Abstract
Theranostic approach provides us a platform where diagnosis and treatment can be carried out simultaneously. Biosynthesis of theranostic‐capable nanoparticles (NPs) can be carried out by phytoconstituents present inside the plants that can act as capping as well as stabilising agents by offering several advantages over chemical and physical methods. This article highlights the theranostic role of NPs with emphasis on potential of plants to produce these NPs through ecofriendly approach that is called ‘Green synthesis’. Biosynthesis, advantages, and disadvantages of plant‐based theronostics have been discussed for better understanding. Moreover, this article has highlighted the approaches required to optimise the plant‐mediated synthesis of NPs and to avoid the toxicity of these agents. Anticipating all of the challenges, the authors expect biogenic NPs can appear as potential diagnostic and therapeutic agents in near future.
Inspec keywords: nanofabrication, nanomedicine, nanoparticles, biomedical imaging, patient treatment
Other keywords: bioinspired tool, biosynthesis, theranostic‐capable nanoparticles, Green synthesis, plant‐based theronostics, plant‐mediated synthesis, potential diagnostic agents, therapeutic agents, theranostics agents, plant‐based metallic nanoparticles
1 Introduction
Nanotechnology is defined as the science and engineering that involve design, synthesis, characterisation, and application of materials and devices whose smallest organisation, in at least one dimension is on nanometre scale or 1 billion of a metre [1]. Nanoparticles (NPs) possess approximate size of 100 nm or less. Nanotechnology involves application of science to control the matter at molecular level. It is an emerging field which is gaining importance in various fields such as mechanics, optics, biomedical science, drug gene delivery, non‐linear optical devices etc. Small size and large surface to volume ratio of NPs make them excellent candidates for biomedical applications [2, 3]. NPs have brought revolution in biomedical field, healthcare, drug as well as gene deliver, and optical devices [4]. Theranostics is a concept that involves incorporation of diagnostic as well as therapeutic function on a single platform [5]. Theranostic nanoparticles (TNPs) contain polymers in which diagnostic as well as therapeutic agents have been incorporated simultaneously having capabilities such as controlled drug release, targeted drug release, multimodality diagnosis, as well as therapy [6]. They can be applied in various diagnostic techniques such as computerised tomography (CT), magnetic resonance imaging (MRI), ultrasound, radiation therapy etc. [7]. TNPs have brought revolution in biomedical field and various products have become commercially available while some are undergoing different phases of clinical trials [8]. Various physical and chemical approaches are available for synthesis of these NPs, but recently, biological systems arranging from prokaryotes to eukaryotes are gaining more attention. By using biological systems, NPs of desired shape and characteristics can be obtained if we ensure selection of appropriate organism, optimal condition for cell growth and enzyme activity, and optimal reaction conditions. Plants are natural nanofactory that contains capping as well reducing agents such as enzymes, proteins, and polysaccharides for synthesis and stabilisation of NPs. Up till now, a wide variety of TNPs has been prepared from plant extracts through green synthesis (Fig. 1) that offer more appropriate platform with significant advantages over other biological entities [3]. Green bionanomaterials involve metals such as silver, copper, gold, titanium, and iron that can be prepared from various biological entities with potential to be applied for various biomedical applications [18]. Metal NPs are promising biological detection tools because of their enhanced optical detection capabilities where incident light is associated with plasmon excitation of the metal resulting in million fold greater light scattering than any other molecule [19]. Green synthesis approach has resulted in initiation of new era of biotechnology [4].
Fig. 1.
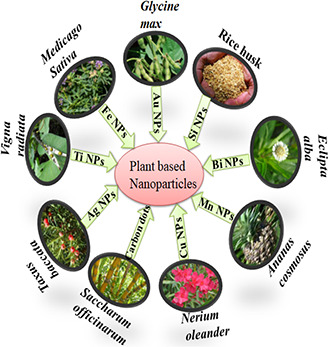
Representation of types of nanoparticles synthesise via different plants [9, 10, 11, 12, 13, 14, 15, 16, 17]
2 Bio‐synthesised NPs as theranostics
Among a number of plants‐based green synthesised metallic NPs, few are studied for their theranostic potential as shown in Fig. 2.
Fig. 2.
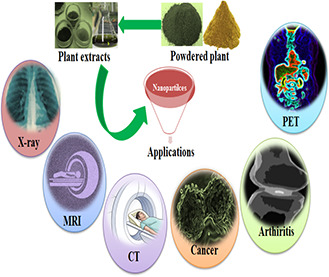
Applications of plant‐based nanomaterials in disease diagnosis and treatment [20, 21, 22, 23]. MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography
2.1 Gold NPs as theranostics
Gold nanoparticles (Au NPs) have been studied as potential candidate as theranostic agents. Photoacoustic (PA) imaging is a technique that generates three‐dimensional image of an anatomical structure due to absorption in near infrared region (NIR). Gold nanocages are ideal agents for PA imaging [24]. Au NPs can be used in optical imaging probes due to their properties such as supermagnetism, fluorescence, and surface plasmon resonance (SPR). When compared with bismuth sulphide and iodinated NPs, their size and shape can be easily controlled and modification can be done by introducing different functional groups [25]. Most of the applications of Au NPs are based on a phenomenon known as localised surface plasmon resonance (LSPR). SPR is a state of oscillation of electrons when they are irradiated by incident light, creating intense peaks at resonant wavelengths. Au nanostructures have LSRP in the NIR ranging from 700 to 900 nm. Nanostructures made up of other metals also exhibit strong LSPR but due to bioinert nature, Au NPs are potential candidate for these applications [26]. Light absorption and scattering of property of Au NPs enable them to diagnose and treat cancer. Moreover, their surface can be modified by attaching sulphur, phosphorus, and nitrogen ligands. In contrast to Au NPs, quantum dots (QDs) do not possess surface functionalisation property and can cause more toxicity [27]. Au NPs accumulate at target site and cause irreversible cell destruction and imaging by irradiating light due to SPR [28]. Hyperthermia is non‐invasive approach for treatment of cancer in which tissues are exposed at high temperature for selective damage of cancerous cells. Owing to high metabolic rate, cancerous cells are more sensitive to high temperature which cause production of heat shock proteins and disrupts cellular metabolism resulting in apoptosis of cancerous cells [29]. Au NPs can be designed to absorb light at SPR wavelength (808 nm) which is then converted into heat, thus producing hyperthermic effect [30]. The viability of human pulmonary carcinoma cell line A459 was inhibited while bronchial epithelial cell line 16 HBE and primary adult stem cell MSC exhibited little cytotoxicity after incubation with Au NPs. It was believed that interaction between Au‐nanorods and lysosome membrane facilitates the release of Au NPs resulting in apoptosis of cell lines. More selective targeting of cancerous cells can be achieved by attachment of cytokines and hormone receptors. TNF‐α is a cytokine that synergise antitumour response when attached with Au NPs promote accumulation of conjugates at the site of tumour because TNF receptors facilitate accumulation of TNF‐α in cancerous cells [31].
Plant‐based green synthesised Au NPs have been revealed to inhibit the growth of cancerous cells [32]. Up till now Au NPs have been produced from many plants those are capable to play role in various biomedical fields as shown in Table 1. It has been reported that Au NPs with theranostic potential can be produced from various plants such as Nyctanthes arbortristis [38], Centella asiatica leaf extract [22], Cinnamomum zeylanicum [35], Rosa hybrida [39], Chrysopogon zizanioides leaf extract [40], and Glycine max [9]. Gum Arabica has been used to produce and stabilise Au NPs that possessed potential to play role in diagnostic techniques [33]. In addition, it was revealed that tea contain capping agents for preparation of Au NPs [34]. In addition to that Butea monosperma [44], Amaranthus spinosus [21], Spheranthus amaranthoides [41], Moringa olifera [43], Syzgium aromaticum [36], Crocus sativus [37], and Punica granatum [42] can also produce Au NPs with theranostic potential.
Table 1.
Characteristics and applications of plant‐based Au NPs acting as theranostic agents
| Plant name | Size, nm | Shape | Diagnosis | Treatment | References |
|---|---|---|---|---|---|
| Acacia senegal | 151 | — | CT | cancer therapy | [33] |
| Glycine max | 15 ± 4 | spherical | molecular imaging | cancer therapy | [9] |
| Camellia sinensis | 40 | spherical | bio imaging | — | [34] |
| Cinnamomum zeylanicum | 25 | spherical | NIR absorber (cancer diagnosis) | — | [35] |
| Syzgium aromaticum | 5–10 | spherical, elliptical | biomedical imaging | photo thermal therapy | [36] |
| Centella asiatica | 9.3, 10.9 | spherical | biomolecular imaging | cancer therapy | [22] |
| Crocus sativus | 15 ± 5 | spherical, triangular | — | cancer therapy | [37] |
| Nyctanthes arbortrist | 19.8 ± 5 | triangular, pentagonal, rod shaped, spherical | contrasting agents in bioimaging | cancer therapy | [38] |
| Rosa hybrida | 10 | cubic | bio imaging | cancer therapy | [39] |
| Chrysopogon zizanioides | 20–50 | cubic | — | biosensor | [40] |
| Amaranthus spinosus | 10.74 | spherical, triangular | molecular imaging, i.e. PET, MRI, SPECT | — | [21] |
| Spheranthus amaranthoides | 39–47 | spherical | cancer diagnosis | cancer therapy | [41] |
| Punica granatum | 70.90 ± 8.42 | — | — | carrier for drug delivery targeting breast cancer | [42] |
| Moringa olifera | 3–5 | hexagonal, triangular | — | cancer therapy | [43] |
| Butea monosperma |
10–30 50–75 30–100 |
spherical triangular hexagonal |
— | efficient drug delivery for future cancer therapy | [44] |
CT, computed tomography; NIR, near infrared region; PET, positron emission tomography; MRI, magnetic.
2.2 Iron oxide nanoparticles as theranostics
Iron oxide nanoparticles (IONPs) possess imaging and therapeutic capabilities due to super magnetic behaviour and surface modification ability; therefore, they are potential candidates for non‐invasive diagnosis as well as treatment of cancer [45]. Large surface area of IONPs allows attachment to different ligands and receptors ensuring site‐specific and controlled drug release. While attachment of molecular markers to IONPs enables them to visualise and detect changes in metabolic pathways; thus have potential for its manipulation in bioimaging. In addition, these factors can ensure site‐specific controlled drug release with reduced drug wastage, thus minimising frequency of drug administration with fewer side effects [46]. In short, IONPs are biodegradable, biocompatible coating allows incorporation of multiple moieties, high drug loading capability, ease of surface modification by ligands ensuring targeted drug delivery [47]. When IONPs are used for imaging of cancerous tissues; different mechanisms have been reported behind differentiation of cancerous cells from normal cells. First, passive targeting of super iron oxide nanoparticles (SIONPs) is done through permeability and retention effect (EPR) effect in which cancerous cells because of damaged vasculature are more permeable due to enhanced EPR so they uptake more NPs when compared with normal cells. Second method of passive targeting is liver and spleen imaging via reticular endothelial system targeting, NPs can be internalised by macrophage‐enriched organs such as liver, spleen, and bone marrow.
When tumorous tissues are present in these organs, they contain less macrophages that results in decreased internalised NPs when compared with normal tissue and hence, week MRI signal in these organs are prone. IONPs provide advantages over other contrast agents such as high magnetic signal strength, low toxicity, long‐lasting contrast enhancement, and improved delineation of tumour margins [48]. Biomedical applications of IONPs have been depicted in Fig. 3.
Fig. 3.
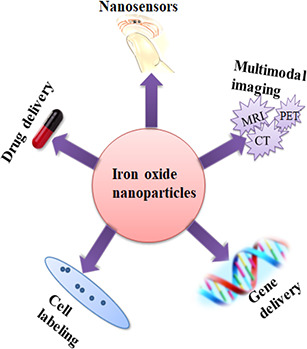
Representation of biomedical applications of IONPs [49, 50, 51]
The best approach for synthesis of IONPs involves their production from plants that can produce stable NPs with a variety of shapes through environment‐friendly process [3]. It has been reported that IONPs with theranostic potential can be synthesised by various plants (Table 2) such as grape seed [20], brown seaweed [52, 53], plantain peel extract [54], aloe vera extract [55], and alfalfa [17]. In addition, synthesis of IONPs from various other plants such as Camellia sinensis [56], eucalyptus [57], Sorghum bran extract [58], Hordeum vulgare and Rumex acetosa [59], and Sageretia thea [60] has also been reported but their potential as theranostic agents. However, all these IONPs need to be further studied for their practical applications.
Table 2.
Characteristics and applications of bioinspired IONPs as theranostic agents
| Plant name | Size, nm | Shape | Therapy | Diagnosis | References |
|---|---|---|---|---|---|
| Medicago sativa | 3.1 | cubic | targeted drug delivery, substrate in cancer treatment | — | [17] |
| Vitis vinifera | 35 | spherical | — | X‐ray contrast agents, MRI, CT bioimaging | [20] |
| Sargassum muticum | 18 ± 4 | cubic | cancer therapy with anticancer activity against Jurkat cells, MCF‐7 cells, HeLa cells, HepG2 cell lines | — | [52, 53] |
| plantain peel | 50 | spherical | applicable in biomedical fields and efficient site‐specific drug delivery | — | [54] |
| Aloe barbadensis | 6–30 | cubic | tumour hyperthermia | MRI | [55] |
MRI, magnetic resonance imaging; CT, computed tomography.
2.3 Titanium dioxide NPs as tool for theranostics
Photothermal therapy (PTT) involves the use of a photosensitiser with strong optical absorption property which converts light energy into heat, thus generating hyperthermia which eventually causes selective damage to cancerous cells with minimum side effects. Photodynamic therapy (PDT) involves the use of photosensitiser that undergoes excitation after absorbing photons from visible light and transfer the absorbed energy to oxygen and reactive oxygen species (ROS). Titanium dioxide nanoparticles (TiO2 NPs) can produce ROS when used as photosensitiser during PDT. TiO2 NPs have recently emerged as potential photosensitiser acting as a multicomponent nanocomposite for PTT and PDT. In addition to that TiO2 NPs after conjugation with folic acid can be used for site‐specific delivery of anticancer drugs [61, 62]. TiO2 NPs are used as candidate for cell imaging and as a contrast agent in MRI [63, 64] and optical coherence tomography [65]. TiO2 NPs has been used for diagnostic and therapeutic purpose for rheumatoid arthritis [23]. PTT when applied alone can cause damage of healthy tissues; therefore, TiO2 multicomponent nanocomposites for PTT and PDT triggered by NIR light allow synergistic tumour treatment and also overcome the obstacles of UV light excitation [61]. Green synthesis of TiO2 NPs has been carried out by using various plants such as Annona squamosa peel [66], N. arbortristis leaf extract [67], Jatropha curcas aqueous [68], Euphorbia prostrata leaf extract [69], Hibiscus flower [70], and Vigna radiata [16] which have potential to be used in various biomedical applications as shown in Table 3.
Table 3.
Characteristics and applications of bioinspired TiO2 NPs acting as theranostic agent
| Plant name | Size, nm | Shape | Applications | References |
|---|---|---|---|---|
| Nyctanthe arbortristis | 100–150 | spherical | TiO2 NPs can be applicable for all biomedical applications including cancer therapy as well as cell imaging | [63, 67] |
| Annona squamosa | 23 ± 2 | spherical | TiO2 NPs have potential for biomedical applications such as photodynamic therapy for cancer treatment, cell bioimaging, and targeted drug delivery | [63, 66] |
| Jatropha curcas L. | 25–50 | spherical | TiO2 NPs produced by this plant can be used for all biomedical application including cancer treatment and cell imaging | [63, 68] |
| Vigna radiata | — | oval | TiO2 NPs showed cytotoxic activity against osteosarcoma cell lines thus could be tested for its potential as anticancer agent | [16, 63] |
| Hibiscus flower | — | — | TiO2 NPs possessed potential for various biomedical applications including their role as theranostic | [63, 70] |
2.4 Silver nanoparticles as theranostic agents
Silver nanoparticles (Ag NPs) brought revolution in biomedical field by acting as diagnostic as well as therapeutic modality. Antitumour potential of Ag NPs has been reported against Dalton's lymphoma ascites. The study reveals that activation of caspase 3 enzyme is responsible for apoptosis in the target cells [71]. Cytotoxic activity of Ag NPs showed mitotic arrest and chromosome instability against normal human lung fibroblasts (IMR‐90) and human glioblastoma (U251) [72]. Ag NPs have also been reported for their cytotoxic activity against acute myeloid leukaemia [73], A549 (human lung cancer cell line), B16 (mouse melanoma cell line), and MCF7 (human breast cancer cells) [74]. Advances in the applications of Ag NPs led their utilisation in various applications such as biosensing, diagnostic imaging, and cancer diagnosis as well as cancer therapy thus acting as theranostic agents [75].
Aptamer‐based silver nanoprobes can be used for intracellular protein imaging as well as for single protein spectral analysis. They can act as a contrast imaging agent for dark field light scattering microscopy as well as allow intracellular microenvironment analysis [76]. Biosensor array has been developed using Ag NPs to indicate serum levels of p53 protein in patients with head and neck squamous cell carcinoma. This biosensor provides promising tool for tumour diagnosis [77]. Biomedical applications of Ag NPs have been represented in Fig. 4. Green synthesis of Ag NPs with theranostic potential has been carried out from various plants (Table 4) such as Olax scandens leaf extract [74], Ablemoschus esculentus pulp extract [81], Allophylus cobbe, Artemisia princeps, and Typha angustifolia [86], oak fruit [82], Sargassum vulgare [83], Plumeria alba [84], Cyamopsis tetragonaloba [78], Taxus baccata extract [15], Dimocarpus Longan Lour [85], Alternanthera sessilis [79], and Ganoderma neo‐japonicum imazeki extract [80].
Fig. 4.
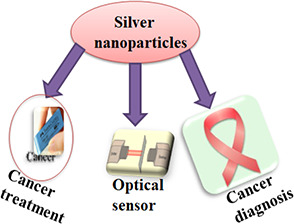
Table 4.
Characteristics and applications of plant‐based Ag NPs acting as theranostic agent
| Plant name | Size, nm | Shape | Therapy | Diagnosis | References |
|---|---|---|---|---|---|
| Cyamopsis tetragonaloba | 10 | cubic | — | optical sensors can be used for detection ammonia content in biological fluids | [78] |
| Alternanthera sessilis | <100 | cubic | therapy for prostate cancer, breast cancer | — | [79] |
| Ganoderma neo‐japonicum imazeki | 5 | spherical | cancer therapy, arthiritis, and neovascularisation | — | [80] |
| Olax scandens | 0–60 | spherical | anticancer agent1 | can be used as diagnostic tool for cancer | [74] |
| Taxus baccata | 75.1 | spherical | these NPs can be used for tracking and imaging techniques for cancer diagnosis | cancer therapy | [15] |
| Ablemoschus esculentus | 6.7 | — | cytotoxic activity against Jurkat cell lines, having potential to be applied as anticancer agents | — | [81] |
| Oak Fruit Hull (Jaft) | 40 | spherical | anticancer agents against MCF‐7 cell lines | — | [82] |
| Sargassum vulgare | 10 | — | showed anticancer activity against cancerous human myeloblastic leukaemic cells HL60 and cervical cancer cells lines | — | [83] |
| Plumeria alba | 36.19 | spherical | showed anticancer activity against COLO 205 cells | — | [84] |
| Dimocarpus Longan Lour. | 9–32 | cubic | therapy for prostate cancer | developing role as a diagnostic agent | [85] |
| Allophylus cobbe, Artemisia princeps, Typha angustifolia | 2–100 | — | anticancer activity | diagnose tumour | [86, 87] |
2.5 QDs and carbon nanotubes
QDs are actually NPs made up of semiconductor and luminescent materials. QDs exhibit narrow, symmetric, and size‐tunable emission spectra which is responsible for multicolour applications. In addition, they also give strong fluorescence string for prolong time period which makes them superior for bioimaging. QDs generate fluorescence through Froster resonance energy transfer because of their unique properties, i.e. broad absorption, size‐dependent narrow emission, and resistance to photobleaching [88]. Several plants like Aloe [89], Orange peel [90], Trapa bispinosa [91], Jinhua bergamot [92], and Sccharum officinarum [14] etc. have been utilised for green syntheses of carbon nanotube (Table 5). However, further studies are needed to explore theranostic potential of these agents.
Table 5.
Characteristics and applications of bioinspired carbon dot
| Plant name | Size, nm | Shape | Applications and Research gaps | References |
|---|---|---|---|---|
| orange peel | 2–7 | spherical shape | theranostic potential was not evaluated | [90] |
| Trapa bispinosa | 5–10 | spherical shape | these can be used in biomedical applications like delivery of active pharmaceutical ingredient and genes inside the cells | [91] |
| Sccharum officinarum | 3 | — | can be used in fluorescent imaging probes for bioimaging applications | [14] |
| aloe | 5 | spherical shape | theranostic potential was not evaluated | [89] |
| Jinhua bergamot | — | — | these probes showed photoluminisence so there potential for bioimaging should be explored | [92] |
3 NPs produced by plants whose theranostic potential required to be explored
3.1 Copper nanoparticles
Copper nanoparticles (Cu NPs) are also considered potential candidate as theranostic agents (Fig. 5) because of their properties such as strong absorption in NIR region and high heat to light transformation capacity; they can cause selective damage to tumour cells. Moreover, they exhibit fluorescence signal and are capable to be applied for optical imaging and image‐guided phototherapy [94], for ultrasound and MRI with high spatial resolution scan [95]. Green synthesis of Cu NPs has been reported from many plants. Crystalline nature Cu NPs up to the size range of 15–30 and 10–60 nm were prepared from Aloe barbadensis [96] and Citrus medica Linn. [97], respectively. Similarly, Glorosia superba L. extract was used as a fuel for synthesis of Cu NPs [98] and rod‐shaped nanoparticles were obtained from Carica papaya [99]. Highly stable Cu NPs were prepared from Tabernaemontana divaricate leaves [100] and Euphorbia esula L. [101]. It has been revealed that Ginkgo biloba [102] and Nerium oleander [13] contain reducing and stabilising agents for copper resulting in Cu NPs formation. Spherical‐shaped Cu NPs up to the size range of 26–30 nm were obtained from Acalphya indica [103] while ultra‐small Cu NPs up to 2.90 nm were prepared from Lemon grass tea [104]. Well‐dispersed and crystalline nature Cu NPs were fabricated by using Calotropis gigantea extract [105]. Herbal extracts such as tamarind and lemon juice were used to produce cubic NPs up to 20–50 nm size [106]. Amorphous Cu NPs with anticancer potential were produced by using Ficus religiosa leaves [107]. Moreover, Tinosporia cardifolia extract produced Cu NPs with sponge‐like structure and 6–8 nm size [108]. Microwave‐assisted synthesis of Cu NPs was carried out by using Terminalia arjuna bark extract [109], but theranostic potential of all these NPs still needs to be explored.
Fig. 5.
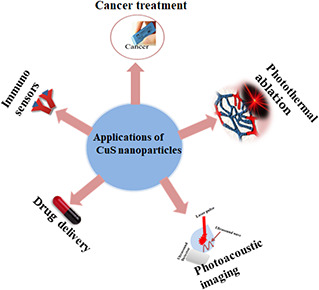
Biomedical applications of CuS NPs [93]
3.2 Manganese oxide nanoparticles
Manganese oxide (MnO2) nanoparticles NPs also have magnetic property so are considered potential candidate as theranostic agent. MnO2 nanorods have been recently reported as radiofrequency responsive materials. Their ability to heat up upon irradiation of specific radiofrequency waves elevates temperature of surrounding tissues therefore can be used as a hyperthermic agent to induce cancerous cell death [110, 111]. Typically, gadolinium contrast agents are used for T1MR imaging, but they cause nephrogenic systemic fibrosis.
MnO2 NPs with paramagnetic behaviour provides an MRI contrast agent for lung cancer bioimaging as well also ensures targeted drug delivery to cancerous cells [112]. Spherical‐shaped MnO2 NPs with 40–50 nm size were fabricated from Ananas comosus [12]. Needle‐shaped MnO2 NPs <100 nm size were synthesised from Sapindus mukorossi [113]. Similarly, Cucurbita pepo ‐mediated MnO2 NPs synthesis was also carried out [114]. A reduction in metal ions was achieved through lemon while curcumin was used as stabilise resulting in spherical‐ and eclipsed‐shaped MnO2 NPs formation [115]. However, theranostic potential of all these NPs is still needed to be evaluated.
3.3 Bismuth nanoparticles
Bismuth nanoparticles (Bi NPs) because of their properties such as small carrier effective masses, long Fermi wavelength, and small bond overlap energy have attracted the attention of researchers to evaluate their potential as theranostic agent. It has been revealed that Bi NPs can be used as radio sensitiser and as contrast agent for CT. Bi NPs has been developed as theranostic moiety with potential to be applied for dual modal CT/PA imaging and PTT for treatment of tumour [116]. Bi NPs with nanoscale spherical sponge morphology have been reported as potential agents for image‐guided PTT against tumour [117]. Commonly used contrast agents for CT of GIT such as BaSO4 suspension and iodinated molecules (meglumine diatrizoateare) can give false‐positive results due to intrinsic insolubility and iodine hypersensitivity, respectively. Moreover, anatomy of GIT including complex loops and peristalsis also contribute to difficulty in diagnosis. Bi NPs are most appropriate candidates to be applied for CT visualisation of GIT that overcome all above‐mentioned problems, in addition to that they offer low cost, biocompatibility, and high sensitivity [118]. In addition, Cu3 BiS3 nanocrystals can serve as new generation of theranostics which has been reported with strong NIR absorbance and exhibits CT imaging response due to large X‐ray attenuation coefficient of bismuth. Therefore, cancerous cells can be efficiently diagnosed and killed upon irradiation of NIR and X‐ray [119]. Bi‐gadolinium NPs are potential candidates for CT‐guided radiotherapy with prolong circulation causing selective death of cancerous cells without damaging surrounding healthy tissues, thus providing more effective cancer therapy [120]. Spherical‐shaped Bi NPs was from Eclipta alba with 40 nm size; however, theranostic potential of these NPs was not evaluated [11].
3.4 Silica (metalloid) nanoparticles
Silica nanoparticles (Si NPs) are novel prognostic biomarkers for cancer diagnosis and delivery of chemotherapeutic moiety to cancerous cells [121]. They are optically transparent, biologically inert, relatively non‐toxic elements with surface modification properties. These properties make Si NPs an appropriate candidate for incorporation of various dyes such as polymethines, indocyanine green, Alexa Flour 750 etc. Moreover, incorporation of more than one moieties can be achieved by making mesoporus silica nanoparticles (MSNs) [122]. The presence of uniform pores, surface modification properties allow simultaneous incorporation of multiple moieties inside distinct domains including contrast agent for bioimaging, therapeutic agent, and a ligand that ensures targeted drug delivery thus acting as ideal candidate as theranostic agent [123]. MSNs ensure delivery of many hydrophobic drugs such as paclitaxel and camptothecin which if administered through intravenous route require large amount of organic solvents such as DMSO for solubility which may cause toxicity. The presence of silanol groups on MSNs make them soluble in aqueous environment and porosity provides increased surface area with high incorporation efficiency while surface modification property provides more strong control over holding and releasing drug molecules [124]. By taking advantage of surface modification property of MSNs, Cheng et al. have suggested a novel platform for their use as cancer theranostics [125]. Toxicity study on MSNs reveals that they are less toxic and do not cause any type of hypersensitivity and immunogenic sensitisation reactions [126]. Green synthesis of Si NPs is commonly carried out from rice husk but theranostic potential is still not evaluated. The waste material has been revealed as silica precursor [10]. More studies are required to explore and further develop Si NPs for clinical and diagnostics purposes.
4 Mechanism behind plant‐based green synthesis of NPs
Phytoconstituents such as terpenoids, flavonoids, sugars, proteins, and alkaloids are responsible for reduction in metal ions resulting in NP formation. Terpenoids are organic polymers produced in plants exhibiting strong antioxidant potential. Eugenol is an important terpenoid responsible for reduction in metal ions. It has been revealed that breakdown of proton from the OH group of eugenol forms resonance structure having potential for further oxidation. It is followed by reduction in metal ions responsible for NP formation [127]. Flavonoids are polyphenolic compounds found in plants having potential to chelate and reduce metal ions. Tautomeric transformation of flavonoids from enol‐form to keto‐form release hydrogen atom that reduce metal ions and forms NPs [128]. Sugars are an important constituent found in plants that also assist in NP formation. It has been revealed that aldehyde group of sugar is oxidised into carboxyl group via nucleophilic addition of OH group which results in reduction in metal ions and NP formation. Proteins are another important class of phytoconstituents involved in NP formation. Amino acids that are building blocks of proteins contain binding sites for metal ions such as carboxyl group (aspartic and glutamic acid), nitrogen atom of imidazole ring (histidine), thiol (cysteine), thioether (methionine), hydroxyl (serine, threonine, tyrosine), carbonyl group (asparagine and glutamine) etc. Amino acids can also reduce metal ions such as hydroxyl group (tyrosine), carbonyl group (glutamine and asparagine), and thiol (cysteine) resulting in NP formation. General mechanism involved in plant‐based NP formation is proposed as: (i) first of all reduction in metal ions takes place by the phytoconstituents followed by nucleation of reduced metal atoms that is called activation phase; (ii) during growth phase, small NPs adhere to form large size NP (Ostwald ripening); (iii) termination is the last phase during which NPs attain their shape [127].
5 General fabrication process for plant‐based green synthesis of NPs
Green synthesis of metal NPs can be carried out in a single‐step process. However, appropriate solvent system, environment‐friendly reducing agent, and non‐toxic stabilising agent should be selected for this approach [129]. Preparation of metal NPs through plant‐mediated green chemistry approach is usually carried out by mixing plant extract that act as reducing agent and a metal precursor under suitable conditions as shown in Fig. 6. Hydrogen tetrachloroaurate (III) hydrate (HAuCl4 . 3H2 O) and sodium tetrachloroaurate (NaAuCl4) are used as gold precursor for synthesis of Au NPs. Reduction process takes place within 1–1.5 h that is indicated by the appearance of stable light violet or purple colour [9, 130]. In the case of IONPs, most commonly used iron precursors are iron (III) chloride hexahydrate (FeCl3 . 6H2 O) and ferric ammonium sulphate dodecahydrate FeNH4 (SO4)2 . 12H2 O etc. Appearance of black‐coloured solution indicates the completion of reduction process [17, 54]. Preparation of TiO2 NPs is carried out by using metatitanic acid TiO(OH)2, titanium oxysulphate TiOSO4, titanium teteraisopropoxide (C12 H28 O4 Ti) etc. as precursors of titanium which undergoes reduction by phytoconstituents under defined conditions. The completion of reduction process is indicated by the deposition of coalescent white crystals at the bottom of container [66, 67, 68, 70]. Silver nitrate AgNO3 is a commonly used silver precursor for fabrication of Ag NPs and completion of silver reduction is indicated by reddish brown colour solution [79]. Green synthesis of carbon nanotubes can be carried out by autoclaving Saccharum officinarum as a carbon source until dark brown‐coloured solution forms [14]. Plant‐based green synthesis of NPs offers several attractive features as shown in Table 6.
Fig. 6.
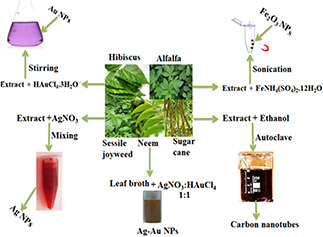
Schematic illustration of general fabrication process for plant‐based green synthesis of nanoparticles [14, 17, 79, 130, 131]
Table 6.
Features of plant‐based fabrication of nanoparticles compared to traditional methods
| Plant‐based fabrication | Traditional methods | References |
|---|---|---|
| Plant‐based CuO NPs were found to be more stable with less ion releasing capability and less toxic | engineered CuO NPs were more soluble, less stable with high ion releasing capability, and more toxic | [132] |
| Plant‐based Ni NPs were found to be non‐toxic and possessed magnetic property, good colloidal stability, better antioxidant, and antimicrobial potential. | Ni NPs synthesised through chemical methods did not possessed magnetic property and they were found to have poor colloidal stability, antioxidant and antimicrobial potential, and showed toxicity | [133] |
| plant‐based TiO2 NPs exhibited better dispersibility, stability, and smaller size resulting in enhanced therapeutic potential | chemically synthesised TiO2 NPs were unable to exhibit equivalent dispersibilty, stability, and size resulting in poor therapeutic potential | [70] |
| green synthesised nanoparticles prepared from peanut shell exhibited the size range 10–50 nm, pharmacological potential similar to commercially available Ag NPs | peanut‐based synthesis can be scaled up to large scale as it offers the same features as commercially available nanoparticles at low cost | [134] |
| plant‐based synthesis of nanoparticles is a one‐step process, safe to handle, offer minimum side effects, and plants are readily available | chemical syntheses of nanoparticles require toxic solvents, high energy, and produce many byproducts | [135] |
| plant‐based nanoparticles have tested only in small number of biomedical applications and used only small‐scale production. Therefore, this research area needs significant attention of researchers | nanoparticles synthesised through physical and chemical methods have been widely tested and applied for various biomedical applications | [127] |
6 Advantages offered by green synthesised NPs and toxicity concerns of theranostics
NPs of well‐defined shape with desired characteristics are commonly prepared by chemical methods, but these methods are quite expensive and involve the use of chemicals that may pollute our environment and NPs produced by these methods may produce harmful effects on human beings [136]. Green synthesis is an alternative echo‐friendly approach for synthesis of NPs that may be carried out by using plant extracts as well as microorganisms like bacteria and fungi. NPs produced by these methods are usually economical, biocompatible, and possess relatively low toxic effects on human health. Moreover, in contrast to chemical methods, there is no need to apply high pressure, temperature, and energy; and usually do not involve toxic chemicals [137]. Plants are considered as most appropriate platform for synthesis of NPs with naturally occurring phytoconstituents that act as capping and stabilising agents for synthesis of NPs [138]. Plant‐mediated synthesis of NPs offer several benefits over microorganisms such as ease of improvement, less biohazards, elaborate process of maintaining cell culture, reduce the cost of microorganisms isolation, and their culture media.
Moreover, plant‐mediated synthesis of NPs can be easily upgraded to industrial scale [136, 139, 140]. In the case of physical and chemical methods, it is sometimes challenging to prevent aggregation of NPs and to achieve stable NPs of desired shape and size while, green synthesised NPs have been reported to be more stable and can be reduced at faster rate when compared with other methods [3]. On the other hand, major drawback of synthesis of NPs using plants is varying chemical composition of plant extracts of same species collected from different geographical regions, thus sometimes making it challenging to produce reproducible results [139].
Although TNPs are being widely applied to treat and diagnose cancers, however, they may impart potentially toxic impact on human health due to different reasons. For example, raw materials (plant products) used for the synthesis of NPs should be tested for its toxicity or side effects. In addition, certain and small sized NPs have potential to penetrate cell membrane where they disturb normal cellular functions; mainly resulting in necrosis, cell degeneration, and apoptosis in Na+ –K+ ATPase cell membrane [141]. Large amounts of bismuth oxide NPs are released in the atmosphere as a result of volcanic eruptions and are found associated with eco‐toxic impacts [142]. Ag NPs possess strong antibacterial activity but unable to differentiate beneficial bacterial strains, which can eliminate environment‐friendly bacteria; for example, nitrogen‐fixing bacteria thus impeding plant growth and resulting in eutrophication of rivers and lakes [143, 144]. Ag NPs have been reported to deposit inside the testes and effect sperm cells imparting toxicity on male reproductive cells [145]. It has been revealed that Ag NPs become more toxic when stored for prolong time period [146]. They cause osmoregulation disturbances in fish by inhibiting basolateral activity inside gills [147]. Moreover, release of silver ions into the environment can result in bluish to grey skin and eye discoloration, kidney and liver damage, and respiratory and intestinal tract irritation [143]. In the case of IONPs, commercially available products such as Feridex and Resovist are biocompatible and non‐cytotoxic. However, emerging studies have reported DNA damage, oxidative stress, and mitochondrial membrane dysfunction caused by SIONPs. Therefore, further studies should be conducted to ensure safety of these NPs [148]. TiO2 NPs produce inflammatory responses, lung cancer, and productive and developmental toxicities in animals have been revealed, so further studies should be conducted to evaluate toxicity in humans [149]. Au NPs are biocompatible relatively less toxic and non‐immunogenic [150]. Toxicity study has been carried out on MnO2 NPs by using rats where they caused DNA damage in leucocytes, micronuclei, and chromosomal aberrations thus causing genetic damage and histopathological changes in the liver, kidney, spleen, and brain [151]. Cu NPs have been reported to cause increased DNA damage and oxidative DNA lesions when compared with control resulting in non‐viable cells [152]. Si NPs cause cytotoxicity in human bronchiolar carcinoma derived cells imposing risk for lung cancer [153]. Therefore, appropriate precautions should be taken before introducing NPs as potential theranostic agents; especially those based on heavy metals [141].
7 Proteins and peptides as potential candidates for biomimetic synthesis of theranostic capable NPs
Protein and peptide‐based biomimetic synthesis of NPs is another emerging approach that has achieved increasing attention by offering several unique features. NPs obtained from this approach can be applied for in vivo imaging and therapy, bio‐sensing, and bio‐labelling [154]. Bovine serum albumin is a commercially available protein that is most commonly applied for preparation of NPs. Gadolinium‐based hybride NPs prepared from bovine serum albumin can be employed as effective blood pool contrast agents [155]. Paramagnetic gadolinium is also effective tool for MRI [156]. A wide variety of theranostic capable NPs have been prepared through this approach, i.e. QDs, Fe2 O3 NPs, Cu NPs, MnO2 NPs, Bi2 S3 NPs etc. Protein‐based synthesis of NPs offers several attractive features such as good stability, reproducibility, compatibility, minimum non‐specific absorption, better pharmacokinetics, and green processing can be carried out at room temperature [157, 158, 159]. Peptides are products obtained after hydrolysis of proteins that are composed of three or more proteins linked through peptide bond. When compared with amino acids; peptides possess less molecular weight, clear structure, and composition. Peptides can be designed especially as they are more liable to change in sequence, thus providing opportunity to produce multifunctional NPs. Most commonly used peptide templates are CCY‐based linear peptide, cyclopeptide while Glutathione and CLEDININE are some other peptides. Various types of NPs can be prepared by using peptides, i.e. Au NPs, Ag NPs, Cu NPs etc. Glutathione‐based NPs offer several advantages such as better accumulation at tumour site, CT imaging, improved therapeutic efficiency, and increased renal clearance. However, peptides offer several limitations when compared with proteins such as non‐human endogenousness, less bioactivity, and reaction efficiency [154, 160]. The exact mechanism involved behind protein or peptide‐mediated NP synthesis is not completely explored; however, it has been revealed that proteins contain metal binding sites, i.e. N‐terminal amine, Cys residues that help them in metal adherence. Proteins because of their enormous charged groups and molecular chain flexibility exhibit the confirmation of nanocages in basic environment, thus forming nanoclusters with metal ions. In the case of peptides, some functional groups such as phenolic group of tyrosine converts into phenoxide in basic environment which reduce metal ion to metallic NP [154].
8 Conclusion and future prospects
In the light of above discussion, we can conclude that NPs‐based theranostics is promising approach to increase the quality of clinical care and treatment [161]. Synthesis and development of NPs can be carried out using economical approach (i.e. plant‐mediated synthesis of TNPs) which could provide stable TNPs of desired shape, physical, and chemical characteristics in a single‐step process. Although green synthesis approach gained significant attention of researchers and a wide variety of plant extract‐based NPs have been prepared and evaluated till now, still this research area need to be explored for the synthesis of more efficient theranostics. Further efforts are needed to optimise the reaction conditions for synthesis of bioinspired NPs and engineering of recombinant organisms and gene modification of plants in order achieve high amount of phytoconstituents such as proteins, enzymes, phenols that are natural capping and reducing agents present in plants. Productivity of plants to prepare NPs can be further improved by exploring the pathways involved in NP synthesis and heavy metal accumulation and detoxification. Plants can also produce complex nanohybrides. It has been revealed that bimetallic synthesis of NPs with Au core–Ag shell can be carried out by using Neem (Azadirachta indica) leaf broth [131]. Moreover, Brassica juncea ‐mediated trimetallic Au–Ag–Cu NP synthesis has also been reported [162]. However, still there is an intense need to further explore the potential of plants to produce complex nanomaterials. Some toxic impacts of TNPs have been revealed; therefore, further studies should be conducted to investigate underlying mechanisms of toxicity and to establish appropriate dose in order to get maximum therapeutic benefits with least side effects. In the view of above discussion, we can conclude that green synthesised nanomaterials can appear as potential therapeutic and diagnostic agents in near future, but currently, it is inceptive and needs intensive efforts to overcome all the research gaps.
9 References
- 1. Silva G.A.: ‘Introduction to nanotechnology and its applications to medicine’, Surg. Neurol., 2004, 61, (3), pp. 216 –220 [DOI] [PubMed] [Google Scholar]
- 2. Mody V.V. Siwale R. Singh A. et al.: ‘Introduction to metallic nanoparticles’, J. Pharm. Bioallied. Sci., 2010, 2, (4), p. 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green. Chem., 2011, 13, (10), pp. 2638 –2650 [Google Scholar]
- 4. Balasooriya E.R. Jayasinghe C.D. Jayawardena U.A. et al.: ‘Honey mediated green synthesis of nanoparticles: new era of safe nanotechnology’, J. Nanomater., 2017, 2017, (3), pp. 1 –10 [Google Scholar]
- 5. Kim T.H. Lee S. Chen X.: ‘Nanotheranostics for personalized medicine’, Expert. Rev. Mol. Diag., 2013, 13, (3), pp. 257 –269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muthu M.S. Leong D.T. Mei L. et al.: ‘Nanotheranostics – application and further development of nanomedicine strategies for advanced theranostics’, Theranostics, 2014, 4, (6), p. 660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jokerst J.V. Gambhir S.S.: ‘Molecular imaging with theranostic nanoparticles’, Acc. Chem. Res., 2011, 44, (10), pp. 1050 –1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wagner V. Dullaart A. Bock A.‐K. et al.: ‘The emerging nanomedicine landscape’, Nat. Biotechnol., 2006, 24, (10), p. 1211 [DOI] [PubMed] [Google Scholar]
- 9. Shukla R. Nune S.K. Chanda N. et al.: ‘Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles’, Small, 2008, 4, (9), pp. 1425 –1436 [DOI] [PubMed] [Google Scholar]
- 10. Vijayalakshmi U. Vaibhav V. Chellappa M. et al.: ‘Green synthesis of silica nanoparticles and its corrosion resistance behavior on mild steel’, J. Indian Chem. Soc., 2015, 92, (5), pp. 675 –678 [Google Scholar]
- 11. Prabhusaran N.: ‘Exploration of herbal bismuth nanoparticles using Eclipta alba and in vitro antimicrobial activity against pathogenic bacterial strains’, Int. J. Pharm. Pharm. Res., (Human), 2016, 6, (1), pp. 126 –139 [Google Scholar]
- 12. Asaikkutti A. Bhavan P.S. Vimala K. et al.: ‘Dietary supplementation of green synthesized manganese‐oxide nanoparticles and its effect on growth performance, muscle composition and digestive enzyme activities of the giant freshwater prawn Macrobrachium rosenbergii’, J. Trace Elem. Med. Biol., 2016, 35, pp. 7 –17 [DOI] [PubMed] [Google Scholar]
- 13. Gopinath M. Subbaiya R. Selvam M.M. et al.: ‘Synthesis of copper nanoparticles from Nerium oleander leaf aqueous extract and its antibacterial activity’, Int. J. Curr. Microbiol. App. Sci., 2014, 3, (9), pp. 814 –818 [Google Scholar]
- 14. Mehta V.N. Jha S. Kailasa S.K.: ‘One‐pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells’, Mater. Sci. Eng. C, 2014, 38, pp. 20 –27 [DOI] [PubMed] [Google Scholar]
- 15. Kajani A.A. Bordbar A.‐K. Esfahani S.H.Z. et al.: ‘Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract’, RSC Adv., 2014, 4, (106), pp. 61394 –61403 [Google Scholar]
- 16. Chatterjee A. Nishanthini D. Sandhiya N. et al.: ‘Biosynthesis of titanium dioxide nanoparticles using Vigna radiata ’, Asian J. Pharm. Clin. Res., 2016, 9, (4), pp. 85 –88 [Google Scholar]
- 17. Herrera‐Becerra R. Zorrilla C. Ascencio J.A.: ‘Production of iron oxide nanoparticles by a biosynthesis method: an environmentally friendly route’, J. Phys. Chem. C, 2007, 111, (44), pp. 16147 –16153 [Google Scholar]
- 18. Barabadi H. Ovais M. Shinwari Z.K. et al.: ‘Anti‐cancer green bionanomaterials: present status and future prospects’, Green Chem. Lett. Rev., 2017, 10, (4), pp. 285 –314 [Google Scholar]
- 19. Alivisatos P.: ‘The use of nanocrystals in biological detection’, Nat. Biotechnol., 2004, 22, (1), pp. 47 –52 [DOI] [PubMed] [Google Scholar]
- 20. Narayanan S. Sathy B.N. Mony U. et al.: ‘Biocompatible magnetite/gold nanohybrid contrast agents via green chemistry for Mri and Ct bioimaging’, ACS Appl. Mater. Interfaces, 2011, 4, (1), pp. 251 –260 [DOI] [PubMed] [Google Scholar]
- 21. Das R.K. Gogoi N. Babu P.J. et al.: ‘The synthesis of gold nanoparticles using Amaranthus Spinosus leaf extract and study of their optical properties’, Adv. Mater. Phys. Chem., 2012, 2, (04), p. 275 [Google Scholar]
- 22. Das R.K. Borthakur B.B. Bora U.: ‘Green synthesis of gold nanoparticles using ethanolic leaf extract of Centella Asiatica ’, Mater. Lett, 2010, 64, (13), pp. 1445 –1447 [Google Scholar]
- 23. Zhao C. Rehman F.U. Yang Y. et al.: ‘Bio‐imaging and photodynamic therapy with tetra sulphonatophenyl porphyrin (Tspp)‐Tio 2 nanowhiskers: new approaches in rheumatoid arthritis theranostics’, Sci. Rep., 2015, 5, p. 11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mallidi S. Larson T. Tam J. et al.: ‘Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer’, Nano Lett., 2009, 9, (8), pp. 2825 –2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim D. Park S. Lee J.H. et al.: ‘Antibiofouling polymer‐coated gold nanoparticles as a contrast agent for in vivo X‐ray computed tomography imaging’, J. Am. Chem. Soc., 2007, 129, (24), pp. 7661 –7665 [DOI] [PubMed] [Google Scholar]
- 26. Xia Y. Li W. Cobley C.M. et al.: ‘Gold nanocages: from synthesis to theranostic applications’, Acc. Chem. Res, 2011, 44, (10), pp. 914 –924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang X. Jain P.K. El‐Sayed I.H. et al.: ‘Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy’, Nanomedicine, 2007, 5, (10), pp. 681 –693 [DOI] [PubMed] [Google Scholar]
- 28. Akhter S. Ahmad M.Z. Ahmad F.J. et al.: ‘Gold nanoparticles in theranostic oncology: current state‐of‐the‐art’, Expert. Opin. Drug Deliv., 2012, 9, (10), pp. 1225 –1243 [DOI] [PubMed] [Google Scholar]
- 29. Huff T.B. Tong L. Zhao Y. et al.: ‘Hyperthermic effects of gold nanorods on tumor cells’, Nanomedicine, 2007, 2, (1), pp. 125 –132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao J. Lee P. Wallace M.J. et al.: ‘Gold nanoparticles in cancer therapy: efficacy, biodistribution, and toxicity’, Curr. Pharm. Des., 2015, 21, (29), pp. 4240 –4251 [DOI] [PubMed] [Google Scholar]
- 31. Cabral R.M. Baptista P.V.: ‘Anti‐cancer precision theranostics: a focus on multifunctional gold nanoparticles’, Expert. Rev. Mol. Diagn., 2014, 14, (8), pp. 1041 –1052 [DOI] [PubMed] [Google Scholar]
- 32. Ovais M. Raza A. Naz S. et al.: ‘Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics’, Appl. Microbiol. Biotechnol., 2017, 101, (9), pp. 3551 –3565 [DOI] [PubMed] [Google Scholar]
- 33. Kattumuri V. Katti K. Bhaskaran S. et al.: ‘Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: in vivo pharmacokinetics and X‐ray‐contrast‐imaging studies’, Small, 2007, 3, (2), pp. 333 –341 [DOI] [PubMed] [Google Scholar]
- 34. Vilchis‐Nestor A.R. Sánchez‐Mendieta V. Camacho‐López M.A. et al.: ‘Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract’, Mater. Lett., 2008, 62, (17–18), pp. 3103 –3105 [Google Scholar]
- 35. Smitha S. Philip D. Gopchandran K.: ‘Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth’, Spectrochim. Acta A., 2009, 74, (3), pp. 735 –739 [DOI] [PubMed] [Google Scholar]
- 36. Raghunandan D. Bedre M.D. Basavaraja S. et al.: ‘Rapid biosynthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution’, Colloids Surf. B, 2010, 79, (1), pp. 235 –240 [DOI] [PubMed] [Google Scholar]
- 37. Vijayakumar R. Devi V. Adavallan K. et al.: ‘Green synthesis and characterization of gold nanoparticles using extract of anti‐tumor potent Crocus sativus ’, Physica E Low Dimens Syst. Nanostruct., 2011, 44, (3), pp. 665 –671 [Google Scholar]
- 38. Das R.K. Gogoi N. Bora U.: ‘Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract’, Bioprocess. Biosyst. Eng., 2011, 34, (5), pp. 615 –619 [DOI] [PubMed] [Google Scholar]
- 39. Noruzi M. Zare D. Khoshnevisan K. et al.: ‘Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature’, Spectrochim. Acta A, 2011, 79, (5), pp. 1461 –1465 [DOI] [PubMed] [Google Scholar]
- 40. Arunachalam K.D. Annamalai S.K.: ‘ Chrysopogon zizanioides aqueous extract mediated synthesis, characterization of crystalline silver and gold nanoparticles for biomedical applications’, Int. J. Nanomed., 2013, 8, p. 2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nellore J. Pauline P.C. Amarnath K.: ‘Biogenic synthesis by Sphearanthus amaranthoids; towards the efficient production of the biocompatible gold nanoparticles’, Dig. J. Nanomater. Biostruct., 2012, 7, (1), pp. 123 –133 [Google Scholar]
- 42. Ganeshkumar M. Sathishkumar M. Ponrasu T. et al.: ‘Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery’, Colloids Surf. B, 2013, 106, pp. 208 –216 [DOI] [PubMed] [Google Scholar]
- 43. Anand K. Gengan R. Phulukdaree A. et al.: ‘Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti‐cancer and catalytic activity’, Ind. Eng. Chem. Res., 2015, 21, pp. 1105 –1111 [Google Scholar]
- 44. Patra S. Mukherjee S. Barui A.K. et al.: ‘Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics’, Mater. Sci. Eng. C., 2015, 53, pp. 298 –309 [DOI] [PubMed] [Google Scholar]
- 45. Santra S. Kaittanis C. Grimm J. et al.: ‘Drug/dye‐loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging’, Small, 2009, 5, (16), pp. 1862 –1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Santhosh P.B. Ulrih N.P.: ‘Multifunctional superparamagnetic iron oxide nanoparticles: promising tools in cancer theranostics’, Cancer Lett., 2013, 336, (1), pp. 8 –17 [DOI] [PubMed] [Google Scholar]
- 47. Kievit F.M. Zhang M.: ‘Surface engineering of iron oxide nanoparticles for targeted cancer therapy’, Acc. Chem. Res., 2011, 44, (10), pp. 853 –862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosen J.E. Chan L. Shieh D.‐B. et al.: ‘Iron oxide nanoparticles for targeted cancer imaging and diagnostics’, Nanomed., Nanotechnol. Biol. Med., 2012, 8, (3), pp. 275 –290 [DOI] [PubMed] [Google Scholar]
- 49. Gupta A.K. Gupta M.: ‘Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications’, Biomaterials, 2005, 26, (18), pp. 3995 –4021 [DOI] [PubMed] [Google Scholar]
- 50. Frank J.A. Miller B.R. Arbab A.S. et al.: ‘Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents’, Radiology, 2003, 228, (2), pp. 480 –487 [DOI] [PubMed] [Google Scholar]
- 51. McBain S.C. Yiu H.H. Dobson J.: ‘Magnetic nanoparticles for gene and drug delivery’, Int. J. Nanomed., 2008, 3, (2), p. 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mahdavi M. Namvar F. Ahmad M.B. et al.: ‘Green biosynthesis and characterization of magnetic iron oxide (Fe3 O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract’, Molecules, 2013, 18, (5), pp. 5954 –5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Namvar F. Rahman H.S. Mohamad R. et al.: ‘Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract’, Int. J. Nanomed., 2014, 9, p. 2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Venkateswarlu S. Rao Y.S. Balaji T. et al.: ‘Biogenic synthesis of Fe3 O4 magnetic nanoparticles using plantain peel extract’, Mater. Lett., 2013, 100, pp. 241 –244 [Google Scholar]
- 55. Phumying S. Labuayai S. Thomas C. et al.: ‘Aloe vera plant‐extracted solution hydrothermal synthesis and magnetic properties of magnetite (Fe3 O4) nanoparticles’, Appl. Phys. A, 2013, 111, (4), pp. 1187 –1193 [Google Scholar]
- 56. Huang L. Weng X. Chen Z. et al.: ‘Green synthesis of iron nanoparticles by various tea extracts: comparative study of the reactivity’, Spectrochim. Acta A, 2014, 130, pp. 295 –301 [DOI] [PubMed] [Google Scholar]
- 57. Wang T. Jin X. Chen Z. et al.: ‘Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater’, Sci. Total Environ., 2014, 466, pp. 210 –213 [DOI] [PubMed] [Google Scholar]
- 58. Njagi E.C. Huang H. Stafford L. et al.: ‘Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts’, Langmuir, 2010, 27, (1), pp. 264 –271 [DOI] [PubMed] [Google Scholar]
- 59. Makarov V.V. Makarova S.S. Love A.J. et al.: ‘Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants’, Langmuir, 2014, 30, (20), pp. 5982 –5988 [DOI] [PubMed] [Google Scholar]
- 60. Khalil A.T. Ovais M. Ullah I. et al.: ‘Biosynthesis of iron oxide (Fe2 O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties’, Green Chem. Lett. Rev., 2017, 10, (4), pp. 186 –201 [Google Scholar]
- 61. Mou J. Lin T. Huang F. et al.: ‘Black titania‐based theranostic nanoplatform for single Nir laser induced dual‐modal imaging‐guided Ptt/Pdt’, Biomaterials, 2016, 84, pp. 13 –24 [DOI] [PubMed] [Google Scholar]
- 62. Rehman F. Zhao C. Jiang H. et al.: ‘Biomedical applications of nano‐titania in theranostics and photodynamic therapy’, Biomater. Sci., 2016, 4, (1), pp. 40 –54 [DOI] [PubMed] [Google Scholar]
- 63. Yin Z.F. Wu L. Yang H.G. et al.: ‘Recent progress in biomedical applications of titanium dioxide’, Phys. Chem. Chem. Phys., 2013, 15, (14), pp. 4844 –4858 [DOI] [PubMed] [Google Scholar]
- 64. Řehoř I. Vilímová V. Jendelová P. et al.: ‘Phosphonate–titanium dioxide assemblies: platform for multimodal diagnostic–therapeutic nanoprobes’, J. Med. Chem., 2011, 54, (14), pp. 5185 –5194 [DOI] [PubMed] [Google Scholar]
- 65. Kirillin M.Y. Shirmanova M.V. Sirotkina M.A. et al.: ‘Contrasting properties of gold nanoshells and titanium dioxide nanoparticles for optical coherence tomography imaging of skin: Monte Carlo simulations and in vivo study’, Biomed. Opt., 2009, 14, (2), p. 021017 [DOI] [PubMed] [Google Scholar]
- 66. Roopan S.M. Bharathi A. Prabhakarn A. et al.: ‘Efficient phyto‐synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract’, Spectrochim. Acta A, 2012, 98, pp. 86 –90 [DOI] [PubMed] [Google Scholar]
- 67. Sundrarajan M. Gowri S.: ‘Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor‐tristis leaves extract’, Chalcogenide Lett., 2011, 8, (8), pp. 447 –451 [Google Scholar]
- 68. Hudlikar M. Joglekar S. Dhaygude M. et al.: ‘Green synthesis of TiO2 nanoparticles by using aqueous extract of Jatropha curcas L. Latex’, Mater. Lett., 2012, 75, pp. 196 –199 [Google Scholar]
- 69. Zahir A.A. Chauhan I.S. Bagavan A. et al.: ‘Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in leishmania donovani’, Antimicrob. Agents Chemother., 2015, 59, (8), pp. 4782 –4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar P.S.M. Francis A.P. Devasena T.: ‘Comparative studies on green synthesized and chemically synthesized titanium oxide nanoparticles. A validation for green synthesis protocol using hibiscus flower’, J. Environ. Nanotechnol., 2014, 3, (4), pp. 78 –85 [Google Scholar]
- 71. Sriram M.I. Kanth S.B.M. Kalishwaralal K. et al.: ‘Antitumor activity of silver nanoparticles in Dalton's lymphoma ascites tumor model’, Int. J. Nanomed., 2010, 5, p. 753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Asharani P. Hande M.P. Valiyaveettil S.: ‘Anti‐proliferative activity of silver nanoparticles’, BMC Cell Biol., 2009, 10, (1), p. 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guo D. Zhu L. Huang Z. et al.: ‘Anti‐leukemia activity of Pvp‐coated silver nanoparticles via generation of reactive oxygen species and release of silver ions’, Biomaterials, 2013, 34, (32), pp. 7884 –7894 [DOI] [PubMed] [Google Scholar]
- 74. Mukherjee S. Chowdhury D. Kotcherlakota R. et al.: ‘Potential theranostics application of bio‐synthesized silver nanoparticles (4‐in‐1 system)’, Theranostics, 2014, 4, (3), p. 316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Majdalawieh A. Kanan M.C. El‐Kadri O. et al.: ‘Recent advances in gold and silver nanoparticles: synthesis and applications’, J. Nanosci. Nanotechnol., 2014, 14, (7), pp. 4757 –4780 [DOI] [PubMed] [Google Scholar]
- 76. Chen L.Q. Xiao S.J. Peng L. et al.: ‘Aptamer‐based silver nanoparticles used for intracellular protein imaging and single nanoparticle spectral analysis’, J. Phys. Chem. B, 2010, 114, (10), pp. 3655 –3659 [DOI] [PubMed] [Google Scholar]
- 77. Zhou W. Ma Y. Yang H. et al.: ‘A label‐free biosensor based on silver nanoparticles array for clinical detection of serum P53 in head and neck squamous cell carcinoma’, Int. J. Nanomed., 2011, 6, p. 381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pandey S. Goswami G.K. Nanda K.K.: ‘Green synthesis of biopolymer–silver nanoparticle nanocomposite: an optical sensor for ammonia detection’, Int. J. Biol. Macromol., 2012, 51, (4), pp. 583 –589 [DOI] [PubMed] [Google Scholar]
- 79. Firdhouse M.J. Lalitha P.: ‘Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis – antiproliferative effect against prostate cancer cells’, Cancer Nanotechnol., 2013, 4, (6), pp. 137 –143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gurunathan S. Raman J. Malek S.N.A. et al.: ‘Green synthesis of silver nanoparticles using Ganoderma Neo‐Japonicum Imazeki: a potential cytotoxic agent against breast cancer cells’, Int. J. Nanomed., 2013, 8, p. 4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mollick M.M.R. Rana D. Dash S.K. et al.: ‘Studies on green synthesized silver nanoparticles using abelmoschus esculentus (L.) pulp extract having anticancer (in vitro) and antimicrobial applications’, ARAB J. Chem. J., 2015, 5, (4), pp. 1 –13 [Google Scholar]
- 82. Heydari R. Rashidipour M.: ‘Green synthesis of silver nanoparticles using extract of oak fruit hull (JAFT): synthesis and in vitro cytotoxic effect on Mcf‐7 cells’, Int. J. Breast Cancer, 2015, 2015, (12), pp. 1 –6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Govindaraju K. Krishnamoorthy K. Alsagaby S.A. et al.: ‘Green synthesis of silver nanoparticles for selective toxicity towards cancer cells’, IET Nanobiotechnol., 2015, 9, (6), pp. 325 –330 [DOI] [PubMed] [Google Scholar]
- 84. Mata R. Nakkala J.R. Sadras S.R.: ‘Catalytic and biological activities of green silver nanoparticles synthesized from Plumeria Alba (Frangipani) flower extract’, Mater. Sci., 2015, 51, pp. 216 –225 [DOI] [PubMed] [Google Scholar]
- 85. He Y. Du Z. Ma S. et al.: ‘Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (Pc‐3) cells of silver nanoparticles using Dimocarpus Longan Lour. peel extract’, Nanoscale Res. Lett., 2016, 11, (1), p. 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang X.‐F. Liu Z.‐G. Shen W. et al.: ‘Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches’, Int. J. Mol. Sci., 2016, 17, (9), p. 1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gurunathan S. Jeong J.‐K. Han J.W. et al.: ‘Multidimensional effects of biologically synthesized silver nanoparticles in helicobacter pylori, helicobacter felis, and human lung (L132) and lung carcinoma A549 cells’, Nanoscale Res. Lett., 2015, 10, (1), p. 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ho Y.‐P. Leong K.W.: ‘Quantum dot‐based theranostics’, Nanoscale, 2010, 2, (1), pp. 60 –68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xu H. Yang X. Li G. et al.: ‘Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples’, J. Agric. Food Chem., 2015, 63, (30), pp. 6707 –6714 [DOI] [PubMed] [Google Scholar]
- 90. Prasannan A. Imae T.: ‘One‐pot synthesis of fluorescent carbon dots from orange waste peels’, Ind. Eng. Chem., 2013, 52, (44), pp. 15673 –15678 [Google Scholar]
- 91. Mewada A. Pandey S. Shinde S. et al.: ‘Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel’, Mater. Sci., 2013, 33, (5), pp. 2914 –2917 [DOI] [PubMed] [Google Scholar]
- 92. Yu J. Song N. Zhang Y.‐K. et al.: ‘Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+ ’, Sensor Actuat. B Chem., 2015, 214, pp. 29 –35 [Google Scholar]
- 93. Goel S. Chen F. Cai W.: ‘Synthesis and biomedical applications of copper sulfide nanoparticles: from sensors to theranostics’, Small, 2014, 10, (4), pp. 631 –645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhou M. Tian M. Li C.: ‘Copper‐based nanomaterials for cancer imaging and therapy’, Bioconjug. Chem., 2016, 27, (5), pp. 1188 –1199 [DOI] [PubMed] [Google Scholar]
- 95. Perlman O. Weitz I.S. Azhari H.: ‘Copper oxide nanoparticles as contrast agents for Mri and ultrasound dual‐modality imaging’, Phys. Med. Biol., 2015, 60, (15), p. 5767 [DOI] [PubMed] [Google Scholar]
- 96. Gunalan S. Sivaraj R. Venckatesh R.: ‘Aloe barbadensis miller mediated green synthesis of mono‐disperse copper oxide nanoparticles: optical properties’, Spectrochim. Acta A, 2012, 97, pp. 1140 –1144 [DOI] [PubMed] [Google Scholar]
- 97. Shende S. Ingle A.P. Gade A. et al.: ‘Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity’, World J. Microbiol. Biotechnol., 2015, 31, (6), pp. 865 –873 [DOI] [PubMed] [Google Scholar]
- 98. Naika H.R. Lingaraju K. Manjunath K. et al.: ‘Green synthesis of Cuo nanoparticles using Gloriosa Superba L. extract and their antibacterial activity’, J. Taibah. Univ. Sci., 2015, 9, (1), pp. 7 –12 [Google Scholar]
- 99. Sankar R. Manikandan P. Malarvizhi V. et al.: ‘Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation’, Spectrochim. Acta A, 2014, 121, pp. 746 –750 [DOI] [PubMed] [Google Scholar]
- 100. Sivaraj R. Rahman P.K. Rajiv P. et al.: ‘Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen’, Spectrochim. Acta A, 2014, 133, pp. 178 –181 [DOI] [PubMed] [Google Scholar]
- 101. Nasrollahzadeh M. Sajadi S.M. Khalaj M.: ‘Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand‐free ullmann‐coupling reaction and reduction of 4‐nitrophenol’, RSC Adv., 2014, 4, (88), pp. 47313 –47318 [Google Scholar]
- 102. Nasrollahzadeh M. Sajadi S.M.: ‘Green synthesis of copper nanoparticles using Ginkgo biloba L. Leaf extract and their catalytic activity for the huisgen [3 + 2] cycloaddition of azides and alkynes at room temperature’, J. Colloid Interface Sci., 2015, 457, pp. 141 –147 [DOI] [PubMed] [Google Scholar]
- 103. Sivaraj R. Rahman P.K. Rajiv P. et al.: ‘Biosynthesis and characterization of acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity’, Spectrochim. Acta A, 2014, 129, pp. 255 –258 [DOI] [PubMed] [Google Scholar]
- 104. Brumbaugh A.D. Cohen K.A. St. Angelo S.K.: ‘Ultrasmall copper nanoparticles synthesized with a plant tea reducing agent’, ACS Sustain. Chem. Eng., 2014, 2, (8), pp. 1933 –1939 [Google Scholar]
- 105. Sharma J.K. Akhtar M.S. Ameen S. et al.: ‘Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye‐sensitized solar cells applications’, J. Alloys. Compd., 2015, 632, pp. 321 –325 [Google Scholar]
- 106. Sastry A. Aamanchi R.K. Prasad C.S.R.L. et al.: ‘Large‐scale green synthesis of Cu nanoparticles’, Environ. Chem., 2013, 11, (2), pp. 183 –187 [Google Scholar]
- 107. Sankar R. Maheswari R. Karthik S. et al.: ‘Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles’, Mater. Sci., 2014, 44, pp. 234 –239 [DOI] [PubMed] [Google Scholar]
- 108. Nethravathi P. Kumar M.P. Suresh D. et al.: ‘ Tinospora cordifolia mediated facile green synthesis of cupric oxide nanoparticles and their photocatalytic, antioxidant and antibacterial properties’, Mat. Sci. Semicon. Proc., 2015, 33, pp. 81 –88 [Google Scholar]
- 109. Yallappa S. Manjanna J. Sindhe M. et al.: ‘Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract’, Spectrochim. Acta A, 2013, 110, pp. 108 –115 [DOI] [PubMed] [Google Scholar]
- 110. Rejinold N.S. Jayakumar R. Kim Y.‐C.: ‘Radio frequency responsive nano‐biomaterials for cancer therapy’, J. Control. Release, 2015, 204, pp. 85 –97 [DOI] [PubMed] [Google Scholar]
- 111. Kulkarni V.M. Bodas D. Paknikar K.M.: ‘Lanthanum strontium manganese oxide (LSMO) nanoparticles: a versatile platform for anticancer therapy’, RSC Adv., 2015, 5, (74), pp. 60254 –60263 [Google Scholar]
- 112. Howell M. Mallela J. Wang C. et al.: ‘Manganese‐loaded lipid‐micellar theranostics for simultaneous drug and gene delivery to lungs’, J. Control. Release, 2013, 167, (2), pp. 210 –218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jassal V. Shanker U. Gahlot S. et al.: ‘ Sapindus mukorossi mediated green synthesis of some manganese oxide nanoparticles interaction with aromatic amines’, Appl. Phys. A, 2016, 122, (4), p. 271 [Google Scholar]
- 114. Krishnaraj C. Ji B.‐J. Harper S.L. et al.: ‘Plant extract‐mediated biogenic synthesis of silver, manganese dioxide, silver‐doped manganese dioxide nanoparticles and their antibacterial activity against food‐and water‐borne pathogens’, Bioprocess. Biosyst. Eng., 2016, 39, (5), pp. 759 –772 [DOI] [PubMed] [Google Scholar]
- 115. Jayandran M. Haneefa M.M. Balasubramanian V.: ‘Green synthesis and characterization of manganese nanoparticles using natural plant extracts and its evaluation of antimicrobial activity’, J. Appl. Pharm. Sci., 2015, 5, (12), pp. 105 –110 [Google Scholar]
- 116. Yu X. Li A. Zhao C. et al.: ‘Ultrasmall semimetal nanoparticles of bismuth for dual‐modal computed tomography/photoacoustic imaging and synergistic thermoradiotherapy’, ACS Nano., 2017, 11, (4), pp. 3990 –4001 [DOI] [PubMed] [Google Scholar]
- 117. Li Z. Liu J. Hu Y. et al.: ‘Multimodal imaging‐guided antitumor photothermal therapy and drug delivery using bismuth selenide spherical sponge’, ACS Nano., 2016, 10, (10), pp. 9646 –9658 [DOI] [PubMed] [Google Scholar]
- 118. Wei B. Zhang X. Zhang C. et al.: ‘Facile synthesis of uniform‐sized bismuth nanoparticles for Ct visualization of gastrointestinal tract in vivo’, ACS Appl. Mater. Interfaces, 2016, 8, (20), pp. 12720 –12726 [DOI] [PubMed] [Google Scholar]
- 119. Li B. Ye K. Zhang Y. et al.: ‘Photothermal theragnosis synergistic therapy based on bimetal sulphide nanocrystals rather than nanocomposites’, Adv. Mater., 2015, 27, (8), pp. 1339 –1345 [DOI] [PubMed] [Google Scholar]
- 120. Detappe A. Thomas E. Tibbitt M.W. et al.: ‘Ultrasmall silica‐based bismuth gadolinium nanoparticles for dual magnetic resonance–computed tomography image guided radiation therapy’, Nano Lett., 2017, 17, (3), pp. 1733 –1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wu X. Wu M. Zhao J.X.: ‘Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy’, Nanomedicine, 2014, 10, (2), pp. 297 –312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hahn M.A. Singh A.K. Sharma P. et al.: ‘Nanoparticles as contrast agents for in‐vivo bioimaging: current status and future perspectives’, Anal. Bioanal. Chem., 2011, 399, (1), pp. 3 –27 [DOI] [PubMed] [Google Scholar]
- 123. Wu S.‐H. Hung Y. Mou C.‐Y.: ‘Mesoporous silica nanoparticles as nanocarriers’, Chem. Commun., 2011, 47, (36), pp. 9972 –9985 [DOI] [PubMed] [Google Scholar]
- 124. Li Z. Barnes J.C. Bosoy A. et al.: ‘Mesoporous silica nanoparticles in biomedical applications’, Chem. Soc. Rev., 2012, 41, (7), pp. 2590 –2605 [DOI] [PubMed] [Google Scholar]
- 125. Cheng S.‐H. Lee C.‐H. Yang C.‐S. et al.: ‘Mesoporous silica nanoparticles functionalized with an oxygen‐sensing probe for cell photodynamic therapy: potential cancer theranostics’, J. Mater. Chem., 2009, 19, (9), pp. 1252 –1257 [Google Scholar]
- 126. Rosenholm J.M. Mamaeva V. Sahlgren C. et al.: ‘Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage’, Nanomedicine, 2012, 7, (1), pp. 111 –120 [DOI] [PubMed] [Google Scholar]
- 127. Makarov V. Love A. Sinitsyna O. et al.: ‘“Green” nanotechnologies: synthesis of metal nanoparticles using plants’, Acta Naturae, 2014, 6, (1), pp. 1 –10 [PMC free article] [PubMed] [Google Scholar]
- 128. Ahmad N. Sharma S. Alam M.K. et al.: ‘Rapid synthesis of silver nanoparticles using dried medicinal plant of basil’, Colloids Surf. B Biointerfaces, 2010, 81, (1), pp. 81 –86 [DOI] [PubMed] [Google Scholar]
- 129. Raveendran P. Fu J. Wallen S.L.: ‘Completely ‘Green’ synthesis and stabilization of metal nanoparticles’, J. Am. Chem. Soc., 2003, 125, (46), pp. 13940 –13941 [DOI] [PubMed] [Google Scholar]
- 130. Philip D.: ‘Green synthesis of gold and silver nanoparticles using hibiscus Rosa sinensis ’, Physica E Low Dimens. Syst. Nanostruct., 2010, 42, (5), pp. 1417 –1424 [Google Scholar]
- 131. Shankar S.S. Rai A. Ahmad A. et al.: ‘Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth’, J. Chem. Technol. Biotechnol., 2004, 275, (2), pp. 496 –502 [DOI] [PubMed] [Google Scholar]
- 132. Saif S. Tahir A. Asim T. et al.: ‘Plant mediated green synthesis of Cuo nanoparticles: comparison of toxicity of engineered and plant mediated Cuo nanoparticles towards daphnia magna’, Nanomaterials, 2016, 6, (11), p. 205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sudhasree S. Shakila Banu A. Brindha P. et al.: ‘Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity’, Toxicol. Environ. Chem., 2014, 96, (5), pp. 743 –754 [Google Scholar]
- 134. Velmurugan P. Sivakumar S. Young‐Chae S. et al.: ‘Synthesis and characterization comparison of peanut shell extract silver nanoparticles with commercial silver nanoparticles and their antifungal activity’, Ind. Eng. Chem. Res., 2015, 31, pp. 51 –54 [Google Scholar]
- 135. Kharey P. Tripathi A.K.: ‘Review on conventional and green synthesis methodologies of iron oxide based magnetic nanoparticles’, Adv. Sci. Lett, 2015, 21, (8), pp. 2523 –2528 [Google Scholar]
- 136. Kumar V. Yadav S.K.: ‘Plant‐mediated synthesis of silver and gold nanoparticles and their applications’, J. Chem. Technol. Biotechnol., 2009, 84, (2), pp. 151 –157 [Google Scholar]
- 137. Parashar V. Parashar R. Sharma B. et al.: ‘Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization’, Dig. J. Nanomater. Biostruct., 2009, 4, (1), pp. 45 –50 [Google Scholar]
- 138. Kavitha K. Baker S. Rakshith D. et al.: ‘Plants as green source towards synthesis of nanoparticles’, Int. Res. J. Biol. Sci., 2013, 2, (6), pp. 66 –76 [Google Scholar]
- 139. Ahmed S. Ahmad M. Swami B.L. et al.: ‘A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise’, J. Adv. Res., 2016, 7, (1), pp. 17 –28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mubarakali D. Thajuddin N. Jeganathan K. et al.: ‘Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens’, Colloids Surf. B., 2011, 85, (2), pp. 360 –365 [DOI] [PubMed] [Google Scholar]
- 141. Bystrzejewska‐Piotrowska G. Golimowski J. Urban P.L.: ‘Nanoparticles: their potential toxicity, waste and environmental management’, Waste Manag., 2009, 29, (9), pp. 2587 –2595 [DOI] [PubMed] [Google Scholar]
- 142. Handy R.D. Owen R. Valsami‐Jones E.: ‘The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs’, Ecotoxicology, 2008, 17, (5), pp. 315 –325 [DOI] [PubMed] [Google Scholar]
- 143. Panyala N.R. Peña‐Méndez E.M. Havel J.: ‘Silver or silver nanoparticles: a hazardous threat to the environment and human health?’, J. Appl. Biomed., 2008, 6, (3), pp. 117 –129 [Google Scholar]
- 144. Senjen R.: ‘Nanosilver – a threat to soil, water and human health’. FOE, 2007.
- 145. McAuliffe M.E. Perry M.J.: ‘Are nanoparticles potential male reproductive toxicants? A literature review’, Nanotoxicology, 2007, 1, (3), pp. 204 –210 [Google Scholar]
- 146. Kittler S. Greulich C. Diendorf J. et al.: ‘Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions’, Chem. Mater., 2010, 22, (16), pp. 4548 –4554 [Google Scholar]
- 147. Wood C.M. Playle R.C. Hogstrand C.: ‘Physiology and modeling of mechanisms of silver uptake and toxicity in fish’, Environ. Toxicol. Chem., 1999, 18, (1), pp. 71 –83 [Google Scholar]
- 148. Singh N. Jenkins G.J. Asadi R. et al.: ‘Potential toxicity of superparamagnetic iron oxide nanoparticles (Spion)’, Nano Rev., 2010, 1, (1), p. 5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shi H. Magaye R. Castranova V. et al.: ‘Titanium dioxide nanoparticles: a review of current toxicological data’, Part Fibre Toxicol., 2013, 10, (1), p. 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Boisselier E. Astruc D.: ‘Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity’, Chem. Soc. Rev., 2009, 38, (6), pp. 1759 –1782 [DOI] [PubMed] [Google Scholar]
- 151. Singh S.P. Kumari M. Kumari S.I. et al.: ‘Toxicity assessment of manganese oxide micro and nanoparticles in wistar rats after 28 days of repeated oral exposure’, J. Appl. Toxicol., 2013, 33, (10), pp. 1165 –1179 [DOI] [PubMed] [Google Scholar]
- 152. Karlsson H.L. Cronholm P. Gustafsson J. et al.: ‘Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes’, Chem. Res. Toxicol., 2008, 21, (9), pp. 1726 –1732 [DOI] [PubMed] [Google Scholar]
- 153. Lin W. Huang Y.‐W. Zhou X.‐D. et al.: ‘In vitro toxicity of silica nanoparticles in human lung cancer cells’, Toxicol. Appl. Pharmacol., 2006, 217, (3), pp. 252 –259 [DOI] [PubMed] [Google Scholar]
- 154. Yang W. Guo W. Chang J. et al.: ‘Protein/peptide‐templated biomimetic synthesis of inorganic nanoparticles for biomedical applications’, J. Mater. Chem. B, 2017, 5, (3), pp. 401 –417 [DOI] [PubMed] [Google Scholar]
- 155. Zhang B. Jin H. Li Y. et al.: ‘Bioinspired synthesis of gadolinium‐based hybrid nanoparticles as MRI blood pool contrast agents with high relaxivity’, J. Mater. Chem., 2012, 22, (29), pp. 14494 –14501 [Google Scholar]
- 156. Zhang J. Hao G. Yao C. et al.: ‘Albumin‐mediated biomineralization of paramagnetic Nir Ag2 s Qds for tiny tumor bimodal targeted imaging in vivo’, ACS Appl. Mater. Interfaces, 2016, 8, (26), pp. 16612 –16621 [DOI] [PubMed] [Google Scholar]
- 157. Yang W. Guo W. Le W. et al.: ‘Albumin‐bioinspired Gd: Cus nanotheranostic agent for in vivo photoacoustic/magnetic resonance imaging‐guided tumor‐targeted photothermal therapy’, ACS Nano., 2016, 10, (11), pp. 10245 –10257 [DOI] [PubMed] [Google Scholar]
- 158. Yang W. Wu X. Dou Y. et al.: ‘A human endogenous protein exerts multi‐role biomimetic chemistry in synthesis of paramagnetic gold nanostructures for tumor bimodal imaging’, Biomaterials, 2018, 161, pp. 256 –269 [DOI] [PubMed] [Google Scholar]
- 159. Zhang B. Wang J. Yu J. et al.: ‘Site‐specific biomimetic precision chemistry of bimodal contrast agent with modular peptides for tumor‐targeted imaging’, Bioconjugate Chem., 2017, 28, (2), pp. 330 –335 [DOI] [PubMed] [Google Scholar]
- 160. Söptei B. Nagy L.N. Baranyai P. et al.: ‘On the selection and design of proteins and peptide derivatives for the production of photoluminescent, red‐emitting gold quantum clusters’, Gold Bull.., 2013, 46, (3), pp. 195 –203 [Google Scholar]
- 161. Jeelani S. Reddy R.J. Maheswaran T. et al.: ‘Theranostics: a treasured tailor for tomorrow’, J. Pharm. Bioallied. Sci., 2014, 6, (Suppl 1), p. S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Haverkamp R.G. Marshall A.T. van Agterveld D.: ‘Pick your carats: nanoparticles of gold–silver–copper alloy produced in vivo’, J. Nanopart. Res., 2007, 9, (4), pp. 697 –700 [Google Scholar]


