Abstract
Foley catheters are inevitable in health care unit. Pathogens colonise and form biofilm on catheter causing catheter‐associated urinary tract infection. Therefore, the authors aimed to functionalise catheter to resist biofilm formation. The authors impregnated urinary catheters with a synergistic combination of antibiotics and silver nanoparticles (SNPs) to evaluate antibiofilm efficacy in vitro and in vivo. SNPs were synthesised using Spirulina platensis. Synergy between the SNPs and antibiotics was determined by the checker‐board method. In vivo efficacy of the functionalised catheters was assessed in mice. Liver and kidney function tests of mice were performed. The in vitro anti‐adherence activity of the functionalised catheters was evaluated after 2 years. Nanoparticle sizes were 42–75 nm. Synergistic activity was observed among SNPs (2 µg/ml), amikacin (6.25 µg/ml), and nitrofurantoin (31.25 µg/ml). In mice, catheters functionalised with combinations of antibiotics and SNPs exhibited no colonisation until Day 14. Blood, liver, and kidney tests were normal. After 2 years, catheters functionalised with antibiotics exhibited 25% inhibition of bacterial adhesion, and catheters functionalised with the nanoparticle‐antibiotic combination exhibited 90% inhibition. Impregnation of urinary catheters with a synergistic combination of antibiotics and SNPs is an efficient and promising method for preventing biofilm formation.
Inspec keywords: catheters, drugs, silver, nanoparticles, nanomedicine, liver, kidney, blood, microorganisms, adhesion, biomechanics, cellular biophysics
Other keywords: Foley catheters, synergistic nanoparticle‐antibiotics combination, silver nanoparticles, biofilm formation resitance, health care unit, pathogens, urinary tract infection, SNP, Spirulina platensis, checker‐board method, liver function, kidney function, vitro antiadherence activity, amikacin, nitrofurantoin, blood, bacterial adhesion, size 42 nm to 75 nm, Ag
1 Introduction
Medical device‐associated infection is an emerging issue in modern healthcare. Nosocomial infections such as urinary tract infections (UTIs) associated with urinary catheters confer a predisposition to bacteriuria [1]. Most common catheter colonisers are Escherichia coli, Klebsiella, Pseudomonas, Citrobacter, coagulase‐negative Staphylococci, and Candida. Biofilm‐forming pathogens colonise both external and internal catheter surfaces. These pathogens cause pyelonephritis, local and systemic bacteraemia, urinary calculi, bladder cancer, and mortality. Infections associated with medical devices contribute to the reservoir of multi drug‐resistant pathogens and increase healthcare costs.
Catheter‐associated urinary tract infections (CAUTIs) are prevented by the use of antibiotic prophylaxis and the use of functionalised catheters. Incorporation of antimicrobial agents onto the urinary catheter is an option to reduce infection. Urinary catheters are functionalised with antibiotics [2], antimicrobial enzymes such as cellobiose dehydrogenase [3], silver ions [4], and magnesium fluoride nanoparticles [5]. The excessive administration of antimicrobial drugs has led to the development of drug‐resistant strains [6]. Antimicrobial enzymes and magnesium nanoparticles are commonly used; however, they affect catheter durability. In clinical trials, antibiotic‐coated catheters yield inconsistent outcomes. The prevalence of multidrug‐resistant pathogens in intensive care units poses a serious threat to human health and safety.
A paucity of novel antibiotics in clinical trials motivates research on using antibiotics in synergy with other oligodynamic antimicrobial compounds. Such synergistic use scenarios may feature improved resistance risk profiles. The present work explores the synergistic combination of silver nanoparticles (SNPs) with antibiotics to control CAUTI, and evaluates this combination in a mouse model.
2 Materials and methods
2.1 Collection, confirmation and susceptibility profile of CAUTI Isolate
Ten catheter‐associated uropathogenic Escherichia coli strains were obtained from Inbiotics Clinical Research Laboratory, Nagercoil, Tamil Nadu, India. All chemicals were purchased from Hi Media, India and Qiagen, N.V. The isolates were tested for the presence of chu A genes, which are virulence genes of uropathogenic E. coli. The primer pairs used for PCR were chu A.1 (5′‐GACGAACCAACGGTCAGGAT‐3′) and chu A.2 (5′‐TGCCGCCAGTACCAAAGACA‐3′) [7]. The PCR mixture contained template DNA (2 µl), Taq DNA polymerase (3 µl), dNTP mix (3 µl), forward primer (2 µl), reverse primer (2 µl), 10× assay buffer (2 µl), and nuclease‐free water (1 µl). The PCR programme consisted of denaturation at 94°C for 1 min, annealing at 54°C for 90 s, and extension at 72°C for 2 min. These steps were repeated for 35 cycles. The amplified PCR product was assessed by agarose gel electrophoresis. The susceptibility of the isolates to the antibiotics was evaluated by disc diffusion assay [8].
2.2 Synthesis and characterisation of SNP
SNPs (0.25 mM) were prepared by the green method using the aqueous extract of Spirulina platensis. S. platensis powder (10 mg) was extracted with 25 ml of warm sterile distilled water. The extract was centrifuged at 10,000 rpm for 10 min, and the supernatant was used for the synthesis of SNP. To 100 mL of silver nitrate solution, 5 mL of the S. platensis extract was added; the mixture was kept at room temperature until the colour completely changed to reddish brown. SNP was characterised by scanning electron microscopy (SEM), energy dispersive spectrum (EDS), and UV–Vis spectrophotometer.
2.3 Minimum inhibitory concentration and synergy assay
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the antibacterial agents against E. coli were evaluated by broth dilution assay [9]. Fractional inhibitory concentrations (FICs) were identified by varying the concentrations of SNP (0–10 µg/ml), amikacin (0–100 µg/ml), and nitrofurantoin (0–500 µg/ml). FICs were calculated by dividing the MICs of individual drugs used in combination by the MIC of each drug when used alone. FIC Index (FICI) is calculated from the sum of FIC of each antimicrobial agent. From FICI, the interactions between antimicrobial agents were determined. FICI value of <0.5 indicates synergy, FICI value of >0.5–4 indicates no interaction, and FICI value of >4 indicates antagonism [10].
2.4 Surface functionalisation of urinary catheters with SNPs and antibiotics
Foley catheters of eight French scale were used for functionalisation. The catheters were cut into 1 cm pieces and immersed in chloroform for 30 s. Catheters were immediately transferred to separate beakers containing either 10 µg/ml SNP, antibiotics (31.25 µg/ml of amikacin and 156.25 µg/ml of nitrofurantoin), or a mixture of SNP and antibiotics (10 µg/ml of SNP, 31.25 µg/ml of amikacin, and 156.25 µg/ml of nitrofurantoin). All beakers were shaken for 16 h. The catheter pieces were washed twice with sterile distilled water to remove unbound antibiotics and sterilised by UV radiation for 30 min.
2.5 Determination of the elemental composition and concentration of SNPs on the catheter
The concentration of SNPs attached to the catheter was determined by gravimetry and EDS spectrum analysis. In the gravimetric method, the increase in the weight of the catheter before and after coating was recorded. The coating solution contained antibiotics and SNPs. Using the elemental composition data from the EDS spectrum, the concentration of SNPs among other elements was calculated. The concentration of SNPs retained in the catheter after the silver release study was also determined using a similar method.
2.6 Release kinetics of SNPs from the coated catheters
The concentration of SNPs released from the catheter was quantified by soaking the catheter in saline for 14 days. On Days 7 and 14, the concentrations of released SNPs were analysed by ICPS [11].
2.7 Accumulation of SNPs into collagen matrix
To prove that the uptake of SNPs by the tissue in contact with the catheter was only a few nanograms, the following experiment was performed. Xenoderm was attached to the external surface of the coated catheter. Xenoderm is a self‐adhesive wound dressing made of collagen matrix. The cells inside our body are surrounded by extracellular tissue made up of collagen. Thus, in the present study, xenoderm was used as a representative of the extracellular matrix. Accumulation of SNPs at the point of contact of xenoderm was recorded using SEM and EDS analysis on Days 0, 5, 10, and 15.
2.8 In vitro antibacterial and antibiofilm activity of the functionalised catheter
In vitro antibacterial activity of the functionalised catheter was evaluated using the method of Bauer et al. [8]. The functionalised catheter was placed on a Mueller and Hinton plate swabbed with E. coli (1 × 105 CFU/ml). The plate was incubated at 37°C for 24 h, and the diameter of the zone of inhibition was recorded.
Biofilm formation on the catheter was determined by crystal violet assay [12]. The assay was performed in 12‐well plates by adding 100 µl of culture (105 CFU/mL) and 900 µl of LB broth to each well. Unfunctionalised and functionalised catheters were placed in separate wells and incubated at 37°C for 24 h. After incubation, the planktonic cells were removed by aspirating the medium. The catheters were gently rinsed twice with sterile distilled water to remove loosely bound cells. The cells adhered to the catheters were fixed by adding 500 µl of methanol and drying. Finally, 250 µl of 0.1% crystal violet was added and incubated for 15 min. The stained cells were extracted with 250 µl of 25% glacial acetic acid, and the absorbance was recorded at 570 nm. Biofilm reductions in functionalised catheters were calculated by comparing OD values of functionalised catheters with those of unfunctionalised catheters. The results were recorded as % reduction in biofilm.
2.9 In vitro anti‐adhesive property of catheter
In vitro anti‐adhesive property of the functionalised catheter was assessed using the number of days that the catheter prevented the attachment of pathogens. This assay was performed by incubating a catheter in LB broth containing 105 CFU/mL E. coli for 1 h. The catheter was washed gently to remove loosely bound cells and placed on a sterile nutrient agar plate. If no colonies were observed after 24 h, the above incubation and plating were repeated until colonies were observed.
2.10 In vivo efficiency of functionalised catheter
The in vivo efficiency of the functionalised catheter was assessed as described by Guiton et al. [13]. Female mice were used in these experiments. Animals were kept in a humid environment with 12 h photoperiod (Grouping of mice for assay of in vivo functionalised catheter efficacy is shown in table 1).
Table 1.
Grouping of mice for assay of in vivo functionalised catheter efficacy
| S. no. | Batch | Treatment |
|---|---|---|
| 1. | T1 | Control: Mice without any catheter insertion |
| 2. | T2 | Catheter control: Mice catheterised with unfunctionalised catheter without E. coli |
| 3. | T3 | Uropathogenic E. coli control: Mice catheterised with unfunctionalised catheter inoculated with E. coli |
| 4. | T4 | Antiseptic control (chlorhexidine gluconate – 0.25%): Mice catheterised with catheters dipped in antiseptic solution and inoculated with E. coli |
| 5. | T5 | SNP control: Mice catheterised with catheters functionalised with SNPs and inoculated with E. coli |
| 6. | T6 | Antibiotic control: Mice catheterised with catheters functionalised with a mixture of antibiotics and inoculated with E. coli |
| 7. | T7 | Synergistic combination: Mice catheterised with catheters functionalised with a mixture of SNP and antibiotics and inoculated with E. coli |
2.10.1 Catheterisation of mice
Functionalised catheters were challenged with 100 µl of (105 CFU/ml) E. coli. Micturition was induced to lubricate urethra through mild caudal abdominal massage before catheterisation. Mice were anesthetised by intramuscular injection (0.5 ml/kg body weight) of a mixture of ketamine (70 mg/kg body weight) and acepromazine (2 mg/kg body weight). After anesthetisation, the animals were positioned in dorsal recumbence to facilitate intimate contact with urothelial cells. An animal was held by the base of its tail, and its hind limbs were clasped by the index finger and thumb on both sides to clearly expose the urinary papillae. The catheter was held by the free hand. The catheter was carefully introduced perpendicular to the urethra [13]. Urine samples were collected and used for microbial enumeration. Blood samples were collected and analysed for liver function (total proteins, albumin, globulin urea, Serum Glutamate oxaloacetate transaminase (SGOT), Serum Glutamate pyruvate transaminase (SGPT), and Serum alkaline phosphatase (SAP)) and kidney function tests (creatinine). After Day 14, the mice were euthanised. The catheter segments were removed and assessed for biofilm formation.
2.11 Determination of anti‐adhesive property of functionalised catheter after 2 years
The anti‐adhesive property of functionalised catheter was evaluated after 2 years. The ability of the catheters to resist the attachment of bacteria was evaluated; the results are expressed as % reduction in attachment.
2.12 Statistics
The data presented are means of three replicates, and the standard deviation is depicted by error bars. The Tukey–Kramer multiple comparison test was used to determine the significance between treatments.
3 Results and discussion
3.1 Collection, confirmation, and susceptibility profile of CAUTI isolate
Colonisation of Foley catheter by uropathogenic E. coli is well supported by Jahandeh et al. [14]. Uropathogenic E. coli is different from commensal E. coli strains. Many virulence factors in uropathogens permit their adhesion to catheter and subsequent invasion and infection [15]. The virulence genes code for adhesions, toxins, and iron acquisition systems [16]. One factor essential to the survival of E. coli in a low iron niche such as the urinary tract is the iron binding system encoded by chuA. Hence, the uropathogens collected in the present study were assessed for the presence of chuA genes by PCR amplification (Fig. 1 a). Lane M denotes the 10,000 bp marker. S1–S10 show the PCR products for each of ten uropathogenic E. coli isolates obtained from Inbiotics Laboratory, Nagercoil. All isolates except S1 present a 1400 bp product consistent with the presence of ChuA. This result confirms that these nine isolates were uropathogens. The 250 bp product is an internal control confirming the presence of DNA in each sample. The results corroborate the observations by Jahandeh [14]. chuA encodes an outer membrane protein that aids survival in iron‐limited habitats. The presence of the haeme/haemoglobin gene cluster (Chu) in uropathogenic E. coli is also reported by Jahandeh [14].
Fig. 1.
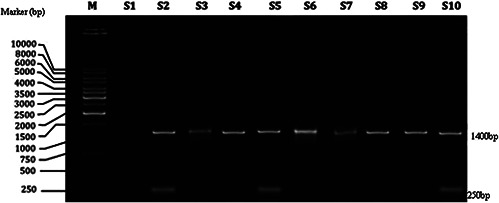
PCR amplification of ChuA gene
The susceptibility of uropathogenic E. coli to different classes of antibiotics was assessed using penicillin, amikacin, gentamycin, ceftobiprole, nitrofurantoin, ciprofloxacin, nalidixic acid, and co‐trimoxazole The susceptibility measurements were interpreted to indicate that an isolate was resistant (R), intermediate (I), or sensitive (S), as per the CLSI guidelines [17]. We inferred that S3, S4, and S8 developed resistance (R), to penicillin, amikacin, and nalidixic acid. S1, S2, S5, S7, S9, and S10 developed resistance (R), to penicillin, amikacin, nalidixic acid, and co‐trimoxazole. S6 developed resistance to all antibiotics except for ciprofloxacin and gentamicin. If a treatment strategy is designed for a most resistant organism, then it is applicable to all other organisms. So S6 is selected for further study. Resistance to antibiotics is supported by the results of Pitout [18]. Amikacin and nitrofurantoin are generally used to treat UTI, and the isolates developed resistance to these drugs. Hence, they were selected to use in synergistic combination with SNP. Since clinical isolate S6 exhibited resistance to the widest extent of tested antibiotics, we selected it for further synergistic study and in vivo experiments.
3.2 Synthesis and characterisation of SNP
In this era of antibiotic resistance and emergence of super bugs, silver regains its momentum in nano size. We used the extract of S. platensis for the synthesis of SNP. The formation of SNP, indicated by the appearance of a brownish red colour, was initiated within 15 min following the addition of Spirulina extract. Previous reports propose that reduction of nitrate to nitrite plays a key role in SNP synthesis associated with S. platensis [19]. Spirulina ‐sourced reducing sugars, or other molecules with OH groups, may reduce silver to SNPs. SEM, EDS, and UV–Vis Spectroscopic characteristics of SNP are depicted in Figs. 2 a –c. The SEM image shows the distribution of particles from 42 to 75 nm. The EDS data indicate the presence of silver. The absorption peak at 420 nm is characteristic of SNPs.
Fig. 2.
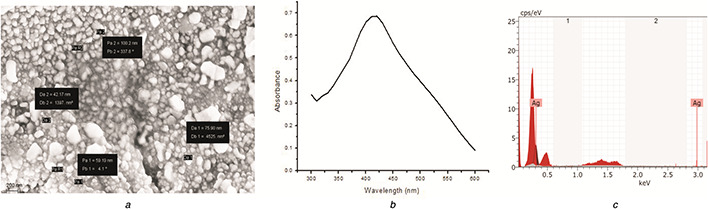
Characteristics of SNP
(a) SEM, (b) UV–Vis spectrum, (c) EDS
3.3 MIC and synergy assay
We assayed MICs and MBCs of antibiotics and SNPs against E. coli (Table 2). The minimum MICs for amikacin, nitrofurantoin, and SNP were 100, 500, and 10 µg/mL, respectively. The MBCs for amikacin, nitrofurantoin, and SNP were 400, 1000, and 10 µg/mL, respectively. The MIC and MBC of SNP against E. coli are reportedly 0.25–2 µg/mL [20]. The observed results were statistically significant at the 99% confidence interval. Miller et al. [21] find that the resistance to antibiotics changes with time and geographical region in response to the increased usage of these drugs.
Table 2.
MICs and MBCs of antimicrobial agents against E. coli
| S. no. | Antimicrobials | MIC, µg/mL | MBC, µg/mL |
|---|---|---|---|
| 1. | Amikacin | 100 | 400 |
| 2. | Nitrofurantoin | 500 | 1000 |
| 3. | SNP | 10 | 10 |
SNPs, amikacin, and nitrofurantoin exhibited synergistic effects (Fig. 3). MIC values are depicted in solid lines with symbols. Above this MIC, the drug shows no interaction. SNP at 2 µg/ml with amikacin at 6.25 µg/ml and nitrofurantoin at 31.25 µg/ml showed maximum synergy with an FICI of 0.325. These results corroborate those of Gonzales et al. [22]. These results indicate that when antibacterial agents are combined judiciously, previously ineffective drugs can be reused.
Fig. 3.

Synergy of SNP with antibiotics
Synergistic effect of SNP with ampicillin is supported by Hwang et al. [20], with an FICI value of 0.375. The synergistic effect of SNP and antibiotics is also supported by the results of Panacek et al. [23]. Pathogens develop resistance to amikacin by making the membrane impermeable to them. In nitrofurantoin resistant strains, the unstable nitrofurantoin metabolite is non‐lethal to bacteria by an unknown mechanism.
Generally, the synergistic effects of two agents result from the combination of two different mechanisms of action. Bactericidal activity of SNP is due to its lytic attack on the bacterial membrane. Furthermore, SNP binds protein sulfhydryl groups, causing the proteins to denature. SNPs also bind proteins of the respiratory chain, inhibiting ATP synthesis. In the present study, SNP increased the permeability of the bacterial membrane, facilitating the entry of amikacin into the bacterial cell. Silver nanomaterials and antibiotics synergistically generate oxidative stress, causing lethality to bacteria [19]. Antibiotics that were once deemed ineffective can be used in synergistic combination with SNP. Multiple components targeting multiple sites are more effective than a single component with a single target.
3.4 Surface functionalisation of urinary catheters with SNP and antibiotics
Impregnation of catheters with bactericidal agent is an attractive mitigant of surface pathogen attachment. We impregnated catheters with antibiotics (31.25 µg/ml of amikacin and 156.25 µg/ml of nitrofurantoin), SNP (10 µg/mL), or a combination of SNP and antibiotics (10 µg/ml SNP, 31.25 µg/ml of amikacin, and 156.25 µg/ml of nitrofurantoin). The present results are consistent with antimicrobial activity of central venous catheters coated with SNP [24]. The concentrations of all antimicrobial agents used for catheter functionalisation were five‐fold greater than the concentrations identified by the synergy assay. We used elevated concentrations to ensure complete impregnation of the catheter and gradual ingredient release during urinary output.
Fig. 4 shows SEM images of unfunctionalised and functionalised catheters. The unfunctionalised catheter (Fig. 4 a) and the antibiotic‐coated catheter (Fig. 4 b) exhibit rough surfaces. Crevices and clefts are preferable sites for microbial attachment and infection‐causing biofilm formation. Fig. 4 c shows SNPs impregnated on the catheter. In the synergistic combination (Fig. 4 d), both SNP and antibiotics were impregnated. The impregnation method that we used has been shown to homogenously distribute antibiotics and nanoparticles.
Fig. 4.
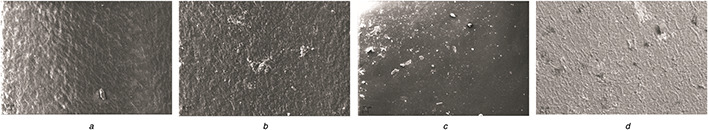
SEM image of functionalised catheter
(a) Control, (b) Antibiotic, (c) SNP, (d) Synergy
The entire external and internal surface of the catheter was functionalised; we observed no uncoated areas. Uniformity of coating has a crucial role in the successful prevention of CAUTI. Similar to the present results, uniform coating by magnesium nanoparticles of both urinary catheter surfaces was documented by Lellouche et al. [5].
3.5 Determination of the elemental composition and concentration of SNPs on the catheter
The elemental composition and concentration of SNPs on the coated catheters are shown in Fig. 5. The coated catheter showed 0.88 weight% of silver among other elements. The concentration of antimicrobial agents in the solution used for coating of the catheter (1 cm) was 397.4 µg/2 ml. The total concentration of antimicrobial agents attached to the catheter was 0.350 mg/cm. From this total weight of antimicrobial agents and EDS, the concentration of SNPs coated on to the catheter was determined. Therefore, the concentration of SNPs coated onto the catheter was 3.08 µg/cm.
Fig. 5.
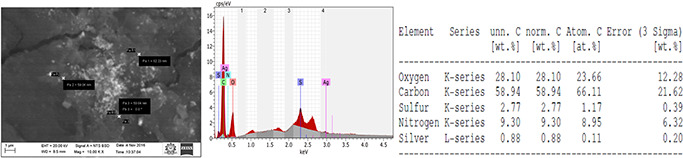
SEM image of the coated catheter
3.6 Release kinetics of SNPs from coated catheter
The concentration of SNPs released from the coated catheter into the saline was determined by ICPS, and the results are presented in Table 3. The concentration of SNPs released into the saline up to Day 7 was 0.260 µg/mL (8.44%), and 0.150 µg/mL (5.31%) was released from Day 7 to Day 14. The release of SNPs was biphasic. In the first 7 days, rapid release was observed; however, the release rate became slower thereafter. On an average, 29.28 ng/cm/day was released. Cumulatively, 13.75% of SNPs were removed from the coated catheter within 14 days. These results are supported by the observations of Roe et al. [25], who demonstrated that 15% of SNPs were released from the coated catheter within 14 days. The previous study also recorded the release of SNPs (45 ng/cm/day).
Table 3.
Release kinetics of SNPs from the coated catheters into saline
| S. no | Days | Concentration of SNPs, µg/mL |
|---|---|---|
| 1. | 0 | 0 |
| 2. | 7 | 0.260 |
| 3. | 14 | 0.150 |
Within the first 7 days, 0.260 µg/µLSNPs was released, and the concentration attached to the catheter was 2.82 µg/cm.
The concentrations of SNPs retained on the catheter after release into saline on Days 7 and 14 are presented in Table 4. In EDS spectrum analysis, the coated catheter on Day 0 showed 0.88 weight% silver among other elements. This corresponded to 3.08 µg/cm on the catheter. The weight% of silver was reduced to 0.81 weight% on Day 7 and 0.74 weight% on Day 14. This corresponded to 2.84 µg/cm (Day 7) and 2.59 µg/cm (Day 14).
Table 4.
Concentrations of SNPs on the coated catheters
| S. no | Days | Concentration of SNPs, µg/cm of catheter |
|---|---|---|
| 1. | 0 | 3.08 |
| 2. | 7 | 2.84 |
| 3. | 14 | 2.59 |
The sum of SNPs released into saline was 0.260 µg/ml and the remaining SNPs attached to the catheter on Day 7 was 2.82 µg/cm. On Day 14, the sum of SNPs released into the saline was (0.150 µg/ml) and the remaining SNPs attached to the catheter was 2.67 µg/cm. Therefore, our results showed that only 13.75% of the total SNPs coated onto the catheter was released, and 86.25% of SNPs remained attached to the catheter. Therefore, the concentration of SNPs released for up to 14 days was not toxic to cells.
3.7 Accumulation of SNPs into collagen matrix
The results of accumulation of SNPs in xenoderm were recorded by EDS analysis, as shown in Fig. 6. The increase in the weight of the xenoderm due to deposition of antimicrobial agents was 7 µg. The weight% of silver accumulated in the xenoderm was 0.01% among all elements on Day 1, 0.07% on Day 7, and 0.12% on Day 14. These values corresponded to 0.7, 4.9, and 8.4 ng/cm, respectively. This concentration was not toxic to the cells. These findings demonstrated that the concentrations of SNPs coating the catheter and found at sites of accumulation were not cytotoxic.
Fig. 6.
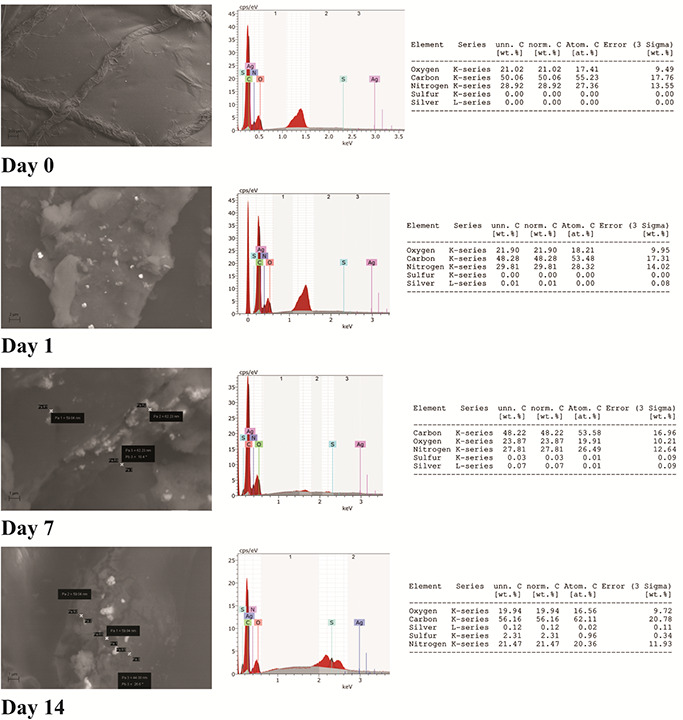
Accumulation of SNPs in xenoderm (collagen matrix)
3.8 In vitro antimicrobial and antibiofilm activity of functionalised catheter
Fig. 7 shows the in vitro antibacterial and antibiofilm activity of functionalised catheters. The inhibition zone was 4 mm for antibiotic‐coated catheters, 25 mm for SNP‐coated catheters, and 32 mm for combination‐coated catheters (Fig. 7 a). Biofilm log reduction for antibiotic‐coated catheters was 1.01; this statistic was 2.51 for SNP‐coated catheters; for combination‐coated catheters, the log reduction was 5.27 (Fig. 7 b). The log reduction for combination‐coated catheters was significantly greater than the log‐reductions for the other treatments. This synergistic effectiveness is due to the prevention of bacterial attachment to the catheter surface. Irreversible attachment of bacteria to a surface is the first stage in the formation of biofilm. The present results are consistent with the antibiofilm activity of urinary catheters coated with magnesium nanoparticles, reported by Lellouche et al. [5].
Fig. 7.
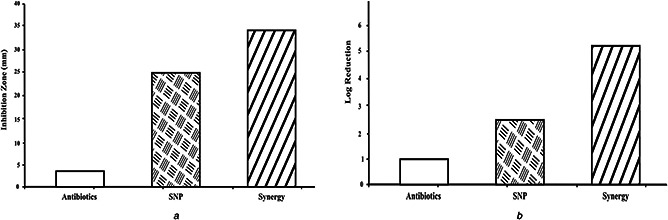
In vitro antimicrobial and antibiofilm activity of functionalised catheter
(a) Antibacterial activity, (b) Crystal violet assay
3.9 In vitro anti‐adhesive property of catheter by modified serial plate transfer assay
The functionalised catheter attachment resistance efficiency was evaluated by a modified serial plate transfer assay (Table 5). Unfunctionalised catheters prevented the attachment of colonies for 2 days. Antibiotic‐coated catheters resisted attachment for 4 days, and SNP‐coated catheters resisted attachment for 8 days. The number of colonies attached to the unfunctionalised catheters on Day 5 was 1 × 103 CFU/cm2. The number of organisms attached to the antibiotics‐coated catheters was 0.027 × 103 CFU/cm2. No colonies were attached to the SNP‐coated catheters or the catheters coasted with a combination of antibiotics and SNP. The magnitudes of bacterial attachment on Day 15 were 12.12 × 103 CFU/cm2 for unfunctionalised catheters, 8.13 × 103 CFU/cm2 for antibiotic‐coated catheters, 3.19 × 103 CFU/cm2 for SNP‐coated catheters, and zero for combination‐coated catheters. The P values for the in vitro challenge assay comparisons between Days 5 and 10, and 10 and 15, were 0.0022 and 0.00113, respectively. All treatment results were statistically distinct. The anti‐attachment activity of nanoparticle‐functionalised catheters is consistent with the results of Lellouche et al. [8]. The latter study confirmed that the anti‐attachment and antibiofilm properties are caused by nanomaterial rather than ions.
Table 5.
In vitro anti‐adhesive assay
| Treatment | Number of colonies, CFU/cm2 | ||
|---|---|---|---|
| 5th day | 10th day | 15th day | |
| control | 1000 ± 38 | 5190 ± 100 | 12,117 ± 240 |
| antibiotics | 27 ± 3 | 2190 ± 54 | 8125 ± 100 |
| SNP | 0 | 425 ± 7 | 3191 ± 10 |
| synergy | 2 ± 0 | 10 ± 0 | 100 ± 2 |
The SNP and antibiotics were efficiently impregnated into the catheters. Efficient impregnation permits the slow release of both SNP and antibiotics upon exposure to urine. Moreover, all antibacterial agents prevented the attachment of bacteria, which is an initial stage in the formation of biofilm. These results were consistent with SEM images of the catheters, recorded on Day 15. Unfunctionalised catheters (Fig. 8 a) presented abundant networks of microbes organised into biofilm. Catheters functionalised with antibiotics (Fig. 8 b) also presented extensive biomass. SNPs‐coated catheters (Fig. 8 c) presented relatively little growth. We observed no such growth on the catheters functionalised with a combination of SNP and antibiotics (Fig. 8 d).
Fig. 8.

SEM of biofilm formation on catheter
(a) Control, (b) Antibiotic, (c) SNP, (d) Synergy
Synergistic combination of SNP and antibiotics efficiently prevented the attachment of bacteria. Cho et al. [26] report that gentamicin‐releasing catheters inhibit pathogen growth until Day 5 only. Minardi et al. [27] studied the impact of tigecycline and rifampin on biofilm formation in urethral stents.
3.10 In vivo efficacy of functionalised catheters
Urine samples were collected from mice on alternate days; Fig. 9 a shows the total number of bacteria present in urine samples for each treatment. Reductions from the urine bacterial counts for mice with unfunctionalised catheters were calculated for each group of mice with differently treated catheters. On Day 3, the reduction in total bacterial count was 50, 78, 90, and 98% for the mice with antiseptic‐coated catheters, antibiotic‐coated catheters, SNP‐coated catheters, and combination‐coated catheters. This observation is consistent with the model that as urine flows through the catheter, the antibacterial agents are released slowly and destroy the bacteria. The efficiency in killing the bacteria decreased over time. On Day 13, antiseptic‐coated catheterised mice presented only a 21% reduction in the total number of bacteria in urine. The antibiotic‐coated catheter group presented a 65% reduction, and the SNP group presented an 85% reduction. In the combination group, 96% of the urine bacteria were absent, relative to the control. The P ‐values for the urine microbial load on Days 3, 5, 7, 9, 11, and 13 were 0.00197, 0.00684, 0.00241, 0.00730, 0.0052, and 0.00289, respectively. The P –value is <0.01, and hence they are statistically significant at the 99.9% confidence interval.
Fig. 9.

Influence of functionalisation on biofilm on catheter
(a) Urine, (b) Catheter
On Day 15, the mice were euthanised and their catheters were removed to assess biofilm growth. The reduction in the biofilm in the functionalised catheter is shown in Fig. 9 b. Antiseptic‐coated catheter exhibited 42% reduction in biofilm, antibiotic‐coated catheter exhibited 55% reduction, and SNP‐coated catheter exhibited 83% reduction in biofilm. The largest biofilm reduction (98%) was measured for the synergistic combination of antibiotics and SNP. The P ‐value for the in vivo biofilm assay was 0.001136, and the values are statistically significant. Earlier study by Webster et al. [28] also reported that antiseptic‐coated catheters were not effective in controlling catheter‐associated bacteuria. Microbicidal properties of SNPs delivered through polymers are supported by Lim et al. [29]. Throughout the experimental period, all mice were normal. These in vivo results indicate that catheters functionalised with a synergistic combination of antibiotics and SNP prevent biofilm formation.
Table 6 shows blood profiles of catheterised mice. To the best of our knowledge, we are the first to report on kidney and liver function in catheterised mice. The concentration of urea was normal in all treatments. In the mice with unfunctionalised catheters (T3), the urea concentration was 33 mg/dl. A slightly higher concentration of 41 mg/dl was observed for the antiseptic‐coated catheter (T4) group. In the group of mice with SNP‐coated catheters (T6), urea was 29 mg/dl, and in the combination (T7) group, urea was 32 mg/dL. Irrespective of the treatment, the serum creatinine and bilirubin concentrations were within the normal range. These observations indicate that kidney function was normal in all animals. In mice with antiseptic‐coated catheters, the creatinine concentration was in the high normal range. The total protein concentration was within the normal range in all treatments.
Table 6.
Blood profiles of mice
| Test name | Normal value | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| urea, mg/dl | 5–40 | 32a ± 2.1 | 33b ± 2.7 | 33b ± 5.0 | 41 ± 2.1 | 29 ± 2.0 | 30 ± 3.0 | 32a ± 3.1 |
| creatinine, mg/ dl | 0.5–1.3 | 0.6c ± 0.0 | 0.7d ± 0.0 | 0.8 ± 0.0 | 1.2 ± 0.2 | 0.7d ± 0.1 | 1.4 ± 0.5 | 0.6c ± 0.3 |
| total bilirubin, mg/dl | 0.3–1 | 0.4e ± 0.0 | 0.6f ± 0.0 | 0.6 ± 0.0 | 0.9 ± 0.0 | 0.4e ± 0.0 | 0.6f ± 0.0 | 0.4 ± 0.0 |
| total protein, g/dl | 6.4–7.8 | 6.5 ± 0.5g | 6.5g ± 0.0 | 6.8 ± 0.8 | 6.2 ± 0.0 | 6.1 ± 0.5 | 6.6 ± 0.5 | 7.48 ± 0.5 |
| albumin, g/dl | 3.5–5.2 | 3.3 ± 0.5 | 3.8 ± 0.0 | 4.0 ± 0.9 | 4.3 ± 0.5 | 3.6 ± 0.1 | 3.2 ± 0.1 | 4.7 ± 0.7 |
| globulin, g/dl | 1.5–2.5 | 2.2 ± 0.3 | 2.6 ± 0.4 | 2.8h ± 0.8 | 1.6 ± 0.1 | 2.1 ± 0.7 | 1.7 ± 0.5 | 2.8h ± 0.1 |
| SGPT, U/L | 0–40 | 21 ± 2.2 | 33 ± 2.70 | 44 ± 3.1 | 77 ± 5.7 | 23 ± 2.6 | 20 ± 1.9 | 15 ± 1.2 |
| SGOT, U/L | 0–40 | 14 ± 1.4 | 21i ± 1.2 | 42 ± 1.5 | 91 ± 14 | 21i ± 0.9 | 17 ± 0.9 | 13 ± 0.8 |
| SAP, U/L | 64–300 | 180 ± 16.6 | 201 ± 11 | 311 ± 22 | 366 ± 29 | 206 ± 15 | 71 ± 1.1 | 76 ± 2.4 |
a,b,c,d,e,f,g,h,i Data with common superscripts in each row are homogenous.
The activities of SGOT, SGPT, and SAP were 77, 91, and 366 U/L, respectively, in the antiseptic group (T4). These T4 activity measurements were high relative to their counterparts in the other groups.
This shows the possible liver damage due to uropathogenic E. coli. Liver damage by UTI is reported by Wyke [30]. Smyk et al. [31] reported the association between UTI and autoimmune liver disease. The present results corroborate the observation of Haque et al. [32], which demonstrates increased enzyme activity in damaged liver. The antiseptic‐coated catheter has a harmful effect on the liver, indicated by the significantly elevated SGOPT, SGPT, and SAP. Catheters functionalised with a combination of SNP, amikacin, and nitrofurantoin offer remarkable anti‐infective properties and have no apparent side effects on liver or kidney function.
3.11 In vitro anti‐adherence property of catheter after 2 years
Functionalised catheters were sealed aseptically and opened after 2 years. These catheters were challenged with 105 CFU/ml for 2 h and subsequently assessed for their anti‐attachment activities. Antibiotic‐coated catheter exhibited 31% attachment reduction, SNP exhibited 75%, and the combination exhibited 91% inhibition of bacterial attachment. These observations indicate that functionalised catheters retain the ability to inhibit biofilm formation for at least 2 years.
4 Conclusion
Multi‐drug‐resistant uropathogens are emerging at an alarmingly increasing rate. SNPs synthesised using S. platensis exerted an oligodynamic effect against uropathogenic E. coli. Antibiotics that have lost their potency can be repurposed for synergistic and efficacious use with SNPs. Surface functionalisation of catheters with this synergistic combination enables superior control of biofilm formation. Impregnation of antibacterial agents on to the catheter is an efficient and simple way of restricting catheter biofilm formation. The functionalised catheter exhibits no adverse effects on the physiological status of mice. This synergistic combination of antibiotics and SNP appears biocompatible.
5 Acknowledgment
The authors sincerely thank the Principal and Management of Mepco Schlenk Engineering College (Autonomous), Sivakasi, for providing the infrastructure and support to carry out the work. We kindly acknowledge CoE, Indutech, Coimbatore, Tamil Nadu, India for the service rendered in recording SEM images.
6 References
- 1. Bonkat G. Widmer A.F. Rieken M. et al.: ‘Microbial biofilm formation and catheter‐associated bacteriuria in patients with suprapubic catheterisation’, World J. Urol., 2013, 31, pp. 565 –571 [DOI] [PubMed] [Google Scholar]
- 2. Goncalves I. Abreu A.S. Matama T. et al.: ‘Enzymatic synthesis of poly(catechin)‐antibiotic conjugates: an antimicrobial approach for indwelling catheters’, Appl. Microbiol. Biotechnol., 2015, 99, pp. 637 –651 [DOI] [PubMed] [Google Scholar]
- 3. Thallinger B. Brandauer M. Burger P. et al.: ‘Cellobiose dehydrogenase functionalized urinary catheter as novel antibiofilm system’, J. Biomed. Mater. Res. B Appl. Biomater., 2016, 104 (7), pp 1448 –56. doi: 10.1002/jbm.b.33491. Epub 2015 Aug 6. [DOI] [PubMed] [Google Scholar]
- 4. Paladini F. Pollini M. Deponti D. et al.: ‘Effect of silver nanocoatings on catheters for haemodialysis in terms of cell viability, proliferation, morphology and antibacterial activity’, J. Mater. Sci. Mater. Med., 2013, 24, pp. 1105 –1112 [DOI] [PubMed] [Google Scholar]
- 5. Lellouche J. Friedman A. Lahmi R. et al.: ‘Antibiofilm surface functionalization of catheters by magnesium fluoride nanoparticles’, Int. J. Nanomed., 2012, 7, pp. 1175 –1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vellinga A. Murphy A.W. Hanahoe B. et al.: ‘A multilevel analysis of trimethoprim and ciprofloxacin prescribing and resistance of uropathogenic Escherichia coli in general practice’, J. Antimicrob. Chemother., 2010, 65, pp. 1514 –1520 [DOI] [PubMed] [Google Scholar]
- 7. Abdallah K.S. Cao Y. We D.‐J.: ‘Epidemiologic investigation of extra‐intestinal pathogenic E. coli (ExPEC) based on PCR phylogenetic group and fimH single nucleotide polymorphisms (SNPs) in China’, Int. J. Mol. Epidemiol. Genet., 2011, 2, (4), pp. 339 –353 [PMC free article] [PubMed] [Google Scholar]
- 8. Bauer A.W. Kirby W.M. Sherris J.C. et al.: ‘Antibiotic susceptibility testing by a standardized single disk method’, Am. J. Clin. Pathol., 1966, 45, pp. 493 –496 [PubMed] [Google Scholar]
- 9. CLSI : ‘Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A6’ (Clinical and Laboratory Standards Institute, Wayne, PA, 2003) [Google Scholar]
- 10. Guo N. Ling G. Liang X. et al.: ‘In vitro synergy of pseudolaric acid B and fluconazole against clinical isolates of Candida albicans ’, Mycoses, 2011, 54e, pp. 400 –406 [DOI] [PubMed] [Google Scholar]
- 11. Hsiao I.L. Bierkandt F.S. Reichardt P. et al.: ‘Quantification and visualization of cellular uptake of TiO2 and Ag nanoparticles: comparison of different ICP‐MS techniques’, J. Nanobiotechnol., 2016, 14, p. 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moryl M. Torzewska A. Jalmuzna P. et al.: ‘Analysis of Proteus mirabilis distribution in multi‐species biofilms on urinary catheters and determination of bacteria resistance to antimicrobial agents’, Polish J. Microbiol., 2013, 62, pp. 377 –384 [PubMed] [Google Scholar]
- 13. Guiton P.S. Hung C.S. Hancock L.E. et al.: ‘Enterococcal biofilm formation and virulence in an optimized murine model of foreign body‐associated urinary tract infections’, Infect. Immun., 2010, 78, pp. 4166 –4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jahandeh N. Ranjbar R. Behzadi P. et al.: ‘Uropathogenic Escherichia coli virulence genes: invaluable approaches for designing DNA microarray probes’, Cent. European J. Urol., 2015, 68, pp. 452 –458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agarwal J. Srivastava S. Singh M.: ‘Pathogenomics of uropathogenic Escherichia coli ’, Indian J. Med. Microbiol., 2012, 30, pp. 141 –149 [DOI] [PubMed] [Google Scholar]
- 16. Johnson J.R.: ‘Virulence factors in Escherichia coli urinary tract infection’, Clin. Microbiol. Rev., 1991, 4, pp. 80 –128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clinical and Laboratory Standards Institute : ‘Performance standards for antimicrobial susceptibility testing’. Twenty‐First Informational Supplement M100‐S21, Wayne, PA, USA, 2011. [Google Scholar]
- 18. Pitout J.D.: ‘Extraintestinal pathogenic Escherichia coli a combination of virulence with antibiotic resistance’, Front. Microbiol., 2012, 3, p. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mala R. Arunachalam P. Sivasankari M.: ‘Synergistic bactericidal activity of silver nanoparticles and ciprofloxacin against phytopathogens’, J. Cell Tissue Res., 2012, 12, (2), pp. 3249 –3254 [Google Scholar]
- 20. Hwang I.S. Hwang J.H. Choi H. et al.: ‘Synergistic effects between silver nanoparticles and asntibiotics and the mechanisms involved’, J. Med. Microbiol., 2012, 61, pp. 1719 –1726 [DOI] [PubMed] [Google Scholar]
- 21. Miller G.H. Sabatelli F.J. Hare R.S. et al.: ‘The most frequent aminoglycoside resistance mechanisms—changes with time and geographic area: a reflection of aminoglycoside usage patterns? Aminoglycoside Resistance Study Groups’, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 1997, 24, (Suppl. 1), pp. S46 –S62 [DOI] [PubMed] [Google Scholar]
- 22. Gonzales P.R. Pesesky M.W. Bouley R. et al.: ‘Synergistic, collaterally sensitive β‐lactam combinations suppress resistance in MRSA’, Nat. Chem. Biol., 2015, published online, 11, (11), pp 855 –861. doi: 10.1038/nchembio.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panácek A. Smékalová M. Kilianová M. et al.: ‘Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect’, Molecules, 2016, 21, p. 26, doi: 10.3390/molecules 2101002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu K. Yang Y. Zhang Y.M. et al.: ‘Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy’, Int. J. Nanomed., 2015, 10, pp. 7241 –7252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roe D. Karandikar B. Savage N.B. et al.: ‘Antimicrobial surface functionalization of plastic catheters by silver nanoparticles’, J. Antimicrob. Chemother., 2008, 61, pp. 869 –876 [DOI] [PubMed] [Google Scholar]
- 26. Cho Y.W. Park J.H. Kim S.H. et al.: ‘Gentamicin‐releasing urethral catheter for short‐term catheterization’, J. Biomater. Sci. Polym. Ed., 2003, 14, (9), pp. 963 –972 [DOI] [PubMed] [Google Scholar]
- 27. Minardi D. Cirioni O. Ghiselli R. et al.: ‘Efficacy of tigecycline and rifampin alone and in combination against Enterococcus faecalis biofilm infection in a rat model of ureteral stent’, J. Surg. Res., 2012, 176, pp. 1 –6 [DOI] [PubMed] [Google Scholar]
- 28. Webster J. Hood R.H. Burridge C.A. et al.: ‘Water or antiseptic for periurethral cleaning before urinary catheterization: a randomized controlled trial’, Am. J. Infect. Control., 2001, 29, (6), pp. 389 –394 [DOI] [PubMed] [Google Scholar]
- 29. Lim Y.H. Tiemann K.M. Heo G.S. et al.: ‘Preparation and in vitro antimicrobial activity of silver‐bearing degradable polymeric nanoparticles of polyphosphoester‐block‐Poly(L‐lactide)’, ACS Nano., 2015, 9, (2), pp. 1995 –2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyke R.J.: ‘Bacterial infections complicating liver disease’, Baillieres Clin. Gastroenterol., 1989, 3, (1), pp. 187 –210 [DOI] [PubMed] [Google Scholar]
- 31. Smyk D.S. Bogdanos D.P. Kriese S. et al.: ‘Urinary tract infection as a risk factor for autoimmune liver disease: from bench to bedside’, Clin. Res. Hepatol. Gastroenterol., 2012, 36, (2), pp. 110 –121 [DOI] [PubMed] [Google Scholar]
- 32. Haque A. Best F.A. Amante F.H.: ‘High parasite burdens cause liver damage in mice following Plasmodium berghei ANKA infection independently of CD8_ T cell‐mediated immune pathology’, Infect. Immun., 2011, 79, (5), pp. 1882 –1888 [DOI] [PMC free article] [PubMed] [Google Scholar]


