Abstract
Metal nanoparticles have generated great interest due to their excellent optical and chemical properties. The widely used chemical method for synthesising nanoparticles involves capping agents for colloidal stability. However, there are scarce reports on the application of metal nanoparticles synthesised without using capping agents. Hence, there is a need to develop pristine nanoparticles devoid of capping that can be used for translational research. Here, the authors developed a facile and rapid method for synthesising bare metal nanoparticles (platinum/silver/gold) that are chemically reactive and stable for a month upon storage. They synthesised bare metal nanoparticles of sub‐15 nm and characterised using standard techniques (UV–VIS‐NIR/DLS/zeta//TEM/XRD). They assessed the safety of the synthesised nanoparticles on the liver carcinoma cell line (HepG2). Bare gold and platinum nanoparticles were non‐toxic in comparison to bare silver nanoparticles. Bare metal nanoparticles were also checked for metal detection wherein antimony, mercury and chromium were detected using bare gold and silver nanoparticles. The spectroscopic shifts of the nanoparticles when bound to metals resulted in blue and red shifting of the plasmon band, indicating the sensing of metals. These results show that bare metal nanoparticles have the potential to emerge as a promising candidate for biomedical and sensing applications.
Inspec keywords: ultraviolet spectra, electrokinetic effects, liver, cellular biophysics, nanoparticles, cancer, toxicology, gold, platinum, X‐ray diffraction, silver, colloids, transmission electron microscopy, plasmonics, visible spectra, nanomedicine
Other keywords: bare plasmonic metal nanoparticles, liver carcinoma cell line, capping agents, pristine nanoparticles, bare metal nanoparticles, synthesised nanoparticles, platinum nanoparticles, silver nanoparticles, XRD, TEM
1 Introduction
Plasmonic metal nanoparticles have gained tremendous potential in the past decades due to their unique physico‐chemical properties. The manipulation of atoms at the nanoscale regime led to technological advancement in the area of nanotechnology. The chemical reactivity is higher for nanoparticles in the size dimension of 1 – 100 nm [1]. These unusual effects can be attributed to their higher surface area‐to‐volume ratio and collective oscillation of conducting electrons coupling with incident light at specific wavelengths. This phenomenon is referred to as localised surface plasmon resonance (LSPR) [2]. LSPR is an indigenous property confined to plasmonic nanoparticles and is not observed in other metallic nanoparticles. Plasmonic nanoparticles are synthesised using noble metals that are considered inert and non‐corrodible in comparison to other metals. This led to extensive research on biological applications of plasmonic nanomaterials over the last two decades. Currently, metal nanoparticles of gold, silver and platinum are widely used in sensing, drug delivery and photonic applications [3].
These noble metal nanoparticles are synthesised using several approaches, but the most traditional method employed is the wet chemical method [4]. The metal nanoparticles’ size/shape is influenced by their capping agent, reaction parameters and nature of the solvent used. The platinum nanoparticles (PtNPs), silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) absorb light in the ultraviolet (UV), visible wavelengths and far visible/near infra‐red (NIR) wavelengths, respectively. The absorption and scattering properties of metal nanoparticles enable applications, such as surface‐enhanced Raman scattering (SERS), optical sensors, fluorescence (SPR) sensor chips, deoxyribonucleic acid (DNA) sensors and electrochemical sensors [5, 6]. Due to such versatility in applications, there is a substantial increase in utilising nanoparticles for commercial, technological and translational research. The wet‐chemical approach of synthesising metal nanoparticles is advantageous in terms of monodispersity and stability. The method implies simple reduction chemistry where a metal salt is reduced, forming nuclei in the presence of a reducing agent and further stabilised using a capping agent. The capping agents depending on their nature, provide stability and functionalisation to nanoparticle surfaces. The most commonly used capping agents for metal nanoparticles are citrate, polymers (PVP/PVA/PEG), quaternary surfactants (CTAB/CTAC/BDAC) and biomolecules (DNA/peptide/vitamin C/GSH). However, Faraday, in the year 1856, accidentally synthesised bare gold nanoparticles using only metal salt and sodium borohydride (NaBH4) as a reducing agent, which showed excellent colloidal stability [7].
Bare metal nanoparticles have a larger surface area and are more reactive than capped metal nanoparticles by virtue of their pristine nature [8]. Due to the absence of a stabilising/capping agent; the stoichiometric volume of the chemicals used, critically determines the colloidal stability of the nanoparticle solution. Apart from stoichiometry, other parameters, such as reaction temperature, reaction time and storage, plays a prudent role in the formation of stable bare metal nanoparticles [9]. However, the bare nanoparticles application is limited as compared to capped nanoparticles due to lack of functionalisation and prudent conditions during synthesis and storage. Despite these limitations, bare metal nanoparticles are advantageous for sensing and biological applications due to their pristine surface and ease of binding. The use of bare metal nanoparticles for cell‐based applications is also not explored to date regarding their fate. It is evident that the cytotoxicity due to the interaction between nanoparticles and cells is highly predominated by the capping agent over the nanoparticle surface. The toxicity is accounted for the electrostatic interaction between the negatively charged cell membrane and the positively charged nanoparticles [10, 11]. The most established capping agents like CTAB and citrate showed cytotoxicity and genotoxicity at lower concentrations, respectively [12]. However, limited studies are reported on cytotoxicity induced by bare plasmonic metal nanoparticles despite possessing promising potential in the area of nanomedicine application [13]. Cytotoxicity assessment due to nanoparticles is often carried out on human cancer cell lines because of the high heterogeneity of tumour cells. Indeed, tumour cell lines are considered as a pertinent model for primary tumour cells that helps in deciphering the molecular mechanisms of nanoparticle toxicity.
Due to sensitive surface chemistry, bare plasmonic metal nanoparticles have potential in sensing applications. In the literature, there are reports of bare metal nanoparticles utilised as sensing probes for the detection of heavy metals via the colorimetric method [8]. Several capping agents, such as surfactants, citrate and polymers, have also been used to stabilise metal nanoparticles applicable in detecting metals [14, 15, 16]. Metal contamination in the environment is substantially increasing due to anthropogenic activities as well as a natural phenomenon. Antimony (Sb), chromium (Cr) and Mercury (Hg) are some of the hazardous metals that are being exposed to the environment and affecting the health of humans. Antimony is being extensively used as a constituent in alloys, paint pigments and the rubber industry. A pure form of antimony is used in devising semiconductor materials such as infra‐red detectors and diodes. Antimony exposure is mostly occupational and workers who get exposed to airborne dust might suffer from respiratory ailments. Chromium is one of the toxic metals which is being mainly released into the environment from metallurgical and chemical industries. Commercially, chromium is being used in chrome plating, industrial welding, leather tanning, dyes and pigments. Chromium is considered a toxic industrial pollutant that is also a human carcinogen, according to various regulatory agencies [17]. Mercury is an environmental toxicant and pollutant that is widespread and produces adverse alterations in the human body when exposed. The major sources of chronic and low‐level exposure to mercury in humans are fish consumption and dental amalgam. Several high throughput techniques, such as HPLC, AAS and ICP‐MS, are being traditionally used for heavy metal detection [18]. Several functional nanomaterials using capping agents as the interacting ligand for heavy metal detection has also been reported. Despite nanoparticles wherein a ligand is used to detect the heavy metal, there is a necessity to develop facile synthetic methods for developing pristine metal nanoparticles without using a capping agent. This facilitates developing rapid, specific, sensitive and cost‐effective nanoprobes for toxic metal detection and exploring biological applications.
Therefore, in this work, we have synthesised bare plasmonic metal nanoparticles, namely gold (Au), silver (Ag) and platinum (Pt) of sizes <15 nm with high monodispersity and yield. The synthesised bare metal nanoparticles were characterised extensively using standard techniques in order to determine their physico‐chemical properties. The toxicity of nanoparticles is critical and required to assess their safety due to their enormous use in consumer applications. As the liver is considered to be the major organ for bioaccumulation of nanoparticles, we assessed their safety on the HepG2 cell line [19, 20]. In this work, we explored the use of label‐free metal nanoparticles as nanoprobes for the qualitative detection of metal cations and assessed their safety for biomedical applications.
2 Materials and methods
Hydrogen tetrachloroaurate trihydrate (HAuCl4 ·3H2 O), silver nitrate (AgNO3), hexachloroplatinic acid hexahydrate (H2 PtCl6 ·6H2 O) were purchased from Finar, Ranbaxy and Sigma‐Aldrich, respectively. The borohydride reducing agents sodium borohydride (NaBH4) and lithium borohydride (LiBH4) were procured from Sigma‐Aldrich. Metal salts of Pb2+, Fe3+, As3+, Ni2+, Mo2+, Hg2+, Cr3+, Sn2+, Mn2+, Co2+, Cu2+, Cd2+, Ba2+, Al3+, Sb3+ were bought from Merck, Germany. All the chemicals were used as received. Dulbecco's Modified Eagle Medium (DMEM), 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT), phosphate buffer saline (PBS), foetal bovine serum (FBS), trypsin and antibiotics were acquired from Sigma‐Aldrich. All the stock solutions were predisposed in double‐distilled water. All the glasswares were cleaned with aqua regia and further rinsed with Milli Q prior to use in the experiments.
2.1 Bare plasmonic metal nanoparticle synthesis using the wet chemical reduction method
Respective metal salts of silver, gold and platinum were reduced using a strong reducing agent to yield bare plasmonic metal nanoparticles. Ice cold NaBH4 was used to reduce HAuCl4 ·3H2 O and AgNO3, whereas LiBH4 (ice‐cold) was used in the case of H2 PtCl6 ·6H2 O reduction. In the case of AgNP synthesis, 25 ml of 0.002 M NaBH4 was added to 10 ml 0.001 M AgNO3 at room temperature. AuNP synthesis was carried out at boiling temperature (100°C). 500 μl of 0.025 M gold salt was added to 7 ml double distilled water followed by the addition of 500 μl NaBH4 (0.1 M, ice‐cold) and left undisturbed until the ruby red colour appeared. However, in the case of PtNP synthesis, 0.2 M LiBH4 was used to reduce 200 μl of 0.06 M platinum salt in 5 ml double distilled water at room temperature. The colour change in case of all the metal nanoparticles indicated the complete reduction of the metal salts into nanoparticles using wet chemical methods. The pH during the synthesis of bare nanoparticles was 3.3 (acidic). The nanoparticles are in the dispersion phase in the colloidal solution consisting of water as a dispersion medium. The synthesised nanoparticles were washed and purified using centrifugation at 10,000g for 10 min. The synthesised nanoparticles were stored at room temperature (25°C) and were checked for 4 weeks (28 days) stability.
2.2 Characterisation techniques
2.2.1 Optical spectroscopy
The plasmon bands of the synthesised bare metal nanoparticles (platinum/gold/silver) were characterised for their optical properties using optical spectroscopy. 1 ml of the sample was transferred into polystyrene cuvette (for AuNPs and AgNPs). However, for platinum metal nanoparticles, a quartz cuvette was used to measure their plasmonic properties in the ultraviolet region (UV). The as‐prepared bare nanoparticles were measured by proceeding 1 ml of the colloidal solution in a polystyrene cuvette. The measurements were performed via UV–vis–NIR spectrophotometer (spectraMax M5, Molecular Devices).
2.2.2 Average hydrodynamic diameter and zeta potential measurement
The average hydrodynamic diameter and surface charge of bare plasmonic metal nanoparticles were measured using ZetaSizer (Nano ZS, Malvern UK). 1 ml each of the samples were transferred into a cuvette (cuvette models‐ZEN1020 and DTS0012) for the dynamic light scattering (DLS) and zeta potential measurements. The absorbance coefficient of 1.030, 3.990, 0.100 and a refractive index of 1.340, 0.135, 1.520 with respect to PtNPs, AgNPs and AuNPs were pre‐filled in the software. All the measurements were carried out at 25°C.
2.2.3 Transmission electron microscopy (TEM)
Bare plasmonic metal nanoparticles were centrifuged at 10,000g for 10 min. After centrifugation, the colourless supernatant was discarded and the pellet was dispersed into 1 ml Milli Q. 10 μl from each sample were suspended onto formvar coated copper grid and left to air‐dry. Finally, the grids were visualised under 120 kV transmission electron microscope Tecnai (FEI, The Netherlands).
2.2.4 X‐ray diffraction (XRD)
The crystal structure of the synthesised bare metallic nanoparticles was determined through Panalytical XRD. 10 mg of the lyophilised metal nanoparticle powders was used for measurement. X‐rays were transmitted through the samples during measurement for determining the crystal structure.
2.2.5 Cell culture
The liver carcinoma cell line (HepG2) was procured from the American Type Cell Culture Collection (ATCC, USA). The cells were incubated in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% (v/v) FBS and 1% Pen‐Strep as an antibiotic to avoid bacterial contamination. The cells were cultured at 37°C with 5% CO2 supply in a 90% humidified incubator.
2.2.6 MTT assay
The standard protocol of MTT assay was followed with slight modification [21]. 1 × 104 cells per well were seeded in a 96 well‐plate and incubated for 24 h at 37°C in a CO2 incubator. Haemocytometer was used for counting cells prior to seeding. The plate consisted of groups denoted as a control group, exposed group and cell‐free group. Cells unexposed to nanoparticles constituted the control group. The samples were bare nanoparticles of Au, Ag and Pt diluted to different concentrations (1 × 1011 – 1 × 109 particles/ml) dispersed in media. The concentration of nanoparticles was achieved through UV–vis measurement as per the previous report using the following formula:
Here μ ext is the extinction coefficient of the samples. Q ext is the extinction efficiency and r is the radius of a nanoparticle [22]. The cells were exposed to nanoparticles for a duration of 24 h. After the exposure duration, 10 μl MTT solution (5 mg/ml in PBS) was added to each well and left for incubation at 37°C in 5% CO2 for 2 h. After forming formazan crystals, the media was pipetted out and subsequently replaced by 100 μl of DMSO in each well. The formazan crystals started dissolving, which was indicated by the appearance of purple colour. The plate was then wrapped in aluminium foil and left for 10 min at room temperature for the complete dissolution of formazan crystals. The absorbance was measured at 570 nm by a spectrophotometer (spectraMax M5, Molecular Devices). The assay was carried out three times in quadruples.
2.2.7 Experiment on metal detection
Corresponding metal salts of Pb2+, Fe3+, As3+, Ni2+, Mo2+, Hg2+, Cr3+, Sn2+, Mn2+, Co2+, Cu2+, Cd2+, Ba2+, Al3+, Sb3+ dissolved in acid were spiked in double‐distilled water and diluted to 0.5 and 2.5 ppm, respectively. Consequently, 900 μl of each concentration of metal salts were titrated against freshly prepared bare metallic nanoparticles. The later were transferred to cuvettes and investigated for the absorption spectrum in 300–900 nm.
3 Results and discussion
3.1 UV–vis measurements
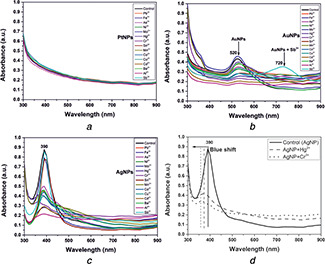
Bare plasmonic metal nanoparticles of platinum, silver and gold were synthesised using the wet chemical method without using any capping/stabilising agent. Fig. 1 a shows the absorption spectra of the synthesised PtNPs, AgNPs and AuNPs in the UV region and vis‐NIR region, respectively. PtNPs exhibited plasmon band in the UV region, whereas AgNPs and AuNPs exhibited plasmon peak in the visible range at 390 and 520 nm, respectively, as shown in Fig. 1 a. The observed characteristic plasmon band of the metallic nanoparticles at specific wavelengths is due to the coherent oscillation of electrons on the nanoparticle surface, which occurs upon an incident of the electromagnetic spectrum. This phenomenon is known as localised surface plasmon resonance (LSPR), which is highly governed by the shape and size of a nanoparticle [2]. The unimodal distribution of the absorption spectrum at specific wavelengths for AgNPs and AuNPs represents the transverse dipole axis of the nanoparticle, indicating the shape to be spherical. The synthesised bare metallic nanoparticles showed good monodispersity, which is evident from the narrow peaks exhibited by the absorption spectra obtained for the respective bare nanoparticles. We also checked the stability of the bare nanoparticles for four weeks and observed no shift or change in the absorption spectra, as shown in Figs. 1 b–d. This indicates good colloidal stability of the synthesised bare metal nanoparticles devoid of any capping/stabilising agent.
Fig. 1.
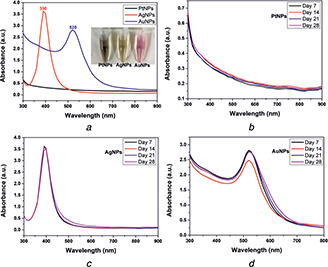
Optical spectroscopy of bare metal nanoparticles and their stability
(a) As‐synthesised metal nanoparticles showed stable absorption bands without any aggregation, (b) , (c) , (d) Stability of the synthesised bare metal nanoparticles of platinum, silver and gold showed stable plasmon bands, indicating their stability over storage for four weeks
3.2 DLS and zeta potential measurements of bare metal nanoparticles
The size and surface charge of bare plasmonic metal nanoparticles corresponding to Figs. 2 a and b. DLS revealed the hydrodynamic diameter of 43.83, 14.85 and 14.69 nm for PtNPs, AgNPs and AuNPs, respectively, with a comparative low polydispersity index (PDI) values of 0.394, 0.368 and 0.330, respectively. The obtained hydrodynamic diameter of AgNPs and AuNPs showed a good agreement with the particle size in the sub‐15 nm regime. Also, the minimum PDI value obtained during DLS measurement indicates the formation of highly monodispersed nanoparticles. However, the hydrodynamic radius obtained for PtNPs is larger in comparison to AgNPs and AuNPs [23]. This might be due to the consideration of more number of PtNPs collectively during DLS measurement, which was confirmed later through TEM analysis; where the nanoparticles were of crystalline size agglomerating into worm‐like structures. Hence, it can be affirmed that the agglomerating PtNPs showed a larger hydrodynamic radius as compared to that of AgNPs and AuNPs, where the later were sparsely dispersed without agglomeration.
Fig. 2.
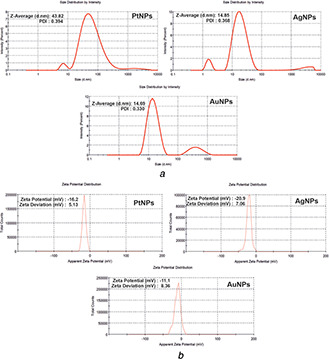
DLS and zeta potential measurements determining
(a), (b) Hydrodynamic radius and surface charge of bare metal nanoparticles. The metal nanoparticles have negatively charged surface and DLS determined the average size and PI of the synthesised bare metal nanoparticles
The surface charge on bare metal nanoparticles was evaluated through zeta potential measurement. The bare PtNPs, AgNPs and AuNPs showed the zeta potential of −16.2, −20 and −11.1 mV, respectively. The zeta potential values indicated the negative surface charge on the nanoparticle surface due to the thin layer of hydride ions from the respective reducing agent used [9]. The stabilisation of the bare nanoparticles involves slow hydrolysis of excess NaBH4 to NaB(OH)4 that is weakly catalysed by the metal nanoparticles. A strong covalent bond between the nanoparticle and H/BH4 forms a bond with the core. Thus the nanoparticles core is surrounded by stabilising H or BH4 ligands. The colloidal stability of these bare metal nanoparticles was evident as no precipitation was observed in the solutions. The obtained zeta potential value is less than −30 mV, which might be due to lack of capping agent as it is well known that surface charge is predominantly governed by the nature of the capping agent used. Also, bare metal nanoparticles with zeta potential less than −20 mV were stable as per a previous report [24]. Therefore, the synthesised bare nanoparticles are in order of AuNPs > AgNPs > PtNPs in terms of anionic strength. However, we were able to synthesise capping‐free stable metal nanoparticles by maintaining stoichiometry of the chemicals and prudent conditions that were critical during the synthesis procedure.
3.3 TEM analysis
The estimation of the size and shape of bare metal nanoparticles was done using TEM. Figs. 3 a–c show the size and shape of PtNPs, AgNPs and AuNPs, respectively. The shapes of the bare nanoparticles were found to be spherical and their average sizes were below 15 nm. The average sizes of bare AgNPs and AuNPs were found to be 13.81 and 13.37 nm, respectively. The nanoparticles were monodisperse and synthesised in high yield. Bare PtNPs were of 3.47 nm size as observed in the electron micrograph. The histograms showed a good Gaussian distribution for the size distribution of nanoparticles. We measured 50 nanoparticles per sample for statistics to determine the average mean size. The smaller size can be attributed due to the use of LiBH4, which has a high reduction potential compared to NaBH4. This leads to rapid nucleation within a short time during synthesis yielding PtNPs of a much smaller size. The PtNPs resembled network‐like structures, possibly due to self‐assembly, as shown in Fig. 3 a.
Fig. 3.
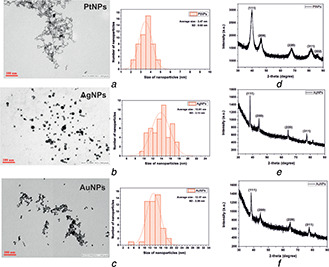
TEM images of
(a) PtNPs, (b) AgNPs, (c) AuNPs showed the average sizes of sub‐15 nm along with histogram for mean size distribution measurement, (d) , (e) , (f) Crystal structure of PtNPs, AgNPs and AuNPs was measured using XRD
3.4 X‐ray diffraction
The structure and growth of the single crystal in the synthesised bare metal nanoparticles were determined using the XRD technique. Figs. 3 d–f show the XRD peaks of PtNPs, AgNPs and AuNPs at 2θ value range 30°–90°, respectively. In the case of PtNPs, all the five peaks corresponded to standard Bragg reflections (111), (200), (220), (311) and (222) of the face centred cubic (fcc) lattice corresponding to a 2θ value of 39.8°, 46.1°, 67.9°, 81.4° and 86.2°, respectively. The obtained XRD pattern for the PtNPs is in good agreement with the standard diffraction pattern of bulk platinum metal (JCPDS File no. 04‐0802) [25]. However, AgNPs and AuNPs exhibited similar diffraction peaks at 2θ value of 38.1°, 44.3°, 64.5° and 77.4° for the corresponding standard Bragg reflections (111), (200), (220) and (311), respectively. The obtained diffraction patterns for AgNPs and AuNPs matched with the standard diffraction pattern of cubic silver (JCPDS File no.‐04‐0783) and gold (JCPDS File no.‐89‐3697) metal [26]. The intense diffraction peak at 39.8° in the case of PtNPs and 38.1° in the case of AgNPs and AuNPs showed fixed preferential growth in the (111) direction [27]. The XRD measurements showed the diffractograms of the metal nanoparticles revealing their respective crystalline structure.
3.5 MTT assay
Cell viability assay for preliminary cytotoxicity assessment of bare plasmonic metal nanoparticles was carried out through MTT assay. Fig. 4 shows the viability of cells exposed to bare metal nanoparticles of platinum (Pt), silver (Ag) and gold (Au) at various concentrations under 24 h exposure. PtNPs and AuNPs induced no significant changes in cell viability irrespective of the concentrations used as compared to the control group after 24 h exposure. This can be attributed to the surface chemistry and interaction between nanoparticle and cell membrane. Since the nanoparticles are devoid of capping, the surface is negatively charged due to hydride linking with the zero‐valent metal. Therefore, the cell membrane which is negatively charged does not show any electrostatic interaction with the bare nanoparticles. However, bare AgNPs reduced the cell viability significantly at the highest concentration only. This is due to the oxidation of zero‐valent Ag, releasing Ag cations into the media [28, 29, 30]. As per the previous report, AgNPs at concentration 1011 particles/ml showed cytotoxicity [31]. The silver ions interact with the negatively charged cell membrane causing cell membrane rupture, leading to decreased cell viability. The decreased cell viability observed at the maximum concentration is due to the higher number of AgNPs that releases a sufficient amount of silver ions to cause the cellular damage since the dissolution of AgNPs into silver ions is high in the initial period and vice versa [29]. MTT assay indicated higher mitochondrial activity in viable cells and vice‐versa, indicating the cytotoxic effect of bare metal nanoparticles.
Fig. 4.
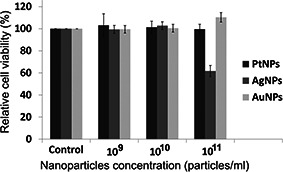
Relative cell viability upon exposure of bare metal nanoparticles at different concentrations (particles/ml) to liver cancer cells
3.6 Sensing of metal cations using bare metal nanoparticles
The bare plasmonic metal nanoparticles were tested with several metal ions in aqueous solutions to investigate their sensing ability using optical spectroscopy. To assess the selectivity, absorption spectra of bare nanoparticles mixed with the metal ions (Pb2+, Fe3+, As3+, Ni2+, Mo2+, Hg2+, Cr3+, Sn2+, Mn2+, Co2+, Cu2+, Cd2+, Ba2+, Al3+, Sb3+) were investigated. The effect of metal ions on the SPR absorbance of the nanoparticles is illustrated in Fig. 5. It was evident from the absorption spectra of the nanoparticles that PtNPs showed no interaction with the metal ions. However, bare AgNPs and AuNPs, when mixed with different metals, showed a selective change in the absorption spectra. AuNPs selectively interacted with antimony resulting in the emergence of a second plasmon peak at the NIR region, as shown in Fig. 5 b. The signature peak at the NIR wavelength indicates the selective interaction between bare AuNPs and Sb3+, which was not observed with other metal ions. Also Figs. 6 a and b show the spectrum and corresponding TEM images of AuNPs and AuNPs + Sb3+ aggregates. It is clearly evident from the TEM images that AuNPs interacted with antimony (AuNPs + Sb3+), resulting in aggregates, which is due to the coupling or sensing of antimony using bare gold nanoparticles. Also, as a signature of transition metal identity, gold can form a co‐ordinate bond by donating d electrons. Therefore, it can be affirmed that Sb3+ ions induced aggregation of AuNPs, which exhibits a prominent plasmon band shift from the visible to NIR regions.
Fig. 5.
Differential behaviour of metal nanoparticle sensing of metal cations
(a) , (b) , (c) , (d) , (e) , (f) Spectroscopic shifts of gold and silver metal nanoparticles due to their selective binding to metal cations. PtNPs have no interaction to metal cations
Fig. 6.
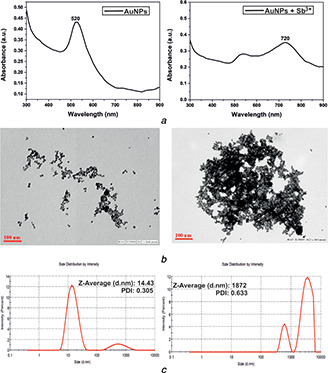
Effect of AgNP binding to antimony
(a) Visible band of AgNPs shifted to the NIR region upon binding to antimony, (b) , (c) Binding of antimony led to the aggregation of metal nanoparticles and also changed the hydrodynamic radius of AuNPs
On the other hand, AgNPs, when mixed with mercury or chromium, resulted in blue‐shifting of plasmon peaks, as shown in Figs. 5 c and d. Among the metal ions considered, Hg2+ and Cr3+ exhibited a considerable amount of blue shift (30 and 40 nm, respectively) in the plasmon band along with the dampening of the peak, as shown in Fig. 5 d. The donation of electrons by the ligands (Hg2+, Cr3+) resulted in a blue‐shift of the plasmon band, decreasing the particle size [32]. Thus, the obtained shift of the SPR from extrinsic to the intrinsic regime is evidence of the particles becoming smaller due to dissolution. Bare AgNPs showed promising potential in detecting two toxic metals using optical spectroscopy. However, a detailed study of the bare AgNPs on heavy metal sensing, including the mechanism, is needed. Since, bare AuNPs were found to be non‐toxic irrespective of concentration and exhibited pronounced selectivity towards antimony; therefore, the current study has been directed towards bare AuNPs in optical spectroscopic detection of antimony.
To further assess the specificity of bare AuNPs towards Sb3+, the remaining trivalent and bivalent metal ions in aqueous solution (Fe3+, Cr3+, As3+, Al3+ /Pb2+, Ni2+, Hg2+, Sn2+, Mn2+, Co2+, Cu2+, Mo2+, Cd2+, Ba2+) were mixed during the study. The concentration for each metal ion in the mixture aqueous solution used for the experiment was 2.5 ppm. We divided the metals into one trivalent and four random bivalent groups, as shown in Table 1.
Table 1.
Grouping of metals on the basis of charge (trivalent and divalent)
| Group | Metal |
|---|---|
| 1 | Fe3+, Cr3+, As3+, Al3+ |
| 2 | Cu2+, Mo2+, Ba2+, Sn2+ |
| 3 | Pb2+, Ni2+, Ba2+, Sn2+ |
| 4 | Hg2+, Cd2+, Pb2+, Ni2+ |
| 5 | Mn2+, Co2+, Hg2+, Cd2+ |
These groups were further assessed for binding to AuNPs.
Antimony was added in each group prior to optical spectroscopic measurement. The absorption spectrum of AuNPs + Sb3+ when mixed with group 1 metals was similar to the absorption spectra exhibited by AuNPs containing Sb3+. Similarly, the SPR peak exhibited at the NIR region by AuNPs + Sb3+ did not change upon the addition of metals present in groups 2 and 3. This indicated specific interaction of AuNPs with antimony even in the presence of different metal ions. However, due to the addition of metals of groups 3 and 4 into AuNPs + Sb3+, the plasmon peak at NIR quenched, which indicated non‐specific interaction due to the metal ions present in the groups (see Fig. 7 a). Also, as a control, we measured the spectra of AuNPs dispersed in metal mixture aqueous solution of groups 1, 2, 3 and 4 separately in the absence of antimony, as shown in Fig. 7 b. Since AuNPs + Sb3+, when mixed with groups 2 and 5 metals consisting of Ba2+, Sn2+ and Pb2+, Ni2+, respectively, showed no deviation in spectra at NIR, therefore we performed individual optical spectroscopy of AuNPs + Sb3+ mixed with Mn2+, Co2+, Hg2+ and Cd2+ separately maintaining the same concentration of 2.5 ppm. Surprisingly, we found a considerable amount of dampening and shifting of the NIR peak in the case of AuNPs + Sb3+ mixed with Mn2+ and Hg2+, respectively, as shown in Fig. 7 c. Co2+ and Cd2+, when added, showed no deviation in the signature absorption spectrum exhibited by AuNPs + Sb3+. Hence, Mn2+ and Hg2+ were confirmed to be deterrent metal ions during the detection of antimony. To further validate our finding, we mixed all the divalent metal ions with AuNPs + Sb3+ except the metal ions – Mn2+, Hg2+ and measured its absorption spectrum, as shown in Fig. 7 d. The absorption spectrum obtained was in accord with AuNPs + Sb3+, exhibiting the signature peak at the NIR wavelength. The signature plasmon peak in the NIR region for the metal ion aqueous solution containing antimony confirmed the specificity of bare AuNPs towards Sb3+. The observed mechanism is, however, not well‐understood, but an area of exploration is required further. One possible hypothesis can be that the dielectric constant of the medium is changed when AuNPs interact with mercury and manganese ions. This is supported by the theory stated regarding the influence of dielectric constant on the SPR band [33]. The dampening of the plasmon band of AuNP + Sb3+ in the NIR wavelength can be due to the rapid dissolution of AuNPs and converting into salts. However, the absence of mercury and manganese showed spectroscopic signature peaks in the NIR wavelength and aggregated nanoparticles, as observed in Fig. 6 b. The conclusive study is limited to qualitative detection of antimony using bare AuNPs wherein this is an emergence of label‐free bare nanoparticles as a promising sensing probe for metal detection. Quantitative study for a limit of detection (LOD) and limit of quantification (LOQ) using bare metal nanoparticles is the future perspective of the current work (Fig. 8).
Fig. 7.
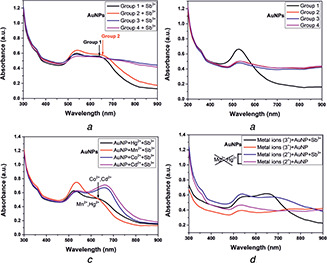
Absorption spectra of
(a) AuNPs containing antimony and metals of groups 1, 2, 3, 4 and 5, (b) AuNPs containing only the group metals (without antimony), (c) AuNPs containing antimony with Hg2+, Mn2+, Co2+, Cd2+ individually and, (d) AuNPs mixed with all the trivalent and bivalent (except Hg2+ and Mn2+) metals containing antimony. The system without antimony served the purpose of a control
Fig. 8.
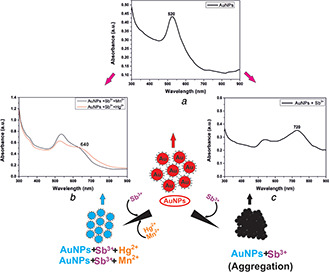
Diagrammatic representation of AuNPs detecting antimony. Absorption spectra of
(a) AuNPs (control), (b) AuNPs containing antimony with Hg2+ and Mn2+, respectively, and, (c) AuNPs containing antimony only
4 Conclusions
We have synthesised stable bare plasmonic metal nanoparticles (platinum/silver/gold) using a wet chemical method of average diameters of sub‐15 nm. The nanoparticles were characterised using standard techniques revealing their size, shape, surface charge and crystal structure. The bare metal nanoparticles are assessed for their safety towards liver carcinoma cells using the cell viability assay. Of all the metal nanoparticles studied, AgNPs showed toxicity to liver cancer cells at the highest concentration only. However, the bare AuNPs and PtNPs were non‐toxic at similar concentrations. Bare AuNPs and AgNPs showed the excellent sensing capability as label‐free probes. AuNPs showed an optical shift towards NIR upon binding to antimony, whereas AgNPs blue‐shifted upon binding to chromium and mercury. In contrast, platinum metal nanoparticles did not show any interaction with metal cations. The selective binding of metal nanoparticles to metal cations will enable futuristic applications in the field of bio‐sensing and nanomedicine. This research shows that bare metal nanoparticles can be improved as sensing probes that are easy to synthesise, cost‐effective and less toxic.
5 Acknowledgment
This research work was supported by the CSIR‐Indian Institute of Toxicology Research (Major Laboratory Project‐MLP‐001) approved by the competent authority of CSIR‐IITR, Lucknow, India. The authors acknowledge Mr. Jai Shankar for TEM imaging, IIT Kanpur, Kanpur, for XRD measurement. Nabojit Das was thankful to CSIR for providing fellowship. They are thankful to CSIR‐IITR, Lucknow, for providing the platform and facilities to carry out this research work. This paper is approved by the CSIR‐IITR publication committee and the communication number is 3639.
6 References
- 1. Auffan M., Rose J., Bottero J. Y. et al.: ‘Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective’, Nat. Nanotechnol., 2009, 4, (10), pp. 634–641 [DOI] [PubMed] [Google Scholar]
- 2. Jia S., Bian C., Sun J. et al.: ‘A wavelength‐modulated localized surface plasmon resonance (LSPR) optical fiber sensor for sensitive detection of mercury (II) ion by gold nanoparticles‐DNA conjugates’, Biosens. Bioelectron., 2018, 114, pp. 15–21 [DOI] [PubMed] [Google Scholar]
- 3. Paramasivam G., Kayambu N., Rabel A. et al.: ‘Anisotropic noble metal nanoparticles: synthesis, surface functionalization and applications in biosensing, bioimaging, drug delivery and theranostics’, Acta Biomat., 2017, 49, pp. 45–65 [DOI] [PubMed] [Google Scholar]
- 4. Ealias A.M., Saravanakumar M.P.: ‘A review on the classification, characterisation, synthesis of nanoparticles and their application’, IOP Conf. Ser. Mater. Sci. Eng., 2017, 26, p. 032019 [Google Scholar]
- 5. Matsui J., Akamatsu K., Hara N. et al.: ‘SPR sensor chip for detection of small molecules using molecularly imprinted polymer with embedded gold nanoparticles’, Anal. Chem., 2005, 77, (13), pp. 4282–4285 [DOI] [PubMed] [Google Scholar]
- 6. Malekzad H., Zangabad P.S., Mirshekari H. et al.: ‘Noble metal nanoparticles in biosensors: recent studies and applications’, Nanotechnol. Rev., 2017, 6, (3), pp. 301–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faraday M.X.: ‘The Bakerian lecture.– experimental relations of gold (and other metals) to light’, Philos. Trans. R. Soc. Lond., 1857, 147, pp. 145–181 [Google Scholar]
- 8. Shellaiah M., Simon T., Sun K. W. et al.: ‘Simple bare gold nanoparticles for rapid colorimetric detection of Cr3+ ions in aqueous medium with real sample applications’, Sens. Actuators B, 2016, 226, pp. 44–51 [Google Scholar]
- 9. Deraedt C., Salmon L., Gatard S. et al.: ‘Sodium borohydride stabilizes very active gold nanoparticle catalysts’, Chem. Commun., 2014, 50, (91), pp. 14194–14196 [DOI] [PubMed] [Google Scholar]
- 10. Torrano A.A., Pereira Â.S., Oliveira O.N Jr. et al.: ‘Probing the interaction of oppositely charged gold nanoparticles with DPPG and DPPC Langmuir monolayers as cell membrane models’, Colloids Surf. B, Biointerfaces, 2013, 108, pp. 120–126 [DOI] [PubMed] [Google Scholar]
- 11. Murphy C.J., Gole A.M., Stone J.W. et al.: ‘Gold nanoparticles in biology: beyond toxicity to cellular imaging’, Acc. Chem. Res., 2008, 41, (12), pp. 1721–1730 [DOI] [PubMed] [Google Scholar]
- 12. Fraga S., Faria H., Soares M.E. et al.: ‘Influence of the surface coating on the cytotoxicity, genotoxicity and uptake of gold nanoparticles in human HepG2 cells’, J. Appl. Toxicol., 2013, 33, (10), pp. 1111–1119 [DOI] [PubMed] [Google Scholar]
- 13. Castiglioni S., Cazzaniga A., Perrotta C. et al.: ‘Silver nanoparticles‐induced cytotoxicity requires ERK activation in human bladder carcinoma cells’, Toxicol. Lett., 2015, 237, (3), pp. 237–243 [DOI] [PubMed] [Google Scholar]
- 14. Leng Y., Li Y., Gong A. et al.: ‘Colorimetric response of dithizone product and hexadecyltrimethyl ammonium bromide modified gold nanoparticle dispersion to 10 types of heavy metal ions: understanding the involved molecules from experiment to simulation’, Langmuir, 2013, 29, (25), pp. 7591–7599 [DOI] [PubMed] [Google Scholar]
- 15. Knecht M.R., Sethi M.: ‘Bio‐inspired colorimetric detection of Hg2+ and Pb2+ heavy metal ions using Au nanoparticles’, Anal. Bioanal. Chem., 2009, 394, (1), pp. 33–46 [DOI] [PubMed] [Google Scholar]
- 16. Bojdi M.K., Mashhadizadeh M.H., Behbahani M. et al.: ‘Synthesis, characterization and application of novel lead imprinted polymer nanoparticles as a high selective electrochemical sensor for ultra‐trace determination of lead ions in complex matrixes’, Electrochim. Acta, 2014, 136, pp. 59–65 [Google Scholar]
- 17. Morrison R.D., Murphy B.L.: ‘Environmental forensics: contaminant specific guide’ (Elsevier, USA, 2010) [Google Scholar]
- 18. Zhu S., Chen B., He M. et al.: ‘Speciation of mercury in water and fish samples by HPLC‐ICP‐MS after magnetic solid phase extraction’, Talanta, 2017, 171, pp. 213–219 [DOI] [PubMed] [Google Scholar]
- 19. Balasubramanian S.K., Jittiwat J., Manikandan J. et al.: ‘Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats’, Biomaterials, 2010, 31, (8), pp. 2034–2042 [DOI] [PubMed] [Google Scholar]
- 20. Sadauskas E., Danscher G., Stoltenberg M. et al.: ‘Protracted elimination of gold nanoparticles from mouse liver’, Nanomed.: Nanotechnol. Biol. Med., 2009, 5, (2), pp. 162–169 [DOI] [PubMed] [Google Scholar]
- 21. Mosmann T.: ‘Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays’, J. Immunol. Methods, 1983, 65, (1–2), pp. 55–63 [DOI] [PubMed] [Google Scholar]
- 22. Rayavarapu R.G., Petersen W., Hartsuiker L. et al.: ‘In vitro toxicity studies of polymer‐coated gold nanorods’, IOP Nanotechnol., 2010, 21, (14), p. 145101 [DOI] [PubMed] [Google Scholar]
- 23. Caputo F., Clogston J., Calzolai L. et al.: ‘Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI‐NCL. A step by step approach combining orthogonal measurements with increasing complexity’, J. Controlled Release, 2019, 299, pp. 31–43 [DOI] [PubMed] [Google Scholar]
- 24. Deng H.H., Li G.W., Hong L. et al.: ‘Colorimetric sensor based on dual‐functional gold nanoparticles: analyte‐recognition and peroxidase‐like activity’, Food Chem., 2014, 147, pp. 257–261 [DOI] [PubMed] [Google Scholar]
- 25. Pandey S., Mishra S.B.: ‘Catalytic reduction of p‐nitrophenol by using platinum nanoparticles stabilised by guar gum’, Carbohydr. Polym., 2014, 113, pp. 525–531 [DOI] [PubMed] [Google Scholar]
- 26. Suvith V.S., Philip D.: ‘Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles’, Spectrochim. Acta, Part A, 2014, 118, pp. 526–532 [DOI] [PubMed] [Google Scholar]
- 27. Krishnamurthy S., Esterle A., Sharma N.C. et al.: ‘Yucca‐derived synthesis of gold nanomaterial and their catalytic potential’, Nanoscale Res. Lett., 2014, 9, (1), p. 627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greulich C., Braun D., Peetsch A. et al.: ‘The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range’, RSC Adv., 2012, 2, (17), pp. 6981–6987 [Google Scholar]
- 29. Loza K., Diendorf J., Sengstock C. et al. ‘The dissolution and biological effects of silver nanoparticles in biological media’, J. Mater. Chem. B, 2014, 2, (12), pp. 1634–1643 [DOI] [PubMed] [Google Scholar]
- 30. Gliga A.R., Skoglund S., Wallinder I.O. et al.: ‘Size‐dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release’, Part. Fibre Toxicol., 2014, 11, (1), pp. 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santoro C.M., Duchsherer N.L., Grainger D.W.: ‘Minimal in vitro antimicrobial efficacy and ocular cell toxicity from silver nanoparticles’, Nanobiotechnology, 2007, 3, (2), pp. 55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mogensen K.B., Kneipp K.: ‘Size‐dependent shifts of plasmon resonance in silver nanoparticle films using controlled dissolution: monitoring the onset of surface screening effects’, J. Phys. Chem. C, 2014, 118, (48), pp. 28075–28083 [Google Scholar]
- 33. Som T., Karmakar B.: ‘Surface plasmon resonance and enhanced fluorescence application of single‐step synthesized elliptical nano gold‐embedded antimony glass dichroic nanocomposites’, Plasmonics, 2010, 5, (2), pp. 149–159 [Google Scholar]


