Abstract
The main goal of this study was to synthesise and characterise different formulations based on alginate and alginate/chitosan microspheres containing nanoselenium (nano‐Se) for controlled delivery applications. Nanosize elemental selenium was produced by using probiotic yogurt bacteria (Lactobacillus casei) in a fermentation procedure. The structural and morphological characterisation of the microspheres was performed by Fourier transform infrared (FTIR), X‐ray diffraction (XRD) and scanning electron microscopy (SEM) analysis. FTIR and XRD pattern indicated that was an effective cross‐linking of selenium nanoparticles within the polymeric matrix in both cases. The SEM images reveal that selenium nanoparticles are mainly exposed on the surface of alginate, in contrast to porous structure of alginate/chitosan/nano‐Se, interconnected in a regular network. This architecture type has a considerable importance in the delivery process, as demonstrated by differential pulse voltammetry. Selenium release from both matrices is pH sensitive. Moreover, chitosan blended with alginate minimise the release of encapsulated selenium, in simulated gastric fluid, and prolong the duration of release in intestinal fluid. The overall effect is the enhancement of total percentage release concomitant with the longer duration of action. The authors’ formulation based on alginate/chitosan is a convenient matrix to be used for selenium delivery in duodenum, caecum and colon.
Inspec keywords: organic‐inorganic hybrid materials, nanocomposites, blending, filled polymers, nanoparticles, nanofabrication, nanomedicine, biomedical materials, drug delivery systems, microorganisms, biological organs, selenium, polymer blends, fermentation, scanning electron microscopy, X‐ray diffraction, Fourier transform infrared spectra, surface morphology, nanoporous materials, porosity, pH, voltammetry (chemical analysis), encapsulation
Other keywords: structural characterisation, hybrid microspheres entrapping nanoselenium, green synthesis, alginate‐chitosan microspheres, controlled delivery applications, nanosize elemental selenium, probiotic yogurt bacteria, Lactobacillus casei, fermentation, scanning electron microscopy, morphological characterisation, SEM, Fourier transform infrared spectra, FTIR, XRD, X‐ray diffraction, selenium nanoparticles, polymeric matrix, porous structure, differential pulse voltammetry, pH, blending, encapsulated selenium, simulated gastric fluid, intestinal fluid, total percentage release concomitant, duodenum, caecum, colon, Se
1 Introduction
Nowadays, oral administration of drugs or supplements is orientated towards developing microspheres prepared from natural polymers as effective drug delivery matrices, owing to its non‐toxicity, biodegradability, biocompatibility, mucoadhesion and antibacterial properties. Such as drug delivery systems are designed to provide a therapeutic agent in the needed amount, at the right time, to the proper location in the body, in a manner that optimises efficacy, increases compliance and minimises side effects [1]. By taking advantage of the specific characteristics, the microspheres could provide a larger surface area and possess an easier estimation of diffusion and mass transfer behaviour, also the encapsulated small molecules could diffuse out of the barrier with precise kinetics modelling and control‐release of drugs to the body fluid. Naturally derived polymers – cellulose, chitosan, alginate and agarose – are highly biocompatible and typically composed of a polymeric network that can contain up to 99% or higher water content. As a result, they have been referred to as ‘hydrogels’, and their swelling capability in water allows them to exhibit an environment that resembles the highly hydrated state of natural tissues [2, 3]. Chitosan, derived from chitin through a deacetylation process, is found in the exoskeleton of marine crustaceans such as shrimps and crabs, as well as insects and the cell walls of fungi [2, 3, 4, 5]. Chitosan forms gels either by raising the pH to 7 or higher or by interacting with a variety of divalent and polyvalent anions being biocompatible, biodegradable, non‐toxic and biofunctional. The biomedical applications of chitosan include tissue engineering, drug delivery, stem cells or growth factor encapsulation [2, 3, 4, 5]. Alginate is a polysaccharide derived from brown algae, certain seaweeds or bacteria. It is a linear polysaccharide copolymer of (1, 4)‐linked b‐D‐mannuronic acid (M) and a‐L‐guluronic acid (G) monomers, and its structure consists of blocks of M or G monomers. Alginate forms readily a gel structure in the presence of divalent ions such as Ca2+, Ba2+ and Sr2+ via ionic cross‐linking. Alginate has been used in various biomedical applications, including drug, antibody or growth factor delivery, cell encapsulation and seeding, and gene delivery in plants and mammals in the form of microspheres [6, 7, 8].
It is widely accepted that selenium, an essential trace element, is necessary to correct and improve organism's functions, being a constituent of one of the main antioxidative enzymes – glutathione peroxidase [9, 10]. The combination of the administered dose and the chemical form of selenium play a fundamental role in determining its activity as toxic or carcinostatic. For example, the selenoenzyme glutathione peroxidase can protect cells from oxidative damage [11] and the selenoenzyme group, glutathione S‐transferases, can repair damaged DNA and prevent mutation [12]. Other forms of selenium, however, can produce reactive oxygen species leading to oxidative stress and cell death [13, 14].
Years ago, it was generally considered about elemental selenium to be biologically inert, but recently, some researchers proved that nanoselenium (nano‐Se) has similar bioavailability to other selenium forms [15, 16, 17] and reported that nano‐Se not only has a higher efficiency in up‐regulating selenoenzymes, but also seem to be less toxic comparing to selenite. Moreover, compared with seleno‐methionine, nano‐Se has lower toxicity and possesses equal efficacy in increasing the activities of selenoenzymes [15]. These results indicated that nano‐Se can serve as an antioxidant with reduced risk of Se toxicity, showing a better absorption into plants, animals, humans and microorganisms. Moreover, one of the most important applications of Se‐nanoparticles is its chemo‐preventive property, by immunological stimulation. For example, the immunostimulatory effect of biogenic selenium nanoparticles was demonstrated in vivo, using a breast cancer model in mice [16, 17]. Another recent in vivo study demonstrated that selenium‐substituted hydroxyapatite nanoparticles show an antitumor effect on hepatocellular carcinoma [18]. Complementary, a recent in vitro study [19] indicated that Se‐nanoparticles are a promising option for treating head and neck squamous cell carcinoma, without adversely affecting healthy cells and without resorting to the use of harmful chemotherapeutics.
Biosynthesis of Se‐nanoparticles can be performed using various biomaterials like bacteria, fungus, algae and plants, by eco‐friendly and potentially economically viable ‘green’ synthesis route towards synthesis of red elemental selenium [9, 20, 21, 22]. Synthesis of elemental nano‐Se employs the reduction of a selenium salt with a reducing agent, usually in the presence of a stabilising agent to prevent the clusters of selenium atoms from growing and to obtain stabilised nanoparticles in colloidal suspension [20, 21, 22].
The aim of our study is to prepare different formulations based on alginate and alginate/chitosan as controlled delivery matrices for nano‐Se, and to evaluate their structural properties and delivery efficiency in simulated gastrointestinal fluids. Moreover, the antioxidant capacity of different formulations was evaluated by FRAP (ferric reducing ability of plasma) assays.
2 Materials and methods
2.1. Preparation of alginate and alginate/chitosan microspheres with nano‐Se
Sodium alginate and chitosan (low molecular weight, 85% deacetylated) were purchased from Sigma Aldrich and used without further treatments. Nano‐Se colloidal solution, in a concentration of 205 ppm, was obtained by using probiotic lactic acid bacteria (Lactobacillus casei) following a method described by Eszenyi et al. [20]. The morphology and size distribution of Se‐nanoparticles, after purification procedure, was investigated by scanning electron microscopy (SEM) (JEOL JSM 7000F) and Malvern Mastersizer 2000 particle size analyser (Malvern Instruments Limited). Alginate and alginate/chitosan microspheres incorporating nano‐Se particles were prepared by classical cross‐linking method, previously described in the literature [1, 23]. Sodium alginate and chitosan polymeric gels were prepared in concentrations of 1.5% w/w in distilled water under magnetic stirring at a temperature of 37°C. On the basis of the similar studies presented in the literature [24, 25] and optimisation tests, the mixed gel with sodium alginate/chitosan ratio 2:1 was chosen as optimum combination and then loaded with 2 ml of Se‐nanocolloidal solution (in a concentration of 205 ppm). The same volume of nano‐Se was incorporated in single alginate hydrogel, for comparison.
The above gels (alginate/nano‐Se and alginate/chitosan/nano‐Se) were dropwised into 4% CaCl2 solution, obtaining a suspension of floating microspheres, red to orange coloured, characteristic for nano‐selenium. Next, the microspheres were filtrated and freeze dried for 24 h using Martin Christ Alpha 1‐2 LD equipment. The efficiency of nano‐Se entrapment in alginate and alginate/chitosan matrix was determined spectrophotometricaly (Shimadzu Mini UV–vis spectrophotometer) by measuring the absorbance at 280 nm, which is the characteristic absorption band of selenium [26]. The absorbance value of the initial blend (with 100% selenium content) as well as of the withdrawn solution (obtained after filtration) was used for percentage calculation.
2.2 Structural and morphological characterisation of alginate/nano‐Se and alginate/chitosan/nano‐Se microspheres
The structural characterisation of alginate/nano‐Se and alginate/chitosan/nano‐Se microspheres was performed by attenuated total reflectance (ATR) Fourier transform infrared (FTIR) (ATR‐FTIR) spectroscopy (Perkin Elmer BX II equipped with MIRACLE device for attenuated total reflection) in the range of 400–4000 cm−1, X‐ray diffraction (Rigaku Miniflex, with Cu‐Kα radiation) and scanning electron microscopy (SEM) (JEOL JSM 7000F). The spectral characteristics of powder alginate and chitosan (as received from the supplier) were emphasised as a reference, along with the FTIR spectrum and the XRD pattern of nano‐Se powder. Prior to electron microscopy investigation (SEM), the samples were sputter coated for 2 min at 25 mA with platinum.
2.3 In vitro nano‐Se release
Cumulative selenium release in simulated gastrointestinal solutions with pH = 1.8 and pH = 7.4 was investigated by using differential pulse voltammetry (DPV), in a three electrode configuration (TRACELAB 150), software controlled [27]. The working electrode was a 3 mm diameter graphite control electrode, reference electrode was an Ag/AgCl/3M KCl and the counter electrode was a Pt wire. To elaborate the calibration curve, the stock nanocolloidal solution (concentration 205 ppm) was used to prepare serial dilutions; the corresponding voltammograms were recorded, measuring the current intensity of serial solutions with concentrations ranging from 0.005 to 0.045 mg/ml. The rate of release was divided into a stomach phase (0–180 min) and a small intestine phase (180–400 min).
Simulated fluids were prepared according to the literature [28] as follows:
(a) Simulated gastric fluid (SGF – an artificial dissolution medium that is intended to represent stomach acid) was prepared by dissolving 2.0 g of sodium chloride and 3.2 g of purified pepsin (derived from porcine stomach mucosa) in 7.0 ml of hydrochloric acid and water up to 1000 ml. The final solution was adjusted to pH = 1.8.
(b) Simulated intestinal fluid (SIF) was prepared by dissolving 6.8 g of monobasic potassium phosphate in 250 ml of water and then adding 77 ml of 0.2 N sodium hydroxide and 500 ml of water. Then, 10 g of pancreatin was added and the resulting solution was adjusted with 0.2 N hydrochloric acid to a pH of 7.4 and finally diluted to 1000 ml. All the reagents were purchased from Fluka.
2.4 In vitro antioxidant capacity by FRAP assay
To assess the synergic or antagonic antioxidant effect of Se‐nanoparticles together with the polymeric matrix, different formulations based on alginate and chitosan (single component) as well as the composites (alginate‐Se and chitosan‐Se) were used in a FRAP assay, according to a method described by Benzie and Strain [29]. For this purpose, the samples were prior dissolved in a mixture of HCl:HNO3 (1:1, v/v), under continuous stirring, until both the polymeric matrix and nano‐Se were completely dissolved. The interpretation of the results is based on the reduction of Fe3+ from tripyridyltriazine Fe(TPTZ)3+ complex, to the blue coloured complex‐Fe(TPTZ)2+ in acidic medium. The stock solutions included: 300 mM acetate buffer; 20 mM FeCl3 ·6H2 O solution; 10 mM TPTZ (2, 4, 6‐tripyridyl‐s‐triazine). The working FRAP solution was freshly prepared by mixing acetate buffer, FeCl3 ·6H2 O and TPTZ solution, in the ratio 10:1:1 (v:v:v). Properly diluted samples (100 μL) were allowed to react with 500 μl working FRAP solution and 2 ml distilled water for 1 h, in dark. Solutions of FeSO4 ·7H2 O ranging from 0.1 to 1 mM were prepared and used for the calibration curve (R 2 = 0.995) of known Fe2+ concentrations. Absorbance was measured at 595 nm (Shimadzu mini UV–vis spectrophotometer) and the results were expressed as μmol ferrous equivalent/g dry weight samples. The results were reported as mean and SD values and for statistical significance; one way analysis of variance (Tukey's multiple comparison test, p < 0.05) was applied.
3 Results
3.1 Preparation, structural and morphological characterisation of alginate/nano‐Se and alginate/chitosan/nano‐Se microspheres
Colloidal solution of nano‐Se, with characteristic red colour, was successfully obtained by using lactic acid bacteria species which are able to reduce the toxical concentration into nanosized, elemental selenium spheres. The morphological details and size distribution of selenium nanoparticles, showing a regular, spherical shape, with average size of 400 nm, are presented in Figs. 1 a and b.
Fig. 1.
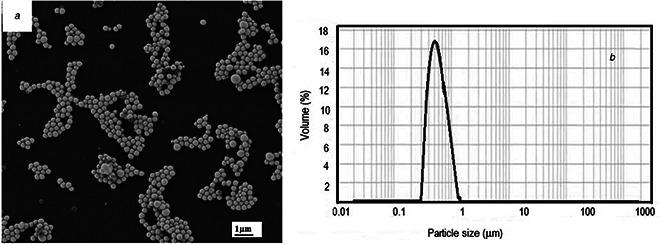
Morphological details and size distribution of Se‐nanoparticles
(a) SEM images of Se‐nanoparticles showing a regular, spherical shape, (b) Particle size distribution of Se‐nanoparticles
By cross‐linking and ionotropic gelation, Se‐nanoparticles were successfully incorporated into alginate and alginate/chitosan microspheres, the characteristic red colour of nano‐Se being emphasised in Fig. 2 by comparing the visual aspect (gross appearance) of alginate microspheres before (a) and after nano‐Se (b) entrapment. 92 and 89% efficiency of selenium entrapment in alginate and alginate/chitosan microspheres was calculated based on UV/vis absorbance values.
Fig. 2.
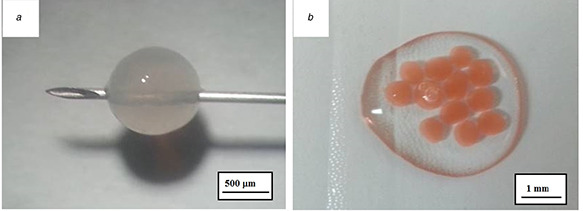
Visual aspect (gross appearance) of alginate microspheres
(a) Single alginate microsphere before nano‐Se entrapment, (b) Several alginate microspheres after nano‐Se entrapment
Upon freeze drying procedure, the morphological details of the microspheres are presented in Fig. 3 with different magnification. The microscopic details clearly show the texture of the microspheres surface, with nano‐Se particles incorporated. By comparing the details in Figs. 3 c and f it seems that Se‐nanoparticles are displayed predominantly on the alginate surface, whereas with respect to alginate/chitosan matrix, the surface is laced with nano‐Se particles, but only few of these are exposed, the majority being deeply embedded. A porous structure, interconnected in a regular network, is also observed. This architecture type might have a considerable importance in the delivery process.
Fig. 3.
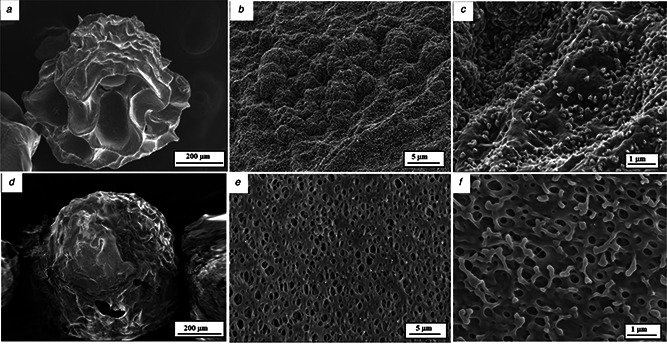
SEM morphological details of the freeze‐dried microspheres, with different magnifications
(a)–(c) Alginate/nano‐Se microsphere, (d)–(f) Alginate/chitosan/nano‐Se microsphere
ATR‐FTIR spectra of the raw polymers, nano‐Se powder and the final composites were recorded in the spectral region 500–4000 cm−1. As a reference, ATR‐FTIR spectra of pure alginate and chitosan (as received from the supplier) were also recorded for comparison. The spectra are displayed in Figs. 4 a –f. The marker bands of nano‐Se (powder) shows, in the high wavelength range, an intense absorption peak at 3290 cm−1 due to OH stretching of the aromatic rings and two sharp peaks at 2850 and 2923 cm−1 representing ether‐methoxy‐OCH3 groups. These peaks show the presence of a biopolymer associated with the Se‐nanoparticles, probably resulted from the bacteria cell walls. In the low wavelength range, 1742 cm−1 (C = O stretch of the estheric group), 1631 (C–H vibration of the aromatic ring), 1530 cm−1 (N–H bending), 1437 cm−1 (C–H asymmetric bending in CH2 and CH3 groups), 1257–1170 cm−1 (secondary OH bending) and 1060 cm−1 (in plane C–H bending) are emphasised.
Fig. 4.
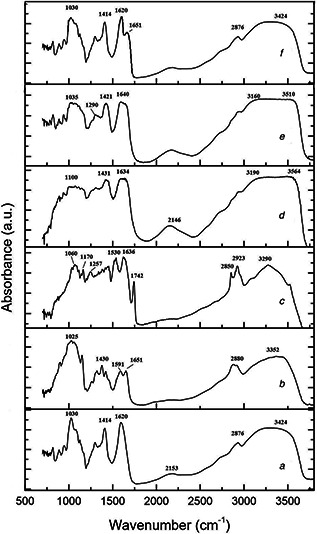
ATR‐FTIR spectra recorded in the spectral region 500–4000 cm−1
(a) Alginate, (b) Chitosan, (c) Nano‐Se, (d) Alginate/nano‐Se, (e) Alginate/chitosan/nano‐Se, (f) Alginate/chitosan
In the spectra of chitosan, the broad band at 3352 cm−1 is assigned to the amine and hydroxyl groups (O–H stretching), 2880 cm−1 represent the aliphatic C–H stretching, 1651 cm−1 is due to C = O stretching of the amide I, 1591 cm−1 is the bending vibration of N–H bond in amide II, while the 1430 cm−1 is due to symmetrical stretching of –COO– groups, 1315 cm−1 is the C–O stretching and 1025 is the C–O–C stretching of the saccharide structure. With respect to alginate, in the high wavelength region, the bands at 3427 and 2878 cm−1 are assigned to O–H and C–H stretching, respectively, while in the low wavelength, the bands at 1620 and 1414 are due to asymmetric and symmetric stretching of carboxylate groups, and 1030 cm−1 is due to C–O–C stretching. Compared the spectrum of pure alginate with alginate/nano‐Se, the two –COO– peaks (asymmetric and symmetric stretching) weakened obviously after crosslinking by Ca2+ and in the same time shifted to higher wavenumbers. The phenomenon is also present in the blended alginate/chitosan/nano‐Se. In the FTIR spectrum of alginate/chitosan/nano‐Se composites, we can observe the asymmetrical stretching of –COO– groups shifted to 1640 cm−1 and the symmetrical stretching of –COO– groups shifted to 1421 cm−1. In addition, the absorption band at 1591 cm−1 of chitosan shifts to 1620 cm−1 after the reaction with alginate, the stretching vibration of –OH and –NH2 at 3424 cm−1 shifts to 3448 cm−1 and becomes broad. All the FTIR results are in agreement with previous reported studies [24, 30]. The observed shifts in frequencies and the appearance of some new peaks confirm that chitosan and sodium alginate are effectively bound with the nano‐Se.
To get information about the crystalline behaviour of the starting polymers, nano‐Se powder and final composites, the XRD analysis were conducted and the results are presented in Figs. 5 a –f. The amorphous structure of alginate [25] is revealed by the intense peak at about 18° and a large band at 42°. XRD pattern of chitosan clearly emphasises two distinct peaks at about 15° and 23°, corresponding to hydrate and anhydrous crystals, respectively, and a broad band at about 40°. This behaviour indicates that the chitosan sample exhibits a semi‐crystalline nature [31]. Nano‐Se shows an intense peak at about 19°, low intensity peaks at 23° and 25° and a large band at around 38°. These fingerprints can be observed also in the blended alginate/nano‐Se, alginate/chitosan and alginate/chitosan/nano‐Se, with different relative intensity. The weakening of the rigid semi‐crystalline nature of chitosan after cross‐linking may be due to the disruption of the NH2 groups present in the glucosamine units of chitosan due to hydrogen bonding. The results indicated that was an effective cross‐linking.
Fig. 5.
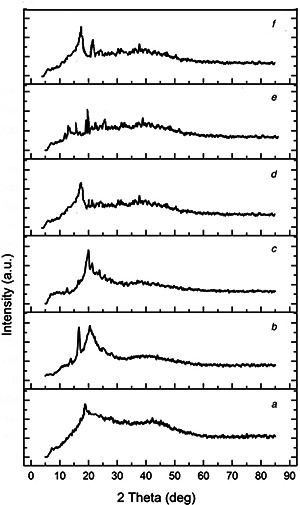
XRD patterns of the raw polymers, nano‐Se powder and the final composites
(a) Alginate, (b) Chitosan, (c) Nano‐Se, (d) Alginate/nano‐Se, (e) Alginate/chitosan/nano‐Se, (f) Alginate/chitosan
3.2 In vitro nano‐Se release
The calibration curve of selenium using DPV is presented in Fig. 6 a along with the corresponding voltammograms in Fig. 6 b. On the basis of this calibration curve, the cumulative release of selenium was measured in both artificial gastric juice (pH = 1.8) and artificial intestinal fluid (pH = 7.4) at different time intervals, to mimic the digestive tract environment. The results are depicted in Fig. 7, for alginate/nano‐Se and alginate/chitosan/nano‐Se microspheres. After the first 180 min in acid medium, the release profile of selenium shows 41 and 24% with respect to alginate and alginate/chitosan matrix. The initial burst release from alginate matrix is due to the adsorbed Se‐nanoparticles on the surface, as evidenced also by SEM images. In the next 3 h, after transferring the microspheres in basic environment, 17% of selenium was released from alginate and 55% from alginate/chitosan matrix.
Fig. 6.
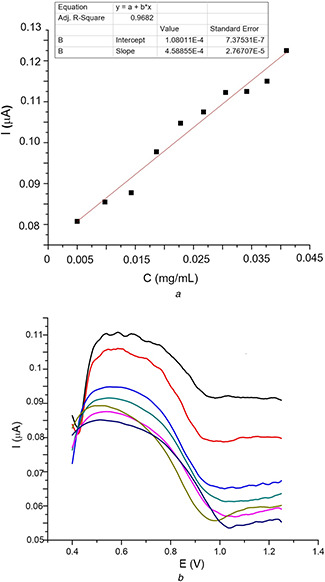
Differential pulse voltammetry
(a) Calibration curve of nano‐Se, (b) Corresponding selenium voltammograms recorded at different concentrations
Fig. 7.
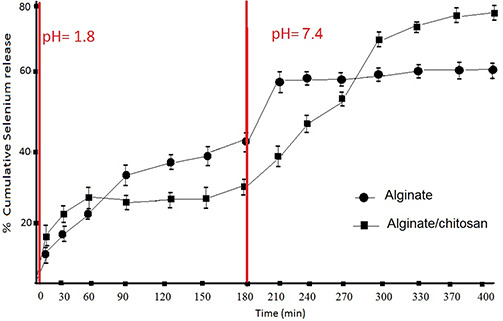
Cumulative release of nano‐Se measured at different time intervals
(a) Artificial gastric juice (pH = 1.8), (b) Artificial intestinal fluid (pH = 7.4)
This result suggests that selenium release from both matrices is pH sensitive. Moreover, chitosan blended with alginate minimise the release of encapsulated selenium in simulated gastric fluid, and prolong the duration of release in intestinal fluid. The overall effect is the enhancement of total percentage release concomitant with the longer duration of action.
3.3 Antioxidant capacity
According to the diagram presented in Fig. 8, the FRAP assay emphasised that chitosan matrix shows a higher antioxidant activity compared to alginate one (2.41 ± 0.41 versus 0.40 ± 0.06 µmol Fe2+ equivalents/g). With respect to Se‐composites, one can notice that alginate with nano‐Se incorporated present a significantly increased antioxidant activity, showing a synergic effect, opposite to chitosan‐Se (2.07 ± 0.27 versus 0.95 ± 0.19 µmol Fe2+ equivalents/g). Alginate/chitosan/nano‐Se formulation keeps a high antioxidant capacity, with no significant difference compared with the maximum effect.
Fig. 8.
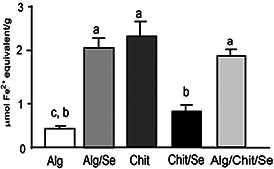
Evaluation of antioxidant capacity of different formulations based on alginate, chitosan and composites with nano‐Se. Data are expressed as mean values±SD with n = 3 according to Tukey's multiple comparison test (same letter means no significant difference, p < 0.05)
4 Discussions
Biomolecule‐mediated nanoparticle synthesis has recently gained the attention of researchers due to its eco‐friendly and non‐toxic nature. In natural environments, live cells, such as bacteria, fungi, yeasts and plants, are known to be capable of converting selenate and selenite to Se0 [9, 11, 21, 22]. Previous studies demonstrated that each bacteria strain produce selenium nanospheres in different size ranges as follows: Lactobacillus sp. 200–400 nm, Bifidobacter sp. 400–500 nm, Streptococcus thermophilus 50–100 nm [11]. This technology seems to be more effective than the chemical synthesis, because it results relatively regular and uniform sized, high purity selenium spheres (100–500 nm, bacterium depending), production process is cheaper, faster and parameters can be controlled better. Probiotic organisms are preferred as natural source of selenium as they are safe for clinical administration and human consumption. Using other organisms (even if some are better in terms of short generation time, ease of culturing, downstream processing and manipulation) is often associated with toxicity concerns due to its nature. The main advantage of using the oral route for the application of Se‐nanoparticles is its potential to modulate the immune response through its interaction with gastrointestinal immune cells and the corresponding milieu [16, 17]. Hence, the challenge to use nano‐Se as food supplements is related to find an appropriate matrix as floating microspheres, to obtain prolonged and sustained release of selenium in gastrointestinal tract. For this purpose, alginate and chitosan were chosen to prepare different formulations based on alginate and alginate/chitosan as controlled delivery matrices for nano‐Se. Preliminary characterisation experiments were undertaken to select the optimum composite alginate/chitosan in a 2:1 ratio. By cross‐linking and ionotropic gelation, Se‐nanoparticles were successfully incorporated into alginate and alginate/chitosan microspheres. The surface morphology of both types of microspheres was very different, as demonstrated by SEM: most of the Se‐nanoparticles are exposed uniformly on the surface of alginate, but laced in the alginate/chitosan matrix concomitant with a porous, tri‐dimensional structure formation.
Similar studies have been reported combining both sodium alginate and chitosan or other natural polymers for protein or peptide delivery, anticancer and anti‐inflammatory drugs, in micro‐ or nano‐particulate systems [5, 7, 25, 30, 31, 32, 33]. Attempting to optimise the formulation, some authors suggested that increasing the amount of chitosan in a composite mucoadhesive tablet formulation resulted in more controlled drug release, while an increase in sodium alginate resulted in improved adhesive properties of the tablet [34]. From structural point of view, the cross‐linking reaction between Ca2+ and carboxylic groups of sodium alginate was demonstrated by ATR‐FTIR analysis. In addition, selenium incorporation in alginate/chitosan matrix was also emphasised by the shifting of vibrational bands corresponding to symmetrical and asymmetrical stretching of –COO– groups concomitant with the broadening of the stretching vibration of –OH and –NH2, which are also shifted to higher wavelengths. The results confirm that the carboxylic groups of alginate associate with ammonium groups of chitosan through electrostatic interactions to form the polyelectrolyte complex. The FTIR spectra of the final composites were affected by blending due to hydrogen bonding [35, 36]. Moreover, the XRD pattern revealed the low crystallinity degree of the blank polymers, nano‐Se and final composite. It seems that nano‐Se maintained a low level of crystallinity within the final composite and implies that it did not change its form during gel formulation in combination with sodium alginate and chitosan as well as freeze drying. Some studies suggested that stable amorphous forms (or even low crystallinity) are advantageous as they exhibit better solubility and therefore higher rates of dissolution which is expected to enhance drug release and subsequent adsorption and bioavailability [25, 31]. Of primary importance is the ability of microsphere formulations to eliminate initial drug burst while modulating the onset of steady drug release. DPV has been proved to be a sensitive electrochemical method frequently used to determine the concentration of trace selenium in pharmaceutical products [37]. To investigate the effect of pH on release mechanism, we have determined the cumulative release percent in both simulated gastric and intestinal fluids, by mean of DPV technique. As reported by previous studies [38], at low pH, alginate beads do not significantly swell or release their content. When the microspheres were placed in SGF with pH = 1.8 for a period of 3 h, the release was due mainly to the Se‐nanoparticles attached on the surfaces; in the same time, alginate undergoes acid‐catalysed hydrolysis to yield low molecular weight alginic acid. Later, on transferring the microspheres into SIF with pH = 7.4, the alginic acid produced previously may now tend to dissolve, resulting in a burst of entrapped drug. The blended alginate/chitosan matrix shows a minimised release in SGF, prolonging the duration of selenium release in SIF. So, selenium release depends upon the nature of the polymer matrix as well as the pH of the media. Interesting, the percentage of selenium loading, 92 and 89% for alginate and alginate/chitosan microspheres, respectively, did not affect significantly the release percent.
An interesting result was obtained with respect to antioxidant capacity of polymeric matrices alone or in combination with nano‐Se. Even if chitosan (alone) shows a higher antioxidant capacity compared with alginate, a synergic effect of nano‐Se together with alginate was obtained, in opposition to antagonic effect of nano‐Se with chitosan. Some previous research papers [39, 40, 41] suggest that the antioxidant mechanism of alginate is mainly based on the hydrogen transfer, rather than electrons transfer. Probably, by this mechanism, the alginate microspheres with nano‐Se encapsulated lead to a significantly increase of the antioxidant power, showing a synergic effect. On the other hand, the chitosan molecule contains both hydroxyl and amino groups that can affect its antioxidant ability [42]. In this case, the antagonic effect of nano‐Se encapsulated in chitosan was demonstrated. To our knowledge, this is the first study based on FRAP assay, assessing the synergic/antagonic antioxidant effect of nano‐Se with respect to different natural polymeric matrices. O the basis of the corroborated results, we consider that the porous architecture of alginate/chitosan matrix is obviously a favourable structure. Our results are in agreement with the general consensus related to particles release from polymeric matrix, which involves three different mechanisms: (i) desorption of molecules from the surface of the matrix; (ii) diffusion through the swollen polysaccharide matrix; and (iii) release due to polymeric network degradation [30, 38]. We consider that alginate/chitosan is a convenient matrix to be used for selenium delivery in the basic media, supported also by previous studies showing that selenium absorption occurs mainly in the duodenum, caecum and colon (more than 85%) [14].
5 Conclusions
We have successfully prepared alginate and alginate/chitosan microspheres incorporating nano‐Se by cross‐linking and ionotropic gelation. Different ultrastructure and morphology of the microspheres was emphasised by SEM: Se‐nanoparticles are mainly exposed on the surface of alginate or laced in a porous alginate/chitosan matrix with a tri‐dimensional structure. An effective cross‐linking in both cases was demonstrated by structural investigations using FTIR spectroscopy and XRD analysis. Nano‐Se maintained a low level of crystallinity within the final composites, which implies an advantageous formulation for the purpose of controlled release. By DPV we have demonstrated that blended alginate/chitosan matrix compared with single alginate matrix shows a minimised release in SGF, prolonging the duration of selenium release in SIF. In the same time, polymeric matrix selection is important with respect to antioxidant effect; a synergic action of alginate and nano‐Se was demonstrated by FRAP assay. Our results demonstrated that nano‐Se release depends upon the nature of the polymer matrix as well as the pH of the media, alginate/chitosan being a convenient matrix to be used for selenium delivery in duodenum, caecum and colon.
6 References
- 1. Khan I. Khan M. Umar N.M. et al.: ‘Nanobiotechnology and its applications in drug delivery system: a review’, IET Nanobiotechnol., 2015, 9, (6), pp. 396 –400 [DOI] [PubMed] [Google Scholar]
- 2. Zeng R. Tu M. Liu H.‐W. et al.: ‘Preparation, structure and drug release behaviour of chitosan‐based nanofibres’, IET Nanobiotechnol., 2009, 3, (1), pp. 8 –13 [DOI] [PubMed] [Google Scholar]
- 3. Zarandi M.A. Zahedi P. Rezaeian I. et al.: ‘Drug release, cell adhesion and wound healing evaluations of electrospun carboxymethyl chitosan/polyethylene oxide nanofibres containing phenytoin sodium and vitamin C’, IET Nanobiotechnol., 2015, 9, (4), pp. 191 –200 [DOI] [PubMed] [Google Scholar]
- 4. Pulavendran S. Rajam M. Rose C. et al.: ‘Hepatocyte growth factor incorporated chitosan nanoparticles differentiate murine bone marrow mesenchymal stem cell into hepatocytes in vitro’, IET Nanobiotechnol., 2015, 4, (3), pp. 51 –60 [DOI] [PubMed] [Google Scholar]
- 5. Shrestha B. Rath J.P.: ‘Poly(vinyl alcohol)‐coated chitosan microparticles act as an effective oral vaccine delivery system for hepatitis B vaccine in rat model’, IET Nanobiotechnol., 2014, 8, (4), pp. 201 –207 [DOI] [PubMed] [Google Scholar]
- 6. Kaygusuz H. Uysal M. Adimcilar V. et al.: ‘Natural alginate biopolymer montmorillonite clay composites for vitamine B2 delivery’, J. Bioact. Compat. Pol., 2015, 30, (1), pp. 48 –56 [Google Scholar]
- 7. Qian Q. Bonani W. Maniglio D. et al.: ‘Modulating the release of drugs from alginate matrices with addition of gelatin microbeads’, J. Bioact. Compat. Pol., 2014, 29, (3), pp. 193 –207 [Google Scholar]
- 8. Gryshkov O. Pogozhykh D. Zernetsch H. et al.: ‘Process engineering of high voltage alginate encapsulation of mesenchymal stem cells’, Mater. Sci. Eng. C, 2014, 36, pp. 77 –83 [DOI] [PubMed] [Google Scholar]
- 9. El‐Ramady H.R. Domokos‐Szabolcsy E. Abdalla N.A. et al.: ‘Selenium and nano‐selenium in agroecosystems’, Environ. Chem. Lett., 2014, 12, pp. 495 –510 [Google Scholar]
- 10. Kipp A.P. Strohm D. Brigelius‐Flohé R. et al.: ‘Revised reference values for selenium intake’, J. Trace Elem. Med. Biol., 2015, 32, pp. 195 –199 [DOI] [PubMed] [Google Scholar]
- 11. Papp L.V. Lu J. Holmgren A. et al.: ‘From selenium to selenoproteins: synthesis, identity, and their role in human health’, Antioxid. Redox Signal., 2007, 9, pp. 775 –782 [DOI] [PubMed] [Google Scholar]
- 12. Zachara B.A. Gromadzinska J. Palus J. et al.: ‘The effect of selenium supplementation in the prevention of DNA damage in white blood cells of hemodialyzed patients: a pilot study’, Biol. Trace Elem. Res., 2011, 142, pp. 274 –283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dumont E. Vanhaecke F. Cornelis R.: ‘Selenium speciation from food source to metabolites: a critical review’, Anal. Bioanal., Chem., 2006, 385, (7), pp. 1304 –1323 [DOI] [PubMed] [Google Scholar]
- 14. Reilly C.: ‘Selenium in food and health’ (Springer, Berlin, 2006, 2nd edn.) [Google Scholar]
- 15. Wang H. Zhang J. Yu H.: ‘Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice’, Free Radic. Biol. Med., 2007, 42, pp. 1524 –1533 [DOI] [PubMed] [Google Scholar]
- 16. Yazdi M.H. Mahdavi M. Varastehmoradi B. et al.: ‘The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: an in vivo study’, Biol. Trace Elem. Res., 2012, 149, pp. 22 –28 [DOI] [PubMed] [Google Scholar]
- 17. Yazdi M.H. Mahdavi M. Setayesh N. et al.: ‘Selenium nanoparticle‐enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer’, DARU J. Pharm. Sci., 2013, 21, (33), pp. 1 –9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yanhua W. Hao H. Li Y. et al.: ‘Selenium‐substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma’, Colloids Surf. B, Biointerfaces, 2016, 140, pp. 297 –306 [DOI] [PubMed] [Google Scholar]
- 19. Hassan C.E. Webster T.J.: ‘The effect of red‐allotrope selenium nanoparticles on head and neck squamous cell viability and growth’, Int. J. Nanomedicine, 2016, 11, pp. 3641 –3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eszenyi P. Sztrik A. Babka B. et al.: ‘Elemental nano‐sized selenium production by probiotic lactic acid bacteria’, Int. J. Biosci. Biochem. Bioinfor., 2011, 1, (2), pp. 148 –152 [Google Scholar]
- 21. Kora A.J. Rastogi L.: ‘Bacteriogenic synthesis of selenium nanoparticles by Escherichia coli ATCC 35218 and its structural characterization’, IET Nanobiotechnol., 2016, doi: 10.1049/iet‐nbt.2016.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li B. Liu N. Li Y. et al.: ‘Reduction of selenite to red elemental selenium by Rhodopseudomonas palustris Strain N’, PLoS ONE, 2014, 9, (4), doi: 10.1371/journal.pone.0095955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim K.K. Pack D.W.: ‘Microspheres for drug delivery’, in Ferrari M. Lee A. Lee J. (Eds.): ‘BIOMEMS and biomedical nanotechnology’ (Springer U.S., 2006), pp. 19 –49 [Google Scholar]
- 24. Liu B. Luo J. Wang X. et al.: ‘Alginate/quaternized carboxymethyl chitosan/clay nanocomposite microspheres: preparation and drug‐controlled release behavior’, J. Biomater. Sci. Polym. Ed., 2013, 24, (5), pp. 589 –605 [DOI] [PubMed] [Google Scholar]
- 25. Boateng J.S. Areago D.: ‘Composite sodium alginate and chitosan based wafers for buccal delivery of macromolecules’, Austin J. Anal. Pharm. Chem., 2014, 1, (5), pp. 1022 –1027 [Google Scholar]
- 26. Zhanga W. Chena Z. Liua H. et al.: ‘Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila ’, Colloids Surf. B, Biointerfaces, 2011, 88, pp. 196 –201 [DOI] [PubMed] [Google Scholar]
- 27. Holak W. Specchio J.J.: ‘Determination of selenium in food supplements by differential pulse cathodic stripping voltammetry in the presence of added copper’, Analyst, 1994, 119, (10), pp. 2179 –2182 [DOI] [PubMed] [Google Scholar]
- 28. Choi A.J. Buisson N. Kim C.T.: ‘Digestion characteristics and kinetic analysis of biomolecules in a simulated human intestinal system’, Integr. Food Nutr. Metab., 2015, 2, (3), pp. 189 –192 [Google Scholar]
- 29. Benzie I.F. Strain J.J.: ‘The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power. The FRAP assay’, Anal. Biochem., 1996, 239, pp. 70 –76 [DOI] [PubMed] [Google Scholar]
- 30. Zorhi M. Nomani A. Gazori T. et al.: ‘Characterization of chitosan/alginate self‐assembled nanoparticles as protein carrier’, J. Dispersion Sci. Technol., 2011, 32, (4), pp. 576 –582 [Google Scholar]
- 31. Vijayalakshmi K. Gomathi T. Sudha P.N.: ‘Preparation and characterization of nanochitosan/sodium alginate/microcrystalline cellulose beads’, Der Pharmacia Lett., 2014, 6, (4), pp. 65 –77 [Google Scholar]
- 32. Reinas A.E. Hoscheid J. Outuki P.M. et al.: ‘Preparation and characterization of microcapsules of Pterodon pubescens benth by using natural polymers’, Brazilian J. Pharm. Sci., 2014, 50, (4), pp. 919 –930 [Google Scholar]
- 33. Bulut E. Sanli O.: ‘Optimization of release conditions of Alzheimer's drug donepezil hydrochloride from sodium alginate/sodium carboxymethyl cellulose blend microspheres’, J. Macromol. Sci. B: Phys., 2014, 53, pp. 902 –917 [Google Scholar]
- 34. Shaikh A.A. Pawar Y.D. Kumbhar S.T.: ‘Effect of chitosan and sodium alginate on mucoadhesion and drug release of itraconazole tablets’, Int. J. Res. Pharm. Biomed. Sci., 2012, 3, pp. 293 –297 [Google Scholar]
- 35. Li P. Dai Y.N. Zhang J.P. et al.: ‘Chitosan alginate nanoparticles as a novel drug delivery system for nifedipine’, Int. J. Biomed. Sci., 2008, 4, (3), pp. 221 –228 [PMC free article] [PubMed] [Google Scholar]
- 36. Riyajan S.A. Nuim J.: ‘Interaction of green polymer blend of modified sodium alginate and carboxylmethyl cellulose encapsulation of turmeric extract’, Int. J. Polym. Sci., 2013, pp. 1 –9, doi: org/10.1155/2013/364253 [Google Scholar]
- 37. Zhang Q. Li X. Hongzhou H.S. et al.: ‘Determination of trace selenium by differential pulse adsorptive stripping voltammetry at a bismuth film electrode’, Electrochim. Acta, 2010, 55, (16), pp. 4717 –4721 [Google Scholar]
- 38. Bajpai S.K. Sharma S.: ‘Dynamic release of riboflavin from ethyl cellulose coated barium alginate beads for gastrointestinal drug delivery: an in vitro study’, J. Macromol. Sci. A, 2007, 42, pp. 649 –661 [Google Scholar]
- 39. Velderrain‐Rodríguez G.R. Ovando‐Martínez M. Villegas‐Ochoa M. et al.: ‘Antioxidant capacity and bioaccessibility of synergic mango peel phenolic compounds in edible coatings applied to fresh‐cut papaya’, J. Food Nutr. Sci., 2015, 6, pp. 365 –373 [Google Scholar]
- 40. Falkeborg M. Cheong L.Z. Gianfico C. et al.: ‘Alginate oligosaccharides: enzymatic preparation and antioxidant property evaluation’, Food Chem., 2014, 164, pp. 185 –194 [DOI] [PubMed] [Google Scholar]
- 41. Hernandez‐Marin E. Martínez A.: ‘Carbohydrates and their free radical scavenging capability: a theoretical study’, J. Phys. Chem. B, 2012, 116, pp. 9668 –9675 [DOI] [PubMed] [Google Scholar]
- 42. Wan A. Xu Q. Sun Y. et al.: ‘Antioxidant activity of high molecular weight chitosan and N,O‐quaternized chitosans’, J. Agric. Food Chem., 2013, 61, (28), pp. 6921 –6928 [DOI] [PubMed] [Google Scholar]


