Abstract
The present study was designed to check the role of silver nanoparticles (AgNPs) on physiological, biochemical parameters and antioxidants of wheat (Triticum aestivum L.) under heat stress. Plant extract of Moringa oleifera was used for AgNPs synthesis followed by characterization through UV–Vis spectroscopy, SEM, XRD and Zeta analyser. Heat stress was applied in range of 35–40°C for 3 hrs/ day for 3 days to wheat plants at trifoliate stage. Heat stress decreased the RWC (13.2%), MSI (16.3%), chl a (5.2%), chl b (4.1%) and TCCs (9.9%). Wheat plants treated with AgNPs showed significant increase in RWC (12.2%), MSI (26.5%), chl a (10%), chl b (16.4%), TCCs (19%), TPC (2.4%), TFC (2.5%), TASC (2.5%), SOD (1.3%), POX (1.5%), CAT (1.8%), APX (1.2%) and GPX (1.4%), under heat stress. Lower concentration of AgNPs (50 mg/l) decreased the sugar (5.8%) and proline contents (4%), while increase was observed in higher AgNPs concentrations. Overall, AgNPs treatment enhanced thermo‐tolerance in wheat plants, but the mechanism of AgNPs action needs further investigation at genome and proteome level in wheat plants under heat stress.
Inspec keywords: crops, ultraviolet spectra, X‐ray diffraction, visible spectra, botany, microorganisms, scanning electron microscopy, solubility, agricultural products, nanoparticles, antibacterial activity, sugar, silver, food products, biochemistry
Other keywords: heat stress, heat‐stress tolerance, wheat plants, AgNPs exposure, wheat antioxidative defence system, Triticum aestivum L, Moringa oleifera, biochemical parameters, silver nanoparticles, ultraviolet–visible spectroscopy, scanning electron microscopy, X‐ray diffraction, zeta analyser, total chlorophyll contents, TCCs, membrane stability index, MSI, malondialdehyde, sugar level, proline concentration, hydrogen peroxide, RWC, relative water content, total flavonoid content, phenolic content, ascorbate POX, guaiacol POX
1 Introduction
Among cereals, wheat (Triticum aestivum L.) is a staple food in many areas of the world including Pakistan, with a global annual yield of about 650,000,000 tons [1]. To ensure food security, there is a need to double the wheat production by 2050 [2]. Currently, the temperature of the present world is increasing as a severe threat due to unnecessary changes in the environment. According to Intergovernmental Panel on Climate Change [3], the overall temperature will be 1.8–4.0°C more in 2100 than the present, with an average rise of 0.2°C per decade. This forecast is a severe threat for the survival of living organisms including microorganisms, plants and animals. Plants being immobile are more susceptible to get affected by heat stress [4]. Many aspects of plants such as growth, development, physiological changes and yield are severely affected by heat tress due to oxidative stress, caused by overproduction of reactive oxygen species (ROS) [5]. Plants are in constant effort to survive under heat stress through altering morphology, metabolic and signalling process and osmolytes production to sustain cell turgidity by osmotic adjustment and modifying the antioxidant system for smooth homeostasis [6]. Exogenous application of various growth substances (osmoprotectants, plant hormones, polyamines and trace elements) are in daily practise for overwhelming damage of heat stress in plants [7].
Recently, engineered nanoparticles (NPs) have been widely used in many industries worldwide [8]. However, the significant impact of metal NPs on plant growth is of major concern for food quality. The plants treated with NPs could absorb and allocate NPs to various plant parts. Various studies showed adverse effects of NPs on seed germination, plant growth, nutrient uptake and photosynthesis in different media such as agar, nutrient solutions and soil [9]. Among NPs, silver NPs (AgNPs) have diverse applications, as a growth enhancer in agriculture [9] and antimicrobial agent in cosmetics, household products and in water filters. In the literature, both positive and negative effects of AgNPs on plant growth have been illustrated [8]. Lower concentrations of AgNPs, up to 30 and 50 mg/l increase the growth of rice, maize, bean and wheat while high concentrations inhibited the growth of these crops [9].
Similarly, AgNPs increase or decrease the plant growth and biomass depending on the size, dose and duration of exposure [10]. AgNPs are very effective in plant protection against environmental factors such as pathogenic bacteria and fungi, salt and drought stress, heavy metals stress in plants and pests incidence. During the past few decades, AgNPs have been recognised as the most important antibacterial agents and prevent plants from bacterial diseases [11]. Few studies suggested that AgNPs applied as biosurfactants and biofilms on plants to protect against environmental bacteria, pathogenic fungi [12, 13] and land snails [14]. The effect of AgNPs has a stimulating role on fenugreek and tomato seed germination under salinity stress [15, 16]. AgNPs promote lentil seed germination under drought stress [17]. Recently, AgNPs have been reported as pesticides for controlling plants pests [18]. Nowadays, AgNPs are involved to detect the heavy metal presence in water absorbed by plants [19], ultimately predicts the level of heavy metal contamination in plants grown at a particular area [20]. At present, AgNPs have been utilised as an efficient antibacterial agent for controlling canker disease in Citrus plants [21]. AgNPs are also very effective against black mould (Stachybotrys chartarum) species commonly grown on plants [22]. Similarly, AgNPs also protect rice plants from aflatoxins production due to Aspergillus species due to their antifungal properties. Similarly, Abou‐Zeid and Ismail [23] reported that AgNPs priming notably improves growth parameters and photosynthetic efficiency as well phytohormones balance suggesting that AgNPs played a role in the improvement of plant tolerance against environmental stresses such as salt stress.
Overall, AgNPs are playing a very effective role in plants protection against various environmental stresses. However, very less work has been done on the role of AgNPs in wheat plants under heat stress [9]. It was hypothesised that AgNPs treatments (in different doses) may alleviate the heat stress in wheat plants by decreasing the oxidative stress through modification of antioxidant enzyme activities. Therefore, the purpose of current study was (i) to assess the role of different concentrations of AgNPs (25, 50, 75 and 100 mg/l) in physiological, biochemical and antioxidant processes in wheat plants under heat stress and (ii) to investigate AgNPs potential in alleviation of ROS‐based oxidative damage in wheat plants under heat stress.
2 Material and methods
2.1 Plant extract and AgNPs preparation
Fresh leaves of the plant (Moringa oleifera) were collected from the main campus of Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, Pakistan. The plant extract was prepared according to the method described by Hussain et al. [24]. AgNPs were prepared by using Ag nitrate (AgNO3) as raw salt. AgNPs synthesis was carried out through the protocol given by Hussain et al. [24]. AgNPs were characterised through ultraviolet–visible (UV–vis) spectrophotometer, scanning electron microscopy (SEM), X‐ray diffraction (XRD) analysis and zeta analyser (Figs. 1, 2, 3, 4).
Fig. 1.
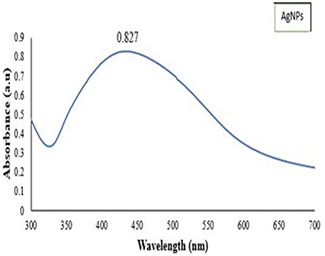
UV–vis absorption spectrum of synthesised AgNPs
Fig. 2.
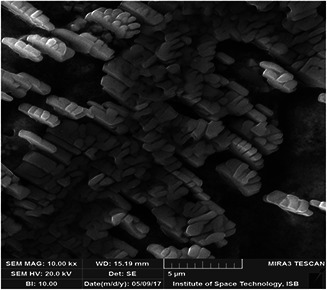
SEM micrograph of synthesised AgNPs
Fig. 3.
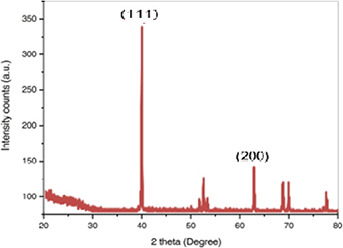
XRD pattern of synthesised AgNPs
Fig. 4.
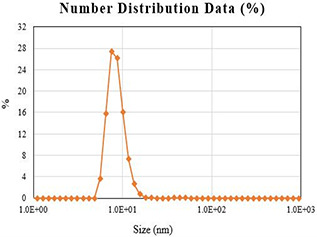
Zeta analyser showing size distribution of synthesised AgNPs
UV–vis spectroscopy was done from the Islamic International University, Islamabad. It is a frequently used technique for initial detection of AgNPs. UV–vis spectrum was recorded from the range of 300–700 nm. The shape of the synthesised AgNPs was checked by using SEM, SIGMA model operated at 5 kV, magnification ×10k from the Institute of Space and Technology, Islamabad. The sample surface images were taken at high magnification. The crystalline nature of the synthesised AgNPs was determined through XRD spectroscopy machine Shimadzu model XRD 6000 in the range of 5–50° in the 2θ angle at the National Centre of Physics, Islamabad. The size of AgNPs was estimated through zeta sizer from Nuclear Institute of Biotechnology and Genetic Engineering, Faisalabad. The synthesised NPs were ultrasonicated for 15 min and then size range was determined through zeta potential.
2.2 Plant material and growth conditions
Wheat seeds of variety Pakistan‐2013 were acquired from Crop Sciences Institute‐National Agricultural Research Centre Islamabad, Pakistan. For sterilisation, viable seeds were immersed in 10% sodium hypochlorite solution for 10 min, followed by three times washing with distilled water [25]. The pot experiment was carried out during a complete growing season (2016–2017) in the experimental area (Glass house) of Department of Botany, Faculty of Sciences, University of Arid Agriculture Rawalpindi (33°38′51″N, 73°4′57.72″E) at temperature 23 ± 2.5°C, relative humidity between 25 and 52% and a mid‐day photosynthetic photon flux density of 405 ± 7.5 μmol m−2 s−1. The wheat seeds were directly sown by hand in sandy loam soil (sand: 52.5%, silt: 2.5% and clay: 45%) with the density of 4–6 seeds per pot. All plants grown in the specified soil media were irrigated with water to avoid water stress and no fertiliser was applied during the entire growing seasons. Weeds were periodically removed by hand. The whole experiment was performed from 15 November 2016 to 20 February 2017.
2.3 AgNPs treatments and heat‐stress exposure
Wheat plants were treated with 25, 50, 75 and 100 mg/l of AgNPs at three leaf stage. The plants were irrigated with 16.7, 33.3, 50, and 66.7 ml of AgNPs as input volume from respective stock solutions (25, 50, 75 and 100 mg/l AgNPs) in the pots. Plants without any treatment were marked as a control. Exposure of heat stress was given according to a method designed by Iqbal et al. [9].
2.4 Analysis of physiological parameters
Relative water contents (RWCs) were analysed according to the method given by Unyayer et al. [26]. Membrane stability index (MSI) was measured by the following method proposed by Sairam [27]. Photosynthetic pigments such as chlorophyll a, b and total chlorophyll contents (TCCs) were estimated through the protocol of Bruinsma [28].
2.5 Estimation of biochemical attributes
2.5.1 Estimation of non‐enzymatic antioxidants
Proline contents were estimated by the process given by Bates et al. [29]. Sugar contents were determined through phenol sulphuric acid method [30]. The protein contents were quantified through the method of Lowry et al. [31]. Malondialdehyde (MDA) contents were determined by the thiobarbituric acid method [32]. The reduced glutathione was determined through the Ellman method [33]. Total ascorbates (TASCs) were estimated by the method of Gossett et al. [34]. Total phenolic contents (TPCs) were evaluated by the method of Waterhouse [35]. Total flavonoid contents (TFCs) were determined using aluminium chloride method [36].
2.5.2 Extraction and assay of antioxidant enzymes activities
For extraction of the antioxidant enzymes, wheat plants were initially grounded with 50 mM sodium phosphate buffer (pH 6.8) for extraction of peroxidase (POX) and catalase (CAT) and 50 mM sodium phosphate buffer (pH 7.2) for ascorbate POX (APX) and 100 mM potassium phosphate buffer (pH 7.6) for superoxide dismutase (SOD) using polyvinyl pyrrolidone in ice cold conditions. The obtained homogenous mixture was centrifuged at 10,000 rpm for 10 min at 48°C for taking supernatants. SOD (SOD: EC 1.15.1.1) activity was done according to the method of Dhindsa et al. [37] and absorbance was taken at 560 nm. CAT (CAT; EC 1.11.3.6) activity was determined by taking absorbance at 240 nm using the method of Aebi [38]. POX (POX: EC 1.11.1) activity was analysed at 470 nm for 10 min [39]. APX (APX: EC.1.11.1.11) activity was measured at 290 nm by the following method of Chen and Asada [40]. For analysis of guaiacol POX (GPX, EC 1.11.1.7) activity method of Zhang [41] was followed.
2.5.3 Quantification of superoxide anion (O2 O2), hydrogen peroxide (H2 O2), superoxide radical (O2) and lipid peroxidation
The O2 O2 concentration was quantified following the method of Doke [42]. Absorbance was taken at 580 nm through UV–vis spectrophotometer. The H2 O2 concentration in the plant tissues was estimated as per the procedure of Bellincampi et al. [43] at an absorbance of 560 nm. Estimation of superoxide radical (O2) was done by the method of Kariola et al. [44]. Lipid peroxidation (LPX) was recorded as per MDA level and the absorbance was taken at 600 nm [32].
2.6 Experimental design and statistical analysis
Each treatment was consisting of three replicates with two times repetition of the experiment. The values were finalised by taking mean standard error (±SE). Statistical analyses of obtained values were carried out through analysis of variance‐one way with the help of SPSS software package (SPSS 16.1). Furthermore, comparison of each value was done with control.
3 Results and discussion
3.1 Green synthesis and characterization of AgNPs
Biosynthesis of AgNPs by using the leaves extract of M. oleifera as the main reducing and stabilising agent was employed in the present study. The aqueous Ag ions were reduced to AgNPs with the addition of prepared M. oleifera leaves extract. The plant extract contains a certain concentration of active constituents, which are intended to play a key role in the synthesis of NPs [45]. The synthesis of AgNPs by utilising the plant extract is of great importance because it eliminates the downstream processing and avoids the difficult maintenance of cell cultures [46]. Fig. 5 illustrates the scheme for the synthesis of the AgNPs.
Fig. 5.
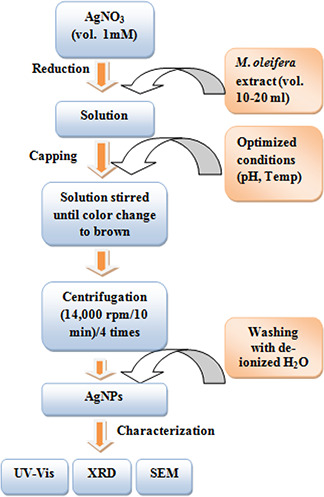
Schematic flowchart diagram showing the process of AgNPs synthesis and characterisation
The synthesised AgNPs were characterised by UV–vis spectroscopy, SEM, XRD and zeta analyser The combination of different techniques is usually required as far as concerned with the characterisation of NPs because a single technique is unable to fully characterise the colloidal NPs. Different characterisation peaks usually in the range of 410–480 nm are obvious for the synthesis of AgNPs [47, 48]. Although, different wavelengths may attribute different sizes and shapes of colloidal AgNPs [49]. The synthesis of AgNPs was monitored by recording a UV–vis spectrum from 300 to 700 nm. As is evident in Fig. 1, plant extract mixed with AgNO3 salt showed a characterisation peak in the range of 413–420 nm for the AgNPs synthesised from M. oleifera.
Morphological analysis of the synthesised AgNPs was performed using SEM. The SEM image represents the cubic to rectangular shapes of the synthesised AgNPs (Fig. 2). The synthesised AgNPs were neutral in charge and compactly aggregated in arrangement with dispersed distribution. The neutral charge of AgNPs was due to the capping of plant extract material, which reduced Ag ions Ag+ into Ago. Similar findings were reported by Hussain et al. [50]. Impurities in the sample are attributed to the organic compounds, which are involved in the reduction of AgNO3 to AgNPs. The crystalline nature of the synthesised AgNPs was confirmed by XRD analysis (Fig. 3). The XRD pattern of the synthesised AgNPs showed diffraction spectra at 25.36°, 37.86° and 48.13°, which can be attributed to 111 and 200 of the face‐centred cubic crystalline Ag.
The synthesised AgNPs were also characterised through zeta potential for determining the size range. The zeta analyser elucidated that the size of synthesised AgNPs ranged from 8 to 28 nm (Fig. 4). Our findings are in line with Mie et al. [51] who reported similar observations regarding size distribution in an attempt to find the effect of AgNPs in the antimicrobial assay.
3.2 Effects of AgNPs on RWCs, MSI and chlorophyll contents
Results of AgNPs treatments on physiological growth of wheat plants under heat stress were taken in terms of plant RWCs, MSI and chlorophyll contents (Fig. 6). Heat stress significantly reduced plant RWC (13.2%), MSI (16.3%), chlorophyll a (5.2%), chlorophyll b (4.1%) and TCCs (9.9%) compared with control. Rizwan et al. [8] elaborated oxidative stress as a major reason behind the disruption of macromolecules and cell membranes, which results in a reduction of green pigments in plants. A significant increase in RWC (12.2%), MSI (26.5%), chlorophyll a (10%), chlorophyll b (16.4%) and TCCs (19%) was observed in response to 50 mg/l of AgNPs treatment under same exposures of heat stress in comparison with control and respective treatments (>50 mg/l AgNPs). Singh et al. [52] reported an increase in physiological attributes is due to changes in cell division and cell elongation. This growth directly related to the protection of membrane integrity and the stabilisation of proteins/enzymes and thus contributes to heat tolerance. Blum [53] stated that production of osmolytes regulates low water potential by increasing more water uptake under stress conditions. Cvjetko et al. [54] observed a decrease in MDA level and electrolyte leakage due to exogenous application of AgNPs in tobacco plants. Iqbal et al. [55] reported an increase in RWC and membrane stability in wheat plants treated with plant‐derived smoke. A profound increase takes place in photosynthetic pigments such as chlorophyll and carotene contents of microalgae (Chlorella vulgaris) due to AgNPs treatment [56]. This increase was due to a reduction in chlorophyll disruption and better growth of microalgae (C. vulgaris). Nwaichi and Anosike [57] also studied variation in chlororphyll contents in response to AgNPs treatments. Similarly, Gupta et al. [58] stated that exposure to AgNPs significantly increases the chlorophyll and carotenoid contents of rice plants. AgNPs priming also notably enhance growth parameters and photosynthetic efficiency as well as phytohormones balance suggesting that AgNPs priming might have a role in the improvement of plant tolerance against environmental stresses [23].
Fig. 6.
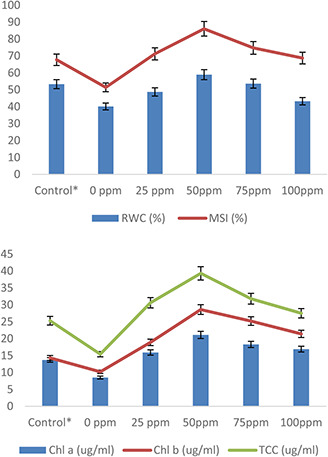
Effect of AgNPs on plant RWCs, MSI, chlorophyll a, b and TCCs of wheat plants exposed to heat stress
3.3 Effects of AgNPs on non‐enzymatic osmolytes production in wheat plants
The concentrations of the studied osmolytes such as proline and total soluble sugar significantly increased in wheat plants under heat stress, while AgNPs treatment directed a reduction in their quantity (Fig. 7). Heat stress alone increases the proline (8.2%) and sugar level (11.6%) as compared with control. The application of AgNPs (50 mg/l) lowered the proline (4%) and sugar level (5.8%) of wheat plants against heat‐stress treatment. Even though the increase in both osmolytes was noted in some AgNPs (〈50〉) treatments with heat stress, but overall this increase was less significant as compared with heat‐stress application only. According to Mishra et al. [59] and Ahmad et al. [60] plants produce different types of osmolytes such as proline and soluble sugars as a counterpart to abiotic stresses and involved in stress tolerance through osmotic adjustment and ROS detoxification. The findings of Tripathi et al. [61] illustrated alleviation of UV‐B stress by increased production of osmolytes (proline/sugar) in response to silicon NPs in wheat. Similarly, Mohamed et al. [62] reported overproduction of proline and soluble sugar in wheat plants. Similarly, Mehmood and Murtaza [63] observed an increase in organic solutes due to AgNPs in pea (Pisum sativum L.) seeds. In addition, Mehrian et al. [64] revealed variations in amino acids contents in tomato plants treated with AgNPs compared with untreated plants.
Fig. 7.
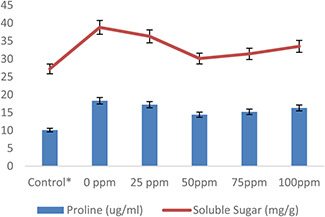
Effect of AgNPs on plant proline contents and soluble sugar contents of wheat plants exposed to heat stress
The substantial difference in protein, MDA and glutathione (GSH) concentration was observed in wheat plants in response to AgNPs treatment under heat stress (Fig. 8). Increase in MDA (1.46%) and GSH (1.73%) was observed in heat‐stress‐treated plants without AgNPs pre‐treatment (Fig. 8). However, the normal MDA level (0.73%) was recorded in pre‐treated wheat plants with 50 mg/l AgNPs under heat stress. Similarly, the maximum protein contents (2.6%) and GSH level (2.3%) were observed at the same treatment of AgNPs (50 mg/l) against heat stress as compared with control and other treatments of AgNPs (Fig. 8). The degradation of membranes lipid under different environmental stresses results in a high level of electrolyte leakage by decreasing membrane integrity. Similarly, protein production and GSH activity also reduced in plants under stress conditions. The application of external agents such as AgNPs increases protein and GSH activity and normalised MDA level by protecting membrane degradation and proteolysis process as observed in the present investigation. Our results are in agreement with findings of researchers [54, 61, 62, 63] as they reported an increase in protein, GSH activity and membrane integrity in plants in response to application of Ag‐based NPs under stress. Similar to this, Mehrian et al. [64] and Barbasz et al. [65] observed decreased LPX in wheat callus and tomato plants in response to AgNP treatments.
Fig. 8.
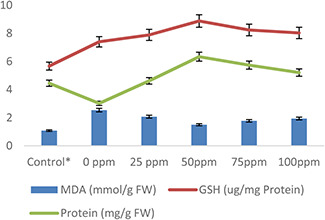
Effect of AgNPs on plant MDA level, GSH activity and protein contents of wheat plants exposed to heat stress
The activities of TPCs, TFCs and TASCs in wheat plants increased significantly under heat stress. However, AgNPs treatment was found to be very effective in the enhancement of non‐antioxidant enzymes under heat stress compared with control (Fig. 9). The maximum level of TPC (2.4%), TFC (2.5%) and TASC (2.5%) were observed in wheat plants pre‐treated with 50 mg/l of AgNPs followed by heat stress. Overall, a gradual decrease was found in non‐antioxidant enzymes with increase or decrease of AgNPs treatment level (Fig. 9). Similar to present results, Krishnaraj et al. [66] and Yasur and Rani [67] noted a high accumulation of total phenolic and total flavonoid in plants under oxidative stress. Some other researchers [21, 68, 69] reported increased production of phenolic compounds in plants and photosynthetic microorganisms with NPs treatment. Similarly, Garcia‐Sanchez et al. [70] observed activation of flavonoid biosynthetic genes in A. thaliana in response to AgNPs. Hussain et al. [21] interpreted the high production of TPC and TFC in citrus plants in response to AgNPs treatments under biotic stress.
Fig. 9.
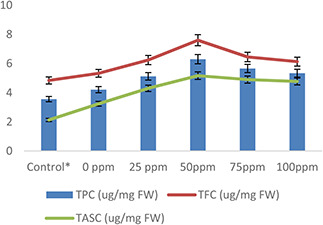
Effect of AgNPs on plant TPCs, TFCs and TASCs of wheat plants exposed to heat stress
3.4 Effects of AgNPs on antioxidant enzymes activities
The antioxidant enzymes such as SOD, POX, CAT, APX and GPX were also studied in wheat plants under normal and heat‐stress conditions. Antioxidant enzymes level increased under heat stress with respect to normal (control) plants (Figs. 10 and 11). However, AgNPs treatments were found to be more influential in increasing the activity of these enzymes under heat stress as compared with normal conditions. The maximum activities of SOD (1.3%), POX (1.5%), CAT (1.8%), APX (1.2%) and GPX (1.4%) were observed in wheat plants pre‐treated with 50 mg/l AgNPs as compared with control and other treatments (Figs. 10 and 11). Plants have a definite mechanism of producing enzymatic compounds to mitigate ROS produced due to oxidative stress [71]. According to Biju et al. [72], various types of antioxidants such as SOD, POX, CAT and APX are produced in plants under different conditions. These are produced either constitutive or induced and act as a resistant tool against oxidative damage. Plants used these antioxidants as their defence system to scavenge ROS produced under various metabolic or stress conditions such as heat or drought. These enzymes are produced in chloroplast, mitochondrion, cytoplasm and peroxisome and protect cell level damage. SOD dismutase O2 O2 − to H2 O2 [72], APX helps in scavenging H2 O2 through the ascorbate–glutathione pathway, GPX and POX also break H2 O2, thus protecting against stress‐mediated cellular injury. Overall, CAT diminishes H2 O2 by splitting it into the water and molecular oxygen thus eliminates the high level of produced H2 O2 [62]. On the other hand, increased SOD, APX and CAT activities were recorded in Spirodela polyrhiza and Vigna subterranean in exposure to AgNPs treatments [57, 73]. Mehrian et al. [64] explained that the level of antioxidants activities (POX, CAT and APX) was significantly high in tomato plants treated with AgNPs as compared with untreated plants. Similarly, few other researchers [17, 21, 64, 73] reported maximum activities of CAT and peroxidase in castor seedlings, S. polyrhiza, Fenugreek and Citrus in response to AgNPs treatment.
Fig. 10.
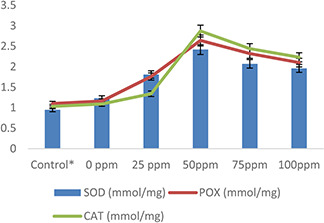
Effect of AgNPs on SOD, POX, and CAT activities of wheat plants exposed to heat stress
Fig. 11.
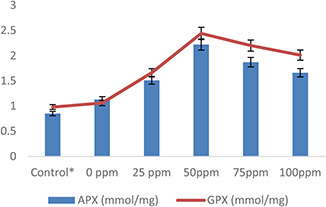
Effect of AgNPs on APX and GPX activities of wheat plants exposed to heat stress
3.5 Effects of AgNPs on superoxide radical (O2), superoxide anion (O2 O2), H2 O2 and LPX
The higher concentrations of SOR, H2 O2, O2 O2 − and LPX were observed in heat‐stress wheat plants, whereas AgNPs treatments lower this concentration (Fig. 12). The increase of 1.2% in SOR content was recorded in heat‐stressed wheat plants, while a decrease of 0.9% was observed in AgNPs (50 mg/l) treated wheat plants. AgNPs dose (50 mg/l) decreased the H2 O2 content in heat‐stressed wheat plants from 1.4 to 1.01%, (Fig. 12). Similarly increase of 1.9% in O2 O2 − content was found in heat‐stressed wheat plants, which decreased up to 1.5% in AgNPs (50 mg/l) treatment. Noteworthy alterations in MDA level took place in heat stress and AgNPs treated wheat plants. Heat‐stress results in a significant increase in LPX (1.8%) in wheat plants. However, application of AgNPs (50 mg/l) under heat stress significantly recovered the membrane damage in wheat plants up to 1%. Overall, AgNPs treatment (50 mg/l) to wheat ants under heat stress revealed significant changes in MDA content (Fig. 12). Thus, the application of AgNPs (50 mg/l) seemed to have a protective effect in terms of these parameters under heat‐stress conditions by lowering the concentration of ROS, SOR, H2 O2, O2 O2 − and LPX levels. ROS production takes place due to metabolism as well as in response to environmental stresses and causes oxidation of lipid membranes, photosynthetic pigments, proteins and nucleic acids [74]. Plants produced antioxidant (SOD, APX, POX and CAT) and non‐antioxidant enzymes (Pro, glycine betaine and sugar) as their defence mechanism to minimise the lethal effects of ROS species inside the cell [52]. For example, Barbasz et al. [65] observed decreased ROS production and LPX in AgNPs treated Arabidopsis seedlings and wheat callus. Similarly, Jiang et al. [73] found an increase in antioxidants such as CAT level in S. polyrhiza when exposed to AgNPs. Recently, Cvjetko et al. [54] reported a reduction in LPX, O2 O2 − and H2 O2 accumulation due to enhanced activity of antioxidant enzymes in treatment to AgNPs in Allium cepa plants.
Fig. 12.
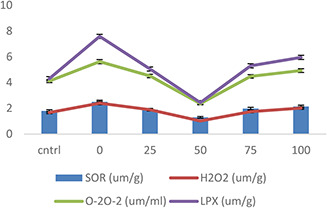
Effect of AgNPs on superoxide radical (O2) superoxide anion (O2 O2), H2 O2 and LPX of wheat plants exposed to heat stress
4 Conclusion
The present paper illustrated AgNPs synthesis by using M. oleifera plant extract, which is a very highly medicinal plant with efficient antioxidant properties. The plant extract acts as a reducing and stabilising agent for the synthesis of eco‐friendly AgNPs, without exploding hazardous substances in the environment. The green synthesised AgNPs played a pivotal role in ameliorating the negative effects of heat stress when applied to wheat plants by enhancing the level of osmolytes, antioxidant and non‐antioxidant enzymes production and declining the MDA concentration, H2 O2 contents and LPX. Furthermore, AgNPs have the potential to reduce oxidative stress in wheat plants due to their unique plasmon‐resonance optical scattering properties against heat stress. Various concentrations of biologically synthesised AgNPs may be applied to wheat plants through the soil and foliar spray for stimulating resistance in wheat crop in heat‐stressed areas. These AgNPs treatments can be applied to wheat crop at vegetative as well as anthesis stage for vigorous plant growth and high grain yield under heat stress. Thus, in future, this environment friendly green synthesised AgNPs can be utilised in various acute hot areas of the world for the plentiful increase of resistance in wheat plants against heat stress. Overall, green synthesised AgNPs treatment could be reflected as an astonishing strategy to grow the wheat crop in high‐temperature zones of the world. However, further efforts are still needed to analyse the role of dose and duration of AgNPs treatment in diminishing severe effects of heat stress on wheat plants in various high‐temperature fields. In addition, there is a dire need to understand the mechanism of AgNPs mediated heat stress tolerance in the wheat crop (at genome and proteome levels), growing in different high‐temperature hot spots in the world.
5 Acknowledgment
We are thankful to the Higher Education Commission (HEC) of Pakistan for their support in the completion of this work.
6 References
- 1. FAO : ‘Food and agriculture organization, FAOSTAT database’. Available at http://faostat.fao.org/site/339/default.aspx, accessed 31/12/2012
- 2. Ray D.K. Mueller N.D. West P.C.. et al.: ‘Yield trends are insufficient to double global crop production by 2050’, PLOS One, 2013, 8, pp. 1 –8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Intergovernmental Panel on Climate Change (IPCC) : ‘Managing the risks of extreme events and disasters to advance climate change adaptation’, in Field C.B. Barros V. Stocker T.F. et al. (Eds.): ‘A special report of working groups I and II of the inter‐governmental panel on climate change’ (Cambridge University Press, Cambridge, 2012), p. 582 [Google Scholar]
- 4. Lobell D.B. Field C.B.: ‘Global scale climate–crop yield relationships and the impacts of recent warming’, Environ. Res. Lett., 2007, 2, pp. 1 –7 [Google Scholar]
- 5. Hasanuzzaman M. Nahar K. Fujita M.: ‘Extreme temperatures, oxidative stress and antioxidant defense in plants’, in Vahdati K. Leslie C. (Eds.): ‘Abiotic stress – plant responses and applications in agriculture’ (In Tech, Rijeka, Croatia, 2013), pp. 169 –205 [Google Scholar]
- 6. Janska A. Marsik P. Zelenkova S. et al.: ‘What is important for metabolic adjustment?’, Plant Biol., 2010, 12, pp. 395 –405 [DOI] [PubMed] [Google Scholar]
- 7. Waraich E.A. Ahmad R. Halim A. et al.: ‘Alleviation of temperature stress by nutrient management in crop plants, a review’, J. Soil Sci. Plant Nutr., 2012, 12, pp. 221 –244 [Google Scholar]
- 8. Rizwan M. Ali S. Qayyum M.F. et al.: ‘Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review’, J. Hazard. Mater., 2017, 322, pp. 2 –16 [DOI] [PubMed] [Google Scholar]
- 9. Iqbal M. Raja N.I. Mashwani Z.R. et al.: ‘Effect of silver nanoparticles on growth of wheat under heat stress’, Iran. J. Sci. Technol. Trans. Sci., 2017. Available at 10.1007/s40995-017-0417-4, accessed 12/12/2017 [DOI] [Google Scholar]
- 10. Nair P.M.G. Chung I.M.: ‘Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of Mung bean (Vigna radiata L.)’, Acta Physiol. Plantarum, 2017, 37, pp. 1 –11 [Google Scholar]
- 11. Niraimathi K. Sudha V. Lavanya R. et al.: ‘Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities’, Colloid Surf. B, 2013, 102, pp. 288 –291 [DOI] [PubMed] [Google Scholar]
- 12. Joanna C. Marcin L. Ewa K. et al.: ‘A nonspecific synergistic effect of biogenic silver nanoparticles and biosurfactant towards environmental bacteria and fungi’, Ecotoxicology, 2018, 27, (3), pp. 352 –359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramachandran R. Sangeetha D.: ‘Antibiofilm efficacy of silver nanoparticles against biofilm forming multidrug resistant clinical isolates’, Pharm. Innov. J., 2017, 6, (11), pp. 36 –43 [Google Scholar]
- 14. Ali S.M. Yousef N.M.H. Nafady N.A. et al.: ‘Application of biosynthesized silver nanoparticles for the control of land snail Eobania vermiculata and some plant pathogenic fungi’, J. Nanomat., 2015, 15, pp. 1 –10 [Google Scholar]
- 15. Hojjat S.S. Kamyab M.: ‘The effect of silver nanoparticle on Fenugreek seed germination under salinity levels’, Russ. Agric. Sci., 2017, 43, (1), pp. 61 –65 [Google Scholar]
- 16. Almutairi Z.M.: ‘Influence of silver nanoparticles on the salt resistance of tomato (Solanum lycopersicum) during germination’, Int. J. Agric. Biol., 2016, 18, pp. 449 –457 [Google Scholar]
- 17. Hojjat S.S.: ‘The effect of silver nanoparticle on lentil seed germination under drought stress’, Int. J. Farming Allied Sci., 2016, 5, (3), pp. 208 –212 [Google Scholar]
- 18. Kodir A. Imawan C. Permana I.S. et al.: ‘Pesticide colorimetric sensor based on silver nanoparticles modified by L‐cysteine’. 2016 Int. Seminar on Sensors, Instrumentation, Measurement and Metrology (ISSIMM), Malang, 2016, pp. 43 –47 [Google Scholar]
- 19. Maiti S. Barman G. Laha K.: ‘Detection of heavy metals (Cu+2, Hg+2) by biosynthesized silver nanoparticles’, J. Appl. Nanosci., 2016, 6, p. 529 [Google Scholar]
- 20. Tao W. Chen G. Zeng G. et al.: ‘Influence of silver nanoparticles on heavy metals of pore water in contaminated river sediments’, Chemosphere, 2016, 162, pp. 117 –124 [DOI] [PubMed] [Google Scholar]
- 21. Hussain M. Raja N.I. Mashwani Z.R. et al.: ‘Green synthesis and characterisation of silver nanoparticles and their effects on antimicrobial efficacy and biochemical profiling in Citrus reticulata ’, IET Nanobiotechnol., 2018, 12, (4), pp. 514 –519, doi: 10.1049/iet‐nbt.2017.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marwah M.B.: ‘Evaluation of silver nanoparticles toxicity against toxic black mold Stachybotrys chartarum ’, J. Plant Pathol. Microbiol., 2017, 8, p. 408 [Google Scholar]
- 23. Abou‐Zeid H.M. Ismail G.S.M.: ‘The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L. to salt stress’, Egypt. J. Bot., 2018, 58, (1), pp. 73 –85 [Google Scholar]
- 24. Hussain M. Raja N.I. Mashwani Z.R. et al.: ‘ In vitro seed germination and biochemical profiling of Artemisia absinthium exposed to various metallic nanoparticles’, 3 Biotech, 2017, 7, (2), pp. 101 –108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iqbal M. Asif S. Ilyas N. et al.: ‘Effect of plant derived smoke on germination and post germination expression of wheat (Triticum aestivum L.)’, Am. J. Plant Sci., 2016, 7, pp. 806 –813 [Google Scholar]
- 26. Unyayer S. Keles Y. Cekic F.O.: ‘The antioxidant response of two tomato species with different tolerances as a result of drought and cadmium stress combination’, Plant Soil Environ., 2005, 51, (2), pp. 57 –64 [Google Scholar]
- 27. Sairam R.K.: ‘Effect of moisture stress on physiological activities of two contrasting wheat genotypes’, Indian J. Exp. Biol., 1994, 32, pp. 584 –593 [Google Scholar]
- 28. Bruinsma J.: ‘The quantitative analysis of chlorophyll a and b in plant extract’, Photochem. Photobiol., 1963, 2, pp. 241 –249 [Google Scholar]
- 29. Bates L.S. Waldren R.P. Tear I.D.: ‘Rapid determination of free proline for water stress studies’, Plant Soil, 1973, 39, pp. 205 –207 [Google Scholar]
- 30. Dubios M.K. Gilles J.K. Robers P.A. et al.: ‘Calorimetric determination of sugar and related substance’, Anal. Chem., 1951, 26, pp. 351 –356 [Google Scholar]
- 31. Lowry O.H. Robebrogh N.J. Farr A.L. et al.: ‘Protein measurement with the folin‐phenol reagent’, J. Biol. Chem., 1951, 193, pp. 265 –275 [PubMed] [Google Scholar]
- 32. Heath R.L. Packer L.: ‘Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation’, Arch. Biochem. Biophys., 1968, 125, pp. 189 –198 [DOI] [PubMed] [Google Scholar]
- 33. Ellman G.L.: ‘A colorimetric method of determining of low concentrations of mercaptans’, Arch. Biochem. Biophys., 1958, 74, (2), pp. 443 –450 [DOI] [PubMed] [Google Scholar]
- 34. Gossett D.R. Millhollon E.P. Cran L.M.: ‘Antioxidant response to NaCl stress in salt sensitive cultivars of cotton’, Crop Sci., 1994, 34, pp. 706 –714 [Google Scholar]
- 35. Waterhouse A.L.: ‘Determination of total phenolics’, in Wrolstad R.E. (Eds.): ‘Handbook of food analytical chemistry, unit I 1.1: Polyphenolics’ (Wiley, New York, 2001), pp. 464 –465 [Google Scholar]
- 36. Zhishen J. Mengcheng T. Jianming W.: ‘The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals’, Food Chem., 1999, 64, pp. 555 –559 [Google Scholar]
- 37. Dhindsa R.S. Plumb‐Dhinds P. Thorpe T.A.: ‘Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase’, J. Exp. Bot., 1981, 32, pp. 93 –101 [Google Scholar]
- 38. Aebi H.: ‘Catalase in vitro ’, Methods Enzymol., 1984, 105, pp. 121 –126 [DOI] [PubMed] [Google Scholar]
- 39. Goliber T.E.: ‘Gravitational stress and lignification in aerial vs. submerged shoots of Hippuris vulgaris ’, Physiol. Plant., 1989, 75, pp. 355 –361 [Google Scholar]
- 40. Chen G.X. Asada K.: ‘Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties’, Plant Cell Physiol., 1989, 30, pp. 987 –998 [Google Scholar]
- 41. Zhang X.Z.: ‘The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system’, in Zhang X.Z. (Ed.): ‘Research methodology of crop physiology’ (Agriculture Press, Beijing, 1992), pp. 208 –211 [Google Scholar]
- 42. Doke N.: ‘Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components’, Physiol. Plant Pathol., 1983, 23, pp. 345 –357 [Google Scholar]
- 43. Bellincampi D. Dipierro N. Salvi G. et al.: ‘Extracellular H2 O2 induced by oligogalacturonides is not involved in the inhibition of the auxin‐regulated rolB gene expression in tobacco leaf explants’, J. Plant Physiol., 2000, 122, pp. 1379 –1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kariola T. Brader G. Helenius E. et al.: ‘Early responsive to dehydration negative regulator of ABA‐responses in Arabidopsis’, Plant Physiol., 2006, 142, pp. 1559 –1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rauwel P. Küünal S. Ferdov S. et al.: ‘A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM’, Adv. Mater. Sci. Eng., 2015, 15, pp. 1 –9 [Google Scholar]
- 46. Shankar S.S. Rai A. Ahmad A. et al.: ‘Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth’, J. Colloid Interface Sci., 2004, 275, pp. 496 –502 [DOI] [PubMed] [Google Scholar]
- 47. Nazeruddin G. Prasad N. Waghmare S.R. et al.: ‘Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti‐microbial activity’, J. Alloys Compd., 2014, 583, pp. 272 –277 [Google Scholar]
- 48. Gogoi N. Babu P.J. Mahanta C. et al.: ‘Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities’, Mater. Sci. Eng. C, 2015, 46, pp. 463 –469 [DOI] [PubMed] [Google Scholar]
- 49. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, pp. 2638 –2650 [Google Scholar]
- 50. Kumar R. Roopan S.M. Prabhakarn A. et al.: ‘Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2012, 90, pp. 173 –176 [DOI] [PubMed] [Google Scholar]
- 51. Mie R. Samsudin M.W. Din L.B. et al.: ‘Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum ’, Int. J. Nanomed., 2014, 9, pp. 121 –127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh M. Kumar J. Singh S. et al.: ‘Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review’, Rev. Environ. Sci. Bio., 2015, 14, pp. 407 –426 [Google Scholar]
- 53. Blum A.: ‘Osmotic adjustment is a prime drought stress adaptive engine in support of plant production’, Plant Cell Environ., 2016, 40, pp. 4 –10 [DOI] [PubMed] [Google Scholar]
- 54. Cvjetko P. Zovko M. Stefanic P.P. et al.: ‘Phytotoxic effects of silver nanoparticles in tobacco plants’, Environ. Sci. Pollut. Res., 2017. Available at 10.1007/s11356-017-0928-8, accessed 08/12/2017 [DOI] [PubMed] [Google Scholar]
- 55. Iqbal M. Asif S. Ilyas N. et al.: ‘Smoke produced from plants waste material elicits growth of wheat (Triticum aestivum L.) by improving morphological, physiological and biochemical activity’, Biotechnol. Rep., 2018, 17, pp. 35 –44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdolsamad S. Younes G. Yaghoobi M.M. et al.: ‘The effect of silver nanoparticles [AgNPs] on chlorophyll A and ÃÂ2 ‐carotene content [as two natural antioxidants] in the microalgae Chlorella vulgaris ’, Res. Rev., J. Ecol. Environ. Sci., 2018, 3, (3), pp. 41 –45 [Google Scholar]
- 57. Nwaichi E.O. Anosike E.O.: ‘Plant response on exposure to Ag nanoparticles: a study with Vigna subterranean ’, Biochem. Anal. Biochem., 2016, 5, (4), pp. 1 –6 [Google Scholar]
- 58. Gupta S.D. Agarwal A. Pradhan S.: ‘Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns’, Ecotoxicol. Environ. Saf., 2018, 161, pp. 624 –633 [DOI] [PubMed] [Google Scholar]
- 59. Mishra B. Srivastava J. Lal J. et al.: ‘Physiological and biochemical adaptations in lentil genotypes under drought stress’, Russ. J. Plant Physiol., 2016, 63, pp. 695 –708 [Google Scholar]
- 60. Ahmed M. Qadeer U. Ahmed Z.I.: ‘Improvement of wheat (Triticum aestivum) drought tolerance by seed priming with silicon’, Arch. Agron. Soil Sci., 2016, 62, pp. 299 –315 [Google Scholar]
- 61. Tripathi D.K. Singh S. Singh S. et al.: ‘Nitric oxide alleviates silver nanoparticles (AgNPs)‐induced phytotoxicity in Pisum sativum seedlings’, Plant Physiol. Biochem., 2017, 110, pp. 167 –177 [DOI] [PubMed] [Google Scholar]
- 62. Mohamed A.K.S.H. Qayyum M.F. Abdel‐Hadi A.M. et al.: ‘Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat’, Arch. Agron. Soil Sci., 2017, 63, (12), pp. 1736 –1747 [Google Scholar]
- 63. Mehmood A. Murtaza G.: ‘Impact of biosynthesized silver nanoparticles on protein and carbohydrate contents in seeds of Pisum sativum L’, Crop Breed. Appl. Biotechnol., 2017, 17, pp. 334 –340 [Google Scholar]
- 64. Mehrian S.K. Heidari R. Rahmani F.: ‘Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants’, Ind. J. Plant Physiol., 2015, 20, pp. 257 –263 [Google Scholar]
- 65. Barbasz A. Kreczmer B. Oćwieja M.: ‘Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO3)’, Acta Physiol. Plant., 2016, 38, (3), p. 76 [Google Scholar]
- 66. Krishnaraj C. Jagan E.G. Ramachandran R. et al.: ‘Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (L) Wettst. Plant metabolism’, Process Biochem., 2012, 47, pp. 651 –658 [Google Scholar]
- 67. Yasur J. Rani P.U.: ‘Environmental effects of nano silver: impact on castor seed germination, seedling growth, and plant physiology’, Environ. Sci. Pollut. Res., 2013, 20, pp. 8636 –8648 [DOI] [PubMed] [Google Scholar]
- 68. Ghorbanpour M. Hadian J.: ‘Multi‐walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro ’, Carbon, 2015, 94, pp. 749 –759 [Google Scholar]
- 69. Vecerova K. Vecera Z. Docekal B. et al.: ‘Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles’, Environ. Pollut., 2016, 218, pp. 207 –218 [DOI] [PubMed] [Google Scholar]
- 70. Garcia‐Sanchez S. Bernales I. Cristobal S.: ‘Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root‐hair development through salicylic acid signaling’, BMC Genomics, 2015, 16, p. 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sewelam N. Kazan K. Schenk P.M.: ‘Global plant stress signaling: reactive oxygen species at the cross‐road’, Front. Plant Sci., 2016, 7, p. 187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Biju S. Fuentes S. Gupta D.: ‘Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system’, Plant Physiol. Biochem., 2017, 119, pp. 250 –264 [DOI] [PubMed] [Google Scholar]
- 73. Jiang H.S. Qiu X.N. Li G.B. et al.: ‘Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza ’, Environ. Toxicol. Chem., 2014, 33, (6), pp. 1398 –1405 [DOI] [PubMed] [Google Scholar]
- 74. Gunes A. Pilbeam D.J. Inal A. et al.: ‘Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress’, J. Plant Interact., 2007, 2, pp. 105 –113 [Google Scholar]


