Abstract
Microbial mediated biological synthesis of metallic nanoparticles was carried out ecofriendly in the present study. Silver nanoparticles (AgNPs) were extracellularly biosynthesised from Streptomyces griseorubens AU2 and extensively characterised by ultraviolet–visible (UV–vis) and Fourier transform infrared spectroscopy, high‐resolution transmission electron microscopy, scanning electron microscopy and X‐ray diffraction analysis. Elemental analysis of nanoparticles was also carried out using energy dispersive X‐ray spectroscopy. The biosynthesised AgNPs showed the characteristic absorption spectra in UV–vis at 422 nm which confirmed the presence of metallic AgNPs. According to the further characterisation analysis, the biosynthesised AgNPs were found to be spherical and crystalline particles with 5–20 nm average size. Antioxidant properties of the biosynthesised AgNPs were determined by 2,2‐diphenyl‐1‐picrylhydrazyl free radical scavenging assay and was found to increase in a dose‐dependent matter. The identification of the strain was determined by molecular characterisation method using 16s rDNA sequencing. The present study is the first report on the microbial biosynthesis of AgNPs using S. griseorubens isolated from soil and provides that the active biological components found in the cell‐free culture supernatant of S. griseorubens AU2 enable the synthesis of AgNPs.
Inspec keywords: silver, microorganisms, nanoparticles, nanofabrication, DNA, molecular biophysics, ultraviolet spectra, visible spectra, scanning electron microscopy, Fourier transform infrared spectra, transmission electron microscopy, X‐ray diffraction, X‐ray chemical analysis, absorption coefficients, cellular biophysics
Other keywords: silver nanoparticles; Streptomyces griseorubens AU2; soil; antioxidant activity; microbial mediated biological synthesis; ultraviolet‐visible spectroscopy; Fourier transform infrared spectroscopy; UV‐vis spectroscopy; high‐resolution transmission electron microscopy; scanning electron microscopy; X‐ray diffraction; elemental analysis; energy dispersive X‐ray spectroscopy; absorption spectra; spherical particles; crystalline particles; 2,2‐diphenyl‐1‐picrylhydrazyl free radical scavenging assay; strain identification; molecular characterisation method; rDNA sequencing; active biological components; cell‐free culture supernatant; wavelength 422 nm; size 5 nm to 20 nm; Ag
1 Introduction
Owing to their strong characteristic features, research have been focusing their attention on different application facilities of silver nanoparticles (AgNPs). Materials developed with AgNP incorporation have great optical, electrical, catalytic and antimicrobial properties with unique physicochemical and biochemical characteristics. Various techniques are being used to synthesise metal nanoparticles many of which are expensive and expose toxic chemical wastes [1].
Biological methods for green nanosynthetic routes are known to be environmentally safe, inexpensive and time saving applications thus they provide appreciable results for nanotechnology. Novel green synthesis strategies using various biological organisms have been recognised as a promising source for reducing metals by specific metabolic pathways [2].
Microorganisms of different taxonomic categories from fungi and prokaryotes such as Fusarium oxysporum [3], Aspergillus flavus [4], Bacillus licheniformis [5] and Klebsiella pneumoniae [6] have been used for AgNP biosynthesis through their cell mass or extracellular components.
Members of Actinobacteria isolated from various ecological environments were recently investigated as potential synthesisers of metallic nanoparticles [7]. Among the class Actinobacteria, the biosynthesis of AgNPs intra/extracellularly has been reported for Thermomonospora sp. [8], Rhodococcus sp. [9] and Streptomyces sp. [1, 10, 11, 12, 13, 14, 15, 16, 17].
Streptomyces is a genus of Gram‐positive aerobic bacteria that grows in various environments, with a filamentous form similar to fungi [18]. The Streptomyces species, members of the bacterial order Actinomycetales, are found worldwide in soil. Organisms from the genus Streptomyces produce effective bioactive compounds and are intensively used in the agricultural, pharmaceutical and enzyme industries. Therefore, they are well known for industrial manufacturing purposes [10, 19]. Though there is only one study about the AgNP biosynthesis from Streptomyces griseorubens isolated from marine sediment [17], the present investigation is the first report of the extracellular AgNP biosynthesis by using S. griseorubens isolated from soil.
This paper indicated that S. griseorubens AU2 isolated from soil have an excellent reducing capacity for the synthesis of AgNPs. The formation and morphology of the biosynthesised AgNPs were analysed and extensively characterised using spectral techniques such as ultraviolet–visible (UV–vis) and Fourier transform infrared spectroscopy (FT‐IR) spectroscopies, transmission electron microscopy (TEM), scanning electron microscopy (SEM) and X‐ray diffraction (XRD) analysis. Elemental analysis of the biosynthesised AgNPs were also performed via TEM and SEM equipped with energy dispersive X‐ray spectroscopy (EDS). The in vitro antioxidative potential of the biosynthesised AgNPs was performed by free radical scavenging activity.
2 Materials and methods
2.1 Isolation and molecular identification of Streptomyces griseorubens AU2
The actinomycete strain was isolated from soil at an orange farm located in Koycegiz district of Mugla Province in the Aegean region of Turkey. Soil sample was taken according to the method of Brown [20]. International Streptomyces Project 2 (ISP‐2) medium (10 g l−1 malt extract, 4 g l−1 yeast extract, 4 g l−1 glucose and 20 g l−1 agar, pH 7.3) with 2 g/l calcium carbonate addition was used to isolate the strain. The isolated S. griseorubens AU2 strain were maintained in 30% glycerol (v/v) at −20°C in the Mugla Sitki Kocman University Collection of Microorganisms (MU). For biosynthesis, pure S. griseorubens AU2 culture were maintained at 4°C in ISP‐2 agar plates and sub‐cultured continuously at regular intervals.
The strain S. griseorubens AU2 was identified with the sequence of 16S rDNA which was amplified by three primer couples as 925F ‐‐‐‐>5'‐AAA CTY AAA KGA ATT GAC GG‐3', 1491R ‐‐‐‐> 5'‐ACG GCT ACC TTG TTA CGA CTT‐3', 529F‐‐‐‐>5'‐GTG CCA GCM GCC GCG G‐3', 1099R‐‐‐‐>5'‐GGG TTG CGC TCG TTG‐3', 27F‐‐‐‐>5'‐AGA GTT TGA TCC TGG CTC AG‐3', 780R‐‐‐‐>5'‐ TAC CAG GGT ATC TAA TCC TGT T‐3'. The polymerase chain reaction (PCR) condition was adjusted accordingly initial denaturing for 2 min at 94°C, 30 s at 94°C, 30 cycles of 30 s at 53°C, 40 s at 72°C and a final 10 min extension at 72°C. Amplicons were purified and sequenced at BM Labosis Company (Ankara, Turkey). After sequencing all the fragments were aligned by using the software MEGA6 and 1484 bp of 16S rDNA was searched at Basic Local Alignment Search Tool algorithm in GenBank (http://www.blast.ncbi.nlm.nih.gov), and the bacteria were identified on the basis of their similarity to the 16S rDNA sequence.
2.1 Biosynthesis of AgNPs
The method described by Zonooz and Salouti [10] was slightly modified for microbial mediated synthesis of AgNPs. The pure S. griseorubens AU2 culture was inoculated on ISP‐2 broth under aseptic conditions and the flasks were incubated at 28°C and 130 rpm for 7 days. After the incubation period of 7 days completed, the culture was centrifuged at 4000 rpm for 20 min and the biomass was used as the main stock for the biosynthesis of AgNPs. For that purpose, the biomass was washed with dH2 O for three times. Then, the biomass was suspended in dH2 O and incubated at 28°C and 130 rpm for 48 h. At the end of the incubation period, the cell‐free culture supernatant that would be used for biosynthesis was obtained by centrifugation at 4000 rpm. About 10 ml of cell‐free supernatant and 50 ml Ag nitrate (AgNO3) solution (1 mM) (filtered through a 0.22 µm filter) were incubated at 28°C and 130 rpm for 48 h. After the incubation period, the solution was observed for visual colour change. The flasks turned from opaque white to brownish yellow indicated the presence of AgNPs.
2.2 UV–vis spectral analysis
Microbiologically synthesised AgNPs by S. griseorubens AU2 cell‐free culture supernatant was monitored by Multiskan GO UV–vis Microplate Spectrophotometer (Thermo Scientific, USA) within a range of 300–700 nm operated at a resolution of 1 nm. About 1 mM AgNO3 solution was used as control.
2.3 FT‐IR spectral analysis
Bioreduction of the Ag ion (Ag+) ions using the cell‐free culture supernatant of S. griseorubens AU2 was monitored by FT‐IR (Thermo Scientific Nicolet iS10‐ATR, USA) at a resolution of 4 cm−1 in potassium bromide (KBr) pellets and the spectra were recorded in the wavelength interval of 4000 and 400 nm−1.
2.4 Transmission electron microscopy
The size and morphology of the biosynthesised AgNPs were studied by high‐resolution TEM (HR‐TEM) using a JEM 2100F electron microscope at an accelerating voltage of 200 kV (JEOL, Japan). For preparing the TEM specimens, a drop of the aqueous suspension of AgNPs was placed onto a carbon‐coated copper grid and allowed to dry at room temperature. Images of the AgNPs were taken with a HR charge‐coupled device camera and the presence of Ag metals in the solution was analysed by EDS system (Oxford Instruments, UK) combined with HR‐TEM.
2.5 Scanning electron microscopy
For taking the SEM images of the biosynthesised AgNPs, a drop of suspension was placed on specimen stubs with double‐sided adhesive carbon tape and allowed to air dry at room temperature. SEM study was performed on a JSM 7600F field emission scanning electron microscope (JEOL, Japan) at an accelerating voltage of 15 kV. Similar to TEM, the presence of metallic Ag in the solution was analysed by EDS system (Oxford Instruments, UK) combined with SEM.
2.6 XRD measurement
To examine the crystal structure of biosynthesised AgNPs, XRD patterns were collected using Cu Kα monochromatic radiation (λ Cu = 1.54018 Å) at 40 kV/20 mA using continuous scanning 2θ mode on an X‐ray diffractometer (Rigaku‐SmartLab, Japan).
2.7 2,2‐Diphenyl‐1‐picrylhydrazyl (DPPH) free radical scavenging activity
The DPPH free radical scavenging assay was carried out to determine the antioxidant properties of the biosynthesised AgNPs [21]. The percentage of inhibition was calculated by the following equation; % DPPH scavenging effect = [(controlOD –sampleOD)/controlOD] × 100, where control was prepared without AgNPs. A strong antioxidant, ascorbic acid, was used as positive control.
3 Results and discussion
According to the morphological characterisation of the strain, it was an aerobic Gram‐positive actinomycete which grew well on ISP‐2 medium that the colour of aerial mycelium was white and the reverse side of the colony was grey (Fig. 1). Sequencing analysis of the strain S. griseorubens AU2 was performed on an ∼1484 bp PCR product and identified as S. griseorubens on the basis of 16S rDNA sequencing, which revealed 99% homology (GenBank Accession Number: KX156364).
Fig. 1.
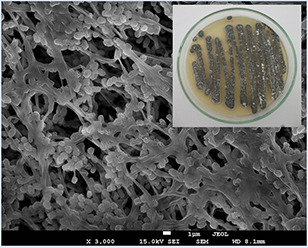
Colony morphology of the S. griseorubens AU2
In the present paper, microbial mediated biosynthesis of AgNPs were achieved extracellularly using the cell‐free supernatant of S. griseorubens AU2. Similar to the previous reports [1, 15, 22, 23], the formation of brown colour in the AgNO3 treated flasks indicated the Ag nanoparticle formation. There was no colour change in the control flasks (cell‐free supernatant without AgNO3 and the AgNO3 solution). The visual colour change may be due to the Ag+ reduction by secondary metabolites present in the cell‐free supernatant of the S. griseorubens AU2. Using the supernatant formed from the autolysed S. griseorubens AU2 biomass caused the formation of nanoparticles to occur in a clear solution, so there were not any mycelial materials, bacteria cell or medium in the reaction mixture [19].
Fig. 2 shows the UV–vis spectrum of the AgNPs following incubation for 24 h. UV–vis spectral analysis indicated that the AgNP solution has a strong and broad absorption peak at 422 nm, which is related to the surface plasmon resonance (SPR) band of AgNPs, as metal AgNPs have free electrons, which give rise to an SPR absorption band [24, 25]. The absorption peak at 422 nm is probably due to the excitation of longitudinal plasmon vibrations and formation of quasi‐linear superstructures of nanoparticles [26].
Fig. 2.
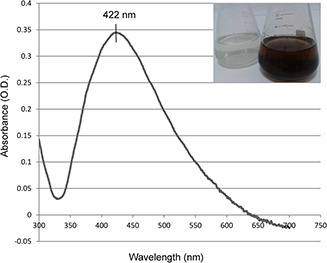
UV–vis spectrum of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2
FT‐IR sprectroscopy were performed to define the biomolecules that may be responsible for reduction of the Ag+ by cell‐free supernatant of S. griseorubens AU2. The FT‐IR spectra of the biosynthesised AgNPs peaks at 3408, 2892, 2360, 1577, 1383, 1020, 637 and 514 cm−1 are shown in Fig. 3. The presence of bands were due to O–H stretching (around 3408 cm−1), aldehydic C–H stretching (2892 cm−1), C = O group (1577 cm−1), C–C and C–N stretching (1383 cm−1) and O–H stretch (1020 cm−1). This FT‐IR result of synthesised AgNPs were quite similar to previous reports by Mohanta and Behera [22] and Karthik et al. [27]. When the metal nanoparticles are formed in the solution, they must be stabilised against the Van der Waals forces of attraction which may otherwise cause coagulation. FT‐IR analysis data confirms the presence of O–H stretching (around 3408 cm−1) which may have a key role of reducing metal ions to their respective nanoparticles [22, 28]. Our results are also in agreement with the results of Shanmugasundaram et al. [15] and Gopinath et al. [28] who suggested that proteins can bind to nanoparticles and stabilise AgNPs.
Fig. 3.
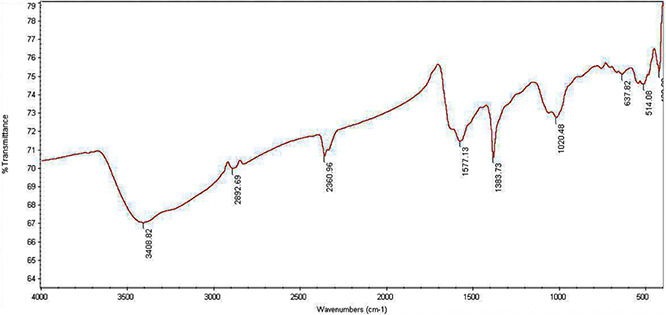
FT‐IR spectrum of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2
XRD pattern of the cell‐free supernatant of S. griseorubens AU2 treated with AgNO3 (Fig. 4) corresponded four intense peaks located over the range of 2θ values (i.e. 30–80°). A comparison of our XRD spectrum with the reported reference values of International Centre for Diffraction Data card number 01‐714‐613 provided by the instrument's library matched well and AgNPs were present as nanocrystals. The peaks at the 2θ values of 37.88°, 43.94°, 64.31° and 77.19° corresponded to the 1 1 1, 2 0 0, 2 2 0 and 3 1 1 cubic crystal lattice planes of Ag, respectively [10, 15, 28].
Fig. 4.
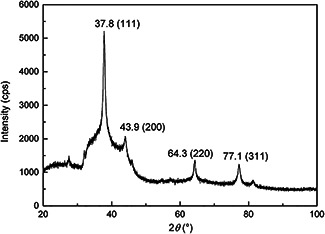
XRD pattern of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2
TEM and SEM are powerful methods to determine the size and morphology of nanostructures. Representative TEM images (Fig. 5) exhibit well‐dispersed AgNPs biosynthesised by cell‐free supernatant of S. griseorubens AU2. The images recorded at higher magnification displayed the detailed morphology and size of the biosynthesised AgNPs. The AgNPs were found to be spherical and exhibit particle sizes ranging from 5 to 20 nm.
Fig. 5.
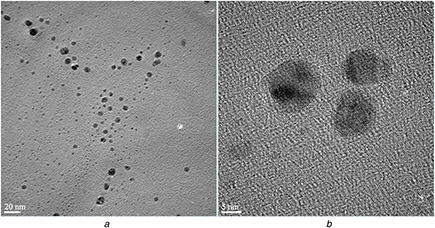
TEM images of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2. The scale bar corresponds
(a) 20 nm, (b) 5 nm
Elemental analysis of the biosynthesised AgNPs was investigated by EDS (Fig. 6) combined with HR‐TEM. The EDS linescan spectrum recorded a strong signal for Ag. The peaks corresponding to C, O, Si and Cu were most likely caused by X‐ray emission from the carbon‐coated copper grid.
Fig. 6.
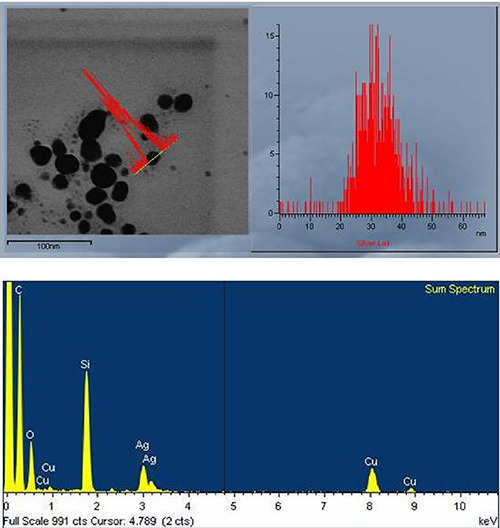
TEM‐EDS spectrum analysis of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2
Fig. 7 shows representative SEM of the AgNPs synthesised by S. griseorubens AU2. As it was confirmed using the TEM images, SEM also showed the presence of homogenous spherical AgNPs that are relatively uniform.
Fig. 7.
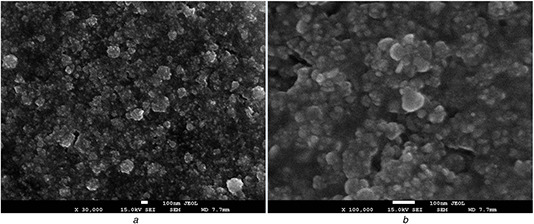
SEM images of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2. The scale bar corresponds
(a) ×30,000, (b) ×100,000
The EDS analysis applied over the SEM showed a strong Ag signal which confirms the presence and crystalline structure of metallic Ag (Fig. 8). Other signals of C, O and N atoms are maybe due to X‐ray emission from the remaining media [29].
Fig. 8.
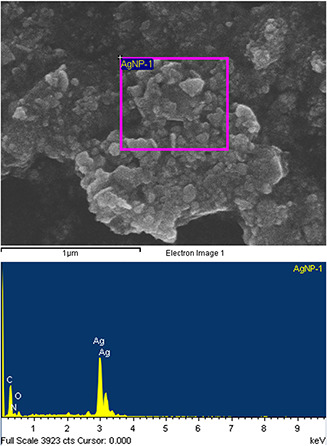
SEM‐EDS spectrum analysis of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2
TEM and SEM images of biosynthesised AgNPs are similar to the reports of Sadhasivam et al. [1] and Shanmugasundaram et al. [15] who biosynthesised AgNPs via Streptomyces hygroscopicus and Streptomyces naganishii MA7, respectively.
DPPH is a stable free radical which has a characteristic absorbance at 517 nm, but reduction by an antioxidant or a radical species decreases its absorption. Lower absorbance of the reaction mixture indicates higher DPPH free radical scavenging activity [30]. In this paper, various concentrations of 20, 40, 60, 80 and 100 µg/ml of biosynthesised AgNPs exhibited 9.66, 14.27, 15.59, 23.46 and 54.99% free radical scavenging activity, respectively (Fig. 9). When compared with standard ascorbic acid, the antioxidant activity of the biosynthesised nanoparticles was found to increase in a dose‐dependent matter. Similar results were also reported by Shanmugasundaram et al. [15]. Researchers who studied about plant‐mediated AgNP synthesis have also indicated that the antioxidant activity increases gradually with the increase in the treatment doses [31, 32, 33].
Fig. 9.
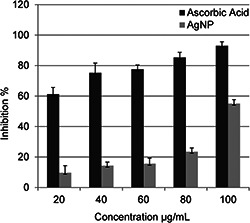
Antioxidant activity of AgNPs synthesised by cell‐free supernatant of S. griseorubens AU2
Among the scientific papers about the biogenic nanoparticle synthesis, it is suggested that DNA [34], sulphur‐containing proteins [35] and nicotinamide adenine dinucleotide (NADH)‐dependent nitrate reductase [3, 9, 36] are involved in the synthesis of AgNPs by the bioreduction of Ag+ to metallic Ag. Ahmad et al. [9] have indicated that NADH and NADH‐dependent nitrate reductase enzymes are important factors in the biosynthesis of metal nanoparticles. The mechanism of the biosynthesised nanoparticles involves the reduction of Ag+ by the electron shuttle enzymatic metal reduction process [36, 37]. In an AgNP biosynthesis study done by using extremophilic actinobacterium S. naganishii (MA7), Shanmugasundaram et al. [15] figured out that the partially purified enzyme (nitrate reductase) from the cell biomass of F. oxysporum was determined to be ∼44 kDa and their results showed that this enzyme was responsible for the synthesis of AgNPs. Chun et al. [38] reported that P450 (cytochrome P450) enzymes of Streptomyces genus may have key roles in biosynthesis and biotransformation reactions. Vaidyanathan et al. [39] explained that α ‐nicotinamide adenine dinucleotide phosphate‐dependent nitrate reductase in the microorganisms has been found to be responsible for the synthesis of metallic Ag and this enzyme is induced by nitrate ions and reduces Ag+ to metallic Ag. Alani et al. [19] suggest that different NADH‐dependent reductases may also be produced by Streptomyces sp. In the present paper, cell‐free supernatant of S. griseorubens AU2 could induce the synthesis of AgNPs from AgNO3. Thus, it is stated that the factors which took part in the biosynthesis of Ag nanoparticles are present in the cell‐free supernatant of S. griseorubens AU2.
4 Conclusion
Biosynthesis methods developed by utilisation of microorganisms have appeared as a non‐toxic and ecofriendly alternative to physical and chemical methods. This paper indicated that S. griseorubens AU2 has the ability to reduce Ag+ into Ag nanoparticles extracellularly. AgNPs were ecofriendly synthesised by the active metabolites of S. griseorubens AU2. It could be concluded that S. griseorubens AU2 cell‐free extract has a great potential to be used for antioxidant AgNP synthesis for industrial and pharmaceutical applications effectively. To our knowledge, this is the first report of microbial nanoparticle biosynthesis by S. griseorubens isolated from soil. Further research based on the S. griseorubens AU2 will involve investigation of a great number of useful bioactive products and diverse nanomaterials besides AgNPs.
5 Acknowledgment
This study is a part of Tuba Baygar's Ph.D. Thesis. Authors thank Assoc. Prof. Dr. Omer Simsek for molecular identification of the bacterium.
6 References
- 1. Sadhasivam S. Shanmugam P. Yun K.S.: ‘Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganism’, Colloids Surf. B, Biointerferences, 2010, 81, (1), pp. 358 –362 [DOI] [PubMed] [Google Scholar]
- 2. Ali M. Kim B. Belfield K.D. et al.: ‘Green synthesis and characterization of silver nanoparticles using Artemisia absinthium aqueous extract – a comprehensive study’, Mater. Sci. Eng. C, 2016, 58, pp. 359 –365 [DOI] [PubMed] [Google Scholar]
- 3. Ahmad A. Mukherjee P. Senapati S. et al.: ‘Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum ’, Colloids Surf. B, Biointerfaces, 2003a, 28, (4), pp. 313 –318 [Google Scholar]
- 4. Vigneshwaran N. Ashtaputre N.M. Varadarajan P.V. et al.: ‘Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus ’, Mater. Lett., 2007, 61, (6), pp. 1413 –1418 [Google Scholar]
- 5. Kalishwaralal K. Deepak V. Ramkumarpandian S. et al.: ‘Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis ’, Mater. Lett., 2008, 62, (29), pp. 4411 –4413 [Google Scholar]
- 6. Shahverdi A.R. Fakhimi A. Shahverdi H.R. et al.: ‘Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli ’, Nanomed. Nanotechnol. Biol. Med., 2007, 3, (2), pp. 168 –171 [DOI] [PubMed] [Google Scholar]
- 7. Verma V.C. Kharwar R.N. Gange A.C.: ‘Biosynthesis of noble metal nanoparticles and their application’, CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour., 2009, 4, (026), pp. 1 –17 [Google Scholar]
- 8. Ahmad A. Senapati S. Khan M.I. et al.: ‘Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp.’, Langmuir, 2003b, 19, (8), pp. 3550 –3553 [Google Scholar]
- 9. Ahmad A. Senapati S. Khan M.I. et al.: ‘Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species’, Nanotechnology, 2003c, 14, (7), p. 824 [Google Scholar]
- 10. Zonooz N.F. Salouti M.: ‘Extracellular biosynthesis of silver nanoparticles using cell filtrate of Streptomyces sp. ERI‐3’, Sci. Iranica, 2011, 18, (6), pp. 1631 –1635 [Google Scholar]
- 11. Kumar P.S. Balachandran C. Duraipandiyan V. et al.: ‘Extracellular biosynthesis of silver nanoparticle using Streptomyces sp. 09 PBT 005 and its antibacterial and cytotoxic properties’, Appl. Nanosci., 2015, 5, (2), pp. 169 –180 [Google Scholar]
- 12. Mohamedin A. El‐Naggar N.E.A. Shawqi Hamza S. et al.: ‘Green synthesis, characterization and antimicrobial activities of silver nanoparticles by Streptomyces viridodiastaticus SSHH‐1 as a living nanofactory: statistical optimization of process variables’, Curr. Nanosci., 2015, 11, (5), pp. 640 –654 [Google Scholar]
- 13. Samundeeswari A. Dhas S.P. Nirmala J. et al.: ‘Biosynthesis of silver nanoparticles using actinobacterium Streptomyces albogriseolus and its antibacterial activity’, Biotechnol. Appl. Biochem., 2012, 59, (6), pp. 503 –507 [DOI] [PubMed] [Google Scholar]
- 14. Shetty P.R. Kumar Y.S.: ‘Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus ’, J. Microbiol. Biotechnol., 2012, 22, (5), pp. 614 –621 [DOI] [PubMed] [Google Scholar]
- 15. Shanmugasundaram T. Radhakrishnan M. Gopikrishnan V. et al.: ‘A study of the bactericidal, anti‐biofouling, cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles’, Colloids Surf. B, Biointerfaces, 2013, 111, pp. 680 –687 [DOI] [PubMed] [Google Scholar]
- 16. Shanmugaiah V. Harikrishnan H. Al‐Harbi N.S. et al.: ‘Facile synthesis of silver nanoparticles using Streptomyces sp. VSMGT1014 and their antimicrobial efficiency’, Dig. J. Nanomater. Biostruct., 2015, 10, pp. 179 –187 [Google Scholar]
- 17. Vidhyashree N. Yamini Sudha Lakshmi S.: ‘Screening, isolation, identification, characterisation and applications of silver nanoparticles synthesized from marine actinomycetes (Streptomyces grieseorubens)’, World J. Pharma. Res., 2015, 4, (8), pp. 1801 –1820 [Google Scholar]
- 18. de Lima Procópio R.E. da Silva I.R. Martins M.K. et al.: ‘Antibiotics produced by Streptomyces’, Braz. J. Infect. Dis., 2012, 16, (5), pp. 466 –471 [DOI] [PubMed] [Google Scholar]
- 19. Alani F. Moo‐Young M. Anderson W.: ‘Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus’, World J. Microbiol. Biotechnol., 2012, 28, (3), pp. 1081 –1086 [DOI] [PubMed] [Google Scholar]
- 20. Brown J.C.: ‘Soil fungi of some British sand dunes in relation to soil type and succession’, Ecology, 1958, 46, pp. 641 –664 [Google Scholar]
- 21. Blois M.S.: ‘Antioxidant determinations by the use of a stable free radical’, Nature, 1958, 181, pp. 1199 –1200 [Google Scholar]
- 22. Mohanta Y.K. Behera S.K.: ‘Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by Streptomyces sp. SS2’, Bioprocess Biosyst. Eng., 2014, 37, (11), pp. 2263 –2269 [DOI] [PubMed] [Google Scholar]
- 23. Singh P. Kim Y.J. Singh H. et al.: ‘Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles’, Int. J. Nanomed., 2015, (10), pp. 2567 –2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noginov M.A. Zhu G. Bahoura M. et al.: ‘The effect of gain and absorption on surface plasmons in metal nanoparticles’, Appl. Phys. B, 2007, 86, (3), pp. 455 –460 [Google Scholar]
- 25. Wang C. Kim Y.J. Singh P. et al.: ‘Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity’, Artif. Cells Nanomed. Biotechnol., 2015, 44, (2), pp. 1127 –1132 [DOI] [PubMed] [Google Scholar]
- 26. Shankar S.S. Ahmad A. Sastry M.: ‘Geranium leaf assisted biosynthesis of silver nanoparticles’, Biotechnol. Progress, 2003, 19, (6), pp. 1627 –1631 [DOI] [PubMed] [Google Scholar]
- 27. Karthik L. Kumar G. Kirthi A.V. et al.: ‘ Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application’, Bioprocess Biosyst. Eng., 2014, 37, (2), pp. 261 –267 [DOI] [PubMed] [Google Scholar]
- 28. Gopinath V. MubarakAli D. Priyadarshini S. et al.: ‘Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach’, Colloids Surf. B, Biointerfaces, 2012, 96, pp. 69 –74 [DOI] [PubMed] [Google Scholar]
- 29. Zaki S. El Kady M.F. Abd‐El‐Haleem D.: ‘Biosynthesis and structural characterization of silver nanoparticles from bacterial isolates’, Mater. Res. Bull., 2011, 46, (10), pp. 1571 –1576 [Google Scholar]
- 30. Bhakya S. Muthukrishnan S. Sukumaran M. et al.: ‘Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity’, Appl. Nanosci., 2015, 6, (5), pp. 755 –766 [Google Scholar]
- 31. Dipankar C. Murugan S.: ‘The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts’, Colloids Surf. B, Biointerfaces, 2012, 98, pp. 112 –119 [DOI] [PubMed] [Google Scholar]
- 32. Swamy M.K. Sudipta K.M. Jayanta K. et al.: ‘The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract’, Appl. Nanosci., 2015a, 5, (1), pp. 73 –81 [Google Scholar]
- 33. Swamy M.K. Akhtar M.S. Mohanty S.K. et al.: ‘Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2015b, 151, pp. 939 –944 [DOI] [PubMed] [Google Scholar]
- 34. Feng Q.L. Wu J. Chen G.Q. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus’, J. Biomed. Mater. Res., 2000, 52, (4), pp. 662 –668 [DOI] [PubMed] [Google Scholar]
- 35. Morones J.R. Elechiguerra J.L. Camacho A. et al.: ‘The bactericidal effect of silver nanoparticles’, Nanotechnology, 2005, 16, (10), p. 2346 [DOI] [PubMed] [Google Scholar]
- 36. Kalimuthu K. Babu R.S. Venkataraman D. et al.: ‘Biosynthesis of silver nanocrystals by Bacillus licheniformis ’, Colloids Surf B, Biointerfaces, 2008, 65, pp. 150 –153 [DOI] [PubMed] [Google Scholar]
- 37. Chauhan R. Kumar A. Abraham J.: ‘A biological approach to the synthesis of silver nanoparticles with Streptomyces sp JAR1 and its antimicrobial activity’, Sci. Pharm., 2013, 81, (2), p. 607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chun Y. Shimada T. Waterman M.R. et al.: ‘Understanding electron transport systems of Streptomyces cytochrome P450’, Biochem. Soc. Trans., 2006, 34, (6), pp. 1183 –1185 [DOI] [PubMed] [Google Scholar]
- 39. Vaidyanathan R. Gopalram S. Kalishwaralal K. et al.: ‘Enhanced silver nanoparticle synthesis by optimization of nitrate reductase activity’, Colloids Surf. B, Biointerfaces, 2010, 75, (1), pp. 335 –341 [DOI] [PubMed] [Google Scholar]


