Abstract
Fluconazole (FLZ) application as a highly successful commercial antifungal azole agent to treat the fungal infections is limited due to emergence of FLZ‐resistant candida. In this study, the potential of green synthesised silver nanoparticles (NPs) as an antifungal agent against Candida albicans fungal pathogen is investigated. The extract of ginger (Zingiber officinale) and thyme (Thymus vulgaris) plays as reducing agent, capping agent and antifungal agent. The UV–visible spectroscopy shows the peak of surface plasmon resonance of synthesised Ag NPs after a period of time. The synthesised Ag NPs are spherical, with average sizes of 12 and 18 nm based on ginger and thyme extract, respectively. Fourier transform infrared spectroscopy confirms the adsorption of the plant extract on the surface of the as‐prepared Ag NPs. Based on the minimum inhibitory concentration (MIC) method against Candida albicans, the antifungal activity of as‐prepared green synthesised Ag NPs shows higher inhibitory in comparison to FLZ. Finally, the Ag NPs synthesised via thyme extract shows no cytotoxicity with concentration below 3.5 ppm, which can be considered as an appropriate candidate instead of FLZ to treat the superficial fungal infections.
Inspec keywords: nanoparticles, surface plasmon resonance, adsorption, nanofabrication, particle size, silver, ultraviolet spectra, antibacterial activity, visible spectra, microorganisms, nanomedicine, Fourier transform infrared spectra, biomedical materials, diseases, materials preparation, cellular biophysics
Other keywords: green synthesis, cell cytotoxicity, antifungal activity, fluconazole application, FLZ‐resistant candida, green synthesised silver nanoparticles, antifungal agent, surface coating, surface plasmon resonance, superficial fungal infections, Zingiber officinale, UV‐visible spectroscopy, Thymus vulgaris extracts, antifungal azole agent, Candida albicans fungal pathogen, plant extracts, ginger, Fourier transform infrared spectroscopy, minimum inhibitory concentration method, Ag
1 Introduction
Superficial fungal infections (SFIs) are increasing worldwide due to direct contact by cross‐border travel. SFIs are generally caused by various fungal organisms such as Candida albicans, which is the most common fungal pathogen for most of the fungal infections in humans [1, 2, 3]. Topical and oral (systemic) therapies are used for the treatment of SFIs [4, 5]. Topical treatment offers several advantages such as avoiding extensive drug absorption, decreasing prolonged systemic exposure, and reducing drug interactions [6]. Nevertheless, oral therapy achieves comparable higher cure rates than do topical treatment [7]. The most commonly used antifungal agents to treat SFIs are azole agents. There are several effective azole agents in different types of formulation [7]. One of the highly successful antifungal azole agents to treat the superficial and invasive fungal infections of C. albicans is fluconazole (FLZ). However, FLZ application is limited due to emergence of FLZ‐resistant candida by the wide spread use of FLZ [8].
By developing nanotechnology, several nanostructures have been introduced as antifungal agents [9, 10, 11]. Among these nanomaterials, silver (Ag) nanoparticles (NPs) have been studied extensively more than others as antibacterial and antifungal agents [12, 13, 14, 15, 16, 17]. Although there are a variety of chemical methods [18, 19, 20, 21] in the preparation of Ag NPs, the ‘green synthesis’ has attracted a lot of interest due to avoiding hazardous waste, synthesising high purified product, economic benefits, and low‐energy consumption [22].
The extract of different parts of various plants such as root, leaf, stem, and fruit can be used in the preparation of Ag NPs as green synthesis method [22, 23, 24, 25, 26]. It has been clarified that the biomolecules extracted from plants are responsible for metal ion reduction, forming the shape of synthesised NPs [27, 28], and colloidal stability by making capping [29, 30, 31] on the particles’ surface during the growth processes. Therefore, the antifungal and antibacterial activities of green synthesised Ag NPs can be improved due to the intrinsic antifungal and antibacterial properties of plant extract. For this purpose, the green synthesis of Ag NPs by thyme (Thymus vulgaris) and ginger (Zingiber officinale) extracts and their antifungal properties against C. albicans versus FLZ antifungal agent have been investigated. It should be noted that thyme and ginger extracts demonstrated anti‐inflammatory, antioxidant [32, 33, 34, 35], and antimicrobial [36, 37] properties.
To the best of our knowledge, thyme and ginger extracts have not been used in the synthesis of Ag NPs. The main purpose of this work is the green synthesis of Ag NPs based on the thyme and ginger extracts and investigate its antifungal properties against Candida albicans in comparison to commercial antifungal agent (FLZ). Eventually, cytotoxicity of the as‐synthesised Ag NPs via ginger and thyme on a human dermal fibroblast cell line (HDF‐1) was assessed. Our results show that the as‐prepared Ag NPs can be used instead of FLZ to treat SFIs with much lower concentrations.
2 Materials and methods
2.1 Extract preparation
To prepare the plant extract, in the initial stage, ginger rhizome and thyme's leaves were cleaned and washed with deionised (DI) water. After that, the plants were sliced into fine pieces and dried in the dark for 4 days. A total of 0.2 g of the prepared pieces of each plant were added to 100 mL of DI water and stirred at 80 °C for 40 min. For further purification, the extract solution was filtered through Whatmann No.1 filter paper and centrifuged at 4000 rpm for 5 min. It should be noted that the obtained extract was filtered by a 0.22 µm syringe filter and kept at 4 °C in a dark place for the green synthesis of Ag NPs.
2.2 Green synthesis of Ag NPs
The 1 mL of the prepared extract was added to 20 mL of 2 mM aqueous solution of AgNO3 (0.0068 g AgNO3 into 20 mL of DI water). Silver nitrate was purchased from Merck Company by 99.9% purity). In the beginning, the colour of the solution is light yellow and changes to dark brown by progress of reaction which confirms the formation of Ag NPs. This colour alteration could be attributed to the formation of Ag NPs. To characterise the prepared particle and investigate the antifungal activity, the final solution was dialysed against water for 24 h by 12 kDa dialysis bag and finally filtered by a 0.22 µm syringe filter. The schematic of the extract preparation steps and Ag NPs synthesis are shown in Fig. 1.
Fig. 1.
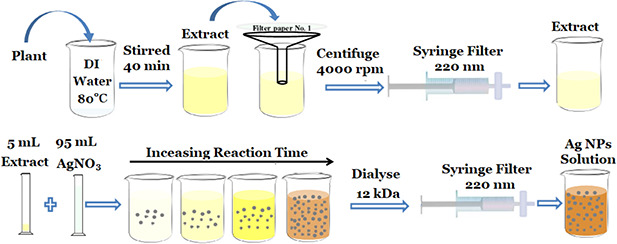
Schematic of the extract preparation and Ag NPs synthesis via plant extract (best viewed online in colour)
2.3 Characterisation of Ag NPs
To detect and confirm the formation of Ag NPs during the synthesis of Ag NPs via ginger and thyme extracts, the UV–visible spectroscopy has been performed at room temperature using a SPUV‐26 SC‐Tech spectrophotometer. Fourier transform infrared spectroscopy (FTIR) was used to confirm the capping of the extract on the surface of Ag NPs by a Frontier PerkinElmer spectrometer. Transmission electron microscopy (TEM) was used to find the morphology of the as‐synthesised Ag NPs via a Leo 912 AB microscope.
2.4 Antifungal study
The antifungal activity was examined by considering the minimum inhibitory concentration (MIC) according to broth micro‐dilution method based on Clinical and Laboratory Standards Institute M27‐A3 guidelines [38]. MIC is the lowest concentration of antifungal agent that prevents the visible growth of particular microorganisms.
A standard strain of C. albicans fungi (ATCC10231obtained from Pasteur Institute of Iran) was grown on Sabouraud Dextrose Agar growth medium containing chloramphenicol and kept in an incubator at 35°C. A total of 100 µL of Ag NPs solution (100 ppm) was added into a 100 µL sterile Roswell Park Memorial Institute (RPMI) medium and then subjected to twofold serial dilution with RPMI medium in a 96‐well plate. Then, 100 µL of C. albicans culture in phosphate‐buffered saline (1 × 106 cell/mL) was introduced into each well containing compound solution. Two wells were considered as positive and negative controls. The positive control well includes 100 µL of the growth medium and 100 µL of fungal suspension, and the negative control well contains 200 µL of the growth medium. After overnight incubation at 28°C, optical density measurements were conducted using a micro‐plate spectrophotometer (Stat Fax 4300, Awareness Company, Elisa Plate Reader). It should be noted that all experiments were conducted in triplicates.
2.5 Cytotoxicity of the as‐synthesised Ag NPs on a human dermal fibroblast cell line
To evaluate the cytotoxicity of Ag NPs, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT)‐based assay was carried out according to the previously described protocol [39]. A HDF‐1 was purchased from Iranian Biological Resource Center (Tehran, Iran). In brief, HDF‐1 cells were transferred to a 96‐well microtitration plate in 200 μl of low glucose Dulbecco's modified Eagle's medium containing 10% foetal bovine serum. The seeding density was 2500 cells per well. After 3 days, the cells entered the logarithmic phase of growth and exposed to green synthesised Ag NPs with various concentrations (0.5, 1.25, 2.5, 5, and 10 ppm). This procedure was repeated for three times to achieve dependable results. Afterward, the Ag NPs were removed from the wells after 72 h. In order to display the regenerative capacity of the exposed cells that survived, 4 days were considered for the recovery period. During this step, fresh medium was harnessed to feed the plates daily. After that, at the end of the recovery period, 50 μl of MTT solution (5 mg/ml) was added to each well.
Then, the plates were further incubated for 4 h. Next, all remained supernatant were effaced. Thereupon, to dissolve the shaped unsolvable formazan crystals, 200 μl of dimethyl sulfoxide (DMSO) was added. To regulate the final pH, 25 μl of glycine buffer was poured to each well. Afterward, by a microtitration plate reader (BioTek®, USA) absorbance was instantly recorded at 570 nm. The utter values of the absorbance changed to surviving fraction data as the percentage of living cells of the control.
3 Results and discussion
3.1 Green synthesis of Ag NPs
The colour changes of synthesis solution are demonstrated in Fig. 2 in which the colour of mixture of AgNO3 and extract solution is changed from colourless to reddish brown and there is no more change in the colour after the reaction goes to completion. To confirm the formation of Ag NPs, UV–visible spectra are recorded against water. The UV–visible spectra related to the synthesis of Ag NPs via ginger and thyme extracts are shown in Figs. 2 a and b. The appeared peak in the range of 400–460 nm is related to the surface plasmon resonance (SPR) of the formed Ag NPs [40]. It can be observed that the SPR intensity increases by increasing the reaction time which confirms the synthesis of more Ag NPs. Indeed, Ag clusters are formed continuously by increasing the reaction. In addition, it can be observed that the rate of reaction for thyme extract is higher than that for ginger. The initial SPR band of Ag NPs appeared after 30 and 60 min in the case of using thyme and ginger as reducing agents of Ag NPs, respectively. Moreover, for the same reaction times, the intensity of SPR for thyme is higher than that for ginger. Indeed, the thyme extract reduces Ag ions faster than ginger in the same concentration. Similar results can be observed for higher concentrations of the extract which is reported in supplementary information 1. It should be noted that at high concentration of the extract, the rate of reaction increased and more Ag NPs are formed due to more reducing agent.
Fig. 2.
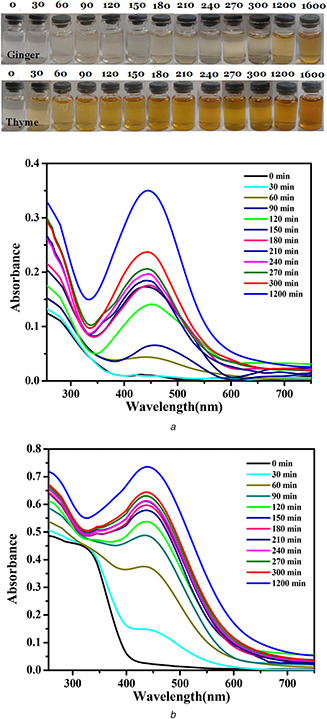
Colour changes of synthesis solution and their corresponding UV–visible spectra of Ag NPs synthesised in different times (in minutes) via
(a) Ginger extracts, (b) Thyme extracts
Fig. 3 shows the typical TEM images of synthesised Ag NPs via ginger (a) and thyme (b) extracts. Ag NPs are spherical in both cases, with the mean sizes of 12 and 18 nm for ginger and thyme, respectively. Here, it should be noted that the ginger extract concentration (5 g of ginger in 100 mL of DI water) is 25 times more than that of the thyme extract. At this condition, Ag ions are reduced more by thyme extract and the final Ag NPs’ size is increased more in comparison to ginger extract which again confirms higher reduction power of the thyme extract.
Fig. 3.
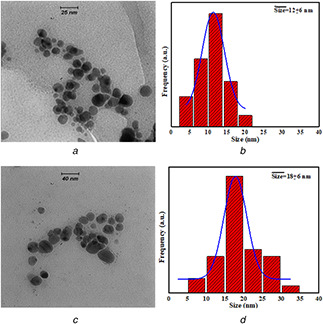
Typical TEM images of Ag NPs synthesised via
(a) Ginger extracts, (b) Their size distribution histograms, (c) Thyme extracts, (d) Their size distribution histograms
Various mechanisms [41, 42, 43] have been proposed to explain the formation of Ag NPs via plant extracts. Releasing or sharing of electrons from the plant extract is responsible for reduction of positive ions, and the phenolic compounds, OH, C=O, and CH group are main sources of electron supplier. Here, we also showed that the light or heating is necessary for reducing Ag ions via plant extract. Indeed, to supply electrons from the plant extract the reaction solution should be in elevated temperature or radiated with environmental light. Fig. 4 shows the effect of light and temperature on the synthesis of Ag NPs after 90 min of reaction. Sample‐A was synthesised in a normal condition, i.e. at day light and room temperature. Both B and C samples were kept in dark, and sample‐C was also heated around 60°C. There is no sign of the synthesis of Ag NPs after 90 min for sample B; however, Ag NPs have been synthesised for both samples A and C, which is an evidence for the catalytic role of light and temperature on the synthesis of Ag NPs via plant extracts. Broader peak in sample‐B in comparison to sample‐A may be due to broader size distribution and or more uneven NPs [44]. It is well established that the effect of temperature on size is dual. It means that as long as there are free electrons and positive ions, temperature promotes the reaction and more Ag NPs are synthesised. In parallel, temperature also increases the size of NPs after nucleation.
Fig. 4.
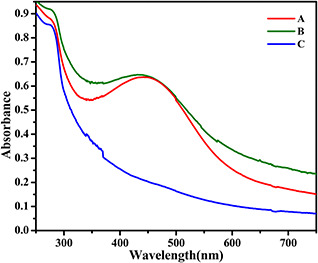
UV–visible spectra of Ag NPs synthesised via ginger extract synthesised
(a) At room temperature in environmental light, (b) At temperature of 60°C in dark, (c) At room temperature in dark
FTIR spectroscopy was carried out to recognise functional groups of extract and its effect as reducing and capping agents on the formation of Ag NPs. Fig. 5 shows FTIR spectra of extracts and synthesised Ag NPs. Fig. 5 a shows the FTIR analysis of ginger extract and Ag NPs synthesised via ginger extract. The spectra at 3288 and 3275 cm−1 belong to OH stretch bonds. The weak peaks at 2936 and 2392 cm−1 are related to OH stretching of carboxylic acid group [45]. The bands at 1644 and 1764 cm−1 are due to C=O stretch of alkyne and the band at 1610 cm−1 are related to C=C stretch [46]. The bands created at 1388 cm−1 are CH3 symmetric bonds of the alkene group. The band at 1077 cm−1 and the weak peaks at 1250 cm−1 could be due to C–O–C ether group. Other weak peaks at 1110 and 1044 cm−1 are related to C–OH stretching [47]. OH phenolic bonds are seen at 617 and 618 cm−1 [46]. The bands below 700 cm−1 can be related to Ag NPs and Ag‐extract vibration.
Fig. 5.
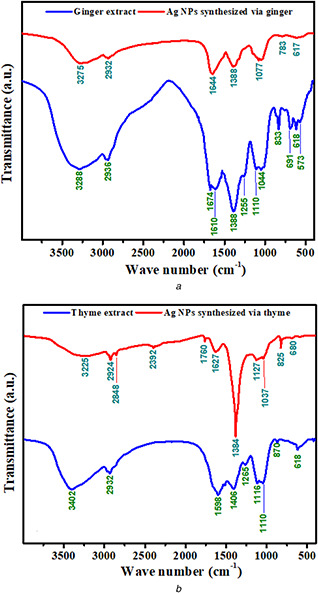
FTIR spectra of extracts and Ag NPs synthesised via
(a) Ginger, (b) Thyme
Similarly, Fig. 5 b shows the bands of thyme extract and synthesised Ag NPs via thyme extract. The bands at 3402 and 3225 cm−1 indicate the OH bond. The peaks at 2932, 2924, and 2848 cm−1 point to CH stretching vibration related to CH2 and CH3. The medium peaks at 1760, 1627, and 1598 cm−1 are due to C=O stretching. The bands at 1403 and 1384 cm−1 are related to CH symmetric stretching of the amide group. Therefore, substantial reduction in peak intensity could indicate the formation of Ag NPs that its peak has occurred at 825 cm−1. The presence of bands with weak strength was also observed at 1037, 1127, 1116, and 1110 cm−1 which confirmed C–O bond of carbohydrates. Finally, the weak peaks at 680 and 617 cm−1 are interrelated to OH bond of the phenolic group.
According to the FTIR results of extracts and the Ag NPs synthesised via extracts, it is obvious that extracts are responsible for Ag NPs synthesis. The bond variation indicates that extracts provide required electrons for Ag NPs formation by the OH bond separation. Furthermore, this bond collapse leads to attach extracts to the Ag NPs. Therefore, FTIR confirms that extracts are adsorbed onto the surface of Ag NPs and play a capping role.
3.2 Antifungal activity
The antifungal activity of Ag NPs, pure extracts, silver nitrate, and FLZ against C. albicans was investigated by MIC method and the results are shown in Table 1. The extract of ginger and thyme demonstrated MIC at 1900 and 1100 ppm, respectively. Silver nitrate demonstrated MIC of 5.2 ppm. The results showed that the MIC of silver nitrate and Ag NPs synthesised by ginger and thyme were 5.2, 0.7, and 0.5 µg/ml, respectively. It can be inferred that the Ag NPs prepared via ginger and thyme extract demonstrated a higher inhibitory effect against C. albicans in comparison to silver nitrate and FLZ with MIC of 16 ppm. Indeed, Ag NPs synthesised with thyme and ginger extract demonstrated lower MIC which can be attributed to the synergetic effect of Ag NPs and plant extract. It has been shown that the antifungal activity of the extract of thyme and ginger may be involved due to their hydrophobic properties of these compounds which can attach to the fungal plasma membrane and interfere fungal proliferation by increasing the membrane permeability or inhibit spore germination and cell respiration [48]. On the other hand, Ag NPs demonstrated antifungal activity by attaching to cell membrane of fungi and interrupting the membrane integrity and finally destructing the membrane structure [49]. As mentioned previously, the FTIR analysis confirmed that the extract of ginger and thyme was coated on the surface of the as‐synthesised Ag NPs. Therefore, prepared Ag NPs attach more to the cell membrane and destruct fungi plasma membrane, and finally prohibit the growth of C. albicans. It should be noticed that the combination of natural extract with Ag NPs may open diverse antifungal mechanisms.
Table 1.
MIC of Ag NPs, pure extracts, silver nitrate, and FLZ against C. albicans
| Sample | Ag NPs via ginger | Ag NPs via thyme | AgNO3 | Ginger extract | Thyme extract | FLZ |
|---|---|---|---|---|---|---|
| concentration in µg/ml, ppm | 0.7 | 0.5 | 5.2 | 1900 | 1100 | 16 |
3.3 MTT‐based cytotoxicity
Fig. 6 shows the cell cytotoxicity of green synthesised Ag NPs via plant extracts which evaluated on a human dermal fibroblast cell line based on MTT assay. The results show that Ag NPs synthesised by ginger extract is toxic at the concentrations above 1.25 ppm, meanwhile, Ag NPs synthesised by thyme extract demonstrated no cytotoxicity effect below the concentration of 3.5 ppm. Indeed, the results indicated that Ag NPs synthesised by thyme extract show lower cytotoxicity in comparison to Ag NPs synthesised by the ginger extract. By considering similar MIC for both Ag NPs synthesised via ginger and thyme extract, and lower toxicity for Ag NPs synthesised via thyme extract, it can be inferred that Ag NPs synthesised via thyme extract can be considered as an appropriate candidate instead of FLZ to treat the SEIs.
Fig. 6.
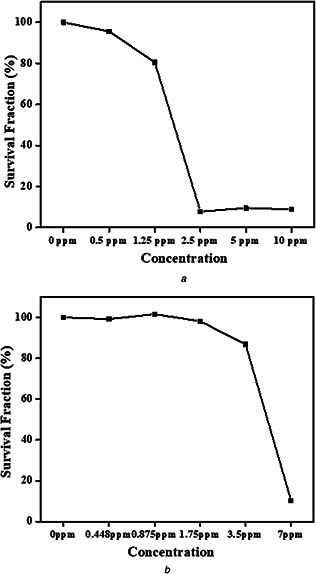
Cytotoxicity study of green synthesised Ag NPs against HDF‐1 via
(a) Ginger extracts, (b) Thyme extracts
4 Conclusion
In this study, Ag NPs were prepared based on the green method by considering the intrinsic antifungal activity of natural extract (ginger and thyme). UV–visible spectra approved the formation of Ag NPs based the formation of SPR peak in the range of 440–450 nm. Moreover, the UV–visible results showed that thyme extract has higher reducing power in comparison to ginger extract. Spherical Ag NPs have been synthesised via ginger and thyme extract with average diameters of 12 and 18 nm, respectively. The Ag NPs prepared via ginger and thyme extract demonstrated higher inhibitory (MIC ∼ 0.5 ppm) in comparison to FLZ (MIC ∼ 16 ppm) against C. albicans. The cell cytotoxicity results demonstrated that Ag NPs are toxic at the concentration above 1.25 and 3.5 ppm based ginger and thyme extracts synthesis, respectively. Ag NPs synthesised via thyme extract can be considered as an appropriate candidate instead of FLZ to treat the SEIs due to its lower toxicity and MIC.
5 References
- 1. Martin M.V.: ‘The use of fluconazole and itraconazole in the treatment of Candida albicans infections: a review’, J. Antimicrob. Chemother., 2000, 45, (4), pp. 555 –555 [DOI] [PubMed] [Google Scholar]
- 2. Nobile C.J. Johnson A.D.: ‘Candida albicans biofilms and human disease’, Annu. Rev. Microbiol., 2015, 69, pp. 71 –92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayer F.L. Wilson D. Hube B.: ‘Candida albicans pathogenicity mechanisms’, Virulence, 2013, 4, (2), pp. 119 –128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hawkins D.M. Smidt A.C.: ‘Superficial fungal infections in children’, Pediatric Clinics, 2014, 61, (2), pp. 443 –455 [DOI] [PubMed] [Google Scholar]
- 5. Hay R.: ‘Superficial fungal infections’, Medicine, 2017, 45, (11), pp. 707 –710 [Google Scholar]
- 6. Das Neves J. Pinto E. Teixeira B. et al.: ‘Local treatment of vulvovaginal candidosis’, IET Nanobiotechnol., 2018, 12, (5), pp. 574 –578 30095415 [Google Scholar]
- 7. Sobel J.D.: ‘Vulvovaginal candidosis’, The Lancet, 2007, 369, (9577), pp. 1961 –1971 [DOI] [PubMed] [Google Scholar]
- 8. Ng S.M.S. Yap Y.Y.A. Cheong J.W.D. et al.: ‘Antifungal peptides: a potential new class of antifungals for treating vulvovaginal candidiasis caused by fluconazole–resistant Candida albicans’, J. Pept. Sci., 2017, 23, (3), pp. 215 –221 [DOI] [PubMed] [Google Scholar]
- 9. Beltrán‐Partida E. Valdes‐Salas B. Curiel‐Alvarez M. et al.: ‘Enhanced antifungal activity by disinfected titanium dioxide nanotubes via reduced nano‐adhesion bonds’, Mater. Sci. Eng., C, 2017, 76, pp. 59 –65 [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro R.F. Motta M.H. Härter A.P.G. et al.: ‘Spray‐dried powders improve the controlled release of antifungal tioconazole‐loaded polymeric nanocapsules compared to with lyophilized products’, Mater. Sci. Eng., C, 2016, 59, pp. 875 –884 [DOI] [PubMed] [Google Scholar]
- 11. Huang W. Bao Y. Duan H. et al.: ‘Antifungal effect of green synthesised silver nanoparticles against setosphaeria turcica’, IET Nanobiotechnol., 2017, 11, (7), pp. 803 –808 [Google Scholar]
- 12. Ahmed S. Ahmad M. Swami B.L. et al.: ‘A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise’, J. Adv. Res., 2016, 7, (1), pp. 17 –28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahadevan S. Vijayakumar S. Arulmozhi P.: ‘Green synthesis of silver nano particles from Atalantia monophylla (L) Correa leaf extract, their antimicrobial activity and sensing capability of H2 O2 ’, Microb. Pathog., 2017, 113, pp. 445 –450 [DOI] [PubMed] [Google Scholar]
- 14. Ahluwalia V. Elumalai S. Kumar V. et al.: ‘Nano silver particle synthesis using Swertia paniculata herbal extract and its antimicrobial activity’, Microb. Pathog., 2018, 114, pp. 402 –408 [DOI] [PubMed] [Google Scholar]
- 15. Kelkawi A.H.A. Kajani A.A. Bordbar A.‐K.: ‘Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity’, IET Nanobiotechnol., 2016, 11, (4), pp. 370 –376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allafchian A. Jalali S.A.H. Aghaei F.: ‘Green synthesis of silver nanoparticles using glaucium corniculatum (L.) curtis extract and evaluation of its antibacterial activity’, IET Nanobiotechnol., 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan C.‐G. Huo C. Gui B. et al.: ‘Facile phyto‐mediated synthesis of silver nanoparticles using Chinese winter jujube (Ziziphus jujuba Mill. cv. Dongzao) extract and their antibacterial/catalytic properties’, IET Nanobiotechnol., 2017, 11, (8), pp. 973 –980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Navaladian S. Viswanathan B. Viswanath R.P. et al.: ‘Thermal decomposition as route for silver nanoparticles’, Nanoscale Res. Lett., 2007, 2, (1), p. 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez‐Sanchez L. Blanco M. Lopez‐Quintela M.: ‘Electrochemical synthesis of silver nanoparticles’, J. Phys. Chem. B, 2000, 104, (41), pp. 9683 –9688 [Google Scholar]
- 20. Hu B. Wang S.B. Wang K. et al.: ‘Microwave‐assisted rapid facile ‘green’ synthesis of uniform silver nanoparticles: self‐assembly into multilayered films and their optical properties’, J. Phys. Chem. C, 2008, 112, (30), pp. 11169 –11174 [Google Scholar]
- 21. Anjugam M. Vaseeharan B. Iswarya A. et al.: ‘Biological synthesis of silver nanoparticles using β‐1, 3 glucan binding protein and their antibacterial, antibiofilm and cytotoxic potential’, Microb. Pathog., 2018, 115, pp. 31 –40 [DOI] [PubMed] [Google Scholar]
- 22. Rafique M. Sadaf I. Rafique M.S. et al.: ‘A review on green synthesis of silver nanoparticles and their applications’, Artif. Cells Nanomed. Biotechnol., 2016, 45, (7), pp. 1 –20 [DOI] [PubMed] [Google Scholar]
- 23. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, (10), pp. 2638 –2650 [Google Scholar]
- 24. Kharissova O.V. Dias H.V.R. Kharisov B.I. et al.: ‘The greener synthesis of nanoparticles’, Trends Biotechnol., 2013, 31, (4), pp. 240 –248 [DOI] [PubMed] [Google Scholar]
- 25. Mittal A.K. Chisti Y. Banerjee U.C.: ‘Synthesis of metallic nanoparticles using plant extracts’, Biotechnol. Adv., 2013, 31, (2), pp. 346 –356 [DOI] [PubMed] [Google Scholar]
- 26. Gao L. Li Q. Zhao Y. et al.: ‘Silver nanoparticles biologically synthesised using tea leaf extracts and their use for extension of fruit shelf life’, IET Nanobiotechnol., 2017, 11, (6), pp. 637 –643 [Google Scholar]
- 27. Dubey S.P. Lahtinen M. Särkkä H. et al.: ‘Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids’, Colloids Surf. B, Biointerfaces, 2010, 80, (1), pp. 26 –33 [DOI] [PubMed] [Google Scholar]
- 28. Chandran S.P. Chaudhary M. Pasricha R. et al.: ‘Synthesis of gold nanotriangles and silver nanoparticles using aloevera plant extract’, Biotechnol. Prog., 2006, 22, (2), pp. 577 –583 [DOI] [PubMed] [Google Scholar]
- 29. Jacob S.J.P. Finub J. Narayanan A.: ‘Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep‐2 cell line’, Colloids Surf. B, Biointerfaces, 2012, 91, pp. 212 –214 [DOI] [PubMed] [Google Scholar]
- 30. Shameli K. Ahmad M. Al‐Mulla E.A. et al.: ‘Green biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extraction’, Molecules, 2012, 17, (7), pp. 8506 –8517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaviya S. Santhanalakshm J. Viswanathan B. et al.: ‘Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity’, Spectrochim. Acta, Part A, 2011, 79, (3), pp. 594 –598 [DOI] [PubMed] [Google Scholar]
- 32. Bellik Y.: ‘Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale roscoe’, Asian Pac. J. Trop. Dis., 2014, 4, (1), pp. 40 –44 [Google Scholar]
- 33. Mashhadi N.S. Ghiasvand R. Askari G. et al.: ‘Anti‐oxidative and anti‐inflammatory effects of ginger in health and physical activity: review of current evidence’, Int. J. Prev. Med., 2013, 4, p. S36 [PMC free article] [PubMed] [Google Scholar]
- 34. Wang S. Zhang C. Yang G. et al.: ‘Biological properties of 6‐gingerol: a brief review’, Nat. Prod. Commun., 2014, 9, (7), pp. 1027 –1030 [PubMed] [Google Scholar]
- 35. Yeh H.‐Y. Chuang C. Chen H. et al.: ‘Bioactive components analysis of two various gingers (Zingiber officinale roscoe) and antioxidant effect of ginger extracts’, LWT‐Food Sci. Technol., 2014, 55, (1), pp. 329 –334 [Google Scholar]
- 36. Karuppiah P. Rajaram S.: ‘Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple‐drug resistant clinical pathogens’, Asian Pac. J. Trop. Biomed., 2012, 2, (8), pp. 597 –601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sulaiman F.A. Kazeem M.O. Waheed A.M. et al.: ‘Antimicrobial and toxic potential of aqueous extracts of Allium sativum, Hibiscus sabdariffa and Zingiber officinale in wistar rats’, J. Taibah Univ. Sci., 2014, 8, (4), pp. 315 –322 [Google Scholar]
- 38. CLSI : ‘Performance standards for antimicrobial susceptibility testing: twenty‐third informational supplement’, 2013.
- 39. Sadighi S. Amanpour S. Behrouzi B. et al.: ‘Lack of metformin effects on different molecular subtypes of breast cancer under normoglycemic conditions: an in vitro study’, Asian Pac. J. Cancer Prev., 2014, 15, (5), pp. 2287 –2290 [DOI] [PubMed] [Google Scholar]
- 40. Budhiraja N. Sharma A. Dahiya S. et al.: ‘Synthesis and optical characteristics of silver nanoparticles on different substrates’, Int. Lett. Chem. Phys. Astron., 2013, 14, p. 80 [Google Scholar]
- 41. Rauwel P. Küünal S. Ferdov S. et al.: ‘A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM’, Adv. Mater. Sci. Eng., 2015, 2015, p. 682749 [Google Scholar]
- 42. Kesharwani J. Yoon K.Y. Hwang J. et al.: ‘Phytofabrication of silver nanoparticles by leaf extract of Datura metel: hypothetical mechanism involved in synthesis’, J. Bionanosci., 2009, 3, (1), pp. 39 –44 [Google Scholar]
- 43. Kumar V. Gundampati R.K. Singh D.K. et al.: ‘Photo‐induced rapid biosynthesis of silver nanoparticle using aqueous extract of Xanthium strumarium and its antibacterial and antileishmanial activity’, J. Ind. Eng. Chem., 2016, 37, (Supplement C), pp. 224 –236 [Google Scholar]
- 44. Liu Y.‐S. Chang Y.‐C. Chen H.‐H.: ‘Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants’, J. Food Drug Anal., 2018, 26, (2), pp. 649 –656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stojanovic R. Belscak‐Cvitanovic A. Manojlovic V. et al.: ‘Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads’, J. Sci. Food Agric., 2012, 92, (3), pp. 685 –696 [DOI] [PubMed] [Google Scholar]
- 46. Devi A. Das V.K. Deka D.: ‘Ginger extract as a nature based robust additive and its influence on the oxidation stability of biodiesel synthesised from non‐edible oil’, Fuel, 2017, 187, pp. 306 –314 [Google Scholar]
- 47. Purnomo H. Jaya F. Widjanarko S.: ‘The effects of type and time of thermal processing on ginger (Zingiber officinale roscoe) rhizome antioxidant compounds and its quality’, Int. Food Res. J., 2010, 17, (2), pp. 335 –347 [Google Scholar]
- 48. Kim K.‐J. Sung W.S. Suh B.K. et al.: ‘Antifungal activity and mode of action of silver nano‐particles on Candida albicans’, Biometals, 2009, 22, (2), pp. 235 –242 [DOI] [PubMed] [Google Scholar]
- 49. De Castro R.D. De Souza T.M.P.A. Bezerra L.M.D. et al.: ‘Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: an in vitro study’, BMC Complement. Altern. Med., 2015, 15, (1), p. 417 [DOI] [PMC free article] [PubMed] [Google Scholar]


