Abstract
Background
International guidelines advocate using daily inhaled corticosteroids (ICS) in the management of children and adults with persistent asthma. However, in real world clinical settings, these medicines are often used at irregular intervals by patients. Recent evidence suggests that the use of intermittent ICS, with treatment initiated at the time of early symptoms, may still have benefits for reducing the severity of an asthma exacerbation.
Objectives
To compare the efficacy and safety of intermittent ICS versus placebo in the management of children and adults diagnosed with, or suspected to have, symptoms of mild persistent asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials (CAGR), the ClinicalTrials.gov website and the World Health Organization (WHO) trials portal in March 2015.
Selection criteria
We included randomised controlled trials (RCTs) that compared intermittent ICS versus placebo in children and adults with symptoms of persistent asthma. No co‐interventions were permitted other than rescue relievers and oral corticosteroids used during exacerbations.
Data collection and analysis
Two review authors independently assessed trials for inclusion, methodological quality and extracted data. The primary efficacy outcome was the risk of asthma exacerbations requiring oral corticosteroids and the primary safety outcome was serious adverse health events. Secondary outcomes included exacerbations, lung function tests, asthma control, adverse effects, and withdrawal rates. Quality of the evidence was assessed using the GRADE criteria.
Main results
Six trials (representing 490 preschool children, 145 school‐aged children and 240 adults) met the inclusion criteria. Study durations were 12 to 52 weeks. Results for preschool children were presented in a separate analysis as this represents a distinct clinical condition, not necessarily related to the development of long term asthma.
There was a reduction in the risk of patients experiencing one or more exacerbations requiring oral corticosteroids in older children (145 participants, odds ratio (OR) 0.57; 95% confidence interval (CI) 0.29 to 1.12, low quality evidence) and adults with asthma (240 participants, OR 0.10; 95% CI 0.01 to 1.95, low quality evidence). These analyses were each based on the findings of a single study. No group difference was observed in the risk of serious adverse health events (385 participants; OR 1.00; 95% CI 0.14 to 7.25, moderate quality evidence). Compared to the placebo group, there was an insufficient number of participants to make firm conclusions whether the intermittent ICS group displayed any reduction in the rate of hospitalisations, day time and night time symptoms scores, or adverse events. Lung function tests reported by a single study favoured the use of ICS. There was no significant group difference in growth rate of children, or overall withdrawals.
In preschool children with frequent wheezing episodes, the use of intermittent ICS at the onset of early symptoms reduced the likelihood of requiring rescue oral corticosteroids by half (490 participants; OR: 0.48; 95% CI 0.31 to 0.73, moderate quality evidence with minimal heterogeneity). Intermittent therapy was associated with fewer serious adverse events (439 participants; OR 0.42; 95% CI 0.17 to 1.02, low quality evidence). There was no significant difference in hospitalisations or in a single study measuring parent perceived quality of life. However, intermittent therapy was associated with improvements in both day time and night time symptoms. There was no increase in the rates of withdrawals, and overall and treatment‐specific adverse events.
Authors' conclusions
In children and adults with mild persistent asthma, two studies have shown that the use of intermittent ICS at the time of exacerbation reduced the chances of needing oral corticosteroids by half. This result is statistically significant if we assume that the effect size is the same for each study population (fixed effects model), but is not statistically significant when using a random effects model. However, the paucity of published evidence limits our conclusions towards the 'as‐needed' use of this medication. The small number of studies and participants were the major reasons for downgrading the overall quality of the findings. A corresponding result was found in preschool children with wheeze. In this age group, an improvement in day time and night time asthma symptoms score and parental perceived quality of life of children similarly favoured the ICS group. However, there was no statistical difference in hospitalisation rates in any group. This treatment was not associated with any significant increase in adverse events. There was no growth suppression noted with the use of intermittent ICS in either preschool or school‐aged children. Considering the limited number of available studies, we emphasise the need for more randomised controlled studies in order to confirm these findings.
Plain language summary
Can taking inhaled corticosteroids when needed for symptoms help people with mild asthma from becoming more unwell?
Daily inhaled corticosteroids (ICS) are the mainstay of medications prescribed for people with asthma who have ongoing difficulty with their breathing. However, for those with a milder form of the condition, it is hard to predict when their asthma will get worse and so many people do not use their inhaler regularly. In this review, we compared the use of ICS used intermittently at the start of an asthma episode with placebo treatment in children and adults with mild asthma (two trials representing 385 participants) and in preschool children deemed to be at risk of developing asthma symptoms in the future (four trials representing 490 participants).
We found taking ICS intermittently reduced the number of people with the need for oral steroids to manage their asthma symptoms. This was also associated with an improvement in lung tests in adults. While the greatest benefits were observed in adults who used a combined inhaler device when symptoms were developing, this was based on the results of just one published study. There were no increased safety concerns for ICS used in this way, although, there was not enough data to look for differences in hospitalisations, asthma symptoms or adverse events.
These findings only represent a subset of all people with asthma. In particular, people with frequent or severe symptoms need to be taking their medication every day to reduce ongoing inflammation in the airways of the lungs. The results looked separately at preschool children as young as one year, when it is harder to predict if asthma will continue into older age. In addition, it is still uncertain which type and dose of ICS is most effective, as well as the best pattern or delivery for intermittent use of the medication. Nevertheless, combining an ICS with a reliever medicine may offer physicians and patients a new approach for milder symptoms if used appropriately.
Summary of findings
Background
Description of the condition
Asthma is estimated to affect approximately 334 million people around the world and is a leading cause of respiratory‐related morbidity and mortality (Vos 2013). This is a significant health problem where the prevalence has been increasing in both developing and developed countries (Global Asthma Report 2014; Lai 2009; Pearce 2007). The recently updated Global Initiative for Asthma (GINA 2014) guideline has revised the definition of asthma as "a heterogeneous disease, usually characterized by chronic airway inflammation. It is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness, and cough that vary over time and in intensity, together with variable expiratory airflow limitation" . Clinicians are guided to make a diagnosis based on an individual's symptoms. There may be no abnormal signs on physical examination in the interval phase, or, if the asthma is persistent and not optimally controlled there may be features of chronic airway limitation. Other supportive features may include a personal history of atopy (the genetic tendency to develop allergic diseases) or a family history of atopy or asthma, as well as information provided from lung function or provocation tests. Classification of severity can then be assessed according to symptom pattern, which helps to determine the need for a controller medication (GINA 2014).
Wheeze is a common symptom in the preschool age group. Almost 50% of children report at least one episode of wheeze in the first six years of life (Martinez 1995). Persistent wheeze is associated with poorer lung function and airway resistance by the age of four years (Brussee 2004), as well as deficits until adulthood if symptoms are present at the age of six years (Horak 2003; Phelan 2002). This can be related to chronic inflammation and reversible flow obstruction within the airways, consistent with the underlying features of asthma in older children and adults. In these instances, the diagnosis of asthma can be confirmed by a therapeutic trial of inhaled bronchodilators for symptomatic relief as suggested by the GINA 2014 guidelines . There has been some hesitancy in making the diagnosis in children under the age of six years as it is difficult to predict long term prognosis. This was suggested by the findings of the longitudinal Tuscon cohort study (Martinez 1995). Some recent reports have continued to reassess the terminology and prognosis in preschool wheeze (Brand 2008; Ducharme 2014). Thus, clinical trials in preschoolers with frequent wheeze are represented as children with wheeze but not as asthmatics.
Description of the intervention
International guidelines recommend daily ICS in adults and children who have persistent asthma (BTS 2012; GINA 2014). This class of medication has been found to improve control over asthma symptoms by suppressing airway inflammation and reducing exacerbations, with demonstrated efficacy in promoting overall quality of life and improving clinical measures of lung function (Barnes 1998; Pauwels 2003; The Childhood Asthma Program Research Group 2000).
In preschool children, defined as children up to five years of age, individuals diagnosed with asthma have disproportionately higher rates of hospital attendances and acute care visits. According to international comparisons, nearly half of children have a reported asthma exacerbation in the preceding year (Garner 2008). However, this figure does not take into account the significant proportion of cases where the diagnosis is not confirmed in early age. The diagnostic challenge remains in children who have recurrent episodes of wheeze without interval symptoms (Bush 2014; Ducharme 2014). This has been the subject of ongoing discussion and research. This is important as early recognition of problematic preschool wheeze and availability of treatment options could help to reduce the burden in childhood of preschool asthma.
How the intervention might work
Introduction and appropriate titration of the dose of ICS has been associated with a reduction in the rates of mild and severe asthma exacerbations (Pauwels 1997). However, in patients with mild persistent symptoms (once per day) with few if any exacerbations, the optimal approach has not been fully studied. People may be inclined to discontinue their recommended daily ICS treatment when they are asymptomatic and therefore the benefits of a preventer inhaler are less well perceived (Rand 2005).
There are established adverse effects associated with prolonged ICS therapy (Zhang 2011). In children, reports have shown an impact on growth trajectory and final adult height (Kelly 2012; Pruteanu 2014; Sharek 1999; Zhang 2014).
Recent evidence has suggested that ICS, used intermittently at the onset of exacerbations and continued for a short duration in combination with a short‐acting beta2‐agonist, could be an effective early step regimen in adults (Papi 2007) and children (Martinez 2011) with mild persistent asthma. A recent Cochrane Review found low quality evidence that intermittent and daily ICS strategies were similarly effective in terms of requirements for the use of rescue oral corticosteroids and the rate of severe adverse health events (Chauhan 2013). However, the strength of this evidence limited conclusions that these two regimes were equivalent and there was no comparison with placebo. Furthermore, there is evidence to support the premise that short courses of high‐dose ICS administered at the onset of symptoms, compared to regular lower‐dose ICS use, provide similar results in terms of the number of exacerbations in studies of adults (Foresi 2000). Thus, timely use of ICS may help to prevent people from experiencing more severe exacerbations.
Why it is important to do this review
While there is a strong dose‐related relationship between regular ICS adherence and treatment outcomes, many reports suggest that fewer than 50% of adults and children are taking the full extent of therapy that has been prescribed for them (Bender 2002; Burgess 2008; Rand 2007). On the one hand this may result in individuals having more symptoms, and increased healthcare costs associated with hospital admissions for avoidable exacerbations. On the other hand this could reflect how health professionals' beliefs about medication‐taking behaviour differ from the general population and misrepresent the response to therapy (Bender 2003).
A number of trials have compared intermittent ICS use versus placebo, but our understanding is that the question about the benefits of intermittent ICS use on exacerbations and patient outcomes has yet to be formally analysed as part of a systematic review. There may also be a dose‐dependent level for clinical response as well as differences when distinguishing episodic wheeze versus persistent asthma in younger children (Ducharme 2009; McKean 2000). These results will be relevant to patients and policy makers as an approach that may be better tolerated, involve less medication costs and potentially be more closely adhered to.
Objectives
To compare the efficacy and safety of intermittent ICS versus placebo in the management of children and adults diagnosed with or suspected to have mild persistent asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) with a parallel group design conducted for at least 12 weeks duration. We did not exclude studies on the basis of blinding. Cross‐over trials were not included to attempt to minimise the effects of treatment group cross contamination. The number of events may also have varied greatly across participants and at different times of the year. We included studies reported as full‐text, those published as abstract only, and unpublished data.
Types of participants
We included children aged 1 to 18 years and adults diagnosed with persistent asthma. We included preschool children with suspected asthma symptoms (and at least one episode documented by a health professional in the past 6 months) in a separate analysis.
Types of interventions
In each trial, participants were randomised to patient‐initiated intermittent ICS therapy used at the onset of an exacerbation (recognised as separate or combined treatment with a short‐acting beta2‐agonist reliever) and compared with placebo. ICS was allowed in any formulation. No co‐interventions were permitted other than rescue relievers and oral corticosteroids used during exacerbations. Studies allowing concomitant medication use (apart from regular ICS) were included if the dose of the medication was stable before and during the intervention period.
Types of outcome measures
Primary outcomes
Asthma exacerbations: patients experience one or more exacerbations requiring rescue oral corticosteroids.
Serious adverse events.
Secondary outcomes
Hospital admissions.
Quality of life (validated questionnaires only).
Symptom scores.
Study withdrawals.
Measures of lung function (forced expiratory volume in one second (FEV1), peak expiratory flow rate (PEFR)).
Adverse events/side effects.
Reporting of one or more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We included trials from the Cochrane Airways Group's Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy in Appendix 2.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). We searched all databases from their inception to March 2015, and imposed no restriction on language or type of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We also searched relevant manufacturers' websites for trial information. We checked for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed) on 25 March 2015.
Data collection and analysis
Selection of studies
Two review authors (JC, CH) independently screened titles and abstracts for inclusion of all the potential studies and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We then retrieved the full‐text study reports/publication and the same two review authors independently screened the full‐text, identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, consulted a third person (IA). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009) and 'Characteristics of included studies' table.
Data extraction and management
We used a data collection form for study characteristics and outcome data which already piloted on more than one study in the review. Two review authors (CH, BC) then extracted study characteristics from included studies and this was cross checked by a third author (JC). We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number, mean age, age range, gender, severity of condition, asthma diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (CH, BC) then independently extracted outcome data from included studies. Study characteristics were recorded in the 'Characteristics of included studies' table. We resolved disagreements by consensus or by involving a separate author (JC). One review author (JC) transferred data into the Review Manager (RevMan 2014) file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (BC) then spot‐checked data for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another author (JC, IA). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgment in the 'Risk of bias' table. We then summarised the risk of bias judgements for all included studies for each of the domains listed.
When considering treatment effects, we took into account the risk of bias for the studies that may have contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR) and continuous data as mean difference (MD) or standardised mean difference (SMD). We entered data presented by the study authors as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, that is if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
We analysed dichotomous data using participants as the unit of analysis (rather than events) to avoid counting the same participant more than once. This is a specific issue with repeated events such as exacerbations. For continuous data, the MD based on change from baseline was preferred over MD based on absolute values.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data was thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis (Higgins 2011). If we identified substantial heterogeneity (considered to be an I² statistic >50%) we reported it and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
If we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible small study and publication biases (Sterne 2011).
Data synthesis
We used a fixed‐effect model and performed a sensitivity analysis with random‐effects model especially in the presence of heterogeneity, because in this situation, a random‐effects meta‐analysis weights the studies relatively more equally than a fixed‐effect analysis.
Summary of findings table
We created a 'Summary of findings' table including outcomes for exacerbations, serious adverse events, quality of life, symptom scores and adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Schünemann 2012) using GRADEpro software (GRADE 2012). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes and made comments to aid reader's understanding of the review, where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Type of ICS used and dose.
Single versus separate ICS and short‐acting beta2‐agonist devices.
Severity of disease at baseline.
Adults versus school‐aged children versus preschool children.
Type of device used or spacer.
Concomitant medication.
Study duration.
We used only primary outcomes in subgroup analyses and used the formal test for subgroup interactions in Review Manager (RevMan 2014).
Sensitivity analysis
We assessed the sensitivity of our primary outcomes to degree of bias by comparing the overall results with those exclusively from trials assessed as being at low risk of bias. This was performed by excluding studies at high risk of bias for blinding, randomisation/allocation concealment and then a second analysis to exclude studies that may have inadequate reporting of outcomes.
Results
Description of studies
Results of the search
From a search in March 2015, we identified 786 abstracts of potentially relevant trials. Concurrently, we conducted a search of trials registered on the Clinicaltrials.gov database in March 2015 which generated a list of 980 registered trials and 113 trials identified from the WHO international clinical trials platform registry using the search strategy listed in Appendix 3. Two review authors (JC and CH) then agreed upon 34 references that were appropriate for further appraisal. We excluded duplicate studies using the official trial numbers, trial duration, together with other identifiable study features. Following the screening of abstracts, we identified 15 studies where the full texts were sought and determined to be eligible for this review. However, after relevant exclusions, six eligible studies were included in the meta‐analysis. The study flow diagram is presented in Figure 1.
1.
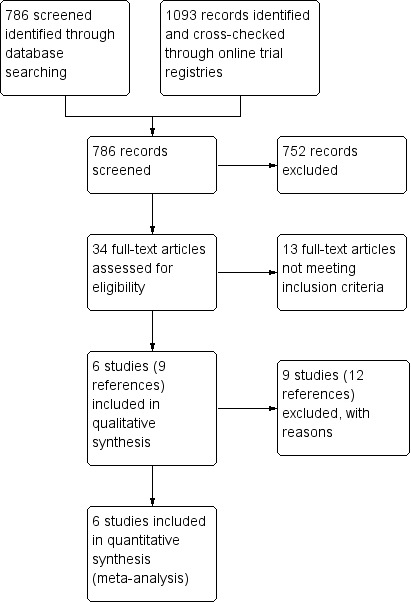
Study flow diagram.
Included studies
Six studies (representing 490 preschool children, 145 school‐aged children and 240 adults) which evaluated the effects of as‐required ICS compared with placebo (as‐required short‐acting beta2‐agonist) met the inclusion criteria for this review. Study duration varied from 12 to 52 weeks. One study recruited older children of 5‐18 years (Martinez 2011); while another study exclusively recruited adults aged 18‐65 years (Papi 2007) with mild persistent asthma. For more details see Characteristics of included studies. For the study including children of school age and older, mild persistent asthma was defined as having symptoms (e.g. wheezing) on average, more than two days a week, or reliance on use of a daily controller treatment to maintain good control.
Four studies recruited preschool children aged 1‐3 years (Svedmyr 1999), 1‐4 years (Papi 2009), 1‐5 years (Bacharier 2008) or 1‐6 years (Ducharme 2009). Different criteria were used across the preschool studies, but were included under a broader assessment of wheezing disorders in the evidence‐based approach outlined by the European Respiratory Society (ERS) Task Force (Brand 2008). Svedmyr 1999 relied on a doctor's diagnosis of asthma based on the finding of reversible variable airway obstruction, but had the occurrence of persistent symptoms between upper respiratory tract infection episodes as an exclusion factor. Whereas Papi 2009 and Bacharier 2008 did not clearly define this, instead using the frequency of wheezing episodes severe enough to need medical attention to identify the condition. Similarly, children were eligible for the Ducharme 2009 study if they had had three or more wheezing episodes in their lifetime associated with upper respiratory tract infections and recently required a dose of oral corticosteroids. There was no indication in any of these studies of which children had been previously trialled and if a therapeutic benefit to inhaled bronchodilators was observed. However, the inclusion criteria for each of these studies required a history of frequent episodes of wheezing needing medical attention. In the study by Papi 2009, participants were stratified at randomisation based on family history and other atopic features associated with persistent asthma.
The studies varied widely in the type and administration of ICS. Two of the studies conducted in preschool children prescribed nebulised medication with either budesonide 1.0 mg, given twice a day for up to seven days (Bacharier 2008) or beclomethasone 800 µg as required for symptoms (Papi 2009). In contrast, Svedmyr 1999 used a step‐down regimen of budesonide 400 µg four times a day for the first three days, reduced to twice a day for the remaining seven days, delivered via a spacer. The highest dosing regimen was seen in Ducharme 2009, where fluticasone 750 µg was given twice a day via an inhaler delivery device, for up to ten days, at the onset of defined upper respiratory tract infective symptoms. The studies involving older children and adults used as‐required dosing of beclomethasone for symptoms, whereas the distinguishing feature in the Papi 2007 study was the use of a combined steroid/bronchodilator inhaler device. Two of the studies (Bacharier 2008; Svedmyr 1999) initiated therapy pre‐emptively at the onset of an episode, whereas the others administered medications on an as‐needed basis alongside a bronchodilator used for symptom relief.
Excluded studies
See Characteristics of excluded studies.
Studies were excluded according to age since there is insufficient evidence showing that the pathophysiology of conditions in infancy, such as bronchiolitis, are similar to that of asthma in older children and adults. For example, Durmaz 2011 and Bisgaard 2006 assigned treatment for infants, before the age of one year, who had an episode of wheeze. Five studies were excluded as there was no placebo group for comparison with intermittent ICS use (Boushey 2005; Hamada 2008; Jenkins 2006; Turpeinen 2008; Zeiger 2011). Haahtela 2006 published a study with the addition of as‐needed budesonide to formoterol (long‐acting beta2‐agonist), therefore this represents a separate class of medications with varying recommended uses and effects.
Risk of bias in included studies
Full details of our 'Risk of bias' judgements are presented in Characteristics of included studies and the summary graphic in Figure 2.
2.
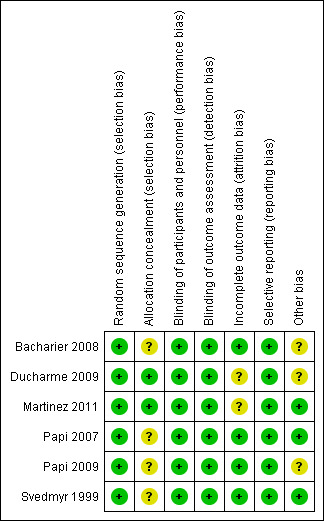
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was sufficiently described within the main texts or online supplements for five of the six included studies (Ducharme 2009; Martinez 2011; Papi 2007; Papi 2009 and Svedmyr 1999). Further information was provided by the contact author for Bacharier 2008. However, no further information was provided to judge allocation concealment, except for the Martinez 2011 and Ducharme 2009 studies.
Blinding
Double‐blinding of participants, study personnel and outcome assessors was reported in all included studies.
Incomplete outcome data
Withdrawals ranged from 3% to 21% for the ICS and 2% to 34% in the placebo‐treated groups. In both the Ducharme 2009 and Martinez 2011 studies, the number of withdrawals in the ICS group was approximately half compared to placebo. The reasons cited for this were mainly lack of perceived efficacy and chronic or severe symptoms. Nonetheless, all participants were included within the intention to treat (ITT) final analysis. When these studies were combined, there was no statistically significant difference between the two comparison groups (see Analysis 1.7).
1.7. Analysis.
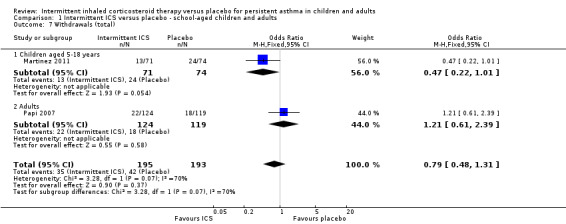
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 7 Withdrawals (total).
Selective reporting
Each of the studies reported all primary and secondary outcomes.
Other potential sources of bias
There was a slightly greater proportion of participants who were male or exposed to tobacco smoke in the treatment group from the Bacharier 2008 study. The study was randomised, but no further information about sequence generation was reported. This difference, when considering the overall study design and otherwise similar asthma history between the groups, suggests this alone is unlikely to contribute to bias in the results.
Oral corticosteroids were made available for children in all of the studies. They were started using a specific criteria/algorithm in three studies (Bacharier 2008; Ducharme 2009 and Martinez 2011) and not clearly defined but judged by the investigator in others (Papi 2007; Papi 2009; Svedmyr 1999). The definition for exacerbations was generally recognised with an increased use of rescue medication as compared with the baseline period for two or more consecutive days for children older than school age and for adults. For the preschool group, this was either defined subjectively as episodes of progressive increase in shortness of breath, cough or wheezing that required a change in medication (Papi 2009), or persistent respiratory symptoms lasting for five or more days (Bacharier 2008). Svedmyr 1999 was the only study to allow continuation of asthma concomitant medication with sodium cromoglycate for participants already taking this as a fixed dose and theophylline allowed when needed.
Effects of interventions
Summary of findings for the main comparison. Intermittent ICS versus placebo for children and adults with persistent asthma.
| Intermittent ICS versus placebo for persistent asthma in children and adults | ||||||
|
Patient or population: School‐aged children and adults
Settings: Community
Intervention: Intermittent ICS Control: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exacerbations requiring oral corticosteroids ‐ School‐age children Follow‐up: 44 weeks | 486 per 1000 | 351 per 1000 (216 to 515) | OR 0.57 (0.29 to 1.12) | 145 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Exacerbations requiring oral corticosteroids ‐ adults Follow‐up: 6 months | 34 per 1000 | 3 per 1000 (0 to 64) | OR 0.10 (0.01 to 1.95) | 240 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Serious adverse events Follow‐up: 26‐44 weeks | 5 per 1000 | 5 per 1000 (1 to 37) | OR 1 (0.14 to 7.25) | 385 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Day time asthma score Mean difference Follow‐up: 6 months | The mean daytime asthma score in the intervention groups was 0.20 lower (0.57 lower to 0.17 higher) | 240 (1 study) | ⊕⊕⊕⊝ moderate2 | |||
| Night time asthma score Mean difference Follow‐up: 6 months | The mean nighttime asthma score in the intervention groups was 0.07 higher (0.31 lower to 0.45 higher) | 240 (1 study) | ⊕⊕⊕⊝ moderate2 | |||
| Adverse events Follow‐up: 6 months | 0 per 1000 | 0 per 1000 (0 to 0) | OR 2.90 (0.12 to 71.96) | 243 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There were very few events leading to wide confidence intervals.
2 This is based on the results of one study, where the confidence intervals crossed the midline.
Summary of findings 2. Intermittent ICS versus placebo for preschool children with wheeze.
| Intermittent ICS versus placebo for preschool children with wheeze | ||||||
|
Patient or population: Preschool children
Settings: Community
Intervention: Intermittent ICS Control: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exacerbations requiring oral corticosteroids Follow‐up: 12‐52 weeks | 434 per 1000 | 269 per 1000 (192 to 359) | OR 0.48 (0.31 to 0.73) | 490 (4 studies) | ⊕⊕⊕⊝ moderate1 | |
| Serious adverse events Follow‐up: 3 to 12 months | 82 per 1000 | 36 per 1000 (15 to 83) | OR 0.42 (0.17 to 1.02) | 439 (3 studies) | ⊕⊕⊝⊝ low1,2 | |
| Hospitalisations Follow‐up: 6 to 12 months | 149 per 1000 | 113 per 1000 (39 to 286) | OR 0.73 (0.23 to 2.29) | 327 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Quality of Life PedsQL total score Follow‐up: mean 12 months | The mean quality of life in the intervention groups was 3.28 higher (‐2.13 to 8.69 higher) | 143 (1 study) | ⊕⊕⊝⊝ low3,4 | |||
| Day time asthma score Standardised mean difference Follow‐up: 12‐52 weeks | The mean daytime asthma score in the intervention groups was 0.35 standard deviations lower (0.57 to 0.13 lower) | 364 (3 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.35 (‐0.57 to ‐0.13); where higher symptom scores indicate worse symptoms | ||
| Night time asthma score Standardised mean difference Follow‐up: 12‐52 weeks | The mean nighttime asthma score in the intervention groups was 0.28 standard deviations lower (0.50 to 0.06 lower) | 364 (3 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.28 (‐0.50 to ‐0.06); where higher symptom scores indicate worse symptoms | ||
| Adverse events Follow‐up: 6 months | 304 per 1000 | 273 per 1000 (155 to 433) | OR 0.86 (0.42 to 1.75) | 166 (1 studies) | ⊕⊕⊝⊝ low2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The included studies vary significantly in their inclusion criteria and risk factors for developing asthma. 2 There were very few events leading to wide confidence intervals.
3 The confidence interval crosses no difference and does not rule out a small increase.
4 This is based on the results of only one study.
See: Table 1 for the main comparison
Primary outcomes
Asthma exacerbations
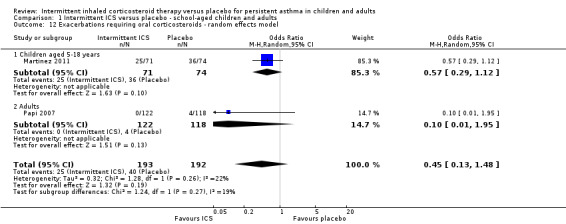
All included studies (representing 490 preschool children, 145 school‐aged children and 240 adults) compared intermittent ICS therapy with placebo. However, as mentioned above, due to the heterogeneous nature of studies in preschool children with wheeze and absence of a clear and consistent diagnosis of persistent asthma, these data were not presented together in the main analysis. In two studies with clear inclusion criteria meeting the definition of persistent asthma, there was a reduction in the rate of exacerbation requiring use of oral corticosteroids in older children (OR 0.57; 95% CI 0.29 to 1.12) and adults diagnosed with mild asthma (OR 0.10; 95% CI 0.01 to 1.95). For Analysis 1.1, the number needed to treat for an additional beneficial outcome (NNTB) was 11 (95% CI 7 to 100). This estimate was based on the combined analysis of both of these studies (OR 0.50; 95% CI 0.26 to 0.94; fixed effects model) and is further illustrated in Figure 3, where the average baseline risk of having an exacerbation requiring oral corticosteroids in the placebo group is 21%, compared to 12% (95% CI 6 to 20) for the active treatment group. By contrast, this comparison was not statistically significant when using the random effects model (Analysis 1.12). The only adult study (Papi 2007) showed the greatest treatment effect and in addition showed a fewer number of participants in the treatment group experiencing exacerbations (Analysis 1.3). The remaining subgroup analyses were not able to be carried out due to the small number of included studies (see Included studies).
1.1. Analysis.
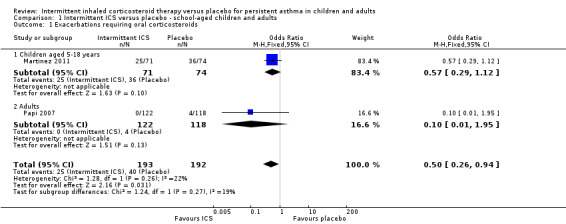
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 1 Exacerbations requiring oral corticosteroids.
3.
In the placebo group 21 people out of 100 had a exacerbation requiring oral corticosteroids over 44 weeks, compared to 12 (95% CI 6 to 20) out of 100 for the inhaled corticosteroids treatment group.
1.12. Analysis.
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 12 Exacerbations requiring oral corticosteroids ‐ random effects model.
1.3. Analysis.
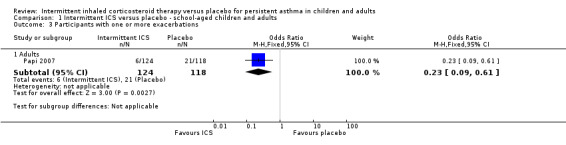
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 3 Participants with one or more exacerbations.
Among all studies of preschool children with wheezing episodes, intermittent ICS use was associated with a significant decrease in the number of participants requiring one or more courses of oral corticosteroids (OR 0.48; 95% CI 0.31 to 0.73, Analysis 2.1). This is equivalent to a NNTB of 7 (95% CI 5 to 14). There were no identified subgroup differences according to type of device used (Analysis 2.14). Sensitivity analyses based on concomitant medication use (Svedmyr 1999), trial duration of less than six months (Papi 2009), and for studies with higher risk of selection bias did not reveal any significant difference in this outcome.
2.1. Analysis.
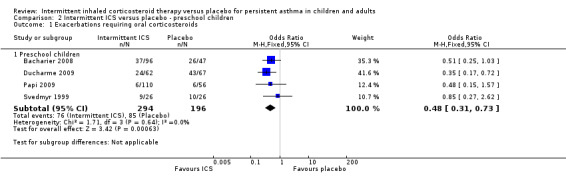
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 1 Exacerbations requiring oral corticosteroids.
2.14. Analysis.
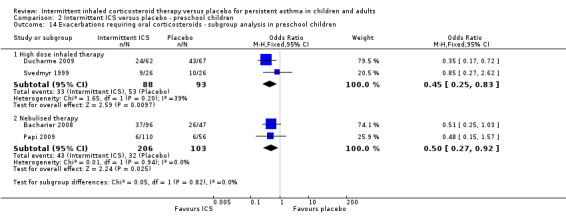
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 14 Exacerbations requiring oral corticosteroids ‐ subgroup analysis in preschool children.
Serious adverse events
There was no statistically significant difference in the rate of serious adverse events in studies of children and adults diagnosed with mild asthma (OR 1.00; 95% CI 0.14 to 7.25, Analysis 1.2), with 1/193 people in the ICS group compared with 1/192 with placebo. Again, further subgroup and sensitivity analyses could not be carried out due to the limited number studies.
1.2. Analysis.
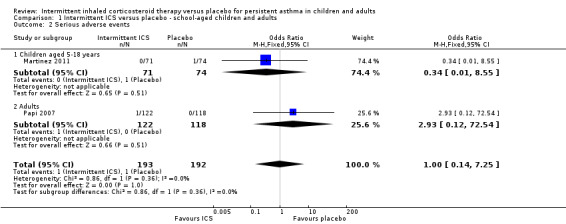
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 2 Serious adverse events.
There were fewer events recorded in preschool children who received ICS compared with the placebo group (OR 0.42; 95% CI 0.17 to 1.02, Analysis 2.2), with no difference between the studies using inhaled versus nebulised therapy (Analysis 2.15).
2.2. Analysis.
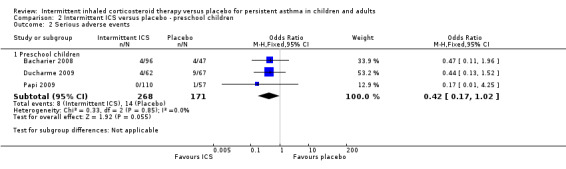
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 2 Serious adverse events.
2.15. Analysis.
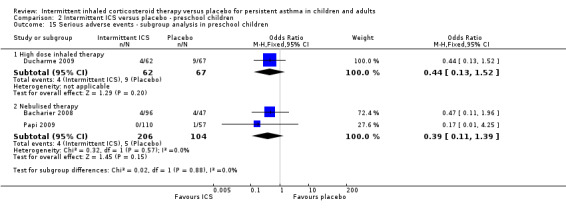
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 15 Serious adverse events ‐ subgroup analysis in preschool children.
Secondary outcomes
Hospitalisations
There was no significant difference in the number of participants requiring hospitalisations comparing active treatment and placebo groups in children (Analysis 1.4) or preschoolers with wheeze (OR 0.73; 95% CI 0.23 to 2.29, Analysis 2.5).
1.4. Analysis.
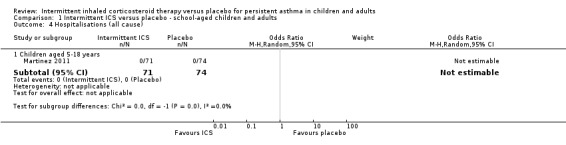
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 4 Hospitalisations (all cause).
2.5. Analysis.
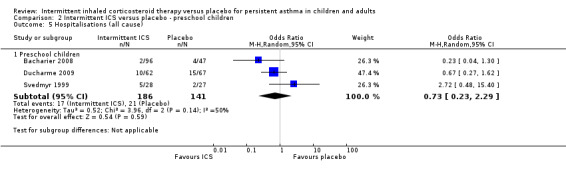
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 5 Hospitalisations (all cause).
Quality of life
Bacharier 2008 was the only study that reported change in quality of life of participants, measured using the Pediatric Quality of Life Inventory (PedsQL) scale scores (as rated by the parent) in a preschool population. In this study, there was a small improvement favouring ICS treatment, however this was not statistically significant (Analysis 2.3).
2.3. Analysis.
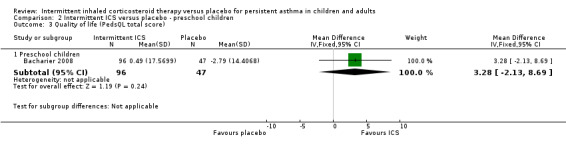
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 3 Quality of life (PedsQL total score).
Symptom scores
There was no significant difference in day time or night time symptom scores according to one study in adults with mild asthma (Analysis 1.5 and Analysis 1.6). While in preschool children, ICS treatment was associated with lower day time symptoms score (SMD ‐0.35; 95% CI ‐0.57 to ‐0.13, Analysis 2.6) and night time symptoms score (SMD ‐0.28; 95% CI ‐0.50 to ‐0.06, Analysis 2.7). This was presented as a standardised mean difference to account for the different outcome measures reported. This is further explored in the in the Table 2.
1.5. Analysis.
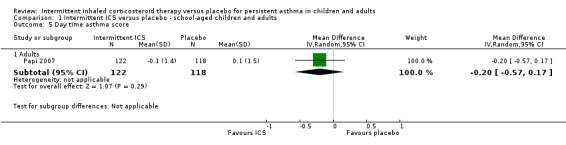
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 5 Day time asthma score.
1.6. Analysis.
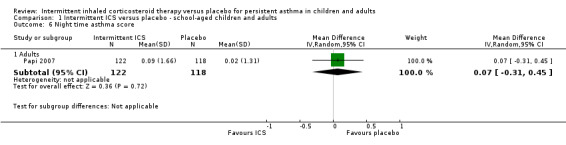
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 6 Night time asthma score.
2.6. Analysis.
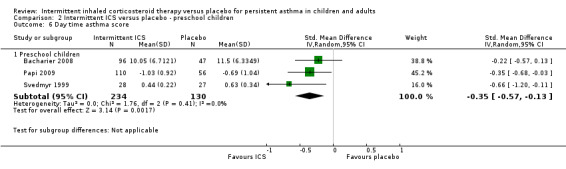
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 6 Day time asthma score.
2.7. Analysis.
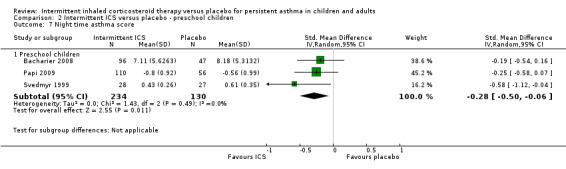
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 7 Night time asthma score.
Study withdrawals
There were 18% and 22% of participants who withdrew from the study in the ICS and placebo groups respectively with no statistical difference between both groups (OR 0.79; 95% CI 0.48 to 1.31, Analysis 1.7). A similar result was observed in the preschool age group (OR 0.67; 95% CI 0.37 to 1.19, Analysis 2.8).
2.8. Analysis.
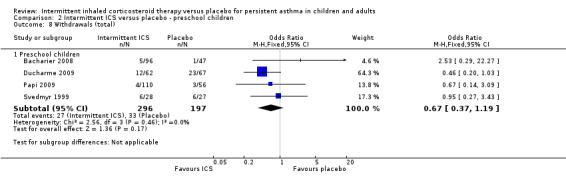
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 8 Withdrawals (total).
Measures of lung function
A single study by Papi 2007 reported change in peak expiratory flow (PEF) (Analysis 1.8) and forced expiratory volume in one second FEV1 (Analysis 1.9).
1.8. Analysis.
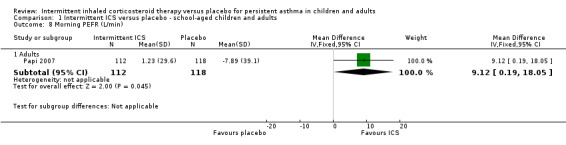
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 8 Morning PEFR (L/min).
1.9. Analysis.
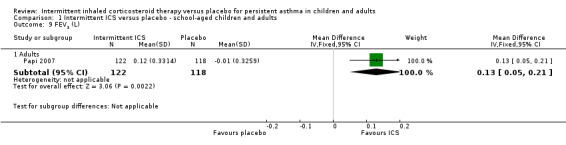
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 9 FEV1 (L).
Adverse events
Total adverse events were recorded in a single study of adults (Analysis 1.10), as well as in preschoolers (Analysis 2.9). The rates of hoarseness of voice and pneumonia, known adverse effects of ICS use, did not show an increased incidence with ICS compared to the placebo (Analysis 2.10 and Analysis 2.11, respectively). In addition, there were no deleterious effects on change in height (Analysis 1.11 and Analysis 2.12) in children of all ages or weight from baseline (Analysis 2.13) in preschool children.
1.10. Analysis.
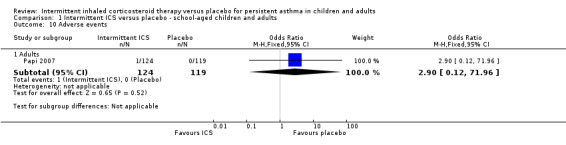
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 10 Adverse events.
2.9. Analysis.
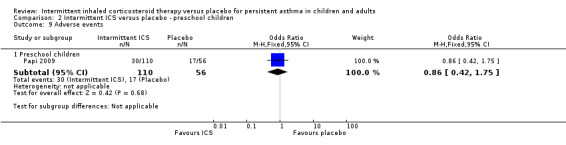
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 9 Adverse events.
2.10. Analysis.
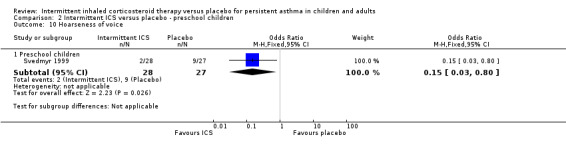
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 10 Hoarseness of voice.
2.11. Analysis.
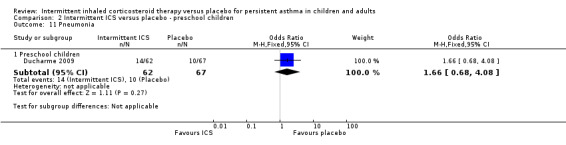
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 11 Pneumonia.
1.11. Analysis.
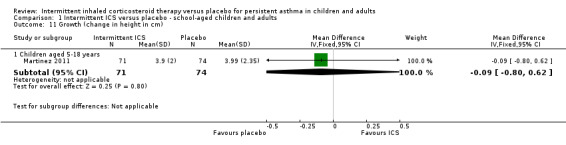
Comparison 1 Intermittent ICS versus placebo ‐ school‐aged children and adults, Outcome 11 Growth (change in height in cm).
2.12. Analysis.
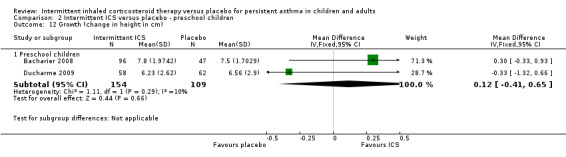
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 12 Growth (change in height in cm).
2.13. Analysis.
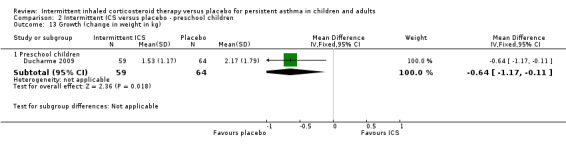
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 13 Growth (change in weight in kg).
Discussion
Summary of main results
One paediatric and one adult asthma study contributed the major findings in the assessment of intermittent ICS use against placebo, used alongside short‐acting bronchodilator therapy. Compared to no treatment, the presented approach of using intermittent ICS at the onset of symptoms, reduced the odds of exacerbation requiring oral corticosteroid by approximately half. The greatest benefit was observed in a study of adults with mild asthma if they were prescribed intermittent ICS before experiencing regular symptoms (Papi 2007), however there was greater uncertainty about these results. Interestingly, as‐needed ICS treatment in the same study was associated with fewer participants experiencing even milder exacerbations during the study period (Analysis 1.3).
The primary efficacy outcome was supported by improvements in lung function in adults favouring intermittent ICS use. The absence of significant overall adverse events and withdrawals for any reason was also observed. The rate of exacerbations requiring oral steroid (21%) and hospitalisation (nil recorded) in the control group indirectly confirms the categorisation of the asthma as mild. There was no significant difference in the rate of serious adverse events noted with the use of intermittent ICS. There was no statistically significant difference when comparing the rate of hospitalisations, although overall the number of events in either the treatment or placebo group was small.
Four studies in preschool children with wheezing symptoms were included for comparison in this systematic review and found similar results to the main analysis. One study conducted in preschool children, reported a mean difference in quality of life of 3.28 units in favour of the ICS group by the end of the 12 month period (Bacharier 2008). The change in PedsQL total scale score was based on parental proxy reports and represents improved perceptions of the child's physical, mental and social health. This was associated with improvements in both day time and night time symptom score. However, neither the number of respiratory tract infections nor the proportion of episode‐free days per participant in this study varied significantly between the treatment and placebo groups (3.7 versus 3.6 and 0.76 vs 0.74 respectively); nor could we compare this against other measures of clinical change (e.g. lung function).
Overall completeness and applicability of evidence
The review authors suggest that more research is needed in order to confirm the role of intermittent ICS as a way to prevent or manage early symptoms in patients with mild but persistent asthma. At present, there remains some uncertainty about which subgroup of patients this will be most suited for. As demonstrated by comparing results using a random effects model, the true effect size may vary from one study to the next. Although it appears that therapy may reduce the number of events requiring courses of oral corticosteroid treatment, it remains unclear whether short‐term high dose versus as‐needed low dose, or spacer versus nebuliser delivery is the best mode for providing optimal benefits. Similarly, it is not yet established if treatment is a means to improve overall asthma control as well as reduce exacerbation severity. Longer term and pragmatic design studies would help to assess the influence of individual medical adherence and patient illness beliefs.
Although several classifications of wheeze phenotypes have been proposed so far in preschool children, each has its own limitations in predicting risk or remission or persistence. Because of the difficulty in predicting long‐term outcomes with the group of preschool children with wheeze, this remains an area of ongoing uncertainty and debate. We therefore emphasised inclusion of participants based on current symptoms at the time and based on clinical judgement. These findings raise the question whether all children with more frequent symptoms and the need for rescue systemic treatment, as a marker of moderate exacerbation, can be managed in the same way.
Quality of the evidence
The strength of this review process was the methods in which studies were identified and data extracted by two review authors independently. Inclusion and exclusion criteria were clearly specified to provide a standardised way of approaching search results. This included a search of both published reports from international journals as well as information available on online clinical trial registries. This was to minimise the number of trials that had been missed or did not have published results (risk of publication bias). We contacted study authors and pharmaceutical companies to request additional study information to better assess risk of bias and include data that had not been published. A limitation of this systematic review is that we only included studies that followed participants over a minimum of 12 weeks and therefore limited the number of eligible studies for comparison. However, as asthma symptoms may vary seasonally, between people and in the same person over time, we felt that because the control rate for events was relatively infrequent, shorter studies would tend to underestimate the difference in treatment effects.
The quality of findings ranks from moderate to very low across the different outcomes. The main limiting factor, with only a small number of studies included for few outcomes, is the potential impact on the size and direction of the average effect due to variations between individual studies. There were substantial differences noted between the intermittent ICS and placebo groups in some of the studies. This is useful to bear in mind given that a greater number of control participants withdrew due to lack of perceived efficacy, which would tend to underestimate the overall measured effects of treatment. See the Table 1 and Table 2 for further details.
Potential biases in the review process
Randomisation and blinding were used in all studies, however a minority of methods described appointment of a separate system to conceal allocation of upcoming treatment assignments. There were no identified issues in the selective reporting of outcomes. Baseline predicted lung function and exposure to cigarette smoke varied across the studies, and in one study participants who were receiving a stable dose of sodium cromoglycate prior to the study commencement were permitted to continue using this. Nonetheless, in general the groups within each study were well matched for characteristics at baseline. There were no other clear sources of potential bias identified.
Limited subgroup analyses were conducted because of the small number of studies that were identified for this review. All studies included participants deemed to have mild disease, except for two studies which included the diagnostic criteria of preschool children with moderate to severe intermittent wheezing (Bacharier 2008; Ducharme 2009). Sensitivity analyses did not reveal significant changes in result heterogeneity when investigating this or for studies using concomitant medication or of shorter study duration.
Agreements and disagreements with other studies or reviews
To our knowledge, this systematic review is the first to examine the efficacy and safety of use of intermittent ICS for children and adults with persistent asthma and to include new, relevant studies for preschool children with moderate to severe wheezing as a separate analysis.
Our findings support the outcomes of the Cochrane Review, Chauhan 2013 suggesting that intermittent and daily ICS strategies may be similarly effective in reducing the use of rescue oral corticosteroids. In addition, there also appears to be no increase in the rate of severe or treatment‐related adverse effects compared with as‐required beta2‐agonist reliever therapy.
Authors' conclusions
Implications for practice.
Based on limited evidence, a reduction in the risk of asthma exacerbations requiring oral corticosteroids was noted with the use of intermittent ICS initiated at the time of exacerbation to manage school‐aged children, adolescents and adults with mild persistent asthma and preschoolers with wheezing. This is supported by improvements in lung function in adults, as well as day and night time asthma symptoms and parent‐perceived quality of life in preschool children with moderate to severe wheezing. However, there were no differences in hospitalisations noted in any groups. There were no signs of growth suppression in terms of change in height, or other obvious safety concerns. These conclusions were derived from only a small number of studies and it is uncertain if there is greater benefit in particular subgroups of patients.
The challenge will be to add recommendations to the existing treatment guidelines that have adopted a well‐defined step‐wise scheme for managing asthma symptoms. This would need an open approach towards the class of ICS as being both suitable for short‐term as well as regular, preventative use for more severe cases. In addition, data on cost‐effectiveness and healthcare utilisation would need to support the implementation over established alternatives (i.e. low dose regular ICS and leukotriene receptor antagonists (LTRA)). Fundamentally, encouraging people with asthma to use two medications, instead of one, in the absence of immediate significant symptomatic relief, may be difficult. The data on preschool wheeze, without a confirmed diagnosis of asthma, is unlikely to weigh heavily on clinical decisions made for older children and adults, although this may provide a further option for children without interval symptoms, not already on regular preventer therapy, to minimise the potential adverse effects from repeated doses of oral corticosteroids.
Implications for research.
This review has highlighted several possible areas for further study. Further RCTs using several different types of ICS would be helpful to confirm the main findings of this review. In addition, there is a need to conduct trials to be able to demonstrate the likely advantages and challenges to patients in routine clinical practice settings. Longer duration studies would also help to illustrate the stability of benefits over time and better explain the nature of the effect on the exacerbations that do occur. More research into combined inhaler of bronchodilator and corticosteroid would be necessary along with a direct comparison with oral anti‐leukotrienes.
In terms of outcomes, quality of life, hospitalisations, withdrawals and adverse events should be reported in all studies. There also remains some inconsistency in terms of terminology used to describe preschool children with persistent wheezing symptoms.
In practice, patients may not take their medications as directed and may have other co‐morbid medical conditions. The use of new technologies such as data loggers may offer additional feedback to both patients and health practitioners to identify the early signs of an exacerbation and better monitor their long term condition.
Acknowledgements
We acknowledge Dr Emma Welsh, Dr Christopher Cates and Mrs Elizabeth Stovold of the Cochrane Airways Group for their advice and support. We are thankful to Gabriele Nicolini, CHIESI FARMACEUTICI S.p.A., Italy and Professor Leonard B. Bacharier for providing additional data and confirmation of methodology assessment upon request.
Rebecca Normansell was the Editor for this review and commented critically on the review.
The background and methods section of this review is based on a standard template used by Cochrane Airways Group.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group's Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Cochrane Register of Studies (CRS) search
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 intermittent*
#6 as‐needed*
#7 "as needed"
#8 prn
#9 irregular*
#10 occasional*
#11 sporadic*
#12 short‐course*
#13 pre‐emptive or preemptive
#14 prevent*:TI,AB
#15 ((steroid* or corticosteroid*) NEAR (inhal*)) OR ICS:TI,AB
#16 fluticasone* or budesonide* or beclomethasone or beclometasone or flunisolide or mometasone or ciclesonide or triamcinolone
#17 #14 and (#15 or #16)
#18 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 OR #13 OR #17
#19 #4 and #18
[Note: in search line #1 MISC1 refers to the field in which the record has been coded for condition, in this case, asthma]
Appendix 3. Clinicaltrial.gov and WHO trial registry search
#1 asthma
#2 wheeze
#3 #1 or #2
#4 required
#5 intermittent
#6 needed
#7 PRN
#8 rescue
#9 #4 or #5 or #6 or #7 or #8
#10 #3 and #9
Data and analyses
Comparison 1. Intermittent ICS versus placebo ‐ school‐aged children and adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations requiring oral corticosteroids | 2 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.26, 0.94] |
| 1.1 Children aged 5‐18 years | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.12] |
| 1.2 Adults | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.95] |
| 2 Serious adverse events | 2 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.25] |
| 2.1 Children aged 5‐18 years | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.55] |
| 2.2 Adults | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 72.54] |
| 3 Participants with one or more exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Adults | 1 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.61] |
| 4 Hospitalisations (all cause) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Children aged 5‐18 years | 1 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Day time asthma score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Adults | 1 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.2 [‐0.57, 0.17] |
| 6 Night time asthma score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Adults | 1 | 240 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.31, 0.45] |
| 7 Withdrawals (total) | 2 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 7.1 Children aged 5‐18 years | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 1.01] |
| 7.2 Adults | 1 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.61, 2.39] |
| 8 Morning PEFR (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Adults | 1 | 230 | Mean Difference (IV, Fixed, 95% CI) | 9.12 [0.19, 18.05] |
| 9 FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Adults | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.05, 0.21] |
| 10 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Adults | 1 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 71.96] |
| 11 Growth (change in height in cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Children aged 5‐18 years | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.80, 0.62] |
| 12 Exacerbations requiring oral corticosteroids ‐ random effects model | 2 | 385 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.13, 1.48] |
| 12.1 Children aged 5‐18 years | 1 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.12] |
| 12.2 Adults | 1 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.95] |
Comparison 2. Intermittent ICS versus placebo ‐ preschool children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations requiring oral corticosteroids | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Preschool children | 4 | 490 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.31, 0.73] |
| 2 Serious adverse events | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Preschool children | 3 | 439 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.02] |
| 3 Quality of life (PedsQL total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Preschool children | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | 3.28 [‐2.13, 8.69] |
| 4 Participants with one or more exacerbations | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Preschool children | 2 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.33, 1.20] |
| 5 Hospitalisations (all cause) | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Preschool children | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.23, 2.29] |
| 6 Day time asthma score | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Preschool children | 3 | 364 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.57, ‐0.13] |
| 7 Night time asthma score | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Preschool children | 3 | 364 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.50, ‐0.06] |
| 8 Withdrawals (total) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Preschool children | 4 | 493 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.19] |
| 9 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Preschool children | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.75] |
| 10 Hoarseness of voice | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Preschool children | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.80] |
| 11 Pneumonia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Preschool children | 1 | 129 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.68, 4.08] |
| 12 Growth (change in height in cm) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Preschool children | 2 | 263 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.41, 0.65] |
| 13 Growth (change in weight in kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Preschool children | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.17, ‐0.11] |
| 14 Exacerbations requiring oral corticosteroids ‐ subgroup analysis in preschool children | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 High dose inhaled therapy | 2 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.25, 0.83] |
| 14.2 Nebulised therapy | 2 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.27, 0.92] |
| 15 Serious adverse events ‐ subgroup analysis in preschool children | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 High dose inhaled therapy | 1 | 129 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.13, 1.52] |
| 15.2 Nebulised therapy | 2 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.11, 1.39] |
2.4. Analysis.
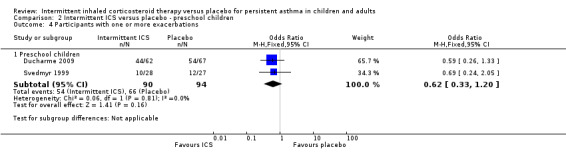
Comparison 2 Intermittent ICS versus placebo ‐ preschool children, Outcome 4 Participants with one or more exacerbations.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bacharier 2008.
| Methods |
Design: randomised, double‐blind placebo‐controlled twelve‐month trial. Setting: 5 clinical centres monitored by the CARE Network Data and Safety Monitoring Board. Date of study: recruited from February 2004 and October 2004. |
|
| Participants |
Participants: 238 randomised (96 received budesonide: 47 received placebo (conventional therapy)) Baseline characteristics: mean age (intermittent ICS: 3.06 years, placebo 2.98 years; range 1‐ 4.92 years); gender ‐ male (intermittent ICS 72.9%, placebo 48.9%); exposure to tobacco smoke (intermittent ICS 4.2%, placebo 1.7%), withdrawal (including dropouts and treatment failures) (intermittent ICS 14, placebo 8). Diagnostic criteria: Preschool children with moderate to severe intermittent wheezing. Asthma severity: N/A Inclusion criteria: Age 12‐59 months, experienced at least two episodes of wheezing in the context of respiratory tract infection (RTI) within the past year (one episode within past six months and one documented by healthcare provider), two urgent care visits for acute wheezing within the past year, or two wheezing episodes for which oral corticosteroids were prescribed, or one episode requiring urgent care and one episode requiring oral corticosteroids. Exclusion criteria: Over the past year, received > 6 courses of oral corticosteroids, hospitalised more than twice for wheezing, or used asthma controller medications (including inhaled corticosteroids (ICS), leukotriene receptor antagonists (LRTA), cromolyn/nedocromil, or theophylline) for 4 or more months cumulative or within the preceding 2 weeks, before 36 weeks gestational age, presence of other significant lung or other medical conditions, gastroesophageal reflux under medical therapy, current antibiotic use for sinusitis, or history of life threatening wheezing episode. |
|
| Interventions |
Interventions: (7‐day course of the study medication at onset of the individualised set of symptoms.) 1. Budesonide group [budesonide inhalation suspension (1.0 mg twice daily) and placebo LTRA once daily]. 2. Conventional therapy group [placebo ICS twice daily and placebo LTRA once daily]. 3. Montelukast group [montelukast (4mg once daily) and placebo ICS twice daily]. Concomitant medication Nil |
|
| Outcomes |
Primary outcome(s): Proportion of episode‐free days (EFD) Secondary outcome(s): 1. Severity of lower respiratory tract symptoms as reflected by the area under the curve (AUC) for symptom scores. 2. Time to initiation of the first course of oral corticosteroids. 3. Total number of oral corticosteroid courses. 4. Number of wheezing episodes. 5. Days missed from daycare and parental work. 6. Caregiver quality of life. 7. Number of unscheduled visits for acute wheezing episodes (primary care office, urgent care, and ED/hospitalisation). 8. Linear growth. |
|
| Notes |
Funding: Nil external. Clinicaltrials.gov study code NCT00000622 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children between the ages of 12 and 59 months who satisfied the eligibility criteria during the run‐in period were randomised to one of three treatment arms (placebo (conventional therapy), ICS, or LTRA), with clinical centre, age (12‐23 months or 24‐59 months), and atopic status (negative API or positive API) serving as stratifying variables. Permuted block sizes of 3 children will be used within each stratum |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated to be a double blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated to be a double blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Both treatment groups had a withdrawal rate of between 10‐20% and were similar. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported |
| Other bias | Unclear risk | A greater proportion of participants in the exposure group were male and were exposed to tobacco smoke at home or daycare. |
Ducharme 2009.
| Methods |
Design: 6 to 12 months, randomised, parallel‐group, triple blind (parents, physicians and nurses, and biostatisticians), placebo‐controlled trial. Pre‐emptive study. Setting: 5 Institutions in Quebec Date of study: November 1999 to April 2005 |
|
| Participants |
Participants: n = 184 (62 received fluticasone: 67 received placebo) Baseline characteristics: mean age (intermittent ICS: 2.60 years, placebo 2.86 years; range 1‐6 years); gender ‐ male (intermittent ICS 52% to placebo 69%); exposure to tobacco smoke (intermittent ICS 23%, placebo 21%). Diagnostic criteria: children with moderate to severe virus‐induced wheezing. Asthma severity: N/A Inclusion criteria: Children aged 1 to 6 years of age, had three or more wheezing episodes in lifetime, [seemingly triggered exclusively by upper respiratory tract infections; if they had no intercurrent symptoms], received at least one course of rescue systemic corticosteroids in previous 6 months (or two in the preceding 12 months), if parents were fluent in French or English. Exclusion criteria: prior intubation for a respiratory illness, neonatal respiratory conditions, other chronic diseases, suspected allergic rhinitis, and allergies to aeroallergens, documented by a positive skin test or elevated specific IgE levels. |
|
| Interventions |
Run‐in period: 7‐14 days to confirm the recent use of systemic corticosteroids and the absence of intercurrent symptoms and beta2‐agonist use. This period started 21 days after the last dose of maintenance asthma medication or oral corticosteroid was given. Interventions: 1. Three inhalations of 250ug of fluticasone propionate twice daily following first sign of URTI until 48 hours had elapsed with no symptoms of cough or wheezing 2. Three inhalations of placebo twice daily following first sign of URTI Concomitant medication Nil |
|
| Outcomes |
Primary outcome(s): Rescue oral corticosteroid use. Secondary outcome(s):
|
|
| Notes |
Funding: GlaxoSmithKline (Canada), Réseau en Santé Respiratoire du Fonds de la Recherche en Santé du Québec; the Fonds de la Recherche en Santé du Québec. Clinicaltrials.gov study code NCT00238927 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised procedure was adopted to prepare randomisation in permuted blocks of four, stratified according to centre (of which there were five) and type of spacer (mouthpiece or mask. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes contained the assignment code. Coded metred‐dose inhaler containing placebo, which looked identical in all respects to the fluticasone inhaler (GlaxoSmith‐ Kline). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple blinding (parents, physicians and nurses, and biostatisticians). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple blinding (parents, physicians and nurses, and biostatisticians). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition rate however intention to treat analysis (19% in the intervention group and 34% in the control group) . Reasons for withdrawals were reported per group. |
| Selective reporting (reporting bias) | Low risk | No apparent bias was noted. |
| Other bias | Unclear risk | Over a median period of 40 weeks. On the recommendation of an independent advisory board, the study period was extended from 6 months to 12 months because of slow recruitment |
Martinez 2011.
| Methods |
Design: 44‐week, randomised, double‐blind, placebo‐controlled trial Setting: 5clinical centres in the USA: Denver, CO; Madison, WI; Saint Louis, MO; San Diego, CA; and Tucson, AZ (satellite centres in Milwaukee, WI, and Albuquerque, NM, also recruited participants). Date of study: January 2007 to May 2009 |
|
| Participants |
Participants: n = 288 (58 received rescue beclomethasone: 50 received placebo) Baseline characteristics: mean age (intermittent ICS: 10.4 years, placebo 10.4 years; range 5‐18 years); gender ‐ male (intermittent ICS 52%, placebo 55%); mean % predicted FEV1 (intermittent ICS 101.4%, placebo 100.4%); mean asthma history (mild persistent asthma during previous 2 years); mean daily dose of inhaled corticosteroids (not stated); rescue medication (intermittent ICS 0.4, placebo 0.5 puffs/day). Diagnostic criteria: US National Asthma Education and Prevention Program asthma care guidelines Asthma severity: mild persistent Inclusion criteria: Naive to controller treatment and history of one to two exacerbations in previous year, if treated for previous 8 weeks with monotherapy other than inhaled corticosteroids, if illness was controlled for the previous 8 weeks on low‐dose corticosteroids as monotherapy. No exacerbations during run in period. Exclusion criteria: pre‐bronchodilator forced expiratory volume in 1 s (FEV1) of less than 60% predicted at the first visit; admitted to hospital for asthma in previous year; asthma exacerbation in the previous 3 months or more than two in previous year; history of life‐threatening asthma exacerbations that required intubation or mechanical ventilation, or that resulted in a hypoxic seizure. |
|
| Interventions |
Run‐in period: twice daily treatment with one puff of beclomethasone dipropionate and rescue treatment with a placebo inhaler added to rescue albuterol every time they needed albuterol. Interventions: 1. twice daily placebo with beclomethasone plus albuterol as rescue (rescue beclomethasone group); and 2. twice daily placebo with placebo plus albuterol as rescue (placebo group) Concomitant medication Nil |
|
| Outcomes |
Primary outcome(s): time to first exacerbation that required treatment with prednisone Secondary outcome(s):
Criteria for assigning treatment failure during treatment period. a. Hospitalization due to asthma. b. Hypoxic seizure due to asthma. c. Intubation due to asthma. d. Requirement for a second burst of prednisone within any 6 month period. e. Significant adverse event related to the use of a study medication. The only criterion for assignment of treatment failure during the trial was the requirement for a second burst of prednisone within any 6 month period. |
|
| Notes |
Funding: this study was funded by National Heart, Lung and Blood Institute. Clinicaltrials.gov study code NCT00394329 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Data Coordinating Center (DCC; Penn State Hershey College, PA, USA) generated the random allocation sequence. |
| Allocation concealment (selection bias) | Low risk | When a clinical centre deemed that a participant was eligible for randomisation, the clinical centre coordinator logged onto the secure CARE Network website, entered the relevant information to confirm participant eligibility, and received the appropriate drug packet code to be assigned to the participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drug groups were labelled as A, B, C, and D to mask statisticians to treatment group during the first complete run‐through of data analyses. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The DCC had no interaction with participants,but was responsible for management of data and statistical analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | May tend to underestimate results. 13 withdrawals (out of 71) in the intermittent ICS group, compared with 24 (out of 74) in the placebo group. A greater number, 17 of treatment failures in the placebo group compared with 6 in the intermittent ICS group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil identified |
Papi 2007.
| Methods |
Design: 6 month, double blind, double dummy, parallel group randomised controlled trial Setting: Multinational, multicenter in 25 centres Date of study: August 2002 to September 2004 |
|
| Participants |
Participants: n = 466 (124 received as‐needed combination therapy: 119 received as‐needed albuterol therapy) Baseline characteristics: mean age (intermittent ICS: 36.8 years, placebo 40.6 years; recruitment of patients between ages of 18‐65 years); gender ‐ male (intermittent ICS 50%, placebo 49%); mean % predicted FEV1 (intermittent ICS 88.5%, placebo 88.9%); mean asthma history (not stated); mean daily dose of inhaled corticosteroids (intermittent ICS 458.8 μg of beclomethasone equivalent, placebo 468.6 μg); rescue medication (intermittent ICS 0.4, placebo 0.5 puffs/day). Diagnostic criteria: National Asthma Education and Prevention Program guidelines Asthma severity: mild persistent asthma Inclusion criteria: History of mild persistent asthma for at least 6 months, prebronchodilator FEV1 >75% associated with ≥ 12% reversibility or positive methacholine challenge. Exclusion criteria: Current or ex‐smoker, diagnosis of COPD, or history of near‐fatal asthma and/or admission to emergency room/intensive care unit because of asthma, 3 or more courses of oral corticosteroids or hospitalisation for asthma during the previous year, or regular treatment for > 6 months with ≥ 500 μg/day of beclomethasone or equivalent. |
|
| Interventions |
Run‐in period: 250 μg of inhaled beclomethasone dipropionate twice daily and albuterol on an as‐needed basis for the relief of symptoms Interventions: Patients were not given a written plan of action to guide the as‐needed use of study drugs but were simply instructed orally to use them any time they were needed for relief of symptoms. 1. placebo twice daily plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler as‐needed (as‐needed combination therapy) 2. placebo twice daily plus 100 μg of albuterol as needed (as‐needed albuterol therapy) Concomitant medication Nil |
|
| Outcomes |
Primary outcome(s): mean rate of morning peak expiratory flow during weeks 23 and 24. Secondary outcome(s): 1. other functional variables; 2. symptom scores; 3. number and severity of exacerbations. "A mild exacerbation was defined as awakening at night owing to asthma or as a decrease in the morning peak expiratory flow rate to more than 20% below the baseline value, the use of more than three additional puffs per day of rescue medication (either albuterol or beclomethasone and albuterol) as compared with during the baseline period (the last week of the run‐in period) for 2 or more consecutive days, or both. Single, isolated days on which mild exacerbation occurred were not counted. A severe exacerbation was defined as a decrease in the morning peak expiratory flow rate to more than 30% below the baseline value on 2 consecutive days or more than eight puffs per day of rescue medication for 3 consecutive days or the need for treatment with oral corticosteroids, as judged by the investigator. Days on which severe exacerbations occurred were excluded from the count of days with mild exacerbations". |
|
| Notes |
Funding: this study was funded by Chiesi Farmaceutici Clinicaltrials.gov study code NCT00382889 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was prepared with the use of a random‐number generator and a balanced‐block design stratified according to centre |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Well balanced attrition rate. Reasons for withdrawals were reported per group. Intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | No apparent risk of bias noted |
| Other bias | Low risk | No apparent risk of bias noted |
Papi 2009.
| Methods |
Design: 12 weeks, randomised, parallel‐group, three‐group, double blind, double‐dummy, placebo‐controlled trial Setting: Multicenter (19 paediatric specialist care units) Date of study: March 2006 to January 2007 |
|
| Participants |
Participants: n = 276 (110 received as‐needed combination: 56 received as‐needed salbutamol) Baseline characteristics: mean age (intermittent ICS: 2.26 years, placebo 2.29 years; range 1‐4 years); gender ‐ male (intermittent ICS 61.8% to placebo 60.7%); daytime symptom score (intermittent ICS 1.37, placebo 1.23); night time symptom score (intermittent ICS 1.00, placebo 1.00); asthma history (not stated); mean daily dose of inhaled corticosteroids (not stated); daytime rescue medication (intermittent ICS 0.26, placebo 0.25 no. of uses), night time rescue medication (intermittent ICS 0.16, placebo 0.17 no. of uses). Diagnostic criteria: preschool children with frequent wheeze. Asthma severity: N/A Inclusion criteria: children aged 1–4 years, frequent wheezing (documented history of at least three episodes of wheezing requiring medical attention in the previous 6 months), after run‐in period (wheeze and/or cough, and/or shortness of breath, and/or required relief medication on at least 7 days of the 2‐week run‐in period) Exclusion criteria: children with a history of severe exacerbations requiring systemic glucocorticoid, chest infection, hospitalisation for asthma, treatment with inhaled glucocorticoids or methyl‐xanthine during the previous 4 weeks or with oral glucocorticoid in the previous 8 weeks. |
|
| Interventions |
Run‐in period: nebulised placebo bid plus 2500 μg prn nebulised salbutamol for symptom relief. Interventions: 1. Prn combination group beclomethasone/salbutamol): placebo 1 vial bid, plus fixed combination of beclomethasone and salbutamol, 800 μg beclomethasone + 1600 μg salbutamol/vial, one vial prn 2. Prn salbutamol group: placebo one vial bid plus salbutamol 2500 μg/vial, one vial prn. Concomitant medication: Nil |
|
| Outcomes |
Primary outcome(s): percentage of symptom‐free days during the 12‐week study period. Secondary outcome(s): 1. daily (24 h), daytime and nighttime wheezing, coughing and shortness of breath score; 2. number of nocturnal awakenings (children and parents); daily (24 h); 3. daytime and night‐time number of doses of relief medication; 4. percentage of days without the need for relief medication; 5. frequency and number of exacerbations and time to first exacerbation. |
|
| Notes |
Funding: this study was funded by Chiesi Farmaceutici Clinicaltrials.gov study code NCT00497523 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Well balanced withdrawals. Primary and secondary outcomes well specified. Intention to treat analysis. |
| Selective reporting (reporting bias) | Low risk | No apparent bias was noted. |
| Other bias | Unclear risk | Occasional use of regular treatment with sodium cromoglycate was allowed prior and during the study, as long as this was a fixed dose and a separate spacer was used. |
Svedmyr 1999.
| Methods |
Design: 12 months (or maximum of 6 treatments), randomised, parallel‐group, double blind placebo‐controlled trial. Pre‐emptive study. Setting: Multicentre, (Recruited from 4 paediatric departments in Sweden) Date of study: Not reported |
|
| Participants |
Participants: n = 55 (28 received budesonide: 27 received placebo) Baseline characteristics: mean age (intermittent ICS: 2.08 years, placebo 2.17 years; range 1‐3.92 years); gender ‐ male (intermittent ICS 60.7% to placebo 77.8%). Diagnostic criteria: Doctor's diagnosis of wheezy bronchitis or asthma. Asthma severity: N/A Inclusion criteria: Children aged 1‐3 years, at least three episodes of wheezing during URTI and asthma symptoms during the last two airway infections; asthma symptoms lasted for at least 3 days during the URTI. Exclusion criteria: If the first symptom did not develop into an URTI within 24 hours, the study period was withheld. |
|
| Interventions |
Run‐in period: not stated. Interventions: 1. treatment started at the first sign of an URTI and lasted for 10 d. The dosage regimen was a nominal dose of budesonide 400 µg q.i.d. for the first 3 d and budesonide 400 µg b.i.d. for the following 7 d. 2. Placebo equivalent. Concomitant medication Treatment with additional beta2‐agonists via the spacer and/or theophylline was allowed when needed. When cromoglycate was used, the dose was fixed and a separate spacer was used. |
|
| Outcomes |
Primary outcome(s): asthma symptom scored by parents twice daily on a 4‐graded scale (0–3). Secondary outcome(s): 1. sleep disturbance; 2. use of rescue medication (oral corticosteroids); 3. need for medical care; 4. adverse events. |
|
| Notes | Funding: Unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Inadequate details were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No high attrition rate. Reasons for withdrawal were reported. The primary outcome was clearly specified. |
| Selective reporting (reporting bias) | Low risk | No apparent bias was noted. |
| Other bias | Low risk | No apparent bias was noted. |
AUC = area under the curve.
EFD = proportion of episode free days.
FEV1 = forced expiratory volume in one second.
ICS = inhaled corticosteroids.
LTRA = leukotriene receptor antagonists.
RTI = respiratory tract infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bisgaard 2006 | Treatments assigned and initiated at less than 1 year of age |
| Boushey 2005 | No placebo group |
| Durmaz 2011 | Treatments assigned and initiated at less than 1 year of age. |
| Haahtela 2006 | Excluded as formoterol is recognised as having long‐acting effects as a beta‐agonist |
| Hamada 2008 | No placebo group |
| Jenkins 2006 | No placebo group |
| Papi 2011 | Duration of follow up < 12 weeks, this is the same study at Papi 2009 with only 1 week outcomes reported |
| Turpeinen 2008 | No placebo group and crossover |
| Zeiger 2011 | No placebo group |
Differences between protocol and review
A change from the original published protocol was to consider all‐cause hospitalisations as a secondary outcome for this review, as we could not derive data for hospitalisations attributed to exacerbations consistently across studies. We also included studies allowing concomitant medication use (apart from regular ICS) if the dose of the medication was stable before and during the intervention period.
Contributions of authors
Jimmy Chong contributed to protocol development, screened the searches and identified RCTs for inclusion, extracted data and wrote the review.
Cheyaanthan Haran screened the searches and identified RCTs for inclusion, extracted data, assisted in checking and write‐up of the review.
Bhupendrasinh Chauhan assisted in refinement of the protocol, data extraction, results interpretation, and write‐up of the review.
Innes Asher was involved in protocol initiation and development, result interpretation and write‐up of the review.
Sources of support
Internal sources
The authors declare that no funding from internal sources was received for this systematic review, Other.
External sources
The authors declare that no funding from external sources was received for this systematic review, Other.
Declarations of interest
Jimmy Chong: none to declare. Cheyaanthan Haran: none to declare. Bhupendrasinh Chauhan: none to declare. Innes Asher: none to declare.
New
References
References to studies included in this review
Bacharier 2008 {published data only}
- Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate‐to‐severe intermittent wheezing. Journal of Allergy and Clinical Immunology 2008; Vol. 122, issue 6:1127‐35.e8. [DOI] [PMC free article] [PubMed]
Ducharme 2009 {published data only}
- Ducharme F, Lemire C, Noya F, Davis GM, Alos N, Leblond H, et al. Preemptive use of high‐dose fluticasone for virus‐induced wheezing in young children. New England Journal of Medicine 2009;360(4):339‐353. [DOI] [PubMed] [Google Scholar]
- Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Randomized controlled trial of intermittent high‐dose fluticasone versus placebo in young children with viral‐induced asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2007;175:[A958]. [Google Scholar]
- Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Safety of intermittent high‐dose fluticasone vs. placebo in young children with viral‐induced asthma: a multicenter randomized controlled trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2007;175:[A958]. [Google Scholar]
Martinez 2011 {published data only}
- Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double‐blind, placebo‐controlled trial. Lancet 2011; Vol. 377, issue 9766:650‐7. [DOI] [PMC free article] [PubMed]
Papi 2007 {published data only}
Papi 2009 {published data only}
- Papi A, Nicolini G, Baraldi E, Boner AL, Cutrera R, Rossi GA, et al. Regular vs prn nebulized treatment for wheeze preschool children [Abstract]. American Journal of Respiratory and Critical Care Medicine 2009; Vol. A4818:[Poster #E34].
- Papi A, Nicolini G, Baraldi E, Boner AL, Cutrera R, Rossi GA, et al. Regular vs prn nebulized treatment in wheeze preschool children. Allergy 2009; Vol. 64, issue 10:1463‐71. [DOI] [PubMed]
Svedmyr 1999 {published data only}
References to studies excluded from this review
Bisgaard 2006 {published data only}
- Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. New England Journal of Medicine 2006;354(19):1998‐2005. [DOI] [PubMed] [Google Scholar]
Boushey 2005 {published data only}
- Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as‐needed corticosteroids for mild persistent asthma. New England Journal of Medicine 2005;352(15):1519‐28. [1533‐4406] [DOI] [PubMed] [Google Scholar]
Durmaz 2011 {published data only}
- Durmaz C, Asilsoy S, Usta Guc B. Efficacy of nebulised budesonide in infants with viral wheezing [Abstract]. Allergy 2011;66(s94):563. [Google Scholar]
Haahtela 2006 {published data only}
- Haahtela T, Tamminen K, Malmberg LP, Zetterström O, Karjalainen J, Ylä‐Outinen H, et al. Formoterol as needed with or without budesonide in patients with intermittent asthma and raised NO levels in exhaled air: A SOMA study. European Respiratory Journal 2006;28(4):748‐55. [DOI] [PubMed] [Google Scholar]
- Haahtela T, Tamminen K, Malmberg P, Karjalainen J, Yia‐Outinen H, Zetterstrom O, et al. As‐needed treatment with a b2‐agonist/ corticosteroid combination in mild intermittent asthma (SOMA) [Abstract]. European Respiratory Journal 2005;26:Abstract No. 1722. [Google Scholar]
Hamada 2008 {published data only}
- Hamada K, Yasuba H, Tanimura K, Hiramatu M, Kobayashi Y, Kita H. How can we stop ICS? Risk control therapy by as needed inhaled fluticasone after stepping down [Abstract]. Journal of Allergy and Clinical Immunology 2008;121:S219 [844]. [Google Scholar]
Jenkins 2006 {published data only}
- Jenkins C, Belousova E, Marks G, Reddel H. Intermittent or continuous inhaled corticosteroids for mild asthma?. Respirology (Carlton, Vic.) 2006;11(Suppl 2):Abstract no. 70. [Google Scholar]
Papi 2011 {published data only}
- Papi A, Nicolini G, Boner AL, Baraldi E, Cutrera R, Fabbri LM, et al. Short term efficacy of nebulized beclomethasone in mild‐to‐moderate wheezing episodes in pre‐school children. Italian Journal of Pediatrics 2011;37:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turpeinen 2008 {published data only}
- Turpeinen M, Nikander K, Pelkonen AS, Syvanen P, Sorva R, Raitio H, et al. Daily versus as‐needed inhaled corticosteroid for mild persistent asthma (The Helsinki early intervention childhood asthma study). Archives of Disease in Childhood 2008;93(8):654‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpeinen M, Pelkonen AS, Nikander K, Sorva R, Selroos O, Juntunen‐Backman K, et al. Bone mineral density in children treated with daily or periodical inhaled budesonide: The Helsinki early intervention childhood asthma study. Pediatric Research 2010;68(2):169‐73. [DOI] [PubMed] [Google Scholar]
Zeiger 2011 {published data only}
- Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. New England Journal of Medicine 2011;365(21):1990‐2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barnes 1998
- Barnes P. Efficacy of inhaled corticosteroids in asthma. Journal of Allergy and Clinical Immunology 1998;102:531‐8. [DOI] [PubMed] [Google Scholar]
Bender 2002
- Bender B. Overcoming barriers to non adherence in asthma treatment. Journal of Allergy and Clinical Immunology 2002;119:916‐23. [DOI] [PubMed] [Google Scholar]
Bender 2003
- Bender B, Milgrom H, Apter A. Adherence intervention research: what have we learned and what do we do next?. Journal of Allergy and Clinical Immunology 2003;112:489‐94. [DOI] [PubMed] [Google Scholar]
Brand 2008
- Brand PL, Baraldi E, Bisgaard H, Boner A, Castro‐Rodriguez J, Custovic A, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence‐based approach. European Respiratory Journal 2008;32(4):1096‐110. [DOI] [PubMed] [Google Scholar]
Brussee 2004
BTS 2012
- British Thoracic Society. British guidelines on the management of asthma, 2012. www.brit‐thoracic.org.uk/Guidelines/Asthma‐Guidelines.aspx (accessed 5 December 2013).
Burgess 2008
- Burgess S, Sly P, Morawska A, Devadason S. Assessing adherence and factors associated with adherence in young children with asthma. Respirology 2008;13:559‐63. [DOI] [PubMed] [Google Scholar]
Bush 2014
- Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. BMJ 2014;348:g15. [1756‐1833] [DOI] [PubMed] [Google Scholar]
Chauhan 2013
- Chauhan B, Chartrand C, Ducharme F. Intermittent versus daily inhaled corticosteroids for persistent asthma in children and adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009611.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducharme 2014
Foresi 2000
- Foresi A, Morelli M, Catena E. Low‐dose budesonide with the addition of an increased dose during exacerbations is effective in long‐term asthma control. Chest 2000;117:440‐6. [DOI] [PubMed] [Google Scholar]
Garner 2008
- Garner R, Kohen D. Changes in the prevalence of asthma among Canadian children. Health Reports 2008; Vol. 19, issue 2:45‐50. [0840‐6529] [PubMed]
GINA 2014
- Global Initiative for Asthma (GINA). From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014.pdf (accessed 24 October 2014).
Global Asthma Report 2014
- Global Asthma Network. The Global Asthma Report 2014. http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf (accessed 24 March 2015).
GRADE 2012 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro Version 3.6 for Windows. GRADE Working Group, 2012.
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions. Available from www.cochrane‐handbook.org.
Horak 2003
- Horak E, Lanigan A, Roberts M, Welsh L, Wilson J, Carlin JB, et al. Longitudinal study of childhood wheezy bronchitis and asthma: outcome at age 42. BMJ 2003; Vol. 326, issue 7386:422‐3. [0959‐8138] [DOI] [PMC free article] [PubMed]
Kelly 2012
- Kelly H, Sternberg A, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. New England Journal of Medicine 2012;367(10):904‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2009
- Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64:476‐83. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 1995
McKean 2000
- McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pauwels 1997
- Pauwels R, Lofdahl C, Postma D, Tattersfield A, O'Byrne P, Barnes P, et al. Effect of inhaled formoterol and budesonide on exacerbations in asthma. New England Journal of Medicine 1997;337(20):1405‐11. [DOI] [PubMed] [Google Scholar]
Pauwels 2003
- Pauwels R, Pedersen S, Busse W, Tan W, Chen Y, Ohlsson S, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double‐blind trial. Lancet 2003;361:1071‐6. [DOI] [PubMed] [Google Scholar]
Pearce 2007
- Pearce N, Aїt‐Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007;62:757‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phelan 2002
Pruteanu 2014
- Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose‐response effects on growth. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009878.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 2005
- Rand C. Non‐adherence in asthma therapy: more than just forgetting. Journal of Pediatrics 2005;146:157‐9. [DOI] [PubMed] [Google Scholar]
Rand 2007
- Rand C, Bilderback A, Schiller K, Edelman J, Hustad C, Zeiger R. Adherence with montelukast or fluticasone in a long‐term clinical trial: results from the mild asthma montelukast versus inhaled corticosteroid trial. Journal of Allergy and Clinical Immunology 2007;119(4):916‐23. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2012
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sharek 1999
- Sharek P, Bergman D, Ducharme F. Beclomethasone for asthma in children: effects on linear growth. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
The Childhood Asthma Program Research Group 2000
- The Childhood Asthma Management Program Research Group. Long‐term effects of budesonide or nedocromil in children with asthma. New England Journal of Medicine 2000;343(15):1054‐63. [DOI] [PubMed] [Google Scholar]
Vos 2013
- Vos T, Flaxman A, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2013;380(9859):2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Axelsson I, Chung, M, Lau J. Dose response of inhaled corticosteroids in children with persistent asthma: a systematic review. Pediatrics 2011;127(1):129‐38. [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009471.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


