Abstract
In recent years nanotechnology has become increasingly important in almost every field. The new and improved physical, chemical and biological properties of material at nanoscale have far reaching implications in the fields of science and technology. Nanoparticles’ effect on various plant species must be investigated to develop a comprehensive toxicity profile for nanoparticles. The current study strives to evaluate the effects of nine types of metal nanoparticles including monometallic and bimetallic alloy nanoparticles [Ag, Au, Cu, AgCu (1:3), AgCu (3:1), AuCu (1:3), AuCu (3:1), AgAu (1:3), AgAu (3:1)] on seed germination, root and shoot growth and biochemical profile of Silybum marianum plant. Seed germination was greatly affected and increased significantly upon treatment with nanoparticles’ suspensions and was recorded highest for Ag nanoparticle suspension. Metal nanoparticles also had a significant effect on the biochemical profile of S. marianum. For the first week, the effect on DPPH, total phenolics content, total flavonoids content, total protein content, peroxidase activity and superoxide dismutase activity was enhanced, but declined as the time progressed. Among the nanoparticles being used, the effect of Ag nanoparticle was mostly enhancing. The results obtained are significant in mapping the effects of different monometallic and bimetallic nanoparticles on medicinal plant species.
Inspec keywords: silver, gold, copper, silver alloys, gold alloys, copper alloys, nanoparticles, nanofabrication, nanobiotechnology, biochemistry, bimetals, enzymes, molecular biophysics, suspensions, toxicology
Other keywords: Au, Cu, AgCu, AuCu, AgAu, Ag, medicinal plant species, superoxide dismutase activity, peroxidase activity, total protein content, total flavonoids content, DPPH, total phenolics, nanoparticle suspensions, shoot growth, root growth, comprehensive toxicity profile, plant species, nanoparticle effect, nanoscale material, biological properties, chemical properties, physical properties, nanotechnology, bimetallic alloy nanoparticles, monometallic alloy nanoparticles, Silybum marianum, biochemical profile, seed germination
1 Introduction
Nanotechnology envelopes engineering, science, and application of submicron materials, involves the use of unique chemical, physical and biological properties of materials at nanoscale in fundamentally new and most useful ways. Nanotechnology is a rapidly developing industry with huge potentials in almost every field, whether it is economics, society, environment or medicinal plant biotechnology. As a result of these attributes, nanotechnology generates both negative as well as positive responses from scientists, governments and social media all over the world [1, 2, 3, 4, 5, 6]. It is important to note here that US federal government alone has invested $17.9 billion from financial year (FY) 2001 through FY 2013 in their National Nanotechnology Initiative (NNI) and President Obama has requested $1.7 billion in NNI funding for the year 2014 [7]. Currently many nanotechnology products are available in markets. Nanomaterials that can emerge at a scale of 1 to100 nanometres are increasingly used for the manufacture of fillers, catalysts, cosmetics, drug carriers, semiconductors, opacifiers, catalysts and microelectronics, etc. [8]. However, a key issue regarding nanotechnology is the protection of humans and the safety of the environment as the nanoscale material is increasingly being researched, produced, used and discarded into the soil, air or water.
There are many different ways to classify nanoparticles. According to Risk Management Services of University of North Texas, five main categories into which nanoparticles can be divided are: fullerenes and carbon nanotubes, metal nanoparticles, ceramics, semiconductors (quantum dots) and polymeric nanoparticles. These nanoparticles are also termed as engineered nanoparticles and according to US environmental protection agency USEPA (2005) [9] report the engineered nanoparticles can be grouped into four types: (i) Metal based nanoparticles such as quantum dots, nanogold, nanosilver, nanocopper, nanozinc, and nanoscale metal oxides; (ii) Dendrimers; (iii) Carbon based nanoparticles including single and multi‐walled carbon tube and fullerene (iv) Composites that conjoins nanoparticles with either other types of nanoparticles or with bulk material.
Nanoparticles fall in a transitional zone between atoms and molecules and the corresponding bulk material, which can be the cause of nanoparticles’ unique properties as compared with the bulk material. The effect may range from positive to negative depending upon the sizes, shapes and even the nature of the bulk material from which they are originally synthesised. There has been a considerable amount of research on studying the toxicology of the nanoparticles. A few of the researchers have shown how nanoparticles such as carbon nanotubes, fullerene and metal oxides affect human cells, rodents and bacteria [10, 11, 12, 13, 14]. However, by large the mechanism of action of these nanomaterials is unknown to us, though it is possible that they may cause their toxic effect due in part by the production of reactive oxygen species (ROS) or by producing the oxidative stress [15]. In addition, very little research has been conducted so far to assess the potential toxic effects of metal nanoparticles on terrestrial test species (wildlife, plants, soil invertebrates and soil microorganisms) [9].
As mentioned earlier, the effect of metal nanoparticles on plants can be positive or negative and depends upon many different factors. In most cases the toxic as well as the positive effect of nanoparticles can be due to the chemical toxicity based on the chemical composition (e.g. the release of toxic metal ions), or may be due to the stress or stimuli caused by the surface area, size or shape of the nanoparticles [10]. It is known that at nanoscale the properties of material differ significantly from the corresponding bulk material which may lead to the increased bioavailability and thus toxicity [15]. Furthermore, metal nanoparticles may be toxic because of the production of reactive hydroxyl radicals due to visible light extracellular ROS that in turn can damage the cellular membrane and thus increases the permeability of the membrane which consequently leads to greater probability of entry of the nanoparticles into the cell [16]. In one of the studies positive effects on the seed germination and growth of the seedlings were reported by Yang et al. [17] when the aged seeds of spinach were soaked in high strength TiO2 – nanoparticles‐solution (0.25–4%) while the best results were obtained by application of 2500 mg/dm3 nano‐TiO2. The TiO2 NPs promoted the growth of spinach plant and nitrogen assimilation in these plants. In another study while investigating the phytotoxicity of nanoscale alumina (nano‐Al2 O3) powders with or without phenanthrene coating, Yang and Watts [18] reported that uncoated particles inhibited root elongation of corn, soybean, cucumber, carrot and cabbage. This study had a huge impact as many in media and policy making used this study as a base to conclude that metal nanoparticles have negative effects on plant growth and development.
All the above mentioned studies and many others, expand our knowledge about nanotoxicology to some extent. There still exist many unresolved issues related to the toxic effects of some metal nanoparticles on plants. As mentioned earlier too that some nanoparticles may have positive effects on plants which may lead from enhanced seed germination to better growth of some plant organs. These attributes of nanoparticles can be more or less due to the size, shape, surface area or nature of the material from which they are formed. The experimental design in this regard needs great attention so that a defensible scientific understanding can be produced on biological effect of nanoparticles [19]. Therefore, this study is being conducted with the aim to provide a clear understanding of the effects of some metals, in the form of their monometallic and bimetallic alloy nanoparticles on the seed germination frequency, subsequent growth and biochemical profile of a medicinally important plant Silybum marianum. In this study metal nanoparticles suspensions were used to soak the plant seeds prior to their germination and growth under controlled conditions.
2 Materials and methods
2.1 Seeds
Seeds of S. marianum plant were obtained from Plant Cell Culture laboratory, Quaid‐i‐Azam University, Islamabad. The plant seeds were originally collected from within Quaid‐i‐Azam University's main campus, where wild S. marianum plants are found in abundance. S. marianum is famous for a medicinal compound silymarin. S. marianum and its active compound silymarin is known for its medicinal values in treating liver diseases, several anti‐inflammatory, including prostaglandin synthesis and inhibition of leukotriens, inhibition of neutrophil migration, Kupffer cell inhibition, and mast cell stabilisation [20, 21, 22, 23]. Preliminary experiments were conducted to check the viability of seeds and it was observed that average germination rate of all plant seeds was greater than 85%. The seeds were kept in dry, dark place at room temperature.
2.2 Nanoparticles suspension preparation
The nanoparticles were provided by the department of chemistry, Quaid‐i‐Azam University, Islamabad. Nanoparticles were prepared and characterised by using environment friendly polyol process by Rahman et al. [24] at Peshawar University. Monometallic and bimetallic alloy nanoparticles were synthesised chemically and data regarding the ratios of nanoparticles and its TEM and EDS graphs were published by Rahman et al. [25, 26, 27]. Sizes of the nanoparticles were calculated by Debye scherrer equation and are reported in Table 1. Nanoparticles suspension was prepared by directly suspending 30 µg/ml of monometallic and bimetallic alloy nanoparticles in deionised water (DI‐water) and dispersing them by ultrasonic vibration (100 W, 40 kHz) for 30 min. After dispersing nanoparticles in DI‐water a magnetic bar was placed in the suspension and the suspension was stirred for 5–10 min prior to its use on a magnetic stirrer in order to avoid aggregation of particles [28]. Nine different nanoparticles including monometallic and bimetallic alloy nanoparticles of different sizes were used, namely: Ag, Au, Cu, AgAu (1:3), AgAu (3:1), AuCu (1:3), AuCu (3:1), AgCu (1:3), and AgCu (3:1). Scanning electron microscope (SEM) graphs of all nine nanoparticles are shown in Fig. 1.
Table 1.
Sizes of metallic and bimetallic alloy nanoparticles calculated by Debye Scherrer formula
| Designation in the fig | Nanoparticles | Sizes, nm |
|---|---|---|
| Monometallic | ||
| A | Ag | 30 |
| B | Cu | 24 |
| C | Au | 35 |
| Bimetallic | ||
| D | AgAu 1:3 | 48 |
| E | AgAu 3:1 | 43 |
| F | AuCu 1:3 | 36 |
| G | AuCu 3:1 | 40 |
| H | AgCu 1:3 | 18 |
| I | AgCu 3:1 | 28 |
Fig. 1.
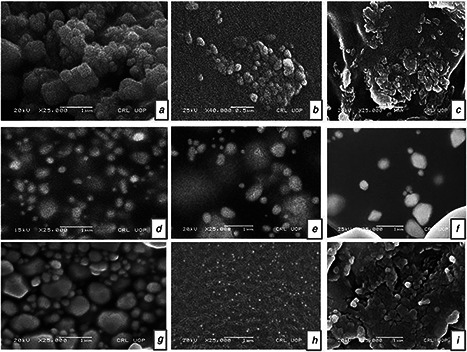
SEM micrograph of metal nanoparticles
a Ag nanoparticles
b Cu nanoparticles
c Au nanoparticles
d AgAu 1:3
e AgAu 3:1
f AuCu 1:3
g AuCu 3:1
h AgCu 1:3
i AgCu 3:1
2.3 Seed germination
Seeds of S. marianum were surface sterilised prior to soaking in nanoparticles suspensions. For sterilisation, seeds were surface sterilised in 70% ethanol for 1 min and then immersed in 0.1% (w/v) mercuric chloride for 60 s and then finally rinsed three times with sterilised DI‐water [29]. The sterilised seeds were then soaked in nanoparticles suspensions for 2 h. After soaking seeds were transferred aseptically onto solidified 4.4 g/l Murashige and Skoog [30] basal medium in laminar flow hood fitted with HEPA filter.
2.4 Plant growth and sample collection
60 flasks were inoculated with each flask carrying 4 S. marianum's seeds in such a way that six flasks were inoculated with seeds that were not presoaked in any of the nine nanoparticles suspensions and were used as control, the rest, that is, 54 flasks contained the seeds that were presoaked in nanoparticles suspensions. The inoculated flasks were then kept in dark for first 14 days to enable seed germination. The experiment was conducted for 6 weeks and samples for biochemical analysis were collected on weekly basis.
2.5 Analytical methods
The seeds of S. marianum were exposed to nine different nanoparticles suspensions (30 µg/ml) in DI‐water to check their effect on seed germination and subsequent growth of the plantlets. The presoaked seeds were raised on MS0 media and germination frequency for different treatments was calculated using the formula: G.F = (number of seeds germinated/total seeds inoculated) × 100.
To prepare extracts for enzyme activities a method proposed by Nayyar and Gupta [31] was used however with some modifications. 1 g of plant tissue was homogenised with 10 ml of extraction buffer [50 mM potassium phosphate buffer containing 1% PVPP, buffer PH 7]. The homogenate thus obtained was centrifuged at 15,000 g at 4°C for 30 min and the supernatant was either directly used for analysis or stored at 4°. The respective absorbance was worked out with a regression curve of standard solutions of various concentrations.
Samples for antioxidant activity were prepared by pulverising dried plant material in chilled pestle and mortar with liquid nitrogen. 0.1 g of powdered plant material mixed with 1 ml of 100% pure methanol. The solution was kept for 5 min and then vortexed for 5 min, sonicated (30 min; Toshiba; Japan) and then centrifuged (13,000 rpm, 10 min). The supernatant was then used for analysis either immediately or was stored at 4°C for use later.
To determine the antioxidant activity resulting from the stress induced by nanoparticles a method as described by Lee et al. [32] was used, though with some modifications. Micro‐plate reader was used for antioxidant activity determination and absorbance was measured at 515 nm.
The radical scavenging activity (RSA) was determined on the basis of percentage DPPH discoloration using the following formula:
% DPPH free RSA = (Ab c −Ab s ⁄Ab c ) × 100
In the above Ab c is the absorbance of negative control, whereas Ab s is the absorbance of the DPPH solution with sample (methanolic extracts) added.
To determine total phenolic content a method described by Velioglu et al. [33] was used by using Folin–Ciocalteu reagent. Absorbance was noted at 630 nm by using UV/VIS–DAD spectrophotometer (Halo DR‐20, UV–VIS spectrophotometer, Dynamica Ltd., Victoria, Australia). A calibration curve (0–50 µg/ml, R2 = 0.968) was worked out using gallic acid as standard. The total phenolic content (TPC) was expressed in µg⁄mg gallic acid equivalents of dry weights and all the determinations were performed in triplicates.
To determine total phenolics content aluminium chloride colorimetric method described by Chang et al. [34] was used. Reaction mixture absorbance was noted at 405 nm using UV/VIS‐DAD spectrophotometer. Quercetine was used as standard in plotting a calibration curve (0–40 µg/ml, R2 = 0.998). The TPC was expressed in terms of µg⁄mg quercetin equivalents of dry weight.
A method of Lowry et al. [35] with some modifications was used to determine the total protein content. Spectrophotometer (Shimadzu, UV‐120‐01) was used to measure the absorbance at 650 nm. A standard curve of BSA was prepared and the unknown protein from the sample was determined from the curve. The extracts for antioxidant enzyme activity were prepared by using a method of Nayyar and Gupta [31]. Peroxidase (POD) activity was performed by using a method of Lagrimini [36] with some modifications and the protease activity was determined by using a method of Mcdonald and Chen [37].
2.6 Statistical analyses
Triplicate samples were used in all experiments, and the experiments were repeated twice. All data were the mean ± standard deviation. The data were subjected to a one‐way analysis of variance. Tukey‐HSD test was used for calculation of significant differences. Using SPSS (for Windows, standard version 7.5.1 by SPSS Inc. Chicago), P < 0.05 value was regarded as significant.
3 Results
3.1 Effects of nanoparticles suspensions on seed germination and average root and shoot length
Germination frequency was calculated after a preliminary 2 week incubation period in complete darkness to boost up seed germination and then subsequent transfer to 16/8 h photoperiod. All seeds were grown on solidified MS0 (Murashige and Skoog) media [30]. The effects of nanoparticles suspensions (30 µg⁄ml) on seed germination and average root and shoot length are shown in Figs. 2 and 3. Seeds without presoaking were taken as control. Percentage germination for control seeds was observed to be significantly low as compared with the seeds being treated with nanoparticles suspensions. Maximum percentage for seed germination was observed in seeds presoaked in Ag nanoparticles suspension, while the minimum percentage of seed germination was seen for control seeds. Besides, the percentage germination frequency was greatly enhanced for presoaked seeds as compared with control seeds.
Fig. 2.
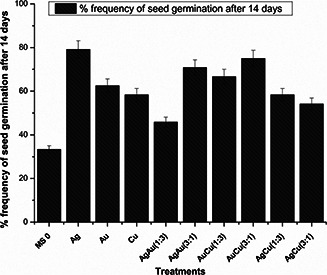
Percentage germination frequency of S. marianum seeds after 14 days
Fig. 3.
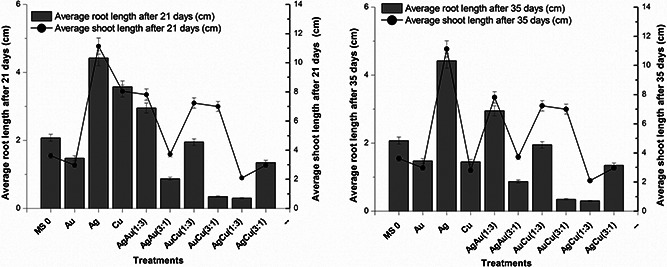
Average root and shoot lengths after 21 and 35 days of S. marianum treated with metallic and bimetallic nanoparticles
The influence of nanoparticles suspensions (30 µg⁄ml) on roots and shoots length varied widely depending upon both the nanoparticles and the number of days of growth of plant passed. As previously stated that the experiment was conducted for 42 days and samples were collected for analysis after 21 and 35 days. After the first 21 days the highest mean shoot length in S. marianum was noted at 4.4 cm for monometallic Ag (silver) nanoparticles suspension, and the lowest value of mean shoot length was observed for bimetallic nanoparticles suspension, AgCu (1:3) that was 0.3 cm. Similarly, average root length after 21 days was also highest for Ag nanoparticles suspension, which was 4.7 cm and average root length was lowest for AgCu (1:3), that is, 0.9 cm. Astoundingly, the pattern remained the same for Ag nanoparticles suspension and AgCu (1:3), which clearly indicates that AgCu (1:3) nanoparticles suspension is phytotoxic in S. marianum and Ag nanoparticles suspension is enhancing in its effect on S. marianum. All in all, after 21 and 35 days monometallic nanoparticles suspensions had positive influence while bimetallic nanoparticles suspensions had negative effect except AgAu (1:3) that had somewhat positive influence on mean root and shoot lengths in S. marianum. Therefore, it is safe to imply that bimetallic nanoparticles exhibited phytotoxicity as compared with the positive effects of monometallic nanoparticles.
3.2 Total flavonoid content (TFC), TPC, and antioxidant activity
In DPPH assay, different nanoparticles suspensions (each, 30 µg/ml) differently affected RSA in S. marianum. Seeds presoaked in AgCu (1:3) nanoparticles suspension showed the greatest 83.85% DPPH activity after 21 days. An increase in % DPPH was also observed after 28 days for all nanoparticles suspensions except for AuCu (3:1). Later after 35 and 42 days the value of % DPPH for control was greater than the nanoparticles suspensions (Fig. 4). Nanoparticles suspensions affected total phenolic and flavonoid content in S. marianum differently. Total phenolics and total flavonoids were studied in relation with the dry mass of the plant material and it was observed that dry mass did not significantly affect the amount of phenolics and flavonoids produced. However, longevity of the experiment significantly affected the amount of total flavonoids and phenolics in S. marianum. It is observed (Fig. 5) that after first 21 days greater phenolics and flavonoids accumulation occurred in S. marianum plantlets, presoaked in bimetallic alloy nanoparticles suspensions, whereas monometallic metal nanoparticles suspensions caused a reduction in total phenolics and flavonoids contents. Maximum total phenolics and flavonoids accumulation was observed after 28 days of experiment which stood at 13.04 µg GA equiv/mg DW and 0.76 µg Qu equiv/mg DW, respectively. Au nanoparticles caused maximum total phenolics accumulation after 28 days while Cu nanoparticles caused maximum total flavonoids accumulation in S. marianum. Hence the maximum total phenolics and flavonoids accumulation was mainly due to monometallic nanoparticle suspensions. As experiment matures further the pattern changes and resembles to some extent with the pattern observed after 21 days. Minimum accumulation of total phenolics and total flavonoids was observed after 42 days of experiment for AuCu (1:3) NPs.
Fig. 4.
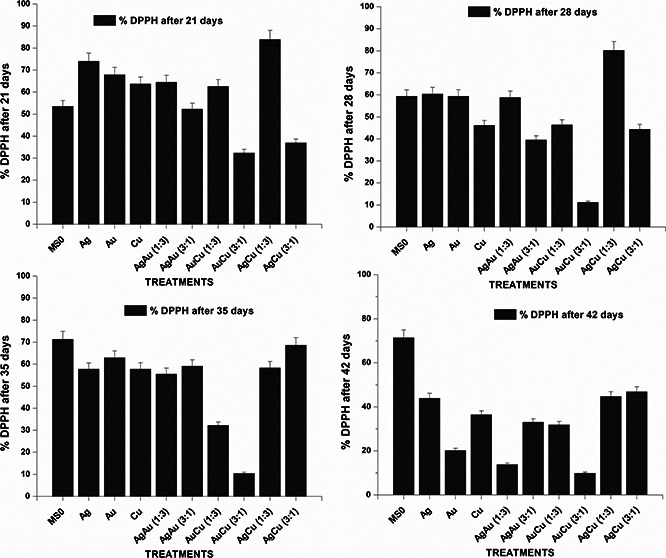
Percentage DPPH RSA of S. marianum plantlets treated with metallic and bimetallic nanoparticles after 21, 28, 35 and 42 days
Fig. 5.

Dry weight in relation to total phenolics and flavonoids content after 21, 28, 35 and 42 days
3.3 Total protein content, POD and protease activity
Greatest amount of total protein content was detected on 28th day for AuCu (1:3) nanoparticle suspensions which was 439.8 µg BSA equiv/mg FW while the lowest 110.6 µg BSA equiv./mg FW for AgCu (1:3) after 42nd day (Fig. 6). Highest protease activity (0.19 nM/min/mg FW) was observed on 28th day of experiment for AgCu (1:3) nanoparticles suspension at 30 µg/ml as compared with the lowest (0.023 nM/min/mg FW) for AgCu (3:1) on the same day. After that the value went down significantly. Interestingly, the highest POD activity was noted on 42nd day (0.278 nM/min/mg FW) for Ag nanoparticle suspension, a monometallic nanoparticle and lowest (0.02 nM/min/mg FW) for AgCu (1:3), a bimetallic alloy nanoparticle, on 28th day (Fig. 7).
Fig. 6.
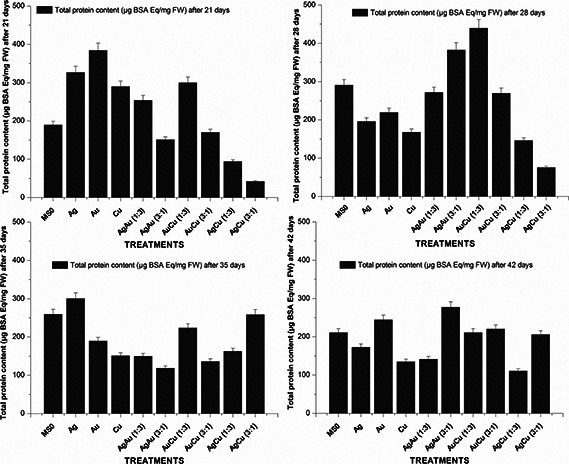
Total protein content of fresh matter of 21, 28, 35 and 42 days old S. marianum plantlets treated with metallic and bimetallic nanoparticles
Fig. 7.
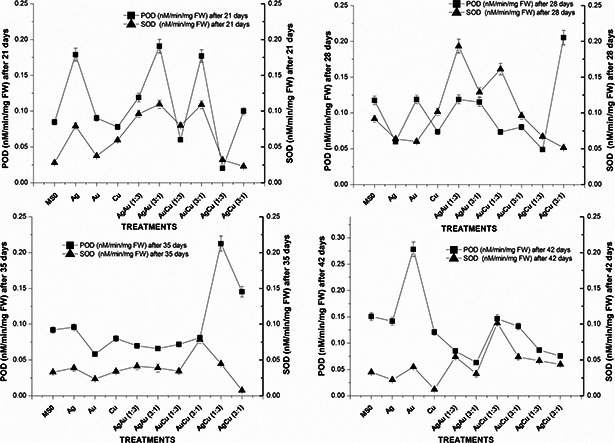
Superoxide dismutase and POD activity of 21, 28, 35, and 42 days old S. marianum plantlets treated with metallic and bimetallic nanoparticles
4 Discussion
In recent years nanotechnology has shown its true potential in almost every field. Nanotoxicology has now emerged as a separate discipline with huge potential as has been originally quoted by Donaldson et al. [38] that the discipline of nanotoxicology would make an important contribution to the development of a sustainable and safe nanotechnology. Very few nanoparticles such as SWCNT, fullerene and TiO2 have been used as test material to reveal their nanotoxicology mechanisms. In one study Lin and Xing [28] analysed the effects of five types of multiwalled nanoparticles on seed germination and root elongation of six higher plants (Raphanus sativus, Brassica napus, Lolium multiflorum, Lactuca sativa, Zea mays and Cucumis sativus). There was no significant effect observed on seed germination except for Zn nanoparticles in Lolium multiflorum and ZnO nanoparticles in Zea mays. Root inhibition varied widely among nanoparticles and plants and it appeared to be partially correlated to nanoparticle concentration [39]. Authors proposed that inhibition of root growth may be due to the seed incubation process rather than presoaking stage. In this study, however, the effects of different nanoparticles suspensions (30 µg/ml, each) on S. marianum are largely positive with Ag nanoparticles as forerunners in this enhancing effect.
There are many ways to assess phytotoxicity of nanoparticles. The germination of seeds and root elongation is a fast and accurate phytotoxicity test with ample advantages: cost effective, rapid, simple, sensitive and more suitable for unstable chemicals [40]. There are many definitions of seed germination depending upon root length: outgrowth of root >1 mm or >5 mm [41, 42, 43]. Seed germination is regarded as a physiological process beginning with the inhibition of water and ending in the emergence of roots [41]. In this study, seeds were considered to be germinated upon emergence of radicles coming out of their seed coats.
In this study the seed germination was greatly affected by nanoparticle suspensions. This is completely in line to suggest that nanoparticles were allowed the seed coats to pass, probably because of their small size. Chemical composition of nanoparticles is key element to evaluate the toxicity of metal nanoparticles on plants and also the stress caused by surface, shape and size of particle [44]. These nanoparticles were taken up by the seeds during presoaking and later boosted the germination frequency to a level higher than the control. All of the nanoparticles used in this study had positive effect on germination frequency; but Ag and AuCu (3:1) were more enhancing in their effects, which can be due to their size. Both are medium sized nanoparticles (Ag, 30 nm and AuCu (3:1), 40 nm). This positive effect of Ag and AuCu (3:1) can also be attributed to the nature of the bulk material of which they are derived, because in case of AuCu (1:3) [36 nm] germination frequency is not that much enhanced. This may be due to the reason that Au is probably more suitable to promote seed germination than Cu‐particularly when in greater proportion as compared with the latter.
Presoaking of S. marianum seeds in nanoparticle suspensions had a significant effect on root and shoot lengths. The data on root and shoot lengths were collected after 21 and 35 days and it were astoundingly similar to each other. Silver (Ag) nanoparticles had the most positive impact on the overall shoot and root development. Another medium‐sized nanoparticle AuCu (1:3) also enhanced the growth of root and shoot in S. marianum; it was followed by Cu nanoparticles‐a small‐sized nanoparticle. However, the overall pattern of root and shoot growth remained similar throughout the longevity of the experiment. Mechanisms underlying both; toxicology of nanoparticles and their positive effect on some plants largely remain unknown; it seems to be closely related to the chemical structure, chemical composition, particle size and surface area of the nanoparticles.
Nanoparticles can be toxic to plants in two different ways: (i) toxic effect based on their chemical composition, for example, release of (toxic) ions; (ii) Stimulus or stress caused by the surface area, and shape of the nanoparticles [12]. As Plants possess cell walls as a primary interacting site for foreign particles so entry of nanoparticles becomes difficult. The mechanism by which nanoparticles enters the plants is still poorly defined. Yet, nanoparticles have ability to magnify changes in cell structures and molecules and also the defensive mechanisms. Nanoparticles effect is based on its physical and chemical properties and ROS production and solubility of toxic nanoparticles is important in this regard [45]. In this study enhanced DPPH activity in the first 2 weeks (i.e. after 21 and 35 days) of the experiment confirms that the nanoparticles induced significant amount of stress in plants in the first few days and effect of stress induced faded out with the passage of days. AgCu (1:3) caused the greatest stress and as a result DPPH activity enhanced. It is important to note that this nanoparticle was the smallest in size, that is, 18 nm. Another important factor is the composition of nanoparticles; bimetallic alloy nanoparticles were less stress inducing than monometallic nanoparticles, however, with exception of AgCu (1:3) nanoparticles whose size was the defining factor in stress induction.
In this study, total phenolics and total flavonoids in relation to dry weight of the plant material were studied and the result varied greatly depending upon the longevity of the experiment. It is clear from Fig. 5 that the dry weight did not have any significant effect on the concentration of total phenolics and total flavonoids. It can be concluded after deliberation that the increase or decrease in the concentration of total phenolics and total flavonoids in the present study were the attributes of the nanoparticles and not the relative increase or decrease in dry weight of the material. Importantly, smallest [AgCu (1:3), size 18 nm] and largest sized [AgAu (1:3), AgAu (3:1), sizes 48 and 43 nm, respectively] nanoparticles resulted in increase in concentration of total phenolics in S. marianum after 21 days. However the case with total flavonoids content was a little different as all the nanoparticles, except for a few, caused the increased accumulation of total flavonoids in dried plant material as compared with control samples. With the passage of days, medium sized nanoparticles started exerting stress and the graph for total phenolics and flavonoids went up for medium sized nanoparticles after 28 days. It was after 28 days that the highest value of total phenolics was recorded in S. marianum plants being treated with Au NPs‐the medium sized nanoparticles. After 35 and 42 days the effect of nanoparticles was reduced significantly on TPC and TFC contents as compared with control. This can be due to the fact that in the beginning of the experiment nanoparticles, during presoaking, exerted stress on the plant embryo and as the plant grew the effect faded. Moreover toxic nanoparticles may increase the production of ROS and hydroxyl radicals that damage the cell membranes and as a result permeability is altered. As a result entry of nanoparticles into plant cells become easier and stress induced by the particles results in secondary metabolites production [16].
In this study, the effect of nanoparticles suspensions on plant total protein content, protease and POD activities was also evaluated for the first time. It was found that the total protein content in S. marianum was greatest‐for a medium‐sized bimetallic alloy AuCu (1:3) nanoparticle suspension‐and remained at 439.8 µg BSA equiv./mg FW after 28 days. It can probably be due to its medium size and its chemical composition. The lowest amount of total protein content was noted after 42 days for AgCu (1:3). It is the smallest among the nanoparticles used in this study. Highest protease activity (0.19 nM/min/mg FW) was observed on 28th day of experiment for AgCu (1:3) nanoparticle suspension at 30 µg/ml as compared with the lowest (0.023 nM/min/mg FW) for AgCu (3:1) on the same day. This can be the possible outcome of their composition due to which a difference in toxic ion production took place. POD activity was also greatly affected by monometallic and bimetallic nanoparticle suspensions. The variation in the pattern is most likely the outcome of their sizes and composition.
5 Conclusions
In summary, the effects of monometallic and bimetallic nanoparticles on the germination, growth and biochemical profile of S. marianum plant were greatly positive. Particle size, surface area and composition played a significant role in this regard. All three factors contributed either singly or in combination to enhance and in some cases inhibit germination and growth. It can also be concluded that nanoparticles, once though seed coat can have a long lasting effect on seed germination and subsequent plant growth. In addition, it can also significantly alter the biochemical profile. However, our knowledge of the underlying mechanisms that dictate such processes is little and is a subject to further investigation. This study will help further the understandings of the interaction between monometallic and bimetallic nanoparticles and medicinal plants.
6 References
- 1. Bai C.L.: ‘Ascent of nanoscience in China’, Science, 2005, 309, pp. 61 –63 (doi: 10.1126/science.1115172) [DOI] [PubMed] [Google Scholar]
- 2. Brumfiel G.: ‘Nanotechnology: a little knowledge’, Nature, 2003, 424, pp. 246 –248 (doi: 10.1038/424246a) [DOI] [PubMed] [Google Scholar]
- 3. Roco M.C.: ‘Environmentally responsible development of nanotechnology’, Environ. Sci. Technol., 2005, 39, pp. 106 –112 (doi: 10.1021/es053199u) [DOI] [PubMed] [Google Scholar]
- 4. Service R.F.: ‘Is nanotechnology dangerous’, Science, 2000, 290, pp. 1526 –1527 (doi: 10.1126/science.290.5496.1526) [DOI] [PubMed] [Google Scholar]
- 5. Service R.F.: ‘Nanomaterials show signs of toxicity’, Science, 2003, 300, pp. 243 –245 (doi: 10.1126/science.300.5617.243a) [DOI] [PubMed] [Google Scholar]
- 6. Yang F. Hong F.S. You W.J. et al.: ‘Influences of nano‐anatase TiO2 on the nitrogen metabolism of growing spinach’, Biol. Trace Elem. Res., 2006a, 110, pp. 179 –190 (doi: 10.1385/BTER:110:2:179) [DOI] [PubMed] [Google Scholar]
- 7. John F. Sargent Jr.: ‘The national nanotechnology initiative: overview, reauthorization, and appropriations issues’, Congressional Res. Service, 2004, 7–5700, p. RL34401 [Google Scholar]
- 8. Biswas P. Wu C.Y.: ‘Critical review: nanoparticles and the environment’, J. Air Waste Manag. Assoc., 2005, 55, pp. 708 –746 (doi: 10.1080/10473289.2005.10464656) [DOI] [PubMed] [Google Scholar]
- 9. USEPA : ‘Nanotechnology white paper e external review draft’ Available at: http://www.epa.gov/osa/pdfs/EPA_nanotechnology_white_paper_external_review_draft_12‐02‐2005.pdf; (United States Environmental Protection Agency, Washington, DC, 2005) [Google Scholar]
- 10. Brunner T.J. Wick P. Manser P. et al.: ‘In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility’, Environ. Sci. Technol., 2006, 40, pp. 4374 –4381 (doi: 10.1021/es052069i) [DOI] [PubMed] [Google Scholar]
- 11. Hussain S.M. Hess K.L. Gearhart J.M. et al.: ‘In vitro toxicity of nanoparticles in BRL 3A rat liver cells’, Toxicol. In Vitro., 2005, 19, pp. 975 –983 (doi: 10.1016/j.tiv.2005.06.034) [DOI] [PubMed] [Google Scholar]
- 12. Jia G. Wang H.F. Yan L. et al.: ‘Cytotoxicity of carbon nanomaterials: single‐wall nanotube, multiwall nanotube, and fullerene’, Environ. Sci. Technol., 2005, 39, pp. 1378 –1383 (doi: 10.1021/es048729l) [DOI] [PubMed] [Google Scholar]
- 13. Lam C.W. James J.T. McCluskey R. et al.: ‘A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks’, Crit. Rev. Toxicol., 2006, 36, pp. 189 –217 (doi: 10.1080/10408440600570233) [DOI] [PubMed] [Google Scholar]
- 14. Soto K.F. Carrasco A. Powell T.G. et al.: ‘Biological effects of nanoparticulate materials’, Mater. Sci. Eng. C., 2006, 26, pp. 1421 –1427 (doi: 10.1016/j.msec.2005.08.002) [DOI] [Google Scholar]
- 15. Nel A. Xia T. Madler L. et al.: ‘Toxic potential of materials at the nanolevel’, Science, 2006, 311, pp. 622 –627 (doi: 10.1126/science.1114397) [DOI] [PubMed] [Google Scholar]
- 16. Kim J.S. Kuk E. Yu K.N. et al.: ‘Antimicrobial effects of silver nanoparticles’, Nanomed. Nanotechnol. Biol. Med., 2007, 3, pp. 95 –101 (doi: 10.1016/j.nano.2006.12.001) [DOI] [PubMed] [Google Scholar]
- 17. Yang K. Wang X.L. Zhu L.Z. et al.: ‘Competitive sorption of pyrene, phenanthrene, and naphthalene on multiwalled carbon nanotubes’, Environ. Sci. Technol., 2006b, 40, pp. 5804 –5810 (doi: 10.1021/es061081n) [DOI] [PubMed] [Google Scholar]
- 18. Yang L. Watts D.J.: ‘Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles’, Toxicol. Lett., 2005, 158, pp. 122 –132 (doi: 10.1016/j.toxlet.2005.03.003) [DOI] [PubMed] [Google Scholar]
- 19. Murashov V.: ‘Comments on ‘Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles’ by Yang, L., Watts, D.J., toxicology letters, 2005, 158, 122–132’, Toxicol. Lett., 2006, 164, pp. 185 –187 (doi: 10.1016/j.toxlet.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 20. Fiebrich F. Koch H.: ‘Silymarin, an inhibitor of lipoxygenase’, Experentia, 1979, 35, pp. 150 –152 [DOI] [PubMed] [Google Scholar]
- 21. Dehmlow C. Erhard J. de Groot H.: ‘Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin’, Hepatology, 1996, 23, pp. 749 –754 (doi: 10.1002/hep.510230415) [DOI] [PubMed] [Google Scholar]
- 22. Fantozzi R. Brunelleschi S. Rubino A. et al.: ‘FMLP‐activated neutrophils evoke histamine release from mast cells’, Agents Actions, 1986, 18, pp. 155 –158 (doi: 10.1007/BF01988009) [DOI] [PubMed] [Google Scholar]
- 23. De La Puerta R. Martinez E. Bravo L.: ‘Effect of silymarin on different acute inflammation models and on leukocyte migration’, J. Pharm. Pharmacol., 1996, 48, pp. 968 –970 (doi: 10.1111/j.2042-7158.1996.tb06014.x) [DOI] [PubMed] [Google Scholar]
- 24. Rahman L.U. Qureshi R. Yasinzai M.M. et al.: ‘Synthesis and spectroscopic characterization of Ag‐Cu alloy nanoparticles prepared in various ratios’, C. R. Chim., 2012, 15, pp. 533 –538 (doi: 10.1016/j.crci.2012.03.012) [DOI] [Google Scholar]
- 25. Rahman L.U. Shah A. Lunsford S.K. et al.: ‘Monitoring of 2‐butanone using a Ag–Cu bimetallic alloy nanoscale electrochemical sensor’, RSC Adv., 2015, 5, pp. 44427 –44434 (doi: 10.1039/C5RA03633J) [DOI] [Google Scholar]
- 26. Rahman L.U. Shah A. Qureshi R. et al.: ‘Spectroscopic analysis of Au‐Cu alloy nanoparticles of various compositions synthesized by a chemical reduction method’, Adv. Mater. Sci. Eng., 2015, 2015, Article ID 638629, 8 pages [Google Scholar]
- 27. Rahman L.U. Shah A. Khan S.B. et al.: ‘Synthesis, characterization, and application of Au–Ag alloy nanoparticles for the sensing of an environmental toxin, pyrene’, J. Appl Electrochem., 2015, 45, pp. 463 –472 (doi: 10.1007/s10800-015-0807-2) [DOI] [Google Scholar]
- 28. Lin D. Xing B.: ‘Phytotoxicity of nanoparticles: inhibition of seed germination and root growth’, Environ. Pollut., 2007, 150, pp. 243 –250 (doi: 10.1016/j.envpol.2007.01.016) [DOI] [PubMed] [Google Scholar]
- 29. Abbasi B.H. Khan M.A. Mahmood T. et al.: ‘Shoot regeneration and free‐radical scavenging activity in Silybum marianum L’, Plant. Cell. Tiss. Org., 2010, 101, pp. 371 –376 (doi: 10.1007/s11240-010-9692-x) [DOI] [Google Scholar]
- 30. Murashige T. Skoog F.: ‘A revised medium for rapid growth and bio assays with tobacco tissue cultures’, Physiol. Plant., 1962, 15, pp. 473 –497 (doi: 10.1111/j.1399-3054.1962.tb08052.x) [DOI] [Google Scholar]
- 31. Nayyar H. Gupta D.: ‘Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants’, Environ. Exp. Bot., 2006, 58, pp. 106 –113 (doi: 10.1016/j.envexpbot.2005.06.021) [DOI] [Google Scholar]
- 32. Lee S.K. Mbwambo Z.H. Chung H.S. et al.: ‘Evaluation of the antioxidant potential of natural products’, Comb. Chem. High T. Scr., 1998, 1, pp. 35 –46 [PubMed] [Google Scholar]
- 33. Velioglu Y.S. Lee S.K. Mbwambo Z.H. et al.: ‘Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products’, J. Agric. Food. Chem., 1998, 46, pp. 4113 –4117 (doi: 10.1021/jf9801973) [DOI] [Google Scholar]
- 34. Chang C.C. Yang M.H. Wen H.M. et al.: ‘Estimation of total flavonoids content in propolis by two complementary colorimetric methods’, J. Food. Drug. Anal., 2002, 10, pp. 178 –182 [Google Scholar]
- 35. Lowry O.H. Rosebrough N.J. Farr A.L. et al.: ‘Protein measurement with the folin phenol reagent’, J. Biol. Chem., 1951, 193, pp. 265 –275 [PubMed] [Google Scholar]
- 36. Lagrimini L.M.: ‘Wound‐induced deposition of polyphenols in transgenic plants overexpressing peroxidase’, Plant. Physiol., 1991, 96, pp. 577 –583 (doi: 10.1104/pp.96.2.577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDonald C.E. Chen L.L.: ‘The lowry modification of the folin reagent for determination of proteinase activity’, Anal. Biochem., 1965, 10, pp. 175 –177 (doi: 10.1016/0003-2697(65)90255-1) [DOI] [PubMed] [Google Scholar]
- 38. Donaldson K. Stone V. Tran C.L. et al.: ‘Nanotoxicology’, Occup. Environ. Med., 2004, 61, pp. 727 –728 (doi: 10.1136/oem.2004.013243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Munzuroglu O. Geckil H.: ‘Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus ’, Arch. Environ. Contam. Toxicol., 2002, 43, pp. 203 –213 (doi: 10.1007/s00244-002-1116-4) [DOI] [PubMed] [Google Scholar]
- 40. Kordan H.A.: ‘Seed viability and germination: a multi‐purpose experimental system’, J. Biol. Educ., 1992, 26, pp. 247 –251 (doi: 10.1080/00219266.1992.9655281) [DOI] [Google Scholar]
- 41. Moore M.T. Huggett D.B. Huddleston G.M. et al.: ‘Herbicide effects on Typhalatifolia (Linneaus) germination and root and shoot development’, Chemosphere, 1999, 38, pp. 3637 –3647 (doi: 10.1016/S0045-6535(98)00561-X) [DOI] [Google Scholar]
- 42. Murata M.R. Hammes P.S. Zharare G.E.: ‘Effect of solution pH and calcium concentration on germination and early growth of groundnut’, J. Plant. Nutr., 2003, 26, pp. 1247 –1262 (doi: 10.1081/PLN-120020368) [DOI] [Google Scholar]
- 43. Ren L. Zeiler L.F. Dixon D.G. et al.: ‘Photoinduced effects of polycyclic aromatic hydrocarbons on Brassica napus (canola) during germination and early seedling development’, Ecotoxicol. Environ. Saf., 1996, 33, pp. 73 –80 (doi: 10.1006/eesa.1996.0008) [DOI] [PubMed] [Google Scholar]
- 44. Navarro E. Baun A. Behra R. et al.: ‘Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi’, Ecotoxicology., 2008, 17, pp. 372 –386 (doi: 10.1007/s10646-008-0214-0) [DOI] [PubMed] [Google Scholar]
- 45. Masarovicova E. Kralova K.: ‘Metal nanoparticles and plants’, Ecol. Chem. Eng. S., 2013, 20, pp. 9 –22 [Google Scholar]


