oil and its active compound β‐elemene’, Am. J. Chin. Med., 2011, 39, pp. 1093 –1102 [DOI] [PubMed] [Google Scholar]
- 3. Chithra P. Sajithal G.B. Chandrakasan G.: ‘Influence of Aloe vera on glycosaminoglycans in the matrix of healing dermal wounds in rats’, J. Ethnopharmacol., 1998, 59, pp. 179 –186 [DOI] [PubMed] [Google Scholar]
- 4. Mariita R.M. Orodho J.A. Okemo P.O. et al.: ‘Methanolic extracts of Aloe Secundiflora Engl. inhibits in vitro growth of tuberculosis and diarrhea‐causing bacteria’, Pharmacognosy Res., 2011, 3, pp. 95 –99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heggers J.P. Pineless G.R. Robson M.C.: ‘Dermaide Aloe/Aloe vera gel: comparison of the antimicrobial effects’, Am. J. Med. Technol., 1979, 41, pp. 293 –294 [Google Scholar]
- 6. Ingale A.G. Chaudhari A.N.: ‘Biogenic synthesis of nanoparticles and potential applications: an eco‐friendly approach’, J. Nanomed. Nanotechnol., 2013, 4, pp. 1 –7 [Google Scholar]
- 7. Kumar S. Yadav J.P. Lorenzetti L.J.: ‘Ethanobotanical and pharmacological properties of Aloe vera: a review’, J. Med. Plants Res., 2014, 8, (48), pp. 1387 –1398 [Google Scholar]
- 8. Zandi K. Zadeh A.: ‘Antiviral activity of Aloe vera against herpes simplex virus type 2: an in vitro study’, Afr. J. Biotechnol., 2007, 6, (15), pp. 1770 –1773 [Google Scholar]
- 9. Doshi S. Mohan P. Prasad Kabra M. et al.: ‘Evaluation of analgesic activity of some polyherbal extracts against acetic acid induced writhing in experimental animals’, World J. Pharm. Sci., 2014, 2, (1), pp. 105 –107 [Google Scholar]
- 10. Heck E. Head M. Nowak D. et al.: ‘ Aloe vera (gel) cream as a topical treatment for outpatient burns’, Burns, 1981, 7, pp. 291 –294 [Google Scholar]
- 11. Robson M.C. Heggers J.P. Hagstrom W. J.: ‘Myth, magic, witchcraft or fact? Aloe vera revisited’, Burn Care Res., 1982, 3, pp. 157 –163 [Google Scholar]
- 12. Ferro V.A. Bradbury F. Cameron P. et al.: ‘In vitro susceptibilities of Shigella flexneri and Streptococcus pyogenes to inner gel of Aloe barbadensis Miller’, Antimicrob. Agents Chemother., 2003, 47, pp. 1137 –1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lalitha Devi D. Srinivas B. Narasinga Rao B.: ‘An evaluation antimicrobial activity of Aloe barbadensis Miller (Aloe vera) gel extract’, J. Pharm. Biomed. Sci., 2012, 21, pp. 1 –4 [Google Scholar]
- 14. Pugh N. Ross S.A. Elsohly M.A. et al.: ‘Characterization of aloeride, a new high molecular weight polysaccharide from Aloe vera with potent immunostimulatory activity’, J. Agric. Food Chem., 2001, 49, pp. 1030 –1034 [DOI] [PubMed] [Google Scholar]
- 15. Rajeswari R. Umadevi M. Sharmila Rahale C. et al.: ‘ Aloe vera: the miracle plant its medicinal and traditional uses in India’, J. Pharmacogn. Phytochem., 2012, 1, pp. 118 –124 [Google Scholar]
- 16. Davis R.H. Leitner M.G. Russo J.M. et al.: ‘Anti inflammatory activity of Aloe vera against a spectrum of irritants’, J. Am. Podiatr. Med. Assoc., 1989, 79, pp. 263 –276 [DOI] [PubMed] [Google Scholar]
- 17. Thompson J.E.: ‘Topical use of Aloe vera derived allantoin gel in otolaryngology’, Ear Nose Throat J., 1991, 70, p. 119 [PubMed] [Google Scholar]
- 18. Heggers J.P. Pelley R.P. Robson M.C.: ‘Beneficial effects of Aloe in wound healing’, Phytother. Res., 1993, 7, pp. 48 –52 [Google Scholar]
- 19. Yagi A. Egusa T. Arase M. Tanabe M. et al.: Isolation and characterization of the glycoprotein fraction with a proliferation‐promoting activity on human and hamster cells in vitro from Aloe vera gel. Planta Med., 1997, 63, pp. 18 –21 [DOI] [PubMed] [Google Scholar]
- 20. Serrano M. Valverde J.M. Guillen F. et al.: ‘Use of Aloe vera gel coating preserves the functional properties of table grapes’, J. Agr. Food Chem., 2006, 54, pp. 3882 –3886 [DOI] [PubMed] [Google Scholar]
- 21. Raymond J.C. Joan W. Bryan C. et al.: ‘Prevention and treatment of acute radiation‐induced skin reactions: a systematic review and meta‐analysis of randomized controlled trials’, BMC Cancer, 2014, 14, p. 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sato Y. Ohta S.: ‘Studies on chemical protectors against radiation XXXI. Protective effects of Aloe ar‐ borescens on skin injury induced by X‐irradiation’, Yakugaku Zasshi, 1990, 110, pp. 876 –884 [DOI] [PubMed] [Google Scholar]
- 23. Byeon S. Pelley R. Ullrich S.E. et al.: ‘Aloe barbadensis extracts reduce the production of interleukin‐10 after exposure to ultraviolet radiation’, J. Invest. Dermatol., 1988, 110, pp. 811 –817 [DOI] [PubMed] [Google Scholar]
- 24. Silva S.S. Caridade S.G. Mano J.F. et al.: ‘Effect of crosslinking in chitosan/aloe vera‐based membranes for biomedical applications’, Carbohydr. Polym., 2013, 98, pp. 581 –588 [DOI] [PubMed] [Google Scholar]
- 25. Ahmed S. Ikram S.: ‘Chitosan based scaffolds and their applications in wound healing’, Aci. Life Sci., 2016, 10, pp. 27 –37 [Google Scholar]
- 26. Karthik S. Vinoth M. Balu et al.: ‘An ecofriendly route to enhance the antibacterial and textural properties of cotton fabrics using herbal nanoparticles from Azadirachta indica (neem)’, J. Alloys Compd., 2017, 723, pp. 698 –707 [Google Scholar]
- 27. Vinoth M. Suriyaprapha R. Arunmetha S. et al.: ‘Synthesis of Nothapodytes nimmoniana leaf nanoparticles for antireflective and self‐cleaning applications’, Synth. React. Inorg. Met.‐Org. Nano‐Met. Chem., 2015, 46, pp. 1445 –1449 [Google Scholar]
- 28. Karthik S. Suriyaprabha R. Balu K.S. et al.: ‘Influence of ball milling on the particle size and antimicrobial properties of Tridax procumbens leaf nanoparticles’, IET Nanobiotechnol., 2017, 11, pp. 12 –17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhineshbabu N.R. Arunmetha S. Manivasakan P.G. et al.: ‘Enhanced functional properties of cotton fabrics using TiO2 /SiO2 nanocomposites’, J. Ind. Text., 2016, 45, pp. 1 –19 [Google Scholar]
- 30. Rajendran R. Radhai R. Balakumar C. et al.: ‘Synthesis and characterization of neem chitosan nanocomposites for development of antimicrobial cotton textiles’, J. Eng. Fiber Fabr., 2012, 7, pp. 136 –141 [Google Scholar]
- 31. Dhineshbabu N.R. Manivasakan P. Karthik A. et al.: ‘Hydrophobicity, flame retardancy and antibacterial properties of cotton fabrics functionalised with MgO/methyl silicate nanocomposites’, RSC Adv., 2014, 4, pp. 432161 –432173 [Google Scholar]
- 32. Dhineshbabu N.R. Manivasakan P. Yuvakkumar R. et al.: ‘Enhanced functional properties of ZrO2/SiO2 hybrid nanosol coated cotton fabrics’, J. Nanosci. Nanotechnol., 2013, 13, pp. 4017 –4024 [DOI] [PubMed] [Google Scholar]
- 33. Rajendran R. Radhai R. Rajalakshmi V.: ‘Development of mosquito repellent fabrics using Vitex negundo loaded nanoparticles’, Malaya. J. Biosci., 2014, 1, (1), pp. 19 –23 [Google Scholar]
- 34. Rajendran R. Rajalakshmi V. Radhai R.: ‘Fabrication of antimicrobial medical textiles using V. negundo loaded nanoparticles’, Int. J. Pharm. Pharm. Sci., 2014, 3, pp. 1394 –1406 [Google Scholar]
- 35. Vinay G.N. Sanjeev R.S.: ‘Antibacterial properties of silk fabric treated with Aloe vera and silver nanoparticles’, J. Text. I., 2017, 108, pp. 385 –396 [Google Scholar]
- 36. Chong K.L. Seong S.H. Young K.S. et al.: ‘Prevention of ultraviolet radiation‐induced suppression of contact hypersensitivity by Aloe vera gel components’, Int. J. Immunopharmacol., 1999, 21, pp. 303 –310 [DOI] [PubMed] [Google Scholar]
- 37. Gupta B.: ‘Textile based smart wound dressings’, Indian J. Fibre Text., 2010, 35, pp. 174 –187 [Google Scholar]
- 38. Karthik S. Suriyaprabha R. Vinoth M. et al.: ‘Larvicidal, super hydrophobic and antibacterial properties of herbal nanoparticles from Acalypha indica for biomedical applications’, RSC Adv., 2017, 7, pp. 41763 –41770 [Google Scholar]
- 39. Chandrasekar S. Vijayakumar S. Rajendran R. et al.: ‘Herbal‐chitosan nanocomposites for durable antibacterial finishing on cotton materials’, Int. J. Biopharm., 2013, 4, (3), pp. 219 –224 [Google Scholar]


